Abstract
Objective
Adipose depot mass is tightly regulated to maintain energy homeostasis. AKT is a critical kinase in the insulin-signaling cascade that is required for the process of adipogenesis in vitro. However, the role of AKT in the maintenance and/or function of mature adipocytes in vivo had not been examined.
Methods
To study this, we deleted Akt1 and Akt2 in adipocytes of mice using the AdipoQ-Cre driver.
Results
Strikingly, mice lacking adipocyte AKT were severely lipodystrophic, having dramatically reduced gonadal adipose and no discernible subcutaneous or brown adipose tissue. As a result, these mice developed severe insulin resistance accompanied by fatty liver, hepatomegaly and with enlarged islets of Langerhans.
Conclusions
These data reveal the critical role of adipocyte AKT and insulin signaling for maintaining adipose tissue mass.
Keywords: Akt, Lipodystrophy, Insulin signaling, Insulin resistance
Highlights
-
•
AKT-action and insulin signaling are required in an adipocyte-autonomous fashion to maintain adipose tissue stores.
-
•
Mice with adipocyte-specific loss of Akt1 and Akt2 display a nearly complete loss of white and brown adipose tissue.
-
•
These lipodystrophic mice have organomegaly, perturbed glucose homeostasis and insulin resistance.
1. Introduction
Adipose tissue functions as a repository for long-term energy storage and as a critical regulator of energy balance, satiety, glucose metabolism, and other physiological processes. White adipose tissue (WAT) stores and releases fatty acids and glycerol in response to the energetic needs of the organism, whereas brown adipose tissue (BAT) produces heat to maintain thermostasis upon cold-exposure. Lipodystrophy is a disease characterized by lack of adipose tissue accompanied by metabolic derangements. The most common form of lipodystrophy is congenital generalized lipodystrophy (CGL), which affects approximately one in ten million individuals and is characterized by a near complete absence of adipose tissue [1]. CGL Type 1 is caused by mutations in 1-acylglycerol-3-phosophate O-acyltransferase 2 (AGPAT2) [1], [2] and mice null for Agpat2 exhibit a complete lipoatrophy [3]. Mutations in Seipin cause CGL Type 2, or Berardinelli-Seip congenital lipodystrophy type 2 (BSCL2), and mice null for Bscl2/Seipin, or with adipose-tissue specific loss, are also lipodystrophic, [4], [5]. Several additional genes have been implicated in CGL and familial partial lipodystrophy, including Akt2 and a subunit of phosphatidylinositol 3 kinase (PI3K) [1], [6], [7], [8], [9], [10]. Hepatomegaly from fatty liver and splenomegaly are common in lipodystrophic patients, with hyperinsulinemia and hypertriglyceridemia often present.
AKT, a Ser/Thr kinase, is activated upon engagement of the insulin receptor and mediates hormonal regulation of many aspects of energy homeostasis in insulin-responsive tissues, including adipose tissue. AKT can also be activated by insulin growth factor 1 (IGF-1) [11], [12], [13]. Three isoforms of AKT have been identified, of which AKT1 and AKT2 account for the majority of AKT in adipocytes [14], [15]. AKT1 is expressed nearly ubiquitously and is critical in the control of growth, while the AKT2 isoform is expressed predominantly in the adult insulin-responsive tissues, including liver, muscle, and adipose. Mice lacking Akt2 are insulin-resistant [16] but spared from severe metabolic abnormalities due to compensation by Akt1 [17], [18], [19].
In this study, we deleted Akt1 and Akt2 selectively in adipocytes of mice, thus interrupting a critical insulin-signaling pathway. We found that loss of these AKT isoforms in adipocytes caused profound lipodystrophy, associated with fatty liver and systemic insulin resistance.
2. Materials and methods
2.1. Adipo-AKT KO mice
All mice were on a C57/B6 background and were maintained on normal chow diets from Laboratory Rodent Diet, Lab Diet 5001. Cre-positive male mice heterozygous for Akt1 and Akt2 floxed alleles were bred to females homozygous for Akt1 and Akt2 floxed alleles. This breeding scheme generated Cre-positive experimental mice homozygous floxed for both genes and Cre-negative male mice homozygous floxed for both genes that served as controls. Cre-positive males heterozygous for both floxed genes were generated and exhibited a wild type phenotype (data not shown). All mice were born at a Mendelian ratio. All mouse experiments were reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee in accordance with the guidelines of the National Institutes of Health.
