Abstract
Cognitive impairment is associated with changes in cerebral metabolites in patients with obstructive sleep apnea (OSA). Several studies have used magnetic resonance spectroscopy (MRS) to detect variations in cerebral metabolites; however, the results have been inconsistent. This meta-analysis summarizes the differences in cerebral metabolites between patients with OSA and controls. Two electronic databases, PubMed and Embase, were searched for articles (published before March 31, 2016) describing studies that used MRS to evaluate the cerebral metabolite changes. The overall effects were measured using the weighted mean difference with a 95% confidence interval. Subgroup analysis and sensitivity analysis were used to explore the sources of between-study heterogeneity and the stability of the results. Publication bias was also evaluated. Thirteen studies were ultimately included. In the hippocampus, the N-acetylaspartate (NAA)/creatine ratio was lower in patients with OSA. In the frontal lobe, only the NAA/choline ratio was lower in patients with OSA. Cerebral metabolites are significantly altered in the hippocampus in patients with OSA. Further clinical studies are needed to explore the underlying mechanisms between OSA and the changes in cerebral metabolites in the brain.
Obstructive sleep apnea (OSA) is one of the most common sleep disturbances, affecting 4% to 9% of the adult population1,2. It is characterized by repetitive complete (apnea) or partial (hypopnea) obstruction of the upper airway during sleep. The pathophysiology of OSA includes oxygen desaturation, micro-arousals, and abnormal ventilation and sleep architecture3. OSA can increase the risk of cardiovascular disease4, stroke5, metabolic disease6, excessive daytime sleepiness7, work-place errors, traffic accidents8, and death9, resulting in significant economic burden. Therefore, it is an important health problem that should not be overlooked10. Altered cognitive functions have recently been reported in patients with OSA, including inattention, poor memory, and general intellectual and executive dysfunctions11,12. These cognitive impairments could affect the quality of life of patients with OSA.
Structural-metabolic changes in some regions of the brain are responsible for the occurrence of cognitive impairment13. Magnetic resonance spectroscopy (MRS) is a noninvasive method commonly used to evaluate local changes in metabolites, such as N-acetylaspartate (NAA), choline (Cho), creatine (Cr), glutamate and glutamine (Glx), and myo-inositol (mI). NAA is a marker of neuronal integrity, while Cho is associated with membrane metabolism14. Cr reflects energy metabolism, Glx regulates neurotransmitter activity, and mI is considered a glial cell marker14. Measurements of these metabolites serve as an indicator of cerebral impairment, including neuronal loss, axonal injury, and gliosis.
Many small-scale studies have sought to determine whether the cerebral metabolites in brain regions differ between patients with OSA and controls to determine the pathophysiology of cognitive impairment in patients with OSA. However, the results have been inconsistent. To the best of our knowledge, no systematic review or meta-analysis has evaluated the changes in cerebral metabolites in patients with OSA. Therefore, we examined this issue in the present study.
Results
Search results
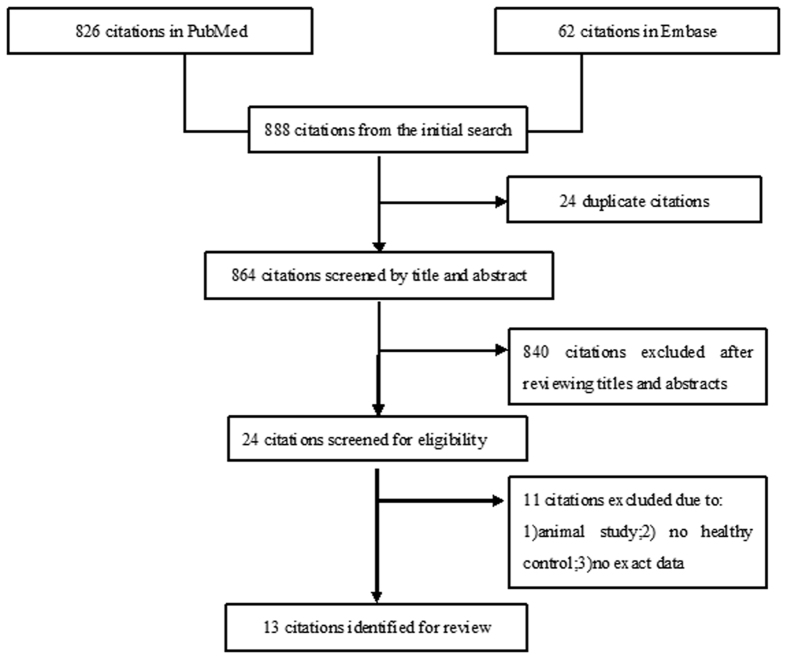
The initial search retrieved 888 references from PubMed (n = 826) and Embase (n = 62). Of these 888 citations, 24 papers were initially removed due to duplication. A further 840 records were removed after screening by titles and abstracts. For the remaining 24 papers, we carefully reviewed the full text and excluded an additional 11 articles due to the following reasons: (1) animal study, (2) control group was not healthy, or (3) no exactable data15,16,17,18,19,20,21,22,23,24,25. Ultimately, 13 articles (269 cases and 233 controls) evaluating changes in cerebral metabolites in OSA were included. The flow diagram of the search procedure is shown in Fig. 1.
Figure 1. Flow diagram of selection procedure in this meta-analysis showing the number of records retrieved and the number of studies included.
Study characteristics
The 13 articles were published between February 1997 and February 2016: three were studies conducted in America26,27,28; two each were studies conducted in Italy29,30, Australia31,32, and Turkey33,34; and the remainder were from Greece35, Egypt36, Japan37, and India38. Most of the subjects were middle-aged, while only one study focused on pediatric OSA26. The mean Epworth Sleep Scale (ESS) score ranged from 8.2 to 11.7, and the mean apnea-hypopnea index (AHI) was 19.0 to 71.5 events per hour in patients with OSA. The percentage of males ranged from 64% to 100% and mean body mass index (BMI) was 26.4 to 47.3 kg/m2 in the OSA groups. According to the Newcastle–Ottawa Scale (NOS) guidelines, three studies scored 6 points, eight scored 5 points, and two scored 4 points. This information is summarized in Table 1.
