Abstract
Objective
Complex local crosstalk amongst endocrine cells within the islet ensures tight coordination of their endocrine output. This is illustrated by the recent demonstration that the negative feedback control by delta cells within pancreatic islets determines the homeostatic set-point for plasma glucose during mouse postnatal development. However, the close association of islet endocrine cells that facilitates paracrine crosstalk also complicates the distinction between effects mediated directly on beta cells from indirect effects mediated via local intermediates, such as somatostatin from delta cells.
Methods
To resolve this problem, we generated reporter mice that allow collection of pure pancreatic delta cells along with alpha and beta cells from the same islets and generated comprehensive transcriptomes for each islet endocrine cell type. These transcriptomes afford an unparalleled view of the receptors expressed by delta, alpha and beta cells, and allow the prediction of which signal targets which endocrine cell type with great accuracy.
Results
From these transcriptomes, we discovered that the ghrelin receptor is expressed exclusively by delta cells within the islet, which was confirmed by fluorescent in situ hybridization and qPCR. Indeed, ghrelin increases intracellular calcium in delta cells in intact mouse islets, measured by GCaMP6 and robustly potentiates glucose-stimulated somatostatin secretion on mouse and human islets in both static and perfusion assays. In contrast, des-acyl-ghrelin at the same dose had no effect on somatostatin secretion and did not block the actions of ghrelin.
Conclusions
These results offer a straightforward explanation for the well-known insulinostatic actions of ghrelin. Rather than engaging beta cells directly, ghrelin engages delta cells to promote local inhibitory feedback that attenuates insulin release. These findings illustrate the power of our approach to resolve some of the long-standing conundrums with regard to the rich feedback that occurs within the islet that is integral to islet physiology and therefore highly relevant to diabetes.
Keywords: Ghrelin, Delta cell, Somatostatin release, Transcriptome, Beta cell, Alpha cell
Abbreviations: Crhr2, Corticotropin-releasing hormone receptor type 2; FISH, Fluorescent in situ hybridization; Ghsr, Growth hormone secretagogue receptor; GSSS, Glucose-stimulated somatostatin secretion; Iapp, Islet amyloid polypeptide; RPKM, Reads per kilobase gene model per million reads sequenced; Trpm2, Transient receptor potential melastatin 2; Ucn3, Urocortin 3; YFP, Yellow fluorescent protein
Graphical abstract
1. Introduction
Pancreatic alpha and beta cells co-localize in an arrangement that facilitates accurate coordination of glucagon and insulin release from islets. While glucagon and insulin, along with Islet amyloid polypeptide (Iapp), are among the few islet signals that meet the classic definition of a hormone as a ‘factor that is released into the general circulation to signal at a distant site in the body’, a rich constellation of paracrine and neural interactions takes place within the islet to ensure tight control over their release [1], [2], [3]. Pancreatic delta cells are the third-most common endocrine cell type in the islets, and the somatostatin they release is an important inhibitor of both insulin and glucagon [4], [5], [6]. We recently described a novel negative feedback loop where the paracrine peptide Urocortin 3 (Ucn3) is co-released with insulin from beta cells and promotes glucose-stimulated somatostatin release via the type 2 corticotropin-releasing hormone (Crhr2) receptor expressed by delta cells [7]. Somatostatin then attenuates further insulin secretion and helps to maintain stable and tight control over plasma glucose. The appearance of Ucn3 across the beta cell mass correlates with the well-known uptick in plasma glucose levels [8], [9], [10]. The onset of Ucn3 is directly responsible for this phenomenon by initiating delta cell-dependent feedback on insulin release, thus determining the homeostatic set-point for plasma glucose [7]. Delta cell-mediated feedback breaks down early in diabetes, which leads to marked increases in plasma glucose fluctuations that resemble the glycemic volatility that impacts patients across the diabetes spectrum [7].
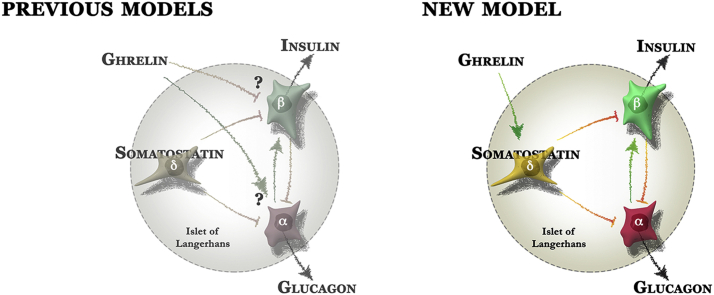
While these observations highlight the physiological importance of delta cell-dependent feedback, we know relatively little about the cues that control delta cells. The intimate co-localization of islet endocrine cells has also made it difficult to distinguish direct effects on beta cells from indirect actions that are mediated by locally produced intermediaries. We previously generated a transgenic mIns1-H2b-mCherry reporter mouse in which beta cells are labeled by the nuclear expression of mCherry driven by the mIns1 promoter and crossed these to S100b-eGFP reporter mice that we serendipitously discovered to label alpha cells [11]. These reporter mice enabled the comparison of mouse and human beta cell transcriptomes but did not fully resolve the transcriptomes of pancreatic alpha and delta cells. To improve substantially on these prior observations and to determine the factors that directly engage delta cells, we now generated a set of triple transgenic mouse models in which mIns1-H2b-mCherry [11] beta cells are crossed to mice with alpha or delta cells are marked by YFP in a Cre-dependent fashion. We used these reporter mice to generate unbiased and comprehensive transcriptomes of pancreatic delta and alpha cells, along with beta cells from the same islets. Our transcriptomes are validated by the strong enrichment for a large panel of known alpha, beta, and delta cell markers in the appropriate cell type. We then use this information to determine the signals that directly engage the delta cell by virtue of selectively expressed receptors and analyze ghrelin stimulation of delta cells as an example to highlight the utility of our approach. We discovered that ghrelin acts directly on mouse and human delta cells to promote somatostatin release. Our observations offer a straightforward explanation for the well-known insulinostatic actions of ghrelin (e.g. [12], [13], [14], [15], [16]) and illustrate the physiological importance of delta cell-mediated feedback within pancreatic islets. An accurate understanding of ghrelin's mechanism of action within pancreatic islets is highly relevant given ghrelin's central role in the regulation of energy and glucose metabolism [17].
2. Research design and methods
2.1. Biological materials and ethics statements
All mouse procedures were approved by the UC Davis or the Salk Institute for Biological Studies Institutional Animals Care and Use Committee and were performed in compliance with the Animal Welfare Act and the Institute for Laboratory Animal Research (ILAR) Guide to the Care and Use of Laboratory Animals. Animals were maintained on a 12-h light/12-h dark cycle with free access to water and standard rodent chow. Static and dynamic hormone secretion experiments were carried out on C57BL/6NHsd mice, between 8 and 16 weeks of age, from Harlan (Indianapolis, IN). We obtained human islets via the Integrated Islet Distribution Program; the receipt was declared exempt from IRB review under 45 CRR 46.101 (b) category (4).