2.2. Histopathology
Organs were harvested for histopathology from 12-week-old mice, fixed in formalin for 24 h, rinsed in 70% ethanol, and stored in 70% ethanol at 4 °C overnight. Sectioning and staining of the liver, spleen, kidney, and pancreas were performed by the Molecular Pathology and Imaging Core at the University of Pennsylvania, supported by NIH grants DK050306, CA098101, and DK049210. Sectioning of the epididymal fat pads was performed by the Pathology Core at the Children's Hospital of Philadelphia Research Institute. Staining of the epididymal fat pads was performed in house. Slides were deparaffinized in xylene (3 × 3 min), then rehydrated in an ethanol series of 100% (3 × 3 min), 95% (1 × 3 min), 80% (1 × 3 min), dH2O (1 × 5 min). Slides were stained in hematoxylin for 30 min, rinsed in dH2O, and rinsed in tap water for 5 min. Slides were dipped in acid ethanol 8–12× to destain them, rinsed in tap water (2 × 1 min), and rinsed in dH2O (1 × 2 min). Excess water was removed and slides were stained in eosin for 1 min. Slides were then dehydrated in an ethanol series: 95% (3 × 5 min), 100% (3 × 5 min), and then in xylene (3 × 15 min) before drying and mounting a coverslip.
2.3. F4/80 staining
Rat anti-mouse F4/80 monoclonal antibody C1:A3-1 from AbD Serotec was used for staining sections from the epididymal fat for macrophage, natural killer cells, and F4/80 positive antigen presenting cell infiltrate. The protocol used was modified from that recommended by the supplier.
2.4. Adipokines
Blood samples were taken from 8 to 12 week old mice between 12 and 2 pm. Adiponectin, leptin, and resistin levels were measured using ELISA kits from Linco by the Radioimmunoassay and Biomarkers Core at the Penn Diabetes Research Center, supported by NIH grant DK19525.
2.5. Liver triglyceride
Liver triglycerides were measured by weighing 100 mg liver and lysing in 400 uL cell lysis buffer (50 mM Tris, pH 7.4, 140 mM NaCl, 0.1% Triton-X) (250 mg/mL homogenate). Samples were diluted 10 fold and incubated in 1% deoxycholate at a 1:1 ratio for 10 min, then incubated for 10 min in Thermo-Scientific Infinity Triglyceride reagent. All incubations were performed at 37 °C. The Stanbio triglyceride standard was used. Samples were read on a Tecan plate reader at 500 nm.
2.6. Glucose homeostasis
For glucose tolerance tests, 8–12 week old male mice were fasted for 16–18 h overnight and injected with 1 g/kg glucose intraperitoneally. Blood samples were taken from the tail in Sarstedt microvette lithium heparin tubes (Ref 16.433.100) at time 0 for fasting insulin measurements and 15 min post injection. Blood glucose levels were taken from the tail on a OneTouch Ultra glucometer at fasting, and post-injection at 15, 30, 60, and 120 min.
For the meal challenges, 8–12 week old male mice were fasted for 16–18 h overnight and then refed normal chow for 2 h.
Crystal Chem Ultra-sensitive Mouse Insulin ELISA kits were used for all insulin measurements. WAKO NEFA assay kits were used for all NEFA measurements.
3. Results
3.1. Loss of AKT1 and AKT2 in adipocytes causes lipodystrophy
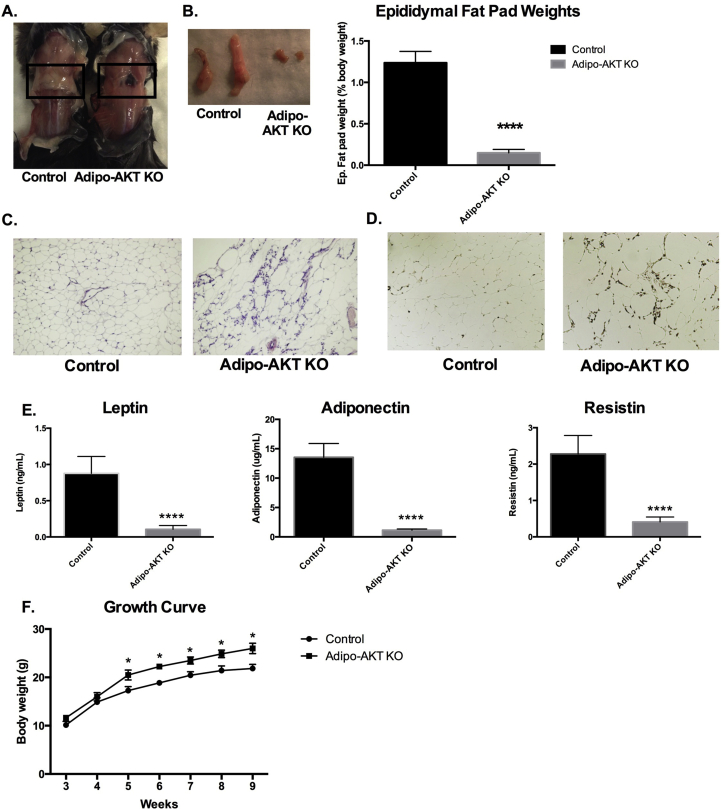
To determine the role of AKT in regulating adipocyte function and survival, we generated mice lacking both Akt1 and Akt2 using the AdipoQ-Cre driver [20]. Adipo-AKT knockout (KO) mice were severely lipodystrophic at 8–12 weeks of age such that all subcutaneous and cutaneous fat depots, including interscapular brown adipose tissue, were completely absent (Figure 1A). The epididymal WAT was about 10× smaller (as a percentage of body weight) in Adipo-AKT KO mice as compared to control mice (Figure 1B). Hematoxylin and Eosin staining of tissue sections showed that the residual epididymal WAT (eWAT) in KO mice contained a modest number of enlarged adipocytes (Figure 1C). The KO eWAT also displayed increased infiltration of F4/80+ macrophages (Figure 1D). The levels of important adipokines, such as leptin, adiponectin, and resistin, were dramatically decreased in Adipo-AKT KO mice relative to controls (Figure 1E). The body weight of Adipo-AKT KO mice was increased about 15% by 5 weeks of age, with controls weighing ∼17.3 g and Adipo-AKT KO mice weighing ∼20.5 g (Figure 1F). This increase in body weight was maintained at 9 weeks of age with control mice weighing ∼21.8 g and Adipo-AKT KO mice weighing ∼26.0 g (Figure 1F). These results show that AKT is required for the maintenance of adipose tissues.