Table 1. Study characteristics.
| |
OSA |
Control |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | No. | Age | Male (%) | BMI | AHI | ESS | No. | Age | Male (%) | BMI | AHI | ESS | NOS |
| Kamba et al.37 | Japan | 23 | 48.5 ± 12.9 | 82.6 | (–) | (–) | (–) | 15 | 45.7 ± 17.6 | 46.7 | (–) | (–) | (–) | 5 |
| Alchanatis et al.35 | Greece | 22 | 49 ± 9.7 | 100 | (–) | 70.6 ± 19.4 | 8.2 ± 3.3 | 10 | 42.9 ± 10.5 | 100 | (–) | 3.4 ± 1.5 | (–) | 6 |
| Alchanatis et al.35 | Greece | 14 | 48 ± 10.1 | 100 | (–) | 70.1 ± 19.8 | 8.4 ± 2.5 | 10 | 42.9 ± 10.5 | 100 | (–) | 3.4 ± 1.5 | (–) | 6 |
| Bartlett et al.31 | Australia | 8 | 47.7 | 100 | 30.8 | (–) | (–) | 5 | 51.8 | (–) | 25.0 | (–) | (–) | 5 |
| Halbower et al.26 | America | 6 | 11.5 ± 3.5 | (–) | 30.0 ± 6.2 | 37.9 ± 8.0 | (–) | 6 | 11.0 ± 2.3 | (–) | 20.9 ± 4.9 | 0.4 ± 0.2 | (–) | 5 |
| Halbower et al.26 | America | 7 | 11.0 ± 3.1 | (–) | 29.4 ± 5.8 | 19.0 ± 9.9 | (–) | 6 | 11.0 ± 2.3 | (–) | 21.0 ± 4.8 | 0.2 ± 0.3 | (–) | 5 |
| Tonon et al.30 | Italy | 14 | 48 ± 7 | 100 | 32.3 ± 4.6 | (–) | 13 ± 2 | 10 | (–) | 100 | 25 ± 2 | (–) | (–) | 5 |
| Sarchielli et al.29 | Italy | 20 | 52.7 ± 11.0 | 65 | 26.4 ± 2.1 | 16.7 ± 15.0 | 11.7 ± 1.7 | 20 | 51.4 ± 13.2 | 70 | 22.1 ± 3.4 | 2.7 ± 0.3 | 4.4 ± 0.8 | 5 |
| Sharma et al.38 | India | 18 | 48.0 ± 8.8 | 66.7 | 30.0 ± 5.5 | 46.7 ± 29.8 | 12 ± 5.6 | 32 | 39.9 ± 10.2 | 65.6 | 27.2 ± 5.9 | 0.9 ± 1.3 | 5.5 ± 4.1 | 5 |
| Algin et al.33 | Turkey | 24 | 52 | 95.8 | (–) | 56 ± 8 | (–) | 9 | 48 | 66.7 | (–) | <5 | (–) | 5 |
| O’Donoghue et al.32 | Australia | 30 | 45.2 ± 9.6 | 100 | 33.3 ± 4.6 | 71.5 ± 16.2 | 12.9 ± 4.3 | 23 | 41.3 ± 9.3 | 100 | 25.6 ± 2.9 | 5.3 ± 3.9 | 5.2 ± 3.4 | 6 |
| Gharraf et al.36 | Egypt | 15 | 48.1 ± 6.9 | 73.3 | 47.3 ± 13.5 | 65.5 ± 20.3 | 17.7 ± 2.5 | 10 | 49.1 ± 7.6 | 70 | 26.2 ± 2.1 | 2.7 ± 1.2 | 3.4 ± 1.2 | 6 |
| Kizilgoz et al.34 | Turkey | 20 | 47.7 ± 11.6 | 75 | (–) | 29.3 ± 21 | (–) | 5 | 43.8 ± 2.6 | 20 | (–) | 4 ± 0.88 | (–) | 4 |
| Yadav et al.28 | America | 36 | 48 ± 9.3 | 77.8 | 30.6 ± 5.9 | 30.6 ± 5.9 | 9.9 ± 4.9 | 53 | 46.8 ± 8.1 | 60.4 | 24.7 ± 3.8 | (–) | 5.7 ± 3.3 | 5 |
| Macey et al.27 | America | 14 | 47.5 ± 10.5 | 64.2 | (–) | 29.5 ± 15.6 | (–) | 22 | 47.5 ± 10.1 | 50 | (–) | (–) | (–) | 4 |
Abbreviations: OSA, Obstructive sleep apnea; No., number of enrolled subjects; Male (%), percentage of male subjects; AHI, Apnea/Hypopnea Index; BMI, Body Mass Index; ESS, Epworth Sleepiness Score; NOS, Newcastle–Ottawa Scale.
