Abstract
Hemichannels are ion channels composed of six connexins (Cxs), and they have the peculiarity to be permeable not only to ions, but also to molecules such as ATP and glutamate. Under physiological conditions they present a low open probability, which is sufficient to enable them to participate in several physiological functions. However, massive and/or prolonged hemichannel opening induces or accelerates cell death. Therefore, the study of the molecular mechanisms that control hemichannel activity appears to be essential for understanding several physiological and pathological processes. Carbon monoxide (CO) is a gaseous transmitter that modulates many cellular processes, some of them through modulation of ion channel activity. CO exerts its biological actions through the activation of guanylate cyclase and/or inducing direct carbonylation of proline, threonine, lysine, and arginine. It is well accepted that guanylate cyclase dependent pathway and direct carbonylation, are not sensitive to reducing agents. However, it is important to point out that CO—through a lipid peroxide dependent process—can also induce a secondary carbonylation in cysteine groups, which is sensitive to reducing agents. Recently, in our laboratory we demonstrated that the application of CO donors to the bath solution inhibited Cx46 hemichannel currents in Xenopus laevis oocytes, a phenomenon that was fully reverted by reducing agents. Therefore, a plausible mechanism of CO-induced Cx46 hemichannel inhibition is through Cx46-lipid oxidation. In this work, I will present current evidence and some preliminary results that support the following hypothesis: Carbon monoxide inhibits Cx46 HCs through a lipid peroxidation-dependent process. The main goal of this paper is to broaden the scientific community interest in studying the relationship between CO-Fatty acids and hemichannels, which will pave the way to more research directed to the understanding of the molecular mechanism(s) that control the opening and closing of hemichannels in both physiological and pathological conditions.
Keywords: hemichannels, connexins, carbon monoxide, lipid peroxides, PUFAs
Introduction
The hypothesis of the present work is: Carbon monoxide modulates connexin function through a lipid peroxidation-dependent process (Figure 1). This hypothesis is supported by the following knowledge and preliminary data.
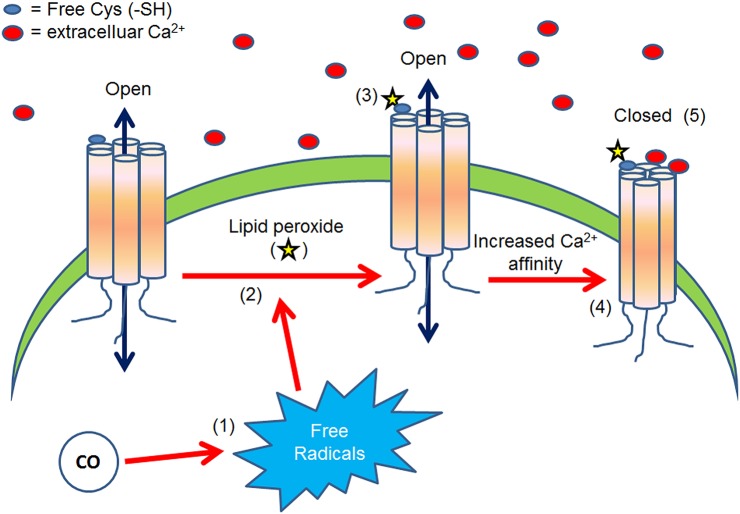
Figure 1.
The above diagram presents what is proposed as a possible molecular mechanism to explain the CO-induced Cx46 inhibition. CO increases free radicals concentration (1) which in turn induces lipid peroxide production (2). Then, lipid peroxides can induce the carbonylation of extracellular cysteine (-SH) (3), increasing Cx46-Ca2+ sensitivity (4) and thus stabilizing the closed state of the loop gating (5).
General characteristics of connexins
Connexins (Cxs) are transmembrane proteins that share a common topology: four transmembrane domains, two extracellular loops, one intracellular loop and the C, and N-termini located both on the cytoplasmic side. Twenty isoforms have been described in mammals (Reviewed in Eiberger et al., 2001), which are named following their expected molecular weight (e.g., Cx26 is expected to have an MW of ~26 kDa). Even when Cx isoforms exhibit considerable homology, the C-terminus is the most variable region which differs in length and number of regulatory sites, which include consensus phosphorylation (Reviewed in Lampe and Lau, 2000), protein-protein interaction (Flores et al., 2008; Reviewed in Hervé et al., 2012) and cleavage sites (Lin et al., 1997). Almost all Cxs (except for Cx23; Lovine et al., 2008) have six conserved extracellular-loop cysteines (Cys), which have been proposed are essential for gap-junction channel (GJC) formation (Dahl et al., 1991). Finally, it is important to note that in mammals, virtually all cell types express at least one Cx type, but there are major differences in expression at the tissue level. Thus, for example, Cx43 is the most ubiquitously expressed (Beyer et al., 1987), whereas Cx46 has been described mostly in the lens (Paul et al., 1991). The widespread expression of Cxs suggests that they are essential for several physiological processes and that, due to their unique properties, they support cellular processes that cannot be replaced by any other Cx type (Wölfle et al., 2007).
