Abstract
Objectives
Phosphatidylinositol-3-OH kinase (PI3K) signalling in the endocrine pancreas contributes to glycaemic control. However, the mechanism by which PI3K modulates insulin secretion from the pancreatic beta cell is poorly understood. Thus, our objective was two-fold; to determine the signalling pathway by which acute PI3K inhibition enhances glucose-stimulated insulin secretion (GSIS) and to examine the role of this pathway in islets from type-2 diabetic (T2D) donors.
Methods
Isolated islets from mice and non-diabetic or T2D human donors, or INS 832/13 cells, were treated with inhibitors of PI3K and/or phosphodiesterases (PDEs). The expression of PI3K-C2β was knocked down using siRNA. We measured insulin release, single-cell exocytosis, intracellular Ca2+ responses ([Ca2+]i) and Ca2+ channel currents, intracellular cAMP concentrations ([cAMP]i), and activation of cAMP-dependent protein kinase A (PKA) and protein kinase B (PKB/AKT).
Results
The non-specific PI3K inhibitor wortmannin amplifies GSIS, raises [cAMP]i and activates PKA, but is without effect in T2D islets. Direct inhibition of specific PDE isoforms demonstrates a role for PDE3 (in humans and mice) and PDE8 (in mice) downstream of PI3K, and restores glucose-responsiveness of T2D islets. We implicate a role for the Class II PI3K catalytic isoform PI3K-C2β in this effect by limiting beta cell exocytosis.
Conclusions
PI3K limits GSIS via PDE3 in human islets. While inhibition of p110α or PIK-C2β signalling per se, may promote nutrient-stimulated insulin release, we now suggest that this signalling pathway is perturbed in islets from T2D donors.
Keywords: PI3K, cAMP, PDE, Beta cells, Insulin secretion, T2D
Highlights
-
•
PI3K limits GSIS via PDE3 in human islets and PDE3/8 in mouse islets.
-
•
PI3K-inhibition does not augment GSIS in islets from T2D donors.
-
•
PDE inhibition restores glucose-responsiveness in islets from T2D donors.
-
•
PI3K-C2β plays a significant role in limiting beta cell exocytosis.
1. Introduction
Type 2 diabetes (T2D) is a metabolic disorder characterized by high blood glucose levels and insulin resistance [1], [2]. Dysfunctional pancreatic beta cells of the islets of Langerhans play a crucial role in the development of T2D, as overt hyperglycemia only develops following inadequate insulin release [3], [4]. Thus, defects in the insulin secreting beta cells are critical to the pathogenesis and progression of T2D.
The preservation of nutrient-stimulated insulin secretion is essential for proper glycaemic control, particularly in the face of insulin resistance [5], [6]. The main trigger for insulin release from the beta cell is glucose. Briefly, the mitochondrial metabolism of glucose mediates closure of ATP-sensitive K+ (KATP) channels, elicits membrane depolarization, and activates voltage-gated Ca2+ channels (VGCCs), triggering Ca2+-dependent exocytosis of insulin containing granules [7], [8], [9].
However, multiple pathways are involved in the modulation of insulin release from the beta cell [10], [11], [12]. For example, both receptor-dependent and constitutively active phosphatidylinositol 3-OH kinase (PI3K) isoforms are important regulators of islet mass and function [13], [14], [15], [16], [17], [18]. It is generally well-accepted that, although specific PI3K isoforms exert either negative [19], [20] or positive [21], [22] regulation of insulin secretion, the net effect of non-selective PI3K inhibition is an amplification of glucose-stimulated insulin secretion (GSIS) [17], [20], [23], [24], consistent with an acute negative feedback role for insulin receptor (IR) signalling [25], [26]. However, the mechanism by which PI3K limits GSIS from pancreatic beta cells is poorly understood. Though a number of theories have been proposed [17], [19], [24], the consensus suggests that non-specific PI3K inhibition amplifies GSIS downstream of Ca2+ entry into the beta cell, and this is likely due to a rise in cAMP resulting from phosphodiesterase (PDE) inhibition [24]. However, the PI3K and PDE isoforms that mediate this, their impact on downstream insulin exocytosis, and a potential role in human T2D have not yet been elucidated.
In the present study, we investigated effectors downstream of PI3K in isolated mouse and human islets. Given that individuals with T2D show perturbed PI3K signalling in both insulin sensing tissues and the islets themselves [27], [28], [29], we also examined the importance of this pathway in islets isolated from T2D donors. Our results indicate that the inhibition of PI3K amplifies GSIS through a cyclic adenosine monophosphate (cAMP)-dependent mechanism. A highly membrane permeable antagonist of cAMP [30] blocked the insulinotropic effect of PI3K-inhibition. We find that PI3K likely promotes the activity of phosphodiesterase 3 (PDE3; and PDE8 in mice) to suppress intracellular cAMP ([cAMP]i) and downstream cAMP-dependent protein kinase A (PKA) signal transduction. Furthermore, we implicate the involvement of specific PI3K catalytic subunits p110α and PI3K-C2β. Finally, we report that for islets of T2D donors in which GSIS is defective, the classical PI3K inhibitor wortmannin fails to raise levels of cAMP while also failing to amplify GSIS. We can, however, restore glucose-responsiveness in these islets by directly inhibiting PDEs, which implies an upstream defect in PI3K signalling. Collectively, these results suggest that the PI3K-PDE-cAMP pathway is impaired in the T2D beta cell, and this signalling pathway impairment may be one mechanism contributing to beta cell dysfunction.
2. Materials and methods
2.1. Cells and cell culture
Islets from male C57/BL6 mice were isolated by collagenase digestion, and subsequently purified by handpicking. Human islets from 31 healthy donors (50+/−15 years of age) and from 7 T2D donors (60+/−8 years of age) were from the Clinical Islet Laboratory at the University of Alberta or the IsletCore program at the Alberta Diabetes Institute. Human islet donor characteristics are summarized in Supplementary Tables 1 and 2. For experiments measuring membrane capacitance and Ca2+ currents, islets were dispersed in Ca2+-free buffer and plated as single cells in 35-mm culture dishes. INS 832/13 cells were a gift of Dr. Christopher Newgard (Duke University). Islet and cell culture methods were described previously [20], [21]. The Animal Care and Use Committee and the Human Research Ethics Board at the University of Alberta approved all studies.
2.2. Pharmacologic inhibitors and peptides
Wortmannin, a covalent inhibitor of PI3Ks (displaying similar potency for Class I, II and III catalytic isoforms [31]), was from Sigma Aldrich Canada (Oakville, ON). PIK-75 (Symansis, Shanghai, China) is a selective p110α inhibitor (IC50 – 8 nM for p110α versus 907 nM for p110β) [32]. TGX-221 (Symansis) is a selective inhibitor for p110β (IC50 – 5 nM for p110β versus 1 μM for p110α). These exhibit no notable activity against a wide array of lipid kinases at 1 μM and 10 μM respectively [33]. A66 (2S-N1-(5-(2-tert-butylthiazol-4-yl)-4-methylthiazol-2-yl)pyrrolidine-1,2-dicarboxamide; Selleckchem, Houston, TX) inhibits both p110α (IC50 – 32 nM) and the class-II PI3K, PI3K-C2β (IC50 – 462 nM), with no notable activity against other lipid kinases or the related kinases DNA-PK and mTOR at 10 μM [34].
IBMX (3-Isobutyl-1-methylxanthine; Santa Cruz Biotechnology, Santa Cruz, CA) is a non-specific inhibitor of PDEs, with activity against PDE1, 2, 3, 4, and 5 with respective IC50 values of 19, 50, 18, 13 and 32 nM. Notably, IBMX does not have activity against PDE8; an isoform shown to be important in GSIS in rat islets and INS1 cells [35]. Milrinone (1,6-Dihydro-2-methyl-6-oxo-(3,4-bipyridine)-5-carbonitrile) (Santa Cruz Biotechnology) is a specific inhibitor of PDE3 with an IC50 value of 0.3 μM. Dipyridamole (2,6-Bis (diethanolamine)-4,8-dipiperidinopyrimido [5,4-d] pyrimidine; Sigma Aldrich Canada) is an inhibitor of PDE5 (IC50 – 0.39 μM) and the IBMX- insensitive PDE8 isoform (IC50 – 4.5 μM) [36].
Rp-8-Br-cAMPS-pAB, a novel, highly membrane permeable prodrug derivative of the cAMP antagonist Rp-cAMPS [30], and the control para-acetoxybenzyl ester (pAB) were from the Biolog Life Science Institute (Bremen, Germany). GLP-1 (7-36) peptide was from AnaSpec (Fermont, CA). Forskolin was from Tocris Bioscience (Bristol, UK).
2.3. siRNA constructs and quantitative PCR
PI3K-C2β and scrambled siRNA constructs were from OriGene (Rockville, MD). An Alexa Fluor 488-modified negative siRNA construct was from Qiagen (Toronto, ON). These were transfected in dissociated mouse islet cells or MIN6 cells using DharmaFECT 1 (GE Healthcare, Mississauga, ON). For quantitative PCR, RNA from MIN6 cells was extracted 48-hrs post transfection using TRIzol Reagent (Life Technologies, Burlington, ON), and cDNA was synthesized using Super Script II and oligodT (Life Technologies) according to the manufacturer's protocol. Real-time PCR to detect PI3K-C2β was performed as previously described [22]. Primers were as follows: left: TCCACCAGACCCTCTGCTAC and right: AACTTGCGGCAATATTGGAT.