2.2. Immunofluorescence and FISH
Immunofluorescence was conducted as previously described [7], [8]. Insulin was detected using guinea pig anti-insulin (Dako #A0564; 1:500), somatostatin using sheep anti-somatostatin (American Research Products Inc. #13-2366, 1:1000), Ucn3 using rabbit anti-Ucn3 (#6570; in house, 1:2000), Glucagon using rabbit anti-glucagon (Abcam #ab11022-1; 1:200), and YFP using goat anti-GFP (Rockland 600-101-215; 1:1000). All secondary antibodies were obtained from Jackson Laboratories Inc. RNA FISH was conducted using RNAscope probes for Ghsr and Sst (Advanced Cell Diagnostics) according to the manufacturers instructions.
2.3. Islet isolation and FACS sorting
Islet isolation was conducted as previously described [7], [11], [18]. Islets from mIns1-H2b-mCherry [11] (deposited with the Jackson laboratories as strain #28589) × Rosa-LSL-YFP [19] × Sst-Cre [20] or Gcg-Cre [21] triple transgenic animals were pooled by sex in 2 (Sst-Cre) or 3 (Gcg-Cre) replicate groups of a dozen animals. FACS sorting was conducted as described previously [7], [11] with each sample collected directly in Trizol to ensure immediate cell lysis and preservation of RNA integrity.
2.4. Next generation sequencing and bioinformatics
RNA was isolated from Trizol-preserved samples by chloroform extraction and cleaned up over an RNeasy microcolumn essentially as previously described [11]. RNA quality was verified by Tapestation (Agilent, Santa Clara, CA). Indexed sequencing libraries were constructed using the TruSeq RNA sample Prep Kit v2 (Illumina Inc. San Diego, CA), sequenced at 50 cycles, and single read on an Illumina HiSeq 2000 platform. Results were validated by qPCR using Sybr chemistry and the primers listed in Table 1. Sequencing reads were mapped to the mouse genome version GenCode M5 (GRCm38.p3) using STAR v2.4 [22]. On average over 33 million reads were sequenced for each library with 89.9% of sequenced reads aligning (>63% unique alignment overall). FeatureCounts [23] was used to create count tables of the sorted bam files using reads aligning to RefSeq-defined exons. EdgeR version 3.12.0 [24] was used to conduct pairwise statistical comparisons. Wordles of transcript abundance were generated on wordle.net. Single cell RNAseq data from [25] were used to generate the violin plots in Figure 2C. Cells that had an RPKM value > 10 k of either Sst, or Ins2, or Gcg were defined as delta, beta, or alpha cells, respectively.
Table 1.
qPCR primer information.
| Ref Seq ID | Gene | Primer | Sequence 5′→3′ | Amplicon size (bp) |
|---|---|---|---|---|
| NM_008100 | Gcg | qrodGcg.fwu1 | TCACAGGGCACATTCACCAG | 121 |
| qrodGcg.rvu1 | CATCATGACGTTTGGCAATGTT | |||
| NM_001185084 | Ins2 | qrodIns2.fwu1 | GCTCTCTACCTGGTGTGTGGG | 128 |
| qrodIns2.rvu1 | CAAGGTCTGAAGGTCACCTGC | |||
| NM_009215 | Sst | qrodSst.fwu1 | GACCCCAGACTCCGTCAGTTT | 112 |
| qrodSst.rvu1 | TCTCTGTCTGGTTGGGCTCG | |||
| NM_021488 | Ghsr | qmGhsr.fwu1 | GACCAGAACCACAAACAGACAG | 113 |
| qmGhsr.rvu1 | GGCTCGAAAGACTTGGAAAA | |||
| NM_013556 | Hprt | qmHPRT.fwu | TCCTCCTCAGACCGCTTTT | 90 |
| qmHPRT.rvu | CCTGGTTCATCATCGCTAATC |
Figure 2.
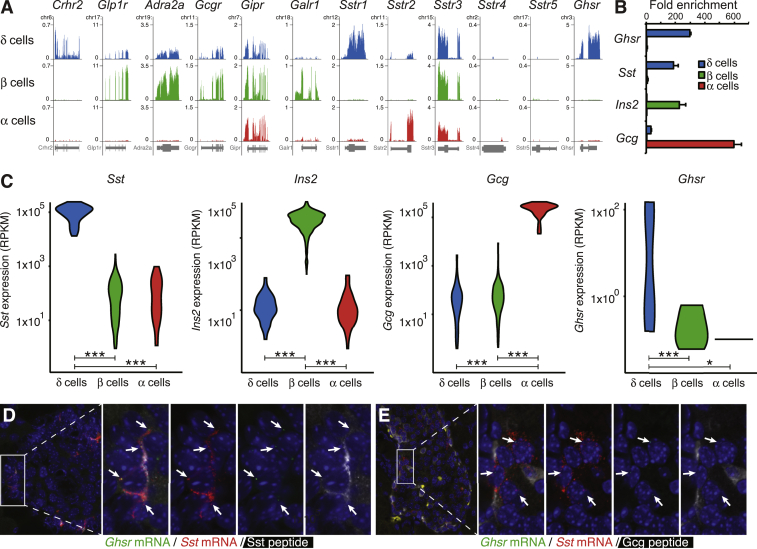
Delta cells selectively express Ghsr. A: Normalized browser plots illustrating the expression of a series of GPCRs in delta, beta and alpha cells of the mouse islet. B: Confirmation by qPCR that Ghsr message is selectively expressed by pancreatic delta cells. C: Violin plots of single cell RNA-seq of wild type mouse pancreatic islet cells [25] confirms that Ghsr expression is detectable only in delta cells. D: FISH confirmation of the expression of Ghsr gene (green dots) in pancreatic delta cells of wild type mice, colocalized with Sst message (red dots) and Sst peptide (white). E: mRNA for Ghsr (green dots) and Sst (red dots) co-localizes in a peripheral islet population that does not express Gcg peptide (white). *P < 0.05; **P < 0.01; ***P < 0.001.
2.5. Functional imaging by GCaMP6
Sst-Cre mice and LSL-GCaMP6 mice (Jackson laboratories strain #24106) were crossed for functional imaging. Intact islets from bitransgenic offspring were plated on poly-D lysine-coated number 1.5 35 mm glass cover slip tissue culture dishes (Mattek) and maintained at 37 °C 5% CO2 in RPMI 10% FBS, 5.5 mM glucose with pen/strep. Islets were allowed to adhere for 24 h prior to imaging using a Nikon A1R confocal microscope. During live cell fluorescence acquisition, islets were continuously superfused with 37 °C KRB bubbled with 95% O2 and 5% CO2. To measure baseline, high glucose, glucose plus ghrelin, and control responses, buffers were switched at the indicated time points. The position of delta cells within the islet in X, Y, and Z were first identified during a brief pulse with 16.8 mM glucose. Afterwards, islets were washed with 2.8 mM glucose for 30 min to allow fluorescence to return to baseline. For fluorescence intensity analysis, GCaMP6 glucose responsive regions of interest corresponding to single delta cells were identified and defined during the maximal depolarization by 30 mM KCl. Data analysis was performed using Nikon Elements software.
