This review discusses neuroprotective interventions currently applied in clinical practice for the treatment of spinal cord injuries, as well as translational therapies and neuroregenerative strategies in development. The need for combinatorial approaches to this multifactorial problem is emphasized.
Keywords: Spinal cord injury, Stem cell, Trauma, Biomaterial, Neuroprotection, Neuroregeneration
Abstract
Spinal cord injuries (SCIs) result in devastating lifelong disability for patients and their families. The initial mechanical trauma is followed by a damaging secondary injury cascade involving proapoptotic signaling, ischemia, and inflammatory cell infiltration. Ongoing cellular necrosis releases ATP, DNA, glutamate, and free radicals to create a cytotoxic postinjury milieu. Long-term regeneration of lost or injured networks is further impeded by cystic cavitation and the formation of an inhibitory glial-chondroitin sulfate proteoglycan scar. In this article, we discuss important neuroprotective interventions currently applied in clinical practice, including surgical decompression, blood pressure augmentation, and i.v. methylprednisolone. We then explore exciting translational therapies on the horizon, such as riluzole, minocycline, fibroblast growth factor, magnesium, and hypothermia. Finally, we summarize the key neuroregenerative strategies of the next decade, including glial scar degradation, Rho-ROCK inhibition, cell-based therapies, and novel bioengineered adjuncts. Throughout, we emphasize the need for combinatorial approaches to this multifactorial problem and discuss relevant studies at the forefront of translation. We conclude by providing our perspectives on the future direction of SCI research.
Significance
Spinal cord injuries (SCIs) result in devastating, lifelong disability for patients and their families. This article discusses important neuroprotective interventions currently applied in clinical practice, including surgical decompression, blood pressure augmentation, and i.v. methylprednisolone. Translational therapies on the horizon are discussed, such as riluzole, minocycline, fibroblast growth factor, magnesium, and hypothermia. The key neuroregenerative strategies of the next decade are summarized, including glial scar degradation, Rho-ROCK inhibition, cell-based therapies, and novel bioengineered adjuncts. The need for combinatorial approaches to this multifactorial problem is emphasized, relevant studies at the forefront of translation are discussed, and perspectives on the future direction of SCI research are presented.
Introduction
The Acute Injury and Postinjury Milieu
Traumatic spinal cord injuries affect 1.4 million North Americans, a disproportionate number of whom are younger than 30 years. Direct lifetime costs are staggering, at $1.1 to $4.6 million per patient [1, 2]. The initial mechanical insult is followed by a secondary injury cascade that generates further permanent damage. Promising neuroprotective strategies to mitigate the secondary injury, and neuroregenerative approaches to restore function, are discussed herein.
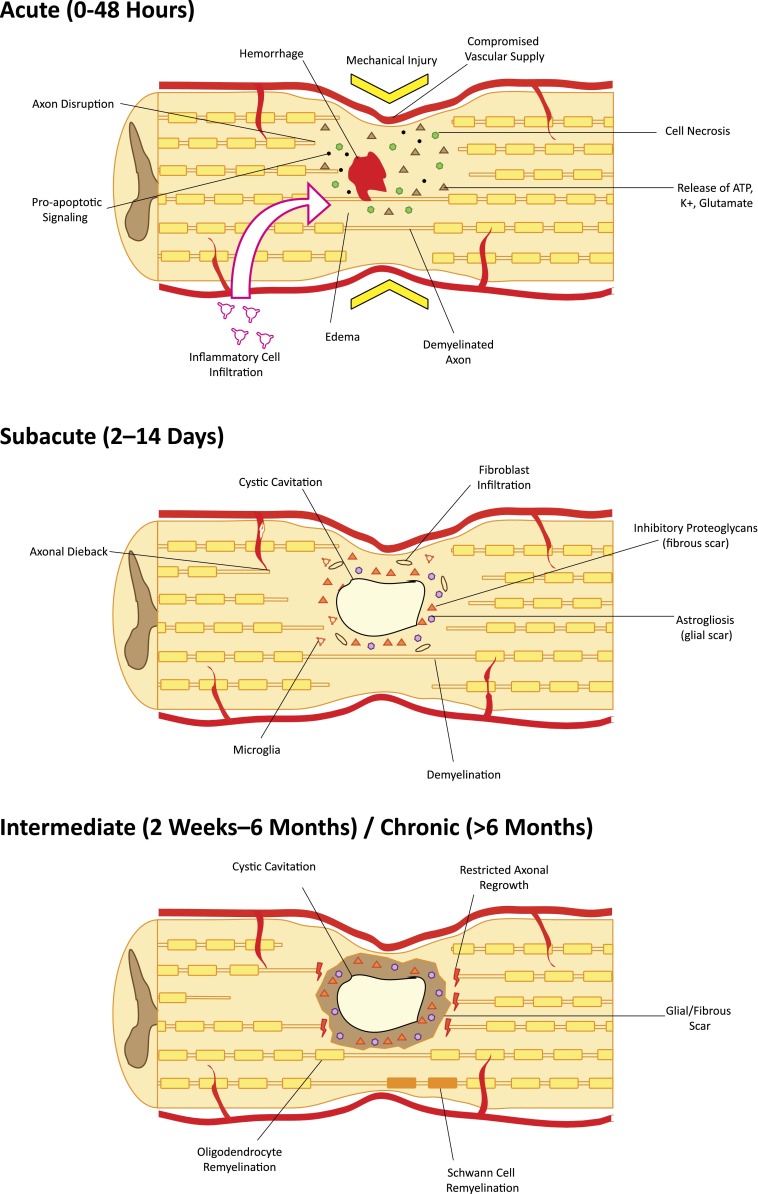
Acute cell dysfunction and death occur via cell permeabilization and initiation of proapoptotic signaling cascades and because of ischemia due to destruction of the sensitive microvascular supply [3, 4]. Furthermore, disruption of the blood-spinal cord barrier exposes the cord to inflammatory cells, cytokines, and vasoactive peptides [5, 6]. In the subsequent hours, hemorrhage and progressive edema cyclically add to the harsh postinjury milieu. Ongoing cellular necrosis releases ATP, DNA, and K+, which activate microglia to secrete proinflammatory cytokines. As a result, dramatic numbers of macrophages, microglia, and polymorphonuclear leukocytes infiltrate the injury site [7]. Engaged phagocytes generate reactive free radicals and cytotoxic by-products that further contribute to cell death. Moreover, release of glutamate via neurons and reuptake failure by astrocytes lead to excitotoxic injury in nearby neurons [8, 9]. At a systemic level, loss of CNS-mediated sympathetic vascular tone results in profound hypotension. Furthermore, impaired local autoregulation makes the cord particularly susceptible to ongoing postinjury ischemia [10, 11] (Fig. 1). Each step in this injury cascade is an important target for combinatorial neuroprotective strategies.
Figure 1.
Pathophysiology of spinal cord injury in the acute, subacute, and chronic setting. Acute traumatic injury causes cell death through ischemia, release of cytotoxic molecules, initiation of apoptotic cascades, hemorrhage, edema, and infiltration of inflammatory cells. In the subacute phase, cystic cavities begin to coalesce and become surrounded by reactive astrocytes, fibroblasts, and inflammatory cells. Inhibitory proteoglycans are secreted into the extracellular matrix. Degeneration/dieback of damaged and denuded axons occurs. In the intermediate/chronic phase, encompassing most patients, mechanical and chemotactic barriers restrict axon regeneration. Limited remyelination by oligodendrocytes and Schwann cells may portend small functional gains during this period.