Connexin hemichannels
Hemichannels are composed of six Cxs monomers and are permeable to molecules up to ~1.2 kDa. These ion channels participate in several physiological functions, such as spreading of calcium waves (Cotrina et al., 1998), Ca2+ permeation across plasma membrane (Sánchez et al., 2010; Schalper et al., 2010), cellular viability (Bellido and Plotkin, 2010), proliferation (Song et al., 2010), migration (Cotrina et al., 2008), light processing by the retina (Kamermans et al., 2001), mechanotransduction (Romanello et al., 2003), glucose uptake (Retamal et al., 2007), and synaptic plasticity (Stehberg et al., 2012), among others. Most of the hemichannel actions are exerted in part by the release of signaling molecules such as ATP (Stout et al., 2002), cyclic ADP-ribose (cADPR) (Bruzzone et al., 2001), prostaglandin E2 (PGE2) (Cherian et al., 2005), glutamate, and aspartate (Ye et al., 2003) to the extracellular media, where they participate in paracrine/autocrine communication. On the other hand, under pathological conditions, massive and/or prolonged hemichannel opening induce and/or accelerate cell death. Nowadays, hemichannels with a gain of activity are known as “leaky hemichannels,” and these have been observed in neurological disorders such as Charcot-Marie-Tooth disease, metabolic alterations such as ischemia, oculodentodigital dysplasia, skin diseases, inflammatory processes, and deafness (Reviewed in Retamal et al., 2015a). Although the mechanism by which hemichannels induce cell death is not well understood, it is highly probable that loss of metabolites (Stridh et al., 2008), ion gradients and membrane potential, as well as the massive entry of Ca2+ (Sánchez et al., 2010; Schalper et al., 2010) are some of the processes involved. Because hemichannels are important in cellular communication and cell survival, cells have several mechanisms to control hemichannel opening/closing, including phosphorylation (Bao et al., 2004), changes in membrane potential (Trexler et al., 1996; Retamal et al., 2010), alterations in extracellular Ca2+ concentration (Gómez-Hernández et al., 2003; Lopez et al., 2014), changes in redox potential (Retamal et al., 2006, 2009; Reviewed in Retamal, 2014) and presence of unsaturated fatty acids (Retamal et al., 2011). In summary, controlled hemichannel opening enables physiological autocrine/paracrine cell communication, but massive and/or uncontrolled hemichannel opening induces or accelerates cell death. Therefore, the study of the molecular mechanisms that control hemichannel activity is essential in order to understand several physiological and pathological processes.
Hemichannel voltage gating
Currently, it is well accepted that Cx channels are voltage dependent (Trexler et al., 1996; Retamal et al., 2010), although the molecular mechanisms that regulates the hemichannel voltage gating are not yet well understood. But, recently it was established by means of exchange different domains between Cx46 and Cx50 that the N-terminus contains the principal components of the hemichannel voltage sensor and unitary conductance (Kronengold et al., 2012). Additionally, there are two gating mechanisms that enable hemichannels to open and close, which are known as fast and slow/loop-gating (Reviewed in Oh and Bargiello, 2015). The first seems to depend on C-terminus docking to the intracellular loop and results in fast transitions from the open state to various substates (Reviewed in Oh and Bargiello, 2015). In contrast, slow or loop-gating is formed by the interface between the first transmembrane domain (TM1) and the first extracellular loop (EL1; Tang et al., 2009). Their distinctive feature is the slow time constant from fully open to fully closed states (tens to hundreds of milliseconds; Reviewed in Oh and Bargiello, 2015). Large conformational changes reduce the pore diameter from ~20 Å to less than 4 Å (Verselis et al., 2009). It is widely accepted that extracellular divalent cations reduce the open probability of hemichannels in the plasma membrane (Gómez-Hernández et al., 2003; Lopez et al., 2014). Physiological concentrations of Ca2+ have been shown to stabilize the loop-gate closed state of Cx46 hemichannels (Verselis et al., 2009; Reviewed in Oh and Bargiello, 2015). Furthermore, atomic force microscopy studies of Cx26 hemichannels have indicated a narrowing of the extracellular channel entrance with 2 mM Ca2+ (Müller et al., 2002).
