Abstract
Currently, there are few reports on the isolation of microorganisms from goat milk and goat cheese that have antibacterial activity. In particular, there are no reports on the isolation of microorganisms with antibacterial activity from these products in central Mexico. Our objective was to isolate bacteria, from goat products, that synthesized antimicrobial peptides with activity against a variety of clinically significant bacteria. We isolated and identified Lactobacillus rhamnosus, L. plantarum, L. pentosus, L. helveticus and Enterococcus faecium from goat cheese, and Aquabacterium fontiphilum, Methylibium petroleiphilum, Piscinobacter aquaticus and Staphylococcus xylosus from goat milk. These bacteria isolated from goat cheese were able to inhibit Staphylococcus aureus, Bacillus cereus, Escherichia coli, Listeria monocytogenes, L. inoccua, Pseudomona aeruginosa, Shigella flexneri, Serratia marcescens, Enterobacter cloacae and Klebsiella pneumoniae. In addition, bacteria from goat milk showed inhibitory activity against B. cereus, L. lactis, E. coli, S. flexneri, E. cloacae and K. pneumonia; S. aureus, L. innocua, S. agalactiae and S. marcescens. The bacteriocins produced by these isolates were shown to be acid stable (pH 2–6) and thermotolerant (up to 100 °C), but were susceptible to proteinases. When screened by PCR for the presence of nisin, pediocin and enterocin A genes, none was found in isolates recovered from goat milk, and only the enterocin A gene was found in isolates from goat cheese.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-016-0587-3) contains supplementary material, which is available to authorized users.
Keywords: Goat milk, Goat cheese, Bacteriocins, Enterocin A, Pathogenic bacteria
Introduction
Different types of commercial milk, including goat, cow and sheep milk, are produced worldwide for human consumption. Goat milk is consumed less than cow milk and represents ~2 % of the global milk source. In 2012, goat milk production worldwide was ~16.2 billion liters, with México being the largest producer (~155.5 million liters) in Latin America [1]. Although goat milk is mainly consumed as a raw product, it has been used for production of cheese and yogurt, usually at the farm level, in small dairies and in informal retail sales [2]. Recently, goat milk has gained interest mainly because of its iron bioavailability, higher concentration of fatty acids and lower allergenicity [3].
It has been reported that the microbiota in goat milk is composed primarily of Lactobacillus, Lactococcus, Leuconostoc, Enterococcus and Streptococcus species, bacteria with known probiotic and bacteriocinogenic properties. In addition, goat milk samples have been shown to harbor halophilic bacteria (e.g. Jeotgalicoccus psychrophilus and Salinicoccus sp.), though this finding may be atypical [3–5]. The isolation of potential pathogenic bacteria in raw goat milk, such as Staphylococcus aureus and Escherichia coli [6], have also been reported. The antimicrobial activity of bacteria isolated from goat milk has been linked to different compounds, including lactic acid, hydrogen peroxide, and bacteriocins (antimicrobial peptides) [7]. Bacteriocins, in particular, are ribosomally synthesized proteins with known biopreservative and antimicrobial activities. Recently, nisin, lacticin, and enterocin AS-48 were found to be produced by lactic bacteria isolated from raw milk of ewes, goats and cows, collected from different farms in Central Spain [8].
In Mexico, only a few reports on the microbiota of goats, and the physical and chemical properties of goat milk have been published [2, 9]. These include an analysis of cheese produced in a desert rangeland and activity of bacteriocins against potential etiological agents of mastitis [2, 10]. Although it has been shown that the dominant bacterial population in goat milk is lactic bacteria, the microbiota can vary depending on goat breeds, nutrition, weather conditions and animal health [11]. To our knowledge there are no reports on the isolation of microorganisms with antibacterial activity from goat milk and cheese produced in central Mexico. Here we report on the isolation of bacteria from these products, and show that they produce peptides active against a wide variety of clinically significant bacteria.
Materials and Methods
Goat Milk and Cheese Sampling
Samples of raw goat milk were collected using aseptic conditions directly from the udder of Saneen goats in dairy farms located in Apaseo el Alto, Guanajuato, México, and samples were stored at 4 °C until further testing. Goat cheese samples (natural and ash type) were obtained from local supermarkets in the state of Guanajuato, but they were manufactured in a national company located in the state of Querétaro, México. As our purpose was to isolate bacteriocinogenic bacteria, we only obtained two samples of each type of cheese. Ten grams of each cheese was aseptically collected and homogenized in 90 mL of sterile 0.85 % (w/v) NaCl and used for further microbiological analysis.