Figure 1.
Adipo-AKT KO mice are lipodystrophic. A. Control and Adipo-AKT KO mice lack subscapular white and brown fat depots (black box). B. Epididymal fat pads and fat pad weights from control and Adipo-AKT KO mice (control n = 4, Adipo-AKT KO n = 6). C. H&E staining of the epididymal fat pad depots. D. F4/80 staining of the epididymal fat pad depots. E. Circulating canonical adipokines leptin, adiponectin, and resistin (control n = 9, Adipo-AKT KO n = 8). F. Growth curve of control and Adipo-AKT KO mice (control n = 10, Adipo-AKT KO n = 12). All data are presented as mean ± s.e.m. (*, p < .05; ****, p < .0001).
3.2. Hepatomegaly
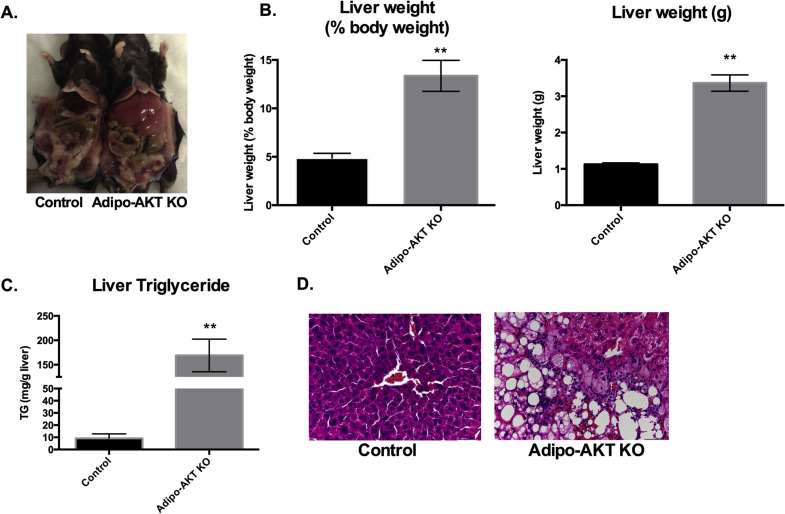
Adipo-AKT KO mice had livers weighing 2.7 times that of control animals when normalized for body weight at 8–12 weeks of age. Livers in Adipo-AKT mice constituted 13% of body weight, and were 3.5 g on average, as compared to 4.9% of body weight and about 1 g in controls (Figure 2A,B). The greater size of KO livers was associated with a large increase in liver triglyceride levels. Control mice had an average of 9.2 mg triglyceride/g of liver whereas Adipo-AKT KO livers had 169.1 mg triglyceride/g of liver (Figure 2C). Histopathological analysis of livers from Adipo-AKT KO mice revealed a disorganized morphology with an increase in both the number and size of lipid droplets (Figure 2D).
Figure 2.
Adipo-AKT KO mice have hepatomegaly. A. Adipo-AKT KO mice have enlarged and pale livers. B. Liver weights in control and Adipo-AKT KO mice. (control n = 4, Adipo-AKT KO n = 6). C. Stored liver triglycerides in liver of control and Adipo-AKT KO mice (n = 6). D. H&E staining of the liver from control and Adipo-AKT KO mice (20×). All data are presented as mean ± s.e.m. (*, p < .05; ****, p < .0001).