Carbon monoxide
There are at least four gaseous transmitters, carbon monoxide (CO), nitric oxide (NO), hydrogen sulfide (H2S; Reviewed in Farrugia and Szurszewski, 2014), and sulfur dioxide (SO2; Chen et al., 2015), which are important modulators of the redox status and redox signaling. Under physiological conditions, CO is produced by two heme oxygenases (HO-1 and HO-2), which catalyze the catabolism of heme groups (Poss and Tonegawa, 1997). Both HO-1 and HO-2 are expressed in several cell types, and while HO-2 is constitutively expressed, HO-1 is inducible by several factors such as hypoxia and inflammation (Reviewed in Wu and Wang, 2005). Under physiological conditions, the human body produces 16.4 μmoles/h (Coburn, 1970), mainly by the action of HO-2 (Reviewed in Wu and Wang, 2005). Once CO is produced, it can be trapped by the hemoglobin, released by expiration (Reviewed in Wu and Wang, 2005) or act as a signaling molecule. In spite of the low concentration (nanomolar) of CO under physiological conditions, it has important roles in normal cardiac function, vascular contractility, platelet aggregation, monocyte activation, hypothalamic-pituitary-adrenal axis, odor response adaptation, nociception and chemoreception, among many other functions (for more details see, Wu and Wang, 2005). Additionally, CO production under physiological conditions can be increased by the induction of HO-1 controlled by physiological signaling molecules, such as transforming growth factor-β (Kutty et al., 1994), platelet-derived growth factor (Durante et al., 1999), and nitric oxide (Durante et al., 1997). Or, it can be decreased by angiotensin II (Ishizaka and Griendling, 1997). On the other hand, under pathological conditions HO-1 can be highly expressed and thus drastically increasing the CO levels (Reviewed in Wu and Wang, 2005). The expression of HO-1 has been implicated in diseases such as atherosclerosis, hypertension, transplant rejection, acute renal injury hyperoxia and hypoxia-induced lung injury, cancer, and neurodegeneration, among others diseases (Deshane et al., 2005; Reviewed in Wu and Wang, 2005). Although CO does not have free electrons as nitric oxide does, it can indirectly increase the oxidative stage of a cell. Thus, at high levels (>1000 ppm), CO increases protein and lipid oxidation (Reviewed in Wu and Wang, 2005), most likely as a result nitric oxide derived molecule production and dysregulation of GSH/GSSG relationship (Reviewed in Wu and Wang, 2005).
CO can act as a signaling molecule through two possible cellular pathways. First, the direct activation of guanylate cyclase, increases cGMP levels, which in turn activate PKG; and secondly, CO acts by direct carbonylation of amino acids, such as proline, threonine, lysine, and arginine (Reviewed in Cattaruzza and Hecker, 2008). However, CO can also induce an indirect carbonylation of cysteine residues through a lipid peroxidation dependent process (Reviewed in Wong et al., 2013). Thom (1990) showed that CO-dependent lipid peroxidation is reduced by the inhibition of xanthine oxidase or superoxide dismutase and iron chelators. Additionally, high concentration of CO was associated with increases in hydroxyl radical production and decreases in the reduced -oxidized glutathione ratio (GSH/GSSG; Lautier et al., 1992; Piantadosi et al., 1995; Reviewed in Wu and Wang, 2005). Therefore, CO can induce an oxidative intracellular environment, which in turn can favor the lipid peroxidation production rate. The process of lipid peroxidation is mediated through both enzymatic and non-enzymatic oxidation of poly unsaturated fatty acids (PUFAs; Reviewed in Higdon et al., 2012). Enzymatic sources of lipid peroxides comprise both COXs (cyclo-oxygenases) that produce PG (prostaglandins) and LOXs (lipoxygenases) that produce leukotrienes (Reviewed in Higdon et al., 2012). On the other hand, non-enzymatic production is mediated by direct oxidation of PUFAs and comprises the production of 4-HNE (4-hydroxynonenal), malondialdehyde (MDA), and acrolein (Reviewed in Higdon et al., 2012). Interestingly, the secondary carbonylation of the Toll-like receptor by 4-HNE can be prevented by reducing agents (Kim et al., 2009), demonstrating that lipid peroxide-induced carbonylation is a dynamic process that can be modulated by the cellular redox status.
Carbon monoxide modulates ion channels
In the early nineteens was described that mice exposure to high levels of CO-gas present degeneration of hippocampal CA1 pyramidal cells by a NMDA-dependent process, measured with hematoxylin-eosin staining (Ishimaru et al., 1992). This suggests that CO may induce neuronal cell death through changes of ion-channel activity. From this work, several reports strongly supported the notion that CO acts as an ion-channel modulator. Thus, it has been shown that CO increases the open probability of calcium-activated K+ (KCa) channels in vascular smooth muscle cells (Wang et al., 1997) and human umbilical vein endothelial cells (Dong et al., 2007). The molecular mechanism of this phenomenon is not well understood, but it has been proposed that depends on the increase of the number of Ca2+ binding sites (Wang et al., 1997), expression of the alpha subunit (Wu et al., 2002), modulation by NO (Wang and Wu, 2003), and a metal-dependent like coordination of CO by Cys at position 911 (C911) (Williams et al., 2008; Telezhkin et al., 2011). Other ion channels are also affected by CO, such as a 70-pS K+ channel in the thick ascending limb of Henle's loop (Liu et al., 1999), Kv2.1 (Dallas et al., 2011), hTREK-1 (Dallas et al., 2008), the amiloride-sensitive Na+ channel (Althaus et al., 2009), Nav1.5 channels (Elies et al., 2014), Cav3.2 (Boycott et al., 2013), and P2X2 receptors (Wilkinson et al., 2009). In the case of Cav3.2 channels, CO induced-inhibition was dependent on the activation of an extracellular thioredoxin-dependent mechanism (Scragg et al., 2008). Together, these data suggest that CO exerts many of its effects through ion-channel modulation.
