Abstract
Aspartoacylase (AspA) gene mutations cause the pediatric lethal neurodegenerative Canavan disease (CD). There is emerging promise of successful gene therapy for CD using recombinant adeno-associated viruses (rAAVs). Here, we report an intracerebroventricularly delivered AspA gene therapy regime using three serotypes of rAAVs at a 20-fold reduced dose than previously described in AspA−/− mice, a bona-fide mouse model of CD. Interestingly, central nervous system (CNS)-restricted therapy prolonged survival over systemic therapy in CD mice but failed to sustain motor functions seen in systemically treated mice. Importantly, we reveal through histological and functional examination of untreated CD mice that AspA deficiency in peripheral tissues causes morphological and functional abnormalities in this heretofore CNS-defined disorder. We demonstrate for the first time that AspA deficiency, possibly through excessive N-acetyl aspartic acid accumulation, elicits both a peripheral and CNS immune response in CD mice. Our data establish a role for peripheral tissues in CD pathology and serve to aid the development of more efficacious and sustained gene therapy for this disease.
Introduction
Canavan disease (CD) is a fatal pediatric leukodystrophy characterized by progressive spongy white matter degeneration and edema in the central nervous system (CNS).1 CD is caused by autosomal recessive loss-of-function mutations in the aspartoacylase enzyme (AspA) (EC 3.5.1.15), which normally metabolizes N-acetyl aspartic acid (NAA) into acetate, a probable myelination substrate.2,3,4,5 NAA is produced by the enzyme aspartate N-acetyl transferase in neurons and transported for metabolism by AspA in oligodendrocytes to facilitate their differentiation, energy production, and lipid synthesis.6,7 AspA mutations inactivate the metabolic activity of AspA, resulting in an excess of NAA. Injection of NAA into rats induces seizures.8 Excess NAA from its defective metabolism in the brain may cause pathology through several mechanisms, including the generation of reactive oxygen species9 and myelin defects from molecular water pump gradient disruption, resulting in intramyelinic edema.10 Alternatively, pathology may be driven by the acetate depletion that stems from impaired NAA metabolism.11 Myelin defects may occur, for instance, through lipid synthesis impairment without sufficient NAA-derived acetate. Impairments in protein folding and chromatin modifications from a lack of NAA-derived acetate in oligodendrocytes may also contribute to disease12,13 It remains unknown if the defect in NAA metabolism causes peripheral tissue pathology. The AspA knockout mouse mimics human CD symptoms,14,15 making it an ideal model system for developing proof-of-concept preclinical gene therapy approaches.
A promising gene therapy for CD is achieved via recombinant adeno-associated viruses (rAAVs), some of which readily infect a wide range of tissues including the CNS by crossing the blood–brain barrier (BBB) after systemic injection.16,17 An early report described the ability of the viral serotype rAAV9 to mediate CNS transduction by intravascular (IV) delivery, and subsequent reports showed similar, but more efficient, activity for rAAVrh.8 and rh.10.16,17,18 These serotypes also effectively treat several rodent models of neurodegenerative diseases, including CD, via systemic delivery.15,19 IV administration of therapeutics requires high doses to reach effective vector concentrations at CNS disease sites due to peripheral tissue uptake and the BBB. The consequences for viral therapy by IV delivery include a high systemic viral load, safety concerns regarding viral capsid immunotoxicity and genotoxicity, and a manufacturing burden. Therefore, safer and more CNS-targeted viral delivery methods are imperative.
To this end, we sought to test a gene therapy regimen that is physically limited to the CNS. Since some rAAVs cross the immature ependymal lining at cerebral ventricles, widely transducing the bulk brain parenchyma within the first 24 hours of birth;20 we chose intracerebroventricular (ICV) vector delivery as our primary avenue rather than the IV route for CD gene therapy. We expected the ICV approach to reintroduce functional AspA exclusively to the CNS at a much lower vector dose, reducing NAA levels in the CNS more efficiently at per vector genome basis. Moreover, this CNS-restricted gene therapy regimen allowed us to explore the role of peripheral tissues in CD pathology, as our previous work revealed motor function impairment in a peripheral tissue-detargeted vector therapy in AspA knockout mice relative to a globally gene-corrected model.15
In this report, we established a CNS-restricted therapeutic rAAV dosing regimen that uses three different viral serotypes, rAAV rh.8, 9, and rh.10, at 20-fold lower doses than previously reported15 in AspA knockout mice but achieved treatment efficacy similar to IV regimen at early time points. This modified dosing regimen, delivered rAAVs primarily to the CNS, ameliorates edema and prolongs survival but fails to maintain correction of motor impairments at later time points. We further characterized peripheral tissue morphology and function in both wild-type (WT) and untreated CD mice and determined that AspA levels correlate with pathology in these tissues. Finally, we studied the effects of normal physiological and pathological NAA concentrations on the neuronal cell types in vitro to explore the role of NAA in neuronal CD pathogenesis. Importantly, our data extend a role for peripheral tissues in CD pathology building on a previous observation of upper airway defects in CD patients21 and an immune response to pathological NAA elevation, highlighting the critical importance of including peripheral tissues in studying CD pathophysiology and as targets for efficacious and sustained CD gene therapy.
Results
CNS-restricted rAAV gene therapy prolongs survival in CD mice compared to systemic targeting, but normalizes motor function only transiently
CD mice exhibit rapid neurodegeneration and perinatal lethality (around 28 days), mimicking the timeline of human pathology in a subset of Canavan patients. We therefore first assessed the efficacy of our gene therapy approach by monitoring the lifespan of treated animals. Long-term survival was monitored with a Kaplan–Meier growth curve for >150 gene-corrected CD mice injected either intravenously (Figure 1a, top panel) or intracerebroventricularly (Figure 1a, bottom panel) with rAAVs rh.8, 9 or rh.10hAspa. (n = 7 for IV and n = 20 for ICV groups). Body weight was recorded every other day for the first month (Figure 1b, top panel) and every 2 weeks thereafter (Figure 1b, bottom panel) to compare differences between the routes of rAAV9 delivery until the animals reached the same weight as their WT littermates. All rAAV-treated CD mice exhibited extended growth and survival up to 2 years with significant differences in weight gain between the IV and ICV administration routes of gene therapy (Figure 1b, right panel, ***P = 0.002). CD animals treated with serotypes rh.8 and 10 showed similar growth curve trends to that of rAAV9 (data not shown). These data establish a uniform rescue of perinatal lethality in all treated animals, confirming the efficacy of ICV treatment for CD.