2.4. Insulin secretion measurements
Insulin secretion was measured in either static or perifusion secretion assays at 37 °C in KRB solution as described previously [20], [21], [37]. For static secretion experiments 12 mouse (or human) islets per replicate were treated with wortmannin (1 nM–1 μM), A66 (1 nM–10 μM) or equivolume DMSO (0.1%) during the 2.8 mM (or 1 mM) glucose KRB 2-hr pre-incubation; these compounds were present for the duration of the experiment. For some experiments, islets were stimulated with GLP-1 (100 nM), forskolin (100 nM or 1 μM), IBMX (100 μM), dipyridamole (50 μM), or milrinone (10 μM) during the 1-hr low glucose (1 mM or 2.8 mM) and 1-hr high glucose (16.7 mM) KRB stimulation as indicated. For perifusion secretion experiments, 15 islets per lane were perifused (0.25 ml/min) with 2.8 mM glucose KRB for 24 min and then with the indicated condition.
For the secretion experiments with the Rp-8-Br-cAMPS-pAB (10 μM) cAMP antagonist, or the pAB control ester (10 μM), mouse islets were treated with wortmannin (100 nM) or equivolume DMSO during the second 1-hr 2.8 mM glucose pre-incubation. Rp-8-Br-cAMPS-pAB or the pAB control ester was present during both the 1-hr low glucose (2.8 mM) and 1-hr high glucose (16.7 mM) KRB stimulation as indicated. Samples were stored at −20 °C and were assayed for insulin via enzyme-linked immunosorbent assay (MSD, Rockville, MD).
2.5. Electrophysiology
Mouse and human islets were dissociated into single cells. Experiments were performed at 32–35 °C. We used the standard whole cell technique with the sine + DC lockin function of an EPC10 amplifier and Patchmaster software (HEKA Electronics, Lambrecht/Pfalz, Germany). Solutions used for capacitance measurements are previously described [20], [21]. Cells were treated with wortmannin (100 nM) or equivolume DMSO (0.1%) 1-hr prior to the recordings. Capacitance increases and Ca2+ currents were normalized to initial whole-cell capacitance and expressed as femtofarad per picofarad (fF/pF), and picoamperes per picofarad (pA/pF). We identified mouse beta cells by size, whereas human beta cells were identified by positive insulin immunostaining.
2.6. Immunoblotting
INS 832/13 cells were grown to 100% confluence in 6-well plates. Cells were treated with wortmannin (100 nM) or equivolume DMSO (0.1%) in KRB at the beginning of the 2.8 mM glucose 2-hr pre-incubation at 37 °C; these compounds were present for the duration of the experiment. Cells were treated with insulin (200 nM) or forskolin (1 μM) for 15 min in 16.7 mM KRB. Cells were then washed once with PBS, and then lysed using a standard RIPA lysis buffer supplemented with phosphodiesterase and protease inhibitor cocktails (Millipore, Etobicoke, ON).
Cell lysates were subjected to SDS-PAGE on Mini-PROTEAN TGX Stain-Free gels (Bio-Rad, Mississauga, ON) transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA), and probed with primary antibodies: anti-phospho-(Ser/Thr) AKT substrate; #9611, anti-phospho-(Ser/Thr) PKA substrate; #9621, anti-AKT; #9272, anti-PKA C-α; #4782 (Cell Signaling Technology, Beverly, MA) and anti-β-actin; sc-4778 (Santa Cruz Biotechnology). Detection was with peroxidase-conjugated secondary anti-rabbit (NA934V) and anti-mouse antibodies (NA931V) (GE Healthcare), and visualization by chemiluminescence with ECL-Plus (GE Healthcare). Images were acquired using a ChemiDoc MP System (Bio-Rad) and analyzed using Image Lab Software 5.2.1 (Bio-Rad).
2.7. Intracellular cAMP measurements
Islets were treated with wortmannin (100 nM) or equivolume DMSO (0.1%) in KRB at the beginning of the 2.8 mM (or 1 mM) glucose 2-hr pre-incubation; these compounds were present for the duration of the experiment. Islets (30 mouse islets or 50 human islets per triplicate) were then handpicked into 1.7 ml microcentrifuge tubes in 500 μl of 2.8 mM (or 1 mM) glucose KRB and transferred to the 37 °C incubator. Following a 1-hr incubation period, the low glucose KRB solution was replaced with 500 μl of 16.7 mM high glucose KRB. For the forskolin-stimulated experiments, 1 μM forskolin was included in the 16.7 mM KRB. Following a subsequent 1-hr incubation, the KRB solution was removed and islets were snap-frozen in liquid nitrogen and stored at −80 °C. On day of cAMP assay 1 mM phenylmethylsulfonyl fluoride (PMSF) was added to cell lysates; intracellular cAMP levels were measured using a Cyclic AMP XP assay kit from Cell Signaling as per manufacture's protocol.
2.8. Fluorescence measurement of intracellular Ca2+ and PKA activation
For the measurement of intracellular Ca2+ ([Ca2+]i), mouse islets were incubated for 45 min with 10 μM Fura Red-AM (Invitrogen, Burlington, ON), 0.08% pluronic acid (Life Technologies), and wortmannin (100 nM) or equivolume DMSO (0.1%) in an extracellular imaging solution as described previously [20]. Islets were imaged in fresh imaging solution (containing wortmannin or DMSO) with 0.5 mM glucose at 37 °C; the glucose concentration was increased as indicated. Fluorescence was measured every 5 s at 440 nm and 490 nm (excitation). Emission was collected using a 660/50 nm bandpass filter. Images were analyzed with Ratio Cam software (Metamorph, Sunnyvale, CA, USA).
For fluorescence measurements of PKA activation, INS 832/13 cells were transduced for 16-hrs with adenovirus directing expression of the FRET reporter AKAR3 [38]. Briefly, the culture media was replaced by 170 μl/well of saline containing 11.1 mM glucose and 0.1% BSA so that assays could be performed using a FlexStation 3 microplate reader. Excitation was at 435/9 nm using a 455 nm cut-off dichroic mirror, and emitted light was detected at 485/15 nm (CFP) or 535/15 nm (YFP). For each well, the average emission intensity was calculated from 12 excitation flashes for each time point. Test solutions dissolved in saline containing 0.1% DMSO were placed in V-bottom 96-well plates (Greiner Bio-One, Monroe, NC) and an automated pipetting procedure was used to transfer 30 μl of each test solution to the assay plate containing cells. The CFP/YFP emission ratio was calculated for each well, and the values for 8 wells were averaged. The time course of the change of FRET ratio was plotted after exporting these data to Origin v.8.0 (Northampton, MA).
2.9. Statistical analysis
For single-cell studies, the n-values represent the number of cells studied from at least three separate experiments or human islet donors. For measurement of [Ca2+]i, n-values represent the number of islets studied from three experiments. For GSIS studies, n-values represent the numbers of replicates from at least three separate experiments or human islet donors. Electrophysiology data were analyzed using FitMaster v2.32 (HEKA Electronik). All data were analyzed by paired t-test or by a two-way ANOVA followed by a post hoc t-test using the Tukey HSD method. Outliers were identified and removed using Grubb's test for outliers. Data are expressed as means ± SEM, and p < 0.05 was considered significant.
3. Results
3.1. PI3K inhibition amplifies GLP-1 and forskolin-potentiated insulin secretion in mouse pancreatic islets
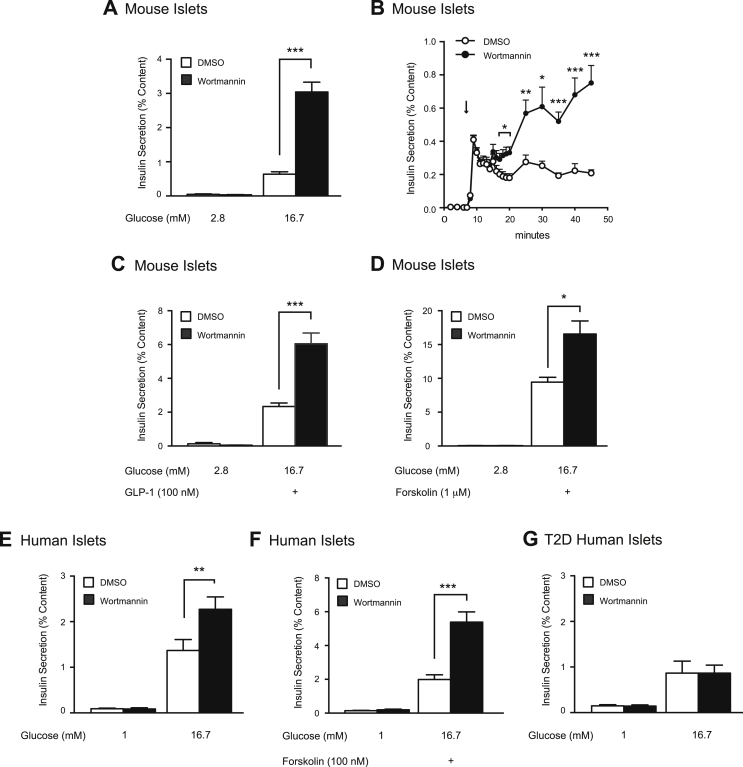
We, and others, have shown that the non-specific PI3K inhibitor wortmannin potentiates GSIS in insulin-secreting cell lines and rodent islets, suggesting that PI3K plays a limiting role in insulin secretion [20], [23], [24]. Here we confirm that wortmannin amplifies GSIS in mouse islets (n = 12,12; p < 0.001; Figure 1A), via effects on the second phase of insulin secretion (n = 7,7; Figure 1B). This was concentration-dependent with an EC50 of 3.2 nM (Supplementary Figure 1), which is consistent with the known IC50 for PI3K inhibition by wortmannin [39], [40]. Wortmannin treatment had no effect on insulin secretion under low glucose conditions (Figure 1A) or on insulin content (Supplementary Figure 2A).