2.6. Hormone secretion
Secretion experiments were conducted in Krebs–Ringer Buffer (KRB). We hand-picked the required number of islets from a pool of all islets and assigned these as the next replicate to each subsequent treatment. Static somatostatin secretion was carried out using 50 or 100 islets per well for mouse islets under high or low glucose, respectively. 100 islets/well were used for human islet release. Dynamic somatostatin release was determined using a custom-built perfusion setup using 200 islets in 6 parallel chambers. We measured somatostatin by RIA [26] using anti-Sst14 antiserum (S201; diluted 1:50,000) [27] as described [7]. EC50 and minimal detectable dose are 12 pg/tube and 1 pg/tube, respectively. Insulin was measured by commercial RIA (Millipore) as previously described [18]. Ghrelin, des-acyl-ghrelin, Astressin2b, and the Sstr3 antagonist Sst3−ODN−8 (Carbamoyl−des−AA1,2,4,5,12,13[d−Cys3,Tyr7,d−AgI8 (Me,2−napthoyl)]−SS; #315−260−15) [28] were generously synthesized and provided by Drs. Michael Beyermann and Jean Rivier.
2.7. Statistical analyses
Statistical analyses were carried out in Prism 6.0e for Mac (GraphPad Software, Inc.). We reported all values as mean values across bio-logical replicates and assumed normality, unless otherwise noted. We evaluated experiments with more than two treatments by ANOVA followed by Student's t-test to determine which means differed statistically, and we applied Welch's correction for unequal variance when necessary. We evaluated perfusion data by two-way ANOVA for treatment and the interaction of treatment and time for each block.
3. Results
3.1. Generation and validation of alpha, beta and delta cell transcriptomes
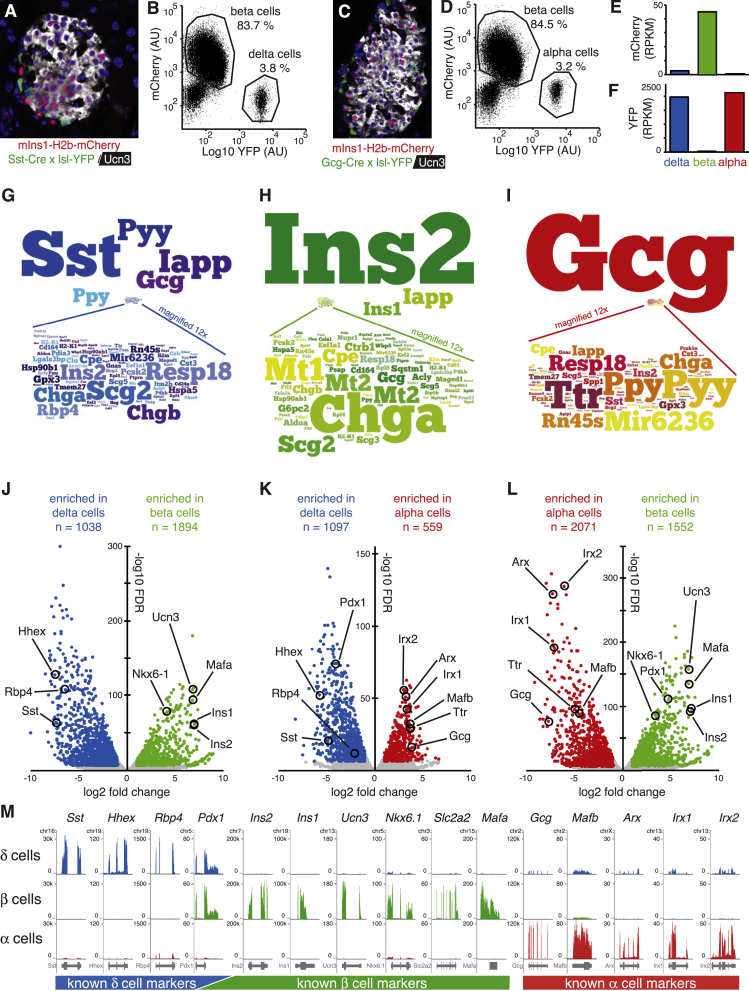
To obtain transcriptomes of mouse alpha, beta and delta cells, we crossed our mIns1-H2b-mCherry mice [11] to either Sst-Cre [20] or Gcg-Cre [21] mice and a Rosa-LSL-YFP reporter [19] (Figure 1A–D). The resulting triple transgenic offspring enabled FACS purification of highly pure beta cells and delta or alpha cells that we used to generate comprehensive transcriptomes of mouse delta, beta, and alpha cells. Beta cell transcriptomes were markedly enriched for mCherry expression (Figure 1E), while alpha and delta cell transcriptomes were each marked by strong enrichment for YFP (Figure 1F), underpinning our robust FACS-based purification. Alpha, beta, and delta cells are all endocrine cells most noted for the release of glucagon, insulin, and somatostatin, respectively. Indeed, somatostatin transcripts are by far the most abundant transcripts in delta cells and account for 3.4 ± 0.25% of all aligned reads in delta cells (Figure 1G). Insulin (encoded by Ins2 and Ins1 genes in mouse) makes up 18.9 ± 2.4% of all reads in the beta cell transcriptome (Figure 1H), while glucagon transcripts make up 27.8 ± 0.9% of all reads in alpha cells (Figure 1I). Iapp is the second-most abundant transcript in our delta cell transcriptomes and while Iapp is best-known as a beta cell hormone, delta cells also express Iapp [29].
Figure 1.
Generation and validation of the delta, beta, and alpha cell transcriptomes. A: Islet of a mIns1-H2b-mCherry × Sst-Cre × LSL-YFP triple transgenic mouse labels all beta cells with nuclear mCherry (counterstained in white with Ucn3) and labels all delta with YFP (stained in green). B: FACS plots of dissociated beta and delta cells from this same cross. C: Islet of a mIns1-H2b-mCherry × Gcg-Cre × LSL-YFP triple transgenic mouse labels all beta cells with nuclear mCherry (counterstained in white with Ucn3) and labels alpha cells with YFP (stained in green). D: FACS plots of dissociated alpha and delta cells from this same cross. E, F: Beta cell transcriptomes are highly enriched for mCherry reads while delta and alpha cell transcriptomes are highly enriched for YFP reads, confirming our transgenic purification strategy. RPKM = reads per kilobase gene model per million reads sequenced. G–I: Graphical representation of the relative expression of the 100 most-abundantly detected transcripts in each cell type. Note that most of these genes are expressed at levels that are so much lower compared to the most abundant transcript in each endocrine cell type that they were magnified 12x to maintain legibility. J–L: Volcano plots listing the number of significantly enriched genes for each pairwise comparison. Selected markers of alpha, beta, and delta cell identity are highlighted for visual reference. M: Genome browser plots comparing the expression of a large panel of markers with well-established expression patterns validate the high degree of purity achieved in these delta, beta, and alpha cells transcriptomes.