Figure 1.
rAAV-mediated gene therapy delivered via intracerebroventricular injection in CD mice mimics the lethality and morphology correction seen in systemic delivery but does not restore motor function. (a) Kaplan–Meier survival curves for all groups treated with rAAV9, rAAVrh.8, and rAAVrh.10 at P0 treated intravenously (IV; top panel) and intracerebroventricularly (ICV; bottom panel). (b) Early growth rate was assessed by taking weights at 2-day intervals for the first month (top panel) and at 2-week intervals up to 24 weeks (bottom panel) and plotted as a function of time. rAAV9-treated animals were shown as a representative of the serotypes. ***P = 0.002. (c) Semiquantitative neuropathology analyses were normalized to CD animals using Image J. (d) Representative T2-weighted MRI images showing water accumulation (white) in P25 CD/PBS and P60 WT and rAAV9-treated CD animals. (e) Scotopic ERGs of overnight dark-adapted WT and CD mice (n = 4 each) to test the response of rods recorded as waveforms (µV) to flashes of light in increasing intensity called steps (upper panel, a wave; lower panel, b wave). (f) Motor functions of study groups were tested at P30 and P90 based on their performance on the rotarod moving at fixed (3 rpm) and accelerated speed (4–40 rpm in 5 minutes), balance beam, and inverted screen. The experiments had a cutoff of 120 seconds for fixed rotarod and 300 seconds for accelerated rotarod. CD/PBS, CD/rAAV, CD mice treated with PBS or rAAV, respectively; Het, heterozygote; IV and ICV, intravenous and intracerebroventricular routes of delivery; MRI, magnetic resonance imaging; rAAV, recombinant adeno-associated viruses; WT, wild-type.
ICV gene therapy also mitigated neuropathology, which presents as vacuoles in multiple brain regions in the brains of CD mice by postnatal day 25 (P25), indicative of neurodegeneration.15 However, these vacuoles were significantly diminished in most regions in CD mice treated with ICV delivered gene-correcting rAAV serotypes, regardless of administration route, except in the striatum and cerebellum (Figure 1c, Supplementary Figure S1a).
It has been established that NAA accumulation in the brain and cerebral edema is a pathological consequence of CD in humans; hence, we utilized magnetic resonance spectroscopy and magnetic resonance imaging to track edema in living study animals at P60. ICV injections relieved edema to a greater extent than did IV injections (Figure 1d, Supplementary Figure S1b,c).
We previously reported that untreated CD mice develop vision impairment.15 Scotopic electroretinography studies were performed on all groups of mice (n = 4) at P90 to evaluate retinal response to light flashes, as described earlier.15 Both IV and ICV delivery routes partially restore a-wave and b-wave amplitudes in CD mice to a similar extent regardless of serotype or delivery route (represented here with rAAV9 data, Figure 1e), even though the retina is transduced by IV but not by ICV injections (see Supplementary Figure S1d). It is likely that these particular optic symptoms in CD are rooted not in the eye, but in the brain centers, where transduction using EGFP vectors of the corresponding serotypes occurred via both delivery methods (see Supplementary Figure S2a,b).
Motor functions were next evaluated in both the IV- and ICV-injected CD mice. IV-treated animals improved in all tests to nearly WT levels by 3 months. Conversely, the ICV group performed significantly better on three of the four motor tests by 1 month of age, even performing as well as WT animals on the balance beam test (Figure 1f). However, the ICV motor corrections were only transient and gradually diminished by 3 months in all tests. Our findings here reproduced what were observed in our previously published study where IV-delivered rAAVhAspA vector was engineered to be tissue-specific endogenous micro RNA (miRNA) sensitive for detargeting from peripheral tissues.15 In other words, both sets of study animals showed that they could not sustain motor function correction over time (data not shown), suggesting possible involvement of peripheral tissues in CD pathology, which may be worsened developmentally.
Deficiency of AspA leads to morphological and functional abnormalities in multiple peripheral tissues
To investigate the role of AspA in peripheral tissues during CD pathogenesis, we first confirmed the presence of the AspA mRNA in peripheral tissues from 3-week-old WT mice (n = 4) via qPCR (Figure 2a). We also compared the ratios of weights of the tissues to body weight between CD and WT mice at P25 and noted that several CD mouse organs differed in such ratios compared to WT, including the brain (Figure 2b; Supplementary Figure S3a). We confirmed that the increased CD brain mass was caused due to water retention by lyophilizing brain tissues (n = 7; Supplementary Figure S3b). Furthermore, comparison of clinical hematology (i.e., the complete differential count of blood (n = 5; Figure 2c)) and serum chemistry (n = 5, Figure 2d) profiles between 25-day-old WT and untreated CD mice revealed that the AspA-deficient CD mice exhibit lymphocytopenia (low lymphocyte count), granulocytosis (high count of granulocytes) hypoglycemia (low glucose), and defective pancreatic function (low amylase levels) (Figure 2c,d). We note that these effects are unlikely to be due to starvation, as the observed feeding behavior appeared to be normal. We then examined the effect of AspA deficiency on different neuronal types by examining the morphology of the optic (sensory), sciatic (motor), and vagus (mixed) nerves via EM studies in P25 mice. Compared to WT mice, the myelin sheath is loosely arranged in the optic nerves, with several gaps present between bilayers. The sciatic nerve shows isolated regions of myelin extrusions, while the vagus nerve contains several lesions (Figure 2e, arrows). It is interesting to note that murine sciatic nerves contain high levels of AspA22 and, however more importantly, X-ray diffraction studies revealed no difference in myelin profiles between WT, untreated, and treated CD animals at this site (see Supplementary Figure S3c). These data suggest that myelin pathology in peripheral nerves of CD mice is more complicated than expected.
Figure 2.
Physiological impacts of aspartoacylase (AspA) deficiency in peripheral tissues in addition to CNS pathology in CD mice. (a) qPCR of aspartoacylase mRNA from fresh tissue homogenates from WT at P30 (n = 4) showing extensive expression normalized to GAPDH. (b) Ratio of the weight of peripheral organs normalized to body weight of the WT or CD animals (n = 4) from which the tissues were collected. (c) Complete differential count of blood for CD and WT mice (n = 4). Data normalized to WT animals. (d) Serum of CD and WT mice (n = 5) was tested for various biochemical markers. Data normalized to WT animals. (e) Electron microscopy sections of nerves in order of relative size (optic, vagus, and sciatic) of WT and CD mice reveal abnormalities in CD mice (arrows). Scale bar, 200 nm. CD, untreated CD; CNS, central nervous system; WT, wild-type.