Barriers to Recovery
Regeneration after spinal cord injury (SCI) requires targeted axon growth and remyelination of long tracts. Loss of the cord’s structural framework, including cystic cavitation, impairs directed axonal regrowth and free cell migration [12]. In addition to architectural disruption, uncontrolled reactive astrogliosis generates inhibitory glial scarring by creating a physical barrier of irregular mesh-like astrocytic processes in the perilesional zone [13]. Extracellular matrix in the glial scar is predominantly composed of chondroitin sulfate proteoglycans (CSPGs) [14, 15], tenascin [16, 17], and neural/glial antigen 2 (NG2) proteoglycans [18, 19], which further restrict axonal regeneration and inhibit neurite outgrowth through membrane tyrosine phosphatase, PTPσ [20, 21]. Furthermore, existing myelin- and neuron-related signals neurite outgrowth inhibitor (NOGO) [22], oligodendrocyte myelin glycoprotein, semaphorin 3A, semaphorin 4D [23], and myelin-associated glycoprotein [24] bind to NOGO receptor (NgR) (or neuropilin-1/plexin-A1 for semaphorin 3A) to activate Rho GTPase and its downstream effector, Rho-associated protein kinase (ROCK) [21]. Together, these result in growth cone collapse and potent inhibition of regeneration [20].
In addition to reforming neural circuits, myelination is an important component of regeneration. Demyelination of axons by oligodendroglial apoptosis along with minimal oligodendrocyte precursor cell (OPC) proliferation after injury contribute to poor functional recovery. Denuded axons lose rapid saltatory conduction and are particularly vulnerable to nonfunctional electrogenesis [25, 26]. Preserving and regenerating functional, myelinated circuits is key to SCI recovery.
Neuroprotection
Sodium Channel Blockade
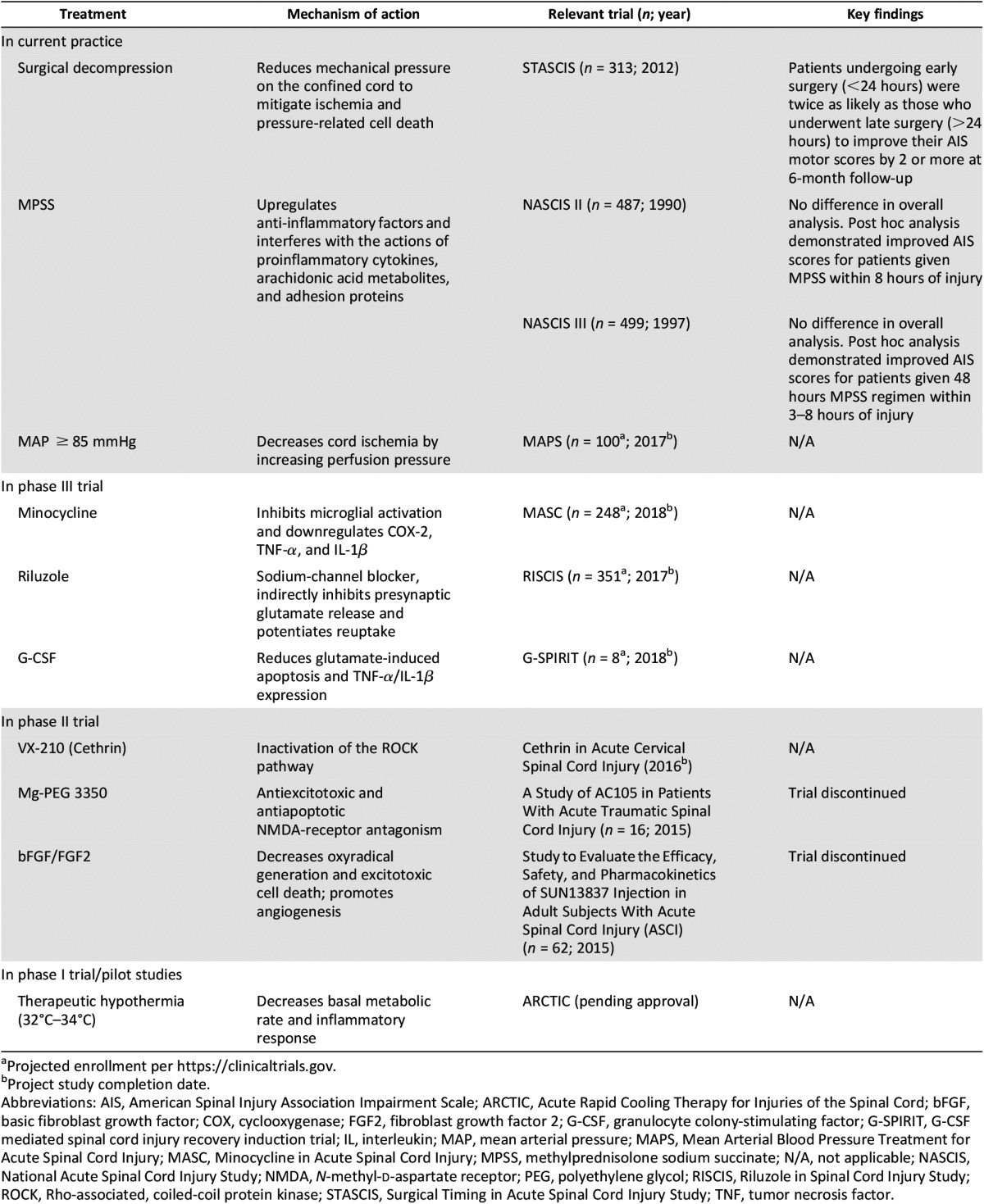
Riluzole is a U.S. Food and Drug Administration (FDA)-approved benzothiazole antiepileptic used in amyotrophic lateral sclerosis (ALS) [27]. Riluzole selectively blocks tetrodotoxin-sodium channels associated with injured neurons (Table 1). It also inhibits presynaptic glutamate release and increases reuptake to potentially mitigate excitotoxicity [28]. Its approval by regulatory bodies and its demonstrated safety in ALS make it a particularly appealing drug for translation in SCI. In preclinical studies, treatment with riluzole has resulted in dramatic sensorimotor improvements functionally and electrophysiologically [29, 30]. A consortium (led by senior author M.F. and including AOSpine, the North American Clinical Trials Network, the Rick Hansen Institute, and the Ontario Neurotrauma Foundation) is conducting a phase II/III randomized controlled trial (NCT01597518) entitled “Riluzole in Spinal Cord Injury Study” (RISCIS) to assess the effects of riluzole using the American Spinal Injury Association (ASIA) Impairment Scale (AIS), Spinal Cord Independence Measure, and brief pain inventory outcomes [31]. The trial is recruiting patients with C4–C8 level injuries and is expected to conclude in December 2018.
Table 1.
Current neuroprotective strategies for spinal cord injury
Anti-Inflammatory Drugs
Minocycline is a CNS-penetrating tetracycline antibiotic that inhibits microglial activation and downregulates proinflammatory cyclooxygenase-2, tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β). Preclinical studies of acute minocycline treatment showed decreased inflammatory cell infiltration, reduced cystic cavitation, and improved behavioral outcomes [32]. A phase II randomized controlled trial (RCT) (n = 52) examining the effects of 7 days of i.v. minocycline versus placebo demonstrated safety, stable cerebrospinal fluid (CSF) drug levels, and a trend toward improved motor scores [33]. These exciting results have led to the phase III Minocycline in Acute Spinal Cord Injury trial (NCT01828203) in which 7 days of i.v. minocycline is compared with placebo in 248 patients. Completion of the trial is expected in 2018 [34].