3.2. Transcriptome validation
We observed on average 1369 genes that were differentially enriched in each pairwise comparison, as defined by a Log2-fold expression > 1 or < −1 and a FDR < 0.00001. Known delta cell markers such as Sst, Rbp4, and Hhex [30], [31] were highly enriched in pairwise comparisons to beta and alpha cells (Figure 1J,K). Known beta cell markers such as Ucn3, Mafa, Ins1, Ins2, and Nkx6-1 [11] were markedly enriched in beta compared to delta and alpha cells (Figure 1J,L). Alpha cell markers such as Gcg, Ttr, Irx1, Irx2, Arx, and Mafb [30], [32], [33] were enriched in alpha cells compared to delta and beta cells (Figure 1K,L). As Pdx1 is expressed in beta and delta cells (Figure 1M), it was enriched in neither cell type upon pairwise comparison (Figure 1J).
3.3. Islet cell transcriptomes reveal GPCR expression profiles
Given the importance of paracrine interactions to control islet insulin and glucagon output [7], we assessed the expression of GPCRs in our islet transcriptomes in more detail. As noted, Crhr2 is expressed exclusively by delta cells, but several GPCRs that are well-known for their beta cell expression are also expressed to a lesser extent by delta cells but absent from alpha cells (Figure 2A). This group includes glucagon-like peptide 1 receptor (Glp1r), the alpha 2 adrenergic receptor (Adra2a), and the glucagon receptor (Gcgr). In contrast, the gastric inhibitory polypeptide receptor (Gipr) is ubiquitously expressed in the islet and is detected in approximately equal measures by alpha and delta cells as well. In contrast, the galanin receptor 1 (Galr1) is expressed selectively in beta cells. In searching for GPCRs that, like Crhr2, are expressed selectively by delta cells, we noted the delta cell-specific expression of Sstr1. Alpha cells selectively express Sstr2, while Sstr3 is expressed by alpha, beta and delta cells. There is no detectable expression of Sstr4 or Sstr5 in any of the endocrine cells of the mouse islets. Another example of a GPCR that caught our attention as expressed selectively by delta cells is the growth hormone secretagogue receptor (Ghsr), which encodes the GHS-1R that responds to ghrelin (Figure 2A). We validated the selective expression of Ghsr in delta cells by qPCR (Figure 2B). A recently published data set of high quality, single cell RNAseq of dissociated wild type mouse islets that contains populations of Sst-expression delta cells, Ins2-expression beta cells and Gcg-expression alpha cells [25] supported these observations, as Ghsr in these data was also detectable only in delta cells (Figure 2C). We further confirmed Ghsr mRNA expression in delta cells by FISH. We found that Ghsr mRNA (green) colocalizes with Sst mRNA (red) in cells at the islet periphery that co-express SST peptide (white) (Figure 2D) but not GCG peptide (white) (Figure 2E).
3.4. Ghrelin directly activates calcium in delta cells
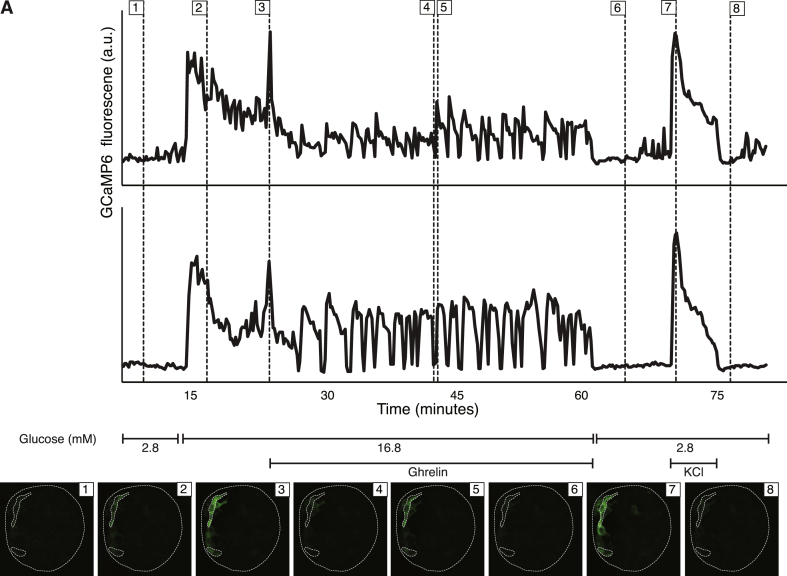
GHS-1R primarily couples through Gαq [34], which results in phospholipase C-mediated intracellular calcium release. Thus, we evaluated the calcium response of delta cells to ghrelin in intact islets. We used islets from bitransgenic Sst-Cre [20] × LSL-GCaMP6 mice that express the Ca2+ sensor GCaMP6 [35] specifically and selectively in delta cells. After establishing baseline fluorescence under KRB with 2.8 mM glucose, increasing glucose to 16.8 mM glucose led to a marked increase in delta cell GCaMP6 fluorescence intensity, which gradually tapered down (Figure 3; Supplementary Video 1). Upon subsequent addition of 100 nM ghrelin delta cells responded with a large initial Ca2+ peak that was followed by a distinct oscillatory response (Figure 3). The Ca2+ signal returned to baseline upon cessation of stimulation. Ghrelin stimulation under 2.8 mM glucose produced no increases GCaMP6 signal (not shown). Depolarization by 30 mM KCl at the conclusion of the experiment resulted in a robust GCaMP6 calcium peak, confirming delta cell viability and responsiveness throughout the experiment.
Figure 3.
Ghrelin activates an intracellular calcium response as measured by GCaMP6 in intact islets. A: Calcium responses were measured over time in intact islets using the genetically encoded calcium sensor GCaMP6 expressed selectively in delta cells (Sst-Cre × LSL-GCaMP6). Calcium traces of two representative cells in response to changes in glucose concentration and stimulation with 100 nM ghrelin or 30 mM KCl, as indicated. Thumbnails of the islet at the indicated time points in each graph are given, with the top and bottom region of interest corresponding to the top and bottom trace, respectively. Movie of the full calcium trace for this figure is included as Supplemental Video 1.