Figure 1.
PI3K inhibition amplifies insulin secretion in mouse and human islets, but not in islets from T2D donors. A, Static GSIS was measured from mouse islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars). B, GSIS was measured via perifusion of mouse islets treated with DMSO (0.1%) (open circle) or wortmannin (100 nM) (black circle). C, GLP-1 (100 nM)-amplified GSIS was measured from mouse islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars). D, Forskolin (1 μM)-amplified GSIS was measured from mouse islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars). E, shows the same as A, but in human islets from non-diabetic donors. F, Forskolin (100 nM)-amplified GSIS was measured from non-diabetic human islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars). G, shows the same as E, but in human islets from T2D donors. *-p < 0.05, **-p < 0.01, ***-p < 0.001.
Wortmannin also amplifies the secretory response following treatment with common insulin secretagogues: GLP-1 and forskolin. In the presence of GLP-1 (100 nM), wortmannin potentiates GSIS by 2.6-fold (n = 12,12; p < 0.001; Figure 1C) and in the presence of forskolin (1 μM) by 1.8-fold (n = 12,12; p < 0.05; Figure 1D). Neither treatment condition significantly affected insulin content (Supplementary Figure 2B, C).
3.2. PI3K inhibition amplifies GSIS in non-diabetic, but not T2D human islet donors
Rodent islets are crucial to diabetes research. However, many studies overlook species specific disparities in islet responses to glucose [41], [42]. We now show that wortmannin potentiates GSIS by 1.7-fold in isolated human islets (n = 16,17 from 6 unique donors; p < 0.01; Figure 1E). Wortmannin also amplifies GSIS as potentiated by forskolin (100 nM) (n = 18,18 from 6 unique donors; p < 0.001; Figure 1F). Insulin content was unchanged in both experiments (Supplementary Figure 2D, E).
We next examined the ability of wortmannin to potentiate GSIS in islets isolated from T2D donors. We show that wortmannin does not amplify GSIS in these islets (n = 14, 14 from 5 unique donors; Figure 1G), a finding consistent with impaired PI3K signalling in islets from T2D donors [18], [29] and failure of wortmannin to amplify GSIS in isolated islets from ob/ob mice [23]. Insulin content was again unchanged (Supplementary Figure 2F).
3.3. PI3K inhibition amplifies beta cell exocytosis downstream of [Ca2+]i
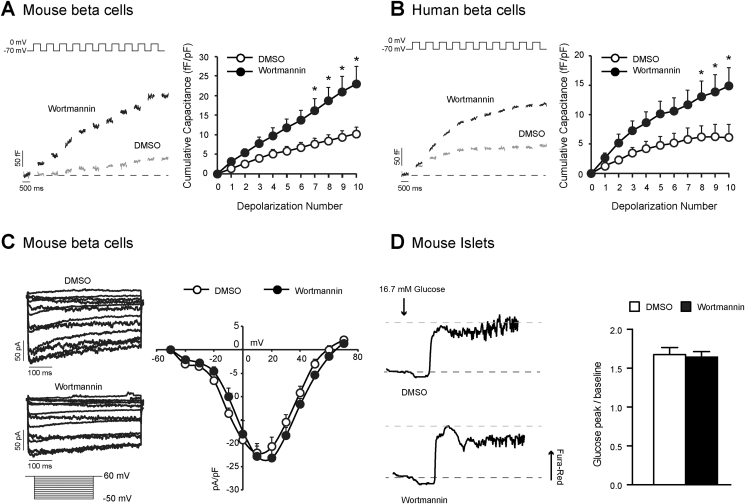
While we and others have demonstrated the importance of islet PI3K signalling in glycaemic control, the molecular mechanism by which PI3K antagonizes insulin secretion is not well understood. Previous work suggests that PI3K suppresses GSIS downstream of the glucose-stimulated rise in [Ca2+]i [17]. Thus, we examined whole-cell membrane capacitance changes and voltage-dependent Ca2+ channel activity in single beta cells from mouse and human islets. The total exocytotic response was increased by 2.3-fold in mouse beta cells following a 1-hr treatment with wortmannin (100 nM) (n = 16,18; p < 0.05; Figure 2A). Similarly, the exocytotic response in human beta cells was increased by 2.4-fold following wortmannin (100 nM) treatment (n = 34,25 from 4 unique donors; p < 0.05; Figure 2B). Furthermore, we were unable to detect significant changes in beta cell Ca2+ channel activity (n = 15,19; Figure 2C), or whole cell intracellular Ca2+ responses following wortmannin treatment (n = 11,9; Figure 2D).
Figure 2.
Wortmannin amplifies exocytosis downstream of Ca2+entry in to the beta cell. A, (left) Representative capacitance recordings from mouse beta cells treated with DMSO (0.1%) (grey lines) or wortmannin (100 nM) (black lines). (right) Average cumulative capacitance response during each depolarization step is shown. B, shows the same as A, but in beta cell from non-diabetic human donors. C, (left) Representative voltage-dependent Ca2+ channel recordings are shown. (right) Average cumulative voltage-dependent Ca2+ channel responses during each voltage-step was measured in mouse beta cells treated with DMSO (white circles) or wortmannin (100 nM) (black circles). D, Representative responses to the glucose-stimulated increase in the Fura Red-AM ratio in mouse islets treated with DMSO or wortmannin (100 nM) are shown. (right) The average ratio of the glucose peak/baseline response is shown. *-p < 0.05.
3.4. PI3K is a negative regulator of intracellular cAMP
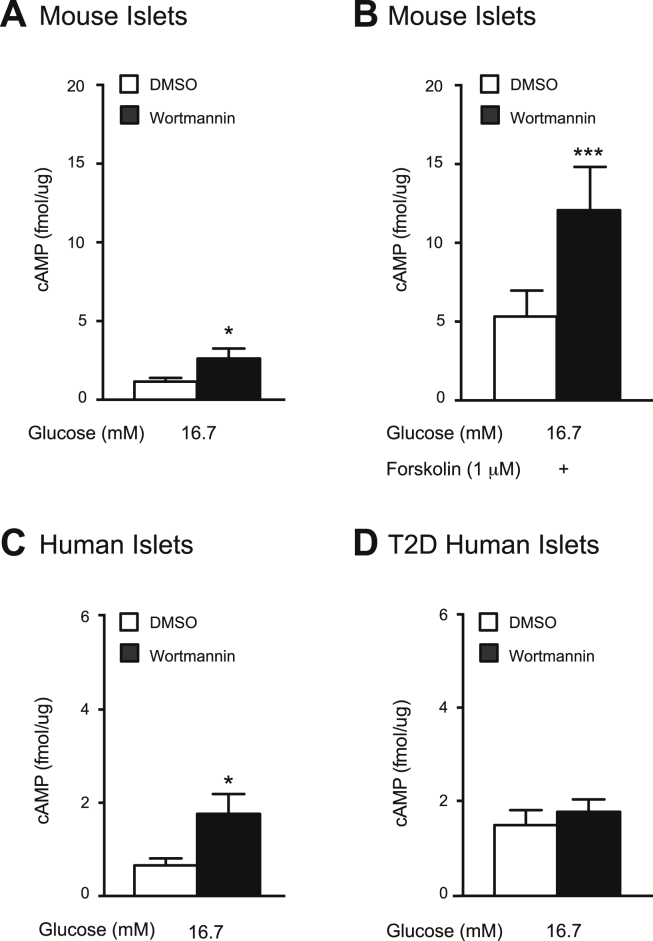
Wortmannin has previously been suggested to raise [cAMP]i in rat islets [24]. Thus, we assessed the effects of PI3K inhibition on [cAMP]i in mouse and human islets. In mouse islets, wortmannin increases glucose-stimulated [cAMP]i by 2.2-fold (n = 13,14; p < 0.05; Figure 3A) and forskolin-amplified [cAMP]i by 2.3-fold (n = 15,15; p < 0.001; Figure 3B). Similarly, in human islets, wortmannin increases [cAMP]i by 2.8-fold (n = 9,9 from 3 unique donors; p < 0.05; Figure 3C), but this response is absent in islets from T2D donors (n = 12,12 from 4 unique donors; Figure 3D).
Figure 3.
PI3K inhibition increases glucose and forskolin-stimulated intracellular cAMP content in mouse and human islets, but not in islets from T2D donors. A, Glucose-stimulated intracellular cAMP content was measured from mouse islets treated with DMSO (0.1%) (open bars) or wortmannin (100 nM) (black bars). B, Forskolin (1 μM)- stimulated intracellular cAMP content was measured from mouse islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars). C, shows the same as A, but in islets from non-diabetic human donors. D, shows the same as A, but in islets from T2D human donors. *-p < 0.05, ***-p < 0.001.