Methylprednisolone (MPSS) is a synthetic glucocorticoid that acts on cytoplasmic receptors to upregulate anti-inflammatory factors and interfere with the actions of proinflammatory cytokines, arachidonic acid metabolites, and adhesion proteins. In animal models, MPSS has also been shown to mitigate oxidative stress and enhance oligodendrocyte and motor neuron survival [35]. A series of clinical trials and meta-analyses over the last 3 decades have collectively demonstrated improvements in motor scores for patients administered i.v. MPSS within 8 hours of injury [36–38]. Providing MPSS to this subset of patients will be recommended in the upcoming 2016 AOSpine guidelines, developed by an international and interdisciplinary committee of expert physicians, allied health workers, patients, and independent consultants applying the rigorous Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool [39–44].
Therapeutic Hypothermia
Therapeutic hypothermia has been successfully used for neuroprotection in patients after resuscitated cardiac arrest [45] and neonatal hypoxic-ischemia encephalopathy [46]. Hypothermia significantly decreases the basal metabolic rate of the CNS and diminishes the systemic inflammatory response [47]. In SCI, pilot studies of systemic hypothermia have demonstrated that it may show promise as a neuroprotective intervention [48]. The Acute Rapid Cooling Therapy for Injuries of the Spinal Cord (ARCTIC) phase II/III trial, which aims to evaluate the safety and efficacy of cooling within 6 hours of injury, is pending approval [49].
Surgical Decompression
After injury, progressive edema and hemorrhage generate mechanical pressure on the confined spinal cord. Surgical decompression relieves this pressure to mitigate further secondary injury. The Surgical Timing in Acute Spinal Cord Injury (STASCIS) trial, published in 2012, was a prospective observational study of 222 patients undergoing early (<24 hours) versus late (>24 hours) decompression. Patients receiving early surgical intervention were twice as likely to improve by 2 or more AIS grades at 6 months [50]. A prospective Canadian cohort study similarly demonstrated that a significantly greater proportion of patients who underwent early decompression improved by two or more AIS grades [51]. Furthermore, Dvorak et al. reported shorter lengths of hospital stay after early decompression for patients with ASIA A (complete) or ASIA B (complete motor; incomplete sensory) injuries [52]. Early decompression in acute SCI is now a widely adopted practice that we strongly advocate.
Other Neuroprotective Strategies
Numerous other neuroprotective approaches have been translated into recently concluded or ongoing clinical trials. Granulocyte colony-stimulating factor (G-CSF) is a signaling glycoprotein that has been shown to enhance the survival of ischemic CNS cells, protect against glutamate-induced apoptosis, and reduce TNF-α and IL-1β expression in vivo [53, 54]. Two phase I/IIa nonrandomized trials have demonstrated improved AIS scores after G-CSF administration, without significant adverse events [55, 56]. A phase III RCT of i.v. G-CSF, the G-CSF-Mediated Spinal Cord Injury Recovery Induction Trial (G-SPIRIT; n = 88) is recruiting patients with acute cervical SCI in Japan. The study began in May 2015 and is expected to conclude in 2018.
Vascular compression, intraluminal thrombosis, loss of autoregulation, and system hypotension contribute to ongoing postinjury cord ischemia. Trials of blood pressure augmentation to reduce ischemia have demonstrated improved ASIA grade outcomes for patients with mean arterial pressures (MAPs) held above 85–90 mmHg [57]. The American Association of Neurological Surgeons and Congress of Neurological Surgeons provide level III recommendations to maintain MAP for 7 days after injury [58]. Building on this, the Canadian Multicentre CSF Monitoring and Biomarker Study (CAMPER; NCT01279811) is recruiting participants to assess the effects of cord perfusion pressure (MAP minus intrathecal CSF pressure) on AIS scores and neuropathic pain inventories. CAMPER will also provide insight into the temporal profiles and prognostic value of CSF biomarkers after SCI [34].
Magnesium is an N-methyl-d-aspartate receptor receptor antagonist with antiexcitotoxic and antiapoptotic properties successfully used in the neuroprotection of animals after traumatic brain injury, myocardial infarction, and SCI [53–56, 59]. Sustaining sufficiently high CSF levels of Mg requires an excipient such as polyethylene glycol (PEG). A phase I/II placebo-controlled RCT (n = 40) of AC105 (Acorda Therapeutics, Ardsley, NY, http://www.acorda.com) was started but subsequently discontinued [34].
Basic fibroblast growth factor (bFGF) is a heparin-binding protein involved with wound healing, angiogenesis, embryogenesis, and the in vitro maintenance of stem cell pluripotency. It has also been shown to decrease oxyradical generation and excitotoxic cell death in preclinical models of neurodegenerative diseases and SCI [60]. A phase I/II RCT (n = 164) of an FGF analog, SUN13837 (Asubio Pharmaceuticals, Edison, NJ, http://www.asubio.co.jp), was discontinued early [34].
Neuroregeneration
The Glial Scar
CSPGs in the Glial Scar
Chondroitinase ABC (ChABC) is a bacterial enzyme shown to effectively degrade CSPGs, including NG2, promoting functional gains in mouse models after intrathecal administration using an osmotic minipump [61, 62]. Evidence also shows that coadministration of ChABC with neural precursor cells enhances transplant survival and remyelination of host axons [63, 64]. More recently, large-scale CSPG digestion by direct lentiviral ChABC gene delivery into rat spinal cords demonstrated reduced cavitation volume and enhanced axon preservation. Treated rats also displayed improved sensorimotor function on behavioral and electrophysiological assessments [65]. ChABC is an exciting therapy for which the optimal modality for delivery remains to be elucidated. Future avenues of research may include exploration of human CNS-specific analogs of ChABC and development of novel, safe, and effective delivery techniques.
Anti-NOGO/RhoA-ROCK
Another promising field of study is the NOGO-A/RhoA-Rock pathway. Neurite outgrowth inhibitor A (NOGO-A) is an integral membrane protein in oligodendrocytes that binds NgR. NgR phosphorylates the small GTPase RhoA, which subsequently activates ROCK to inhibit neurite outgrowth and collapse the growth cone [66]. Blocking the function of myelin protein NOGO-A with NOGO-receptor antagonists, anti-NOGO-A antibodies, or inhibition of downstream RhoA-ROCK has been shown to enhance neurite growth and axonal regeneration in animal studies [67–69]. A phase II clinical trial of a monoclonal NOGO-A antibody is now under way in ALS, the results of which may portend a trial in SCI [70]. Furthermore, VX-210 (Cethrin; BioAxone BioSciences, Cambridge, MA, http://bioaxonebio.com) is a Rho GTPase antagonist that demonstrated significant motor improvement and no safety concerns in a phase I/IIa trial (NCT00500812) in SCI [71]. A phase IIb trial, supported by Vertex Pharmaceuticals (Boston, MA, http://www.vrtx.com), is expected to begin in the near future to further quantify efficacy and safety. The results of these trials will be important in determining the course of investigation of these pathways as therapeutic approaches for SCI.