Carbon monoxide modulates Cx-hemichannels
Recently, was demonstrated that CO is a new hemichannel modulator (León-Paravic et al., 2014; Reviewed in Retamal et al., 2015b). The application of CO donors (CORM-A1, CORM-2, and CORM-3) to the bath solution inhibited the currents of Cx46 hemichannels expressed in Xenopus laevis oocytes (X. oocytes). The inhibition has an IC50 of approximated 3.4 μM, making Cx46 hemichannels an excellent CO sensor under pathological (>10 μM) condition (Kajimura et al., 2010). Moreover, CORM-2 effect was fully prevented by the addition of hemoglobin (a CO scavenger) to the bath solution and was correlated with Cx46 carbonylation, which in turn, produced important protein structural rearrangements in vitro (León-Paravic et al., 2014). Interestingly, the effect of CO did not involve changes in voltage dependency or modifications of the C-terminus. Additionally, hemichannels formed by Cx46 lacking extracellular-loop Cys were much less sensitive to CORM-2 compared to wild type Cx46 hemichannels. Moreover, hemichannel inhibition was fully recovered by addition of reducing agents to the bath solution (e.g., GSH and DTT; León-Paravic et al., 2014). The extracellular cysteine redox status potentially could affect the conformational disposition of the loop-gating, which in turn, is known to affect the loop- gating (Reviewed in Retamal et al., 2016). From these data it can be proposed that CO could inhibit Cx46 hemichannels through changes of the loop-gating properties, likely enhancing the effect of Ca2+ (Figure 1).
For many years, protein carbonylation was considered synonymous with protein degradation (Reviewed in Wong et al., 2013). However, recent evidence suggests that there is a naturally occurring process of protein decarbonylation (Wong et al., 2008; Reviewed in Wong et al., 2013). This mechanism involves an unknown thiol-dependent enzymatic process, in which the enzymes thioredoxin (Trx) and glutaredoxin (Grx1) seem to play important roles (Wong et al., 2008; Reviewed in Wong et al., 2013). Therefore, based on the current knowledge, the effect of CO (most likely secondary carbonylation) upon protein activity can be reversed and controlled by the redox status of a cell. Nevertheless, the exact molecular mechanism of decarbonylation is poorly understood. In our study (León-Paravic et al., 2014) we blocked TRx with aurarofin and a small recovery of hemichannel current was observed, which suggests that TRx does not play an important role in the recovery of hemichannel current induced by reducing agents. The question still remains as to whether GRx could participate. As indicated, CO can also act indirectly through lipid peroxidation of Cys groups (Reviewed in Wong et al., 2013; Milic et al., 2015). Lipid-induced protein oxidation can be reverted by glutathione peroxidase (GPx4) and glutathione S-transferase (GST; Reviewed in Ribas et al., 2014), which are activated by reducing agents (Reviewed in Ribas et al., 2014). Therefore, a plausible mechanism of CO-induced Cx46 hemichannel inhibition is through Cx46-lipid oxidation.
In support of the role of oxidized lipids on Cx46 hemichannel inhibition, polyunsaturated fatty acids (PUFAs), such as linoleic acid and arachidonic acids, which can be easily oxidized (Reviewed in Ribas et al., 2014), inhibits Cx46 hemichannels in vitro (Retamal et al., 2011). Therefore, it cannot be ruled out that PUFAs may exert their inhibitory effects upon hemichannels through their oxidized-derived molecules. Moreover, preliminary results have shown that 4-Hydroxy-2-nonenal (4-HNE), a reactive aldehyde derived from oxidized lipids (100 μM), inhibits by 60 ± 12% Cx46 hemichannels in X. oocytes (Figure 2) and Vitamin C—a lipid peroxide inhibitor—reduced by ~50% the effect of CO upon Cx46 hemichannels (Figure 2). Moreover, the presence of Ca2+ in the extracellular media is fundamental for observing CO-induced inhibition of Cx46 hemichannels, suggesting that the CO-induced inhibition/extracellular Cys-lipid peroxidation involve certain conformational changes that alter loop gating properties (Figure 2).
Figure 2.
CO effect appears to be mediated by lipid peroxides. (A) Representative control of Cx46 hemichannel currents in Xenopus laevis oocytes recorded in ND96 solution (containing 1.8 mM Ca2+ and 1.0 mM Mg2+) by means of dual whole cell voltage clamp technique. The presence of 100 μM CORM-2 induces a dramatic drop in the current amplitude. Most of the inhibition induced by CORM-2 was prevented by the co-addition of 100 μM ascorbic acid to the bath solution. This suggests that the effect of CO needs free radical production into the Xenopus oocytes. In parallel experiments, oocytes expressing Cx46 were exposed to the lipid peroxide 4-HNE (100 μM), and an evident hemichannel current inhibition was observed. n = 3 for each condition. (B) Representative recordings of oocytes expressing Cx46 placed in a DCFS, without (control) or with 100 μM CORM-2 (n = 3).
