Abstract
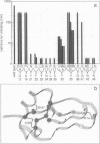
In a previous study, a genetic screening procedure was used to identify variants of bovine pancreatic trypsin inhibitor that can fold to an active conformation but that are inactivated much more rapidly than the wild-type protein in the presence of dithiothreitol (DTT). The mechanisms by which 30 of these DTT-sensitive variants are inactivated have now been investigated. Some of the amino acid replacements cause rapid inactivation in the presence of DTT because the three disulfides of the native protein are reduced up to 300-fold faster than for the wild-type protein, leading to complete unfolding. Other substitutions, however, do not greatly increase the rate of complete reduction and unfolding but lead to accumulation of an inactive two-disulfide species. There is a striking correlation between the locations of the DTT-sensitive amino acid replacements in the three-dimensional structure of the protein and the mechanisms by which the variants are inactivated. All of the substitutions that cause rapid unfolding are clustered at one end of the folded protein, in the vicinity of the two disulfides that are reduced most slowly during unfolding of the wild-type protein, while substitutions of the other class are all located at the other end of the protein, near the trypsin binding site. These results indicate that the kinetic stability of native bovine pancreatic trypsin inhibitor and its ability to function as a protease inhibitor are largely influenced by residues in two distinguishable regions of the folded protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coplen L. J., Frieden R. W., Goldenberg D. P. A genetic screen to identify variants of bovine pancreatic trypsin inhibitor with altered folding energetics. Proteins. 1990;7(1):16–31. doi: 10.1002/prot.340070103. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Energetics of folding and unfolding of pancreatic trypsin inhibitor. J Mol Biol. 1977 Jun 25;113(2):295–312. doi: 10.1016/0022-2836(77)90143-7. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Goldenberg D. P. Kinetic role of a meta-stable native-like two-disulphide species in the folding transition of bovine pancreatic trypsin inhibitor. J Mol Biol. 1984 Nov 5;179(3):497–526. doi: 10.1016/0022-2836(84)90077-9. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Protein folding. Biochem J. 1990 Aug 15;270(1):1–16. doi: 10.1042/bj2700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D. P., Frieden R. W., Haack J. A., Morrison T. B. Mutational analysis of a protein-folding pathway. Nature. 1989 Mar 9;338(6211):127–132. doi: 10.1038/338127a0. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. P. Kinetic analysis of the folding and unfolding of a mutant form of bovine pancreatic trypsin inhibitor lacking the cysteine-14 and -38 thiols. Biochemistry. 1988 Apr 5;27(7):2481–2489. doi: 10.1021/bi00407a034. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Housset D., Kim K. S., Fuchs J., Woodward C., Wlodawer A. Crystal structure of a Y35G mutant of bovine pancreatic trypsin inhibitor. J Mol Biol. 1991 Aug 5;220(3):757–770. doi: 10.1016/0022-2836(91)90115-m. [DOI] [PubMed] [Google Scholar]
- Hurle M. R., Marks C. B., Kosen P. A., Anderson S., Kuntz I. D. Denaturant-dependent folding of bovine pancreatic trypsin inhibitor mutants with two intact disulfide bonds. Biochemistry. 1990 May 8;29(18):4410–4419. doi: 10.1021/bi00470a021. [DOI] [PubMed] [Google Scholar]
- Jering H., Tschesche H. Preparation and characterization of the active derivative bovine trypsin-kallikrein inhibitor (Kunitz) with the reactive site lysine-15 -- alanine-16 hydrolyzed. Eur J Biochem. 1976 Jan 15;61(2):443–452. doi: 10.1111/j.1432-1033.1976.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Kress L. F., Laskowski M., Sr The basic trypsin inhibitor of bovine pancreas. VII. Reduction with borohydride of disulfide bond linking half-cystine residues 14 and 38. J Biol Chem. 1967 Nov 10;242(21):4925–4929. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marks C. B., Naderi H., Kosen P. A., Kuntz I. D., Anderson S. Mutants of bovine pancreatic trypsin inhibitor lacking cysteines 14 and 38 can fold properly. Science. 1987 Mar 13;235(4794):1370–1373. doi: 10.1126/science.2435002. [DOI] [PubMed] [Google Scholar]
- Oas T. G., Kim P. S. A peptide model of a protein folding intermediate. Nature. 1988 Nov 3;336(6194):42–48. doi: 10.1038/336042a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Tüchsen E., Woodward C. Assignment of asparagine-44 side-chain primary amide 1H NMR resonances and the peptide amide N1H resonance of glycine-37 in basic pancreatic trypsin inhibitor. Biochemistry. 1987 Apr 7;26(7):1918–1925. doi: 10.1021/bi00381a020. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Amide protein exchange and surface conformation of the basic pancreatic trypsin inhibitor in solution. Studies with two-dimensional nuclear magnetic resonance. J Mol Biol. 1982 Sep 15;160(2):343–361. doi: 10.1016/0022-2836(82)90180-2. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kim P. S. Reexamination of the folding of BPTI: predominance of native intermediates. Science. 1991 Sep 20;253(5026):1386–1393. doi: 10.1126/science.1716783. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Deisenhofer J., Huber R. Comparison of two highly refined structures of bovine pancreatic trypsin inhibitor. J Mol Biol. 1987 Jan 5;193(1):145–156. doi: 10.1016/0022-2836(87)90633-4. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Nachman J., Gilliland G. L., Gallagher W., Woodward C. Structure of form III crystals of bovine pancreatic trypsin inhibitor. J Mol Biol. 1987 Dec 5;198(3):469–480. doi: 10.1016/0022-2836(87)90294-4. [DOI] [PubMed] [Google Scholar]