Cell-Based Approaches
Cell therapies using pluripotent sources are an exciting strategy based on the grafts’ ability to be pro-oligodendrogliogenic [72, 73], pro-neuronogenic [74], immunomodulatory [75, 76], and/or antigliotic [77]. Furthermore, transplanted cells may be capable of modifying the microenvironment and regenerating/remyelinating damaged circuits. However, to successfully use these strategies, we must optimize the cell source, differentiation protocols, and graft survival.
Stem Cell Inc. (Newark, CA, http://www.stemcellsinc.com) is conducting an international phase I/II clinical trial (NCT02163876) of human CNS stem cell injections for cervical SCI that is expected to conclude in 2017. Ongoing follow-up for a similar phase I/II thoracic injury trial (NCT01321333) has demonstrated patient improvement in multiple sensory modalities with no safety concerns thus far [78]. Neuralstem (Germantown, MD, http://www.neuralstem.com) began a phase I safety trial (NCT01772810) at the University of California San Diego of NSI-566 neural stem cell transplants for chronic thoracic SCI in 2014, with expected completion in February 2016. These are important clinical proof-of-concept steps in the path to widespread translation of cell therapies.
Cell Sources
Endogenous neural stem cells may be mobilized from local reservoirs, particularly the ependymal layer of the spinal cord central canal [79]. Techniques are being developed to achieve this using growth factor infusions [80], transplanted hydrogels [81], or electrical fields [82]. In parallel, grafts of exogenous human embryonic- and induced pluripotent stem (iPS)-derived cells are being investigated. Human embryonic stem (ES) cells allow consistent differentiation compared with human iPS cells but present ethical challenges, possible karyotypic instability, and are in limited supply [83, 84]. Additionally, the prospect of an autologous pluripotent cell therapy with induced pluripotent stem cell (iPSC) technology is enticing in SCI, where the immune response is always at the forefront. Human iPSC technology offers a highly translatable and potentially unlimited source of pluripotent cells; however, logistical issues surrounding low reprogramming efficiency and insertion mutagenesis exist with viral derivation [85, 86]. Nonviral iPSC generation, such as using the piggyBac transposon, affords an effective and reproducible alternative [87, 88]. iPS technology continues to evolve as potential early senescence and the variable yield of neural progeny of iPS compared with ES cells are investigated [89, 90]. Furthermore, the effect of residual epigenetic memory in DNA methylation and histone modification remains to be fully understood [77, 91].
Several other important cell types are being investigated for SCI, including mesenchymal stem cells (MSCs), olfactory ensheathing cells (OECs), and bone marrow nucleated cells (BMNCs). MSCs are multipotent stromal cells capable of differentiating along connective tissue lineages, allowing them to play a key role in tissue repair [92]. They are also capable of potently modulating the local and systemic immune response [93–95]. In preclinical models, MSCs have been associated with neural tissue sparing, increased levels of prosurvival trophic factors, and neovascularization [96, 97]. Several phase I and II trials of autologous MSCs, transplanted into the parenchyma or intrathecal space, are ongoing worldwide. Two phase III trials have also been registered (NCT02481440, NCT01676441), with results expected in the next 1–2 years [34]. BMNCs are being studied for their similar supportive properties. They have been shown to positively modulate the microenvironment in SCI, possibly mediated by the small fraction (0.01%–0.001%) of MSCs in BMNCs. A recent study of intraparenchymal and intravenous autologous BMNC administration in children with chronic SCI showed no significant adverse events [98]. Further preclinical and clinical work is required to better understand the mechanism of action of BMNCs.
OECs are glia that ensheathe olfactory neurons in a manner similar to the way Schwann cells ensheathe peripheral neurons. They support exposed olfactory cells in the nasal mucosa by phagocytosing bacteria and debris, secreting neurotrophic factors, and facilitating axon regeneration after loss [99, 100]. OECs are harvested from the olfactory bulb or mucosa and, when transplanted into the injured cord, promote remyelination, axonal regeneration, and improve behavioral outcomes [101]. At least 10 studies of patients with chronic SCI treated with OECs have been described (cumulative n = 1,193). A recent meta-analysis found no significant increase in adverse events after OEC transplant; however, higher-quality studies are still required to define efficacy [102].
Remyelinating the Injured Cord
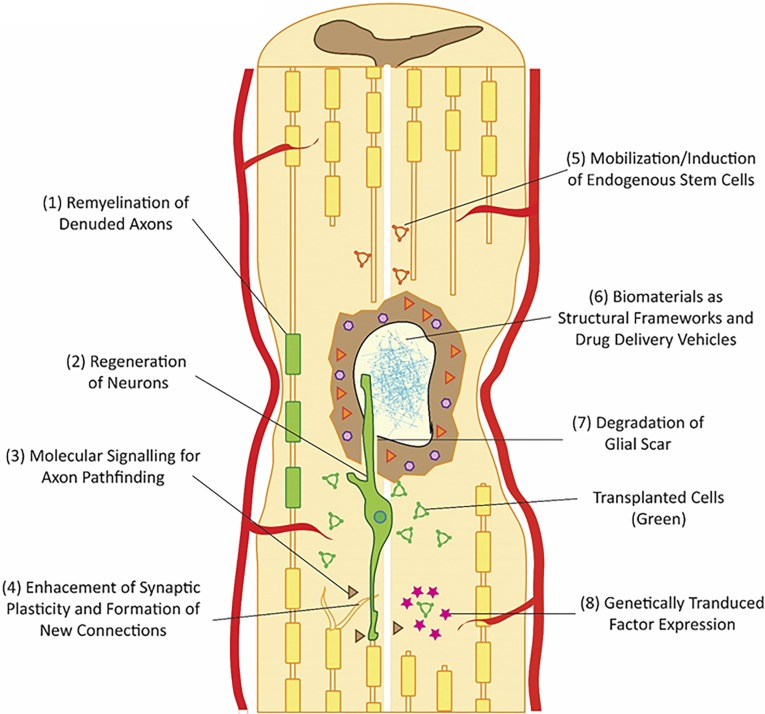
Oligodendrocytes are particularly vulnerable to traumatic injury, leaving behind demyelinated, dysfunctional axons. Exogenous intraparenchymal injections of neural precursor cells (NPCs) and OPCs have been shown to produce well-differentiated, myelinating oligodendrocytes in vivo. Moreover, rodents transplanted with human OPCs and NPCs weeks after SCI have shown significant functional recovery [103, 104]. However, differentiation protocols can be complex and provide heterogeneous results. Evolving differentiation protocols include Noggin (bone morphogenetic protein inhibition), SB431542 (downstream Smad inhibition), and Sonic hedgehog [105]. Protocol refinement and better molecular characterization of the cells being generated are critical advancements on the path to definitive translation (Fig. 2).
Figure 2.
Schematic highlighting promising primary and adjunctive neuroregenerative strategies in spinal cord injury. Transplanted auto/allogenic cells can be differentiated to (1) oligodendrocytes to remyelinate denuded axons or (2) neurons for restoration of functional neural circuits. (3) Promoting this is likely to require molecular signaling techniques for axon guidance. (4) Enhancement of synaptic plasticity with formation of new connections may be a key mechanism of recovery. (5) Another important approach is induction of endogenous neural stem cells, particularly from the central canal. (6) The disruption of the structural architecture of the cord can be overcome by cotransplants of biomaterials that can form a framework for growth. (7) The glial scar barrier can be degraded by enzymes such as chondroitinase ABC. (8) Finally, transplanted cells can be genetically modified to secrete prosurvival, promigration, or other important factors.