Future directions
Hemichannels are relevant players in the development and progression of several diseases, and they are now used as targets for developing new molecules for disease treatments (Reviewed in Retamal et al., 2015a). However, in spite of years of research, the molecular mechanisms that control the opening and closing of these channels are still not well understood. Thus, it is highly relevant to understand these mechanisms and project this knowledge to produce new agonist(s)/antagonist(s) against Cx- hemichannels, as well as to understand why hemichannels become lethal under certain pathological conditions. Although the effect of CO upon GJC has not been studied, it is possible to propose that CO may not have a relevant impact in GJC properties. It because, CO/lipid peroxides seem to act through modifications of extracellular Cys, which in GJC are not accessible for modifications by reducing nor oxidant molecules.
CO has been used for the treatment of several diseases, but many of its effects are far from being well understood. Therefore, the current knowledge is insufficient for understanding how CO exerts its action at the cellular level and, thus, to find possible side effects of this treatment. Also, this knowledge may help to develop new strategies in the therapeutic use of CO, e.g., under metabolic stress, where hemichannels become massively open, which accelerates cell death. Therefore, a possible application of this research would be to pursue the use of CO as a hemichannel inhibitor in preventing or limiting stroke-induced cell death.
Author contributions
MR wrote this paper and did all the figures.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by FONDECYT project 1160227 to Dr. MR and thanks to Dr Anne Bliss for her grammar edition.
References
- Althaus M., Fronius M., Buchäckert Y., Vadász I., Clauss W. G., Seeger W., et al. (2009). Carbon monoxide rapidly impairs alveolar fluid clearance by inhibiting epithelial sodium channels. Am. J. Respir. Cell Mol. Biol. 41, 639–650. 10.1165/rcmb.2008-0458OC [DOI] [PubMed] [Google Scholar]
- Bao X., Altenberg G. A., Reuss L. (2004). Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am. J. Physiol. Cell Physiol. 286, C647–C654. 10.1152/ajpcell.00295.2003 [DOI] [PubMed] [Google Scholar]
- Bellido T., Plotkin L. I. (2010). Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability. Bone 49, 50–55. 10.1016/j.bone.2010.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. (1987). Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J. Cell Biol. 105, 2621–2629. 10.1083/jcb.105.6.2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott H. E., Dallas M. L., Elies J., Pettinger L., Boyle J. P., Scragg J. L., et al. (2013). Carbon monoxideinhibition of Cav3.2 T-type Ca2+ channels reveals tonic modulation by thioredoxin. FASEB J. 27, 3395–3407. 10.1096/fj.13-227249 [DOI] [PubMed] [Google Scholar]
- Bruzzone S., Guida L., Zocchi E., Franco L., De Flora A. (2001). Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 15, 10–12. 10.1096/fj.00-0566fje [DOI] [PubMed] [Google Scholar]
- Cattaruzza M., Hecker M. (2008). Protein carbonylation and decarboylation: a new twist to the complex response of vascular cells to oxidative stress. Circ. Res. 102, 273–274. 10.1161/CIRCRESAHA.108.172148 [DOI] [PubMed] [Google Scholar]
- Chen S., Zheng S., Liu Z., Tang C., Zhao B., Du J., et al. (2015). Endogeous sulfur dioxide protects against oleic acid-induced acute lung injury in association with inhibition of oxidative stress in rats. Lab. Invest. 95, 142–156. 10.1038/labinvest.2014.147 [DOI] [PubMed] [Google Scholar]
- Cherian P. P., Siller-Jackson A. J., Gu S., Wang X., Bonewald L. F., Sprague E., et al. (2005). Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol. Biol. Cell. 16, 3100–3106. 10.1091/mbc.E04-10-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F. (1970). The carbon monoxide body stores. Ann. N.Y. Acad. Sci. 174, 11–22. 10.1111/j.1749-6632.1970.tb49768.x [DOI] [PubMed] [Google Scholar]
- Cotrina M. L., Lin J. H., Alves-Rodrigues A., Liu S., Li J., Azmi-Ghadimi H., et al. (1998). Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. U.S.A. 95, 15735–15740. 10.1073/pnas.95.26.15735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina M. L., Lin J. H., Nedergaard M. (2008). Adhesive properties of connexin hemichannels. Glia 56, 1791–1798. 10.1002/glia.20728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G., Levine E., Rabadan-Diehl C., Werner R. (1991). Cell/cell channel formation involves disulfide exchange. Eur. J. Biochem. 197, 141–144. 10.1111/j.1432-1033.1991.tb15892.x [DOI] [PubMed] [Google Scholar]
- Dallas M. L., Boyle J. P., Milligan C. J., Sayer R., Kerrigan T. L., McKinstry C., et al. (2011). Carbon monoxide protects against oxidant-induced apoptosis via inhibition of Kv2.1. FASEB J. 25, 1519–1530. 10.1096/fj.10-173450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas M. L., Scragg J. L., Peers C. (2008). Modulation of hTREK-1 by carbon monoxide. Neuroreport 19, 345–348. 10.1097/WNR.0b013e3282f51045 [DOI] [PubMed] [Google Scholar]
