Abstract
In adult mammals, massive sudden loss of cardiomyocytes following infarction overwhelms the limited regenerative capacity of the myocardium, resulting in formation of a collagen-based scar. Necrotic cells release danger signals, activating innate immune pathways and triggering an intense inflammatory response. Stimulation of toll-like receptor signaling and complement activation induces expression of pro-inflammatory cytokines (such as interleukin-1 and tumor necrosis factor-α) and chemokines (such as monocyte chemoattractant protein-1/CCL2). Inflammatory signals promote adhesive interactions between leukocytes and endothelial cells, leading to extravasation of neutrophils and monocytes. As infiltrating leukocytes clear the infarct from dead cells, mediators repressing inflammation are released, and anti-inflammatory mononuclear cell subsets predominate. Suppression of the inflammatory response is associated with activation of reparative cells. Fibroblasts proliferate, undergo myofibroblast transdifferentiation, and deposit large amounts of extracellular matrix proteins maintaining the structural integrity of the infarcted ventricle. The renin-angiotensin-aldosterone system and members of the transforming growth factor-β family play an important role in activation of infarct myofibroblasts. Maturation of the scar follows, as a network of cross-linked collagenous matrix is formed and granulation tissue cells become apoptotic. This review discusses the cellular effectors and molecular signals regulating the inflammatory and reparative response following myocardial infarction. Dysregulation of immune pathways, impaired suppression of post-infarction inflammation, perturbed spatial containment of the inflammatory response, and overactive fibrosis may cause adverse remodeling in patients with infarction contributing to the pathogenesis of heart failure. Therapeutic modulation of the inflammatory and reparative response may hold promise for prevention of post-infarction heart failure.
Keywords: Myocardial Infarction, Inflammation, Fibrosis, Immune Cells, Remodeling
Subject Terms: Heart Failure
1. Introduction
Adverse left ventricular (LV) remodeling following myocardial infarction (MI) constitutes the structural basis for ischemic heart failure (HF), and is comprised of complex short- and long-term changes in LV size, shape, function, and cellular and molecular composition.1, 2 While multiple pathophysiological factors converge to remodel the heart after MI, the fundamental determinants of this process (and its progression to clinical HF) are the extent of the initial infarction and the sufficiency of the post-MI reparative process. In clinical practice, limiting infarction extent is routinely addressed by timely coronary reperfusion. In contrast, therapeutic manipulation of the ensuing repair process, which is driven principally by robust tissue inflammation and subsequently by its active suppression and resolution, has proved much more challenging and elusive. Nonetheless, recent studies have suggested a large number of potential therapeutic targets that may favorably influence cardiac wound healing and repair. In this review, we will broadly consider the multiplicity of cellular and molecular factors that influence post-MI repair, highlighting the translational implications for these events in the amelioration of adverse remodeling and the development of ischemic HF.
2. The phases of cardiac repair after myocardial infarction
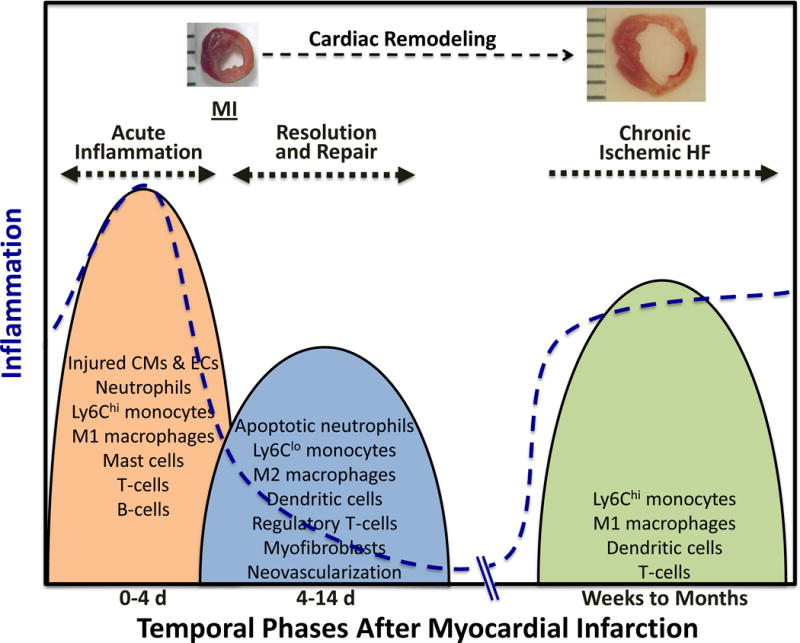
Cardiac repair after MI results from a finely orchestrated and complex series of events, initiated by intense sterile inflammation and immune cell infiltration (inflammatory phase) that serve to digest and clear damaged cells and extracellular matrix tissue (~3–4 d in mice), followed by a reparative phase with resolution of inflammation, (myo)fibroblast proliferation, scar formation, and neovascularization over the next several days (Figure 1).3, 4 Early inflammatory activation is a necessary event for the transition to later reparative and proliferative programs. Appropriate and timely containment and resolution of inflammation are further determinants of the quality of wound healing; a proper physiologic balance needs to be achieved between these two phases for optimal repair.5, 6 An inflammatory phase that is disproportionately prolonged, of excessive magnitude, or insufficiently suppressed can lead to sustained tissue damage and improper healing, defective scar formation, and heightened cell loss and contractile dysfunction, thereby promoting infarct expansion, adverse remodeling and chamber dilatation. To date, there has been no large-scale immunomodulatory or anti-inflammatory therapeutic strategy post-MI that has been successfully translated into clinical practice, no doubt a reflection of both the exquisite complexity and our incomplete understanding of the healing process.
Figure 1.
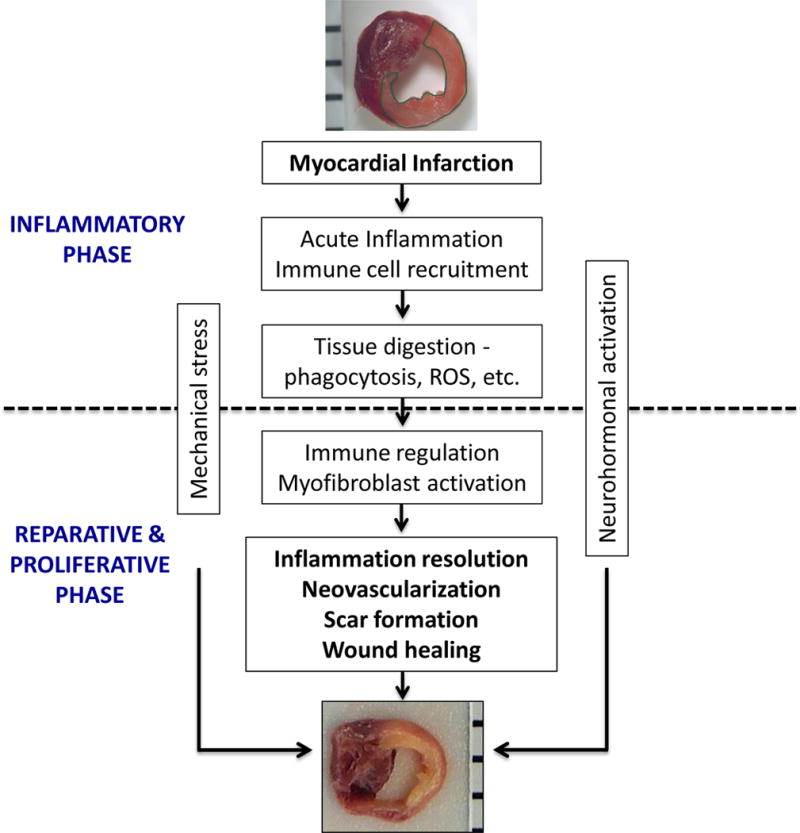
Biphasic nature of cardiac repair after myocardial infarction (MI). Early after MI, tissue injury and necrosis initiates the inflammatory phase, consisting of intense sterile inflammation, and the dynamic recruitment of several immune cell subtypes including neutrophils, monocyte/macrophages, dendritic cells, and lymphocytes. After ~4 days in murine models, this transitions to a reparative and proliferative phase, with shift of immune cell polarity toward immunomodulation and resolution, myofibroblast proliferation, collagen deposition and scar formation, and neovascularization, thereby resulting in wound healing. Neurohormonal activation and mechanical stress are other factors that influence this healing process. ROS, reactive oxygen species.
3. The inflammatory phase
3.1. Molecular cascades implicated in the post-infarction inflammatory response
Hypoxia during ischemia impairs vascular endothelial cell integrity and its barrier function thereby augmenting vessel permeability, facilitating leukocyte infiltration.7 If the ischemic period is sufficiently prolonged, parenchymal and cardiomyocyte cell death programs are activated, primarily due to cell necrosis, but also secondary to apoptosis and autophagic mechanisms.3, 7 Restoration of blood flow may further augment tissue damage via reperfusion injury, due to abrupt re-oxygenation, reactive oxygen species (ROS) generation, and activation of the complement pathway.3, 5, 7–9 Necrotic and stressed/injured cells, and the damaged extracellular matrix, release substances that act as danger signals, termed danger-associated molecular patterns (DAMPs). DAMPs bind to cognate pattern recognition receptors (PRRs) of the innate immune system on surviving parenchymal cells and infiltrating leukocytes (and also activate the complement pathway) to robustly activate a cascade of inflammatory mediators, including inflammatory cytokines, chemokines, and cell adhesion molecules.8, 10–14 In addition to being passively released upon cell necrosis or matrix damage, select DAMPs may also be upregulated and secreted by stressed cardiomyocytes and fibroblasts, and by activated leukocytes.8, 11, 12, 15–17
Several DAMPs can trigger the inflammatory response during MI (Table 1). These including high mobility group box-1 (HMGB1), S100 proteins, fibronectin extra domain A, interleukin(IL)-1α, heat shock proteins (HSPs), low molecular weight hyaluronic acid, ATP, uric acid, mitochondrial DNA, dsRNA, ssRNA, and complement, among others.7, 8, 11, 12, 14 The PRRs are primarily the membrane bound toll-like receptor/IL-1 receptors (TLR/IL-1Rs), as well as cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and the cell-surface receptor for advanced glycation end-products (RAGE). The signaling pathways downstream of these PRRs have been comprehensively detailed in recent reviews13, 14, 18, 19 and briefly considered below. In the context of MI, downstream signaling converges on the activation of mitogen-activated protein kinases (MAPKs) and nuclear factor (NF)-κB. These pathways (NF-κB in particular) drive the expression of a large panel of pro-inflammatory genes including inflammatory cytokines (e.g, tumor necrosis factor-α [TNF], IL-1β, IL-6, IL-18); CXC chemokines containing the glutamic acid-leucine-arginine (ELR) motif that act predominantly as neutrophil chemoattractants; CC chemokines that attract monocytes and T-lymphocytes; cell adhesion molecules (e.g., VCAM, ICAM, selectins), and complement factor B.14, 20, 21 Subsequent leukocyte recruitment further amplifies the inflammatory response, augments the production of DAMPs, and promotes both efferocytosis of dying cells and tissue digestion via the release of proteases and oxidases.3, 22 Efficient efferocytosis of apoptotic cardiomyocytes is particularly important for transitioning to the phase of inflammation resolution and wound healing, and is mediated in part by macrophages expressing the myeloid-epithelial-reproductive tyrosine kinase (Mertk).23
Table 1.
Select DAMPs and Corresponding Pattern Recognition Receptors
| DAMP | Receptor(s) | Reference(s) |
|---|---|---|
| HMGB1 | TLR2, TLR4, TLR9, RAGE | 8, 10–12, 14, 15, 25–40, 83 |
| S100A8/A9 | TLR4, NLRP3, RAGE | 11, 14, 42–47, 83 |
| S100A1 | Endolysosomal TLR4 | 48 |
| Fibronectin-EDA | TLR2, TLR4 | 8, 10, 11, 51–53 |
| IL-1α | IL-1R | 14, 54, 83 |
| HSP-60, HSP-70 | TLR2, TLR4/6 | 8, 10, 11, 83 |
| LMW hyaluronic acid | TLR2, TLR4, NLRP3 | 8, 10, 11, 18, 83 |
| ATP | P2X7/NLRP3 | 11, 14, 18, 83, 87, 88 |
| Uric acid | TLR2, TLR4, NLRP3 | 11, 14, 18, 83 |
| Mitochondrial DNA | TLR9, NLRP3 | 8, 10, 11, 14, 83 |
| dsRNA | TLR3 | 8, 14, 83 |
| ssRNA | TLR7, TLR8 | 8, 10, 11, 14 |
LMW, low molecular weight. Some references are in-depth review articles
3.1.1. DAMPs
HMGB1
HMGB1, a loosely-associated chromatin protein involved in DNA stabilization and gene control,24 is a potent mediator of inflammation following tissue injury.25 HMGB1 is passively released from necrotic cells (but not apoptotic cells),25 actively secreted by stimulated monocytes and macrophages,26 and induced by peroxynitrite and oxidative stress in ischemic cardiomyocytes.27 HGMB1 engages and activates several TLRs (including TLR2, TLR4, TLR9) and RAGE,8, 15, 28, 29 to induce NF-κB nuclear translocation and pro-inflammatory signaling. HMGB1 also promotes monocyte recruitment in a TLR- and RAGE-independent manner via direct binding to CXCL12 (stromal cell-derived factor-1 [SDF-1]) and the formation of HMGB1-CXCL12 heterocomplexes that synergistically enhance CXCR4 signaling in inflammatory cells.30
In humans with acute MI, serum HMGB1 levels are elevated and predictive of subsequent mortality, LV dysfunction, and effort intolerance.31–33 In rodents with reperfused15, 34 and non-reperfused33 MI, serum levels and myocardial HMGB1 expression increase very early after injury. In reperfused MI, HMGB1 plays a pivotal role in the activation of MAPK and NF-κB pathways, increasing leukocyte infiltration, and augmenting tissue injury, apoptosis, and infarct size, in part via RAGE-dependent signaling.15, 34 Interestingly and in contrast, however, in acute and chronic non-reperfused MI models, augmenting HMGB1 via either exogenous administration35–37 or cardiomyocyte-specific overexpression38, 39 improved LV remodeling and function with augmented c-kit+ progenitor cell infiltration, reduced dendritic cell accumulation, less collagen deposition, and better tissue angiogenesis. Exogenous administration of HMGB1 prior to ischemia/reperfusion (I/R) also induces preconditioning and cardioprotection.40 Interestingly, antibody-mediated HMGB1 neutralization in non-reperfused MI reduced tissue inflammatory cytokine expression and macrophage infiltration at day 3, but induced scar thinning and more pronounced LV remodeling, underscoring the concept that inflammation, properly regulated and controlled, is essential for optimal wound healing in the heart.33 Hence, these results indicate that while HMGB1 is an inflammatory mediator, the ultimate effects of HMGB1 modulation depends on the underlying disease, its temporal context, and the degree of inflammatory response referable to the pathophysiology. Compared to non-reperfused MI hearts, I/R hearts exhibit greater magnitude but shorter duration of inflammatory cell infiltration.9, 41 This profile of augmented inflammation may explain the beneficial results with HMGB1 inhibition in reperfused MI, and the divergence in response from non-reperfused MI in which angiogenic effects may predominate.