Axon Regeneration
Human neural stem cells have shown mature neuronal differentiation in animal models with long-distance axonal growth and integrated connectivity with the host CNS [106]. Host axons have been shown to reciprocally connect with transplanted neural stem cells, creating relay circuits that can potentially bridge disrupted tracts [107, 108]. Emerging in vitro and in vivo protocols for generating direct induced and transpluripotent pathway mature neurons hold the potential to rebuild host circuits [109, 110]. However, mechanisms to direct axonal growth and synapse development in functionally targeted areas are lacking. This represents a critical barrier to recovery. Axon pathfinding strategies have predominantly focused on the role of chemotactic and adhesive cues in guiding the neuronal growth cone [111]. In vitro and in vivo studies demonstrating the importance of cell adhesion molecules, including nerve growth factor-inducible large external glycoprotein [112], neural cell adhesion molecule [112, 113], transient axonal glycoprotein-1 (TAG-1) [114], calcium-dependent cadherins [115], semaphorin 3A [23, 116], and netrin [117] have advanced our understanding of embryonic development of the CNS [111]. Further discovery and exploitation of the underlying molecular pathways may yield potent adjunctive methods of generating functional neuronal circuits through chemotactic signaling of transplanted cells (Fig. 2).
Improving Cell Survival
Transplanted xenograft cells have poor survival rates in animal SCI models. Continued progress in the field will require improvements in cell survival by modifying cells, the injured cord milieu, and the host animals. Growth factors (platelet-derived growth factor, FGF2, and epidermal growth factor) and anti-inflammatory agents (minocycline) have been shown to improve cell survival but can be logistically challenging in animal models (e.g., osmotic minipumps) [118, 119]. Alternate interventions to increase growth factor levels or decrease the immune response will be required to continue improving cell survival. One approach is the genetic modification of grafted cells to inducibly express the necessary proteins. Fibroblasts and mesenchymal stem cells have been successfully modified to secrete bFGF [120], hepatocyte growth factor [121], NT3 [122, 123], brain-derived neurotrophic factor (BDNF) [122, 124], and glial cell-derived neurotrophic factor (GDNF) [125, 126] in vivo. Safe and efficient methods of transducing human ES/iPS cells in a similar fashion are being studied (Fig. 2).
Other strategies of interest are the development of bioengineered cell transplant vehicles to gradually deliver signaling proteins either spontaneously or after an exogenous stimulus. This has been achieved with success with fibrin matrices [106], hyaluronan/methylcellulose [127], and other bioengineered materials. Growth factor-secreting hydrogels have been shown to decrease cavity volume and improve behavioral measures after injury. Furthermore, hydrogels can be used to mitigate immunorejection of transplanted cells through encapsulation to form a temporary physical barrier to the immune response [128, 129].
An often overlooked but critical additional method is the mobilization of endogenous growth factors through noninvasive interventions such as physical rehabilitation. While rehabilitation is an integral component of the care provided to patients with SCI, it is often overlooked in preclinical trials. Host animals that undergo treadmill locomotor training postinjury show significantly enhanced NPC survival mediated by insulin-like growth factor-1 signaling [130]. This finding highlights the necessity of adjunctive therapies in SCI and underscores the importance of reciprocal knowledge exchange between the clinical and preclinical worlds.
Biomaterials
Drug-Eluting Hydrogels and Self-Assembling Peptides
Transplantable hydrogel polymers are an attractive medium to fill cavitation defects. Their porous construction allows cell migration and nutrient diffusion [131]. Hydrogels can also function as cell-delivery vehicles to improve graft survival and migration [132, 133]. Furthermore, engineered needle-injectable hydrogels can promote cell differentiation and form a barrier against the immune response. Multiple biomaterial substrates have been evaluated in SCI, including agarose [134, 135], collagen [136], hyaluronan/methylcellulose [137], fibrin [138], and alginate [139], all of which have shown promising results in supporting regeneration. Further modification to integrate growth factors [140] or immunomodulatory drugs [141] provides additional high-yield combinatorial opportunities for exploration (Fig. 2). This has been successfully performed for BDNF [142], NT-3 [143], and GDNF [139].
Synthetic self-assembling peptide (SAP) hydrogels are a unique class of engineered proteins that can associate into highly stable organized tissue scaffolds in situ [144, 145]. While liquid at ambient temperature, when exposed to the mammalian body, they begin to assemble into a biocompatible nanofiber structure similar to native extracellular matrix [146]. SAPs grafted into injured cords have demonstrated reduced astrogliosis and cell death with enhanced axonal regeneration [147]. Furthermore, cotransplants with neural stem cells have been shown to promote injury repair and functional recovery of the forelimbs in cervical SCI [148]. The technology behind drug-eluting hydrogels and SAPs is rapidly evolving and we foresee this becoming an increasingly important adjunct to cell-based therapies moving forward.
Future Directions
The multifactorial nature of SCI and neural repair necessitates a combinatorial approach if we are to translate experimental therapeutics into significant functional gains for patients. While acute neuroprotective interventions are crucial to mitigate secondary injury, therapeutic neuroregenerative approaches are required to help the millions of patients living with chronic postinjury disability. Pluripotent cell-based therapies will play a central role but require further advancements in genetic engineering, biomaterials, and a deeper understanding of SCI at a molecular level. Furthermore, the development of less-toxic immunosuppressive drugs or consistent methods of generating autologous iPS cells is an important milestone on the path to translation. Careful targeting of these treatment strategies to individual subsets of patients is an important avenue of further investigation requiring a better understanding of injury heterogeneity. In defining these groups, a critical need exists for validated biomarkers of injury severity and recovery trajectory through magnetic resonance imaging [149] and serum/CSF biochemistry [150].
Successful future therapies will require these and other synergistic approaches to address the persistent barriers to regeneration, including the glial scar, the loss of structural framework, and immunorejection. Ongoing clinical trials underscore the excitement and progress we have made in investigating therapeutic approaches to SCI and highlight the importance of the work being done by thousands of scientists in regenerative medicine.
Acknowledgments
M.F. is supported by the Gerald and Tootsie Halbert Chair in Neural Repair and Regeneration and the DeZwirek Family Foundation.
Author Contributions
C.S.A.: manuscript writing; M.F.: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
M.F. is an investigator in the Stem Cells Inc. phase I/II clinical trial (NCT02163876) of human CNS stem cell injections for cervical SCI and the lead investigator in the upcoming phase IIb trial, supported by Vertex Pharmaceuticals Inc. The other author indicated no potential conflicts of interest.
References
- 1.Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2014;37:117–118. doi: 10.1179/1079026813Z.000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christopher and Dana Reeve Foundation. One degree of separation: Paralysis and spinal cord injury in the United States. 2010. Available at http://www.christopherreeve.org/site/c.ddJFKRNoFiG/b.5091685/k.58BD/One_Degree_of_Separation.htm. Accessed March 31, 2016.