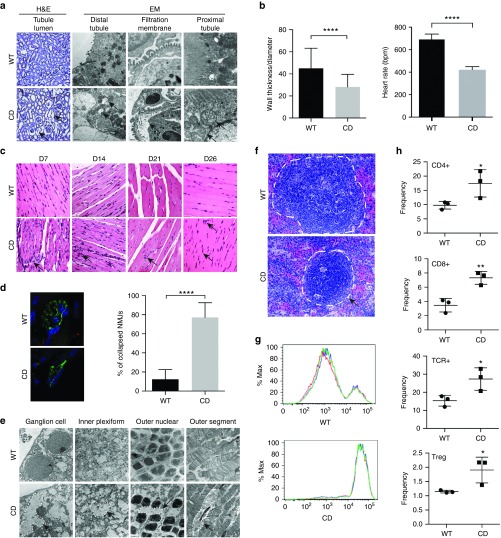
We then examined peripheral tissues in P25 CD mice beginning with the kidney, which showed AspA levels that are equal to or even higher than the brain (Figure 2a). Morphologically, H&E stain showed noticeably larger lumens in the kidney tubules, while EM studies revealed large vacuoles in the distal convoluted tubules as well as several breaks in the filtration membrane and injury in the microvillous brush border of the proximal convoluted tubules (Figure 3a, arrows). These data suggest that the kidney filtration apparatus may be impaired in CD, which could potentially explain the excessively high NAA levels in the urine and serum.3,15 Next we examined the heart, liver, and muscle since, in our published IV gene therapy study, one version of the therapeutic vectors contains miRNA binding sites for miRNA expressed in these tissues, preventing therapeutic AspA expression in these organs.15 We found significantly thinner cardiac walls in CD mice compared to WT at P25 (Figure 3b, left panel). Noninvasive electrocardiography (ECG) revealed profound bradycardia in awake P18 CD mice (Figure 3b, right panel), and the size of the heart was larger relative to body weight (Figure 2b). H&E staining in the liver did not show any morphological abnormalities, however, we did find evidence of mild transaminitis in the serum (Figure 2d). Skeletal muscle morphology showed increased nucleation in CD mice over several developmental stages (Figure 3c) and staining at the neuromuscular junction (NMJ) revealed vast denervation in P25 CD mice accompanied by a significant breakdown of healthy pretzel-shaped morphology in favor of a collapsed form (Figure 3d). These data indicate that several peripheral tissues are affected in the diseased model. Defective vision is another important symptom of CD patients and untreated CD mice.23,24 Optical coherence tomography studies did not show gross degeneration of retinal cell layers in the eye of P25 CD mice (data not shown), but higher resolution EM analyses of the diseased mice retina revealed several abnormalities. We observed extensive loss of basal ganglion cells in CD animals (Figure 3e), which correlates with thinner optic nerves. We also observed increased vacuolation in the inner plexiform and outer nuclear layers, suggestive of an osmotic imbalance exerted by excess NAA, and widespread disruption of the retinal cellular matrix in the outer nuclear layer (Figure 3e). The small intestine, reported to contain large amounts of AspA,22 also displayed changes in the villi and mucosa along with the reduction in the number of crypts via H&E staining (see Supplementary Figure S4a). We lastly examined the spleen, the major lymphatic organ, which was abnormally small in CD mice relative to body mass (Figure 2b). To our surprise, though the thymus in CD mice is morphologically identical to WT (data not shown), the spleen displayed distinct morphological abnormalities, including a smaller white pulp (Figure 3f) that correlates with low B-cell numbers as well as lymphocytopenia in P25 mice (see Supplementary Figure S4b, Figure 2c). Carboxyfluorescein succinimidyl ester (CFSE) CellTrace staining revealed that splenocytes of CD mice are unable to proliferate when challenged by CD28 (Figure 3g), and fluorescence-activated cell sorting of spleen cells in P18 CD mice revealed a significantly increased count of several immune cell types in splenocytes, including several T-cell subtypes (Figure 3h). No major abnormalities were found in thymus cell composition (data not shown). These data provide strong evidence that the immune system in CD mice is affected.
Figure 3.
Peripheral organs reveal abnormalities in morphology and functions contributing to additional pathology in CD mice. (a) Representative images from H&E-stained paraffin sections showing increased lumen size (arrow; left panel) (n = 4) and electron microscopy of the renal cortex and medulla of P21 age-matched mice showing vacuolated distal tubules, disrupted filtration membrane, and lysed organelles in the proximal tubules (arrows; n = 3 for each cohort) (right panel). (b) Quantitation of the heart wall thickness using Image J of H&E-stained sections (left panel; three sections at different levels each for n = 3 animals per cohort) and quantitation of heart rate of WT and CD mice (n = 4) at P27 via electrocardiography (ECG) showing evidence of bradycardia (right). (c) Representative H&E-stained sections through perinatal developmental ages showing increased density of nuclei in muscle fibers of the tibialis anterior (TA) muscle in CD animals over WT animals (arrows; n = 3). (d) Neuromuscular junction terminals of the TA muscle visualized by staining neurofilament (green) and bungarotoxin (red) show several collapsed NMJs in CD mice compared to WT animals (n = 3). Scale bars, 20 μm. (e) Electron microscopy of different layers of the retina in P21 age-matched mice (n = 3 for WT and CD mice) showing morphological changes in several layers including, but not limited to, shrinkage of cells, accumulation of vacuoles, and disintegration of the matrix (arrows). (f) Representative images from H&E-stained paraffin sections for spleen of WT and CD mice revealing a smaller white pulp in CD mice (dotted line). (g) Splenocyte proliferation assay using the CFSE dye in CD versus WT mice (n = 3 for CD and WT mice). The x axis is the fluorescence intensity on a log10 scale. The colored lines represent individual technical replicates. Three replicates were performed for every mouse. (h) Quantification of immune cell types in the spleen of WT and CD mice using FACS (n = 3 mice). CD, untreated CD; CFSE, carboxyfluorescein succinimidyl ester; FACS, fluorescence activated cell sorting; NMJ, neuromuscular junction; TCR, T-cell receptor; WT, wild-type.
Role of NAA as an inflammatory stimulant
We have thus far demonstrated, stemming from our use of CNS-isolated CD treatment, that immune cells outside of the CNS are impaired in CD mice. These findings reveal a potential role of the peripheral immune system during CD pathogenesis. We sought to expand the exploration of an immune response within the brain and looked for evidence of CNS-centered immune responses in untreated CD mice. A CNS-specific immunological response like microglial activation may mediate neuronal death via CNS inflammation, if unchecked.25 We thus examined the presence of activated microglia with a CD45RO antibody and, indeed, detected remarkable microglial activation in the CD brain compared to WT at P25 (Figure 4a). We then checked the brain homogenates for proportion of viable cells based on quantitation of Adenosine Tri Phosphate (ATP), an indicator of metabolically active cells (see Supplementary Figure S5a). We found no significant difference between the WT and CD animals at P25, suggesting that cells may not die under pathological conditions. We then quantified numbers of neurons in the brain sections of WT and CD mice stained with fluorescent neuronal marker for comparison. Our result showed no significant difference in the abundance of neurons in all regions except the brain stem (see Supplementary Figure S5b). However, our data could not rule out that NAA-associated neuroinflammation caused possible reductions in other cell types and/or formation of vacuoles in the brain parenchyma without causing neuronal cell death in the CD brains.
Figure 4.
Role of NAA-induced inflammation in contributing to CD pathology. (a) Representative images of avidin–biotin complex (ABC)-stained brain sections for CD45 (n = 3) for both WT and CD animals. Brown indicates activated microglia. (b) qPCR for AspA and the inflammatory IL6 gene in macrophages isolated from the bone marrow of P18 WT mice treated with indicated amounts of NAA (n = 3). (c) qPCR of inflammatory TNF-α in bone marrow-derived macrophage cultures of P18 WT and CD mice treated with different doses of NAA. CD mice start to show disease symptoms post P18. (d) qPCR of AspA and inflammatory genes GATA6, TNF-α in WT and CD mouse brains. CD, untreated CD; NAA, N-acetyl aspartic acid; WT, wild-type.