- Deshane J., Wright M., Agarwal A. (2005). Heme oxygenase-1 expression in disease states. Acta Biochim. Pol. 52, 273–284. [PubMed] [Google Scholar]
- Dong D. L., Zhang Y., Lin D. H., Chen J., Patschan S., Goligorsky M. S., et al. (2007). Carbon monoxide stimulates the Ca2(+)-activated big conductance k channels in cultured human endothelial cells. Hypertension 50, 643–651. 10.1161/HYPERTENSIONAHA.107.096057 [DOI] [PubMed] [Google Scholar]
- Durante W., Kroll M. H., Christodoulides N., Peyton K. J., Schafer A. I. (1997). Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ. Res. 80, 557–564. 10.1161/01.RES.80.4.557 [DOI] [PubMed] [Google Scholar]
- Durante W., Peyton K. J., Schafer A. I. (1999). Platelet-derived growth factor stimulates heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 19, 2666–2672. 10.1161/01.ATV.19.11.2666 [DOI] [PubMed] [Google Scholar]
- Eiberger J., Degen J., Romualdi A., Deutsch U., Willecke K., Söhl G. (2001). Connexin genes in the mouse and human genome. Cell Commun. Adhes. 8, 163–165. 10.3109/15419060109080717 [DOI] [PubMed] [Google Scholar]
- Elies J., Dallas M. L., Boyle J. P., Scragg J. L., Duke A., Steele D. S., et al. (2014). Inhibition of the cardiac Na+ channel Nav1.5 by carbon monoxide. J. Biol. Chem. 289, 16421–16429. 10.1074/jbc.M114.569996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G., Szurszewski J. H. (2014). Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology 147, 303–313. 10.1053/j.gastro.2014.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C. E., Li X., Bennett M. V., Nagy J. I., Pereda A. E. (2008). Interaction between connexin35 and zonula occludens-1 and its potential role in the regulation of electrical synapses. Proc. Natl. Acad. Sci. U.S.A. 105, 12545–12550. 10.1073/pnas.0804793105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Hernández J. M., de Miguel M., Larrosa B., González D., Barrio L. C. (2003). Molecular basis of calcium regulation in connexin-32 hemichannels. Proc. Natl. Acad. Sci. U.S.A. 100, 16030–16035. 10.1073/pnas.2530348100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé J. C., Derangeon M., Sarrouilhe D., Giepmans B. N., Bourmeyster N. (2012). Gap junctional channels are parts of multiprotein complexes. Biochim. Biophys. Acta 1818, 1844–1865. 10.1016/j.bbamem.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Higdon A., Diers A. R., Oh J. Y., Landar A., Darley-Usmar V. M. (2012). Cell signaling by reactive lipid species: new concepts and molecular mechanism. Biochem. J. 442, 453–464. 10.1042/BJ20111752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru H., Katoh A., Suzuki H., Fukuta T., Kameyama T., Nabeshima T. (1992). Effects of N-methyl-D-aspartate receptor antagonists on carbon monoxide-induced brain damage in mice. J. Pharmacol. Exp. Ther. 261, 349–352. [PubMed] [Google Scholar]
- Ishizaka N., Griendling K. K. (1997). Heme oxygenase-1 is regulated by angiotensin II in rat vascular smooth muscle cells. Hypertension 29, 790–795. 10.1161/01.HYP.29.3.790 [DOI] [PubMed] [Google Scholar]
- Kajimura M., Fukuda R., Bateman R. M., Yamamoto T., Suematsu M. (2010). Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 13, 157–192. 10.1089/ars.2009.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M., Fahrenfort I., Schultz K., Janssen-Bienhold U., Sjoerdsma T., Weiler R. (2001). Hemichannel-mediated inhibition in the outer retina. Science 292, 1178–1180. 10.1126/science.1060101 [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Park Z. Y., Kim S. Y., Jeong E., Lee J. Y. (2009). Alteration of Toll-like receptor 4 activation by 4-hydroxy-2-nonenal mediated by the suppression of receptor homodimerization. Chem. Biol. Interact. 182, 59–66. 10.1016/j.cbi.2009.07.009 [DOI] [PubMed] [Google Scholar]
- Kronengold J., Srinivas M., Verselis V. K. (2012). The N-terminal half of the connexin protein contains the core elements of the pore and voltage gates. J. Membr. Biol. 245, 453–463. 10.1007/s00232-012-9457-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty R. K., Nagineni C. N., Kutty G., Hooks J. J., Chader G. J., Wiggert B. (1994). Increased expression of heme oxygenase-1 in human retinal pigment epithelial cells by transforming growth factor-beta. J. Cell. Physiol. 159, 371–378. 10.1002/jcp.1041590221 [DOI] [PubMed] [Google Scholar]
- Lampe P. D., Lau A. F. (2000). Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 384, 205–215. 10.1006/abbi.2000.2131 [DOI] [PubMed] [Google Scholar]
- Lautier D., Luscher P., Tyrrell R. M. (1992). Endogenous glutathione levels modulate both constitutive UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis 13, 227–232. 10.1093/carcin/13.2.227 [DOI] [PubMed] [Google Scholar]