- 3.LaPlaca MC, Simon CM, Prado GR, et al. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- 4.Choo AM, Liu J, Lam CK, et al. Contusion, dislocation, and distraction: Primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J Neurosurg Spine. 2007;6:255–266. doi: 10.3171/spi.2007.6.3.255. [DOI] [PubMed] [Google Scholar]
- 5.Whetstone WD, Hsu JY, Eisenberg M, et al. Blood-spinal cord barrier after spinal cord injury: Relation to revascularization and wound healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mautes AE, Weinzierl MR, Donovan F, et al. Vascular events after spinal cord injury: Contribution to secondary pathogenesis. Phys Ther. 2000;80:673–687. [PubMed] [Google Scholar]
- 7.Waxman SG. Demyelination in spinal cord injury. J Neurol Sci. 1989;91:1–14. doi: 10.1016/0022-510x(89)90072-5. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Mealing GA, Morley P, et al. Novel injury mechanism in anoxia and trauma of spinal cord white matter: Glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19:RC16. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–1198. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guha A, Tator CH, Rochon J. Spinal cord blood flow and systemic blood pressure after experimental spinal cord injury in rats. Stroke. 1989;20:372–377. doi: 10.1161/01.str.20.3.372. [DOI] [PubMed] [Google Scholar]
- 11.Guha A, Tator CH. Acute cardiovascular effects of experimental spinal cord injury. J Trauma. 1988;28:481–490. doi: 10.1097/00005373-198804000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Milhorat TH, Capocelli AL, Jr, Anzil AP, et al. Pathological basis of spinal cord cavitation in syringomyelia: Analysis of 105 autopsy cases. J Neurosurg. 1995;82:802–812. doi: 10.3171/jns.1995.82.5.0802. [DOI] [PubMed] [Google Scholar]
- 13.Yuan YM, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. 2013;29:421–435. doi: 10.1007/s12264-013-1358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snow DM, Lemmon V, Carrino DA, et al. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 15.Höke A, Silver J. Proteoglycans and other repulsive molecules in glial boundaries during development and regeneration of the nervous system. Prog Brain Res. 1996;108:149–163. doi: 10.1016/s0079-6123(08)62538-8. [DOI] [PubMed] [Google Scholar]
- 16.Becker T, Anliker B, Becker CG, et al. Tenascin-R inhibits regrowth of optic fibers in vitro and persists in the optic nerve of mice after injury. Glia. 2000;29:330–346. doi: 10.1002/(sici)1098-1136(20000215)29:4<330::aid-glia4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Silver J. Inhibitory molecules in development and regeneration. J Neurol. 1994;242(suppl 1):S22–S24. doi: 10.1007/BF00939236. [DOI] [PubMed] [Google Scholar]
- 18.Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- 19.Jones LL, Yamaguchi Y, Stallcup WB, et al. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeon RJ, Schreiber RC, Rudge JS, et al. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forgione N, Fehlings MG. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014;82:e535–e539. doi: 10.1016/j.wneu.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Chen MS, Huber AB, van der Haar ME, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 23.Moreau-Fauvarque C, Kumanogoh A, Camand E, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cafferty WB, Duffy P, Huebner E, et al. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: With an emphasis on the role of voltage-gated potassium channels. Brain Res Brain Res Rev. 2001;38:165–191. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 26.Nashmi R, Fehlings MG. Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience. 2001;104:235–251. doi: 10.1016/s0306-4522(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt JM, Gordon PH. Current clinical trials in amyotrophic lateral sclerosis. Expert Opin Investig Drugs. 2007;16:1197–1207. doi: 10.1517/13543784.16.8.1197. [DOI] [PubMed] [Google Scholar]
- 28.Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000;871:175–180. doi: 10.1016/s0006-8993(00)02430-6. [DOI] [PubMed] [Google Scholar]
- 29.Nógrádi A, Szabó A, Pintér S, et al. Delayed riluzole treatment is able to rescue injured rat spinal motoneurons. Neuroscience. 2007;144:431–438. doi: 10.1016/j.neuroscience.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 30.Stutzmann JM, Pratt J, Boraud T, et al. The effect of riluzole on post-traumatic spinal cord injury in the rat. Neuroreport. 1996;7:387–392. doi: 10.1097/00001756-199601310-00003. [DOI] [PubMed] [Google Scholar]
- 31.Fehlings MG, Wilson JR, Frankowski RF, et al. Riluzole for the treatment of acute traumatic spinal cord injury: Rationale for and design of the NACTN Phase I clinical trial. J Neurosurg Spine. 2012;17(1 suppl):151–156. doi: 10.3171/2012.4.AOSPINE1259. [DOI] [PubMed] [Google Scholar]
- 32.Wells JE, Hurlbert RJ, Fehlings MG, et al. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 33.Casha S, Zygun D, McGowan MD, et al. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135:1224–1236. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 34.Minocycline in acute spinal cord injury (MASC), NCT identifier: NCT01828203. Available at https://clinicaltrials.gov/ct2/show/NCT01828203?term=NCT01828203&rank=1. Accessed July 21, 2015.
- 35.Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(suppl 1):36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 36.Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- 37.Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 38.Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 39.Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–1051. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guyatt GH, Oxman AD, Kunz R, et al. Incorporating considerations of resources use into grading recommendations. BMJ. 2008;336:1170–1173. doi: 10.1136/bmj.39504.506319.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. doi: 10.1136/bmj.a744. [DOI] [PubMed] [Google Scholar]
- 44.Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 46.Papile LA, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146–1150. doi: 10.1542/peds.2014-0899. [DOI] [PubMed] [Google Scholar]
- 47.Kwon BK, Mann C, Sohn HM, et al. Hypothermia for spinal cord injury. Spine J. 2008;8:859–874. doi: 10.1016/j.spinee.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26:407–415. doi: 10.1089/neu.2008.0745. [DOI] [PubMed] [Google Scholar]
- 49.The Miami Project to Cure Paralysis. Neuroprotection—therapeutic hypothermia. 2014. Available at http://www.themiamiproject.org/research/what-are-clinical-trials/clinical-trials/therapeutic-hypothermia-acute/. Accessed October 15, 2015.