3.5. Ghrelin promotes somatostatin secretion
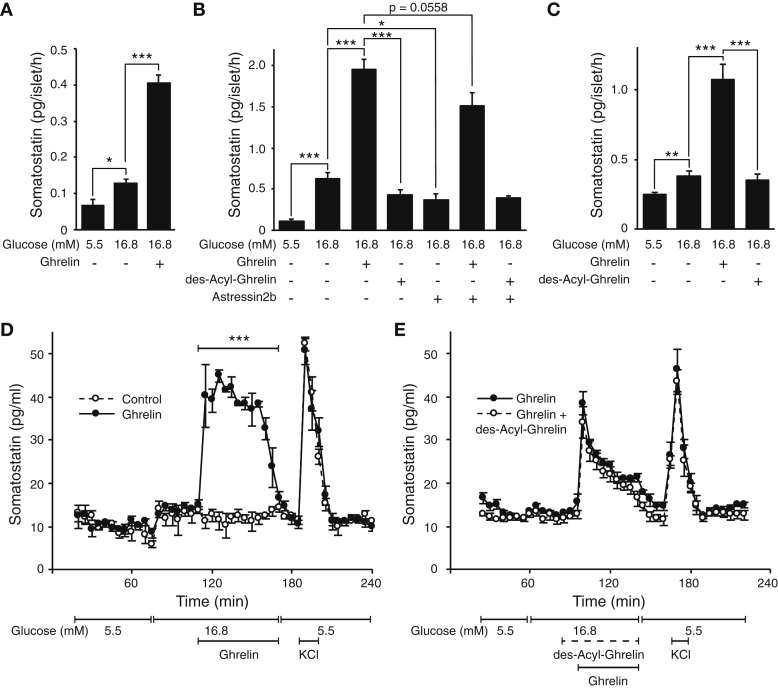
Given the increase in intracellular calcium upon activation of GHS-1R on delta cells and the importance of calcium in hormone secretion, we proceeded to determine if ghrelin promotes somatostatin secretion. Indeed, stimulation with ghrelin strongly potentiated glucose-stimulated somatostatin secretion (GSSS) in mouse islets (Figure 4A). Des-acyl-ghrelin, which lacks the octanyl post-translation modification at the Ser3 position and does not activate GHS-1R, did not potentiate GSSS. To determine if the actions ghrelin depended on the feedback mediated by beta cell-derived Ucn3 [7], we blocked the actions of endogenous Ucn3 with the CRHR2-selective antagonist Astressin2b. Astressin2b attenuated GSSS as previously reported [7], but did not prevent the ghrelin-induced potentiation of GSSS (Figure 4B). This indicates that ghrelin engages delta cells directly, independent of Ucn3-mediated potentiation of GSSS [7]. Ghrelin, but not des-acyl-ghrelin, robustly potentiated GSSS from human islets in vitro, confirming that its actions are similar across rodents and primates (Figure 4C). We next tested the actions of ghrelin on somatostatin secretion in an islet perfusion setup and observed that islets demonstrated robust potentiation of GSSS in response to 100 nM ghrelin. Islets that did not receive ghrelin continued to maintain glucose-stimulated somatostatin levels (Figure 4D). Des-acyl-ghrelin did not elicit any response following a 15-minute pre-incubation and did not block the potentiation of GSSS by ghrelin (Figure 4E). Islets in all perfusion experiments responded to brief depolarization with 30 mM KCl with a strong somatostatin peak, confirming islet viability.
Figure 4.
Ghrelin potentiates glucose-stimulated somatostatin secretion. A: Ghrelin significantly increases somatostatin release from mouse islets in vitro. B: Ghrelin, but not des-acyl-ghrelin, promotes somatostatin release. Astressin2b attenuates glucose-stimulated somatostatin release by preventing beta cell-derived Ucn3 from stimulating delta cells. Astressin2b does not prevent ghrelin from stimulating somatostatin release, indicating that ghrelin's actions are independent from the feedback mediated by Ucn3. C: Ghrelin, but not des-acyl-ghrelin, promotes glucose stimulated somatostatin secretion from human islets in vitro. D: Perfusion of mouse islets under high glucose with ghrelin (black circles) acutely potentiates glucose-stimulated somatostatin secretion compared to control (open circles). E: Application of des-acyl-ghrelin before and during stimulation does not affect the ability of ghrelin to potentiate glucose-stimulated somatostatin secretion. All mice were wild type, and all peptides were applied at 100 nM final concentration. Values represent the mean ± SEM for 8 (A), 7 (B) or 6 (C) wells per group (A–C) or 3 parallel perfusion chambers (D, E). *P < 0.05; **P < 0.01; ***P < 0.001.
3.6. Ghrelin attenuates insulin release in a somatostatin-dependent manner
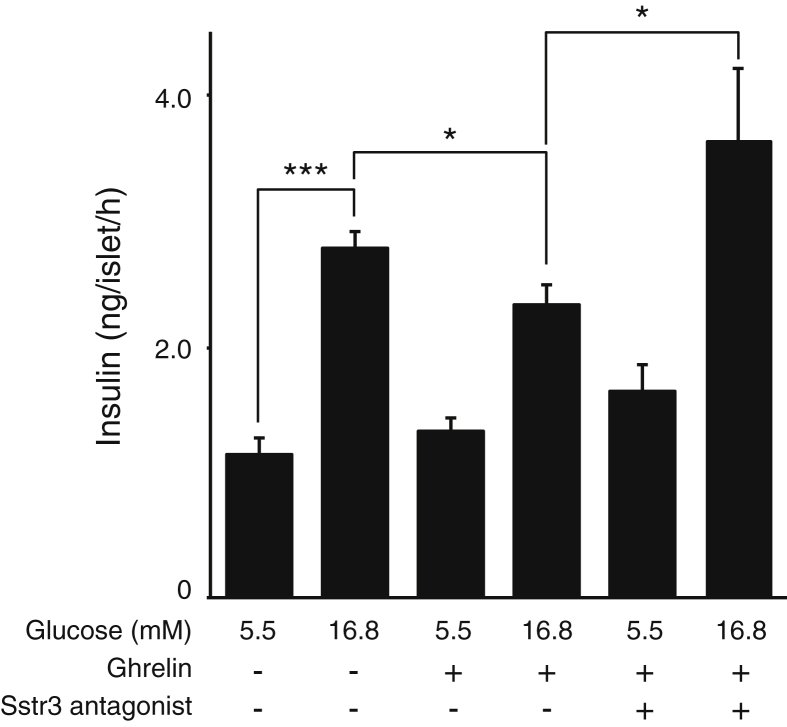
We next assessed whether the insulinostatic effect of ghrelin depended on intact somatostatin-mediated feedback within the islet. Stimulation of wild type mouse islets with ghrelin attenuated glucose-stimulated insulin secretion (Figure 5), in line with most published reports. Co-administration with an antagonist to the Sstr3 receptor [28], which is the only Sst receptor that is detectably expressed by primary mouse beta cells (Figure 2A), blocked the insulinostatic actions of ghrelin completely (Figure 5). These observations demonstrate that ghrelin's insulinostatic actions depend on intact Sstr3-dependent feedback within the islet. The fact that Sstr3 blockade promoted glucose-stimulated insulin secretion suggest that this Sstr3-mediated feedback exerts a tonic inhibitory effect on glucose-stimulated somatostatin secretion.
Figure 5.
Attenuation of glucose-stimulated somatostatin secretion by ghrelin depends on local feedback mediated by Sst and Sst3r. Ghrelin significantly attenuates glucose-stimulated insulin secretion from wild type mouse islets in vitro. Co-stimulation with an Sstr3 antagonist fully prevents the insulinostatic actions of ghrelin. All peptides were applied at 100 nM final concentration. Values represent the mean ± SEM for 4 wells per group. *P < 0.05; **P < 0.01; ***P < 0.001.