Other DAMPs
S100A8 (calgranulin A) and S100A9 (calgranulin B) are members of the calcium-binding S100 family expressed in phagocytic cells.42 S100A8 and A9 rapidly associate to form the S100A8/A9 heterodimer. S100A8/A9 complexes function as DAMPs secreted by neutrophils and monocytes/macrophages during inflammatory conditions and signal via RAGE and TLR4 receptors.43, 44 Humans with acute MI exhibit elevated serum S100A8/A9 levels that correlate with circulating neutrophil counts and the risk of cardiovascular death and subsequent MI.45, 46 In mice with reperfused MI, S100A8/A9 is rapidly expressed and released after ischemia, primarily by inflammatory cells and fibroblasts, and induces pro-inflammatory signaling, leukocyte infiltration, and cardiac dysfunction in a RAGE-dependent manner,47 suggesting that these DAMPs are central to post-MI inflammation. Interestingly, S100A1, the S100 protein most abundant in cardiomyocytes, is also released from damaged cardiomyocytes in both humans and mice with acute MI.48 However, rather than promoting generalized inflammation, S100A1 is taken up by endocytosis in adjacent cardiac fibroblasts to transiently activate TLR4-endolysosomal signaling, resulting in an immunomodulatory and anti-fibrotic phenotype with beneficial effects on post-MI LV remodeling in vivo.48 This suggests that specific DAMPs have unique cell targets and functional roles in the cardiac repair process that can be either pro- or anti-inflammatory. In this regard, the β-galactoside binding lectin galectin-1 is expressed by hypoxic cardiomyocytes and infiltrating leukocytes after MI, and also imparts anti-inflammatory and cardioprotective effects in the remodeling heart.49
Fibronectin is an extracellular matrix protein secreted by fibroblasts in response to tissue injury and pro-inflammatory cytokines,50 and includes an alternatively spliced exon coding type III repeat extra domain A (EDA) that binds to TLR-4 to activate mast cells and leukocytes.51, 52 Mice with parenchymal myocardium-localized fibronectin-EDA deficiency exhibited improved LV remodeling and function, less monocyte recruitment, and reduced remote zone fibrosis after non-reperfused MI as compared with wild-type mice, indicating a critical role for fibronectin-EDA in tissue inflammation and remodeling.53 Conversely, recent studies have demonstrated that necrotic cardiomyocytes (but not fibroblasts) release IL-1α as danger signal that activates pro-inflammatory MAPK and NF-κB signaling in cardiac fibroblasts in a MyD88-dependent but NLRP3- and TLR-independent manner, via activation of the IL-1R pathway.54 Hence, multiple danger signals act in a concerted fashion on parenchymal and inflammatory cells in the infarcted heart to drive and/or modulate inflammation. For a further discussion of DAMPs, the reader is referred to several comprehensive reviews.8, 10–12
3.1.2. TLRs, NLRs, and RAGE
TLRs
The TLRs comprise the major PRRs on mammalian cells.14 Expressed most prominently on leukocytes, TLRs are also expressed by parenchymal cells, including cardiomyocytes, fibroblasts, and endothelial cells. Thus far, 13 functional mammalian TLRs have been identified (10 in humans, TLRs 1–10)13, 14 that recognize a variety of pathogen-associated molecular patterns14 and DAMPs11, 12 to trigger innate immune responses. Of these, TLRs 1, 2, 4, 5, 6, and 11 are cell-surface receptors, whereas TLR3 and TLRs 7–10 are expressed in endolysosomes.13, 14 Signal transduction by TLRs and IL-1Rs occurs through a conserved cytoplasmic Toll/IL-1R (TIR) domain that serves as the docking site for TIR-containing cytoplasmic adaptor proteins (Figure 2). Except for TLR3, all TLRs (and IL-1Rs) engage with the adaptor MyD88 (myeloid differentiation factor 88) either directly, or, for TLR2 and TLR4, in combination with the adaptor TIRAP (TIR domain-containing adaptor protein) to trigger receptor complex interactions with IRAKs (IL-1R associated kinases) 4, 1 and 2, TRAF6 (TNF receptor associated factor 6), and the MAPKKK transforming growth factor activated kinase (TAK)1. As fully reviewed elsewhere,13, 14 these signaling cascades ultimately activate NF-κB (p65 and p50) and MAPK pathways to upregulate a broad array of pro-inflammatory mediators (Figure 2). TLR4 is also endocytosed after ligand binding; endolysosomal TLR4 signals in a MyD88-independent manner via the cytoplasmic adaptor TRIF (TIR domain-containing adaptor inducing interferon [IFN]-β) and the bridging adaptor TRAM (TRIF-related adaptor molecule). This pathway results in NF-κB nuclear translocation, and the induction of type I IFN via activation of TBK1 (TANK-binding kinase) and the transcription factor IRF3 (interferon-regulatory factor 3). In the heart, the most highly expressed TLRs are TLR4, TLR2, TLR3, and TLR5,13 with TLR4 and TLR2 the most studied in the context of myocardial injury.
Figure 2.
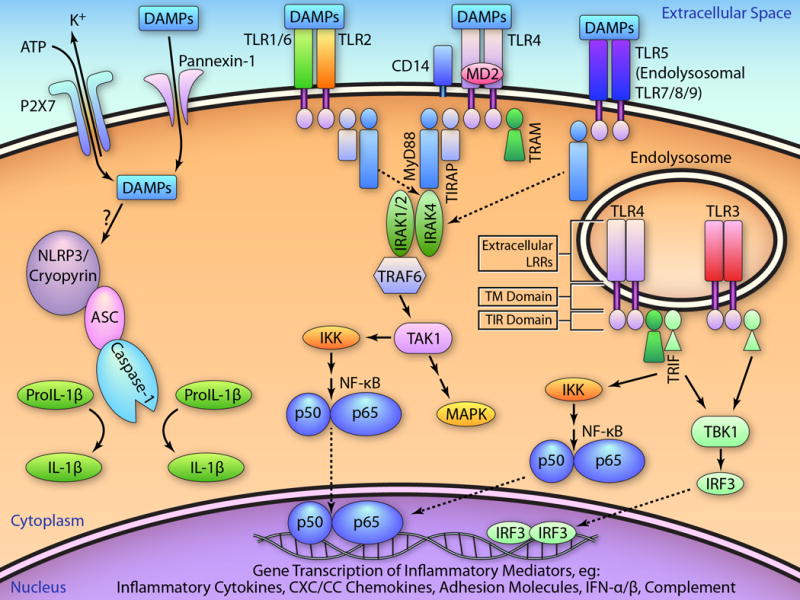
TLR and NLR signaling. DAMPs released from necrotic and damaged cells and extracellular matrix bind to the TLRs, which are comprised of an extracellular leucine rich repeat (LRR) domain, a transmembrane (TM) domain, and a conserved cytoplasmic Toll/IL-1R (TIR) domain, which serves as docking site for other TIR-containing cytoplasmic adaptor proteins (top left box). The binding of DAMPs, and often co-receptors such as CD14 and MD2, to TLR4 engages the adaptor MyD88 via the adaptor TIRAP. MyD88 recruits IRAK4 and the IRAK1/2 and TRAF6 complex, which activates TAK1. TAK1, a MAPKKK, then activates the MAPK cascade and also phosphorylates IκB kinase (IKK), that leads to NF-κB p65 and p50 nuclear translocation and the transcription of a panel of inflammatory genes, including cytokines, chemokines, cell adhesion molecules, and complement factor B. Further, after endocytosis, TLR4 signals in a MyD88-independent manner via the cytoplasmic adaptor TRIF, in turn recruited through the bridging adaptor TRAM. This pathway results in non-canonical IKK and NF-κB activation, as well as the induction of type I IFN via TBK1 and the transcription factor IRF3. Except for TLR3, the other TLRs also signal, directly or indirectly as depicted, via MyD88. Endolysosomal TLR3 activates IRF3 signaling via TRIF and TBK1, whereas endolysosomal TLR7/8 and TLR9 also induce type I IFN via TRAF3 and IRF7 in a MyD88-dependent manner (not pictured). In addition to TLRs, intracellular NLRs respond to a variety of PAMPs via the formation of multiprotein inflammasomes, comprised of an activated NLR protein, the adaptor protein ASC, and procaspase-1. One proposed model of NLRP3 inflammasome activation results from stimulation of the P2X7 ion channel by extracellular ATP, resulting in K+ efflux, recruitment of the pannexin-1 pore, DAMP entry into cytosol, and activation of NLRP3. This activates caspase-1, which then converts pro-IL18 and pro-IL1β to active IL-18 and IL-1β. As discussed in the text, cell type-specific innate immune signaling can result in distinctive, and sometimes divergent, in vivo physiological responses during acute MI. Schema adapted from references 13, 14, 18, 76, and 83. (Illustration credit: Ben Smith).
Augmented TLR4 activation, and increased expression of pro-inflammatory mediators downstream of TLR4 signaling, has been demonstrated in circulating leukocytes from humans with acute MI,55–57 and correlated with the development of HF.56 Similar findings have also been reported for TLR2 in circulating monocytes.58 Moreover, cardiac TLR4 expression increases both after acute MI59 and in chronic HF.60, 61 Mice with genetic disruption or deficiency of TLR4,62, 63 TLR2,64, 65 MyD88,66 or TLR367 exhibit reduced infarct size following I/R, and amelioration of pathological remodeling after non-reperfused MI.59, 67–70 Moreover, pre-treatment with eritoran, a specific TLR4 antagonist, in mice,71 or with an anti-TLR2 antibody in mice64 and pigs72 reduced infarct size after myocardial I/R. In both MI models, sustained TLR-mediated signaling generally augmented cell apoptosis, inflammation, interstitial fibrosis, oxidative stress, and leukocyte recruitment, indicating maladaptive responses triggered by TLR2, TLR4, and TLR3 after MI. Interestingly, and in contrast, beneficial effects were referable to TLR5 after I/R, as TLR5 deficiency increased infarct size, oxidant stress, inflammation, and LV dysfunction after reperfused MI.73
Interestingly, short-term TLR2,74, 75 TLR4,13, 76, 77 and TLR978 activation prior to I/R induces preconditioning and cardioprotection (with reductions in infarct size) in part via TIRAP- and PI3K/Akt-dependent mechanisms. Analogous short-term protective effects in cardiac myocytes have similarly been observed for several innate immune signaling mediators, including NF-κB,79 inflammatory cytokines,80 and chemokines.81, 82 These observations suggest a paradigm whereby short-term activation of innate immune pathways, primarily localized to cardiomyocytes, yield cytoprotective and pro-survival effects via mitochondrial stabilization, whereas activation that is more prolonged or of greater magnitude and involving immune cells results in more robust inflammatory responses and leukocyte recruitment that induce tissue injury.13, 79, 80, 83 In this regard, in vivo chimeric mouse models64 and ex vivo isolated perfused heart studies (in which circulating leukocytes are eliminated)84 have demonstrated that after I/R, leukocyte-localized TLR2 is responsible for inducing myocardial injury and determines infarct size, whereas parenchymal TLR2 signaling induces contractile dysfunction without affecting infarct size. In contrast, both parenchymal and leukocytic TLR2 are needed to mediate endothelial injury and dysfunction after I/R.65 Interestingly, divergent effects of leukocyte vis-à-vis cardiomyocyte TLR4 signaling on cardiomyocyte impairment have also been shown in a model of systemic sepsis.85 Hence, the effects of TLR signaling, and pro-inflammatory mediators in general, are complex and graded, rather than all-or-none, and depend heavily on the cellular and disease context.
NLRs
The NLR family of intracellular PRRs responds to a variety of DAMPs (e.g., ATP and uric acid), and include NOD receptors (NOD1 and NOD2) that activate NF-κB, and the NLRs (NLRP1, NLRP3/cryopyrin, NLRC4) that augment IL-1β and IL-18 secretion via the formation of multiprotein signaling complexes termed inflammasomes. The NLRs contain a CARD (caspase activation and recruitment) or pyrin domain at the N-terminus, a central NACHT domain, and a C-terminal leucine-rich repeat region. The inflammasome consists of an activated NLR protein, the adaptor protein ASC (apoptosis speck-like protein containing a caspase-recruitment domain), and procaspase-1. The complex facilitates caspase-1 activation, which converts pro-IL18 and pro-IL1β (generated by MyD88-dependent TLR pathways) to active IL-18 and IL-1β (Figure 2). One proposed model of activation of NLRP3 involves extracellular ATP stimulation of the purogenic P2X7 ion channel; this triggers K+ efflux, recruitment of the pannexin-1 membrane pore, and entry of DAMPs into cytosol to access NLRP3 (Figure 2).18
The major components of the NLRP3 inflammasome (ASC, cryopyrin, caspase-1) are upregulated and/or activated early after MI in a variety of cell types in the heart, primarily infiltrating leukocytes, fibroblasts, and endothelial cells, but also border zone cardiomyocytes.86–88 IL-1β and IL-18, the cytokine end-products of inflammasome activation, are also increased early after MI.88–90 Inflammasome activation has been suggested to occur initially in cardiac fibroblasts during reperfused MI, in response to ROS production and K+ efflux.86 Global targeted genetic disruption of ASC or caspase-1,86 as well as antibody neutralization of IL-18,90 has been shown to reduce infarct size in vivo after I/R in mice. ASC loss-of function also improved post-MI cardiac remodeling and fibrosis, and decreased leukocyte infiltration and the expression of pro-inflammatory cytokines and chemokines. Studies using chimeric mice indicate that inflammasome activation in leukocytes and resident cardiac cells both contribute to ischemic injury.86 Similarly, isolated perfused NLRP3−/− murine hearts exhibit reduced infarct size and improved contractile function during ex vivo I/R,88 whereas gene silencing of cryopyrin ameliorates cardiac remodeling and dysfunction in vivo after non-reperfused MI in mice.87 Hence, the NLRP3 inflammasome is a key mediator of the post-MI inflammatory response and tissue injury.
RAGE
RAGE is a PRR expressed by a variety of immune and parenchymal cell types that interacts with several DAMPs such as HMGB1 and S100A8/A9. RAGE activation triggers a number of intracellular signaling pathways, including NF-κB- and MAPK-dependent inflammatory genes.19 RAGE deficient mice exhibited reduced infarct size, less leukocyte infiltration and inflammatory cytokine expression, and improved cardiac remodeling and function after reperfused MI.15, 47 Studies of chimeric mice indicate that RAGE signaling in infiltrating leukocytes, rather than resident cardiac cells, is primarily responsible for these adverse pro-inflammatory and remodeling responses post-MI.47 Thus, inflammatory cell-localized RAGE in particular amplifies and promotes tissue injury during MI.
3.1.3. NF-κB
NF-κB is a central transcriptional effector of inflammatory signaling. NF-κB activation and its subsequent nuclear translocation after MI trigger transcription of a large portfolio of genes including inflammatory cytokines, CXC and CC chemokines, and adhesion molecules. The spatial and temporal expression of these mediators in resident and infiltrating cells choreographs events that further amplify the inflammatory response (cytokines) and attract and recruit specific leukocyte populations (chemokines and adhesion molecules) to injured myocardium. The signaling pathways and outputs linked to NF-κB activation, as well as its effects in cardiac diseases, have been reviewed extensively.14, 79, 91, 92 Five subunits – p65 (RelA), RelB, c-Rel, p50, and p52 – comprise the NF-κB family; these subunits form homo- or heterodimers to modulate gene transcription. Classically, p65/p50 heterodimers are bound to inhibitor of κBα (IκBα) in the cytoplasm. Upon IκBα phosphorylation by IκB kinase (IKK), IκBα is rapidly ubiquitinated and degraded by the 26S proteasome, allowing for subunit nuclear translocation. Activation can also occur via IκBα-independent mechanisms to release p50/RelB or p52/RelB. Importantly, only p65, RelB, and c-Rel contain transactivation domains; hence, p50 and p52 homodimers can repress rather than activate gene transcription. Therefore, subunit-dependent differences in target gene specificity impact the transcriptional response, and may contribute to the spectrum of effects observed upon NF-κB activation, ranging from cardioprotection to injury and cell death.
