Abstract
Escherichia coli O157:H7 is a highly acid-resistant food-borne pathogen that survives in the bovine and human gastrointestinal tracts and in acidic foods such as apple cider. This property is thought to contribute to the low infectious dose of the organism. Three acid resistance (AR) systems are expressed in stationary-phase cells. AR system 1 is σS dependent, while AR systems 2 and 3 are glutamate and arginine dependent, respectively. In this study, we sought to determine which AR systems are important for survival in acidic foods and which are required for survival in the bovine intestinal tract. Wild-type and mutant E. coli O157:H7 strains deficient in AR system 1, 2, or 3 were challenged with apple cider and inoculated into calves. Wild-type cells, adapted at pH 5.5 in the absence of glucose (AR system 1 induced), survived well in apple cider. Conversely, the mutant deficient in AR system 1, shown previously to survive poorly in calves, was susceptible to apple cider (pH 3.5), and this sensitivity was shown to be caused by low pH. Interestingly, the AR system 2-deficient mutant survived in apple cider at high levels, but its shedding from calves was significantly decreased compared to that of wild-type cells. AR system 3-deficient cells survived well in both apple cider and calves. Taken together, these results indicate that E. coli O157:H7 utilizes different acid resistance systems based on the type of acidic environment encountered.
A particularly virulent form of Escherichia coli designated serotype O157:H7 has emerged as an important pathogen over the last two decades (20, 26). Numerous outbreaks of E. coli O157:H7-related disease have been recorded since 1982, the largest of which occurred in Japan in 1997 (33, 50). As a member of the enterohemorrhagic group of E. coli, this organism causes a variety of diseases, including hemorrhagic colitis, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura (8, 12, 32, 35, 41).
E. coli O157:H7 is a major food-borne pathogen that threatens many aspects of the food industry (36). The major reservoir of E. coli O157:H7 generally is perceived to be the bovine gastrointestinal tract, thus providing ample opportunity for contamination of ground beef products, a common source of infection (21, 40, 43, 49). However, human infections also have been associated with foods other than hamburger. These include acidic foods, such as apple cider and salami, although the source of infection can usually be traced to bovine fecal contamination (1, 5, 23).
Contamination of recreational and drinking water with infected feces has caused waterborne outbreaks of E. coli O157:H7 disease, too, although the specific source of the fecal contamination is sometimes unclear. For instance, a well-publicized outbreak at a county fair in 1999 is thought to have resulted from contaminated well water (10). However, it is debatable whether this outbreak resulted from contamination of well water with E. coli O157:H7-infected bovine or human feces or a combination of both (6).
E. coli O157:H7 is thought to have a very low infectious dose (<700 organisms) (19, 46) due, in part, to very efficient mechanisms of stress resistance. To colonize or cause disease, an infecting microorganism must breech an impressive array of environmental insults imposed by a host (e.g., acid pH, bile salts, high osmolarity, defensins). Survival is achieved through the induction of defensive microbial stress response systems. Because of its low infectious dose, an extremely important component of E. coli O157:H7 pathogenesis is thought to be its outstanding ability to survive in extremely acidic environments, such as the stomach or in areas of the intestine that contain high levels of organic acids (15, 38). A variety of published reports have documented this acid resistance (AR) (2, 4, 7, 16, 25, 27, 42).
Gorden and Small (18) and Small et al. (44), using laboratory strains of E. coli, first reported the presence of low-pH-induced, stationary-phase AR that protects cells exposed to extremely low pH (pH 2.5). Their seminal work in this area revealed that there are σS dependent and independent systems of AR. Our investigations have determined that AR in E. coli is comprised of three efficient systems. Two of these systems are present in Shigella flexneri, but none of these systems occur in Salmonella enterica serovar Typhimurium (28). Expression of AR system 1 requires growth to stationary phase in acid-buffered complex media (Luria-Bertani [LB]) and successfully protects cells to pH 2.5 in amino-acid-free minimal medium. Glucose represses induction of this AR mechanism and exposes two other acid survival systems that require the addition of an amino acid during pH 2.5 acid challenge (28). One system is glutamate dependent (AR system 2), while the other requires arginine (AR system 3). We have shown that the alternate RNA polymerase sigma factor σS, important to stationary-phase physiology, is not required for the glutamate- or arginine-dependent acid survival systems but is essential for expression of AR system 1 (29). Essential components of AR system 2 include glutamate decarboxylase (GAD; two isoforms), encoded by gadA and gadB, and a putative glutamate:γ-amino butyric acid antiporter, encoded by gadC (9, 22, 24). The product of the adiA locus, arginine decarboxylase (3), was proven to be responsible for system 3 arginine-based acid survival (28). These three systems account for the pH 2.5 AR phenotype originally described by Gorden and Small for E. coli K-12 (18).