3.3. Organomegaly
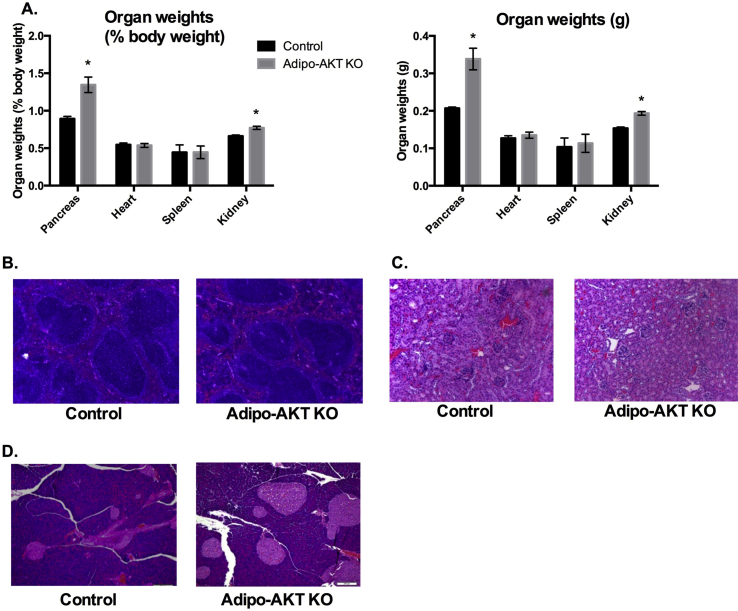
In addition to hepatomegaly, the Adipo-AKT KO mice displayed enlarged pancreases and kidneys (Figure 3A). On the other hand, Adipo-AKT KO mice did not exhibit splenomegaly, which is often associated with lipodystrophy [1], [21], [22], [23] (Figure 3A). Histopathology of both the spleen and kidney revealed no obvious abnormalities (Figure 3B,C). In contrast, the pancreases of Adipo-AKT KO mice had enlarged islets (Figure 3D).
Figure 3.
Adipo-AKT KO mice have enlarged pancreases and renomegaly. A. Organ weights of control and Adipo-AKT KO mice (control n = 4, Adipo-AKT KO n = 6). B. H&E staining of the spleen (10×). C. H&E staining of the kidney (10×). D. H&E staining of the pancreas (10×). All data are presented as mean ± s.e.m. (*, p < .05).
3.4. Glucose homeostasis
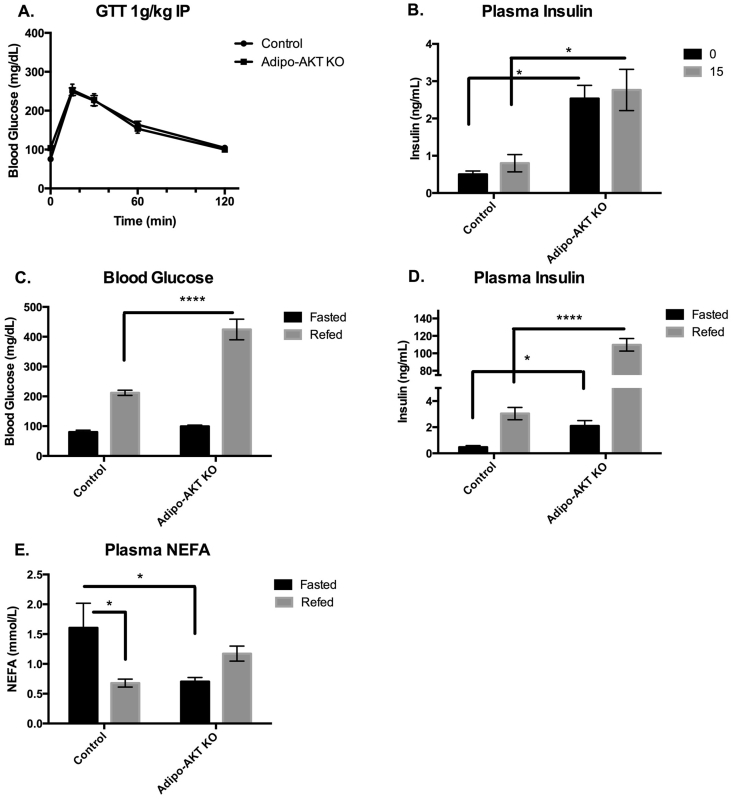
We next assessed glucose homeostasis in control and Adipo-AKT KO mice. Adipo-AKT KO mice had glucose tolerance comparable to control mice at 8–12 weeks of age (Figure 4A). However, while control mice had fasting insulin levels of ∼0.5 ng/mL, levels in the KO mice were appreciably higher at 2.5 ng/mL, indicative of significant insulin resistance (Figure 4B). To further investigate the ability of Adipo-AKT KO mice to regulate blood glucose in the face of insulin resistance, we fasted mice overnight and challenged them with a meal of normal chow. Two hours after refeeding, blood glucose levels were significantly greater in the Adipo-AKT KO mice than the controls (Figure 4C). Consistent with the results from GTT, the insulin levels were elevated in fasted Adipo-AKT KO and increased even further following feeding (Figure 4D).
Figure 4.