Bacterial Isolation
Milk and cheese samples were diluted (10−5–10−6) using sterile 0.85 % (w/v) NaCl, plated on MRS (Man, Rogosa and Sharpe) agar (Difco, Becton–Dickinson, Franklin Lakes, NJ, USA), MRS-1 % (w/v) glucose, MRS-1 % (w/v) lactose, and peptonized milk agar. Cultures were incubated at 37 °C for 24–72 h under both aerobic and anaerobic conditions. For primary selection, 300 colonies were selected and grown on MRS agar at 37 °C for 24 h. Then based on colony morphology, Gram stain, and catalase test, 44 colonies (15 from milk, 20 from ash style cheese and 9 from natural cheese) were selected for further study.
Preliminary Identification of Bacteria by the Overlay Method
A single colony of the 44 isolates was taken and inoculated in duplicate parallel streaks (1 cm) on MRS agar plates at 37 °C. One of the MRS plates was overlaid with trypticase soy agar (0.75 % w/v) containing 105 μL of each indicator strain with ~1 × 108 CFU/mL of the indicator bacterium and incubated at 37 °C for 24 h. Antibacterial activities were detected by the formation of zones of inhibition around the colonies. The other plate was used to select bacteriocinogenic bacteria that showed inhibitory effects in the overlay assay [12]. Indicator bacteria were: Bacillus cereus 183, Listeria monocytogenes, L. innocua, Micrococcus luteus, S. aureus and E. coli ATCC 25922 (Gram-negative bacterium commonly used as control strain for antimicrobial susceptibility test) (www.atcc.org). From the 44 colonies, we selected 12 isolates (7 from cheese and 5 from milk), which showed the relatively higher inhibitory activity against the inhibitor bacteria. These isolates were labeled as MAe1, MAe2, MAe5, LPAe1, LPAn2, LC20, LC22, MN24, MN26, MN27, MN28, and MN29 (Table S1), which were subsequently identified by sequencing of the 16S rDNA.
Bacterial Identification Amplification and Sequencing of the 16S rDNA
The twelve bacteria selected were cultivated in 3 mL of MRS at 37 °C overnight and DNA was extracted as described before [13]. Oligonucleotides UBF (F: 5′-AGAGTTTGATCCTGGCTGAG-3′) and 1492 (R: 5′- GGTTACCTTGTTACGACTT-3′) [14] were used to amplify 16S rDNA sequences with the following conditions: 5 min at 95 °C; 30 cycles of 30 s at 95 °C, 30 s at 58 °C and 90 s at 72 °C, with a final extension of 5 min at 72 °C in a C1000 Touch™ thermocycler (Bio-Rad). Amplicons were purified from agarose gels using the QIAprep Spin Miniprep Kit (250) (Qiagen) and sequenced at the National Laboratory of Genomics for Biodiversity (Langebio, at CINVESTAV-Irapuato, México). Once sequences were obtained, the ambiguous bases from the 5′ and 3′ terminal were deleted, and the resultant sequences were submitted to the GenBank nucleotide database (www.ncbi.nlm.nih.gov) for comparison.
Screening for Nisin, Enterocin A, Pediocin Genes by Polymerase Chain Reaction (PCR)
DNA from the twelve bacteria was used for PCR amplification of nisin, enterocin A and pediocin genes using Taq polymerase (Invitrogen, Carlsbad CA, USA) and gene specific primers; for nisin, nisRF (5′-CTATGAAGTTGCGACGCATCA-3′), nisRR (5′-CATGCCACTGATACCCAAGT-3′); for enterocin A, entAF (5′-GGGTACCACTCATAGTGGAA-3′); enterocin, entAR (5′-CCAGCAGTTCTTCCAATTTCA-3′); and pediocin, pedF 5′-GGTAAGGCTACCACTTGCAT-3′), pedR (5′-CTACTAACGCTTGGCTGGCA-3′) [15]. Amplification was performed in a C1000 Touch Thermal Cycler (Bio-Rad) using the following conditions: 5 min at 95 °C; 30 cycles of 30 s at 95 °C, 30 s at 58 °C and 90 s at 72 °C, with a final extension of 5 min at 72 °C. Amplicon sizes were compared in an agarose gel with those obtained from Lactococcus lactis subsp. lactis ATCC 19435 (Microbial Culture Collection, Micro 500 CINVESTAV Mexico City), Enterococcus faecium UQ1 (provided by Dr. Blanca Garcia Almendarez, Autonomous University of Queretaro, México) and Pediococcus acidilactici (provided by Dr. Blanca Escudero-Abarca, North Carolina State University, USA), which synthesize nisin (608 bp), enterocin A (412 bp) and pediocin (332 bp), respectively. Amplicons were submitted for sequencing to the Langebio laboratory (CINVESTAV-Irapuato, México) and sequences were compared with those reported in the NCBI databases (www.ncbi.nlm.nih.gov).