In humans with acute MI, circulating leukocytes exhibit marked activation of NF-κB.57, 93 In rodents, NF-κB is activated in the heart early after MI in the infarct zone,89, 90, 94 and later (after 24 h) in the remote zone.95 Importantly, whereas there is nuclear translocation of both p65 and p50 in the first 24 h after MI, the profile shifts near exclusively to p65 at later time points.95 Studies regarding a cardioprotective vis-à-vis detrimental role of NF-κB during MI have yielded conflicting results.79 Acute NF-κB activation is essential for late cardioprotection induced by ischemic preconditioning.96, 97 However, mice with cardiomyocyte-restricted overexpression of phosphorylation-resistant IκBα,98 cardiomyocyte-specific p65 deletion,99 IκBα overexpression via gene transfer,100 NF-κB double-stranded decoy DNA transfection,101 and pharmacological blockade of IκBα102 or IKKβ103 have demonstrated that NF-κB inhibition (primarily in cardiomyocytes and perhaps more related to p65) during myocardial I/R decreases infarct size, reduces inflammatory responses including leukocyte infiltration, and improves cardiac function. Studies of non-reperfused MI in mice with cardiomyocyte-restricted overexpression of phosphorylation-resistant IκBα95 or A20 (NF-κB signaling inhibitor),104 and IκBα gene transfer105 have similarly shown that blocking NF-κB (mainly p65 based on time course studies95) attenuates long-term adverse cardiac remodeling, dysfunction, and inflammation.
The p50 subunit lacks a transactivation domain and thus can inhibit NF-κB transcriptional activity. A study using cardiac MRI imaging in p50−/− mice demonstrated that leukocyte p50 expression imparts beneficial effects on remodeling after non-reperfused MI, by improving scar stability and matrix remodeling, and attenuating leukocyte infiltration and cytokine expression.106 These results contrasted with prior I/R and permanent ligation studies of p50−/− mice that reported the opposite results,107, 108 but were consistent with a more recent study showing no effects of myocardial p50 deficiency on infarct size during ex vivo I/R in the isolated perfused heart when the influence of circulating leukocytes was removed.109 Taken together, while further study is clearly required, the prevailing evidence underscores the importance of subunit-specificity and cellular localization as determinants of NF-κB-mediated responses after MI. One possible scenario is that cardiomyocyte p65 activation imparts tissue injurious effects, whereas leukocyte p50 may provide a counterbalance to temper excessive inflammation.
3.1.3. Cytokines and chemokines
A variety of pro-inflammatory cytokines and chemokines are upregulated after MI as a result of innate immune activation. The effects of cytokines and chemokines in normal and injured hearts have been extensively reviewed;3, 79, 80, 83, 110–112 hence, only a few points will be highlighted in this review. Pro-inflammatory cytokines such as IL-1, TNF, IL-6, and IL-18 are upregulated and secreted early after MI and are key regulators and propagators of the inflammatory response. This occurs due to: 1) downstream signaling that further amplifies the initial inflammatory responses via MAPK- and NF-κB pathways, 2) spatial, paracrine extension of the inflammatory response to neighboring parenchymal and infiltrating cells that express cytokine receptors, and 3) recruitment of leukocytes via the upregulation of adhesion molecules in endothelial cells and chemokines in myocardium.3, 22 Chemokines are broadly divided into CC, CXC, and CX3C subtypes.110 In general, the CC chemokines are strong attractants for mononuclear cells, whereas ELR+ CXC chemokines are strong neutrophil chemoattractants.3, 110 The chemokine expression portfolio then regulates the recruitment of leukocyte subpopulations to infarcted myocardium.
The central importance of specific cytokines and chemokines in the inflammatory response after MI is supported by multiple studies, including findings (among others) that: 1) TNF−/− mice (or wild-type mice treated with anti-TNF antibody) exhibit smaller infarcts, attenuated leukocyte infiltration, and lower expression of chemokines and adhesion molecules after I/R,113 2) IL-1R type I deficient mice exhibit less LV dilatation and dysfunction after reperfused MI, and similar reductions in cardiac neutrophil and macrophage infiltration and chemokine/cytokine expression,114 3) mutant mice with augmented activation of gp130, the common receptor subunit for the IL-6 cytokine family, exhibit adverse remodeling and heightened myocardial inflammation,115 4) wild-type mice treated with a competitive CCL2/MCP-1 inhibitor exhibit reduced infarct size and monocyte infiltration after I/R,116 whereas MCP-1 deficient mice have amelioration of adverse post-MI remodeling,117 and 5) mice deficient in CCR2, the cognate receptor for MCP-1, also exhibit reduced infarct size and macrophage infiltration after I/R,118 and amelioration of post-MI remodeling.119
Nonetheless, despite pre-clinical data suggesting that cytokines and chemokines serve to aggravate ischemic injury and remodeling, it should be recognized that several studies conflict with this paradigm, showing that these mediators engender cardioprotective responses and pleiotropic cellular effects on both immune and myocardial resident cells.22, 80, 110, 111 In mice, for example, dual TNF receptor deficiency exacerbated ischemic injury and myocyte apoptosis during I/R,120 IL-6 deficiency did not impact either infarct size or post-MI remodeling,121 activation of signal transducer and activator of transcription 3 (STAT3, a signaling molecule downstream of gp130), either via IL-11 or constitutively, attenuated post-MI remodeling and fibrosis and improved neovascularization,122 and CCR5 deficiency resulted in attenuated recruitment of anti-inflammatory monocytes123, impaired macrophage activation and aggravated post-MI cardiac remodeling.124 Moreover, clinical studies of anti-cytokine (anti-TNF biologics and IL-1R antagonists)83 and anti-chemokine strategies110 in humans with MI or HF have not proven clinical benefit (and in some cases suggested harm), suggesting that indiscriminate cytokine blockade eliminates both the beneficial and detrimental effects of cytokines and chemokines, thereby yielding neutral responses.
Indeed, these and other studies indicate that pro-inflammatory cytokines and chemokines induce effects in the infarcted heart that are not simply gradations of “good” versus “bad”, but rather are complex and variable, depending on such factors as the temporal and disease context, the prevailing cellular composition in the microenvironment, and accompanying pleiotropic influences on multiple (immune and non-immune) cell processes that include inflammatory responses, but also events such as growth, differentiation, apoptosis, oxidative stress, and mitochondrial function. For example, studies in mice deficient for TNF receptor (TNFR) 1 or 2 have shown that after non-reperfused MI, TNF induces divergent TNFR-specific effects on remodeling, hypertrophy, NF-κB activity and inflammation, border zone fibrosis, and apoptosis such that TNFR1 exacerbates, whereas TNFR2 ameliorates, these events.125 This suggests that opposing receptor-specific myocardial responses in vivo95, 125, 126 may explain the negative clinical trial results with global TNF blockade. Also, while much of the current research focus is on the heart-localized effects of pro-inflammatory mediators, extracardiac effects may be of equal import to cardiac repair. In this regard, a recent study has demonstrated that circulating IL-1β induces the proliferation of bone marrow hematopoietic stem cells after MI, thereby enhancing circulating leukocytes and inflammation in the infarcted heart.127 As another example, the chemokine CXCL12/SDF-1 may facilitate cardiac repair after MI by promoting homing and survival of stem cells and neovascularization.128, 129 Lastly, it is important to recognize that the inflammation is a required event for effective tissue repair, and as such suppression of inflammatory activation that is not dysregulated or excessive may not necessarily result in salubrious effects on cardiac remodeling after MI. Moreover, there is evidence that loss-of-function of select pro-inflammatory mediators (e.g., MCP-1,117 IL-1,114 myeloperoxidase130) attenuate inflammation and adverse cardiac remodeling but do not impact cell death and infarct size during I/R. Hence, suppression of the inflammatory cascade during MI need not be accompanied by cardiomyocyte salvage. These caveats are important to consider when designing immunomodulatory therapeutic strategies to enhance post-MI cardiac repair.
3.2. Cellular effectors of the inflammatory response
Cardiomyocytes, immune cells, vascular cells and fibroblasts have been implicated as cellular effectors of the inflammatory reaction in the healing infarct; their relative role in activation of specific inflammatory cascades remains unclear. Resident myocardial cells sense tissue necrosis and trigger the post-infarction inflammatory reaction leading to recruitment of circulating leukocyte subpopulations.
3.2.1. Cardiomyocytes
Necrotic cardiomyocytes provide the main stimulus for the post-infarction inflammatory reaction, by releasing DAMPs in the infarcted area. Surviving cardiomyocytes in the infarct border zone may also trigger inflammatory activation, by producing and secreting cytokines in response to activation with IL-1, TLR ligands, or reactive oxygen species. Immunohistochemical studies and in situ hybridization experiments have suggested that viable cardiomyocytes in the infarct border zone express intercellular adhesion molecule (ICAM)-1131 and may synthesize cytokines16 and chemokines132. The relative contribution of cardiomyocyte-derived inflammatory mediators in progression and extension of post-infarction inflammation remains unknown.
3.2.2. Endothelial cells
The heart is a highly vascular organ; in adult mammals, endothelial cells are the most abundant non-cardiomyocytes.133 Extravasation of leukocytes into the infarcted area requires endothelial activation. Endothelial-specific activation of the transcription factor forkhead box O4 (FoxO4) following infarction has been demonstrated to promote neutrophil infiltration in the infarcted heart134. DAMPs released by dying cardiomyocytes induce rapid upregulation of endothelial adhesion molecules, triggering adhesive interactions with activated leukocytes. Preformed P-selectin is rapidly mobilized from Weibel-Palade bodies135, and E-selectin is upregulated in the ischemic endothelium. Once expressed on the endothelial surface, selectins bind to their leukocyte ligands, capturing neutrophils and monocytes and mediating rolling along the venular endothelium136. Moreover, activated endothelial cells in the infarct zone serve as an important source of cytokines and chemokines137,138.
3.2.3. Neutrophils
Neutrophils are the first immune cell type to infiltrate the infarcted myocardium3, 41 in response to such factors as DAMPs, cytokines and chemokines, endogenous lipid mediators (e.g., prostaglandin E2, leukotriene B4), histamine, and complement components.139–141. Infiltration of the infarct with neutrophils is predominantly localized in the border zone, and is accelerated and accentuated by reperfusion. Neutrophil extravasation in the infarcted heart is dependent on activation of adhesive interactions between the leukocytes and endothelial cells (Figure 3). Circulating neutrophils expressing selectin ligands are captured by the activated endothelium and roll along the endothelial layer. Rolling neutrophils sense chemokines bound to glycosaminoglycans on the endothelial surface. Interactions between CXC chemokines and the CXCR2 receptor expressed by the neutrophils induces conformational changes of leukocyte integrins142,143 strengthening the adhesive interaction, and resulting in arrest and adhesion of the neutrophil to the endothelial surface. Extensive experimental evidence suggests that binding of neutrophil integrins, such as lymphocyte function-associated antigen 1 (LFA1) and macrophage-1 antigen (Mac1), with endothelial ICAMs is essential for firm adhesion144. Neutrophil transmigration follows, as leukocytes actively crawl towards endothelial junctions, then migrate through basement membrane regions with low levels of matrix protein expression145. These regions may overlap with gaps between pericytes that may increase in size in the inflamed myocardium, thus serving as exit points for extravasating neutrophils146. Neutrophil extravasation across the microvasculature requires binding of leukocyte integrins to endothelial adhesion molecules147 and subsequent interactions between endothelial integrin ligands and junctional proteins. Emigrated neutrophils release proteolytic enzymes and contribute to the clearance of the wound from dead cells and matrix debris. Infiltrating neutrophils may also amplify the immune response148. Although both in vitro and in vivo experiments have suggested that neutrophils may exert direct cytotoxic actions on viable cardiomyocytes extending ischemic injury149, 150, the significance of such effects in the clinical context remains controversial.151
Figure 3.
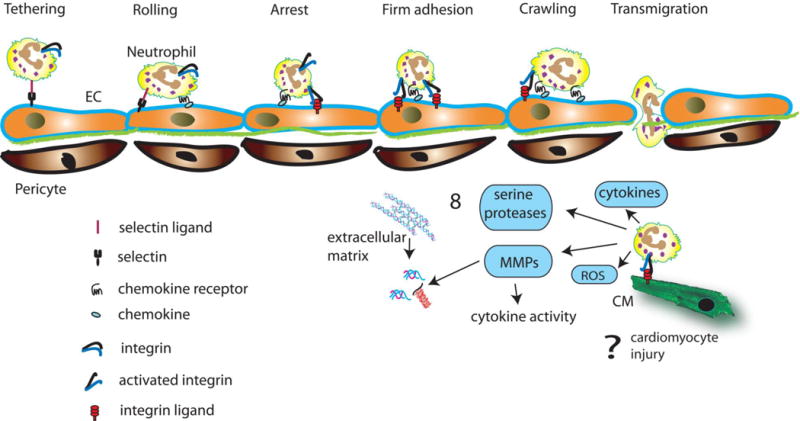
Neutrophil extravasation in the infarcted myocardium is dependent on activation of adhesive interactions between leukocytes and endothelial cells (EC). Pro-inflammatory mediators induce expression of selectins on the endothelial surface, leading to tethering and rolling of circulating neutrophils. Rolling neutrophils sense chemokines bound to glycosaminoglycans on the endothelial surface and exhibit integrin activation. Interactions between leukocyte integrins and their endothelial ligands result in firm adhesion of the neutrophils. Subsequently neutrophils crawl towards endothelial junctions and transmigrate between pericytes, through basement membrane regions with low expression of matrix proteins. Extravasated neutrophils release proteases (both serine proteases and MMPs), and reactive oxygen species (ROS), thus contributing to clearance of the wound. Neutrophils may also modulate inflammatory responses, both by secreting cytokines, and by regulating cytokine activity through release of proteases. Excessive or prolonged neutrophil actions may promote matrix degradation. Because neutrophils are predominantly localized in the infarct border zone, it has been suggested that they may adhere to viable cardiomyocytes, exerting cytotoxic effects. However, the significance of leukocyte-mediated cardiomyocyte injury remains debated.
3.2.4. Monocyte subpopulations
Two distinct waves of monocyte recruitment have been identified in healing myocardial infarcts.152 Early recruitment of pro-inflammatory Ly6Chi monocytes is mediated through activation of the MCP-1/CCR2 axis.117 At a later stage, anti-inflammatory monocyte subpopulations are selectively recruited and may participate in resolution of the post-infarction inflammatory response. During the first few hours following infarction, high levels of IL-1 in the infarct may stimulate a pro-inflammatory program in infarct monocytes. Monocytes infiltrating the infarcted myocardium originate not only from the bone marrow, but also from the spleen, that may serve as a large reservoir of mononuclear cells that can be rapidly deployed to sites of inflammation.153
3.2.5. Lymphocytes
Early infiltration of the infarcted heart with lymphocyte subsets has been extensively documented in both large animal154 and in rodent models41 of MI. Experiments in a rat model of MI suggested that cytotoxic T lymphocytes are activated following infarction; in vitro studies suggested that these cells may exert cytotoxic actions on healthy cardiomyocytes155. Whether infiltrating T cells extend ischemic injury in vivo remains unknown. Emerging evidence suggests that lymphocyte subpopulations may play an important role as orchestrators of the inflammatory response. Using both genetic and antibody-mediated depletion strategies, Zouggari and co-workers demonstrated that B cells promote mobilization of pro-inflammatory Ly6Chi monocytes, thus playing an important role in activation of the inflammatory cascade.156
3.2.6. Fibroblasts
The adult mammalian myocardium contains abundant cardiac fibroblasts;157 in the absence of injury these cells remain quiescent and may play a role in maintaining the extracellular matrix network. However, when stimulated with DAMPs, fibroblasts are capable of secreting large amounts of inflammatory cytokines and chemokines.158 In the infarcted myocardium, fibroblasts may respond to stimulation with reactive oxygen species and IL-1, acquiring a pro-inflammatory phenotype, and serving as an important source of chemokines and cytokines.159 Because several other cell types are capable of pro-inflammatory activation during the early phase of infarct healing, the relative contribution of fibroblasts remains unknown. Activation of IL-1 signaling in cardiac fibroblasts during the inflammatory phase of cardiac repair inhibits α-smooth muscle actin (SMA) expression and delays myofibroblast conversion, promoting a matrix-degrading phenotype (Figure 4).159 Thus, cytokine-driven inflammatory activation of the fibroblasts may prevent premature acquisition of a synthetic myofibroblast phenotype, until the infarct is cleared of dead cells and matrix debris.