Adipo-AKT KO mice are insulin-resistant. A. Glucose tolerance tests were performed after an overnight fast, time 0, and 1 g/kg glucose was injected intraperitoneally with glucose measurements taken at 0, 15, 30, 60, and 120 min (control n = 9, Adipo-AKT KO n = 19). B. Serum insulin levels were collected at time 0 and 15 min after glucose injection. (control n = 8, Adipo-AKT KO n = 14). C. Blood glucose levels following an overnight fast and 2 h meal challenge in control and Adipo-AKT KO mice (control n = 8, Adipo-AKT KO n = 11). D. Serum insulin levels following an overnight fast and 2 h meal challenge in control and Adipo-AKT KO mice (control n = 5, Adipo-AKT KO n = 11). E. Serum non-esterified fatty acids after an overnight fast and 2 h meal challenge (control n = 4, Adipo-AKT KO n = 6). All data are presented as mean +/− s.e.m. (*, p < .05; ****, p < .0001).
Plasma non-esterified fatty acids (NEFA) levels were also measured during fasting and after a 2 h feeding challenge on normal chow. Adipo-AKT KO mice had low basal NEFA levels of 0.7 mmol/L compared to control mice that had fasting NEFA of 1.6 mmol/L. Postprandially, NEFA levels in control mice are suppressed to 0.68 mmol/L while in Adipo-AKT KO mice NEFA levels were not changed (Figure 4E).
4. Discussion
Our findings indicate that AKT1 and/or AKT2 are required for adipose maintenance in vivo. The severity of insulin resistance in the Adipo-AKT KO mice and their inability to regulate blood glucose postprandially follows the pattern observed in human patients with lipodystrophy, many of whom develop frank diabetes following puberty [1], [2], [24]. Multiple mouse models of lipodystrophy have been developed that recapitulate characteristics of human lipodystrophy. Mutations in AGPAT-2 and BSCL-2/seipin account for most human lipodystrophy patients, CGL Type I and CGL Type II, respectively [2], [6], and mice null for these genes have lipodystrophic and insulin resistant phenotypes [3], [4]. Several other genes have been manipulated in mice resulting in lipodystrophy, notably the AZIP/F-1 dominant negative mouse, which interferes with adipocyte development, the lamin A (LMNA) dominant negative mouse that inhibits adipose renewal, and adipocyte-specific loss of peroxisome proliferator-activated receptor gamma (PPARγ), a transcription factor required for adipocyte differentiation and maintenance [10], [25], [26], [27].
Studies performed in vitro established that AKT signaling is necessary for adipogenesis in cultured cells [19], [28], [29], [30], [31]. AKT1 is required for adipogenesis in mouse embryo fibroblasts (MEFs) and 3T3L1 preadipocytes, while AKT2 is dispensable [28], [29], [30]. Mechanistically, AKT functions in precursor cells to suppress Foxo1 activity [28], [31]. The isoform specificity in vitro are in conflict with in vivo data from Akt1 null mice, that have an overall smaller body size but do not exhibit a lipodystrophic phenotype [32], [33]. This suggests that either non-autonomous factors influence adipogenesis, or that AKT2 can support adipogenesis in the whole animal. Additionally, Akt2 null mice have been suggested to develop an age-dependent lipodystrophy [34]. Our data expand on these studies and document a critical role for AKT signaling in maintaining mature adipocytes.
The AdipoQ-Cre transgene is thought to be mainly expressed in mature adipocytes [20], [35], [36], [37]. Mullican et al. (2013) and Lee et al. (2013) showed that recombination does not occur in the stromal vascular fraction of adipose tissue depots [36], [37]. Consistent with this, Berry and Rodeheffer (2013) found that highly purified adipogenic precursor cells in WAT do not express AdipoQ-Cre [35]. Interestingly, a recent study shows that adiponectin is expressed at the embryonic stage in adipocyte precursors of inguinal WAT but not in adult preadipocytes [38]. It is unclear if AdipoQ-Cre is also expressed at sufficient levels in this population to induce efficient recombination. Moreover, it is not known if adipose precursors of other depots, such as the epididymal WAT similarly express adiponectin during embryogenesis. The AdipoQ-Cre mediated deletion of AKT in our model may thus reflect defects in adipocyte differentiation and maintenance, depending on the depot.
There are three AKT isoforms, AKT1-3, that function in the insulin signaling pathway. Each of these isoforms is expressed in adipocytes, but AKT2 is the most abundant isoform [14], [15]. Mice null for Akt1 and Akt2 are born with dwarfism, are not viable, and MEFs derived from these animals are unable to undergo adipogenesis [19]. AKT3 is expressed in differentiated adipocytes [39], but it is clearly not sufficient to preserve adipocyte insulin/IGF signaling in the absence of both AKT1 and AKT2.