Since ICV administration of NAA caused seizures in rats,8 we explored if NAA acts as a CNS inflammatory agent. The primary immune cells in the brain are microglia, the resident macrophages of the brain. We thus performed in vitro studies on CreJ2 retrovirus-immortalized macrophages derived from P18 WT C57BL/6 bone marrow under increasing concentrations of NAA. While excessive NAA abruptly killed off the cells (100 mmol/l; data not shown), decreased doses (i.e., 1 mmol/l) of NAA instead stimulated proinflammatory gene upregulation, most notably IL6 (Figure 4b). Interestingly, AspA was also upregulated in the WT macrophages upon NAA treatment, presumably to regain homeostasis that was disturbed by excess NAA (Figure 4b). We then isolated bone marrow-derived macrophages from WT and CD animals at P18, an age before severe disease onset in CD mice and at which AspA expression peaked in WT animals.26 We treated the macrophages with two doses of NAA, a lower dose that corresponds to physiological NAA levels (25 µmol/l) and a higher dose (25 mmol/l) that corresponds to CD disease levels.7 The CD patients show a variety of NAA levels in the brain and we picked 25 mmol/l to simulate the higher end of the NAA spectrum. We found that TNF-α is significantly upregulated under CD-relevant NAA dose in both WT and CD-derived macrophages (Figure 4c). Analysis of inflammatory gene expression in homogenates of P25 WT and CD brains showed no upregulation of any inflammatory cytokines, including TNF-α and GATA6 (Figure 4d, data not shown). In fact, low levels of the transcription factor GATA6 have been correlated to subfertile animals,27 which bear out findings in the small litter sizes of other animal models that show a Canavan phenotype.28 However, one caveat in our study is that the whole brain homogenates may not accurately represent localized changes.
We then performed in vivo experiments wherein we intraperitoneally injected 25 mmol/l NAA into P18 WT or (4-(2-hydroxyethyl)-1-piperazineethanesulfonate) (HEPES) buffer into P18 CD mice for 1 hour (acute stress) or 3 days (chronic stress) and examined the brains for expression levels of inflammatory genes; such injections have been reported to cause upregulation of reactive oxygen species.29 Since NAA itself is acidic, it could render metabolic stress to cells; hence, we buffered the solution prior to injection. We found that TNF-α levels were not affected by administration of NAA, but IL6 levels were decreased in WT brains just like in CD mice (see Supplementary Figure S5c). ELISA analyses of IL-1B, IL-6, and TNF-α in the brain lysates showed increases with chronic NAA administration in WT animals as compared to untreated age-matched WT and CD brains, indicating the NAA accumulation may give rise to inflammation and subsequent spongy phenotype in the brain (see Supplementary Figure S5d). Increased IL1β levels have also been previously reported in another mouse model for CD.30
Neural cell types show distinct pathology in CD mice
Thus far, our study reveals that accumulation of NAA in the brain during CD pathogenesis appears to induce inflammatory immune responses in CD mice. In addition to an immunological shift, inflammation in the brain also alters the function of the neuronal neighborhood. For instance, the acidic shift in pH in the brain parenchyma caused by excessive NAA accumulation may impair the viability of oligodendrocyte precursors.6 Considering that CD is a leukodystrophy marked by abnormal myelin,15 we first explored if the myelinating abilities of CD oligodendrocytes are intact and can support survival of neurons in spite of the hallmark accumulation of NAA in CD. We isolated oligodendrocyte progenitor cells from CD and WT pups at postnatal day 4 and allowed them to differentiate to oligodendrocytes. We employed an electrophysiologically active motor neuron cell line derived from human embryonic stem cells expressing GFP driven by the motor neuron-specific Hb9 promoter.31 Equal numbers of Hb9-GFP+ motor neurons were added to the oligodendrocytes for coculture, and NAA was added to a final concentration of 25 mmol/l to simulate diseased conditions. The cocultures were then monitored for motor neuron survival at day 5, at which time myelination and neuronal protection by oligodendrocytes would have occurred. Interestingly, treatment with 25 mmol/l NAA resulted in a complete dissolution of the cell membrane not only in the neurons, but in both CD and WT oligodendrocytes, as evidenced by scanning electron microscopy (Figure 5a). Decreasing the dose of NAA to 1 µmol/l reduced motor neuron survival in both cases to an equivalent degree, while oligodendrocytes were unaffected (see Supplementary Figure S5e). These data highlight the sensitivity of neuronal cell types to NAA exposure, within and even below CD-relevant levels, and the subsequent inflammatory responses it induces in CD.
Figure 5.
Contribution of different neural cells types to CD pathology. (a) Representative images of EM sections of Hb9-GFP+ motor neurons alone and added to WT or CD oligodendrocytes for coculture in the presence of 25 mmol/l NAA to simulate diseased conditions showing devastation of cells. Scale bars = 200 nm. (b) Slices of WT and CD mouse brains were stained for cleaved caspase 3 (green) for apoptosis activation, IbaI (red) for activated microglia and DAPI (blue) for nuclei, reveal an increase in apoptosis in CD mice (correlated with the presence of cleaved caspase 3). Scale bars = 20 μm. (c) qPCR shows a decrease in mRNA expression of Bcl2 in untreated CD mice brains normalized to β-actin levels. (d) Cortical brain slices of CD mice were stained for activated microglia and the numbers were normalized relative to WT mice. (e) Electron microscopy of mitochondria on primary cultures of hippocampal neurons and oligodendrocytes reveal abnormal mitochondrial morphology in neurons and oligodendrocytes (arrows). Scale bars = 200 nm. (f) Primary cultures of hippocampal neurons and oligodendrocytes were stained for NeuN or MBP (green) and aspartoacylase (AspA) A (red) to determine localization. Scale bars = 50 µm. NAA, N-acetyl aspartic acid; WT, wild-type.
In addition to immune-related inflammation and myelination impairment, we also investigated if apoptosis mechanisms added to the exacerbation of CNS-centered CD pathogenesis. Using immunofluorescence for the downstream apoptotic cleavage of caspase 3 in whole brain and primary cell cultures, we observed cleaved caspase 3 in P25 CD but not P25 WT mouse cortex (Figure 5b). Further, mRNA expression of Bcl2, an antiapoptotic gene, was also significantly decreased in untreated CD mice brains (Figure 5c), which may contribute to the apoptotic activity seen with increased cleaved caspase 3. Compared to P25 WT mice, we found a higher proportion of activated microglia in the cortex and hippocampus of CD mice (Figure 5d). Since apoptosis requires the central involvement of mitochondria, we examined mitochondrial morphology in primary cultures of CD mice neurons and oligodendrocytes isolated at embryonic day 18 via electron microscopy. Our studies revealed apparently lysed mitochondrial morphology in both neurons and oligodendrocytes, consistent with previous reports (Figure 5e, arrows).15,32 Intriguingly, immunofluorescence staining in these cultures showed a differentiated localization of AspA in WT oligodendrocytes (nuclear) compared to WT neurons (diffuse) (Figure 5f), though the functional consequence of this observation is unknown. Cumulatively, our data indicate a heretofore unknown involvement of both inflammatory and apoptotic mechanisms driven by CD conditions in the brain.