Bacteriocins Production
To study the kinetics of bacteriocins production in the twelve selected bacteria, and to detect the time where the highest inhibitory activities were produced in growth curves, strains were cultured in MRS for 24 h at 37 °C to achieve ~108 cells/mL. Then 250 μL of the culture was added to 250 mL fresh MRS and incubated at 37 °C, and duplicate samples were collected at 2 h intervals over a 24-h period. One of the samples was monitored spectrophotometrically at O.D. = 600 nm. The second sample was centrifuged and the pH of the supernatant was adjusted to 6.8 with 5 N NaOH, filtered through a 0.20-mm filter and the antibacterial activity was evaluated using the well-diffusion method [12, 16]. Twenty-five millilitre of TSB with soft agar 0.7 % (w/v) was mixed with 50 μL (1 × 109 cells/mL) of the indicator bacteria and plated. As E. coli ATCC 25922 was one of the most susceptible bacterium in this work, it was used as indicator bacterium to study the kinetics of the bacteriocins production. Once the medium solidified, wells, 8 mm diameter, were dug into the agar under sterile conditions. Plates were stored 2 h at 27 °C to dehumidify, and 90 μL of the supernatants were added to the wells and incubated for 12 h at 4 °C to allow diffusion of the liquid, followed by incubation for 24 h at 37 °C before diameters of zones of inhibition were measured. The minimum detectable zone measured was 1 mm beyond the well diameter. Assays were repeated in triplicate and the average was recorded. One arbitrary unit of bacteriocin activity (U) was defined as equal to 1 mm2 of the zone of inhibition of growth of the indicator bacterium [12].
Partial Purification of Proteins with Antimicrobial Activity
The twelve bacteria were cultivated in 200 mL of MRS broth for the time where the highest inhibitory activities were detected in growth curves. Cell-free culture supernatants were concentrated with ammonium sulfate to 80 % saturation at 4 °C with constant stirring overnight. Precipitated proteins were pelleted by centrifugation at 16,000×g for 30 min at 4 °C, resuspended in 100 mM phosphate buffer (pH 6.8), and dialyzed overnight against the same buffer using a mini-dialysis kit with a 1 kDa cut-off (Amersham Biosciences). Protein concentration was determined using the Quick Start Bradford 1× Dye reagent (BioRad, Hercules CA, USA) in a SINERGY HTX multimode reader™ (BioTek, USA). Antimicrobial activity was determined by the well-diffusion method against different bacteria (e.g. M. luteus, B. cereus, Streptococcus agalactiae, L. lactis, E. coli ATCC 25922, Shigella flexneri, Serratia marcescens, Enterobacter cloacae and Klebsiella pneumoniae, among others) (Tables S2 and S3), as previously reported [12].
Sensitivity to Enzymes, Heat and pH
To confirm the proteinaceous nature inhibitory substances, partially purified protein samples were treated with different enzymes, including proteinase K (New England BioLabs, Ipswich, MA), peptidase (Sigma-Aldrich Co., St. Louis, MO), trypsin (Sigma-Aldrich Co., St. Louis, MO), protease (Sigma-Aldrich Co., St. Louis, MO), lysozyme (Sigma-Aldrich Co., St. Louis), and amylase (Sigma-Aldrich Co., St. Louis, MO), each at the final concentration of 1 mg/mL. Untreated samples with buffer, buffer alone or enzyme solutions were used as controls. Reactions were incubated at 37 °C for 2 h according to manufacturer’s protocol. Treated samples and controls were assayed by the well diffusion method against the indicator bacterium [12].
To determine the effect of temperature on inhibitory activity, aliquots of partially purified peptides at pH 4 were stored at different temperatures (60, 70, 80, 90 and 100 °C) for 30 min and then assayed by the well diffusion method. The pH stability of the partially purified substances was estimated after 24 h of storage at 4 °C in 100 mM of citrate (citric acid + sodium citrate, pH 2, 4) and phosphate (KH2PO4 + K2HPO4, pH 6, 8) buffers. Activity was evaluated by the well diffusion assay [12].