- León-Paravic C. G., Figueroa V. A., Guzmán D. J., Valderrama C. F., Vallejos A. A., Fiori M. C., et al. (2014). Carbon Monoxide (CO) is a novel inhibitor of connexin hemichannels. J. Biol. Chem. 289, 36150–36157. 10.1074/jbc.M114.602243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. S., Fitzgerald S., Dong Y., Knight C., Donaldson P., Kistler J. (1997). Processing of the gap junction protein connexin50 in the ocular lens is accomplished by calpain. Eur. J. Cell Biol. 73, 141–149. [PubMed] [Google Scholar]
- Liu H., Mount D. B., Nasjletti A., Wang W. (1999). Carbon monoxide stimulates the apical 70-pS K+ channel of the rat thick ascending limb. J. Clin. Invest. 103, 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez W., Liu Y., Harris A. L., Contreras J. E. (2014). Divalent regulation and intersubunit interactions of human connexin26 (Cx26) hemichannels. Channels (Austin) 8, 1–4. 10.4161/chan.26789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovine M. K., Gumpert A. M., Falk M. M., Mendelson T. C. (2008). Cx23, a connexin with only four extracellular-loop cysteines, forms functional gap junction channels and hemichannels. FEBS Lett. 582, 165–170. 10.1016/j.febslet.2007.11.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milic I., Melo T., Domingues M. R., Domingues P., Fedorova M. (2015). Heterogeneity of peptide adducts with carbonylated lipid peroxidation products. J. Mass Spectrom. 50, 603–612. 10.1002/jms.3568 [DOI] [PubMed] [Google Scholar]
- Müller D. J., Hand G. M., Engel A., Sosinsky G. E. (2002). Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 21, 3598–3607. 10.1093/emboj/cdf365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S., Bargiello T. A. (2015). Voltage regulation of connexin channel conductance. Yonsei Med. J. 56, 1–15. 10.3349/ymj.2015.56.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. L., Ebihara L., Takemoto L. J., Swenson K. I., Goodenough D. A. (1991). Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 115, 1077–1089. 10.1083/jcb.115.4.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi C. A., Tatro L., Zhang J. (1995). Hydroxyl radical production in the brain after CO hypoxia in rats. Free Radic Biol Med. 18, 603–609. 10.1016/0891-5849(95)00168-W [DOI] [PubMed] [Google Scholar]
- Poss K. D., Tonegawa S. (1997). Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 94, 10925–10930. 10.1073/pnas.94.20.10925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A. (2014). Connexin and Pannexin hemichannels are regulated by redox potential. Front. Physiol. 5:80. 10.3389/fphys.2014.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., Cortés C. J., Reuss L., Bennett M. V., Sáez J. C. (2006). S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. U.S.A. 103, 4475–4480. 10.1073/pnas.0511118103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., Evangelista-Martínez F., León-Paravic C. G., Altenberg G. A., Reuss L. (2011). Biphasic effect of linoleic acid on connexin 46 hemichannels. Pflugers Arch. 461, 635–643. 10.1007/s00424-011-0936-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., Froger N., Palacios-Prado N., Ezan P., Sáez P. J., Sáez J. C., et al. (2007). Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J. Neurosci. 27, 13781–13792. 10.1523/JNEUROSCI.2042-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., García I. E., Pinto B. I., Pupo A., Báez D., Stehberg J., et al. (2016). Extracellular cysteine in connexins: role as redox sensors. Front. Physiol. 7:1. 10.3389/fphys.2016.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., León-Paravic C. G., Ezquer M., Ezquer F., Rio R. D., Pupo A., et al. (2015b). Carbon monoxide: a new player in the redox regulation of connexin hemichannels. IUBMB Life 67, 428–437. 10.1002/iub.1388 [DOI] [PubMed] [Google Scholar]
- Retamal M. A., Reyes E. P., Garcia I. E., Pinto B., Martinez A. D., Gonzalez C. (2015a). Diseases associated with leaky hemichannels. Front. Cell. Neurosci. 9:267 10.3389/fncel.2015.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., Yin S., Altenberg G. A., Reuss L. (2009). Modulation of Cx46 hemichannels by nitric oxide. Am. J. Physiol. Cell Physiol. 296, C1356–C1363. 10.1152/ajpcell.00054.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., Yin S., Altenberg G. A., Reuss L. (2010). Voltage-dependent facilitation of Cx46 hemichannels. Am. J. Physiol. Cell Physiol. 298, C132–C139. 10.1152/ajpcell.00258.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas V., García-Ruiz C., Fernández-Checa J. C. (2014). Glutathione and mitochondria. Front. Pharmacol. 5:151. 10.3389/fphar.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanello M., Veronesi V., D'Andrea P. (2003). Mechanosensitivity and intercellular communication in HOBIT osteoblastic cells: a possible role for gap junction hemichannels. Biorheology 40, 119–121. [PubMed] [Google Scholar]