Figure 4.
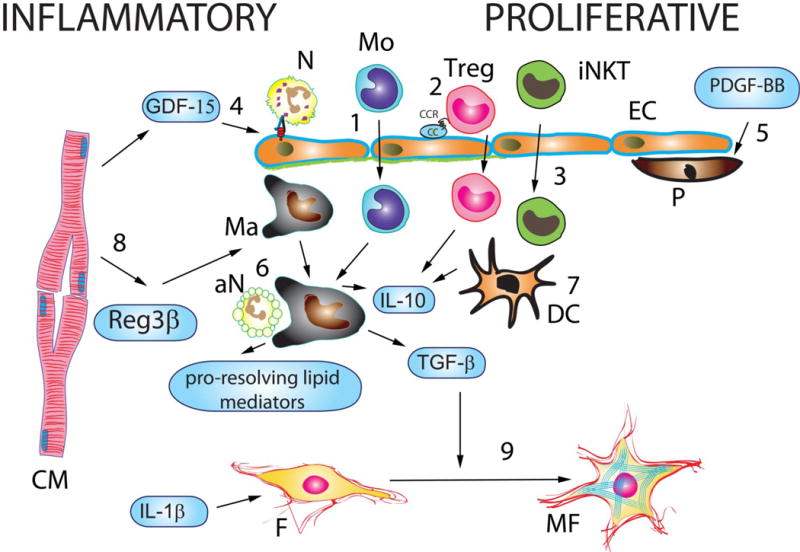
Cellular effectors and molecular signals that repress and resolve inflammation following myocardial infarction leading to the transition from the inflammatory to the proliferative phase of cardiac repair. Recruitment of anti-inflammatory monocyte (Mo) subsets (1), T cell subpopulations, such as regulatory T cells (Tregs) (2) and invariant Natural Killer T cells (iNKT) (3) contributes to repression of the post-infarction inflammatory response. Moreover, members of the TGF-β family (such as TGF-β1 and GDF-15) inhibit neutrophil transmigration by attenuating expression of adhesion molecules by endothelial cells (EC) (4). Recruitment of pericytes (P) by microvascular ECs is mediated through PDGFRβ actions and may also contribute to suppression of post-infarction inflammation (5). Macrophages (Ma) acquire an anti-inflammatory phenotype, secreting TGF-β, IL-10 and pro-resolving lipid mediators, upon ingestion of apoptotic neutrophils (aN) (6). Dendritic cells are also activated following infarction and secrete anti-inflammatory cytokines (7). Cardiomyocytes (CM) in the border zone may contribute to suppression and spatial containment of the post-infarction inflammatory response by secreting mediators that promote an anti-inflammatory macrophage phenotype (such as Reg-3β) (8). Fibroblasts (F) also exhibit dynamic phenotypic alterations that mark the transition from the inflammatory to the proliferative phase (9). During the inflammatory phase, inflammatory cytokines (such as IL-1β and TNF-α) and activation of TLR-dependent signaling by matrix fragments may activate a pro-inflammatory fibroblast phenotype. Stimulation of fibroblasts with IL-1β induces MMP expression and chemokine synthesis, while reducing α-SMA levels. During the proliferative phase, activation of TGF-β-dependent cascades stimulates a matrix-preserving myofibroblast (MF) phenotype.
3.2.7. Resident mast cells and macrophages
The heart contains resident populations of mast cells and macrophages that may play an important role in activation of the inflammatory cascade. Mast cells are strategically located in perivascular areas and contain preformed stores of inflammatory mediators, such as TNF, histamine and tryptase160, 161. Cytokine stimulation, adenosine, reactive oxygen species, and activation of the complement cascade induce mast cell degranulation. TNF and histamine released by resident mast cells may play an important role in triggering the post-infarction inflammatory response.
Recent studies have characterized the resident macrophage population in mouse myocardium162, 163, 164, 165. Using flow cytometry and lineage tracing approaches, Epelman and co-workers found significant heterogeneity in macrophage populations in adult mouse hearts. At steady state, 2 distinct macrophage pools were identified: a CCR2-negative subset that represented an embryonically established lineage that originated from yolk sac macrophages and fetal monocytes and a second (much smaller) pool derived from circulating CCR2+ monocytes.162 The fate of these subpopulations following infarction and their contribution in regulation of the post-infarction inflammatory reaction remains unclear. Heidt and co-workers suggested that, at least in non-reperfused infarcts, resident cardiac macrophages die and may be replaced by monocyte-derived CCR2-expressing cells with potent pro-inflammatory properties.163 Reperfusion may protect resident macrophage subpopulations in the infarcted area; thus, in models of reperfused infarction, these cells may play an important role in activation of the inflammatory cascade. During progression of the inflammatory cascade, recruitment of large numbers of monocytes and proliferation of resident macrophage subsets result in marked expansion of the cardiac macrophage population. The abundant, dynamic and highly plastic population of infarct macrophages plays an important role in regulation of the inflammatory and reparative response following myocardial infarction.
3.2.8. The extracellular matrix
Both cardiomyocytes and non-cardiomyocytes are enmeshed in a network of extracellular matrix proteins. The cardiac interstitial matrix does not simply serve as a structural scaffold, but also transduces molecular signals and plays an active role in regulation of inflammatory and reparative responses166, 167. Fragmentation of the extracellular matrix provides a key stimulus for activation of the inflammatory cascade following infarction. Generation and release of collagen and fibronectin fragments have been implicated in activation of pro-inflammatory signaling.168 Hyaluronan degradation may result in release of high molecular weight fragments with potent pro-inflammatory properties, capable of inducing cytokine and chemokine synthesis by endothelial and immune cells.169
4. The reparative and proliferative phase
4.1. Inhibition and resolution of the inflammatory response
4.1.1 Cell types involved in suppression of the inflammatory response (Figure 4)
Neutrophils
The transition from the inflammatory to the reparative and proliferative phase after MI is driven by changes in the cardiac microenvironment. While their survival can be prolonged by DAMPs, pro-inflammatory cytokines, hypoxia, and acidosis, neutrophils are short-lived cells that rapidly undergo cell death, primarily by apoptosis but also secondary necrosis.139, 170 In various models of acute inflammation, late-stage and apoptotic neutrophils are critical for ushering inflammation resolution by several mechanisms: 1) the release of mediators that promote inflammation resolution such as pro-resolving lipid mediators (e.g., lipoxins and resolvins), annexin A1, and lactoferrin that dampen neutrophil transmigration and entry, and promote neutrophil apoptosis and the phagocytic uptake of apoptotic neutrophils by macrophages,139–141 2) the expression of decoy and scavenging chemokine and cytokine receptors on apoptotic neutrophils that results in tissue depletion of these mediators,139, 141 and 3) the expression of “eat-me” signals (e.g., phosphatidylserine) that facilitate the ingestion of apoptotic neutrophils by macrophages.139, 141. The subsequent phagocytic clearance of these apoptotic cells, induces a pro-resolving M2 phenotype in macrophages, and secretion of anti-inflammatory and pro-fibrotic cytokines such as IL-10 and transforming growth factor (TGF)-β that suppress inflammation and promote tissue repair. It should be noted that while these are fundamental aspects of inflammatory cell biology, they have not been widely tested in the infarcted heart. A recent study demonstrated that neutrophils, via secreted neutrophil gelatinase-associated lipocalin, polarize macrophages toward a reparative phenotype, thereby orchestrating tissue healing.171
Monocytes, macrophages, and dendritic cells
Following the early appearance of neutrophils, monocytes and macrophages (Mo/Mϕ) comprise the most abundant cells in the infarcted heart.3, 4, 41 Seminal studies by Nahrendorf, Swirski, and co-workers4, 152, 172–174 demonstrated that Mo/Mϕ display phasic functional heterogeneity that serve to guide proper wound healing. The initial phase (peak day ~3–4 post-MI) promotes tissue digestion, and is characterized by Ly6Chi monocytes and M1 macrophages that are proteolytic, with augmented expression of proteinases (e.g., cathepsins and MMPs), and pro-inflammatory, with augmented TNF expression. The second phase (peak day ~7 post-MI) promotes tissue repair, with a predominance of Ly6Clo monocytes and M2-like macrophages with augmented expression of anti-inflammatory, pro-fibrotic, and angiogenic factors (e.g., IL-10, TGF-β, and VEGF). It should be emphasized that in the healing infarct, macrophages cannot be simply categorized as polarized M1/M2 cells, but exhibit a wide range of nuanced phenotypes. Moreover, the repertoire of reparative monocytes/macrophages is not limited to traditional cytokines and growth factors. A recent investigation identified a novel secreted protein called myeloid-derived growth factor (MYDGF), as an essential monocyte-derived mediator that may promote repair of the infarcted heart175.
Initial studies4, 152 suggested that the two phases resulted from separate waves of circulating monocyte infiltration – early recruitment of Ly6ChiCCR2+CX3CR1lo monocytes in response to augmented myocardial CCL-2/MCP-1 expression, and later recruitment of Ly6CloCCR2− CX3CR1hi monocytes in response to augmented myocardial expression of fractalkine, the ligand for CX3CR1. However, more recent work173 using chimeric mice deficient for hematopoietic cell Nr4a1 (an orphan nuclear hormone receptor essential for patrolling Ly6Clo monocyte development176) indicated that both phases derive from pro-inflammatory Ly6Chi monocytes, and that during the reparative phase, recruited Ly6Chi monocytes switch their phenotype to Ly-6Clo anti-inflammatory macrophages that proliferate locally to effect inflammation resolution and wound healing. While molecular regulators such as Nr4a1173 and IRF5172 may serve as important modulators of inflammatory vis-à-vis reparative polarity in macrophages, the specific microenvironmental cues that induce this switch remain poorly defined.
In addition to Mo/Mϕ, CD11c+ dendritic cells (DCs) infiltrate the infarcted heart, predominantly during the reparative phase.41, 177 DCs are essential for proper inflammation resolution, scar formation, and angiogenesis post-MI, as DC ablation resulted in persistent cardiac accumulation of Ly6Chigh monocytes and CD206− macrophages, sustained pro-inflammatory cytokine expression, reduced endothelial cell proliferation, and deterioration of LV function post-MI.177
T-lymphocytes
T-lymphocytes, including CD4+ and CD8+ T-cells, Foxp3+ regulatory cells (Tregs), invariant natural killer (iNK) T-cells, and γδT-cells, infiltrate the heart after MI, most robustly during the reparative phase.41, 178–182 Studies using CD4+ T-cell deficient mice, and OT-II mice that exhibit defective T-cell antigen recognition, have demonstrated that CD4+ helper T-cells are activated after MI likely in response to released cardiac autoantigens, and that these T-cells promote wound healing, resolution of inflammation and pro-inflammatory monocyte infiltration, and proper collagen matrix formation and scar formation, thereby limiting adverse remodeling.178 However, the specific identity of these autoantigens remain unclear. Notably, in a hindlimb ischemia model, CD4+ T-cells were also shown to be essential for the recruitment of pro-angiogenic macrophages and collateral artery formation.183 A recent study using both genetic and antibody-based approaches to modulate Tregs early post-MI showed that CD4+Foxp3+ Tregs are also essential for favorable wound healing, scar formation, and inflammation resolution after MI, in part by modulating macrophage differentiation toward an M2-like phenotype.181 Moreover, the activation of iNKT cells after reperfused179 or non-reperfused MI180 has been shown to reduce leukocyte infiltration, myocardial injury, and adverse remodeling, in part by enhancing the expression of anti-inflammatory cytokines such as IL-10. Hence, multiple T-lymphocyte subsets contribute to suppression of the inflammatory response. In contrast, CD4−γδT-cells appear to promote neutrophil and macrophage infiltration and impart detrimental effects on post-MI remodeling.182
Vascular cells
Tissue neovascularization is essential for supplying the healing infarct with nutrients and oxygen. A robust angiogenic response occurs after MI with rapid upregulation of vascular endothelial growth factor (VEGF) in viable border zone cardiomyocytes, and upregulation of VEGF receptors in border zone vasculature and in new vessels extending into the infarct zone.3, 184 Initially, the neovessels are enlarged and lack a pericyte and smooth muscle cell mural coating; these features promote vessel hyperpermeability and inflammatory cell extravasation into the infarct tissue.185, 186 During later phases of infarct healing, however, these neovessels mature and become invested with a mural coat. This process is dependent on endothelial cell platelet-derived growth factor (PDGF) and PDGF receptor-β (PDGFR-β) signaling in pericytes and smooth muscle cells.186 Defects in the formation of the mural coat results in prolonged inflammatory cell infiltration in the infarct zone and reduced collagen deposition, suggesting that PDGF-β-mediated pericyte investment of neovessels is critical for the proper resolution of inflammation post-MI. The importance of pericytes is further supported by the findings that in vivo human pericyte transplantation into the peri-infarct zone of mice attenuates vascular permeability, reduces tissue leukocyte infiltration, augments angiogenesis, and improves cardiac remodeling.187, 188
Cardiomyocytes
It is tempting to hypothesize that viable border zone cardiomyocytes may secrete mediators that limit extension of the inflammatory response, protecting non-infarcted myocardium from the unwanted effects of unrestrained inflammation3,189. However, information on specific cardiomyocyte-derived signals that may contribute to containment of inflammation following infarction remains limited. Recent evidence suggested that, in the infarcted myocardium, cardiomyocytes may secrete regenerating islet-derived-3β (Reg-3β), a mediator that regulates macrophage recruitment and inhibits inflammatory activation, preventing cardiac rupture and expansion of injury.190
4.1.2. The molecular signals implicated in resolution of post-infarction inflammation
Timely suppression and spatial containment of the post-infarction inflammatory reaction is dependent on release of secreted anti-inflammatory mediators (such as IL-10, members of the TGF-β family, and lipid-derived pro-resolving mediators) and on activation of intracellular STOP signals that inhibit the innate immune response. Defects in the molecular pathways responsible for suppression and resolution of the inflammatory response may be involved in the pathogenesis of adverse remodeling and HF following MI191.
IL-10
IL-10 exerts potent anti-inflammatory actions, suppressing synthesis of pro-inflammatory cytokines and chemokines in macrophages192 through activation of STAT3 signaling193. IL-10 upregulation has been documented in both rodent and large animal models of myocardial infarction154, 194. Although the late timing of IL-10 upregulation is consistent with a possible role in suppression of pro-inflammatory signaling, experiments using IL-10 null animals have produced conflicting results. In a mouse model of coronary occlusion/reperfusion, genetic loss of IL-10 was associated with increased early mortality and accentuated expression of pro-inflammatory genes.195 However, another study with a much higher sample size did not confirm these observations, demonstrating comparable mortality and dilative remodeling in IL-10 null and WT animals undergoing reperfused infarction protocols, and suggesting that IL-10 loss is associated with relatively subtle alterations (including increased myocardial levels of TNF and MCP-1).196 Interestingly, in human patients, high plasma levels of IL-10 predict adverse outcome in patients with acute coronary syndromes197 and ST elevation MI (STEMI).198 This observation may reflect a compensatory accentuation of anti-inflammatory cytokine synthesis in high-risk patients.