Results
Bacterial Identification and Determination of the Time with the Highest Inhibitory Activity
We isolated seven bacterial strains from goat cheese and five bacterial strains from goat milk (Table S1). Based on 16S rDNA sequences, isolates from goat cheese were identified as Lactobacillus rhamnosus (LC20), L. plantarum (LC22), L. pentosus (MN24), L. plantarum (MN26, MN29), L. helveticus (MN27) and E. faecium (MN28). In addition, isolates from goat milk were identified as Aquabacterium fontiphilum (strain MAe1), A. fontiphilum (MAe2), Methylibium petroleiphilum (MAe5), Staphylococcus xylosus (LPAe1) and Piscinibacter aquaticus (LPAn2) (Table S1).
When bacteria were cultivated and assayed for inhibitory activity at different times, each isolate showed highest inhibitory effect against E. coli ATCC 25922 at ~22 h. Bacterial isolates from goat cheese showed similar behavior in the growth curve. For example, with L. helveticus (MN27), E. faecium (MN28), and L. plantarum (MN29), the bacteriocin activity was observed in sample collected at middle of the logarithmic phase and achieved the highest level at the stationary period. Alternatively, bacteria isolated from goat milk also showed the highest inhibitory effect against E. coli in the stationary phase (data not shown), except M. petroleiphilum (MAe5), which showed the highest inhibitory activity at the death phase (Fig. 1).
Fig. 1.
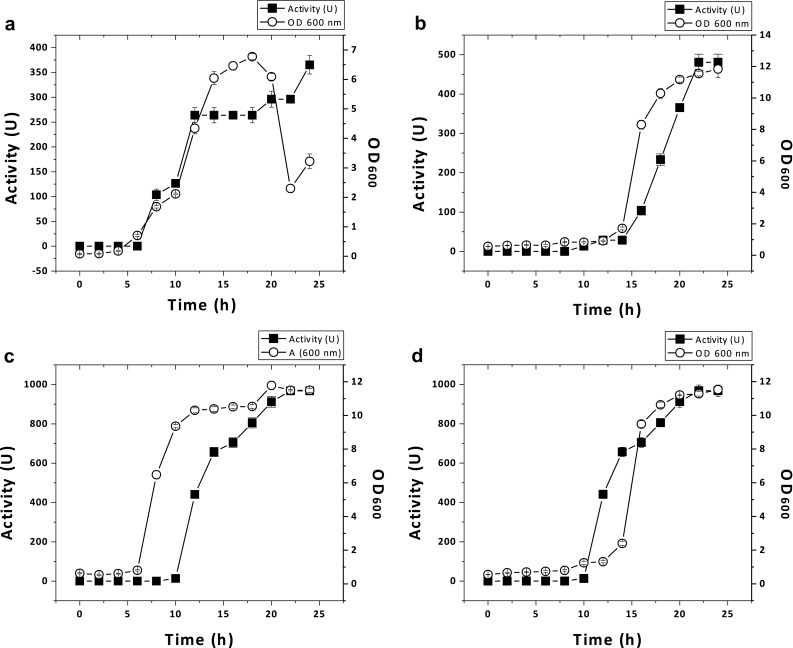
Correlation between growth and the appearance of inhibitory activity in culture medium. Bacteria were grown in MRS broth and duplicate samples were collected at ~2 h intervals and the inhibitory activity was tested against E. coli ATCC 25922. a M. petroleiphilum MAe5, b Lactobacillus helveticus MN27, c E. faecium MN28, and d L. plantarum MN29
Screening of Bacteriocin Genes
When we amplified genes from the seven isolates obtained from goat cheese, enterocin A amplicons were detected (~0.4 kbp) (Fig. S1). Amplicons of nisin (0.608 kbp) or pediocin (0.412 kbp) genes were not observed in these bacterial strains. Additionally, amplicons of nisin, enterocin A and pediocin were not obtained from the five isolates from goat milk.