Discussion
In this study, we utilized a direct ICV route of rAAV gene therapy in CD mice with the intention to deliver higher vector concentrations localized in the CNS with minimal systemic exposure and vector-related toxicity. With the ICV route, we successfully rescued the early lethality of diseased mice with a 20-fold reduced viral load than our previous work with IV injections.15 It is important to note that ICV and IV injections have been used independently in various rAAV gene therapy studies19,33,34,35 including ours to treat experimental animal models of CD. The major drawback of ICV delivery is the limited diffusion of the vector within the brain parenchyma while IV injections targeted to the CNS using rAAVs that cross the BBB require large doses to be significantly efficacious. It is worth stating that a higher dose via the ICV route may be more efficacious benefit therapy, while still at a considerably lower dose than what was given via the IV route. This dosing route may become even more attractive as a recently published study demonstrated that levels of anti-AAV antibodies found typically in the general population does not negatively influence subsequent AAV-mediated gene transfer to the mouse CNS or prime a potentially neurotoxic neuroinflammatory response.36
In most clinical parameters evaluated, including survival (Figure 1a), body weight (Figure 1b), and vision acuity (Figure 1e), we observed no difference between the ICV and IV routes of delivery over the duration of the study. In addition, ICV injections showed a remarkably enhanced rescue of edema in the brain evidenced by change in magnetic resonance imaging signal (Figure 1d). This evidence initially supported the idea that CNS restriction in CD therapy is advantageous over systemic delivery. However, in stark contrast to systemic IV therapy, CNS-restricted ICV-treated CD failed to maintain motor function rescue over a short duration (Figure 1f). A similar result occurred in animals injected with an IV-delivered vector designed to detarget expression from the heart, liver, and skeletal muscle by endogenous miRNA regulation, as described earlier.15 These data implicate a role for peripheral tissues in disease pathology that must be addressed to design effective long-term treatment.
CD is currently defined as a CNS-restricted disorder and patient data for peripheral defects are limited.21 AspA is normally expressed across multiple tissues (Figure 2a),22 so we scrutinized the CD mice for evidence of pathology outside the CNS. While tissues were not universally altered in CD mice, as seen in liver, intestinal, and thymus data, we noted several changes. In addition to organ-size deviations (Figure 2b, Supplementary Figure S3a), untreated CD mice also exhibited renal tubular defects (Figure 3a), bradycardia (Figure 3b), skeletal muscle abnormalities (Figure 3c), collapsed NMJs (Figure 3d), retinal and spleen deterioration (Figure 3e,f), all indicative of a role for peripheral tissues in CD pathogenesis. Of note is the finding that renal tubules show dilation in CD mice. While this dilation could arise due to obstruction in the latter part of the nephron, it is more likely a function of intrinsic tubular injury, as we observed such morphology in the tubular epithelium of diseased mice (Figure 3a). It is possible that the tubular epithelium experiences a similar accumulation of NAA that we observed in neurons, leading to abnormal reabsorption and high NAA efflux in urine. Moreover the filtration process by the glomeruli may expose more regions of the nephron to excess NAA. Similarly, our data also suggest that deficient NAA metabolism in the eye, coupled with the proximal exposure of excess NAA from the brain, may promote optic pathology (Figure 3e). Reducing NAA in the brain may therefore help to alleviate symptoms in the eye.
It is widely accepted that CD pathology arises from a deficiency of AspA. The damage perpetrated by the subsequent accumulation of NAA cannot be underestimated, despite evidence that WT levels of NAA does not cross the BBB.37 Stemming from the observed structural abnormalities in the spleen (Figure 3f) and significantly decreased B-cell counts in the spleens of untreated CD mice (Supplementary Figure S4b), we examined CD-relevant NAA levels and its subsequent generation of free radicals29 and found upregulated IL6 levels in immortalized WT macrophages (Figure 4b), which is known to induce autoimmunity.38 We do, however, observe that we saw effects with 1 mmol/l NAA but not with 10 mmol/l NAA, there is lack of a dose response which would preclude us from making strong conclusions from the data. Additionally, exposure of macrophages derived from the bone marrow of CD and WT animals to CD-relevant NAA in vitro induced upregulation of the inflammatory factor TNF-α (Figure 4c). Moreover, IL1β upregulation detected by ELISA (Supplementary Figure S5d) also bears out a previous observation,30 indicating a propensity of matrix destruction in the diseased brain to induce a positive feedback loop for IL1β and matrix metalloproteinases.39 In the light of the recent discovery of lymphatic drainage system in the CNS,40 the NAA-induced immune response may attract systemic immune cells to accelerate the neurodegeneration seen in CD patients. Examination of peripheral immune cell status also revealed an inability of the splenocytes to proliferate when challenged by CD28 (Figure 3g); however, we should be mindful that stress from disease pathology could also produce such a phenotype. Overall, our data implicate a systemic immune response to NAA stimulation in mouse pathophysiology that may be translated to humans.
Excessive NAA administration to the rodent brain leads to seizures,8 which is a common symptom in CD patients.1 Though we recently tested CD mice for permeability of the BBB using Evans Blue injections and found no significant change in BBB permeability overall,41 our data on reduced neuron counts in the relatively exposed brain stem still support the possibility of toxicity via excess NAA in CD brain (Supplementary Figure S5b). Absence of significant differences between the proportion of viable cells between WT and CD animals (Supplementary Figure S5a) do not agree with previously reported data42 and could potentially reflect the effect of age and mouse model used.
The current study detected inflammatory responses in CD brains and tested the role of these responses on white matter degeneration and vacuolation in CD. Ex vivo brain homogenates of WT and CD animals did not display a systemic increase in TNF-α seen in peripherally derived macrophages, indicating that such a response in the CNS is likely localized rather than generalized (Figure 4d). Indeed, brain sections of untreated CD mice contained abundant activated microglia (Figures 4a and 5d) and localized apoptotic caspase activity (Figure 5c). Administration of the caspase inhibitor minocycline failed to rescue the neurodegenerative phenotype in CD mice (data not shown), a result that might point to upstream causative factors in the failed toxicity response. Interestingly, when WT mice were treated with buffered NAA for 3 days, their brains did not show massive vacuolation (data not shown), but rather an elevation of inflammatory cytokines (Supplementary Figure S5d). These data illustrate that the effects of NAA in WT conditions may be masked by homeostatic adjustments, such as upregulation of AspA in macrophages (Figure 4d) not available to CD brains. Our results introduce the novel potential for immunological neuroinflammation in the acceleration of CNS cell death in CD, but follow-up studies are necessary to further elucidate this notion.
In conclusion, our study establishes the efficiency of ICV-delivered gene therapy vectors for CD, which rescue lethality and extend survival at a 20-fold decreased dose compared to our published IV gene therapy study. We simultaneously extend involvement of several peripheral tissues in the pathology of this heretofore neurologically limited disorder except for a report on the lungs21 and finally propose NAA-induced immune responses as a probable factor in CD pathogenesis. Our findings are informative and important, providing important guidelines to be considered when designing optimized therapeutics to target this fatal disease.
Materials and Methods
Viral production. All rAAV vectors were produced by transient transfection of 293 cells and CsCl gradient sedimentation as previously described, and formulated in a buffer consisting of 5% sorbital and 0.001% pluronic F-68 in PBS, pH7.6.24 Vector preparations were titered by quantitative PCR, purity of vectors was assessed by 4–12% sodium dodecyl sulfate (SDS)-acrylamide gel electrophoresis and silver staining (Invitrogen, Carlsbad, CA) and morphological integrity of virions was assessed by transmission electron microscopy of negative-stained recombinant AAV virions24 at Electron Microscopy Core, UMass Medical School, Worcester, MA.