E. coli O157:H7 also possesses these three systems of AR (9, 28, 29). While levels of AR in E. coli O157:H7 strains generally are more robust than many laboratory strains of E. coli, the levels are equivalent to what can be observed in commensal isolates. In a previous study it was shown that, as with K-12, the rpoS gene of E. coli O157:H7 also controls expression of AR system 1 (39). In addition, it was shown that a mutant of E. coli O157:H7 lacking rpoS was shed in lower numbers in experimentally infected mice and calves and that this reduced shedding, at least in mice, was due to decreased AR (39).
In the present work we continued the examination of AR in E. coli O157:H7 by selectively removing the three determinants of AR to ask which systems are important for survival in acidic foods and which are required in the bovine gastrointestinal tract. Surprisingly, it was found that different AR systems are needed for survival in these two environments.
MATERIALS AND METHODS
Bacterial strains.
The E. coli strains used in this study are listed in Table 1. E. coli O157:H7 strain ATCC 43895 was used as the parent, wild-type strain throughout the study, and all of the mutants described below were derived from this strain. This strain was originally isolated from contaminated ground beef in a 1982 outbreak of hemorrhagic colitis (strain EDL933) (48), and it has been sequenced (37). Construction of an rpoS derivative of this strain involved insertional inactivation with pRR10, which carries the gene encoding β-lactamase (FRIK 816-3 [11]). Mutants of ATCC 43895 disrupted in adiA, gadA, or gadC were constructed in the same way (9). The disruption of these genes does not have polar effects on other genes, and therefore the observed phenotypes are specific to these mutations. Genetic evidence for this has been revealed in E. coli K-12, whose operon structure has been shown by genome sequencing to be equivalent to that of E. coli O157:H7. This evidence includes the following: (i) the gadA mutant is not acid sensitive (AS), indicating that no polar effect affecting AR occurred on downstream genes (39); (ii) Northern blot analysis indicates that the gadC gene is contained within the bicistronic operon gadBC that forms a transcript of specified length (30, 31)—no genes are found downstream from gadC in the operon, and therefore no polar effects could have occurred; (iii) adiA is part of the adiAY operon as shown by Northern blot analysis (17). AdiY is a putative positive regulator of adiA, but an adiY deletion mutant showed no alteration in AR, indicating that the acid sensitivity of the adiA mutant described in this work was not due to polar effects on the downstream adiY gene (17).
TABLE 1.
E. coli strains used in this study
| Strain | Serotype or genotypea | Source or reference |
|---|---|---|
| ATCC 43895 | O157:H7 | 48 |
| EK274 | ATCC 43895 Nar Rfr | 39 |
| FRIK 816-3 | ATCC 43895 rpoS::pRR10 (Ap) | 11 |
| EK275 | FRIK 816-3 Nar Rfr | 39 |
| EF484 | 43895 gadC::pRR10 (Ap) Nar Rfr | This work |
| EF489 | 43895 adiA::pRR10 (Ap) Nar Rfr | This work |
| EF501 | 43895 gadA::pRR10 (Ap) Nar Rfr | 39 |
Na, nalidixic acid; Rf, rifampin; Ap, ampicillin.
Spontaneous mutants of the adiA, gadA, or gadC strains resistant to nalidixic acid and rifampin were generated to aid in recovery from calf fecal specimens. These mutants had survival and growth characteristics similar to those of the parent strain EK274, which was also nalidixic acid and rifampin resistant. Ampicillin (50 μg/ml) was added to cultures of the rpoS, gadA, gadC, and adiA mutants to maintain pRR10.
AR assays.
Cells were grown overnight in one of several media, including LBG (LB plus 0.4% glucose), BHIG (brain-heart infusion [BHI] plus 0.4% glucose), buffered LB (either 100 mM morpholinepropanesulfonic acid [pH 8] or 100 mM morpholineethanesulfonic acid [MES; pH 5.5]) (34), and minimal E salts glucose (EG) (47). Cultures were grown in 3 ml of the appropriate medium in 13-mm-diameter test tubes with shaking (240 rpm) at 37°C to stationary phase (22 h, 1 × 109 to 5 × 109 CFU/ml). The oxidative system was tested with cells grown overnight in pH 5.5 LB or BHI-buffered medium followed by 1:1,000 dilution into prewarmed (37°C) pH 2.5 EG (adjusted with HCl). The glutamate and arginine systems were tested by using stationary-phase cells grown in LBG or BHIG followed by 1:1,000 dilution into prewarmed pH 2.5 EG supplemented with 1.5 mM glutamate or 0.6 mM arginine, respectively. Viable cell counts were determined at 0, 2, and 4 h post-acid challenge by diluting cells in LB, plating cells onto LB agar, and incubating them for 20 h at 37°C. Results presented are representative of triplicate experiments. Survival in acidic (pH 3.5) apple cider (contains pulp, single lot, pasteurized; Thrifty Maid) was assayed similarly. Cultures were grown in appropriate media to stationary phase and diluted 1:1,000 into cider prewarmed to 25°C, and viable counts were determined as described above.