Inhibitory Activity of Partial Purified Antimicrobial Peptides
The seven bacteria isolated from goat cheese inhibited M. luteus, S. aureus, B. cereus and E. coli, but were not active against E. faecalis, S. pyogenes and Salmonella spp. (Table S2). Strains also showed inhibitory activity against S. aureus, L. monocytogenes, L. inoccua, Pseudomona aeruginosa, S. flexneri, S. marcescens, E. cloacae and K. pneumoniae. In addition, all bacteria isolated from goat milk showed inhibitory activity against M. luteus, B. cereus, L. lactis, E. coli, S. flexneri, E. cloacae and K. pneumoniae. Only A. fontiphilum Mae2 (or Mae1), M. petroleiphilum MAe5 and P. aquaticus LPAn2 showed activity against S. aureus, L. innocua, S. agalactiae and S. marcescens (Table S3).
To compare the activity of crude bacteriocins, we determined the protein concentration in each sample and then tested them against E. coli ATCC 25922 using approximately the same protein concentration. Activity was evaluated using arbitrary units (U). As explained previously, E. coli ATCC 25922 was selected for this assay, as it was one of the most susceptible bacterium observed in this work. When we compared the activity of crude bacteriocins of A. fontiphilum Mae1, S. xylosus LPAe1 and P. aquaticus LPAn2, the highest activity (~126 U) was observed with A. fontiphilum Mae1. Antimicrobial peptides produced by bacteria isolated from goat cheese had activities of ~200 U, but the highest value (~296 U) was observed with sample from L. pentosus MN24 (Table 1).
Table 1.
Comparative inhibitory activity of partially purified bacteriocinsa against E. coli ATCC 25922 using different protein concentrations
| Source | Strains | mg protein/mLb | Activity (U)c |
|---|---|---|---|
| Goat’s milk | A. fontiphilum Mae1 | 15 | 126.4494 ± 7.6 |
| A. fontiphilum MAe2 | 8.32 | 44.7678 ± 5.5 | |
| M. petroleiphilum MAe5 | 10 | 28.2744 ± 5 | |
| S. xylosus LPAe1 | 15 | 28.2744 ± 5 | |
| P. aquaticus LPAn2 | 15 | 82.467 ± 6.5 | |
| Goat’s cheese | L. rhamnosus LC20 | 5 | 263.8944 ± 10.2 |
| L. plantarum LC22 | 5 | 204.204 ± 9.1 | |
| L. pentosus MN24 | 5 | 296.0958 ± 8 | |
| L. plantarum MN26 | 5 | 263.8944 ± 10.2 | |
| L. helveticus MN27 | 5 | 263.8944 ± 7.6 | |
| E. faecium MN28 | 5 | 204.204 ± 6.8 | |
| L. plantarum MN29 | 5 | 204.204 ± 9.1 |
aBacteria were cultivated for the time where the highest inhibitory activities were detected in growth curves and supernatants were concentrated with ammonium sulfate to obtain unpurified bacteriocins
bProtein concentration was determined by the Bradford method (BioRad)
cOne unit is defined as 1 mm2 of the zone of inhibition as determined by the well-diffusion method (see text)
Effect of Enzymes, Temperature, pH and Solvents in the Inhibitory Activity
To determine the nature of partially purified antimicrobial peptides, preparations were digested with various enzymes and treated at different temperatures, pH and chemicals, and then the inhibitory activity were tested against E. coli ATCC 25922. The inhibitory effect were completely lost or drastically reduced after treatment with protease K, and trypsin indicating the proteinaceous nature of these bactericidal compounds. Antimicrobial peptides were thermoresistant, even at 100 °C. Most of the crude bacteriocin samples had activity at low pH but lost their inhibitory effect at pH 8. Antimicrobial peptides were resistant to methanol and ethanol (Table 2).
Table 2.