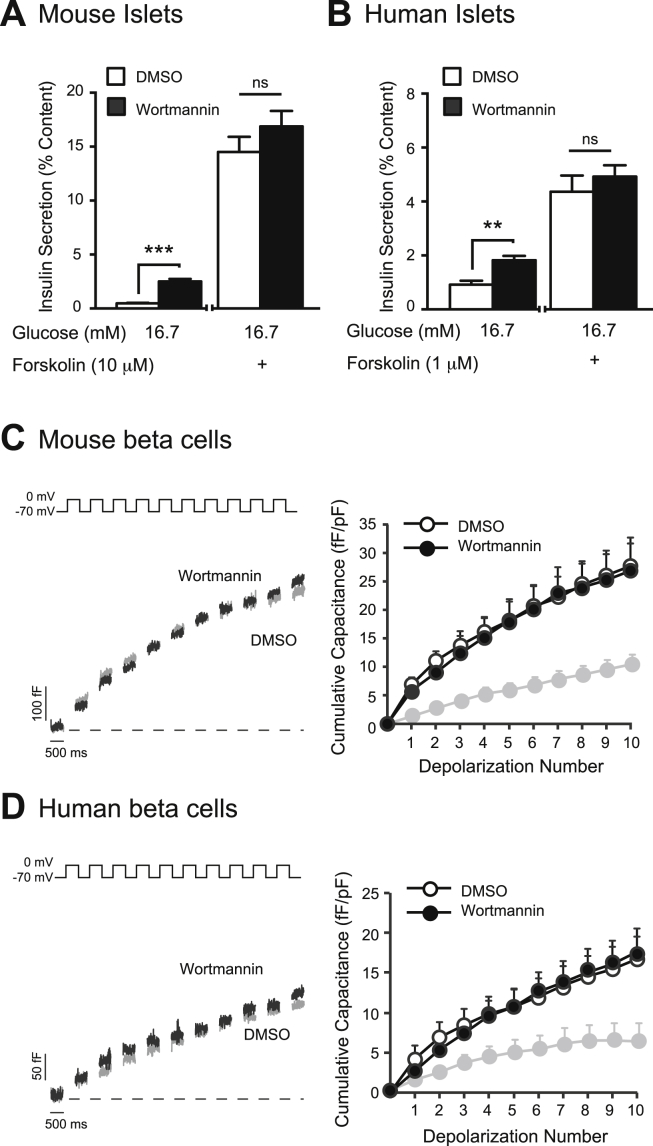
To further support this link between PI3K-cAMP signalling, we show that when forskolin is applied at high concentrations, (>1 μM in mouse islets, and >100 nM in human islets), the amplifying effect of PI3K-inhibition on insulin secretion is obscured (n = 15,15; Figure 4A) and (n = 14,14 from 5 unique donors; Figure 4B). Furthermore, when cAMP is infused via the pipette during patch-clamp experiments, the amplifying effect of wortmannin on exocytosis is also obscured in mouse (n = 20,22; Figure 4C) and in human beta cells (n = 22,22 from 5 unique donors; Figure 4D).
Figure 4.
The amplifying effect of PI3K-inhibition on insulin secretion and exocytosis is obscured with high [cAMP]i. A, GSIS was measured from mouse islets treated with DMSO (0.1%) (open bars) or wortmannin (100 nM) (black bars), together with a high concentration of forskolin (10 μM) B, GSIS was measured from non-diabetic human islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars), together with a moderate concentration of forskolin (100 nM). C, (left) Representative capacitance recordings from mouse beta cells. (right) Average cumulative capacitance response during each depolarization step for beta cells treated with DMSO (open circles) or wortmannin (100 nM) (black circles) and with cAMP infused via the patch pipette. For comparison, the grey line representing the cumulative capacitance response in absence of cAMP infusion (in DMSO-treated mouse beta cells) is shown. D, shows the same as C, but in beta cell from non-diabetic human donors. **-p < 0.01, ***-p < 0.001. ns- non significant.
3.5. PI3K is a negative regulator of PKA
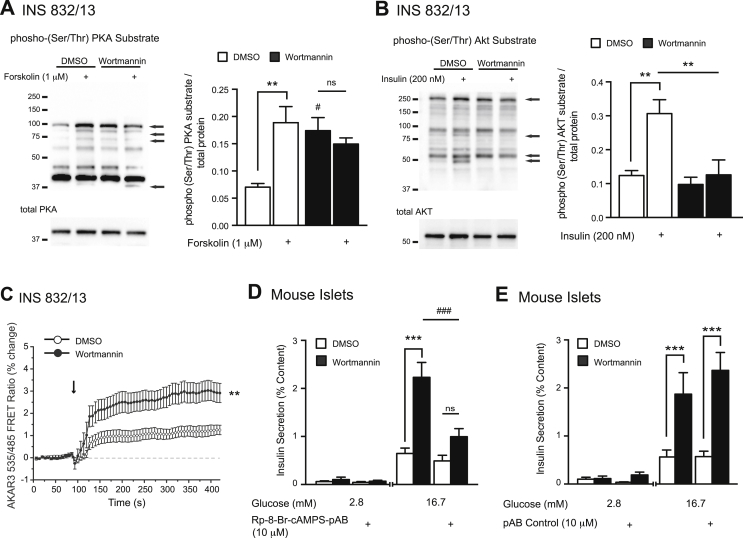
cAMP amplifies GSIS in large part via activation of PKA and subsequent phosphorylation of substrate proteins [43], [44], [45]. Thus, we measured changes in PKA-substrate phosphorylation using western blot and direct PKA activity using a FRET-based reporter assay. Compared to vehicle, wortmannin increases phosphorylation of a number of PKA substrates (n = 4; Figure 5A, black arrows) in INS 832/13 cells. Notably, these changes parallel those seen with forskolin-stimulation (1 μM) in control cells (n = 4; Figure 5A, black arrows). In all treatment groups, total PKA protein levels were unchanged (n = 4; Figure 5A). Importantly, we show that these effects are seen in the face of reduced PKB/AKT activity, as measured via changes in PKB/AKT-substrate protein phosphorylation using western blot (n = 4; Figure 5B). Furthermore, using the PKA-regulated AKAR3 biosensor, we directly show activation of PKA by wortmannin, as inferred from its ability to produce a change in FRET in INS 832/13 cells (n = 3 separate experiments; Figure 5C).
Figure 5.
PI3K inhibition increases PKA substrate (Ser/Thr)- phosphorylation, and amplifies GSIS in a cAMP- dependent manner. A, (left) Representative western blot showing PKA substrate (Ser/Thr)- phosphorylation (top) and total PKA (bottom) in response to forskolin (1 μM) (15 min) in INS 832/13 cells treated with DMSO (0.1%) or wortmannin (100 nM). Black arrows point to changes in protein phosphorylation. (right) Averaged quantified data (normalized to total protein). B, (left) Representative western blot showing AKT Substrate (Ser/Thr)- phosphorylation (top) and total AKT (bottom) in response to 200 nM insulin (15 min) in INS 832/13 cells treated with either vehicle DMSO (0.1%) or wortmannin (100 nM). Black arrows point to changes in protein phosphorylation. (right) Averaged quantified data (normalized to total protein) of proteins identified by black arrows. C, FRET ratio (%485/535) from INS 832/13 cells infected with adenovirus expressing the AKAR3 biosensor demonstrates activation of PKA by 100 nM wortmannin vs. DMSO (at arrow). Control response to 2 μM forskolin and 100 μM IBMX was 0.170+/−0.006 %485/535. D, GSIS was measured from mouse islets acutely treated with DMSO (open bars) or wortmannin (100 nM) (black bars), with the presence of the cAMP antagonist Rp-8-Br-cAMPS-pAB (10 μM). E, shows the same as D, except with the presence of the pAB control compound (10 μM) #-p < 0.05, **-p < 0.01, ***, ###-p < 0.001, ns- non significant.
3.6. Pharmacological antagonism of cAMP ameliorates wortmannin-amplified GSIS
To confirm that increased PKA activity is directly responsible for the wortmannin-mediated amplification of GSIS, we used pharmacological antagonists of PKA. We initially used the well-characterized PKA inhibitor Rp-cAMPS [46]; however, we were unable to block either wortmannin-mediated amplification of GSIS (Supplementary Figure 3A) or forskolin-mediated amplification of GSIS (Supplementary Figure 3B). Given that Rp-cAMPS is a poor PKA antagonist when applied extracellularly on account of its poor membrane permeability [46], we instead used Rp-8-Br-cAMPS-pAB, a novel, highly cell-membrane permeable cAMP antagonist [30]. Rp-8-Br-cAMPS-pAB (10 μM) effectively prevents wortmannin-amplified GSIS in mouse islets (n = 12,12; p < 0.001; Figure 5D). This is in contrast to the pAB control ester (10 μM) (no notable activity against PKA [30]), which has no effect on GSIS amplified by wortmannin (n = 12,12; Figure 5E). We were unable to use this approach in human islets under conditions of static incubation, however, since the pAB control ester (10 μM) itself increased insulin secretion through a mechanism that is likely non-specific (Supplementary Figure 4). Evidently, build up of pAB-derived metabolites under conditions of static incubation leads to an increase of basal insulin secretion from human but not mouse islets. These findings contrasts with the previous report that no such increase of basal insulin secretion occurs under conditions of human islet or rat islet rapid perifusion in which metabolites are quickly cleared from the perifusate [30].