E. coli O157:H7 shedding studies.
Six- to eight-week-old weaned dairy calves were acclimated for 2 weeks before inoculation, the first week outdoors followed by a second week in a climate-controlled BL-2 containment facility. Each calf was fecal cultured three times during acclimation for E. coli O157:H7 to assure that only E. coli O157:H7-negative calves were inoculated in the study. Pairs of calves were housed together on pine shavings, given water ad libitum, and fed grain and hay twice daily. The calves were euthanized with sodium pentobarbital and incinerated at the conclusion of each experiment. Protocols approved by the Auburn University Institutional Animal Care and Use Committee were followed to ensure the welfare of the calves, and strict containment precautions were followed to prevent the release of E. coli O157:H7 and to guarantee the safety of laboratory personnel.
Bacterial strains for the calf inoculations were grown in BHI broth (pH 5.5) to stationary phase (22 h, 1 × 109 to 5 × 109 CFU/ml). Cell pellets were harvested by centrifugation, washed, and suspended in 0.85% NaCl. Four calves were inoculated by gastric lavage with a 50-ml inoculum of 0.85% NaCl containing 1010 total CFU of an equal number of EK274 and either EF484 (gadC) or EF489 (adiA), followed by 500 ml of 0.85% NaCl. In a separate experiment, 1010 total CFU of an equal number of stationary-phase-grown EK274 and logarithmic-phase-grown EF501 (gadA) (2.5 h; 1 × 108 to 5 × 108 CFU/ml) were inoculated into two calves. Conversely, 1010 total CFU of an equal number of stationary-phase-grown EF501 and logarithmic-phase-grown EK274 were inoculated into two calves.
Following inoculation, fecal samples were cultured daily for 16 days for enumeration of E. coli O157:H7. Fifty-gram specimens were collected each morning and were immediately transported to the laboratory for culture. Quantitative culture of the samples was performed by adding 1 g of feces to 9 ml of phosphate buffer followed by serial 10-fold dilution in phosphate buffer. A 0.1-ml volume of each dilution was plated in duplicate onto sorbitol-MacConkey agar (Difco, Detroit, Mich.) containing nalidixic acid (35 μg/ml), which selected for the inoculated E. coli O157:H7 strains, or agar containing both nalidixic acid and ampicillin (50 μg/ml), which selected for the insertion mutants. The CFU per gram of EK274 present in specimens was calculated by subtracting the combined number of nalidixic acid-resistant and ampicillin-resistant colonies from the number of nalidixic acid-resistant colonies. Serologic confirmation of E. coli O157:H7 suspect colonies was made by using a commercial latex agglutination kit (RIM E. coli O157:H7; Remel, Inc., Lenexa, Kans.).
Statistical methods.
Data were entered into a computer spreadsheet program (Excel version 5.0; Microsoft) and analyzed by using statistical software (Statistical Analysis System [SAS] version 6.12 software; SAS Institute, Cary, N.C.). Differences between strains (wild-type versus mutant or AR versus AS) were analyzed by analysis of variance for repeated measures at a significance level of P < 0.05.
RESULTS
AR mutants of E. coli O157:H7.
The E. coli O157:H7 parent (EK274) and derivative rpoS (EK275), gadC (EF484), and adiA (EF489) mutants were tested for the three AR systems previously identified (29). The data presented in Table 2 show that insertional inactivation of rpoS eliminated the oxidative system and partially reduced the arginine- and glutamate-dependent systems when cells were adapted in LB pH 5.5 and BHIG, respectively. These results agree with those reported earlier (39). It is important to note that, although an rpoS mutation lowers glutamate- and arginine-dependent AR, these systems do not have an absolute requirement for RpoS. The extent of RpoS involvement varies with growth condition. This was particularly evident, as noted previously, when adaptation of the rpoS mutant was made in BHIG rather than LBG. After growth in BHIG, EK275 (rpoS) exhibited near wild-type levels of glutamate- and arginine-dependent AR (Table 2), but, when adapted in BHI pH 5.5, it remained defective in oxidative AR (39). The gadC and adiA mutants, however, retained wild-type oxidative AR but were clearly defective in the glutamate- and arginine-dependent systems, respectively.
TABLE 2.