Main results
Five studies26,31,32,33,34 evaluated the changes in the NAA/Cr ratio in the hippocampus [weighted mean difference (WMD), −0.08; 95% confidence interval (CI), −0.14 to −0.02; p < 0.01). There was no evidence of significant between-study heterogeneity (I2 = 0%, p = 0.52). Four studies26,32,33,34 evaluated changes in the NAA/Cho ratio in the hippocampus (WMD, −0.12; 95% CI, −0.36 to 0.11; p = 0.30); however, there was significant between-study heterogeneity (I2 = 77%, p < 0.01). Four studies26,32,33,34 evaluated changes in the Cho/Cr ratio in the hippocampus (WMD, −0.01; 95% CI, −0.10 to 0.08; p = 0.78) and showed significant heterogeneity (I2 = 63%, p = 0.05). Six studies26,29,32,33,35,36 evaluated changes in the NAA/Cr ratio in the frontal lobes (WMD, −0.36; 95% CI, −0.77 to 0.06; p = 0.09), with heterogeneity (I2 = 97%, p < 0.01). Four studies26,32,33,35 evaluated changes in the NAA/Cho ratio in the frontal lobes (WMD, −0.32; 95% CI, −0.64 to −0.01; p = 0.05), with heterogeneity (I2 = 86%, p < 0.01). Five studies26,29,32,33,35 evaluated changes in the Cho/Cr ratio in the frontal lobes (WMD, −0.02; 95% CI, −0.14 to 0.09; p = 0.68), with heterogeneity (I2 = 94%, p < 0.01). This information is shown in Table 2. Because the units of measurement for NAA, Cr, and Cho varied among the included studies, we were unable to pool the results; we have presented the data in Supplementary Table 1.
Table 2. Main results for metabolites in different brain regions.
| Study | OSA | Control | Weight% | >MD [95%CI] | p | I2% | p | PBegg | PEgger |
|---|---|---|---|---|---|---|---|---|---|
| NAA/Cr in hippocampus | |||||||||
| Algin et al.33 | 1.16 ± 0.1 | 1.24 ± 0.1 | 61.0 | −0.08 [−0.16, −0.00] | |||||
| Bartlett et al.31 | 1.73 ± 1.46 | 1.25 ± 0.56 | 0.3 | 0.48 [−0.64, 0.60] | |||||
| Halbower et al.26 | 1.22 ± 0.38 | 1.21 ± 0.23 | 2.8 | 0.01 [−0.35, 0.37] | |||||
| Kizilgoz et al.34 | 2.00 ± 0.95 | 2.40 ± 0.53 | 1.8 | −0.40 [−0.84, 0.04] | |||||
| O’Donoghue et al.32 | 1.58 ± 0.23 | 1.66 ± 0.15 | 34.0 | −0.08 [−0.18, 0.02] | |||||
| Pooled | 100 | −0.08 [−0.14, −0.02] | 0.52 | 0 | <0.01 | 0.81 | 0.28 | ||
| NAA/Cho in hippocampus | |||||||||
| Algin et al.33 | 1.07 ± 0.1 | 1.11 ± 0.2 | 32.0 | −0.04 [−0.18, 0.10] | |||||
| Halbower et al.26 | 0.91 ± 0.05 | 1.29 ± 0.21 | 30.1 | −0.38 [−0.55, −0.21] | |||||
| Kizilgoz et al.34 | 2.06 ± 0.99 | 2.25 ± 0.67 | 13.1 | −0.19 [−0.71, 0.33] | |||||
| O’Donoghue et al.32 | 4.15 ± 0.52 | 4.04 ± 0.45 | 24.9 | 0.11 [−0.15, 0.37] | |||||
| Pooled | 100 | −0.12 [−0.36, 0.11] | <0.01 | 77 | 0.30 | 0.09 | 0.01 | ||
| Cho/Cr in hippocampus | |||||||||
| Algin et al.33 | 1.09 ± 0.1 | 1.15 ± 0.1 | 38.7 | −0.06 [−0.14, 0.02] | |||||
| Halbower et al.26 | 1.37 ± 0.41 | 0.94 ± 0.05 | 6.5 | −0.03 [−0.05, −0.01] | |||||
| Kizilgoz et al.34 | 1.18 ± 0.92 | 1.25 ± 0.78 | 2.4 | −0.07 [−0.63, 0.49] | |||||
| O’Donoghue et al.32 | 0.38 ± 0.04 | 0.41 ± 0.04 | 52.3 | 0.43 [0.10, 0.76] | |||||
| Pooled | 100 | −0.01 [−0.10, 0.08] | 0.05 | 63 | 0.78 | 0.31 | 0.11 | ||
| NAA/Cr in frontal lobe | |||||||||
| Alchanatis et al.35 | 1.58 ± 0.14 | 1.66 ± 0.17 | 23.5 | −0.08 [−0.17, 0.01] | |||||
| Algin et al.33 | 1.61 ± 0.64 | 1.98 ± 0.2 | 22.4 | −0.37 [−0.57, −0.17] | |||||
| Gharraf et al.36 | 1.33 ± 0.13 | 2.08 ± 0.15 | 23.3 | −0.95 [−1.06, −0.84] | |||||
| Halbower et al.26 | 3.1 ± 1.7 | 2.7 ± 0.6 | 6.7 | 0.40 [−0.95, 1.75] | |||||
| O’Donoghue et al.32 | 1.59 ± 0.13 | 1.65 ± 0.18 | 23.5 | −0.06 [−0.15, 0.03] | |||||
| Sarchielli et al.29 | 138.09 ± 6.9 | 147.09 ± 11 | 0.5 | −9.00 [−14.69, −3.31] | |||||
| Pooled | 100 | −0.36 [−0.77, 0.06] | <0.01 | 97 | 0.09 | 0.45 | 0.15 | ||
| NAA/Cho in frontal lobe | |||||||||
| Alchanatis et al.35 | 1.89 ± 0.43 | 1.78 ± 0.42 | 26.4 | 0.11 [−0.11, 0.33] | |||||
| Algin et al.33 | 1.48 ± 0.08 | 1.99 ± 0.36 | 28.0 | −0.51 [−0.68, −0.34] | |||||
| Halbower et al.26 | 1.6 ± 0.4 | 2.2 ± 0.4 | 19.3 | −0.60 [−1.04, −0.16] | |||||
| O’Donoghue et al.32 | 4.56 ± 0.41 | 4.92 ± 0.44 | 26.2 | −0.36 [−0.59, −0.13] | |||||
| Pooled | 100 | −0.32 [−0.64, −0.01] | <0.01 | 86 | 0.05 | 0.31 | 0.50 | ||
| Cho/Cr in frontal lobe | |||||||||
| Alchanatis et al.35 | 0.88 ± 0.19 | 0.99 ± 0.43 | 0.4 | −0.11 [−0.31, 0.09] | |||||
| Algin et al.33 | 1.23 ± 0.14 | 1.03 ± 0.08 | 5.8 | 0.09 [0.04, 0.14] | |||||
| Gharraf et al.36 | 0.99 ± 0.08 | 1.17 ± 0.03 | 8.6 | −0.18 [−0.22, −0.14] | |||||
| Halbower et al.26 | 1.9 ± 1.1 | 1.3 ± 0.3 | 0.0 | 0.60 [−0.25, 1.45] | |||||
| O’Donoghue et al.32 | 0.35 ± 0.02 | 0.33 ± 0.03 | 85.1 | 0.02 [0.01, 0.03] | |||||
| Sarchielli et al.29 | 96.99 ± 12.7 | 94.88 ± 5.19 | 0.0 | 2.11 [−3.90, 8.12] | |||||
| Pooled | 100 | −0.02 [−0.14, 0.09] | <0.01 | 94 | 0.68 | 0.99 | 0.35 | ||
Abbreviations: NAA, N-acetylaspartate; Cho, choline; Cr, creatine; OSA, Obstructive sleep apnea; MD, Mean difference; CI, Confidence Interval; PBegg, p-value for Begg’s test; PEgger, p-value for Egger test.