4. Discussion
The importance of the neural, endocrine, and paracrine inputs to pancreatic islets that collectively determine the output of insulin and glucagon has long been recognized. However, the tools and techniques at our disposal to map this complex local crosstalk have lacked the resolution and specificity to accurately determine the origin of many signals and the identity of the islet cell receptive to its message. The recent onset of next-generation sequencing approaches has now made it attainable to conduct an unbiased evaluation of the expression of all genes in any given tissue that is quantitative over many orders of magnitude. The purity and quality of the input sample has become the factor limiting the usefulness of the data achieved in this way. For pancreatic islets, which are often equated with beta cells, but which contain many additional endocrine and non-endocrine cells, we resolved this issue by generating triple transgenic reporter mice that collectively enable the FACS-purification of alpha, beta, and delta cells from the same islets. From these highly pure input samples, we generated comprehensive gene expression compendia of the three principal endocrine cells within the islets. This now enables us to unravel with great accuracy some of the crosstalk pathways that had to date been unknown. Our current transcriptome data are a distinct improvement over the work we published previously [11] and offer a significantly better resolution between pancreatic alpha and delta cells. Our data sets complement a recent, high quality single cell RNAseq data set of dissociated mouse islets [25]. The single cell approach offers valuable insight into the heterogeneity of gene expression amongst the different endocrine populations in the islet and complement our transcriptomes from FACS-purified populations of alpha, beta, and delta cells that demonstrate a far greater depth and dynamic range and therefore reliably detect the expression of a far greater number of genes, as expected.
While the most prevalent transcript in alpha, beta, and delta cell transcriptomes was Gcg, Ins2, and Sst, respectively, the relative abundance of Gcg in alpha cells (27.8%) and Ins2 in beta cells (18.9%) was much greater than the relative abundance of Sst in delta cells, which made up merely 3.5% of all aligned reads. As our transgenic reporter strategy is very similar for delta and alpha cells (Figure 1), there is no technical reason for delta cells to exhibit such a relatively modest gene expression of their principal endocrine signal Sst, in comparison to the expression of Gcg and Ins2 in alpha and beta cells, respectively. Instead, we think that this is a direct reflection of the fact that insulin and glucagon are hormones released into the systemic circulation to act at sites throughout the body, while somatostatin is responsible for local feedback within the islet.
Our transcriptome analyses reinforce the view that delta cells are similar to beta cells. This extends from the mechanistically similar control of glucose-stimulated exocytosis in both beta and delta cells that proceeds via Katp channels, voltage-gated sodium, and calcium channels [7], [36] to include GPCR control that can potentiate glucose-stimulated exocytosis in both cell types. Some of these GPCRs, such as Crhr2 and Ghsr on delta cells and Galr1 on beta cells, are selectively expressed by only one cell type. In contrast, a series of other GPCRs that are routinely associated with beta cells, including Glp1r, Adra2a, and Gcgr, are expressed by delta cells at likely physiologically meaningful levels. We do not think that these reflect contamination by beta cells, as many known beta cell-specific genes are indeed highly selectively detected in our beta cell transcriptomes only and do not demonstrate similar levels of expression by delta cells (Figure 1). These observations betray significant overlap in delta and beta cell transcriptional programs, possibly afforded in part by the shared expression of Pdx1. These similarities likely reflect the close ontogenetic origins of beta and delta cells and are in line with observations that perturbation of a single transcription factor, Nkx6-1, suffices to turn beta cells into delta-like cells [37]. Their overlapping transcriptomes may contribute to the participation of beta and delta cells in a common functional unit that responds in unison to increases over resting glucose with increased exocytosis. This enables pulsatile, delta cell-mediated feedback to oscillate with insulin release, antiparallel to glucagon [38].
The transcriptomes we report here constitute an invaluable repository that informs on the expression, or lack thereof, of all genes in each of the main endocrine cell types of the islet, including delta cells. In addition to confirming that Crhr2 is selectively expressed by delta cells [7], we discovered that the Ghsr gene is abundantly and selectively expressed in delta cells (Figure 2). Stimulation of mouse and human islets with ghrelin indeed leads to a robust increase in somatostatin secretion (Figure 4) and stimulates calcium responses in delta cells in intact islets (Figure 3). Furthermore, at equimolar concentrations, des-acyl-ghrelin in intact islets does not affect somatostatin secretion and does not block ghrelin-induced somatostatin release, in agreement with prior observations that des-acyl-ghrelin is a full agonist of the GHS-R1 only at very high concentrations [39] and does not block ghrelin [40].
Most studies to date have assumed that the Ghsr within islets is expressed by beta cells and thereby inhibits insulin release [41]. This implies that GHS-R1 in islets is not coupled to its canonical Gαq/11 signaling cascade [42], as this would enhance rather than attenuate insulin secretion. Moreover, the insulinostatic effects of ghrelin are pertussis toxin-sensitive [43], indicative of the involvement of Gαi. Our findings resolve this long-standing paradox [17] by demonstrating that ghrelin promotes somatostatin release, likely by engaging the canonical Gαq/11 and Ca2+ cascade in delta cells, which would then inhibit insulin via beta cell somatostatin receptors coupled to Gαi. Such a model is in good agreement with the preponderance of published data on the mechanism by which ghrelin inhibits insulin secretion, but offers a more parsimonious explanation compared to some of the prior models that have been proposed to resolve the discrepancy between ghrelin's well-accepted insulinostatic actions and the fact that its canonical signaling cascade would promote, not inhibit, exocytosis.
It has been suggested that Ghsr is expressed by alpha cells and promotes glucagon release [44]. This was based on Ghsr detection by in situ hybridization at the islet periphery, where alpha cells are intimately associated with delta cells [44], experiments on the alpha-TC-1 alpha cell line in which expression of Ghsr need not imply similar Ghsr expression by primary alpha cells, and studies on isolated islets under 12 mM glucose, a concentration not typically associated with highest alpha cell activity. More importantly, increases in glucagon stimulate insulin [45], [46] and, therefore, cannot explain the insulinostatic actions of ghrelin that are generally accepted [12], [13], [14], [15], [16]. Hetero-dimerization between GHS-R1 and SSTR5 on the beta cell surface has been offered to explain how ghrelin inhibits insulin release in a Gαi-dependent manner [47]. The fact that Ghsr is expressed by delta cells and that Sstr5 is not detected in any mouse islet endocrine cell (Figure 2) makes this model untenable. More recently, coupling of the GHS-1R with transient receptor potential melastatin 2 (TRPM2) was suggested as an alternate explanation to reconcile ghrelin's insulin inhibition with Gαq/11 and phospholipase C-mediated increases in Ca2+ [48]. Attenuation by ghrelin of non-selective glucose-induced cation currents via TRPM2 that promote insulin release led to a model in which TRPM2 was placed downstream of Ghrelin/GHSR-1R on beta cells [48]. The fact that we demonstrate that Ghsr is expressed exclusively by delta not by beta cells would not alter the hierarchy of Trmp2 downstream of ghrelin/GHS-1R but would merely insert somatostatin as an intermediate factor between ghrelin and Trpm2.