- 50.Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson JR, Singh A, Craven C, et al. Early versus late surgery for traumatic spinal cord injury: Rhe results of a prospective Canadian cohort study. Spinal Cord. 2012;50:840–843. doi: 10.1038/sc.2012.59. [DOI] [PubMed] [Google Scholar]
- 52.Dvorak MFNV, Noonan VK, Fallah N, et al. The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: an observational Canadian cohort study. J Neurotrauma. 2015;32:645–654. doi: 10.1089/neu.2014.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallner S, Peters S, Pitzer C, et al. The granulocyte-colony stimulating factor has a dual role in neuronal and vascular plasticity. Front Cell Dev Biol. 2015;3:48. doi: 10.3389/fcell.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishio Y, Koda M, Kamada T, et al. Granulocyte colony-stimulating factor attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2007;66:724–731. doi: 10.1097/nen.0b013e3181257176. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi H, Yamazaki M, Okawa A, et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: A phase I/IIa clinical trial. Eur Spine J. 2012;21:2580–2587. doi: 10.1007/s00586-012-2213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamiya K, Koda M, Furuya T, et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: A comparison with high-dose methylprednisolone as a historical control. Eur Spine J. 2015;24:963–967. doi: 10.1007/s00586-014-3373-0. [DOI] [PubMed] [Google Scholar]
- 57.Wilson JR, Forgione N, Fehlings MG. Emerging therapies for acute traumatic spinal cord injury. CMAJ. 2013;185:485–492. doi: 10.1503/cmaj.121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Resnick DK. Updated guidelines for the management of acute cervical spine and spinal cord injury. Neurosurgery. 2013;72(suppl 2):1. doi: 10.1227/NEU.0b013e318276ee7e. [DOI] [PubMed] [Google Scholar]
- 59.Kaptanoglu E, Beskonakli E, Solaroglu I, et al. Magnesium sulfate treatment in experimental spinal cord injury: Emphasis on vascular changes and early clinical results. Neurosurg Rev. 2003;26:283–287. doi: 10.1007/s10143-003-0272-y. [DOI] [PubMed] [Google Scholar]
- 60.Siddiqui AM, Khazaei M, Fehlings MG. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res. 2015;218:15–54. doi: 10.1016/bs.pbr.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Bradbury EJ, Moon LDF, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 62.Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: A balance of permissiveness and inhibition. J Neurosci. 2003;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ikegami T, Nakamura M, Yamane J, et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur J Neurosci. 2005;22:3036–3046. doi: 10.1111/j.1460-9568.2005.04492.x. [DOI] [PubMed] [Google Scholar]
- 64.Carter LM, McMahon SB, Bradbury EJ. Delayed treatment with chondroitinase ABC reverses chronic atrophy of rubrospinal neurons following spinal cord injury. Exp Neurol. 2011;228:149–156. doi: 10.1016/j.expneurol.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 65.Bartus K, James ND, Didangelos A, et al. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34:4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niederöst B, Oertle T, Fritsche J, et al. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freund P, Schmidlin E, Wannier T, et al. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 68.Gonzenbach RR, Schwab ME. Disinhibition of neurite growth to repair the injured adult CNS: Focusing on Nogo. Cell Mol Life Sci. 2008;65:161–176. doi: 10.1007/s00018-007-7170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liebscher T, Schnell L, Schnell D, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 70.Meininger V, Pradat P-F, Corse A, et al. Safety, pharmacokinetic, and functional effects of the Nogo-a monoclonal antibody in amyotrophic lateral sclerosis: A randomized, first-in-human clinical trial. PLoS One. 2014;9:e97803. doi: 10.1371/journal.pone.0097803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fehlings MG, Theodore N, Harrop J, et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma. 2011;28:787–796. doi: 10.1089/neu.2011.1765. [DOI] [PubMed] [Google Scholar]
- 72.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arriola A, Kiel ME, Shi Y, et al. Adjunctive MSCs enhance myelin formation by xenogenic oligodendrocyte precursors transplanted in the retina. Cell Res. 2010;20:728–731. doi: 10.1038/cr.2010.63. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Wang B, Xiao Z, et al. Olfactory ensheathing cells promote proliferation and inhibit neuronal differentiation of neural progenitor cells through activation of Notch signaling. Neuroscience. 2008;153:406–413. doi: 10.1016/j.neuroscience.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 75.Wang L, Shi J, van Ginkel FW, et al. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp Neurol. 2009;216:177–183. doi: 10.1016/j.expneurol.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 76.Okamura RM, Lebkowski J, Au M, et al. Immunological properties of human embryonic stem cell-derived oligodendrocyte progenitor cells. J Neuroimmunol. 2007;192:134–144. doi: 10.1016/j.jneuroim.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 77.Jäderstad J, Jäderstad LM, Li J, et al. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc Natl Acad Sci USA. 2010;107:5184–5189. doi: 10.1073/pnas.0915134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curt ACS, Fehlings M, Huhn S. Phase I/II clinical trial of HuCNS-SC cells in chronic thoracic spinal cord injury—interim analysis. 2014. http://www.stemcellsinc.com/Presentations/ASIA_FINAL.pdf. Accessed October 15, 2015.
- 79.Hawryluk GW, Fehlings MG. The center of the spinal cord may be central to its repair. Cell Stem Cell. 2008;3:230–232. doi: 10.1016/j.stem.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 80.Martens DJ, Seaberg RM, van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16:1045–1057. doi: 10.1046/j.1460-9568.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- 81.Espinosa-Jeffrey A, Oregel K, Wiggins L, et al. Strategies for endogenous spinal cord repair: HPMA hydrogel to recruit migrating endogenous stem cells. Adv Exp Med Biol. 2012;760:25–52. doi: 10.1007/978-1-4614-4090-1_3. [DOI] [PubMed] [Google Scholar]
- 82.Babona-Pilipos R, Popovic MR, Morshead CM. A galvanotaxis assay for analysis of neural precursor cell migration kinetics in an externally applied direct current electric field. J Vis Exp. 2012 doi: 10.3791/4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Draper JS, Moore HD, Ruban LN, et al. Culture and characterization of human embryonic stem cells. Stem Cells Dev. 2004;13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- 84.Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 85.Brambrink T, Foreman R, Welstead GG, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salewski RP, Eftekharpour E, Fehlings MG. Are induced pluripotent stem cells the future of cell-based regenerative therapies for spinal cord injury? J Cell Physiol. 2010;222:515–521. doi: 10.1002/jcp.21995. [DOI] [PubMed] [Google Scholar]
- 89.Hu BY, Weick JP, Yu J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng Q, Lu S-J, Klimanskaya I, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 91.Vaskova EA, Stekleneva AE, Medvedev SP, et al. “Epigenetic memory” phenomenon in induced pluripotent stem cells. Acta Naturae. 2013;5:15–21. [PMC free article] [PubMed] [Google Scholar]
- 92.Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J Stem Cells. 2014;6:120–133. doi: 10.4252/wjsc.v6.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swartzlander MD, Blakney AK, Amer LD, et al. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials. 2015;41:79–88. doi: 10.1016/j.biomaterials.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bessout R, Sémont A, Demarquay C, et al. Mesenchymal stem cell therapy induces glucocorticoid synthesis in colonic mucosa and suppresses radiation-activated T cells: New insights into MSC immunomodulation. Mucosal Immunol. 2014;7:656–669. doi: 10.1038/mi.2013.85. [DOI] [PubMed] [Google Scholar]
- 95.Lim JH, Kim JS, Yoon IH, et al. Immunomodulation of delayed-type hypersensitivity responses by mesenchymal stem cells is associated with bystander T cell apoptosis in the draining lymph node. J Immunol. 2010;185:4022–4029. doi: 10.4049/jimmunol.0902723. [DOI] [PubMed] [Google Scholar]
- 96.Quertainmont R, Cantinieaux D, Botman O, et al. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One. 2012;7:e39500. doi: 10.1371/journal.pone.0039500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim JW, Ha KY, Molon JN, et al. Bone marrow-derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats: comparative study between intralesional and intravenous transplantation. Spine. 2013;38:E1065–E1074. doi: 10.1097/BRS.0b013e31829839fa. [DOI] [PubMed] [Google Scholar]