Members of the TGF-β family
Several members of the TGF-β family have been implicated in negative regulation of the inflammatory reaction. Unfortunately, dissection of the role of these mediators in post-infarction inflammation and repair has been hampered by the complex biology of their regulation and activation, by their pleiotropic and context-dependent actions on all cell types involved in infarct healing, and by the complexity of their downstream signaling effectors. Considering its actions on immune and reparative cells and the time course of its upregulation following MI, TGF-β1 may serve as the master switch regulating the transition from inflammation to fibrosis199. Neutralization experiments using gene therapy with the extracellular domain of the type II TGF-β receptor in a model of MI suggested that early inhibition may worsen dysfunction accentuating the inflammatory response, whereas late disruption of TGF-β signaling may protect from interstitial fibrosis and hypertrophic remodeling.200 A recent study suggested that, while broad inhibition of TGF-β following infarction causes early mortality due to cardiac rupture, cardiomyocyte-specific disruption of the TGF-β receptors was protective and stimulated a wide range of anti-inflammatory and cytoprotective signals.201 Thus, the detrimental actions of early TGF-β inhibition on the infarcted myocardium may not be due to direct actions on cardiomyocyte survival, but may reflect loss of anti-inflammatory actions on inflammatory cells, endothelial cells, or fibroblasts.
Growth Differentiation Factor (GDF)-15, a member of the TGF-β family, is also implicated in suppression of the inflammatory response following infarction. GDF-15 exerts potent anti-inflammatory actions by counteracting chemokine-triggered leukocyte integrin activation. Thus, GDF-15 loss in mice is associated with accentuated post-infarction inflammation and fatal cardiac rupture following MI.202 In patients with STEMI, elevated plasma GDF-15 levels are associated with increased mortality,203 likely reflecting activation of a protective anti-inflammatory pathway in patients with an accentuated post-infarction inflammatory reaction.
Lipid-derived pro-resolving mediators
Pro-resolving lipid mediators (including the lipoxins, resolvins, protectins and maresins)204 have potent anti-inflammatory properties and may play an important role in resolution of the inflammatory infiltrate following infarction. Protective effects of exogenous resolvin E1 and resolvin D1 administration have been demonstrated in rodent models of I/R and non-reperfused MI205, 206; however, the potential role of endogenous pro-resolving lipid mediators in suppression and resolution of the post-infarction inflammatory response has not been investigated.
4.1.3. Do chemokine-mediated effects suppress inflammation by recruiting anti-inflammatory leukocytes?
Although traditionally viewed as pro-inflammatory mediators, certain members of the chemokine family may suppress inflammation by recruiting anti-inflammatory monocyte and lymphocyte subsets to the infarcted myocardium. In a mouse model of reperfused MI, genetic disruption of the CC chemokine receptor CCR5 caused enhanced inflammation and accentuated dilative remodeling, associated with decreased infiltration of the infarct by regulatory T cells.123 Specific chemokine-chemokine receptor pairs may mediate recruitment of anti-inflammatory monocyte and lymphocyte subsets, thus protecting the infarcted myocardium from unrestrained inflammation.
4.1.4. Intracellular pathways involved in negative regulation of the inflammatory cascade
Activation of intracellular pathways that restrain the innate immune response may also play a crucial role in timely suppression of the post-infarction inflammatory response, protecting from adverse remodeling. Expression of IRAK-M, a negative regulator of the innate immune response, is upregulated in infarct macrophages and fibroblasts and inhibits macrophage-derived cytokine expression, while promoting a matrix-preserving myofibroblast phenotype in cardiac fibroblasts.207, 208
4.2. Fibroblast activation and formation of the scar
4.2.1. Myofibroblast transdifferentiation and acquisition of a synthetic phenotype
Expansion of the cardiac fibroblast population and conversion into synthetic myofibroblasts are hallmarks of the proliferative phase of cardiac repair209, 210, 211. Myofibroblasts are phenotypically modulated fibroblasts that develop stress fibers and express contractile proteins, such as α-SMA and the embryonal isoform of smooth muscle myosin212, 210. The origin of infarct myofibroblasts remains a debated issue. Experimental studies using bone marrow transplantation strategies have produced conflicting results, suggesting that either resident fibroblasts,213 or circulating bone marrow progenitors214 may be the main source of myofibroblasts in the infarct. Endothelial cells undergoing mesenchymal transdifferentiation,215 epicardial epithelial cells, and pericytes may represent additional sources of myofibroblasts in the healing infarct.216 Recent studies using lineage tracing approaches in a model of MI have demonstrated that epicardial-derived cells that colonize the adult mammalian cardiac interstitium massively transdifferentiate into myofibroblasts in the infarcted heart.217 Thus, following MI, interstitial fibroblasts that survive the ischemic insult, or cells recruited from neighboring viable areas, may undergo myofibroblast transdifferentiation, in response to increased levels of bioactive TGF-β and to the changes in the composition of the surrounding extracellular matrix. Moreover, in the healing infarct, marked induction of chemokines in response to extensive cardiomyocyte necrosis may result in recruitment and activation of additional subsets of reparative fibroblasts that may play an important role in scar formation.
4.2.2. Mediators involved in myofibroblast activation
Conversion of fibroblasts into myofibroblasts requires the cooperation of both soluble mediators and specialized matrix components. TGF-β is induced and activated in the infarcted myocardium and is critically involved in myofibroblast transdifferentiation. In vitro studies have suggested that TGF-β1-induced myofibroblast conversion may be mediated through both canonical Smad-dependent218 and Smad-independent signaling pathways219, 220. Modulation of the extracellular matrix also contributes to myofibroblast conversion. TGF-β-mediated myofibroblast transdifferentiation requires activation of an outside-in signaling pathway transduced by polymerized fibronectin-EDA221, 53. Moreover, secretion and deposition of matricellular proteins, such as thrombospondin (TSP)-1 and osteopontinfamily may contribute to myofibroblast conversion, both by exerting direct actions on cellular phenotype and by accentuating growth factor-mediated responses222, 223, 224.
A growing body of evidence suggests that members of the transient receptor potential (TRP) family of ion channels play an essential role in regulating fibroblast to myofibroblast transition.225 TRP channels are ubiquitously expressed in all cell types, providing calcium entry pathways and regulating a wide range of Ca2+-dependent cell functions226. Although in vitro studies have implicated TRPV4227 and TRPM7228 channels in cardiac myofibroblast transdifferentiation, their in vivo role is unclear. On the other hand, both in vitro and in vivo studies documented a critical role for TRPC6 in infarct myofibroblast conversion, demonstrating that TRPC6 absence is associated with impaired fibroblast function and increased mortality due to cardiac rupture229.
Acquisition of a myofibroblast phenotype by infarct fibroblasts is associated with increased proliferative activity and stimulation of a matrix-preserving program. Extensive experimental evidence suggests that activation of the renin-angiotensin-aldosterone system (RAAS) promotes myofibroblast proliferation and stimulates matrix synthesis. In addition to the well-described effects of circulating angiotensin II and aldosterone, local generation of angiotensin II in the infarct has also been implicated in fibroblast activation.230 Angiotensin II accentuates proliferative activity in cardiac fibroblasts and induces synthesis of extracellular matrix proteins and integrins,231 through effects that predominantly involve the AT1 receptor.232 Aldosterone also promotes cardiac fibroblast proliferation by stimulating Kirsten Ras-A (Ki-RasA) and MAPK1/2 signaling.233 Some actions of the RAAS in cardiac fibroblasts may be mediated through activation of the TGF-β cascade. In addition to its critical effects on myofibroblast conversion, TGF-β also activates a matrix-preserving program in cardiac fibroblasts through Smad-dependent signaling218, 234. Other fibrogenic mediators, such as thePDGFs235, 236, members of the fibroblast growth factor (FGF) family,237 and the mast cell-derived proteases tryptase238 and chymase239 are also released in the infarcted heart and may activate infarct myofibroblasts.
4.3. The extracellular matrix during the proliferative phase of infarct healing
The proliferative phase of infarct healing is characterized by dynamic alterations in the extracellular matrix network that directly regulate cellular phenotype and activity. As matrix fragments in the infarct zone are phagocytosed, a provisional matrix is formed, comprised predominantly of fibrin and fibronectin.240 This highly plastic matrix network serves as a scaffold for migrating and proliferating cells, facilitating the dynamic changes that occur in the healing wound. The infarct matrix is also enriched through deposition of several members of the matricellular protein family. These structurally unrelated macromolecules are not present in normal myocardium, but are markedly upregulated in the infarcted and remodeling heart. Unlike structural matrix components (such as collagens and elastin), matricellular proteins do not provide mechanical support, but may bind to matrix proteins and cell receptors transducing signaling cascades. TSP-1,241 tenascin-C,242 secreted protein acidic and cysteine-rich (SPARC),243 periostin244, 245, osteopontin,246 osteoglycin,247 and members of the CCN family248 have been implicated in regulation of the inflammatory and reparative response following myocardial infarction. Because matricellular proteins are capable of modulating behavior and function of all cells involved in cardiac repair, remodeling and fibrosis, dissection of specific molecular mechanisms responsible for the observed in vivo effects is particularly challenging. Modulation of growth factor activity and signaling,241, 243 or regulation of collagen fibrillogenesis and maturation247, 246 have been suggested as potential mechanisms for specific matricellular actions.
5. The Maturation Phase
The proliferative phase of cardiac repair is followed by scar maturation, as the extracellular matrix becomes cross-linked, and reparative cells are de-activated, and may undergo apoptosis. The molecular signals implicated in quiescence of infarct myofibroblasts remain unknown. Withdrawal of fibrogenic growth factors, activation of inhibitory STOP signals that terminate TGF-β and angiotensin II signaling, and clearance of matricellular proteins, may suppress proliferation and reduce the matrix-synthetic activity of the fibroblasts. Induction and secretion of anti-fibrotic mediators may also contribute to termination of the matrix-synthetic response. The CXC chemokine Interferon-γ-inducible protein (IP)-10/CXCL10 inhibits fibroblast migration through proteoglycan-mediated actions, is upregulated in the infarcted heart and contributes to spatial containment of the fibrogenic response in the infarcted region.249, 250 However, due to the early timing of its induction in the infarcted myocardium, IP-10 is an unlikely candidate for a role in scar maturation. Although reduction in myofibroblast density during infarct maturation has been well-documented210, 251 the fate of these cells remains unknown. Apoptotic death may mediate fibroblast loss in the scar and in the infarct border zone;252 the mediators responsible for fibroblast-specific activation of pro-apoptotic signaling are unknown.
6. Inflammatory cells in the late phase of post-MI cardiac remodeling
As detailed above and summarized in Figure 5, the two phases of healing after MI are acute inflammation with intense cellular infiltration, lasting up to 4 d in mice, followed by resolution and repair with active resolution of inflammation, quiescence of cell activity, and scar stabilization and maturation over 14 d in mice. While the time frame of these events is well defined in the murine model, larger animal models exhibit comparatively greater persistence of cellular infiltration and slower formation of granulation tissue after MI.194 Following healing, in both humans and animal models with MI, a subset of those afflicted will exhibit late progressive ventricular dilatation and HF, a state characterized by chronic inflammation (Figure 5).80 Among other variables, both larger infarcts253 and greater initial inflammatory activation254 are strong predictors of late cardiac remodeling and HF in humans. Moreover, histopathological studies of human failing hearts with chronic ischemic cardiomyopathy have demonstrated augmented tissue macrophages and T-lymphocytes, and increased adhesion molecule expression in endothelial cells.255 One potential explanation for these findings is that late cardiac remodeling may be driven in part by incomplete or impaired resolution of myocardial inflammation with larger degrees of injury post-MI that amplifies over time. Indeed, the exogenous administration of the pro-resolving lipid mediator resolvin D1 after non-reperfused MI hastened inflammation resolution and improved post-MI ventricular remodeling,206 suggesting that facilitation of active resolution may prevent persistent inflammation, and ameliorate late HF.
Figure 5.
Early and late inflammation after myocardial infarction (MI). The early inflammatory phase after MI (~4 d in mice) is characterized by robust innate and adaptive immune cell infiltration and tissue digestion. This is subsequently followed a phase of resolution, myofibroblast proliferation, and wound repair (lasting ~10–14 d in mice), during which immune cells are polarized toward an anti-inflammatory state. However, larger infarcts with more pronounced inflammatory activation exhibit progressive ventricular dilatation and heart failure (HF) over the long-term, together with persistent inflammation and tissue immune cell infiltration. Chronic inflammation may represent incomplete inflammation resolution during the reparative phase and subsequent amplification with time, or a second wave of resurgent immune activation in response to poorly defined factors.
Alternatively, chronic inflammation, and tissue inflammatory cell infiltration, in ischemic HF may represent late recrudescence of immune activation toward the heart in response to as-of-yet poorly identified factors or antigens.256 In support of this idea, Ismahil et al257 recently demonstrated that in chronic ischemic HF there is local (and systemic) expansion of pro-inflammatory Mo/Mϕ, dendritic cells, and T-cells, together with heightened splenic expression of alarmins and pro-inflammatory mediators, and structural splenic remodeling consistent with heightened antigen processing. Moreover, activated mononuclear splenocytes trafficked to the failing heart to promote apoptosis, fibrosis, and dysfunction, suggesting that adverse LV remodeling was in part immune–mediated, possibly in response to cardiac-derived alarmins. The role of particular immune cell populations in the pathogenesis of chronic remodeling and inflammation in HF require further study, along with their organ-specific derivation (e.g., local proliferation vis-à-vis infiltration from remote sites) and the potential antigens and molecular pathways responsible for their activation. As discussed above, these mechanisms may be separate and distinct from those that promote myocardial salvage after acute MI.
7. Targeting the inflammatory response in myocardial infarction
Over the last thirty years, experimental work has revealed a crucial role for the inflammatory cascade in cardiac repair, remodeling, and fibrosis following MI. Unfortunately, this new knowledge has not yet translated into effective therapy. Because of the close link between inflammation and repair, non-selective inhibition of inflammation following myocardial infarction (using corticosteroids or non-steroidal anti-inflammatory drugs) may have detrimental effects on scar formation, promoting rupture and accentuating adverse remodeling.258 Thus, selective targeting of injurious pro-inflammatory signals is needed. Unfortunately, in clinical studies, approaches targeting several specific pro-inflammatory pathways have produced disappointing results. Despite promising experimental data in large animal models, CD11/CD18 integrin inhibition failed to reduce the size of the infarct in patients with myocardial infarction.259 In a large clinical trial, complement inhibition did not reduce mortality and major adverse events in patients with STEMI.260 P-selectin inhibition in patients with acute coronary syndromes appeared to attenuate cardiomyocyte necrosis, but was associated with trends towards worse clinical outcome.261, 262 What are the causes for these translational failures?
First, inflammatory mediators are notoriously pleiotropic, exerting a wide range of actions on many different cell types. Thus, targeting a specific cytokine, growth factor, or inflammatory pathway may modulate several cellular responses; the clinical implications of these actions cannot always be predicted by investigating animal models. Second, temporal and spatial considerations are critical determinants of the effectiveness of an intervention, as described above. The reparative response following myocardial infarction is a highly dynamic process; the window of therapeutic opportunity is brief. Moreover, considering the spatial heterogeneity of the cellular environment in the remodeling infarcted heart, therapeutic interventions may have distinct effects on the infarct, the border zone, and the remote remodeling myocardium. Thus, a successful strategy requires careful design, optimally exploiting knowledge on the time course and topographic characteristics of the inflammatory response. Third, the pathophysiologic heterogeneity of post-infarction remodeling in humans complicates implementation of therapeutic strategies. The severity and characteristics of the remodeling response following infarction are not dependent only on the size of the acute infarct. Genetic background, concomitant conditions (such as hypertension or diabetes), age and gender have major impact on the inflammatory response and profoundly affect post-MI remodeling and clinical outcome.
The pathophysiologic heterogeneity of patients with MI has important therapeutic implications. Certain patient subpopulations exhibit prolonged accentuation of pro-inflammatory signaling following infarction that may be associated with dilative remodeling and systolic dysfunction. These patients may benefit from interventions targeting the inflammatory cascade, such as IL-1 inhibition.263 Other patients do not exhibit significant post-infarction ventricular dilation, but develop a pronounced hypertrophic and fibrotic response, associated with diastolic dysfunction. This type of adverse remodeling is particularly common in diabetics and may be associated with an overactive TGF-β system.264, 265, 266 Identification of patient subpopulations with distinct pathophysiologic perturbations, using suitable biomarkers or molecular imaging modalities, is needed in order to design effective therapies to attenuate adverse remodeling in patients with MI.