Subgroup analyses
Changes in cerebral metabolites in the frontal white matter
Three studies33,35,36 evaluated changes in the NAA/Cr ratio (WMD, −0.51; 95% CI, −1.11 to 0.09; p = 0.09), and three studies33,35,36 evaluated changes in the Cho/Cr ratio (WMD, −0.06; 95% CI, −0.31 to 0.19; p = 0.64). All showed significant between-study heterogeneity (Table 3).
Table 3. Subgroup analysis of cerebral metabolites in the white matter and frontal cortex.
| Study | OSA | Control | Weight% | WMD [95%CI] | p | I2% | p | PBegg | PEgger |
|---|---|---|---|---|---|---|---|---|---|
| NAA/Cr in frontal white matter | |||||||||
| Alchanatis et al.35 | 1.59 ± 0.16 | 1.74 ± 0.15 | 34.3 | −0.15 [−0.26, −0.04] | |||||
| Algin et al.33 | 1.72 ± 0.8 | 2.15 ± 0.1 | 31.5 | −0.43 [−0.76, −0.10] | |||||
| Gharraf et al.36 | 1.13 ± 0.13 | 2.08 ± 0.15 | 34.3 | −0.95 [−1.06, −0.84] | |||||
| Pooled | 100 | −0.51 [−1.11, 0.09] | <0.01 | 98 | 0.09 | 0.30 | 0.07 | ||
| Cho/Cr in frontal white matter | |||||||||
| Alchanatis et al.35 | 1.01 ± 0.15 | 1.19 ± 0.17 | 32.0 | −0.18 [−0.30, −0.06] | |||||
| Algin et al.33 | 1.23 ± 0.1 | 1.05 ± 0.1 | 33.6 | 0.18 [0.10, 0.26] | |||||
| Gharraf et al.36 | 0.99 ± 0.08 | 1.17 ± 0.03 | 34.3 | −0.18 [−0.22, −0.14] | |||||
| Pooled | 100 | −0.06 [−0.31, 0.19] | <0.01 | 97 | 0.64 | 0.99 | 0.72 | ||
| NAA/Cr in frontal cortex | |||||||||
| Alchanatis et al.35 | 1.56 ± 0.12 | 1.58 ± 0.15 | 51.5 | −0.02 [−0.13, 0.09] | |||||
| Algin et al.33 | 1.5 ± 0.4 | 1.8 ± 0.1 | 45.0 | −0.30 [−0.47, −0.13] | |||||
| Halbower et al.26 | 3.1 ± 1.7 | 2.7 ± 0.6 | 3.4 | 0.40 [−0.95, 1.75] | |||||
| Pooled | 100 | −0.13 [−0.39, 0.13] | 0.02 | 75 | 0.32 | 0.99 | 0.60 | ||
| NAA/Cho in frontal cortex | |||||||||
| Alchanatis et al.35 | 2.18 ± 0.35 | 2.07 ± 0.36 | 34.1 | 0.11 [−0.15, 0.37] | |||||
| Algin et al.33 | 1.44 ± 0. 1 | 2.26 ± 0.3 | 34.9 | −0.82 [−1.02, −0.62] | |||||
| Halbower et al.26 | 1.6 ± 0.4 | 2.2 ± 0.4 | 31.0 | −0.60 [−1.04, −0.16] | |||||
| Pooled | 100 | −0.43 [−1.07, 0.20] | <0.01 | 94 | 0.18 | 0.30 | 0.26 | ||
| Cho/Cr in frontal cortex | |||||||||
| Alchanatis et al.35 | 0.74 ± 0.13 | 0.78 ± 0.13 | 15.9 | −0.04 [−0.14, 0.06] | |||||
| Algin et al.33 | 1 ± 0.04 | 1 ± 0.06 | 83.9 | 0.00 [−0.04, 0.04] | |||||
| Halbower et al.26 | 1.9 ± 1.1 | 1.3 ± 0.3 | 0.2 | 0.60 [−0.25, 1.45] | |||||
| Pooled | 100 | −0.01 [−0.04, 0.03] | 0.29 | 20 | 0.80 | 0.30 | 0.22 | ||
NAA, N-acetylaspartate; Cho, choline; Cr, creatine; OSA, Obstructive sleep apnea; MD, Mean difference; CI, Confidence Interval; PBegg, p-value of Begg’s test; PEgger, p-value of Egger test.