3.7. Amplifying effects of wortmannin on GSIS are mediated by PDE3/8 inhibition
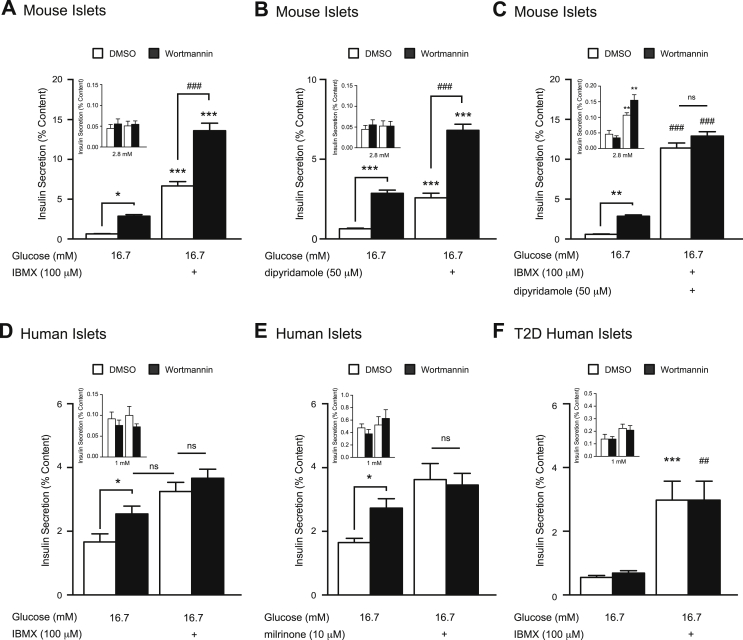
Upstream of PI3K signalling, insulin-like growth factor-1 (IGF1) suppresses GSIS in rat islets by activating cyclic nucleotide phosphodiesterase 3B (PDE3B) [47]. Similarly, outside of the endocrine pancreas, PI3K regulates local [cAMP]i through activation of PDE4 [48]. Given that PDEs are involved in cAMP-PKA signalling, we examined whether the inhibition of PI3K could still amplify insulin secretion in the face of PDE inhibition. In mouse islets, IBMX (100 μM) amplified GSIS by 10-fold compared to vehicle-control (n = 12,12; p < 0.001; Figure 6A), and wortmannin increased GSIS by an additional 2-fold over this (n = 12,12; p < 0.001; Figure 6A) in contrast to a previous report in rat islets [24]. Insulin content was not altered by treatment conditions (Supplementary Figure 2G). This suggests that wortmannin acts either through a PDE-independent mechanism or through PDE isoforms known to be IBMX-insensitive.
Figure 6.
Phosphodiesterase inhibition mimics the effects of PI3K inhibition on GSIS and restores insulin secretion in islets from T2D donors. A, IBMX (100 μM)-amplified GSIS was measured from mouse islets treated with DMSO (0.1%) (open bars) or wortmannin (100 nM) (black bars). (inset) Effects of IBMX (100 μM) under low glucose conditions in the same islets. B, dipyridamole (50 μM)-amplified GSIS was measured from mouse islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars). (inset) Effects of dipyridamole (50 μM) under low glucose conditions in the same islets. C, The effect of IBMX (100 μM) together with dipyridamole (50 μM) was examined on GSIS from mouse islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars). (inset) Effects of IBMX (100 μM) together with dipyridamole (50 μM) under low glucose conditions in the same islets. D, shows the same as A, except in non-diabetic human islets. E, milrinone (10 μM)-amplified GSIS was measured from non-diabetic human islets treated with DMSO (open bars) or wortmannin (100 nM) (black bars). (inset) shows the effects of milrinone (10 μM) under low glucose conditions in the same islets. F, shows the same as D, except in human islets from T2D donors. *-p < 0.05, **, ##-p < 0.01, ***, ###-p < 0.001, ns- non significant.
PDE3 and 4 are the major cAMP degrading enzymes regulating insulin secretion in insulinoma cells, as well as rodent and human islets [47], [49], [50]. However, knockdown studies in rat islets, insulinoma cells, and MIN6 cells suggest that the IBMX-insensitive PDE8B also plays a significant role [35], [36], [49]. While no specific pharmacological inhibitors of PDE8B are commercially available, dipyridamole (50 μM) inhibits this isoform with an IC50 of 4.5 μM, (along with multiple other PDE isoforms, but not PDE3 or 4) [51]. Here, we show that dipyridamole increases GSIS by 4-fold (n = 12,12; p < 0.001; Figure 6B), with no effect under low glucose conditions (Figure 6B inset) or on insulin content (Supplementary Figure 1H). However, wortmannin (100 nM) again amplified GSIS by an additional 2.6-fold (n = 12,12; p < 0.001; Figure 6B) in the presence of dipyridamole (50 μM). When we treated mouse islets with both IBMX (100 μM) and dipyridamole (50 μM), wortmannin-mediated PI3K inhibition no longer amplified GSIS (n = 12,12; Figure 6C), suggesting that multiple PDE isoforms are activated by PI3K in mouse islets to limit GSIS. And while the combined IBMX/dipyridamole treatment also slightly increased insulin secretion under low glucose conditions, this was equally true for both the vehicle and wortmannin-treated islets (n = 12,12; p < 0.01, Figure 6C inset). Additionally, to confirm that the inability of wortmannin to further potentiate insulin secretion in the presence of IBMX and dipyridamole is not simply a result of islet insulin secretion reaching its maximum capacity, we show that GSIS in islets can be further amplified under this condition by forskolin (10 μM) (Supplementary Figure 5).
3.8. Phosphodiesterase inhibition restores glucose responsiveness in T2D islets
Given the reported disparities in islet responses to glucose between species [41], [42], we next examined the effects of combined PI3K and PDE inhibition on GSIS in isolated human islets. Contrary to the mouse data, PI3K inhibition is no longer able to amplify GSIS following IBMX (100 μM)-mediated PDE inhibition in human islets (n = 12,12 from 4 unique donors; Figure 6D), or PDE3 specific inhibition with milrinone (n = 10,10 from 4 unique donors; Figure 6E), which very closely resembles the secretion data obtained with IBMX (Figure 6D). A previous report demonstrated that PDE inhibition can increase insulin secretion from islets from humans with type 2 diabetes [52]. Indeed, IBMX (100 μM)-mediated PDE inhibition restored glucose-responsiveness in islets from type 2 diabetic donors (n = 9,9 from 3 unique donors; p < 0.001 Figure 6D), but this was not associated with a restoration of responsiveness to wortmannin. These findings demonstrate that PDE3 mediates the insulinotropic action of wortmannin in humans, in line with evidence suggesting that PDE3 is the major cAMP metabolizing enzyme in human islets [50], [53], and suggests impaired upstream control of PDE3 in T2D islets.
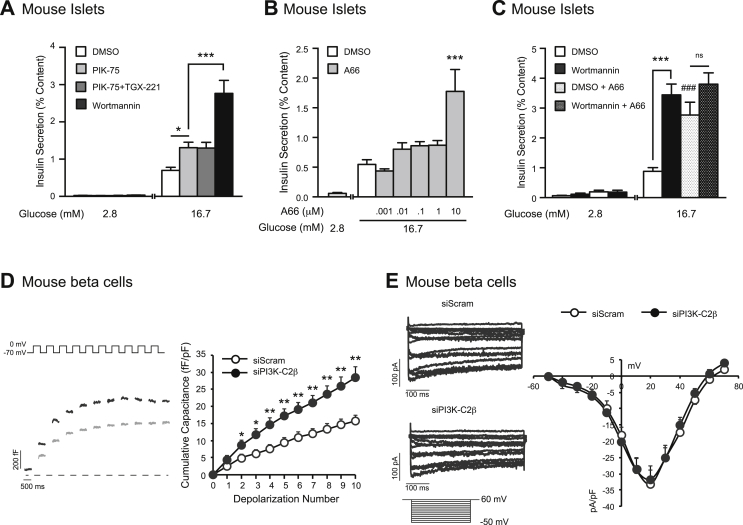
3.9. A class 2 PI3K isoform, PI3K-C2β, limits GSIS
Type 1A PI3K isoforms account for the majority of islet PI3K activity [17]; our previous work showed that the catalytic subunit of the class 1A PI3K isoform PI3Kα (p110α) exerts a negative regulation of insulin secretion by limiting Ca2+-dependent exocytosis [20]. Here we show that PIK-75-mediated inhibition of p110α amplifies GSIS by 2-fold (n = 12,12; p < 0.05; Figure 7A), and this was not modified by dual inhibition of both class 1A isoforms expressed in islets (p110α and p110β) (n = 12,12; p < 0.05; Figure 7A). Since the insulinotropic effect of wortmannin is 2-fold larger than that of the Class 1 PI3K inhibitors (n = 12,12; p < 0.001; Figure 7A) and augments GSIS even in the presence of the p110α inhibitor [20], the ability of wortmannin to amplify insulin secretion is mediated by an additional PI3K isoform(s). A66 is an inhibitor of Class 1 PI3Ks at low nM concentrations that exhibits cross-reactivity with the class-II PI3Ks in the μM range [34]. At concentrations of 1 nM–1 μM (well above the IC50 of 32 nM for p110α), A66 increases GSIS very modestly (n = 14,15; Figure 7B). However at 10 μM, (where class-II PI3K inhibition has been reported [34]), A66 increases GSIS by 3-fold (n = 14,15; p < 0.001; Figure 7B). Furthermore, we find that in the presence of 10 μM A66, wortmannin is no longer able to enhance GSIS (n = 12,12; p < 0.001; Figure 7C). Given that the class II PI3K-C2α is a positive regulator of insulin secretion, and is insensitive to wortmannin treatment [18], we examined the role of the other class II PI3K isoform (PI3K-C2β) in beta cell function. Limited by the lack of specific inhibitors, we examined the effect of siRNA-mediated knockdown of PI3K-C2β on the beta cell exocytotic response. Knockdown was confirmed in mouse insulin secreting cells (Supplementary Figure 6). Loss of PI3K-C2β amplifies the exocytotic response in mouse beta cells (n = 24,18; p < 0.01; Figure 7D), with no effect on VGCC current (n = 11,14; Figure 7E). This essentially replicates the effect of wortmannin on beta cell exocytosis (compare Figure 2, Figure 7).
Figure 7.