Inhibitory effect of bacteriocins produced by different isolates against E. coli ATCC 25922 after treatments with enzymes, temperature, pH and organic chemicals
| Strains | Enzymes | Temp (°C) | pH | Methanol | Ethanol | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase K | Trypsin | 60 | 80 | 100 | 2 | 4 | 6 | 8 | |||
| A. fontiphilum Mae1 | (−) | (+/−) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+/−) | (+) |
| A. fontiphilum MAe2 | (−) | (−) | (+) | (+) | (+/−) | (+) | (+) | (−) | (−) | (−) | (+) |
| M. petroleiphilum MAe5 | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (−) | (−) | (+/−) | (+) |
| S. xylosus LPAe1 | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+/−) | (+) |
| P. aquaticus LPAn2 | (+/−) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (−) | (+) |
| L. rhamnosus LC20 | (+/−) | (+/−) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) |
| L. plantarum LC22 | (+/−) | (+/−) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) |
| L. pentosus MN24 | (+/−) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) |
| L. plantarum MN26 | (+/−) | (+/−) | (+) | (+) | (+) | (+) | (+) | (−) | (−) | (+) | (+) |
| L. helveticus MN27 | (+/−) | (+/−) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) |
| E. faecium MN28 | (+/−) | (+/−) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) |
| L. plantarum MN29 | (+/−) | (+/−) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) |
Bacteriocin samples were subjected to different treatments and then tested against E. coli. (+) indicates that bacteriocins are resistant to the treatment and retained inhibitory activity against E. coli. (−) Signal shows that bacteriocins were susceptible to treatment and did not show inhibitory activity against E. coli. (+/−) indicates that bacteriocins activity was reduced significantly against E. coli. Activity was determined using the well-diffusion method
Discussion
In the present study we isolated an identified bacteria from goat milk and goat cheese produced in the center of Mexico which synthesize bacteriocins that inhibit a wide variety of clinically significant bacteria. From goat cheese, we identified lactic bacteria that synthesize enterocin A, whereas in goat milk we selected microorganisms that may not represent the common microbiota found in this product, and most probably were environmental contaminants. In our initial qualitative assay, we focused only on bacteria with the broadest and highest inhibitory effect against B. cereus 183, L. monocytogenes, L. innocua, M. luteus, S. aureus and E. coli ATCC 25922, without taking into consideration whether they belong to the normal microbiota or they were environmental contaminants. It was interesting that E. coli ATCC 25922 was one of the most susceptible bacterium in this work, as frequently Gram-negative bacteria are resistant to enterocins. However, the susceptibility of E. coli ATCC 25922 to enterocin has been reported previously [17].
From goat cheese, we isolated and identified lactic bacteria such as Lactococcus rhamnosus, L. plantarum, L. helveticus and E. faecium, microorganisms that frequently occur in goat milk [18]. All isolates putatively produced enterocin A, a bacteriocin commonly synthesized by Enterococcus strains [8], although other bacteria, such as L. lactis [19] are known to synthesize this peptide. Additionally, as the nisin gene is commonly found in lactic bacteria [8], we expected to find this gene in the Lactococcus species isolated in this study, but were unable to do so. It is possible that the corresponding nisin gene might be present but have diverged such that it was unable to be amplified with the primers used is the study. Previously it has been reported that bacteria isolated from goat milk of sample obtained in farms from Spain harbor nisin, lacticin and enterocin genes [8]. In general, we found that antimicrobial peptides synthesized by bacteria isolated from goat cheese showed a broader spectrum of inhibitory activity compared with peptides from bacteria obtained from goat milk. When we treated the partial purified bacteriocins at different pH and temperatures, we found that they were acid-stable and thermotolerant, characteristics similar to other enterocins previously reported [20].
Interestingly, we did not isolate lactic bacteria from goat milk, but isolated microorganisms not commonly encountered in milk, i.e. A. fontiphilum, P. aquaticus and M. petroleiphilum; we suggest that these were likely environmental contaminants. To our knowledge these bacteria have not been reported in goat milk. Indeed, this does not mean that goat milk lacks lactic bacteria, as these bacteria are the common microbiota in this product [3–5]. More likely, they were eliminated in our initial screen. In addition, A. fontiphilum and P. aquaticus have been previously isolated from water [21, 22], and M. petroleiphilum has been implicated in metabolism of oxygenate methyl tert-butyl ether [23]. There are no reports on the pathogenicity of A. fontiphilum and M. petroleiphilum, so their impact on human and animal health and safety is not known. We also isolated S. xylosus, a coagulase-negative staphylococcus that has been previously isolated from goat milk and cheese and is able to induce spontaneous infections under special conditions [24]. We were unable to amplify nisin, enterocin A and pediocin genes, and it is probable that these isolates produce other types of antimicrobial peptides or bacteriocins not yet characterized.
In general, the isolates described in this study produced antimicrobial peptides that inhibited known pathogenic microbes, including S. aureus, L. monocytogenes, L. innocua, B. cereus, Str. uberis, P. aeruginosa, E. cloacae and K. pneumonia, among others. Although bacteriocins produced by uncommon and lactic bacteria isolated in this study could play a primary role in suppressing or eliminating potential harmful microbes and commensals present in these natural products, it is possible that other bacteriocins not detected here, and/or other metabolites including lactic, acetic, and formic acids, and 2,3-butadione, acetaldehyde, and hydrogen peroxide could contribute to the biochemical integrity and stability of these products [25].