Animal procedures. All animal procedures were approved by the Institutional Animal Care and Use Committee of UMass Medical School. AspA+/− Sv129/Ev mice littermates were bred using programmatic timing and newborn pups were dosed on P0. Vectors were injected in the facial vein of P0 pups at doses of 4 × 1011 GC/mouse in a final volume of 100 μl and intracerebroventricularly at a dose of 2 × 1010 GC/mouse in a final volume of 2 μl unilaterally, respectively. After the injection pups were cleaned, they were rubbed with bedding, and then returned to their cage. The dam was reintroduced after brief nose numbing using ethanol pads.
Immunohistochemistry. Animals were anesthetized, transcardially perfused with 4% paraformaldehyde (v/v) in PBS. Whole carcasses were postfixed in fixative for 1 day. Organs were extracted, rinsed in PBS, and sagitally sectioned. One half was cryoprotected in 30% sucrose (w/v) in PBS at 4 °C, embedded in optical coherence tomography compound (Sakura Finetek, Torrance, CA) and frozen in a dry ice/ethanol bath. 40-m floating sections of the entire brain were cut in a Cryostat (Thermo Microm HM 550) for immunostaining. The other half was processed for H&E staining for morphological studies.
Floating sections were stained in 12-well plates. Sections were washed once in PBS for 5 minutes, and incubated in blocking solution containing 0.05% Tween-20 (v/v) (Fisher, Pittsburgh, PA) and 10% goat serum (v/v) (Invitrogen, Carlsbad, CA) for 1 hour at room temperature. Sections were incubated with primary antibodies at 4 °C overnight, washed twice in 0.05% Tween-20 in PBS (PBST) for 15 minutes each followed by incubation in appropriate secondary antibodies at room temperature for 1 hour. Sections were washed twice in 0.05% Tween-20 in PBS for 10 minutes each before mounting on glass slides. Vectashield with 4,6-diamidino- 2-phenylindole (Vector Laboratories, Burlingame, CA) was used to mount all slides. Omission of either the primary or the secondary antibody in single-label experiments resulted in no labeling. The primary antibodies used were as follows: mouse aspartoacylase (Abmart, Berkeley Heights, NJ); mouse NeuN (MAB-377, clone A60; Millipore); rabbit NeuN (ABN-78, Millipore) and appropriate secondary antibodies from Invitrogen. For the NMJ staining, 100 NMJs from each muscle were randomly selected and assessed under the microscope to quantify the number of collapsed NMJs for each TA and quadriceps muscle group per animal (n = 3 mice) per group.
Imaging and image analysis. Stained sections were examined using DM 5500B Upright microscope (Leica Microsystems, Wetzlar, Germany), and images were captured with Leica DFC365 FX high-sensitivity monochrome digital camera. Regions of interest were identified according to the mouse brain atlas. Z stack of images was taken with a 63× objective and 1.6× additional magnification for each channel and overlaid to obtain a multicolor image at 100.8×. Deconvolution was performed using Leica Application Suite Advanced Fluorescence software.
Electrocardiography. ECGs were recorded noninvasively in conscious mice similar to a method described previously for mice.43 Briefly, conscious mice were removed from their cages and positioned atop the ECGenie™ recording platform (Mouse Specifics, Boston, MA). The ECG signals were detected passively through the feet of the animals as they rested upon electrodes embedded in the platform floor. Since even modest handling may induce alterations in heart rate, each mouse was permitted to acclimatize for ∼10 minutes prior to collection of data. The signals were digitized at a sampling rate of 2,000 samples/second. When mice were positioned such that a forepaw and hind paw were not uniquely in contact with one of the electrodes, the output from the amplifier was discarded. Only data from continuous recordings were used in the analyses. Each signal was analyzed using EzCG™, which incorporates Fourier analyses and linear time-invariant digital filtering of frequencies below 3 Hz and above 100 Hz to minimize environmental signal disturbances. The software uses a peak detection algorithm to find the peak of the R-waves and to calculate heart rate. The software plots its interpretation of P, Q, R, S, and T for each beat so that spurious data resulting from unfiltered noise or motion artifacts may be rejected.
Western blot. Mice were killed and their tissues were extracted and homogenized as described.44 Protein in equal quantities was loaded into a 12% Tris–HCl gel well (Bio-Rad Laboratories, Hercules, CA) and transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). The membrane was incubated in blocking buffer for 1 hour followed by AspA polyclonal antibody overnight. After three washes in 0.1% of 0.05% Tween-20 in PBS, it was incubated with secondary antibodies conjugated to LI-COR IRDye for 1 hour at room temperature. After two washes with 0.1% of 0.05% Tween-20 in PBS, signal was detected using the Odyssey Imager (LICOR, Lincoln, NE).
Genome copy number. rAAV genomes were quantified in nanograms of genomic DNA extracted from mouse tissues using the QIAamp DNA mini kit for quantitative detection of vector genome copies by Taqman® probes (Applied Biosystems) with a single-copy endogenous GAPDH gene as the diploid cell number reference. The Taqman® real-time PCR kit was run with primer sets which amplified regions of the nRBG poly A (probe, 6FAM-ATG AAG CCC CTT GAG CAT CTG ACT TCT-TAMRA; forward, GCC AAA AAT TAT GGG GAC AT; reverse, ATT CCA ACA CAC TAT TGC AAT G). The sensitivity of the assay was 10 copies/cell.
AspA activity assay. Mice were killed 3 months after injection, and brain homogenates were used for the enzyme assay. Briefly, the brain was homogenized and incubated with 50 mmol/l Tris–HCl (pH 8.0), 0.5% (w/v) NP-40, 50 mmol/l NaCl, 1 mmol/l CaCl2, 2.8 mmol/l NAA in a total volume of 600 µl. After incubation at 37 °C for 3 hours, the assay mixture was centrifuged and the supernatant was incubated with malic dehydrogenase, glutamic oxalacetic transaminase, and NADH for 10 minutes at 37 °C. The amount of l-aspartate released was estimated by decrease in absorbance due to the conversion of NADH to NAD using a spectrophotometer (Shimadzu, Columbia, MD) at 340 nm. One milliunit of aspartoacylase activity is equivalent to 1 nmol of aspartate released in 1 minute. Values were calculated using ANOVA. The value P < 0.05 was considered significant.
1H magnetic resonance imaging and spectroscopy. Mice were anesthetized with 2% isofluorane and constantly monitored for vital signs. All experiments were carried out on a Bruker 4.7-T/40-cm horizontal magnet (Oxford, UK) equipped with 250 mT/m magnetic field gradient and interfaced with a ParaVision 4.0 console. A 1H radiofrequency coil configuration (Insight NeuroImaging Systems, Marlborough, MA) with inner diameter of 4 cm was used for the experiments. T2-weighted anatomical images were acquired using a fast spin-echo sequence (Rapid Imaging with Refocused Echoes (RARE)) (repetition time (TR) = 3,000 msecond; echo time (TE) = 48 msecond, matrix size = 256 × 256; field of view (FOV) = 2.5 cm × 2.5 cm, slice number = 16, slice thickness = 1 mm). 1H magnetic resonance spectroscopy data were acquired using single voxel (point resolved spectroscopy sequence) (TR = 2,500 msecond, TE = 20 msecond, Naverage = 1,024, voxel size = 3 mm × 3 mm × 3 mm).