- 98.Jarocha D, Milczarek O, Kawecki Z, et al. Preliminary study of autologous bone marrow nucleated cells transplantation in children with spinal cord injury. Stem Cells Translational Medicine. 2014;3:395–404. doi: 10.5966/sctm.2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Windus LC, Lineburg KE, Scott SE, et al. Lamellipodia mediate the heterogeneity of central olfactory ensheathing cell interactions. Cell Mol Life Sci. 2010;67:1735–1750. doi: 10.1007/s00018-010-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Silva NA, Cooke MJ, Tam RY, et al. The effects of peptide modified gellan gum and olfactory ensheathing glia cells on neural stem/progenitor cell fate. Biomaterials. 2012;33:6345–6354. doi: 10.1016/j.biomaterials.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 101.Liu J, Chen P, Wang Q, et al. Meta analysis of olfactory ensheathing cell transplantation promoting functional recovery of motor nerves in rats with complete spinal cord transection. Neural Regen Res. 2014;9:1850–1858. doi: 10.4103/1673-5374.143434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li L, Adnan H, Xu B, et al. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: A systematic review and meta-analysis. Eur Spine J. 2015;24:919–930. doi: 10.1007/s00586-014-3416-6. [DOI] [PubMed] [Google Scholar]
- 103.Nistor GI, Totoiu MO, Haque N, et al. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 104.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gil J-E, Woo D-H, Shim J-H, et al. Vitronectin promotes oligodendrocyte differentiation during neurogenesis of human embryonic stem cells. FEBS Lett. 2009;583:561–567. doi: 10.1016/j.febslet.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 106.Lu P, Woodruff G, Wang Y, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bregman BS, Kunkel-Bagden E, Reier PJ, et al. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol. 1993;123:3–16. doi: 10.1006/exnr.1993.1136. [DOI] [PubMed] [Google Scholar]
- 108.Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: A neuroanatomical tracing study of local interactions. J Comp Neurol. 1991;307:311–334. doi: 10.1002/cne.903070211. [DOI] [PubMed] [Google Scholar]
- 109.Torper O, Pfisterer U, Wolf DA, et al. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci USA. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu B-Y, Zhang S-C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol. 2010;2:a001933. doi: 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van den Pol AN, Kim WT. NILE/L1 and NCAM-polysialic acid expression on growing axons of isolated neurons. J Comp Neurol. 1993;332:237–257. doi: 10.1002/cne.903320208. [DOI] [PubMed] [Google Scholar]
- 113.Zhang S, Xia YY, Lim HC, et al. NCAM-mediated locomotor recovery from spinal cord contusion injury involves neuroprotection, axon regeneration, and synaptogenesis. Neurochem Int. 2010;56:919–929. doi: 10.1016/j.neuint.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 114.Wolman MA, Sittaramane VK, Essner JJ, et al. Transient axonal glycoprotein-1 (TAG-1) and laminin-alpha1 regulate dynamic growth cone behaviors and initial axon direction in vivo. Neural Dev. 2008;3:6. doi: 10.1186/1749-8104-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blackmore M, Letourneau PC. L1, beta1 integrin, and cadherins mediate axonal regeneration in the embryonic spinal cord. J Neurobiol. 2006;66:1564–1583. doi: 10.1002/neu.20311. [DOI] [PubMed] [Google Scholar]
- 116.Zhang L, Kaneko S, Kikuchi K, et al. Rewiring of regenerated axons by combining treadmill training with semaphorin3A inhibition. Mol Brain. 2014;7:14. doi: 10.1186/1756-6606-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kennedy TE, Wang H, Marshall W, et al. Axon guidance by diffusible chemoattractants: A gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, et al. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, et al. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu WG, Wang ZY, Huang ZS. Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res. 2011;33:686–693. doi: 10.1179/1743132810Y.0000000031. [DOI] [PubMed] [Google Scholar]
- 121.Jeong SR, Kwon MJ, Lee HG, et al. Hepatocyte growth factor reduces astrocytic scar formation and promotes axonal growth beyond glial scars after spinal cord injury. Exp Neurol. 2012;233:312–322. doi: 10.1016/j.expneurol.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 122.Tobias CA, Shumsky JS, Shibata M, et al. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol. 2003;184:97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- 123.Zhang YJ, Zhang W, Lin CG, et al. Neurotrophin-3 gene modified mesenchymal stem cells promote remyelination and functional recovery in the demyelinated spinal cord of rats. J Neurol Sci. 2012;313:64–74. doi: 10.1016/j.jns.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 124.Sasaki M, Radtke C, Tan AM, et al. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol. 2003;467:403–417. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- 126.Rooney GE, McMahon SS, Ritter T, et al. Neurotrophic factor-expressing mesenchymal stem cells survive transplantation into the contused spinal cord without differentiating into neural cells. Tissue Eng Part A. 2009;15:3049–3059. doi: 10.1089/ten.TEA.2009.0045. [DOI] [PubMed] [Google Scholar]
- 127.Vulic K, Shoichet MS. Tunable growth factor delivery from injectable hydrogels for tissue engineering. J Am Chem Soc. 2012;134:882–885. doi: 10.1021/ja210638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mayfield AE, Tilokee EL, Latham N, et al. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35:133–142. doi: 10.1016/j.biomaterials.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lippens E, Swennen I, Gironès J, et al. Cell survival and proliferation after encapsulation in a chemically modified Pluronic(R) F127 hydrogel. J Biomater Appl. 2013;27:828–839. doi: 10.1177/0885328211427774. [DOI] [PubMed] [Google Scholar]
- 130.Hwang DH, Shin HY, Kwon MJ, et al. Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin-like growth factor-1 signaling. J Neurosci. 2014;34:12788–12800. doi: 10.1523/JNEUROSCI.5359-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 132.Itosaka H, Kuroda S, Shichinohe H, et al. Fibrin matrix provides a suitable scaffold for bone marrow stromal cells transplanted into injured spinal cord: A novel material for CNS tissue engineering. Neuropathology. 2009;29:248–257. doi: 10.1111/j.1440-1789.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- 133.Teng YD, Lavik EB, Qu X, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stokols S, Tuszynski MH. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27:443–451. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 135.Gros T, Sakamoto JS, Blesch A, et al. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials. 2010;31:6719–6729. doi: 10.1016/j.biomaterials.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 136.Tsai EC, Dalton PD, Shoichet MS, et al. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials. 2006;27:519–533. doi: 10.1016/j.biomaterials.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 137.Mothe AJ, Tam RY, Zahir T, et al. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34:3775–3783. doi: 10.1016/j.biomaterials.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 138.Taylor SJ, McDonald JW, 3rd, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98:281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 139.Ansorena E, De Berdt P, Ucakar B, et al. Injectable alginate hydrogel loaded with GDNF promotes functional recovery in a hemisection model of spinal cord injury. Int J Pharm. 2013;455:148–158. doi: 10.1016/j.ijpharm.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 140.Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477–489. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 141.Leipzig ND, Xu C, Zahir T, et al. Functional immobilization of interferon-gamma induces neuronal differentiation of neural stem cells. J Biomed Mater Res A. 2010;93:625–633. doi: 10.1002/jbm.a.32573. [DOI] [PubMed] [Google Scholar]
- 142.Jain A, Kim YT, McKeon RJ, et al. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27:497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 143.Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113:226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Caplan MR, Schwartzfarb EM, Zhang S, et al. Effects of systematic variation of amino acid sequence on the mechanical properties of a self-assembling, oligopeptide biomaterial. J Biomater Sci Polym Ed. 2002;13:225–236. doi: 10.1163/156856202320176493. [DOI] [PubMed] [Google Scholar]
- 145.Segers VF, Lee RT. Local delivery of proteins and the use of self-assembling peptides. Drug Discov Today. 2007;12:561–568. doi: 10.1016/j.drudis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 146.Liu Y, Ye H, Satkunendrarajah K, et al. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013;9:8075–8088. doi: 10.1016/j.actbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 147.Tysseling-Mattiace VM, Sahni V, Niece KL, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iwasaki M, Wilcox JT, Nishimura Y, et al. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35:2617–2629. doi: 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 149.Cadotte DW, Fehlings MG. Will imaging biomarkers transform spinal cord injury trials? Lancet Neurol. 2013;12:843–844. doi: 10.1016/S1474-4422(13)70157-1. [DOI] [PubMed] [Google Scholar]
- 150.Pouw MH, Hosman AJ, van Middendorp JJ, et al. Biomarkers in spinal cord injury. Spinal Cord. 2009;47:519–525. doi: 10.1038/sc.2008.176. [DOI] [PubMed] [Google Scholar]