The inhibition of multiple PI3K isoforms mimics the effect of wortmannin on GSIS and exocytosis. A, GSIS was measured from mouse islets treated with DMSO (0.1%) (open bars), PIK-75 (100 nM) alone (light grey), PIK-75 (100 nM) together with TGX-221 (100 nM) (dark grey), or wortmannin (100 nM) (black bars). B, GSIS was measured from mouse islets treated with DMSO (open bars) or with increasing concentrations of A66 (1 nM–10 μM) (grey bars) C, GSIS was measured from mouse islets treated with DMSO (open bars), wortmannin (100 nM) (black bars), DMSO together with A66 (10 μM) (open bars, dots), or wortmannin (100 nM) together with A66 (10 μM) (black bars, dots). D, (left) Representative capacitance recordings from mouse beta cells expressing siScram (grey lines) or siPI3K-C2β (black lines). (right) Average cumulative capacitance response during each depolarization step) are shown. E, (left) Representative VGCC recordings from mouse beta cells expressing siScram or siPI3K-C2β are shown. (right) Average cumulative VGCC responses during each voltage-step were measured in mouse beta cells expressing siScram (white circles) or siPI3K-C2β (100 nM) (black circles). *-p < 0.05, **-p < 0.01, ***, ###-p < 0.001, ns- non significant.
4. Discussion
We have previously shown that specific PI3K catalytic isoforms have both positive and negative roles in regulating GSIS [20], [21], [22]. For example, p110γ, the lone class 1B catalytic isoform, plays a permissive role via regulation of insulin granule recruitment to the plasma membrane [21], [22]. However, inhibition (or shRNA-mediated knockdown) of p110α, the class 1 PI3K catalytic isoform which accounts for the majority of islet PI3K activity [17], amplifies GSIS [20]. Interestingly, this isoform is unable to account for the majority of the insulinotropic response to non-specific inhibition of PI3Ks with wortmannin (100 nM) [20], suggesting the involvement of one or more additional PI3K isoform(s) in limiting insulin secretion. The importance of PI3K signalling in glycaemic control is highlighted by recent work showing that loss of a Class II PI3K (PI3K-C2β) potentiates insulin signalling and sensitivity in mice [54]. However, we still understand little about the mechanism by which PI3K limits GSIS. While, wortmannin has previously been shown to raise [cAMP] in rat islets [24], the PI3K and PDE isoforms involved, the downstream effects on insulin exocytosis, and the role for this pathway in human islets have not been investigated.
Consistent with most [17], [20], [23], [24], but not all [55], reports we find that wortmannin enhances GSIS from both mouse and human islets. This occurs with an EC50 that closely matches the half-maximal inhibition of PI3K by wortmannin, and, like in rat islets [24], occurs through a cAMP-dependent pathway. Wortmannin-mediated inhibition of PI3K raises [cAMP]i, in response to a glucose (or forskolin) stimulus. It could be argued that the increase in [cAMP]i measured is modest when compared to the dramatic effects on insulin secretion; however, this could simply reflect the inability of our measurements to examine a compartmentalized cAMP signal. Oscillations of cAMP concentration beneath the plasma membrane are essential to the magnitude of insulin secretion [56]. However, we do see an increase in cAMP-dependent protein kinase (PKA) activity, as shown by an increased level of phosphorylated PKA-substrates similar to that induced by forskolin, and an increased FRET signal. PKA potentiates insulin secretion via multiple signal transduction pathways [57], [58] including a direct effect on proteins involved in insulin exocytosis [59], [60]. While we do not directly examine phosphorylation of specific exocytotic proteins upon PI3K-inhibition, we show that wortmannin potentiates the exocytotic response downstream of Ca2+ signalling. As such, we observed no differences in VGCC activity or [Ca2+]i responses to glucose. Furthermore, the amplification of GSIS following wortmannin treatment is absent in the presence of Rp-8-Br-cAMPS-pAB, a novel and highly cell membrane permeable antagonist of cAMP signalling, which was recently shown to blunt GSIS in rat and human islets [30]. Taken together, these findings point to a PI3K-signalling pathway that modulates insulin exocytosis via a cAMP and PKA-dependent mechanism.
We also demonstrate that wortmannin amplifies insulin secretion potentiated by activators of adenylyl cyclase: GLP-1 and forskolin. These findings, together with reports showing that PI3Ks are upstream regulators of phosphodiesterase (PDE) activity in non-insulin secreting cells [61], [62], suggest that wortmannin amplifies GSIS by inhibiting PDEs. Supporting this claim, the non-selective PDE inhibitor IBMX, and the specific PDE3 inhibitor milrinone, both eclipse the amplifying effect of wortmannin on GSIS in human islets. This suggests that PI3K-inhibition increases [cAMP]i and subsequent insulin secretion by inhibiting the activation of PDE3. Our findings are consistent with short-term in vitro insulin treatment which activates PDE3 activity in insulin-secreting cell lines [53].
Furthermore, the importance of this signalling pathway in insulin secretion in humans is shown by the absence of a wortmannin effect on GSIS in islets from T2D donors. While we see a general impairment of GSIS from T2D islets and can restore glucose-responsiveness with IBMX, similar to a previous report [52], our results further suggest an upstream disturbance in the PI3K-dependent regulation of PDE3. It is also worth noting that similar results were seen previously in a mouse model of T2D, whereby inhibitors of PI3K failed to amplify GSIS in islets from ob/ob mice, albeit that study did not connect the inhibition of PI3K to regulation of cAMP [23].
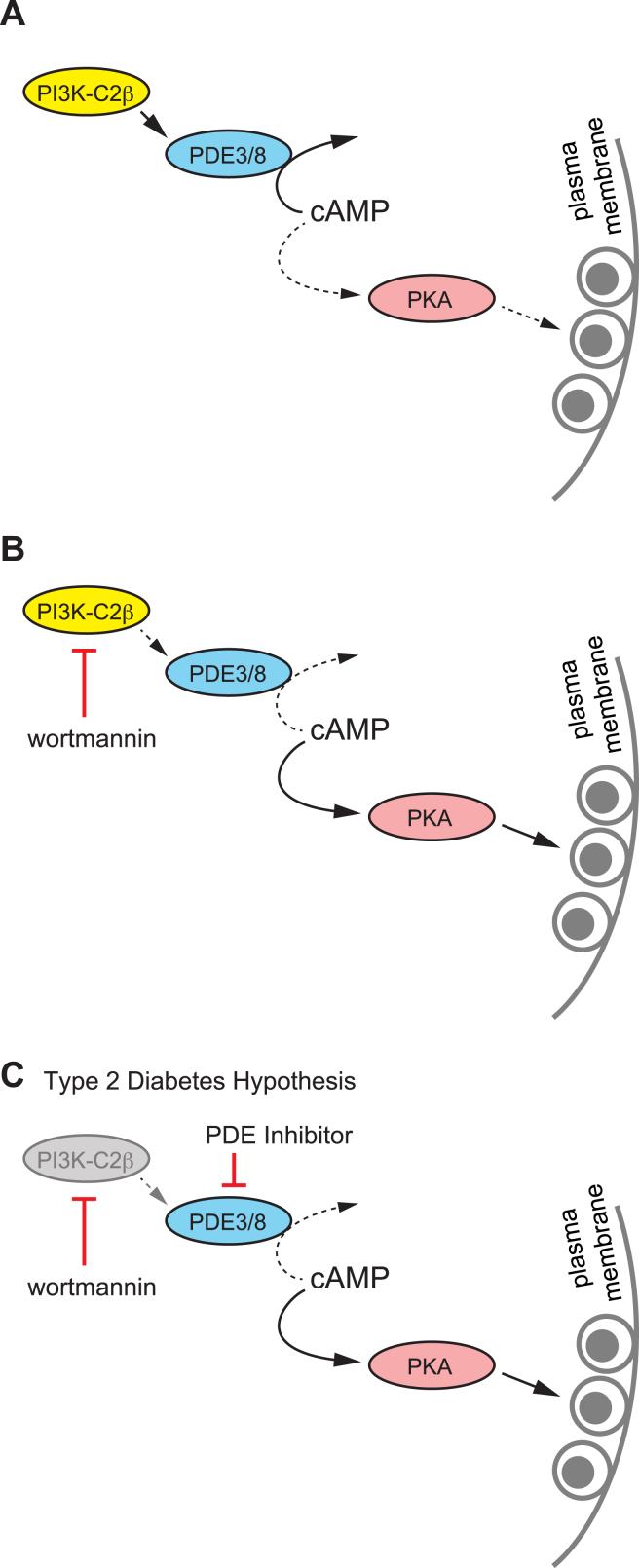
In contrast, the amplifying effect of PI3K-inhibition on GSIS in mouse islets is maintained in the presence of IBMX, suggesting the involvement of IBMX-insensitive PDE isoforms in addition to PDE3. When we block both IBMX-sensitive and -insensitive isoforms (with dipyridamole), the amplifying effect of wortmannin on GSIS is obscured. While we are unable to precisely tease out the specific PDE isoforms involved in mice, PDE8B likely represents this isoform as it is insensitive to IBMX, is shown to modulate [cAMP]i and insulin secretion in rodent islets [35], [36], [49], and appears not to be expressed in human islets [53]. Highlighting the reported disparities in islet responses to glucose between species, we suggest that PDE3 (in humans and mice) and PDE8 (in mice) are downstream of PI3K in beta cells (Figure 8A, B). Furthermore, we hypothesize that this signalling pathway is perturbed in islets from T2D donors (Figure 8C).
Figure 8.
Proposed mechanism for the Class II PI3K-C2β in the negative regulation of insulin secretion. A, Constitutive PI3K-C2β activity promotes cAMP degradation by phosphodiesterase 3 (PDE3) and PDE8 (in mice). This limits cAMP levels under glucose-stimulated (or incretin stimulated) conditions, activation of PKA and subsequent facilitation of insulin exocytosis. B, Inhibition of PI3K-C2β, for example with the non-selective inhibitor wortmannin, reduces PDE3/8 activity to increase cAMP and amplify insulin secretion in a PKA-dependent manner. C, PI3K inhibition does not augment GSIS in islets from T2D donors, but this can be rescued by PDE inhibition.