Proton spectra were fit using LCModel (Version 6.2-2B) which analyzed in vivo proton spectrum as a linear combination of model in vitro spectra from individual metabolite solutions and generated data as absolute fits (in institutional units) and standard deviations (SD%). SD was used as a measure of the reliability of the fit. The spectral inclusion criteria were SD less than 20% for NAA, Cr, and Ins.
Behavioral studies. Neuromuscular function of the mice was assessed by testing grip strength on an inverted screen. Mice were placed in the center of a screen (30 cm2 square-wire mesh, 25 mm2 holes) until they gripped the mesh. The screen was then inverted above a cushioned surface for a 2-second period with the mouse's head declining. Latency to fall from the screen was recorded, with a 2-min time limit for each trial.
Mice were subject to a rotarod test to evaluate motor coordination and balance. Each animal was placed on the rotarod after which speed was adjusted to 3 rpm. The latency to fall off the rotarod within this time period was recorded for fixed speed. Animals were also tested for their latency to fall off at accelerated speeds going from 4 to 40 rpm in 5 minutes.
Animals were tested for each experiment three times with an intertrial interval of approximately 30 minutes for each animal and mean of three trials was used to plot the graphs.
Transmission electron microscopy. Mice were anesthetized and perfused with a solution of 2.5% glutaraldehyde in 0.05 mol/l sodium phosphate buffer, pH 7.2. The brain was extracted and incubated in 2.5% glutaraldehyde in 0.05 mol/l cacodylate buffer overnight at 4 °C. It was sectioned into 1-mm thick sections, rinsed twice in the same buffer and postfixed with 1% osmium tetroxide for 1 hour at room temperature. Sections were washed in DH2O for 20 minutes at 4 °C and then dehydrated in graded ethanol series of 20% increments, before two changes in 100% ethanol. Sections were infiltrated with two changes of 100% propylene oxide and then with a 50%/50% propylene oxide/SPI-Pon 812 resin mixture overnight. After three changes of fresh 100% SPI-Pon 812 resin, sections were polymerized at 68 °C in plastic capsules. Regions of interest were cut out and thick-sectioned for toluidine blue. Chosen regions were reoriented and approximately 70-nm thin sections were placed on copper support grids and contrasted with lead citrate and uranyl acetate. Sections were examined using the FEI Tecani 12 BT with 80-Kv accelerating voltage, and images were captured using a Gatan TEM CCD camera.
X-ray diffraction analysis. X-ray diffraction was performed as described.45 Briefly, optic nerves and spinal cords were dissected from mice killed using isoflurane. Optic nerves and sagitally bisected segments of cervical spinal cord were maintained in physiological saline (154 mmol/l NaCl, 5 mmol/l phosphate, pH 7.4) and loaded into thin-walled, quartz capillary tubes which were then filled with saline and sealed with wax and enamel. X-ray diffraction experiments were carried out using nickel-filtered, single-mirror focused CuKα radiation from a fine-line source on a 3.0-kW Rigaku X-ray generator operated at 40 kV by 10 mA. Exposure times were 30 minutes. Diffraction patterns were collected using a linear, position-sensitive detector (Molecular Metrology, Northampton, MA) and analyzed using PeakFit (Jandel Scientific, San Rafael, CA). Myelin period was calculated from the positions of the intensity maxima in the diffraction patterns. The relative amount of myelin in each sample was calculated by measuring the total integrated intensity of all maxima over background, excluding small-angle scatter around the beam stop and wide-angle scatter.
Statistical analyses. Statistical calculations included Log rank Mantel Cox test for the survival table and one-way ANOVA followed by Tukey's Multiple Comparison test for all other experiments. *P < 0.05; **P < 0.01; ***P < 0.001, NS not significant.
SUPPLEMENTARY MATERIAL Figure S1. Alleviation of neuropathology and retinal transduction compared between IV and ICV modes of delivery. Figure S2. Pattern of EGFP expression in mouse brain after neonatal intracerebroventricular injections. Figure S3. Involvement of the peripheral organs in CD pathology. Figure S4. Effects of CD on peripheral tissues. Figure S5. Effects of NAA accumulation on various cell types.
Acknowledgments
We would like to acknowledge Laura Kaushansky for helping refine the manuscript, Dominic Gessler for helpful suggestions, Lyndsey Braun for her assistance in maintaining mice for the in vitro myelination assay and Claudio Punzo and Vijay Vanguri for their thoughtful insights in interpreting eye and kidney data. The study was funded by an internal grant from University of Massachusetts, grants from Jacob's Cure, NTSAD Foundation, and Canavan Foundation and NIH R01 grant (1R01NS076991) to GG, and partially supported by a grant from National High Technology Research and Development Program (“863”Program) of China (2012AA020810) to GG. The electron microscopy was supported by Award Number S10RR027897 from the National Center for Research Resources. X-ray diffraction analysis was supported by a grant from the European Leukodystrophy Association (ELA), #ELA2010-042C5B (D.A.K.). G.G. is a cofounder of Voyager Therapeutics and holds equity in the company. GG is an inventor on patents with potential royalties licensed to Voyager Therapeutics and other biopharmaceutical companies. B.K. is the Chief Scientific Officer and on the board of directors for Avexis. The other authors declare that they have no financial interests to disclose.