The potential involvement of inflammatory signals in tissue regeneration adds a new perspective to the therapeutic potential of strategies targeting the post-infarction inflammatory response. Recently, neonatal macrophage subsets have been suggested to activate a regenerative program in the infarcted myocardium.267 Moreover, certain chemokines (such as SDF-1/CXCL12), cytokines and growth factors268, 269, 270 may regulate trafficking, activation, differentiation and survival of progenitor cells. Clearly, this field is in its infancy. Whether modulation of the inflammatory response can contribute to activation of a regenerative program in adult mammalian myocardium is unknown.
8. CONCLUDING REMARKS
Our growing knowledge on the role of inflammatory cascades in injury, repair and remodeling of the infarcted heart has revealed new therapeutic targets for patients surviving acute MI. Unfortunately, the pleiotropic actions of inflammatory mediators, and the remarkable pathophysiologic heterogeneity of cardiac remodeling in human patients pose major challenges for clinical implementation of strategies targeting the inflammatory response. A concerted effort is needed to dissect the cellular actions and molecular signals activated by inflammatory mediators, and to identify the pathophysiologic perturbations that may be responsible for adverse remodeling in vulnerable patients. Strategies modulating the inflammatory cascade may not only hold promise as protective measures to prevent remodeling and HF, but may also prove crucial in realizing the visionary goal of myocardial regeneration.271
Supplementary Material
Acknowledgments
SOURCES OF FUNDING: NIH R01 HL76246 and R01 HL85440 (to Dr Frangogiannis); NIH R01 HL125735 and VA Merit I01 BX002706 (to Dr. Prabhu).
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- LV
left ventricular
- MI
myocardial infarction
- HF
heart failure
- ROS
reactive oxygen species
- DAMP
danger-associated molecular pattern
- PRR
pattern recognition receptor
- HMGB-1
high mobility group box-1
- IL
interleukin
- HSP
heat shock protein
- TLR/IL-1R
toll-like receptor/IL-1 receptor
- NOD
nucleotide-binding oligomerization domain
- NLR
NOD-like receptor
- RAGE
receptor for advanced glycation end-products
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-κB
- TNF
tumor necrosis factor-α
- ELR
glutamic acid-leucine-arginine
- VCAM
vascular cell adhesion molecule
- ICAM
intercellular adhesion molecule
- FoxO4
forkhead box O4
- Mertk
myeloid-epithelial-reproductive tyrosine kinase
- SDF-1
stromal cell-derived factor-1
- I/R
ischemia/reperfusion
- EDA
extra domain A
- TIR
Toll/IL-1R
- MyD88
myeloid differentiation factor 88
- TIRAP
TIR domain-containing adaptor protein
- IRAK
IL-1R associated kinase
- TRAF
TNF receptor associated factor
- TAK1
transforming growth factor activated kinase 1
- TRIF
TIR domain-containing adaptor inducing interferon-β
- IFN
interferon
- TRAM
TRIF-related adaptor molecule
- TBK1
TANK-binding kinase
- IRF
interferon-regulatory factor
- CARD
caspase activation and recruitment
- ASC
apoptosis speck-like protein containing a caspase-recruitment domain
- STAT
signal transducer and activator of transcription
- Tregs
regulatory T-cells
- iNK
invariant natural killer
- VEGF
vascular endothelial growth factor
- PDGF
platelet-derived growth factor
- PDGFR-β
PDGF receptor-β
- α-SMA
α-smooth muscle actin
- TGF
transforming growth factor
- MyDGF
myeloid cell-derived growth factor
- DC
dendritic cells
- Reg-3β
regenerating islet-derived-3β
- GDF
growth differentiation factor
- TSP
thrombospondin
- TRP
transient receptor potential
- RAAS
renin-angiotensin-aldosterone system
- FGF
fibroblast growth factor
- SPARC
secreted protein acidic and cysteine-rich
- IP-10
interferon-γ-inducible protein-10
Footnotes
DISCLOSURES: None.
References
- 1.Prabhu SD. Post-infarction ventricular remodeling: an array of molecular events. Journal of molecular and cellular cardiology. 2005;38:547–50. doi: 10.1016/j.yjmcc.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circulation research. 2012;110:159–73. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–45. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic research in cardiology. 2014;109:444. doi: 10.1007/s00395-014-0444-7. [DOI] [PubMed] [Google Scholar]
- 6.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. Journal of the American College of Cardiology. 2010;55:1629–38. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–83. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 9.Vandervelde S, van Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC. Increased inflammatory response and neovascularization in reperfused vs. non-reperfused murine myocardial infarction. Cardiovasc Pathol. 2006;15:83–90. doi: 10.1016/j.carpath.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8:292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 11.de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm. 2013;2013:206039. doi: 10.1155/2013/206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghigo A, Franco I, Morello F, Hirsch E. Myocyte signalling in leucocyte recruitment to the heart. Cardiovasc Res. 2014;102:270–80. doi: 10.1093/cvr/cvu030. [DOI] [PubMed] [Google Scholar]
- 13.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circulation research. 2011;108:1133–45. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 16.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, Entman ML. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–51. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi-Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T. Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation. 1995;91:1520–4. doi: 10.1161/01.cir.91.5.1520. [DOI] [PubMed] [Google Scholar]
- 18.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Xie J, Mendez JD, Mendez-Valenzuela V, Aguilar-Hernandez MM. Cellular signalling of the receptor for advanced glycation end products (RAGE) Cellular signalling. 2013;25:2185–97. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi Y. Neutrophil infiltration and chemokines. Critical reviews in immunology. 2006;26:307–16. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 21.White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation–therapeutic opportunities and pharmacological challenges. Pharmacological reviews. 2013;65:47–89. doi: 10.1124/pr.111.005074. [DOI] [PubMed] [Google Scholar]
- 22.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–53. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 23.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–12. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bianchi ME, Beltrame M. Upwardly mobile proteins. Workshop: the role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO Rep. 2000;1:109–14. doi: 10.1093/embo-reports/kvd030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 27.Loukili N, Rosenblatt-Velin N, Li J, Clerc S, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovascular research. 2011;89:586–94. doi: 10.1093/cvr/cvq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 29.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature immunology. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 30.Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. The Journal of experimental medicine. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cirillo P, Giallauria F, Pacileo M, Petrillo G, D’Agostino M, Vigorito C, Chiariello M. Increased high mobility group box-1 protein levels are associated with impaired cardiopulmonary and echocardiographic findings after acute myocardial infarction. Journal of cardiac failure. 2009;15:362–7. doi: 10.1016/j.cardfail.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen MV, Pedersen S, Mogelvang R, Skov-Jensen J, Flyvbjerg A. Plasma high-mobility group box 1 levels predict mortality after ST-segment elevation myocardial infarction. JACC Cardiovascular interventions. 2011;4:281–6. doi: 10.1016/j.jcin.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahashi T, Yoshikawa T, Ishizaka A, Ogawa S. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res. 2009;81:565–73. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Yao Y, Su Z, Yang Y, Kao R, Martin CM, Rui T. Endogenous HMGB1 contributes to ischemia-reperfusion-induced myocardial apoptosis by potentiating the effect of TNF-α/JNK. American journal of physiology Heart and circulatory physiology. 2011;300:H913–21. doi: 10.1152/ajpheart.00703.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Muller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi K, Fukushima S, Yamahara K, Yashiro K, Shintani Y, Coppen SR, Salem HK, Brouilette SW, Yacoub MH, Suzuki K. Modulated inflammation by injection of high-mobility group box 1 recovers post-infarction chronically failing heart. Circulation. 2008;118:S106–14. doi: 10.1161/CIRCULATIONAHA.107.757443. [DOI] [PubMed] [Google Scholar]
- 37.Limana F, Esposito G, D’Arcangelo D, Di Carlo A, Romani S, Melillo G, Mangoni A, Bertolami C, Pompilio G, Germani A, Capogrossi MC. HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PloS one. 2011;6:e19845. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitahara T, Takeishi Y, Harada M, Niizeki T, Suzuki S, Sasaki T, Ishino M, Bilim O, Nakajima O, Kubota I. High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc Res. 2008;80:40–6. doi: 10.1093/cvr/cvn163. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura Y, Suzuki S, Shimizu T, Miyata M, Shishido T, Ikeda K, Saitoh S, Kubota I, Takeishi Y. High Mobility Group Box 1 Promotes Angiogenesis from Bone Marrow-derived Endothelial Progenitor Cells after Myocardial Infarction. Journal of atherosclerosis and thrombosis. 2015;22:570–81. doi: 10.5551/jat.27235. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, Jiang H, Cui B, Xu C, Lu Z, He B. Preconditioning with high mobility group box 1 protein protects against myocardial ischemia-reperfusion injury. International journal of cardiology. 2010;145:111–2. doi: 10.1016/j.ijcard.2009.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. Journal of leukocyte biology. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 43.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 45.Katashima T, Naruko T, Terasaki F, Fujita M, Otsuka K, Murakami S, Sato A, Hiroe M, Ikura Y, Ueda M, Ikemoto M, Kitaura Y. Enhanced expression of the S100A8/A9 complex in acute myocardial infarction patients. Circulation journal : official journal of the Japanese Circulation Society. 2010;74:741–8. doi: 10.1253/circj.cj-09-0564. [DOI] [PubMed] [Google Scholar]
- 46.Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, Pradhan AD, Healy AM, Buros J, McCabe CH, Libby P, Cannon CP, Braunwald E, Simon DI. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. American heart journal. 2008;155:49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volz HC, Laohachewin D, Seidel C, Lasitschka F, Keilbach K, Wienbrandt AR, Andrassy J, Bierhaus A, Kaya Z, Katus HA, Andrassy M. S100A8/A9 aggravates post-ischemic heart failure through activation of RAGE-dependent NF-kappaB signaling. Basic Res Cardiol. 2012;107:250. doi: 10.1007/s00395-012-0250-z. [DOI] [PubMed] [Google Scholar]
- 48.Rohde D, Schon C, Boerries M, Didrihsone I, Ritterhoff J, Kubatzky KF, Volkers M, Herzog N, Mahler M, Tsoporis JN, Parker TG, Linke B, Giannitsis E, Gao E, Peppel K, Katus HA, Most P. S100A1 is released from ischemic cardiomyocytes and signals myocardial damage via Toll-like receptor 4. EMBO molecular medicine. 2014;6:778–94. doi: 10.15252/emmm.201303498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seropian IM, Cerliani JP, Toldo S, Van Tassell BW, Ilarregui JM, Gonzalez GE, Matoso M, Salloum FN, Melchior R, Gelpi RJ, Stupirski JC, Benatar A, Gomez KA, Morales C, Abbate A, Rabinovich GA. Galectin-1 controls cardiac inflammation and ventricular remodeling during acute myocardial infarction. Am J Pathol. 2013;182:29–40. doi: 10.1016/j.ajpath.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy VS, Harskamp RE, van Ginkel MW, Calhoon J, Baisden CE, Kim IS, Valente AJ, Chandrasekar B. Interleukin-18 stimulates fibronectin expression in primary human cardiac fibroblasts via PI3K-Akt-dependent NF-kappaB activation. Journal of cellular physiology. 2008;215:697–707. doi: 10.1002/jcp.21348. [DOI] [PubMed] [Google Scholar]
- 51.Gondokaryono SP, Ushio H, Niyonsaba F, Hara M, Takenaka H, Jayawardana ST, Ikeda S, Okumura K, Ogawa H. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. Journal of leukocyte biology. 2007;82:657–65. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 52.Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis. 2008;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–92. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 54.Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, Muller O, Vergely C, Zeller M, Tardivel A, Schneider P, Pacher P, Liaudet L. Cutting Edge: IL-1alpha Is a Crucial Danger Signal Triggering Acute Myocardial Inflammation during Myocardial Infarction. J Immunol. 2015;194:499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Methe H, Kim JO, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of circulating Toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111:2654–61. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- 56.Satoh M, Shimoda Y, Maesawa C, Akatsu T, Ishikawa Y, Minami Y, Hiramori K, Nakamura M. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. International journal of cardiology. 2006;109:226–34. doi: 10.1016/j.ijcard.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 57.van der Pouw Kraan TC, Bernink FJ, Yildirim C, Koolwijk P, Baggen JM, Timmers L, Beek AM, Diamant M, Chen WJ, van Rossum AC, van Royen N, Horrevoets AJ, Appelman YE. Systemic toll-like receptor and interleukin-18 pathway activation in patients with acute ST elevation myocardial infarction. Journal of molecular and cellular cardiology. 2014;67:94–102. doi: 10.1016/j.yjmcc.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 58.Selejan S, Poss J, Walter F, Hohl M, Kaiser R, Kazakov A, Bohm M, Link A. Ischaemia-induced up-regulation of Toll-like receptor 2 in circulating monocytes in cardiogenic shock. European heart journal. 2012;33:1085–94. doi: 10.1093/eurheartj/ehr377. [DOI] [PubMed] [Google Scholar]
- 59.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH, Piek JJ, Pasterkamp G, de Kleijn DP. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–64. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 60.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–80. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu L, Wang Y, Cao ZY, Wang MM, Liu XM, Gao T, Hu QK, Yuan WJ, Lin L. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J Cell Mol Med. 2015 doi: 10.1111/jcmm.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–9. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 63.Kim SC, Ghanem A, Stapel H, Tiemann K, Knuefermann P, Hoeft A, Meyer R, Grohe C, Knowlton AA, Baumgarten G. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC physiology. 2007;7:5. doi: 10.1186/1472-6793-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arslan F, Smeets MB, O’Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 65.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–71. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 66.Feng Y, Zhao H, Xu X, Buys ES, Raher MJ, Bopassa JC, Thibault H, Scherrer-Crosbie M, Schmidt U, Chao W. Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. Am J Physiol Heart Circ Physiol. 2008;295:H1311–H1318. doi: 10.1152/ajpheart.00119.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu C, Ren D, Wang X, Ha T, Liu L, Lee EJ, Hu J, Kalbfleisch J, Gao X, Kao R, Williams D, Li C. Toll-like receptor 3 plays a role in myocardial infarction and ischemia/reperfusion injury. Biochim Biophys Acta. 2014;1842:22–31. doi: 10.1016/j.bbadis.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riad A, Jager S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, Karatas A, Heimesaat MM, Bereswill S, Dragun D, Pauschinger M, Schultheiss HP, Tschope C. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954–61. doi: 10.4049/jimmunol.180.10.6954. [DOI] [PubMed] [Google Scholar]
- 69.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108:2905–10. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 70.Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol. 2012;52:1135–44. doi: 10.1016/j.yjmcc.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–4. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 72.Arslan F, Houtgraaf JH, Keogh B, Kazemi K, de Jong R, McCormack WJ, O’Neill LA, McGuirk P, Timmers L, Smeets MB, Akeroyd L, Reilly M, Pasterkamp G, de Kleijn DP. Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ Cardiovasc Interv. 2012;5:279–87. doi: 10.1161/CIRCINTERVENTIONS.111.967596. [DOI] [PubMed] [Google Scholar]
- 73.Parapanov R, Lugrin J, Rosenblatt-Velin N, Feihl F, Waeber B, Milano G, Vergely C, Li N, Pacher P, Liaudet L. Toll-like receptor 5 deficiency exacerbates cardiac injury and inflammation induced by myocardial ischaemia-reperfusion in the mouse. Clin Sci (Lond) 2015;129:187–98. doi: 10.1042/CS20140444. [DOI] [PubMed] [Google Scholar]