Changes in cerebral metabolites in the frontal cortex
Three studies26,33,35 evaluated changes in the NAA/Cr ratio (WMD, −0.13; 95% CI, −0.39 to 0.13; p = 0.32), and the same three evaluated changes in the NAA/Cho ratio (WMD, −0.43; 95% CI, −1.07 to 0.20; p = 0.18); there was significant between-study heterogeneity (see Table 3). These studies26,33,35 also evaluated changes in the Cho/Cr ratio (WMD, −0.01; 95% CI, −0.04 to 0.03; p = 0.80), without heterogeneity (I2 = 20%, p = 0.29).
Sensitivity analysis
A sensitivity analysis was performed by sequentially omitting one study at a time to examine the influence of each study. If significant between-study heterogeneity existed, a sensitivity analysis was performed to assess the stability of the results.
In the hippocampus, after excluding the study by Halbower et al.26, the Cho/Cr ratio significantly decreased in patients with OSA without heterogeneity (I2 = 0%, p = 0.75); the significant heterogeneity of the NAA/Cho ratio also disappeared (I2 = 0%, p = 0.49). Excluding any single study did not influence the pooled results for the NAA/Cho ratio (Supplementary Table 2).
In the frontal lobe, no single study influenced the pooled results for the NAA/Cr and Cho/Cr ratios. The NAA/Cho ratio, after excluding the study by Alchanatis et al.35, significantly decreased in patients with OSA without significant heterogeneity (I2 = 0%, p = 0.49). However, after excluding the studies by Algin33, Halbower26, or O’Donoghue et al.32, there was no significant difference between patients with OSA and controls, but significant heterogeneity was present (Supplementary Table 2).
Publication bias
To assess publication bias among the included cross-sectional studies, Begg’s rank correlation test and the Egger linear regression test were performed. The p-values of these two tests were >0.05, indicating that there was no significant publication bias in evaluating each cerebral metabolite in our meta-analysis.
Discussion
Emotional-cognitive changes and daytime sleepiness are common symptoms of OSA. Structural-metabolic changes in some regions of the brain are responsible for these symptoms13. Neuroimaging studies of patients with OSA might help clarify the biological mechanisms underlying the onset and duration of the disease13. They can also help to identify neural abnormalities associated with brain function in patients with OSA. MRS can provide data on the metabolic status of the tissue by quantifying the cellular metabolites and probing their variation during the disease processes; it can also detect microstructural changes in the brain that are not visible in conventional cranial MRI. Therefore, we combined all MRS studies of cerebral metabolites to achieve a consensus.
Previous studies using MRS have revealed that cerebral metabolites vary in regions of the brain in patients with OSA, including the hippocampus, thalamus, cerebral cortex, white matter, insular cortex, brain stem, cortex, and the frontal, temporal, and occipital lobes. In this meta-analysis, the pooled results showed that OSA was associated with a low NAA/Cr ratio in the hippocampus with no significant between-study heterogeneity and a low Cho/Cr ratio in the hippocampus after excluding the study by Halbower et al.26. In the frontal lobe, OSA was associated with a low NAA/Cho ratio, but this negative relationship was not stable because of varying results in sensitivity analysis. A decrease in NAA is notable in diseases with neuro-axonal loss or dysfunction, such as Alzheimer disease39 and cerebral ischemia40, while an increase in the NAA signal was only seen in Canavan disease41. Cr has neuroprotective properties42 and enhances neurocognitive abilities43. The levels of hippocampal Cr respond to hippocampal exercise44, which increase in hypometabolic states and decrease in hypermetabolic states. A decrease in the Cr signal in the hippocampus indicates the loss of hippocampal function in patients with OSA. An elevated Cho level reflects increased membrane turnover or increased cellular density and has been reported in cases of active demyelination, brain tumors, and glial proliferation14,45,46, whereas lower Cho levels have been reported in mitochondrial, hypomyelination, metabolic, and liver diseases14,45; Grave’s disease47; Lewy body dementia48; and chronic obstructive pulmonary disease49. Increases or decreases in Cho may represent different stages in the evolution of the same pathological process, with increased lipid turnover initially and subsequent decreased turnover and possible apoptosis. Thus, changes in cerebral metabolites in the frontal lobe and hippocampus may demonstrate injury to these regions.
The frontal lobe, especially the prefrontal cortex and the prefrontal-subcortical brain circuits50, is associated with cognitive and executive function. Therefore, lesions in the prefrontal cortex and the prefrontal-subcortical brain circuits can cause cognitive impairment and executive dysfunction, as can lesions in the anterior white matter, by interrupting the prefrontal-subcortical circuits50,51,52. Previous studies have reported that OSA could induce chemical and structural cellular injury and affect the prefrontal cortex53,54,55. In this meta-analysis, cerebral metabolite changes associated with OSA in the frontal lobe might reflect injury to these regions, leading to mild cognitive impairment such as inattention, poor memory, and poor executive function. In addition to the frontal lobes, changes in the cerebral metabolites were also seen in the hippocampal area, which is metabolically active and highly susceptible to hypoxic insult. This brain region is closely associated with learning skills, memory, and advanced mental activity, which might explain some of the cognitive deficits in patients with OSA.