Supplementary Material
References
- Traeger, EC and Rapin, I (1998). The clinical course of Canavan disease. Pediatr Neurol 18: 207–212. [DOI] [PubMed] [Google Scholar]
- Kaul, R, Gao, GP, Balamurugan, K and Matalon, R (1993). Cloning of the human aspartoacylase cDNA and a common missense mutation in Canavan disease. Nat Genet 5: 118–123. [DOI] [PubMed] [Google Scholar]
- Matalon, R, Michals, K, Sebesta, D, Deanching, M, Gashkoff, P and Casanova, J (1988). Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am J Med Genet 29: 463–471. [DOI] [PubMed] [Google Scholar]
- Birnbaum, SM, Levintow, L, Kingsley, RB and Greenstein, JP (1952). Specificity of amino acid acylases. J Biol Chem 194: 455–470. [PubMed] [Google Scholar]
- Mehta, V and Namboodiri, MA (1995). N-acetylaspartate as an acetyl source in the nervous system. Brain Res Mol Brain Res 31: 151–157. [DOI] [PubMed] [Google Scholar]
- Jagielska, A, Wilhite, KD and Van Vliet, KJ (2013). Extracellular acidic pH inhibits oligodendrocyte precursor viability, migration, and differentiation. PLoS One 8: e76048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett, JR, Ross, B, Arun, P, Madhavarao, CN and Namboodiri, AM (2007). N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81: 89–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimitsu, T, Kurisu, K, Hanaya, R, Iida, K, Kiura, Y, Arita, K et al. (2000). Epileptic seizures induced by N-acetyl- l -aspartate in rats: in vivo and in vitro studies. Brain Res 861: 143–150. [Google Scholar]
- Pederzolli, CD, Mescka, CP, Scapin, F, Rockenbach, FJ, Sgaravatti, AM, Sgarbi, MB et al. (2007). N-acetylaspartic acid promotes oxidative stress in cerebral cortex of rats. Int J Dev Neurosci 25: 317–324. [DOI] [PubMed] [Google Scholar]
- Baslow, MH (1999). Molecular water pumps and the aetiology of Canavan disease: a case of the sorcerer's apprentice. J Inherit Metab Dis 22: 99–101. [DOI] [PubMed] [Google Scholar]
- Madhavarao, CN, Arun, P, Moffett, JR, Szucs, S, Surendran, S, Matalon, R et al. (2005). Defective N-acetylaspartate catabolism reduces brain acetate levels and myelin lipid synthesis in Canavan's disease. Proc Natl Acad Sci USA 102: 5221–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W and Popko, B (2009). Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci 12: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray, S, Huynh, JL, Sher, F, Casaccia-Bonnefil, P and Boddeke, E (2009). Epigenetic mechanisms facilitating oligodendrocyte development, maturation, and aging. Glia 57: 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon, R, Rady, PL, Platt, KA, Skinner, HB, Quast, MJ, Campbell, GA et al. (2000). Knock-out mouse for Canavan disease: a model for gene transfer to the central nervous system. J Gene Med 2: 165–175. [DOI] [PubMed] [Google Scholar]
- Ahmed, SS, Li, H, Cao, C, Sikoglu, EM, Denninger, AR, Su, Q et al. (2013). A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS gene therapy in Canavan mice. Mol Ther 21: 2136–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H, Yang, B, Mu, X, Ahmed, SS, Su, Q, He, R et al. (2011). Several rAAV vectors efficiently cross the blood–brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther 19: 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust, KD, Nurre, E, Montgomery, CL, Hernandez, A, Chan, CM and Kaspar, BK (2009). Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B, Li, S, Wang, H, Guo, Y, Gessler, DJ, Cao, C et al. (2014). Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol Ther 22: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust, KD, Wang, X, McGovern, VL, Braun, L, Bevan, AK, Haidet, AM et al. (2010). Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 28: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Passini, MA and Wolfe, JH (2001). Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J Virol 75: 12382–12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois, J and Manaligod, JM (2002). Upper airway abnormalities in Canavan disease. Int J Pediatr Otorhinolaryngol 66: 303–307. [DOI] [PubMed] [Google Scholar]
- Mersmann, N, Tkachev, D, Jelinek, R, Röth, PT, Möbius, W, Ruhwedel, T et al. (2011). Aspartoacylase-lacZ knockin mice: an engineered model of Canavan disease. PLoS One 6: e20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon, R and Michals-Matalon, K (1999). Biochemistry and molecular biology of Canavan disease. Neurochem Res 24: 507–513. [DOI] [PubMed] [Google Scholar]
- Gao, GP and Sena-Esteves, M (2012). Introducing Genes into Mammalian Cells: Viral Vectors. Cold Spring Harbor Laboratory Press: New York. [DOI] [PubMed] [Google Scholar]
- Fischer, HG and Reichmann, G (2001). Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol 166: 2717–2726. [DOI] [PubMed] [Google Scholar]
- Kirmani, BF, Jacobowitz, DM and Namboodiri, MA (2003). Developmental increase of aspartoacylase in oligodendrocytes parallels CNS myelination. Brain Res Dev Brain Res 140: 105–115. [DOI] [PubMed] [Google Scholar]
- Bennett, J, Wu, YG, Gossen, J, Zhou, P and Stocco, C (2012). Loss of GATA-6 and GATA-4 in granulosa cells blocks folliculogenesis, ovulation, and follicle stimulating hormone receptor expression leading to female infertility. Endocrinology 153: 2474–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran, S, Szucs, S, Tyring, SK and Matalon, R (2005). Aspartoacylase gene knockout in the mouse: impact on reproduction. Reprod Toxicol 20: 281–283. [DOI] [PubMed] [Google Scholar]
- Pederzolli, CD, Rosa, AP, de Oliveira, AS, Coelho, JG, Becker, D da L, Dalazen, GR et al. (2010). Neuroprotective role of lipoic acid against acute toxicity of N-acetylaspartic acid. Mol Cell Biochem 344: 231–239. [DOI] [PubMed] [Google Scholar]
- Matalon, R, Michals-Matalon, K, Surendran, S and Tyring, SK (2006). Canavan disease: studies on the knockout mouse. Adv Exp Med Biol 576: 77–93; discussion 361. [DOI] [PubMed] [Google Scholar]
- Karumbayaram, S, Kelly, TK, Paucar, AA, Roe, AJ, Umbach, JA, Charles, A et al. (2009). Human embryonic stem cell-derived motor neurons expressing SOD1 mutants exhibit typical signs of motor neuron degeneration linked to ALS. Dis Model Mech 2: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi, M, Torii, J, Schneck, L and Volk, BW (1972). Electron microscopic and enzyme histochemical studies of the cerebellum in spongy degeneration (van Bogaert and Bertrans type). Acta Neuropathol 20: 22–31. [DOI] [PubMed] [Google Scholar]
- Chai, X, Kong, W, Liu, L, Yu, W, Zhang, Z and Sun, Y (2014). A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis. Neural Regen Res 9: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, KL, Gardiner, JV, Ward, HL, Kong, WM, Murphy, KG, Martin, NM et al. (2008). Overexpression of CART in the PVN increases food intake and weight gain in rats. Obesity (Silver Spring) 16: 2239–2244. [DOI] [PubMed] [Google Scholar]
- Ahmed SS, Li H, Cao C, Sikoglu EM, Denninger AR, Su Q et al. (2013). A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS gene therapy in canavan mice. Mol Ther 21:2136–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treleaven, CM, Tamsett, TJ, Bu, J, Fidler, JA, Sardi, SP, Hurlbut, GD et al. (2012). Gene transfer to the CNS is efficacious in immune-primed mice harboring physiologically relevant titers of anti-AAV antibodies. Mol Ther 20: 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlinguet, L and Laliberté, M (1966). Metabolism of N-acetyl- l -aspartic acid in mice. Can J Biochem 44: 783–789. [Google Scholar]
- Ishihara, K and Hirano, T (2002). IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev 13: 357–368. [DOI] [PubMed] [Google Scholar]
- Tsuzaki, M, Guyton, G, Garrett, W, Archambault, JM, Herzog, W, Almekinders, L et al. (2003). IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res 21: 256–264. [DOI] [PubMed] [Google Scholar]
- Louveau, A, Smirnov, I, Keyes, TJ, Eccles, JD, Rouhani, SJ, Peske, JD et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, SS and Gao, G (2014). Examination of the blood brain barrier integrity in a mouse model of the neurodegenerative Canavan's disease. J Neurol Disord. Nov; 2(6); i105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, JS, Strande, L, Markov, V and Leone, P (2012). Aspartoacylase supports oxidative energy metabolism during myelination. J Cereb Blood Flow Metab 32: 1725–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, V, Otero, JM, Lopez, O, Morgan, JP, Amende, I and Hampton, TG (2001). Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J, Xie, Q, Zhang, H, Ameres, SL, Hung, JH, Su, Q et al. (2011). MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol Ther 19: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila, RL, Inouye, H, Baek, RC, Yin, X, Trapp, BD, Feltri, ML et al. (2005). Structure and stability of internodal myelin in mouse models of hereditary neuropathy. J Neuropathol Exp Neurol 64: 976–990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.