Finally, we examined the specific PI3K catalytic isoforms involved in limiting GSIS. The role of acute PI3K signalling in insulin secretion has previously been examined by us [20], [21], [22] and others [18], [19], [54], [63]. The only PI3K isoform shown to have a limiting role in GSIS thus far is the class 1A PI3K catalytic isoform p110α [19], [20]. However, given that wortmannin amplifies GSIS almost 2-fold more than the specific p110α inhibitor PIK-75, another PI3K isoforms must be involved. While PI3K-related enzymes such as mTOR and myosin light chain kinase could be inhibited by wortmannin [24], [64], we find that knockdown of the class 2 PI3K, PI3K-C2β, also enhances Ca2+ dependent exocytosis. There are no selective PI3K-C2β inhibitors commercially available; however, the effects of wortmannin on GSIS can be mimicked by A66 at concentrations greater than 1 μM that inhibit PI3K-C2β [34]. The observation that A66 had only a small effect on GSIS only at lower concentrations, in which only p110α would be blocked, lends further support to the conclusion that PI3K-C2β mediates most of the wortmannin-dependent amplification of insulin secretion.
Furthermore, in contrast to wortmannin, A66 exhibits no notable inhibitory activity against other lipid kinases or the related kinases DNA-PK and mTOR [34]. While PI3K-C2β signalling has not previously been explored in the beta cell per se, its mRNA expression has been confirmed in human beta cells; albeit with a reported high degree of heterogeneity between donors [65].
In summary, we now provide new insight into the basis for PI3K-mediated regulation of insulin secretion in mouse and human islets. Specifically, PI3K-C2β limits GSIS by suppressing cAMP responses in a manner that requires PDE3 (in humans and mice) and PDE8B (in mice). Furthermore, PI3K inhibition does not augment GSIS in islets from T2D donors. Finally, while multiple catalytic PI3K isoforms may have district and opposing roles in insulin release, the overwhelming net effect of acute non-specific pharmacologic PI3K-inhibition is a rise in glucose-stimulated insulin secretion likely mediated in humans by the alleviation of PI3K-C2β-dependent activation of PDE3 to limit cAMP levels.
Acknowledgements
The authors thank Mrs. Nancy Smith for excellent technical assistance, and Dr. Christopher Newgard (Duke University) for providing INS 832/13 cells. We thank Drs. James Shapiro and Tatsuya Kin at the Clinical Islet Laboratory (University of Alberta) and Mr. James Lyon at the Alberta Diabetes Institute IsletCore (University of Alberta) for the isolation of human islets for research. Human islet isolation in the IsletCore was funded by the Alberta Diabetes Foundation, and the work described in this paper was supported by an operating grant to PEM from the Canadian Diabetes Association (OG-3-14-4565-PM). PEM holds the Canada Research Chair in Islet Biology.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.05.003.
Conflict of interest
There are no conflict of interests to declare.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Danaei G., Finucane M.M., Lu Y., Singh G.M., Cowan M.J., Paciorek C.J. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 3.Kahn B.B. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998;92(5):593–596. doi: 10.1016/s0092-8674(00)81125-3. [DOI] [PubMed] [Google Scholar]
- 4.Porte D., Kahn S.E. beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes. 2001;50(Suppl 1):S160–S163. doi: 10.2337/diabetes.50.2007.s160. [DOI] [PubMed] [Google Scholar]
- 5.Tibaldi J. Preserving insulin secretion in Type 2 diabetes mellitus. Expert Review of Endocrinology & Metabolism. 2008;3(2):147–159. doi: 10.1586/17446651.3.2.147. [DOI] [PubMed] [Google Scholar]
- 6.RISE Consortium Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care. 2014;37(3):780–788. doi: 10.2337/dc13-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashcroft F.M., Rorsman P. ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion. Biochemical Society Transactions. 1990;18(1):109–111. doi: 10.1042/bst0180109. [DOI] [PubMed] [Google Scholar]
- 8.Ashcroft F.M., Proks P., Smith P.A., Ammälä C., Bokvist K., Rorsman P. Stimulus-secretion coupling in pancreatic beta cells. Journal of Cellular Biochemistry. 1994;55(Suppl):54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- 9.Satin L.S., Cook D.L. Voltage-gated Ca2+ current in pancreatic B-cells. Pflügers Archiv: European Journal of Physiology. 1985;404(4):385–387. doi: 10.1007/BF00585354. [DOI] [PubMed] [Google Scholar]
- 10.Henquin J.-C. Pathways in beta-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes. 2004;53(Suppl 3):S48–S58. doi: 10.2337/diabetes.53.suppl_3.s48. [DOI] [PubMed] [Google Scholar]
- 11.Henquin J.-C. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Research and Clinical Practice. 2011;93(Suppl 1):S27–S31. doi: 10.1016/S0168-8227(11)70010-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferdaoussi M., Bergeron V., Zarrouki B., Kolic J., Cantley J., Fielitz J. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia. 2012;55(10):2682–2692. doi: 10.1007/s00125-012-2650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni R.N., Brüning J.C., Winnay J.N., Postic C., Magnuson M.A., Kahn C.R. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96(3):329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni R.N., Winnay J.N., Daniels M., Brüning J.C., Flier S.N., Hanahan D. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. The Journal of Clinical Investigation. 1999;104(12):R69–R75. doi: 10.1172/JCI8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni R.N., Holzenberger M., Shih D.Q., Ozcan U., Stoffel M., Magnuson M.A. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nature Genetics. 2002;31(1):111–115. doi: 10.1038/ng872. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko K., Ueki K., Takahashi N., Hashimoto S., Okamoto M., Awazawa M. Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metabolism. 2010;12(6):619–632. doi: 10.1016/j.cmet.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eto K., Yamashita T., Tsubamoto Y., Terauchi Y., Hirose K., Kubota N. Phosphatidylinositol 3-kinase suppresses glucose-stimulated insulin secretion by affecting post-cytosolic [Ca(2+)] elevation signals. Diabetes. 2002;51(1):87–97. doi: 10.2337/diabetes.51.1.87. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez V., Raimondi C., Somanath S., Bugliani M., Loder M.K., Edling C.E. Class II phosphoinositide 3-kinase regulates exocytosis of insulin granules in pancreatic beta cells. The Journal of Biological Chemistry. 2011;286(6):4216–4225. doi: 10.1074/jbc.M110.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoyagi K., Ohara-Imaizumi M., Nishiwaki C., Nakamichi Y., Ueki K., Kadowaki T. Acute inhibition of PI3K-PDK1-Akt pathway potentiates insulin secretion through upregulation of newcomer granule fusions in pancreatic β-cells. PloS One. 2012;7(10):e47381. doi: 10.1371/journal.pone.0047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolic J., Spigelman A.F., Plummer G., Leung E., Hajmrle C., Kin T. Distinct and opposing roles for the phosphatidylinositol 3-OH kinase catalytic subunits p110α and p110β in the regulation of insulin secretion from rodent and human beta cells. Diabetologia. 2013;56(6):1339–1349. doi: 10.1007/s00125-013-2882-4. [DOI] [PubMed] [Google Scholar]
- 21.Pigeau G.M., Kolic J., Ball B.J., Hoppa M.B., Wang Y.W., Rückle T. Insulin granule recruitment and exocytosis is dependent on p110gamma in insulinoma and human beta-cells. Diabetes. 2009;58(9):2084–2092. doi: 10.2337/db08-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolic J., Spigelman A.F., Smith A.M., Manning Fox J.E., MacDonald P.E. Insulin secretion induced by glucose-dependent insulinotropic polypeptide requires phosphatidylinositol 3-kinase γ in rodent and human β-cells. The Journal of Biological Chemistry. 2014;289(46):32109–32120. doi: 10.1074/jbc.M114.577510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zawalich W.S., Tesz G.J., Zawalich K.C. Inhibitors of phosphatidylinositol 3-kinase amplify insulin release from islets of lean but not obese mice. The Journal of Endocrinology. 2002;174(2):247–258. doi: 10.1677/joe.0.1740247. [DOI] [PubMed] [Google Scholar]
- 24.Nunoi K., Yasuda K., Tanaka H., Kubota A., Okamoto Y., Adachi T. Wortmannin, a PI3-kinase inhibitor: promoting effect on insulin secretion from pancreatic beta cells through a cAMP-dependent pathway. Biochemical and Biophysical Research Communications. 2000;270(3):798–805. doi: 10.1006/bbrc.2000.2514. [DOI] [PubMed] [Google Scholar]
- 25.Persaud S.J., Asare-Anane H., Jones P.M. Insulin receptor activation inhibits insulin secretion from human islets of Langerhans. FEBS Letters. 2002;510(3):225–228. doi: 10.1016/s0014-5793(01)03268-9. [DOI] [PubMed] [Google Scholar]
- 26.Nunemaker C.S., Zhang M., Satin L.S. Insulin feedback alters mitochondrial activity through an ATP-sensitive K+ channel-dependent pathway in mouse islets and beta-cells. Diabetes. 2004;53(7):1765–1772. doi: 10.2337/diabetes.53.7.1765. [DOI] [PubMed] [Google Scholar]
- 27.Pirola L., Bonnafous S., Johnston A.M., Chaussade C., Portis F., Van Obberghen E. Phosphoinositide 3-kinase-mediated reduction of insulin receptor substrate-1/2 protein expression via different mechanisms contributes to the insulin-induced desensitization of its signaling pathways in L6 muscle cells. The Journal of Biological Chemistry. 2003;278(18):15641–15651. doi: 10.1074/jbc.M208984200. [DOI] [PubMed] [Google Scholar]