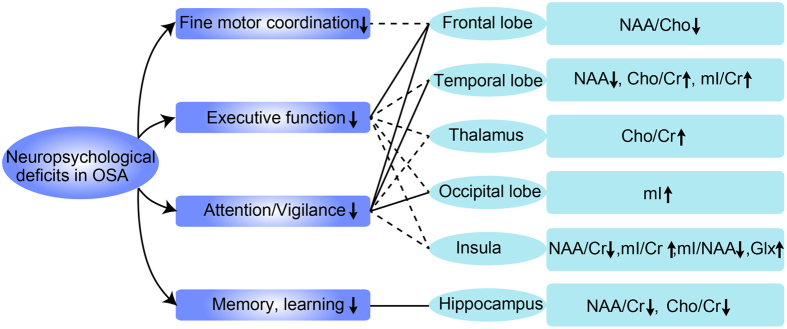
To clearly understand the variations in the metabolites in different cerebral regions, we have summarized all of the results from the different studies in Fig. 2. First, in the thalamus, the Cho/Cr ratio increased in patients with OSA, while the NAA/Cr and NAA/Cho ratio did not. Second, in the parietal-occipital white matter, the levels of NAA, Cho, Cr, and mI and the ratios of NAA/Cr, NAA/Cho, Cho/Cr, and mI/Cr were unchanged. Third, in the brain stem, the ratios of NAA/Cho, NAA/Cr, and Cho/Cr were also unchanged. Fourth, in the temporal lobe, the level of NAA decreased; the ratios of Cho/Cr and mI/Cr increased; and the Cr, Cho, Glx, and mI levels and the NAA/Cr ratio were unchanged. Fifth, the NAA/Cr and mI/NAA ratios decreased and the mI/Cr ratio and Glx level increased in the insular cortex, while the Cho/Cr ratio was unchanged. Finally, in the occipital lobe, only the mI level increased, while the NAA, Cr, Cho, and Glx levels were unchanged. We conclude that the neuropsychological deficits in patients with OSA, including impaired executive dysfunction, fine motor coordination, memory and learning skills, and attention/vigilance, may be related to the metabolite changes in specific cerebral regions.
Figure 2. Neuropsychological deficits and cerebral metabolite changes in respective regions in OSA.
Abbreviation: OSA, obstructive sleep apnea; NAA, N-acetylaspartate; Cho, choline; Cr, creatine; mI, myo-inosito.
To the best of our knowledge, this is the first meta-analysis to investigate the changes in cerebral metabolites in patients with OSA. Despite the interesting findings mentioned above, some limitations should be addressed. First, no firm conclusions could be drawn because of the relatively small sample size and varying results in the sensitivity analysis. Second, we included only English-language studies, which might have resulted in selection bias. Third, although we stratified the changes in cerebral metabolites according to brain region, there was significant between-study heterogeneity. This may have been due in part to the small number of participants and unadjusted confounding factors.
In conclusion, cerebral metabolites are significantly altered in the hippocampus in patients with OSA as evidenced by the low NAA/Cr ratios in the hippocampus and the low NAA/Cho ratio in the frontal lobe. These abnormalities of cerebral metabolites might be a pivotal bridge connecting cognitive deficits and OSA.
Materials and Methods
This systemic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement56. Ethical approval was not necessary for this meta-analysis because each included study had already received ethical approval.
Search strategy
A systematic search was performed using two electronic databases, PubMed and Embase, and the final search was conducted on March 31, 2016. The search terms were as follows: (obstructive sleep apnea) OR (sleep apnea syndromes) OR (OSA) OR (sleep apnea) combined with (magnetic resonance spectroscopy) OR (MRS) OR (magnetic resonance imaging). Additional reports were added when discovered by citation tracking. This search was performed separately by Drs. Xia and Fu.
Inclusion and exclusion criteria
We included studies that satisfied the following inclusion criteria: (1) OSA was confirmed by polysomnography, (2) MRS was used to detect cerebral metabolites in patients with OSA, (3) the results were published in English, and (4) there were sufficient data to extract and estimate the WMD and the 95% CI for differences in cerebral metabolites between patients with OSA and controls. We excluded studies if they were nonclinical studies or had no control group.
Data extraction
All studies that met the criteria were retrieved and the required information was extracted by both reviewers. The details extracted from each study included the first author, publication year, number of enrolled subjects, percentage of males, BMI, AHI, and ESS in subjects and controls. Details on metabolites were also extracted, such as the NAA, Cho, Cr, Glx, and mI levels and the NAA/Cr, Cho/Cr, NAA/Cho, mI/Cr, and Glx/Cr ratios. Disagreements between reviewers were resolved by consensus through a group discussion.
Assessment of study quality
The NOS score was used to assess the quality of the included studies26,27,28,29,30,31,32,33,34,35,36,37,38. The NOS score includes three categories (selection, comparability, and exposure) with a total of eight items57. For each category, a study was given a maximum of two points. Studies that were awarded 0–3, 4–6, or 7–9 points were recognized as low-, intermediate-, and high-quality studies, respectively.
Statistical analysis
RevMan (ver. 5.3, Cochrane Collaboration, Oxford, UK) and Stata (ver. 12.0, StataCorp, College Station, TX, USA) were used for the statistical analyses. The pooled estimates of the WMD and 95% CI for continuous data were calculated using the generic inverse variance method according to Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The heterogeneity across the included studies was evaluated using the Q-test and I2 statistic58. A p-value of >0.1 indicated that there was no heterogeneity. If between-study heterogeneity existed, the DerSimonian and Laird random-effects model was applied; otherwise, a Mantel–Haenszel fixed-effects model was used. Subgroup analysis was performed to explore the source of the heterogeneity. Sensitivity analysis was performed to assess the stability of the results. Begg’s and Egger’s tests were used to evaluate publication bias59,60.
Additional Information
How to cite this article: Xia, Y. et al. Changes in cerebral metabolites in obstructive sleep apnea: a systemic review and meta-analysis. Sci. Rep. 6, 28712; doi: 10.1038/srep28712 (2016).
Supplementary Material
Acknowledgments
This study was supported by grants-in-aid from multi-center clinical research project from school of medicine, Shanghai Jiao Tong University (DLY201502) and Shanghai Shen-Kang Hospital Management Center Project of Shanghai (SHDC12015101).