Conclusion
We report the isolation and identification of cultivable microbiota present in goat milk and cheese produced in the center of México, and show that they are able to synthesize antimicrobial peptides with activity against pathogenic bacteria significant in human and animal health. Although it is obvious that bacteria obtained from goat milk are environmental contaminants, it would be interesting to identify in future studies what kind of bacteriocins are synthesize by these bacteria, probably they represent novel antimicrobial peptides not reported yet.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This research was partially supported by Grant from “Universidad de Guanajuato” (project 262/2013) to J. E. Barboza-Corona, and “Fundación Guanajuato Produce AC” (project FGP 583/12) to M. Valencia-Posadas, respectively. O.F. Hernández-Saldaña is a graduate student supported by CONACyT-México. We appreciate the technical support of Daniela Lisseth Contreras-González and Dr. Luz E. Casados-Vázquez from “Universidad de Guanajuato” during the process of this work.
References
- 1.Escareño L, Salinas-González H, Wurzinger M, Iñiguez L, Solkner J, Meza-Herrera C. Dairy goat production system. Status quo, perspectives and challenges. Trop Anim Health Prod. 2013;45:17–34. doi: 10.1007/s11250-012-0246-6. [DOI] [PubMed] [Google Scholar]
- 2.Gómez-Ruiz WJ, Pinos-Rodríguez JM, Aguirre-Rivera JR, García-López JC. Analysis of a goat milk cheese industry in a desert rangeland of Mexico. Pastor Res Policy Pract. 2012;2:2–11. doi: 10.1186/2041-7136-2-5. [DOI] [Google Scholar]
- 3.Quigley L, O’Sullivan O, Stanton C, Beresford TP, Ross RP, Fitzgerald GF, Cotter PD. The complex microbiota of raw milk. FEMS Microbiol Rev. 2013;37:664–698. doi: 10.1111/1574-6976.12030. [DOI] [PubMed] [Google Scholar]
- 4.Nikolic M, Terzic-Vidojevic A, Jovcic B, Begovic J, Golic N, Topisirovic L. Characterization of lactic acid bacteria isolated from Bukuljac, a homemade goat’s milk cheese. Int J Food Microbiol. 2008;122:162–170. doi: 10.1016/j.ijfoodmicro.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 5.Perin LM, Nero LA. Antagonistic lactic acid bacteria isolated from goat milk and identification of a novel nisin variant Lactococcus lactis. BMC Microbiol. 2014;14:36. doi: 10.1186/1471-2180-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekici K, Bozkurt H, Isleyici O. Isolation of some pathogens from raw milk of different milch animals. Pak J Nutr. 2004;3:161–162. doi: 10.3923/pjn.2004.161.162. [DOI] [Google Scholar]
- 7.Drider D, Fimland G, Hechard Y, McMullen LM, Prevost H. The continuing story of class IIa bacteriocins. Microbiol Mol Biol Rev. 2006;70:564–582. doi: 10.1128/MMBR.00016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez E, González B, Gaya P, Nuñez M, Medina M. Diversity of bacteriocins produced by lactic acid bacteria isolated from raw milk. Int Dairy J. 2000;10:7–15. doi: 10.1016/S0958-6946(00)00017-0. [DOI] [Google Scholar]
- 9.Barrón-Bravo OG, Gutierrez-Chavez AJ, Angel Sahagún CA, Montaldo HH, Shepard L, Valencia-Posadas M. Losses in milk yield, fat and protein contents according to different levels of somatic cell count in dairy goats. Small Rumin Res. 2013;113:421–431. doi: 10.1016/j.smallrumres.2013.04.003. [DOI] [Google Scholar]
- 10.León-Galván MF, Barboza-Corona JE, Lechuga-Arana A, Valencia-Posadas M, Aguayo DD, Cedillo-Pelaez C, Martínez-Ortega EA, Gutierrez-Chavez AJ. Molecular detection and sensitivity to antibiotics and bacteriocins of pathogens isolated from bovine mastitis in family dairy herds of Central Mexico. Biomed Res Int. 2015;2015:615153. doi: 10.1155/2015/615153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callon C, Duthoit F, Delbes C, Ferrand M, Le Frileux Y, de Cremoux R, Montel MC. Stability of microbiol communities in goat milk during a lactation year: molecular approaches. Syst Appl Microbiol. 2007;30:547–560. doi: 10.1016/j.syapm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Barboza-Corona JE, Vázquez-Acosta H, Bideshi-Dennis K, Salcedo-Hernández R. Bacteriocin-like inhibitor substances produced by Mexican strains of Bacillus thuringiensis. Arch Microbiol. 2007;187:117–126. doi: 10.1007/s00203-006-0178-5. [DOI] [PubMed] [Google Scholar]