- 28.Fröjdö S., Vidal H., Pirola L. Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochimica Et Biophysica Acta. 2009;1792(2):83–92. doi: 10.1016/j.bbadis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti P., Lupi R., Federici M., Marselli L., Masini M., Boggi U. Insulin secretory function is impaired in isolated human islets carrying the Gly(972)-->Arg IRS-1 polymorphism. Diabetes. 2002;51(5):1419–1424. doi: 10.2337/diabetes.51.5.1419. [DOI] [PubMed] [Google Scholar]
- 30.Schwede F., Chepurny O.G., Kaufholz M., Bertinetti D., Leech C.A., Cabrera O. Rp-cAMPS prodrugs reveal the cAMP dependence of first-phase glucose-stimulated insulin secretion. Molecular Endocrinology (Baltimore, Md.) 2015;29(7):988–1005. doi: 10.1210/me.2014-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wymann M.P., Bulgarelli-Leva G., Zvelebil M.J., Pirola L., Vanhaesebroeck B., Waterfield M.D. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Molecular and Cellular Biology. 1996;16(4):1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaussade C., Rewcastle G.W., Kendall J.D., Denny W.A., Cho K., Grønning L.M. Evidence for functional redundancy of class IA PI3K isoforms in insulin signalling. The Biochemical Journal. 2007;404(3):449–458. doi: 10.1042/BJ20070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson S.P., Schoenwaelder S.M., Goncalves I., Nesbitt W.S., Yap C.L., Wright C.E. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nature Medicine. 2005;11(5):507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson S., Flanagan J.U., Kolekar S., Buchanan C., Kendall J.D., Lee W.-J. A drug targeting only p110α can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types. The Biochemical Journal. 2011;438(1):53–62. doi: 10.1042/BJ20110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dov A., Abramovitch E., Warwar N., Nesher R. Diminished phosphodiesterase-8B potentiates biphasic insulin response to glucose. Endocrinology. 2008;149(2):741–748. doi: 10.1210/en.2007-0968. [DOI] [PubMed] [Google Scholar]
- 36.Tian G., Sågetorp J., Xu Y., Shuai H., Degerman E., Tengholm A. Role of phosphodiesterases in the shaping of sub-plasma-membrane cAMP oscillations and pulsatile insulin secretion. Journal of Cell Science. 2012;125(Pt 21):5084–5095. doi: 10.1242/jcs.107201. [DOI] [PubMed] [Google Scholar]
- 37.Ferdaoussi M., Dai X., Jensen M.V., Wang R., Peterson B.S., Huang C. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. The Journal of Clinical Investigation. 2015;125(10):3847–3860. doi: 10.1172/JCI82498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen M.D., Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochemical and Biophysical Research Communications. 2006;348(2):716–721. doi: 10.1016/j.bbrc.2006.07.136. [DOI] [PubMed] [Google Scholar]
- 39.Powis G., Bonjouklian R., Berggren M.M., Gallegos A., Abraham R., Ashendel C. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Research. 1994;54(9):2419–2423. [PubMed] [Google Scholar]
- 40.Walker E.H., Pacold M.E., Perisic O., Stephens L., Hawkins P.T., Wymann M.P. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Molecular Cell. 2000;6(4):909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 41.Zawalich W.S., Yamazaki H., Zawalich K.C. Biphasic insulin secretion from freshly isolated or cultured, perifused rodent islets: comparative studies with rats and mice. Metabolism: Clinical and Experimental. 2008;57(1):30–39. doi: 10.1016/j.metabol.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandrasekera P.C., Pippin J.J. Of rodents and men: species-specific glucose regulation and type 2 diabetes research. Altex. 2014;31(2):157–176. doi: 10.14573/altex.1309231. [DOI] [PubMed] [Google Scholar]
- 43.Jones P.M., Persaud S.J. Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic beta-cells. Endocrine Reviews. 1998;19(4):429–461. doi: 10.1210/edrv.19.4.0339. [DOI] [PubMed] [Google Scholar]
- 44.Wan Q.-F., Dong Y., Yang H., Lou X., Ding J., Xu T. Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+ The Journal of General Physiology. 2004;124(6):653–662. doi: 10.1085/jgp.200409082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idevall-Hagren O., Barg S., Gylfe E., Tengholm A. cAMP mediators of pulsatile insulin secretion from glucose-stimulated single beta-cells. The Journal of Biological Chemistry. 2010;285(30):23007–23018. doi: 10.1074/jbc.M109.095992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwede F., Maronde E., Genieser H., Jastorff B. Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacology & Therapeutics. 2000;87(2–3):199–226. doi: 10.1016/s0163-7258(00)00051-6. [DOI] [PubMed] [Google Scholar]
- 47.Zhao A.Z., Zhao H., Teague J., Fujimoto W., Beavo J.A. Attenuation of insulin secretion by insulin-like growth factor 1 is mediated through activation of phosphodiesterase 3B. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(7):3223–3228. doi: 10.1073/pnas.94.7.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerfant B.-G., Zhao D., Lorenzen-Schmidt I., Wilson L.S., Cai S., Chen S.R.W. PI3Kgamma is required for PDE4, not PDE3, activity in subcellular microdomains containing the sarcoplasmic reticular calcium ATPase in cardiomyocytes. Circulation Research. 2007;101(4):400–408. doi: 10.1161/CIRCRESAHA.107.156422. [DOI] [PubMed] [Google Scholar]
- 49.Waddleton D., Wu W., Feng Y., Thompson C., Wu M., Zhou Y.-P. Phosphodiesterase 3 and 4 comprise the major cAMP metabolizing enzymes responsible for insulin secretion in INS-1 (832/13) cells and rat islets. Biochemical Pharmacology. 2008;76(7):884–893. doi: 10.1016/j.bcp.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 50.Parker J.C., VanVolkenburg M.A., Ketchum R.J., Brayman K.L., Andrews K.M. Cyclic AMP phosphodiesterases of human and rat islets of Langerhans: contributions of types III and IV to the modulation of insulin secretion. Biochemical and Biophysical Research Communications. 1995;217(3):916–923. doi: 10.1006/bbrc.1995.2858. [DOI] [PubMed] [Google Scholar]
- 51.Soderling S.H., Bayuga S.J., Beavo J.A. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(15):8991–8996. doi: 10.1073/pnas.95.15.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muhammed S.J., Lundquist I., Salehi A. Pancreatic β-cell dysfunction, expression of iNOS and the effect of phosphodiesterase inhibitors in human pancreatic islets of type 2 diabetes. Diabetes. Obesity & Metabolism. 2012;14(11):1010–1019. doi: 10.1111/j.1463-1326.2012.01632.x. [DOI] [PubMed] [Google Scholar]
- 53.Heimann E., Jones H.A., Resjö S., Manganiello V.C., Stenson L., Degerman E. Expression and regulation of cyclic nucleotide phosphodiesterases in human and rat pancreatic islets. PloS One. 2010;5(12):e14191. doi: 10.1371/journal.pone.0014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alliouachene S., Bilanges B., Chicanne G., Anderson K.E., Pearce W., Ali K. Inactivation of the class II PI3K-C2β potentiates insulin signaling and sensitivity. Cell Reports. 2015;13(9):1881–1894. doi: 10.1016/j.celrep.2015.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdhury A., Dyachok O., Tengholm A., Sandler S., Bergsten P. Functional differences between aggregated and dispersed insulin-producing cells. Diabetologia. 2013;56(7):1557–1568. doi: 10.1007/s00125-013-2903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dyachok O., Idevall-Hagren O., Sågetorp J., Tian G., Wuttke A., Arrieumerlou C. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metabolism. 2008;8(1):26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Kolic J., MacDonald P.E. cAMP-independent effects of GLP-1 on β cells. The Journal of Clinical Investigation. 2015;125(12):4327–4330. doi: 10.1172/JCI85004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seino S., Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiological Reviews. 2005;85(4):1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 59.Wu B., Wei S., Petersen N., Ali Y., Wang X., Bacaj T. Synaptotagmin-7 phosphorylation mediates GLP-1-dependent potentiation of insulin secretion from β-cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(32):9996–10001. doi: 10.1073/pnas.1513004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seino S., Takahashi H., Fujimoto W., Shibasaki T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes, Obesity & Metabolism. 2009;11(Suppl 4):180–188. doi: 10.1111/j.1463-1326.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 61.Sahu A., Koshinaka K., Sahu M. Phosphatidylinositol 3-kinase is an upstream regulator of the phosphodiesterase 3B pathway of leptin signalling that may not involve activation of Akt in the rat hypothalamus. Journal of Neuroendocrinology. 2013;25(2):168–179. doi: 10.1111/j.1365-2826.2012.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao A.Z., Huan J.-N., Gupta S., Pal R., Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nature Neuroscience. 2002;5(8):727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 63.Leibiger B., Moede T., Paschen M., Yunn N.-O., Lim J.H., Ryu S.H. PI3K-C2α knockdown results in rerouting of insulin signaling and pancreatic beta cell proliferation. Cell Reports. 2015;13(1):15–22. doi: 10.1016/j.celrep.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 64.Collier J.J., White S.M., Dick G.M., Scott D.K. Phosphatidylinositol 3-kinase inhibitors reveal a unique mechanism of enhancing insulin secretion in 832/13 rat insulinoma cells. Biochemical and Biophysical Research Communications. 2004;324(3):1018–1023. doi: 10.1016/j.bbrc.2004.09.149. [DOI] [PubMed] [Google Scholar]
- 65.Muller D., Huang G.C., Amiel S., Jones P.M., Persaud S.J. Gene expression heterogeneity in human islet endocrine cells in vitro: the insulin signalling cascade. Diabetologia. 2007;50(6):1239–1242. doi: 10.1007/s00125-007-0671-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.