Our observation that beta cells do not express Ghsr is in agreement with a prior report that detected no Ghsr on HIT-T15 beta cells [49]. This study observed that ghrelin promotes beta cell proliferation and prevents beta cell apoptosis. Since these effects were also observed in response to des-acyl-ghrelin and depended on adenyl cyclase and protein kinase A, it was suggested that they are mediated via an as yet unidentified receptor distinct from GHS-R1 [49]. This is certainly an intriguing possibility, and we do not rule out the presence of an alternate receptor with high affinity for ghrelin and des-acyl-ghrelin in the islet. If an alternate ghrelin receptor indeed exists within the islet, this suggests the possibility of secondary effects of ghrelin stimulation that are mediated indirectly via local intermediates. Nevertheless, we report here that the actions of ghrelin (and not des-acyl-ghrelin) are in full agreement with the selective activation of delta cell GHS-R1 by ghrelin. There has been a prior report that suggested ghrelin had no effect on basal somatostatin release but modestly inhibited arginine-induced somatostatin secretion in perfused rat pancreas [50]. We are unable to offer an explanation that reconciles this observation with our current data.
We think that many of the discrepancies with regard to the mechanistic understanding of ghrelin's insulinostatic actions can be traced to observations that place Ghsr expression on alpha or beta cell lines. As the canonical signaling cascade in response to ghrelin would promote exocytosis, stimulation of insulin (from beta cell lines) or glucagon (from alpha cell lines) in response to ghrelin is the default response. However, caution should be exercised when extrapolating these observations on cell lines to the primary endocrine cells they resemble most closely. All islet endocrine cells derive from a common lineage. Key characteristics of primary islet endocrine cells are often lost in cell lines, with traits normally restricted to one particular cell type reappearing in cell lines that are thought to resemble another islet endocrine cell. The transcriptomes we report here constitute a valuable repository to validate the cell type that expresses any gene that is detectably expressed within pancreatic islets.
5. Conclusions
We contribute significantly to the understanding of the physiological role of ghrelin by offering an alternate mechanistic explanation how ghrelin achieves its well-known insulinostatic actions, which is relevant given ghrelin's central role in metabolism [17]. Moreover, our observations underscore that delta cells play an underappreciated but important physiological role in controlling insulin output by local feedback within pancreatic islets [7]. The high quality transcriptomes from delta, beta, and alpha cells that were instrumental in this discovery will be invaluable to unravel the complex crosstalk that tightly balances insulin and glucagon release from healthy islets and breaks down in diabetes.
Funding
This research was supported by by a Career Development Award from the Juvenile Diabetes Research Foundation (2-2013-54) and an Individual Biomedical Research Award from the Hartwell Foundation (201500731), both to MOH.
Author contributions
MRD conducted functional imaging on intact islets and contributed to writing and discussions. AM and CC-Z conducted all bioinformatics analyses and contributed to discussions. CJD and JV measured somatostatin secretion, generated tracers and and maintained the RIA for somatostatin. GN conducted immunofluorescence and FISH experiments. TvdM conducted experiments and contributed to the writing, editing and discussions. MOH designed the study and directed the project, generated delta, beta and alpha cell transcriptomes, conducted static and dynamic somatostatin secretion experiments and wrote the paper.
Acknowledgments
The authors thank the members of the Huising lab for constructive input and discussion. Human islets were obtained through the NIDDK-supported Integrated Islet Distribution Program (IIDP). Dr. Michael Beyermann and Jean Rivier (Salk Institute for Biological Studies) are gratefully acknowledged for providing ghrelin and des-acyl-ghrelin.
Footnotes
The transcriptome data reported in this paper can be accessed at http://huisinglab.com/islet_txomes_2016/ and have been deposited in the Gene Expression Omnibus (GEO) repository under accession number GSE80673.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.04.007.
Contributor Information
Michael R. DiGruccio, Email: mrdigruccio@ucdavis.edu.
Alex M. Mawla, Email: ammawla@ucdavis.edu.
Cynthia J. Donaldson, Email: donaldson@salk.edu.
Glyn M. Noguchi, Email: gmnoguchi@ucdavis.edu.
Joan Vaughan, Email: vaughan@salk.edu.
Talitha van der Meulen, Email: tvandermeulen@ucdavis.edu.
Mark O. Huising, Email: mhuising@ucdavis.edu.
Conflict of interest
The authors have no conflicts to declare.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Seminars in Cell & Developmental Biology. 2013;24(1):11–21. doi: 10.1016/j.semcdb.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y.H., Szabat M., Bragagnini C., Kott K., Helgason C.D., Hoffman B.G. Paracrine signalling loops in adult human and mouse pancreatic islets: netrins modulate beta cell apoptosis signalling via dependence receptors. Diabetologia. 2011;54(4):828–842. doi: 10.1007/s00125-010-2012-5. [DOI] [PubMed] [Google Scholar]
- 3.Amisten S., Salehi A., Rorsman P., Jones P.M., Persaud S.J. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacology & Therapeutics. 2013;139(3):359–391. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Hauge-Evans A.C., King A.J., Carmignac D., Richardson C.C., Robinson I.C., Low M.J. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58(2):403–411. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taborsky G.J., Jr., Smith P.H., Porte D., Jr. Interaction of somatostatin with the A and B cells of the endocrine pancreas. Metabolism. 1978;27(9 Suppl. 1):1299–1302. doi: 10.1016/0026-0495(78)90062-8. [DOI] [PubMed] [Google Scholar]
- 6.Brown M., Rivier J., Vale W. Biological activity of somatostatin and somatostatin analogs on inhibtion of arginine-induced insulin and glucagon release in the rat. Endocrinology. 1976;98(2):336–343. doi: 10.1210/endo-98-2-336. [DOI] [PubMed] [Google Scholar]
- 7.van der Meulen T., Donaldson C.J., Caceres E., Hunter A.E., Cowing-Zitron C., Pound L.D. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nature Medicine. 2015;21(7):769–776. doi: 10.1038/nm.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Meulen T., Xie R., Kelly O.G., Vale W.W., Sander M., Huising M.O. Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic alpha and beta cells. PLoS One. 2012;7(12):e52181. doi: 10.1371/journal.pone.0052181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum B., Hrvatin S.S., Schuetz C., Bonal C., Rezania A., Melton D.A. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nature Biotechnology. 2012;30(3):261–264. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozzo A., Meneghel-Rozzo T., Delakorda S.L., Yang S.B., Rupnik M. Exocytosis of insulin: in vivo maturation of mouse endocrine pancreas. Annals of the New York Academy of Sciences. 2009;1152:53–62. doi: 10.1111/j.1749-6632.2008.04003.x. [DOI] [PubMed] [Google Scholar]