The insulin and insulin-like growth factor 1 (IGF-1) pathways converge on the insulin receptor substrate and phosphoinositide-3-kinase (PI3K), upstream of AKT [40], [41], [42]. Loss of AKT1 and AKT2 in adipocytes likely ablates both insulin signaling and IGF-1 signaling, potentially contributing to the severity of the lipodystrophy. Concurrent deletion of the insulin receptor and insulin growth factor 1 receptor (IGF1R) using aP2-Cre causes lipodystrophy with a greater reduction in BAT size than WAT when compared to the IR knockout alone, suggesting that IGF1R signaling has a more important role in BAT than in WAT [43], [44]. Interpretation of these data is confounded by the use of the aP2-Cre. While the requirement for IR and IGF1R signaling may differ between depots, all depots depend on AKT-function.
The Adipo-AKT KO mice display profound hepatomegaly and a small but significant renomegaly along with increased pancreas weight (Figure 2, Figure 3D). This organomegaly is also observed in some human lipodystrophic patients, with hepatomegaly often being most prominent [1], [21], [22], [23]. Hyperinsulinemia potentially contributes to the increased body size and organomegaly of Adipo-AKT KO mice through enhanced IR/IGFR-1 signaling. Another possible contributor to organomegaly is increased food intake as a result of reduced circulating levels of leptin (Figure 1E), a critical regulator of energy homeostasis [17], [45], [46], [47], [48]. Increased food intake and decreased movement were observed in the AGPAT −/− lipodystrophy model and normalized with leptin supplementation [46]. Though not measured in this study, it is likely the Adipo-AKT KO mice would exhibit a similar phenotype.
Surprisingly, Adipo-AKT KO mice had low plasma NEFA levels, a phenomenon also observed in some human lipodystrophic patients [9]. These patients exhibit post-prandial plasma NEFA levels unchanged from their fasting levels [9]. Similarly, the Adipo-AKT KO mice had refed NEFA levels that were not significantly differently from either their fasted levels or the fed levels in control mice. In many mouse models of lipodystrophy, there are high fed plasma NEFA levels [25], [26], [27]. Interestingly, genetic background influences lipid profiles in lipodystrophic AZIP/F-1 mice [49]. On a FVB background AZIP/F-1 mice have abnormally high NEFA levels, whereas NEFA levels are similar between control and AZIP/F-1 mice on a C57/B6 background [49]. Similarly, Agpat2 and Bscl-2/seipin null mice, both in the C57/B6 background, have normal NEFA levels [3], [4]. These mice, and the C57/B6 AZIP/F-1 mice, have significantly decreased fasting NEFA levels [25], similar to the Adipo-AKT KO mice. By contrast, adipocyte-specific deletion of Bscl-2/seipin results in a milder lipodystrophy and these mice do not have reduced fasting NEFA levels [5]. Given the severity of lipodystrophy in the Adipo-AKT KO mice, it follows that they have no available adipose stores to undergo lipolysis and release fatty acids in response to a fasting state.
5. Conclusions
Our mouse model recapitulates many of the features observed in a familial lipodystrophy syndrome caused by loss of function mutations in Akt1 and Akt2, including insulin resistance and hepatomegaly [7]. Our study also demonstrates that AKT signaling, likely acting downstream of the insulin and IGF-1 receptors, is critically required for proper adipose expansion and/or maintenance.
Acknowledgments
We would like to acknowledge the Molecular Pathology and Imaging Core at the University of Pennsylvania, supported by NIH grants DK050306, CA098101, and DK049210, the Pathology Core at the Children's Hospital of Philadelphia Research Institute for sectioning of tissues for histopathology, and the Radioimmunoassay and Biomarkers Core at the Penn Diabetes Research Center for adipokine measurements, supported by NIH grant DK19525. We acknowledge NIDDK F30 DK100123 to A.S. for funding support. This research was supported by NIH RO1 DK093959 to M.J.B. and P.S.
Conflict of interest
Morris J. Birnbaum is employed by Pfizer, Inc.