- 13.Pospiech A, Neumann B. A versatil quick-prep of genomic DNA from Gram-positive bacteria. Trends Genet. 1995;11:217–218. doi: 10.1016/S0168-9525(00)89052-6. [DOI] [PubMed] [Google Scholar]
- 14.León-Galván MF, Carbajal N, Frickey T, Santos L. Microbial identification of the Nichupte-Bojorquez coastal lagoon in Cancun, Mexico. Aquat Ecol. 2009;43:197–205. doi: 10.1007/s10452-008-9171-1. [DOI] [Google Scholar]
- 15.Suwanjinda D, Eames C, Panbangred W. Screening of lactic acid bacteria for bacteriocins by microbiological and PCR methods. Biochem Mol Biol Educ. 2007;35:364–369. doi: 10.1002/bmb.84. [DOI] [PubMed] [Google Scholar]
- 16.Rogers AM, Montville TJ. Improved agar diffusion assay for nisin quantification. Food Biotechnol. 1991;5:161–168. doi: 10.1080/08905439109549799. [DOI] [Google Scholar]
- 17.Annamalai N, Manivasagan P, Balasubramanian T, Vijayalakshmi S. Enterocin from Enterococcus faecium isolated from mangrove environment. Afr J Biotechnol. 2009;8:6311–6316. [Google Scholar]
- 18.Alonso-Calleja C, Carballo J, Capita R, Bernardo A, García-López ML. Changes in the microflora of Valdeteja raw goat’s milk cheese throughout manufacturing and ripening. LWT Food Sci Technol. 2002;35:222–232. doi: 10.1006/fstl.2001.0842. [DOI] [Google Scholar]
- 19.Martínez JM, Kok J, Sanders JW, Hernández PE. Heterologous coproduction of enterocin A and pediocin PA-1 by Lactococcus lactis: detection by specific peptide-directed antibodies. Appl Environ Microbiol. 2000;66:3543–3549. doi: 10.1128/AEM.66.8.3543-3549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehaiem A, Pérez Guerra N, Ben Belgacema Z, Fajardo Bernárdez P, Pastrana Castro L, Manaia M. Enhancement of enterocin A production by Enterococcus faecium MMRA and determination of its stability to temperature and pH. Biochem Eng J. 2011;56:94–106. doi: 10.1016/j.bej.2011.05.012. [DOI] [Google Scholar]
- 21.Lin MC, Jiang SR, Chou JH, Arun AB, Young CC, Chen WM. Aquabacterium fontiphilum sp. nov. isolated from spring water. Int J Syst Evol Microbiol. 2009;59:681–685. doi: 10.1099/ijs.0.000745-0. [DOI] [PubMed] [Google Scholar]
- 22.Stackebrandt E, Verbarg S, Fruhling A, Busse HJ, Tindall BJ. Dissection of the genus Methylibium: reclassification of Methylibium fulvum as Rhizobacter fulvus comb. nov., Methylibium aquaticum as Piscinibacter aquaticus gen. nov., comb. nov. and Methylibium subsaxonicum as Rivibacter subsaxonicus gen. nov., comb. nov. and emended descriptions of the genera Rhizobacter and Methylibium. Int J Syst Evol Microbiol. 2009;59:2552–2560. doi: 10.1099/ijs.0.008383-0. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt R, Battaglia V, Scow K, Kane S, Hristova KR. Involvement of a novel enzyme, MdpA, in methyl tert-butyl ether degradation in Methylibium petroleiphilum PM1. Appl Environ Microbiol. 2008;74:6631–6638. doi: 10.1128/AEM.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koop G, De Vliegher S, De Visscher A, Supré K, Haesebrouck F, Nielen M, van Werven T. Differences between coagulase-negative Staphylococcus species in persistence and in effect on somatic cell count and milk yield in dairy goats. J Dairy Sci. 2012;95:5075–5084. doi: 10.3168/jds.2012-5615. [DOI] [PubMed] [Google Scholar]
- 25.Broberg A, Jacobsson K, Ström K, Schnürer J. Metabolite profiles of lactic acid bacteria in grass silage. Appl Environ Microbiol. 2007;73:5547–5552. doi: 10.1128/AEM.02939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