- 74.Ha T, Hu Y, Liu L, Lu C, McMullen JR, Kelley J, Kao RL, Williams DL, Gao X, Li C. TLR2 ligands induce cardioprotection against ischaemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovascular research. 2010;87:694–703. doi: 10.1093/cvr/cvq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong JW, Vallejo JG, Tzeng HP, Thomas JA, Mann DL. Innate immunity mediates myocardial preconditioning through Toll-like receptor 2 and TIRAP-dependent signaling pathways. American journal of physiology Heart and circulatory physiology. 2010;298:H1079–87. doi: 10.1152/ajpheart.00306.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. American journal of physiology Heart and circulatory physiology. 2009;296:H1–12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovascular research. 2008;78:546–53. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 78.Cao Z, Ren D, Ha T, Liu L, Wang X, Kalbfleisch J, Gao X, Kao R, Williams D, Li C. CpG-ODN, the TLR9 agonist, attenuates myocardial ischemia/reperfusion injury: involving activation of PI3K/Akt signaling. Biochimica et biophysica acta. 2013;1832:96–104. doi: 10.1016/j.bbadis.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–32. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 80.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circulation research. 2002;91:988–98. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 81.Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL, Vicencio JM, Yellon DM. Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic research in cardiology. 2013;108:377. doi: 10.1007/s00395-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 82.Morimoto H, Hirose M, Takahashi M, Kawaguchi M, Ise H, Kolattukudy PE, Yamada M, Ikeda U. MCP-1 induces cardioprotection against ischaemia/reperfusion injury: role of reactive oxygen species. Cardiovascular research. 2008;78:554–62. doi: 10.1093/cvr/cvn035. [DOI] [PubMed] [Google Scholar]
- 83.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circulation research. 2015;116:1254–68. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakata Y, Dong JW, Vallejo JG, Huang CH, Baker JS, Tracey KJ, Tacheuchi O, Akira S, Mann DL. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. American journal of physiology Heart and circulatory physiology. 2007;292:H503–9. doi: 10.1152/ajpheart.00642.2006. [DOI] [PubMed] [Google Scholar]
- 85.Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circulation research. 2004;95:700–7. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- 86.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 87.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–30. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovascular research. 2013;99:164–74. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 89.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103:2296–302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 90.Venkatachalam K, Prabhu SD, Reddy VS, Boylston WH, Valente AJ, Chandrasekar B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J Biol Chem. 2009;284:7853–65. doi: 10.1074/jbc.M808824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 92.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 93.Ritchie ME. Nuclear factor-kappaB is selectively and markedly activated in humans with unstable angina pectoris. Circulation. 1998;98:1707–13. doi: 10.1161/01.cir.98.17.1707. [DOI] [PubMed] [Google Scholar]
- 94.Chandrasekar B, Freeman GL. Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Lett. 1997;401:30–4. doi: 10.1016/s0014-5793(96)01426-3. [DOI] [PubMed] [Google Scholar]
- 95.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-kappaB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovascular research. 2011;89:129–38. doi: 10.1093/cvr/cvq274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, Qiu Y, Li JJ, Bolli R. Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circulation research. 1999;84:1095–109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 97.Tranter M, Ren X, Forde T, Wilhide ME, Chen J, Sartor MA, Medvedovic M, Jones WK. NF-kappaB driven cardioprotective gene programs; Hsp70.3 and cardioprotection after late ischemic preconditioning. Journal of molecular and cellular cardiology. 2010;49:664–72. doi: 10.1016/j.yjmcc.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown M, McGuinness M, Wright T, Ren X, Wang Y, Boivin GP, Hahn H, Feldman AM, Jones WK. Cardiac-specific blockade of NF-kappaB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. American journal of physiology Heart and circulatory physiology. 2005;289:H466–76. doi: 10.1152/ajpheart.00170.2004. [DOI] [PubMed] [Google Scholar]
- 99.Zhang XQ, Tang R, Li L, Szucsik A, Javan H, Saegusa N, Spitzer KW, Selzman CH. Cardiomyocyte-specific p65 NF-kappaB deletion protects the injured heart by preservation of calcium handling. Am J Physiol Heart Circ Physiol. 2013;305:H1089–97. doi: 10.1152/ajpheart.00067.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Squadrito F, Deodato B, Squadrito G, Seminara P, Passaniti M, Venuti FS, Giacca M, Minutoli L, Adamo EB, Bellomo M, Marini R, Galeano M, Marini H, Altavilla D. Gene transfer of IkappaBalpha limits infarct size in a mouse model of myocardial ischemia-reperfusion injury. Lab Invest. 2003;83:1097–104. doi: 10.1097/01.lab.0000082060.39079.a6. [DOI] [PubMed] [Google Scholar]
- 101.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J, Ogihara T. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–9. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 102.Onai Y, Suzuki J, Kakuta T, Maejima Y, Haraguchi G, Fukasawa H, Muto S, Itai A, Isobe M. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovascular research. 2004;63:51–9. doi: 10.1016/j.cardiores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH. IKKbeta inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;293:H2248–53. doi: 10.1152/ajpheart.00776.2007. [DOI] [PubMed] [Google Scholar]
- 104.Li HL, Zhuo ML, Wang D, Wang AB, Cai H, Sun LH, Yang Q, Huang Y, Wei YS, Liu PP, Liu DP, Liang CC. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation. 2007;115:1885–94. doi: 10.1161/CIRCULATIONAHA.106.656835. [DOI] [PubMed] [Google Scholar]
- 105.Trescher K, Bernecker O, Fellner B, Gyongyosi M, Schafer R, Aharinejad S, DeMartin R, Wolner E, Podesser BK. Inflammation and postinfarct remodeling: overexpression of IkappaB prevents ventricular dilation via increasing TIMP levels. Cardiovascular research. 2006;69:746–54. doi: 10.1016/j.cardiores.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 106.Timmers L, van Keulen JK, Hoefer IE, Meijs MF, van Middelaar B, den Ouden K, van Echteld CJ, Pasterkamp G, de Kleijn DP. Targeted deletion of nuclear factor kappaB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circulation research. 2009;104:699–706. doi: 10.1161/CIRCRESAHA.108.189746. [DOI] [PubMed] [Google Scholar]
- 107.Frantz S, Tillmanns J, Kuhlencordt PJ, Schmidt I, Adamek A, Dienesch C, Thum T, Gerondakis S, Ertl G, Bauersachs J. Tissue-specific effects of the nuclear factor kappaB subunit p50 on myocardial ischemia-reperfusion injury. The American journal of pathology. 2007;171:507–12. doi: 10.2353/ajpath.2007.061042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frantz S, Hu K, Bayer B, Gerondakis S, Strotmann J, Adamek A, Ertl G, Bauersachs J. Absence of NF-kappaB subunit p50 improves heart failure after myocardial infarction. Faseb J. 2006;20:1918–20. doi: 10.1096/fj.05-5133fje. [DOI] [PubMed] [Google Scholar]
- 109.Zhang LX, Zhao Y, Cheng G, Guo TL, Chin YE, Liu PY, Zhao TC. Targeted deletion of NF-kappaB p50 diminishes the cardioprotection of histone deacetylase inhibition. American journal of physiology Heart and circulatory physiology. 2010;298:H2154–63. doi: 10.1152/ajpheart.01015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cavalera M, Frangogiannis NG. Targeting the chemokines in cardiac repair. Curr Pharm Des. 2014;20:1971–9. doi: 10.2174/13816128113199990449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prabhu SD. Cytokine-induced modulation of cardiac function. Circulation research. 2004;95:1140–53. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 112.Bromage DI, Davidson SM, Yellon DM. Stromal derived factor 1alpha: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther. 2014;143:305–15. doi: 10.1016/j.pharmthera.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maekawa N, Wada H, Kanda T, Niwa T, Yamada Y, Saito K, Fujiwara H, Sekikawa K, Seishima M. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J Am Coll Cardiol. 2002;39:1229–35. doi: 10.1016/s0735-1097(02)01738-2. [DOI] [PubMed] [Google Scholar]
- 114.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hilfiker-Kleiner D, Shukla P, Klein G, Schaefer A, Stapel B, Hoch M, Muller W, Scherr M, Theilmeier G, Ernst M, Hilfiker A, Drexler H. Continuous glycoprotein-130-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation. 2010;122:145–55. doi: 10.1161/CIRCULATIONAHA.109.933127. [DOI] [PubMed] [Google Scholar]
- 116.Liehn EA, Piccinini AM, Koenen RR, Soehnlein O, Adage T, Fatu R, Curaj A, Popescu A, Zernecke A, Kungl AJ, Weber C. A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J Am Coll Cardiol. 2010;56:1847–57. doi: 10.1016/j.jacc.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 117.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–9. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 118.Hayasaki T, Kaikita K, Okuma T, Yamamoto E, Kuziel WA, Ogawa H, Takeya M. CC chemokine receptor-2 deficiency attenuates oxidative stress and infarct size caused by myocardial ischemia-reperfusion in mice. Circulation journal : official journal of the Japanese Circulation Society. 2006;70:342–51. doi: 10.1253/circj.70.342. [DOI] [PubMed] [Google Scholar]
- 119.Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–47. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kurrelmeyer KM, Michael LH, Baumgarten G, Taffet GE, Peschon JJ, Sivasubramanian N, Entman ML, Mann DL. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5456–61. doi: 10.1073/pnas.070036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fuchs M, Hilfiker A, Kaminski K, Hilfiker-Kleiner D, Guener Z, Klein G, Podewski E, Schieffer B, Rose-John S, Drexler H. Role of interleukin-6 for LV remodeling and survival after experimental myocardial infarction. Faseb J. 2003;17:2118–20. doi: 10.1096/fj.03-0331fje. [DOI] [PubMed] [Google Scholar]
- 122.Obana M, Maeda M, Takeda K, Hayama A, Mohri T, Yamashita T, Nakaoka Y, Komuro I, Takeda K, Matsumiya G, Azuma J, Fujio Y. Therapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation. 2010;121:684–91. doi: 10.1161/CIRCULATIONAHA.109.893677. [DOI] [PubMed] [Google Scholar]
- 123.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–87. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zamilpa R, Kanakia R, Cigarroa Jt, Dai Q, Escobar GP, Martinez H, Jimenez F, Ahuja SS, Lindsey ML. CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. American journal of physiology Heart and circulatory physiology. 2011;300:H1418–26. doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–97. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garlie JB, Hamid T, Gu Y, Ismahil MA, Chandrasekar B, Prabhu SD. Tumor necrosis factor receptor 2 signaling limits beta-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic research in cardiology. 2011;106:1193–205. doi: 10.1007/s00395-011-0196-6. [DOI] [PubMed] [Google Scholar]
- 127.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–32. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 129.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–31. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–20. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 131.Kukielka GL, Hawkins HK, Michael L, Manning AM, Youker K, Lane C, Entman ML, Smith CW, Anderson DC. Regulation of intercellular adhesion molecule-1 (ICAM-1) in ischemic and reperfused canine myocardium. J Clin Invest. 1993;92:1504–16. doi: 10.1172/JCI116729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tarzami ST, Cheng R, Miao W, Kitsis RN, Berman JW. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. J Mol Cell Cardiol. 2002;34:209–21. doi: 10.1006/jmcc.2001.1503. [DOI] [PubMed] [Google Scholar]
- 133.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting Cardiac Cellular Composition. Circ Res. 2016;118:400–9. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu M, Goetsch SC, Wang Z, Luo R, Hill JA, Schneider J, Morris SM, Jr, Liu ZP. FoxO4 Promotes Early Inflammatory Response Upon Myocardial Infarction via Endothelial Arg1. Circ Res. 2015;117:967–77. doi: 10.1161/CIRCRESAHA.115.306919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weyrich AS, Ma XY, Lefer DJ, Albertine KH, Lefer AM. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993;91:2620–9. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 137.Kumar AG, Ballantyne CM, Michael LH, Kukielka GL, Youker KA, Lindsey ML, Hawkins HK, Birdsall HH, MacKay CR, LaRosa GJ, Rossen RD, Smith CW, Entman ML. Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation. 1997;95:693–700. doi: 10.1161/01.cir.95.3.693. [DOI] [PubMed] [Google Scholar]
- 138.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 2001;15:1428–30. doi: 10.1096/fj.00-0745fje. [DOI] [PubMed] [Google Scholar]
- 139.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 140.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews Immunology. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–39. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 142.Detmers PA, Lo SK, Olsen-Egbert E, Walz A, Baggiolini M, Cohn ZA. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171:1155–62. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herter J, Zarbock A. Integrin Regulation during Leukocyte Recruitment. J Immunol. 2013;190:4451–7. doi: 10.4049/jimmunol.1203179. [DOI] [PubMed] [Google Scholar]
- 144.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–75. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–32. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–34. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–9. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boufenzer A, Lemarie J, Simon T, Derive M, Bouazza Y, Tran N, Maskali F, Groubatch F, Bonnin P, Bastien C, Bruneval P, Marie PY, Cohen R, Danchin N, Silvestre JS, Ait-Oufella H, Gibot S. TREM-1 Mediates Inflammatory Injury and Cardiac Remodeling Following Myocardial Infarction. Circ Res. 2015;116:1772–82. doi: 10.1161/CIRCRESAHA.116.305628. [DOI] [PubMed] [Google Scholar]
- 149.Simpson PJ, Todd RF, 3rd, Fantone JC, Mickelson JK, Griffin JD, Lucchesi BR. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988;81:624–9. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Entman ML, Youker K, Shoji T, Kukielka G, Shappell SB, Taylor AA, Smith CW. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992;90:1335–45. doi: 10.1172/JCI115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest. 2013;43:986–95. doi: 10.1111/eci.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165:2798–808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 155.Varda-Bloom N, Leor J, Ohad DG, Hasin Y, Amar M, Fixler R, Battler A, Eldar M, Hasin D. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J Mol Cell Cardiol. 2000;32:2141–9. doi: 10.1006/jmcc.2000.1261. [DOI] [PubMed] [Google Scholar]
- 156.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–80. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–76. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J Immunol. 2013;191:4838–48. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 161.Somasundaram P, Ren G, Nagar H, Kraemer D, Mendoza L, Michael LH, Caughey GH, Entman ML, Frangogiannis NG. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol. 2005;205:102–11. doi: 10.1002/path.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heidt T, Courties G, Dutta P, Sager H, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential Contribution of Monocytes to Heart Macrophages in Steady-State and After Myocardial Infarction. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mylonas KJ, Jenkins SJ, Castellan RF, Ruckerl D, McGregor K, Phythian-Adams AT, Hewitson JP, Campbell SM, MacDonald AS, Allen JE, Gray GA. The adult murine heart has a sparse, phagocytically active macrophage population that expands through monocyte recruitment and adopts an ‘M2’ phenotype in response to Th2 immunologic challenge. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S. Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res. 2014;114:872–88. doi: 10.1161/CIRCRESAHA.114.302533. [DOI] [PubMed] [Google Scholar]
- 168.Trial J, Baughn RE, Wygant JN, McIntyre BW, Birdsall HH, Youker KA, Evans A, Entman ML, Rossen RD. Fibronectin fragments modulate monocyte VLA-5 expression and monocyte migration. J Clin Invest. 1999;104:419–30. doi: 10.1172/JCI4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG. CD44 Is Critically Involved in Infarct Healing by Regulating the Inflammatory and Fibrotic Response. J Immunol. 2008;180:2625–33. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- 170.Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends in immunology. 2013;34:398–409. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 171.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 172.Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, Tricot B, Wojtkiewicz G, Dutta P, Sager HB, Borodovsky A, Novobrantseva T, Klebanov B, Fitzgerald K, Anderson DG, Libby P, Swirski FK, Weissleder R, Nahrendorf M. In Vivo Silencing of the Transcription Factor IRF5 Reprograms the Macrophage Phenotype and Improves Infarct Healing. J Am Coll Cardiol. 2014;63:1556–66. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–22. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. The Journal of experimental medicine. 2012;209:123–37. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Korf-Klingebiel M, Reboll MR, Klede S, Brod T, Pich A, Polten F, Napp LC, Bauersachs J, Ganser A, Brinkmann E, Reimann I, Kempf T, Niessen HW, Mizrahi J, Schonfeld HJ, Iglesias A, Bobadilla M, Wang Y, Wollert KC. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat Med. 2015;21:140–9. doi: 10.1038/nm.3778. [DOI] [PubMed] [Google Scholar]