Footnotes
Author Contributions H.X., Y.X. and Y.F. provided the conceptual design of the project, writing and editing final version of the manuscript. J.G., H.Y. and S.Y. participated in writing and editing final version of the manuscript. All listed authors read and approved the final manuscript.
References
- Stradling J. R. & Davies R. J. Sleep. 1: Obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax 59, 73–78 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. et al. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine 328, 1230–1235, 10.1056/nejm199304293281704 (1993). [DOI] [PubMed] [Google Scholar]
- Olson E. J., Moore W. R., Morgenthaler T. I., Gay P. C. & Staats B. A. Obstructive sleep apnea-hypopnea syndrome. Mayo Clinic proceedings 78, 1545–1552, 10.4065/78.12.1545 (2003). [DOI] [PubMed] [Google Scholar]
- Shahar E. et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. American journal of respiratory and critical care medicine 163, 19–25, 10.1164/ajrccm.163.1.2001008 (2001). [DOI] [PubMed] [Google Scholar]
- Arzt M., Young T., Finn L., Skatrud J. B. & Bradley T. D. Association of sleep-disordered breathing and the occurrence of stroke. American journal of respiratory and critical care medicine 172, 1447–1451, 10.1164/rccm.200505-702OC (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmuth K. J., Austin D., Skatrud J. B. & Young T. Association of sleep apnea and type II diabetes: a population-based study. American journal of respiratory and critical care medicine 172, 1590–1595, 10.1164/rccm.200504-637OC (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque F. N. et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest 141, 967–973, 10.1378/chest.11-0975 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. L. et al. Systematic review of motor vehicle crash risk in persons with sleep apnea. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine 2, 193–200 (2006). [PubMed] [Google Scholar]
- Yaggi H. K. et al. Obstructive sleep apnea as a risk factor for stroke and death. The New England journal of medicine 353, 2034–2041, 10.1056/NEJMoa043104 (2005). [DOI] [PubMed] [Google Scholar]
- Knauert M., Naik S., Gillespie M. B. & Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World Journal of Otorhinolaryngology-Head and Neck Surgery 1, 17–27, 10.1016/j.wjorl.2015.08.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary A., Rouleau I. & Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep 23, 369–381 (2000). [PubMed] [Google Scholar]
- Engleman H. M., Kingshott R. N., Martin S. E. & Douglas N. J. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep 23 Suppl 4, S102–S108 (2000). [PubMed] [Google Scholar]
- Zimmerman M. E. & Aloia M. S. A review of neuroimaging in obstructive sleep apnea. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine 2, 461–471 (2006). [PubMed] [Google Scholar]
- Rudkin T. M. & Arnold D. L. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Archives of neurology 56, 919–926 (1999). [DOI] [PubMed] [Google Scholar]
- Alkan A. et al. MR spectroscopy features of brain in patients with mild and severe obstructive sleep apnea syndrome. Clin Imaging 37, 989–992, 10.1016/j.clinimag.2013.07.010 (2013). [DOI] [PubMed] [Google Scholar]
- Ayalon L. & Peterson S. Functional central nervous system imaging in the investigation of obstructive sleep apnea. Current opinion in pulmonary medicine 13, 479–483, 10.1097/MCP.0b013e3282f0e9fb (2007). [DOI] [PubMed] [Google Scholar]
- Birken D. L. & Oldendorf W. H. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neuroscience and biobehavioral reviews 13, 23–31 (1989). [DOI] [PubMed] [Google Scholar]
- Dang-Vu T. T. et al. Neuroimaging in sleep medicine. Sleep medicine 8, 349–372, 10.1016/j.sleep.2007.03.006 (2007). [DOI] [PubMed] [Google Scholar]
- Douglas R. M. et al. Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. American journal of physiology. Regulatory, integrative and comparative physiology 292, R1254–R1259, 10.1152/ajpregu.00404.2006 (2007). [DOI] [PubMed] [Google Scholar]
- Douglas R. M. et al. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. American journal of physiology. Cell physiology 298, C1594–C1602, 10.1152/ajpcell.00298.2009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G. et al. Modest changes in cerebral glucose metabolism in patients with sleep apnea syndrome after continuous positive airway pressure treatment. Respiration; international review of thoracic diseases 84, 212–218, 10.1159/000338117 (2012). [DOI] [PubMed] [Google Scholar]
- Kamba M. et al. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. Journal of neurology, neurosurgery, and psychiatry 71, 334–339 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C. et al. Dynamic changes in brain bioenergetics during obstructive sleep apnea. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 29, 1421–1428, 10.1038/jcbfm.2009.57 (2009). [DOI] [PubMed] [Google Scholar]
- Santarnecchi E. et al. Altered cortical and subcortical local coherence in obstructive sleep apnea: a functional magnetic resonance imaging study. Journal of sleep research 22, 337–347, 10.1111/jsr.12006 (2013). [DOI] [PubMed] [Google Scholar]
- Sarma M. K. et al. Accelerated echo-planar J-resolved spectroscopic imaging in the human brain using compressed sensing: a pilot validation in obstructive sleep apnea. AJNR. American journal of neuroradiology 35, S81–S89, 10.3174/ajnr.A3846 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbower A. C. et al. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS medicine 3, e301, 10.1371/journal.pmed.0030301 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey P. M. et al. Obstructive sleep apnea is associated with low GABA and high glutamate in the insular cortex. Journal of sleep research, 10.1111/jsr.12392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S. K. et al. Insular cortex metabolite changes in obstructive sleep apnea. Sleep 37, 951–958, 10.5665/sleep.3668 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P. et al. A 1H magnetic resonance spectroscopy study in patients with obstructive sleep apnea. European journal of neurology 15, 1058–1064, 10.1111/j.1468-1331.2008.02244.x (2008). [DOI] [PubMed] [Google Scholar]