References
- 1.Garg A. Acquired and inherited lipodystrophies. New England Journal of Medicine. 2004 Mar 18;350(12):1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A.K., Arioglu E., de Almeida S., Akkoc N., Taylor S.I., Bowcock A.M. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nature Genetics. 2002 Apr 22;31(1):21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 3.CortEs V.A., Curtis D.E., Sukumaran S., Shao X., Parameswara V., Rashid S. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metabolism. 2009 Feb 4;9(2):165–176. doi: 10.1016/j.cmet.2009.01.002. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui X., Wang Y., Tang Y., Liu Y., Zhao L., Deng J. Seipin ablation in mice results in severe generalized lipodystrophy. Human Molecular Genetics. 2011 Aug 1;20(15):3022–3030. doi: 10.1093/hmg/ddr205. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- 5.Liu L., Jiang Q., Wang X., Zhang Y., Lin R.C.Y., Lam S.M. Adipose-specific knockout of SEIPIN/BSCL2 results in progressive lipodystrophy. Diabetes. 2014 Jul;63(7):2320–2331. doi: 10.2337/db13-0729. American Diabetes Association. [DOI] [PubMed] [Google Scholar]
- 6.Magré J., Delépine M., Khallouf E., Gedde-Dahl T., Van Maldergem L., Sobel E. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nature Genetics. 2001 Aug;28(4):365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 7.George S., Rochford J.J., Wolfrum C., Gray S.L., Schinner S., Wilson J.C. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004 May 28;304(5675):1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chudasama K.K., Winnay J., Johansson S., Claudi T., König R., Haldorsen I. SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling. American Journal of Human Genetics. 2013 Jul 11;93(1):150–157. doi: 10.1016/j.ajhg.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage D.B., Tan G.D., Acerini C.L., Jebb S.A., Agostini M., Gurnell M. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003 Apr;52(4):910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 10.Wojtanik K.M., Edgemon K., Viswanadha S., Lindsey B., Haluzik M., Chen W. The role of LMNA in adipose: a novel mouse model of lipodystrophy based on the Dunnigan-type familial partial lipodystrophy mutation. The Journal of Lipid Research. 2009 May 15;50(6):1068–1079. doi: 10.1194/jlr.M800491-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alessi D.R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO Journal. 1996 Dec 2;15(23):6541–6551. European Molecular Biology Organization. [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh P.T., Smith L.M., O'Connor R. Insulin-like growth factor-1 activates Akt and Jun N-terminal kinases (JNKs) in promoting the survival of T lymphocytes. Immunology. 2002 Dec;107(4):461–471. doi: 10.1046/j.1365-2567.2002.01525.x. Wiley-Blackwell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng W.-H., Quirion R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neuroscience. 2006;7(1):51. doi: 10.1186/1471-2202-7-51. BioMed Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker K.S., Deak M., Paterson A., Hudson K., Cohen P., Alessi D.R. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochemical Journal. 1998 Apr 1;331(Pt 1):299–308. doi: 10.1042/bj3310299. Portland Press Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altomare D.A., Lyons G.E., Mitsuuchi Y., Cheng J.Q., Testa J.R. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene. 1998 May 7;16(18):2407–2411. doi: 10.1038/sj.onc.1201750. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 16.Cho H., Mu J., Kim J.K., Thorvaldsen J.L., Chu Q., Crenshaw E.B., III Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBbeta) Science. 2001 Jun 1;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 17.Chen W.S., Peng X.D., Wang Y., Xu P.Z., Chen M.L., Luo Y. Leptin deficiency and beta-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Molecular and Cellular Biology. 2009 May 12;29(11):3151–3162. doi: 10.1128/MCB.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer-Posovszky P., Tews D., Horenburg S., Debatin K.-M., Wabitsch M. Differential function of Akt1 and Akt2 in human adipocytes. Molecular and Cellular Endocrinology. 2012 Jul 6;358(1):135–143. doi: 10.1016/j.mce.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Peng X.D., Xu P.Z., Chen M.L., Hahn-Windgassen A., Skeen J., Jacobs J. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes & Development. 2003 Jun 1;17(11):1352–1365. doi: 10.1101/gad.1089403. Cold Spring Harbor Lab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eguchi J., Wang X., Yu S., Kershaw E.E., Chiu P.C., Dushay J. Transcriptional control of adipose lipid handling by IRF4. Cell Metabolism. 2011 Mar 2;13(3):249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler L.H. Lipodystrophies: report of seven cases. Brain. 1928 Jun 1;51(2):147–167. Oxford University Press. [Google Scholar]
- 22.Lawrence R.D. Lipodystrophy and hepatomegaly with diabetes, lipaemia, and other metabolic disturbances; a case throwing new light on the action of insulin. Lancet. 1946 May 25;1(6404):773. doi: 10.1016/s0140-6736(46)91599-1. (concluded) [DOI] [PubMed] [Google Scholar]