- 11.Benner C., van der Meulen T., Caceres E., Tigyi K., Donaldson C.J., Huising M.O. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15(1):620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dezaki K., Hosoda H., Kakei M., Hashiguchi S., Watanabe M., Kangawa K. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004;53(12):3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 13.Dezaki K., Damdindorj B., Sone H., Dyachok O., Tengholm A., Gylfe E. Ghrelin attenuates cAMP-PKA signaling to evoke insulinostatic cascade in islet beta-cells. Diabetes. 2011;60(9):2315–2324. doi: 10.2337/db11-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reimer M.K., Pacini G., Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144(3):916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 15.Tong J., Prigeon R.L., Davis H.W., Bidlingmaier M., Kahn S.E., Cummings D.E. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59(9):2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yada T., Damdindorj B., Rita R.S., Kurashina T., Ando A., Taguchi M. Ghrelin signalling in beta-cells regulates insulin secretion and blood glucose. Diabetes, Obesity and Metabolism. 2014;16(Suppl. 1):111–117. doi: 10.1111/dom.12344. [DOI] [PubMed] [Google Scholar]
- 17.Muller T.D., Nogueiras R., Andermann M.L., Andrews Z.B., Anker S.D., Argente J. Ghrelin. Molecular Metabolism. 2015;4(6):437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huising M.O., van der Meulen T., Vaughan J.M., Matsumoto M., Donaldson C.J., Park H. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proceedings of the National Academy of Sciences U S A. 2010;107(2):912–917. doi: 10.1073/pnas.0913610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi H., He M., Wu P., Kim S., Paik R., Sugino K. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrera P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127(11):2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 22.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 24.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xin Y., Kim J., Ni M., Wei Y., Okamoto H., Lee J. Use of the Fluidigm C1 platform for RNA sequencing of single mouse pancreatic islet cells. Proceedings of the National Academy of Sciences U S A. 2016;113(12):3293–3298. doi: 10.1073/pnas.1602306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 27.Vale W., Rivier J., Ling N., Brown M. Biologic and immunologic activities and applications of somatostatin analogs. Metabolism. 1978;27(9 Suppl. 1):1391–1401. doi: 10.1016/0026-0495(78)90081-1. [DOI] [PubMed] [Google Scholar]
- 28.Reubi J.C., Schaer J.C., Wenger S., Hoeger C., Erchegyi J., Waser B. SST3-selective potent peptidic somatostatin receptor antagonists. Proceedings of the National Academy of Sciences U S A. 2000;97(25):13973–13978. doi: 10.1073/pnas.250483897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulder H., Lindh A.C., Sundler F. Islet amyloid polypeptide gene expression in the endocrine pancreas of the rat: a combined in situ hybridization and immunocytochemical study. Cell and Tissue Research. 1993;274(3):467–474. doi: 10.1007/BF00314543. [DOI] [PubMed] [Google Scholar]
- 30.Artner I., Hang Y., Mazur M., Yamamoto T., Guo M., Lindner J. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59(10):2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., McKenna L.B., Bogue C.W., Kaestner K.H. The diabetes gene Hhex maintains delta-cell differentiation and islet function. Genes & Development. 2014;28(8):829–834. doi: 10.1101/gad.235499.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petri A., Ahnfelt-Ronne J., Frederiksen K.S., Edwards D.G., Madsen D., Serup P. The effect of neurogenin3 deficiency on pancreatic gene expression in embryonic mice. Journal of Molecular Endocrinology. 2006;37(2):301–316. doi: 10.1677/jme.1.02096. [DOI] [PubMed] [Google Scholar]
- 33.Su Y., Jono H., Misumi Y., Senokuchi T., Guo J., Ueda M. Novel function of transthyretin in pancreatic alpha cells. FEBS Letters. 2012;586(23):4215–4222. doi: 10.1016/j.febslet.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Alexander S.P., Mathie A., Peters J.A. Guide to receptors and channels (GRAC), 5th edition. British Journal of Pharmacology. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braun M., Ramracheya R., Amisten S., Bengtsson M., Moritoh Y., Zhang Q. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia. 2009;52(8):1566–1578. doi: 10.1007/s00125-009-1382-z. [DOI] [PubMed] [Google Scholar]
- 37.Schaffer A.E., Taylor B.L., Benthuysen J.R., Liu J., Thorel F., Yuan W. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genetics. 2013;9(1):e1003274. doi: 10.1371/journal.pgen.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salehi A., Qader S.S., Grapengiesser E., Hellman B. Pulses of somatostatin release are slightly delayed compared with insulin and antisynchronous to glucagon. Regulatory Peptides. 2007;144(1–3):43–49. doi: 10.1016/j.regpep.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Gauna C., van de Zande B., van Kerkwijk A., Themmen A.P., van der Lely A.J., Delhanty P.J. Unacylated ghrelin is not a functional antagonist but a full agonist of the type 1a growth hormone secretagogue receptor (GHS-R) Molecular and Cellular Endocrinology. 2007;274(1–2):30–34. doi: 10.1016/j.mce.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Tong J., Davis H.W., Summer S., Benoit S.C., Haque A., Bidlingmaier M. Acute administration of unacylated ghrelin has no effect on basal or stimulated insulin secretion in healthy humans. Diabetes. 2014;63(7):2309–2319. doi: 10.2337/db13-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kageyama H., Funahashi H., Hirayama M., Takenoya F., Kita T., Kato S. Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regulatory Peptides. 2005;126(1–2):67–71. doi: 10.1016/j.regpep.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 42.Howard A.D., Feighner S.D., Cully D.F., Arena J.P., Liberator P.A., Rosenblum C.I. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 43.Dezaki K., Kakei M., Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes. 2007;56(9):2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 44.Chuang J.C., Sakata I., Kohno D., Perello M., Osborne-Lawrence S., Repa J.J. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Molecular Endocrinology. 2011;25(9):1600–1611. doi: 10.1210/me.2011-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samols E., Marri G., Marks V. Promotion of insulin secretion by glucagon. Lancet. 1965;2(7409):415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- 46.Samols E., Marri G., Marks V. Interrelationship of glucagon, insulin and glucose. The insulinogenic effect of glucagon. Diabetes. 1966;15(12):855–866. doi: 10.2337/diab.15.12.855. [DOI] [PubMed] [Google Scholar]
- 47.Park S., Jiang H., Zhang H., Smith R.G. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proceedings of the National Academy of Sciences U S A. 2012;109(46):19003–19008. doi: 10.1073/pnas.1209590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurashina T., Dezaki K., Yoshida M., Sukma Rita R., Ito K., Taguchi M. The beta-cell GHSR and downstream cAMP/TRPM2 signaling account for insulinostatic and glycemic effects of ghrelin. Scientific Reports. 2015;5:14041. doi: 10.1038/srep14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Granata R., Settanni F., Biancone L., Trovato L., Nano R., Bertuzzi F. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology. 2007;148(2):512–529. doi: 10.1210/en.2006-0266. [DOI] [PubMed] [Google Scholar]
- 50.Egido E.M., Rodriguez-Gallardo J., Silvestre R.A., Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. European Journal of Endocrinology. 2002;146(2):241–244. doi: 10.1530/eje.0.1460241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.