- Tonon C. et al. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep 30, 305–311 (2007). [DOI] [PubMed] [Google Scholar]
- Bartlett D. J. et al. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep medicine 5, 593–596, 10.1016/j.sleep.2004.08.004 (2004). [DOI] [PubMed] [Google Scholar]
- O’Donoghue F. J. et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep 35, 41–48, 10.5665/sleep.1582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algin O. et al. Neurochemical-structural changes evaluation of brain in patients with obstructive sleep apnea syndrome. European journal of radiology 81, 491–495, 10.1016/j.ejrad.2010.12.092 (2012). [DOI] [PubMed] [Google Scholar]
- Kizilgoz V. et al. Proton magnetic resonance spectroscopy of periventricular white matter and hippocampus in obstructive sleep apnea patients. Polish journal of radiology / Polish Medical Society of Radiology 78, 7–14, 10.12659/PJR.889923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alchanatis M. et al. Frontal brain lobe impairment in obstructive sleep apnoea: a proton MR spectroscopy study. The European respiratory journal 24, 980–986, 10.1183/09031936.04.00127603 (2004). [DOI] [PubMed] [Google Scholar]
- Gharraf H., Matrawy K. & Reda I. Proton magnetic resonance spectroscopy of brain in obstructive sleep apnea in Egyptian subjects. Egyptian Journal of Chest Diseases and Tuberculosis 63, 395–403, 10.1016/j.ejcdt.2013.12.001 (2014). [DOI] [Google Scholar]
- Kamba M., Suto Y., Ohta Y., Inoue Y. & Matsuda E. Cerebral metabolism in sleep apnea. Evaluation by magnetic resonance spectroscopy. Am J Respir Crit Care Med 156, 296–298, 10.1164/ajrccm.156.1.9611063 (1997). [DOI] [PubMed] [Google Scholar]
- Sharma S. K. et al. Proton magnetic resonance spectroscopy of brain in obstructive sleep apnoea in north Indian Asian subjects. The Indian journal of medical research 132, 278–286 (2010). [PubMed] [Google Scholar]
- Rango M. et al. Central nervous system trans-synaptic effects of acute axonal injury: a 1H magnetic resonance spectroscopy study. Magnetic resonance in medicine 33, 595–600 (1995). [DOI] [PubMed] [Google Scholar]
- Bruhn H. et al. Cerebral metabolism in man after acute stroke: new observations using localized proton NMR spectroscopy. Magnetic resonance in medicine 9, 126–131 (1989). [DOI] [PubMed] [Google Scholar]
- Grodd W., Krageloh-Mann I., Petersen D., Trefz F. K. & Harzer K. In vivo assessment of N-acetylaspartate in brain in spongy degeneration (Canavan’s disease) by proton spectroscopy. Lancet (London, England) 336, 437–438 (1990). [DOI] [PubMed] [Google Scholar]
- Wyss M. & Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiological reviews 80, 1107–1213 (2000). [DOI] [PubMed] [Google Scholar]
- Rae C., Digney A. L., McEwan S. R. & Bates T. C. Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proceedings. Biological sciences / The Royal Society 270, 2147–2150, 10.1098/rspb.2003.2492 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M. J. et al. Memory training alters hippocampal neurochemistry in healthy elderly. Neuroreport 14, 1333–1337, 10.1097/01.wnr.0000077548.91466.05 (2003). [DOI] [PubMed] [Google Scholar]
- Bitsch A. et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR. American journal of neuroradiology 20, 1619–1627 (1999). [PMC free article] [PubMed] [Google Scholar]
- Castillo M., Kwock L. & Mukherji S. K. Clinical applications of proton MR spectroscopy. AJNR. American journal of neuroradiology 17, 1–15 (1996). [PMC free article] [PubMed] [Google Scholar]
- Elberling T. V. et al. Reduced myo-inositol and total choline measured with cerebral MRS in acute thyrotoxic Graves’ disease. Neurology 60, 142–145 (2003). [DOI] [PubMed] [Google Scholar]
- Molina J. A. et al. Proton magnetic resonance spectroscopy in dementia with Lewy bodies. Eur Neurol 48, 158–163, 65520 (2002). [DOI] [PubMed] [Google Scholar]
- Shim T. S. et al. Cerebral metabolic abnormalities in COPD patients detected by localized proton magnetic resonance spectroscopy. Chest 120, 1506–1513 (2001). [DOI] [PubMed] [Google Scholar]
- Cummings J. L. Frontal-subcortical circuits and human behavior. Archives of neurology 50, 873–880 (1993). [DOI] [PubMed] [Google Scholar]
- Kramer J. H., Reed B. R., Mungas D., Weiner M. W. & Chui H. C. Executive dysfunction in subcortical ischaemic vascular disease. Journal of neurology, neurosurgery, and psychiatry 72, 217–220 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall D. R. Executive cognitive impairment: a novel perspective on dementia. Neuroepidemiology 19, 293–299, 26268 (2000). [DOI] [PubMed] [Google Scholar]
- Beebe D. W. & Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. Journal of sleep research 11, 1–16 (2002). [DOI] [PubMed] [Google Scholar]
- Yaouhi K. et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. Journal of sleep research 18, 36–48, 10.1111/j.1365-2869.2008.00705.x (2009). [DOI] [PubMed] [Google Scholar]
- Zhang X., Ma L., Li S., Wang Y. & Wang L. A functional MRI evaluation of frontal dysfunction in patients with severe obstructive sleep apnea. Sleep medicine 12, 335–340, 10.1016/j.sleep.2010.08.015 (2011). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. & Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 6, e1000097, 10.1371/journal.pmed.1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G., Shea B., O’Connell D. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses.(2011). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed April 2015).
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 327, 557–560, 10.1136/bmj.327.7414.557 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.