- 176.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nature immunology. 2011;12:778–85. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, Sugano Y, Takahashi T, Abe H, Mochizuki S, Sano M, Yoshikawa T, Okada Y, Koyasu S, Ogawa S, Fukuda K. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 2012;125:1234–45. doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- 178.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–63. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 179.Homma T, Kinugawa S, Takahashi M, Sobirin MA, Saito A, Fukushima A, Suga T, Takada S, Kadoguchi T, Masaki Y, Furihata T, Taniguchi M, Nakayama T, Ishimori N, Iwabuchi K, Tsutsui H. Activation of invariant natural killer T cells by alpha-galactosylceramide ameliorates myocardial ischemia/reperfusion injury in mice. J Mol Cell Cardiol. 2013;62:179–88. doi: 10.1016/j.yjmcc.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 180.Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, Taniguchi M, Nakayama T, Ishimori N, Iwabuchi K, Tsutsui H. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res. 2012;111:1037–47. doi: 10.1161/CIRCRESAHA.112.270132. [DOI] [PubMed] [Google Scholar]
- 181.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 182.Yan X, Shichita T, Katsumata Y, Matsuhashi T, Ito H, Ito K, Anzai A, Endo J, Tamura Y, Kimura K, Fujita J, Shinmura K, Shen W, Yoshimura A, Fukuda K, Sano M. Deleterious effect of the IL-23/IL-17A axis and gammadeltaT cells on left ventricular remodeling after myocardial infarction. J Am Heart Assoc. 2012;1:e004408. doi: 10.1161/JAHA.112.004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–10. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 184.Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M. VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol. 1996;270:H1803–11. doi: 10.1152/ajpheart.1996.270.5.H1803. [DOI] [PubMed] [Google Scholar]
- 185.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem. 2002;50:71–9. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 186.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–23. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 187.Chen CW, Okada M, Proto JD, Gao X, Sekiya N, Beckman SA, Corselli M, Crisan M, Saparov A, Tobita K, Peault B, Huard J. Human pericytes for ischemic heart repair. Stem Cells. 2013;31:305–16. doi: 10.1002/stem.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circulation research. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Frangogiannis NG. The reparative function of cardiomyocytes in the infarcted myocardium. Cell Metab. 2015;21:797–8. doi: 10.1016/j.cmet.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 190.Lorchner H, Poling J, Gajawada P, Hou Y, Polyakova V, Kostin S, Adrian-Segarra JM, Boettger T, Wietelmann A, Warnecke H, Richter M, Kubin T, Braun T. Myocardial healing requires Reg3beta-dependent accumulation of macrophages in the ischemic heart. Nat Med. 2015;21:353–62. doi: 10.1038/nm.3816. [DOI] [PubMed] [Google Scholar]
- 191.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 193.Matsukawa A, Kudo S, Maeda T, Numata K, Watanabe H, Takeda K, Akira S, Ito T. Stat3 in resident macrophages as a repressor protein of inflammatory response. J Immunol. 2005;175:3354–9. doi: 10.4049/jimmunol.175.5.3354. [DOI] [PubMed] [Google Scholar]
- 194.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–77. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang Z, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation. 2000;101:1019–26. doi: 10.1161/01.cir.101.9.1019. [DOI] [PubMed] [Google Scholar]
- 196.Zymek P, Nah DY, Bujak M, Ren G, Koerting A, Leucker T, Huebener P, Taffet G, Entman M, Frangogiannis NG. Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. Cardiovasc Res. 2007;74:313–22. doi: 10.1016/j.cardiores.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cavusoglu E, Marmur JD, Hojjati MR, Chopra V, Butala M, Subnani R, Huda MS, Yanamadala S, Ruwende C, Eng C, Pinsky DJ. Plasma interleukin-10 levels and adverse outcomes in acute coronary syndrome. Am J Med. 2011;124:724–30. doi: 10.1016/j.amjmed.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 198.Ammirati E, Cannistraci CV, Cristell NA, Vecchio V, Palini AG, Tornvall P, Paganoni AM, Miendlarzewska EA, Sangalli LM, Monello A, Pernow J, Bjornstedt Bennermo M, Marenzi G, Hu D, Uren NG, Cianflone D, Ravasi T, Manfredi AA, Maseri A. Identification and predictive value of interleukin-6+ interleukin-10+ and interleukin-6- interleukin-10+ cytokine patterns in ST-elevation acute myocardial infarction. Circ Res. 2012;111:1336–48. doi: 10.1161/CIRCRESAHA.111.262477. [DOI] [PubMed] [Google Scholar]
- 199.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ikeuchi M, Tsutsui H, Shiomi T, Matsusaka H, Matsushima S, Wen J, Kubota T, Takeshita A. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004;64:526–35. doi: 10.1016/j.cardiores.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 201.Rainer PP, Hao S, Vanhoutte D, Lee DI, Koitabashi N, Molkentin JD, Kass DA. Cardiomyocyte-specific transforming growth factor beta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res. 2014;114:1246–57. doi: 10.1161/CIRCRESAHA.114.302653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–8. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 203.Kempf T, Bjorklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, Tongers J, Wollert KC, Wallentin L. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J. 2007;28:2858–65. doi: 10.1093/eurheartj/ehm465. [DOI] [PubMed] [Google Scholar]
- 204.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H153–64. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 206.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. Journal of molecular and cellular cardiology. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32:2598–608. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Saxena A, Shinde AV, Haque Z, Wu YJ, Chen W, Su Y, Frangogiannis NG. The role of Interleukin Receptor Associated Kinase (IRAK)-M in regulation of myofibroblast phenotype in vitro, and in an experimental model of non-reperfused myocardial infarction. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–75. [PMC free article] [PubMed] [Google Scholar]
- 210.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000;48:89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 211.Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis & tissue repair. 2013;6:5. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, Bedosky KM, Freed DH, Kardami E, Dixon IM. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–84. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 213.Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol. 2005;14:241–6. doi: 10.1016/j.carpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 214.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–71. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 215.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 216.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ruiz-Villalba A, Simon AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, Sanchez-Dominguez R, Segovia JC, Prosper F, Perez-Pomares JM. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol. 2015;65:2057–66. doi: 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 218.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. Faseb J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 220.Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Maruoka S, Horie T. Transforming growth Factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-Jun-NH2-terminal kinase-dependent pathway. Am J Respir Crit Care Med. 2001;163:152–7. doi: 10.1164/ajrccm.163.1.2005069. [DOI] [PubMed] [Google Scholar]
- 221.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lenga Y, Koh A, Perera AS, McCulloch CA, Sodek J, Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ Res. 2008;102:319–27. doi: 10.1161/CIRCRESAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 223.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–11. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Thodeti CK, Paruchuri S, Meszaros JG. A TRP to cardiac fibroblast differentiation. Channels. 2013;7:211–4. doi: 10.4161/chan.24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–12. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 227.Adapala RK, Thoppil RJ, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, Thodeti CK. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol. 2013;54:45–52. doi: 10.1016/j.yjmcc.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–15. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sun Y, Weber KT. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J Mol Cell Cardiol. 1996;28:851–8. doi: 10.1006/jmcc.1996.0080. [DOI] [PubMed] [Google Scholar]
- 231.Booz GW, Baker KM. Molecular signalling mechanisms controlling growth and function of cardiac fibroblasts. Cardiovasc Res. 1995;30:537–43. [PubMed] [Google Scholar]
- 232.McEwan PE, Gray GA, Sherry L, Webb DJ, Kenyon CJ. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation. 1998;98:2765–73. doi: 10.1161/01.cir.98.24.2765. [DOI] [PubMed] [Google Scholar]
- 233.Stockand JD, Meszaros JG. Aldosterone stimulates proliferation of cardiac fibroblasts by activating Ki-RasA and MAPK1/2 signaling. Am J Physiol Heart Circ Physiol. 2003;284:H176–84. doi: 10.1152/ajpheart.00421.2002. [DOI] [PubMed] [Google Scholar]
- 234.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–38. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 235.Zymek P, Cieslak A, Tincey S, Ren G, Michael L, Entman ML, Frangogiannis N. Platelet derived growth factor receptor (PDGFR)-beta signaling regulates vascular maturation in healing myocardial infarcts. J Am Coll Cardiol. 2005;45:5A. [Google Scholar]
- 236.Zhao W, Zhao T, Huang V, Chen Y, Ahokas RA, Sun Y. Platelet-derived growth factor involvement in myocardial remodeling following infarction. J Mol Cell Cardiol. 2011;51:830–8. doi: 10.1016/j.yjmcc.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Santiago JJ, McNaughton LJ, Koleini N, Ma X, Bestvater B, Nickel BE, Fandrich RR, Wigle JT, Freed DH, Arora RC, Kardami E. High molecular weight fibroblast growth factor-2 in the human heart is a potential target for prevention of cardiac remodeling. PLoS One. 2014;9:e97281. doi: 10.1371/journal.pone.0097281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.McLarty JL, Melendez GC, Brower GL, Janicki JS, Levick SP. Tryptase/Protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. 2011;58:264–70. doi: 10.1161/HYPERTENSIONAHA.111.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhao XY, Zhao LY, Zheng QS, Su JL, Guan H, Shang FJ, Niu XL, He YP, Lu XL. Chymase induces profibrotic response via transforming growth factor-beta 1/Smad activation in rat cardiac fibroblasts. Mol Cell Biochem. 2008;310:159–66. doi: 10.1007/s11010-007-9676-2. [DOI] [PubMed] [Google Scholar]
- 240.Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–88. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 241.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 242.Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, Imanaka-Yoshida K. Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2010;298:H1072–8. doi: 10.1152/ajpheart.00255.2009. [DOI] [PubMed] [Google Scholar]
- 243.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d’Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–23. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–21. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, Apstein CS, Colucci WS, Singh K. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88:1080–7. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 247.Van Aelst LN, Voss S, Carai P, Van Leeuwen R, Vanhoutte D, Sanders-van Wijk S, Eurlings L, Swinnen M, Verheyen FK, Verbeken E, Nef H, Troidl C, Cook SA, Brunner-La Rocca HP, Mollmann H, Papageorgiou AP, Heymans S. Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ Res. 2015;116:425–36. doi: 10.1161/CIRCRESAHA.116.304599. [DOI] [PubMed] [Google Scholar]
- 248.Ahmed MS, Gravning J, Martinov VN, von Lueder TG, Edvardsen T, Czibik G, Moe IT, Vinge LE, Oie E, Valen G, Attramadal H. Mechanisms of Novel Cardioprotective Functions of CCN2/CTGF in Myocardial Ischemia/Reperfusion Injury. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00604.2010. [DOI] [PubMed] [Google Scholar]
- 249.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–83. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-Quesada C, Lu B, Gerard C, Frangogiannis NG. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res. 2014;103:217–27. doi: 10.1093/cvr/cvu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem. 2013;61:555–70. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Takemura G, Ohno M, Hayakawa Y, Misao J, Kanoh M, Ohno A, Uno Y, Minatoguchi S, Fujiwara T, Fujiwara H. Role of apoptosis in the disappearance of infiltrated and proliferated interstitial cells after myocardial infarction. Circ Res. 1998;82:1130–8. doi: 10.1161/01.res.82.11.1130. [DOI] [PubMed] [Google Scholar]
- 253.Larose E, Rodes-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM, Dery JP, Gleeton O, Roy L, Noel B, Barbeau G, Rouleau J, Boudreault JR, Amyot M, De Larochelliere R, Bertrand OF. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. Journal of the American College of Cardiology. 2010;55:2459–69. doi: 10.1016/j.jacc.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 254.van Diepen S, Newby LK, Lopes RD, Stebbins A, Hasselblad V, James S, Roe MT, Ezekowitz JA, Moliterno DJ, Neumann FJ, Reist C, Mahaffey KW, Hochman JS, Hamm CW, Armstrong PW, Granger CB, Theroux P, Investigators AA. Prognostic relevance of baseline pro- and anti-inflammatory markers in STEMI: an APEX AMI substudy. International journal of cardiology. 2013;168:2127–33. doi: 10.1016/j.ijcard.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 255.Devaux B, Scholz D, Hirche A, Klovekorn WP, Schaper J. Upregulation of cell adhesion molecules and the presence of low grade inflammation in human chronic heart failure. Eur Heart J. 1997;18:470–9. doi: 10.1093/oxfordjournals.eurheartj.a015268. [DOI] [PubMed] [Google Scholar]
- 256.Prabhu SD. It takes two to tango: monocyte and macrophage duality in the infarcted heart. Circulation research. 2014;114:1558–60. doi: 10.1161/CIRCRESAHA.114.303933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–82. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167:152–66. doi: 10.1016/j.trsl.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Baran KW, Nguyen M, McKendall GR, Lambrew CT, Dykstra G, Palmeri ST, Gibbons RJ, Borzak S, Sobel BE, Gourlay SG, Rundle AC, Gibson CM, Barron HV. Double-blind, randomized trial of an anti-CD18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction: limitation of myocardial infarction following thrombolysis in acute myocardial infarction (LIMIT AMI) study. Circulation. 2001;104:2778–83. doi: 10.1161/hc4801.100236. [DOI] [PubMed] [Google Scholar]
- 260.Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, Jr, O’Neill WW, Todaro TG, Vahanian A, Van de Werf F. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. Jama. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 261.Tardif JC, Tanguay JF, Wright SS, Duchatelle V, Petroni T, Gregoire JC, Ibrahim R, Heinonen TM, Robb S, Bertrand OF, Cournoyer D, Johnson D, Mann J, Guertin MC, L’Allier PL. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J Am Coll Cardiol. 2013;61:2048–55. doi: 10.1016/j.jacc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 262.Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–603. doi: 10.1016/j.jacc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 263.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–1377 e1. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 264.Carrabba N, Valenti R, Parodi G, Santoro GM, Antoniucci D. Left ventricular remodeling and heart failure in diabetic patients treated with primary angioplasty for acute myocardial infarction. Circulation. 2004;110:1974–9. doi: 10.1161/01.CIR.0000143376.64970.4A. [DOI] [PubMed] [Google Scholar]
- 265.Aronson D, Musallam A, Lessick J, Dabbah S, Carasso S, Hammerman H, Reisner S, Agmon Y, Mutlak D. Impact of diastolic dysfunction on the development of heart failure in diabetic patients after acute myocardial infarction. Circ Heart Fail. 2010;3:125–31. doi: 10.1161/CIRCHEARTFAILURE.109.877340. [DOI] [PubMed] [Google Scholar]
- 266.Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW, Wang XF, Frangogiannis NG. Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice. Circ Heart Fail. 2015;8:788–98. doi: 10.1161/CIRCHEARTFAILURE.114.001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–92. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Penn MS, Pastore J, Miller T, Aras R. SDF-1 in myocardial repair. Gene Ther. 2012;19:583–7. doi: 10.1038/gt.2012.32. [DOI] [PubMed] [Google Scholar]
- 269.Beohar N, Rapp J, Pandya S, Losordo DW. Rebuilding the damaged heart: the potential of cytokines and growth factors in the treatment of ischemic heart disease. J Am Coll Cardiol. 2010;56:1287–97. doi: 10.1016/j.jacc.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xiang FL, Lu X, Hammoud L, Zhu P, Chidiac P, Robbins J, Feng Q. Cardiomyocyte-specific overexpression of human stem cell factor improves cardiac function and survival after myocardial infarction in mice. Circulation. 2009;120:1065–74. doi: 10.1161/CIRCULATIONAHA.108.839068. 9 p following 1074. [DOI] [PubMed] [Google Scholar]
- 271.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol. 2015;30:240–5. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