- 23.Misra A., Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003 Mar;82(2):129–146. doi: 10.1097/00005792-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Garg A. Lipodystrophies. American Journal of Medicine. 2000 Feb;108(2):143–152. doi: 10.1016/s0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- 25.Moitra J., Mason M.M., Olive M., Krylov D., Gavrilova O., Marcus-Samuels B. Life without white fat: a transgenic mouse. Genes & Development. 1998 Oct 15;12(20):3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimomura I., Hammer R.E., Richardson J.A., Ikemoto S., Bashmakov Y., Goldstein J.L. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes & Development. 1998 Oct 15;12(20):3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F., Mullican S.E., DiSpirito J.R., Peed L.C., Lazar M.A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proceedings of the National Academy of Sciences of the United States of America. 2013 Nov 12;110(46):18656–18661. doi: 10.1073/pnas.1314863110. National Acad Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yun S.J., Kim E.K., Tucker D.F., Kim C.D., Birnbaum M.J., Bae S.S. Isoform-specific regulation of adipocyte differentiation by Akt/protein kinase Bα. Biochemical and Biophysical Research Communications. 2008 Jun;371(1):138–143. doi: 10.1016/j.bbrc.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Bae S.S., Cho H., Mu J., Birnbaum M.J. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. Journal of Biological Chemistry. 2003 Dec 5;278(49):49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 30.Baudry A., Yang Z.Z., Hemmings B.A. PKB is required for adipose differentiation of mouse embryonic fibroblasts. Journal of Cell Science. 2006 Mar 1;119(5):889–897. doi: 10.1242/jcs.02792. [DOI] [PubMed] [Google Scholar]
- 31.Nakae J., Kitamura T., Kitamura Y., Biggs W.H., Arden K.C., Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Developmental Cell. 2003 Jan;4(1):119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 32.Cho H., Thorvaldsen J.L., Chu Q., Feng F., Birnbaum M.J. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. Journal of Biological Chemistry. 2001 Oct 19;276(42):38349–38352. doi: 10.1074/jbc.C100462200. American Society for Biochemistry and Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 33.Chen W.S., Xu P.Z., Gottlob K., Chen M.L., Sokol K., Shiyanova T. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes & Development. 2001 Sep 1;15(17):2203–2208. doi: 10.1101/gad.913901. Cold Spring Harbor Lab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garofalo R.S., Orena S.J., Rafidi K., Torchia A.J., Stock J.L., Hildebrandt A.L. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. Journal of Clinical Investigation. 2003 Jul 15;112(2):197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry R., Rodeheffer M.S. Characterization of the adipocyte cellular lineage in vivo. Nature Cell Biology. 2013 Mar;15(3):302–308. doi: 10.1038/ncb2696. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullican S.E., Tomaru T., Gaddis C.A., Peed L.C., Sundaram A., Lazar M.A. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Molecular Endocrinology. 2013 Jan;27(1):127–134. doi: 10.1210/me.2012-1267. Endocrine Society Chevy Chase, MD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K.Y., Russell S.J., Ussar S., Boucher J., Vernochet C., Mori M.A. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013 Mar;62(3):864–874. doi: 10.2337/db12-1089. American Diabetes Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong K.Y., Bae H., Park I., Park D.Y., Kim K.H., Kubota Y. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development. 2015 Aug 1;142(15):2623–2632. doi: 10.1242/dev.125336. [DOI] [PubMed] [Google Scholar]
- 39.Easton R.M., Cho H., Roovers K., Shineman D.W., Mizrahi M., Forman M.S. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Molecular and Cellular Biology. 2005 Mar;25(5):1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. American Society for Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stokoe D., Stephens L.R., Copeland T., Gaffney P.R., Reese C.B., Painter G.F. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997 Jul 25;277(5325):567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 41.Withers D.J., Burks D.J., Towery H.H., Altamuro S.L., Flint C.L., White M.F. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nature Genetics. 1999 Sep;23(1):32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- 42.Nakae J., Barr V., Accili D. Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO Journal. 2000 Mar 1;19(5):989–996. doi: 10.1093/emboj/19.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boucher J., Mori M.A., Lee K.Y., Smyth G., Liew C.W., Macotela Y. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nature Communications. 2012 Jun 12;3:902–911. doi: 10.1038/ncomms1905. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blüher M., Michael M.D., Peroni O.D., Ueki K., Carter N., Kahn B.B. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Developmental Cell. 2002 Jul;3(1):25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 45.Asilmaz E., Cohen P., Miyazaki M., Dobrzyn P., Ueki K., Fayzikhodjaeva G. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. Journal of Clinical Investigation. 2004 Feb;113(3):414–424. doi: 10.1172/JCI19511. American Society for Clinical Investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.CortEs V.A., Cautivo K.M., Rong S., Garg A., Horton J.D., Agarwal A.K. Leptin ameliorates insulin resistance and hepatic steatosis in Agpat2-/- lipodystrophic mice independent of hepatocyte leptin receptors. The Journal of Lipid Research. 2014 Feb;55(2):276–288. doi: 10.1194/jlr.M045799. American Society for Biochemistry and Molecular Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebihara K., Ogawa Y., Masuzaki H., Shintani M., Miyanaga F., Aizawa-Abe M. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes. 2001 Jun;50(6):1440–1448. doi: 10.2337/diabetes.50.6.1440. [DOI] [PubMed] [Google Scholar]
- 48.Shimomura I., Hammer R.E., Ikemoto S., Brown M.S., Goldstein J.L. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999 Sep 2;401(6748):73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 49.Colombo C., Haluzik M., Cutson J.J., Dietz K.R., Marcus-Samuels B., Vinson C. Opposite effects of background genotype on muscle and liver insulin sensitivity of lipoatrophic mice. Role of triglyceride clearance. Journal of Biological Chemistry. 2003 Feb 7;278(6):3992–3999. doi: 10.1074/jbc.M207665200. American Society for Biochemistry and Molecular Biology. [DOI] [PubMed] [Google Scholar]