- Sánchez H. A., Mese G., Srinivas M., White T. W., Verselis V. K. (2010). Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J. Gen. Physiol. 136, 47–62. 10.1085/jgp.201010433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalper K. A., Sánchez H. A., Lee S. C., Altenberg G. A., Nathanson M. H., Sáez J. C., et al. (2010). Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am. J. Physiol. Cell Physiol. 299, C1504–C1515. 10.1152/ajpcell.00015.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scragg J. L., Dallas M. L., Wilkinson J. A., Varadi G., Peers C. (2008). Carbon monoxide inhibits Ltype Ca2+ channels via redox modulation of key cysteine residue by mitochiondrial reactive oxygen specias. J. Biol. Chem. 283, 24412–24419. 10.1074/jbc.M803037200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Liu X., Liu R., Yang L., Zuo J., Liu W. (2010). Connexin 43 hemichannel regulates H9c2 cell proliferation by modulating intracellular ATP and [Ca2+]. Acta Biochim. Biophys. Sin. (Shanghai). 42, 472–482. 10.1093/abbs/gmq047 [DOI] [PubMed] [Google Scholar]
- Stehberg J., Moraga-Amaro R., Salazar C., Becerra A., Echeverría C., Orellana J. A., et al. (2012). Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 26, 3649–3657. 10.1096/fj.11-198416 [DOI] [PubMed] [Google Scholar]
- Stout C. E., Costantin J. L., Naus C. C., Charles A. C. (2002). Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 277, 10482–10488. 10.1074/jbc.M109902200 [DOI] [PubMed] [Google Scholar]
- Stridh M. H., Tranberg M., Weber S. G., Blomstrand F., Sandberg M. (2008). Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J. Biol. Chem. 283, 10347–10356. 10.1074/jbc.M704153200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Dowd T. L., Verselis V. K., Bargiello T. A. (2009). Conformational changes in a pore-forming region underlie voltage-dependent “loop gating” of an unapposed connexin hemichannel. J Gen Physiol. 133, 555–570. 10.1085/jgp.200910207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telezhkin V., Brazier S. P., Mears R., Müller C. T., Riccardi D., Kemp P. J. (2011). Cysteine residue 911 in C-terminal tail of human BK(Ca)α channel subunit is crucial for its activation by carbon monoxide. Pflugers Arch. 461, 665–675. 10.1007/s00424-011-0924-7 [DOI] [PubMed] [Google Scholar]
- Thom S. R. (1990). Carbon monoxide mediated brain lipid peroxidation in the rat. J. Appl. Physiol. 68, 997–1003. [DOI] [PubMed] [Google Scholar]
- Trexler E. B., Bennett M. V., Bargiello T. A., Verselis V. K. (1996). Voltage gating and permeation in a gap junction hemichannel. Proc. Natl. Acad. Sci. U.S.A. 93, 5836–5841. 10.1073/pnas.93.12.5836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis V. K., Trelles M. P., Rubinos C., Bargiello T. A., Srinivas M. (2009). Loop gating of connexin hemichannels involves movement of pore-lining residues in the first extracellular loop domain. J. Biol. Chem. 284, 4484–4493. 10.1074/jbc.M807430200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Wu L. (2003). Interaction of selective amino acid residues of K(ca) channels with carbon monoxide. Exp. Biol. Med. (Maywood) 228, 474–480. [DOI] [PubMed] [Google Scholar]
- Wang R., Wu L., Wang Z. (1997). The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 434, 285–291. [DOI] [PubMed] [Google Scholar]
- Wilkinson W. J., Gadeberg H. C., Harrison A. W., Allen N. D., Riccardi D., Kemp P. J. (2009). Carbon monoxide is a rapid modulator of recombinant and native P2X(2) ligand-gated ion channels. Br. J. Pharmacol. 158, 862–871. 10.1111/j.1476-5381.2009.00354.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. E., Brazier S. P., Baban N., Telezhkin V., Müller C. T., Riccardi D., et al. (2008). A structural motif in the C-terminal tail of slo1 confers carbon monoxide sensitivity to human BK Ca channels. Pflugers Arch. 456, 561–572. 10.1007/s00424-007-0439-4 [DOI] [PubMed] [Google Scholar]
- Wölfle S. E., Schmidt V. J., Hoepfl B., Gebert A., Alcoléa S., Gros D., et al. (2007). Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ. Res. 101, 1292–1299. 10.1161/CIRCRESAHA.107.163279 [DOI] [PubMed] [Google Scholar]
- Wong C. M., Cheema A. K., Zhang L., Suzuki Y. J. (2008). Protein carbonylation as a novel mechanism in redox signaling. Circ. Res. 102, 310–318. 10.1161/CIRCRESAHA.107.159814 [DOI] [PubMed] [Google Scholar]
- Wong C. M., Marcocci L., Das D., Wang X., Luo H., Zungu-Edmondson M., et al. (2013). Mechanism of protein decarbonylation. Free Radic. Biol. Med. 65, 1126–1133. 10.1016/j.freeradbiomed.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Cao K., Lu Y., Wang R. (2002). Different mechanisms underlying the stimulation of K(Ca) channels by nitric oxide and carbon monoxide. J. Clin. Invest. 110, 691–700. 10.1172/JCI0215316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Wang R. (2005). Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 57, 585–630. 10.1124/pr.57.4.3 [DOI] [PubMed] [Google Scholar]
- Ye Z. C., Wyeth M. S., Baltan-Tekkok S., Ransom B. R. (2003). Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 23, 3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]