Effect of rpoS, gadC, and adiA on the three AR systems in E. coli O157:H7
| Adaptation mediuma | Induced AR system | Challenge medium (pH 2.5) | % Survivalb
|
|||
|---|---|---|---|---|---|---|
| EK274 | EK275 (rpoS) | EF484 (gadC) | EF489 (adiA) | |||
| LB (pH 8) | Negative control | EG | <0.005 | <0.004 | <0.005 | <0.005 |
| LB (pH 5.5) | AR 1 (RpoS-dependent) | EG | 38 | <0.005 | 40 | 10 |
| LBG | Negative control | EG | <0.004 | <0.006 | <0.005 | <0.007 |
| LBG | AR 2 (Glt-dependent) | EG + Glt | 45 | 5 | <0.007 | 73 |
| LBG | AR 3 (Arg-dependent) | EG + Arg | <0.004 | <0.004 | NDc | <0.006 |
| BHIG | Negative control | EG | <0.004 | <0.005 | <0.004 | <0.005 |
| BHIG | AR 2 (Glt-dependent) | EG + Glt | 64 | 20 | <0.007 | 85 |
| BHIG | AR 3 (Arg-dependent) | EG + Arg | 2 | 1 | 10 | <0.005 |
Adaptation involved overnight growth in the medium indicated. AR 1 is completely inhibited during growth at pH 8, the condition chosen as a negative control. The negative controls for systems 2 and 3 involved adding cells with active systems to pH 2.5 media that lack substrates for the system, i.e., glutamate (Glt) and arginine (Arg).
Percent survival was measured by determining the number of surviving cells after 4 h of acid treatment. Data presented are representative example from triplicate trials.
ND, not determined.
Mechanism of E. coli O157:H7 AR in apple cider.
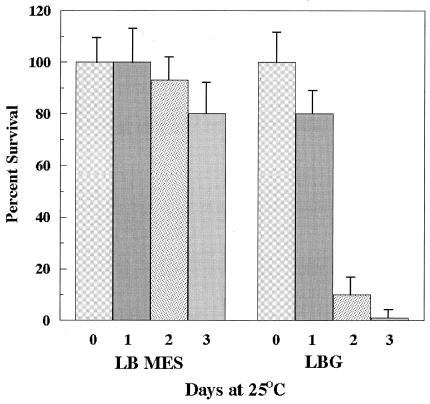
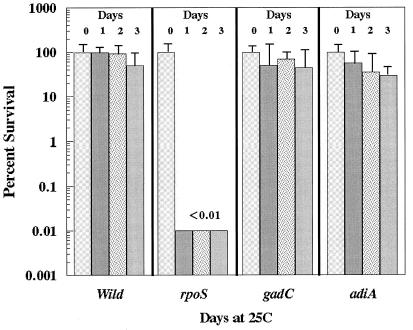
AR of E. coli O157:H7 is believed to contribute to the survival of this microbe in acidic foods, such as unpasteurized apple cider, that have been linked to E. coli O157:H7 infection (1, 5). Having assembled mutants deficient in each individual system, we were able to investigate which AR system was most important for survival in this type of environment. In initial experiments, wild-type E. coli O157:H7 strains were adapted for 18 h in LB MES pH 5.5, which induces AR system 1, and in LBG or BHIG, both of which repress AR system 1 but induce the other two systems. The adapted cells were diluted to 106 CFU/ml in apple cider (pH 3.5), and survival was monitored over time at 25°C. The results immediately suggested that AR system 1 was most important in this environment. Cells adapted in LBG, repressed for AR system 1, did not survive in pH 3.5 apple cider to the same extent as cells grown in LB MES pH 5.5 (Fig. 1). We then tested various mutants for survival. The results clearly demonstrated that an rpoS mutant, lacking AR system 1 but exhibiting normal resistance via systems 2 and 3, survived very poorly, becoming undetectable after only 24 h (Fig. 2). In contrast, mutants defective in AR system 2 (gadC) or AR system 3 (adiA) survived at high levels for at least 3 days. Because rpoS mutants are susceptible to a variety of different stresses, we needed to prove that acidic pH was the reason this mutant succumbed to apple cider. This was accomplished by showing that the rpoS mutant grew if the pH of apple cider was neutralized (data not shown).
FIG. 1.
Survival of E. coli O157:H7 in pH 3.5 apple cider. Wild-type E. coli O157:H7 (EK274) cells were grown overnight in either LB MES (pH 5.5) or LBG. These stationary-phase cultures were diluted 1:1,000 into Thrifty Maid apple cider with a measured pH of 3.5. The cultures were incubated at 25°C for the times indicated. Survival was measured by diluting the cultures and plating samples onto LB agar. One-hundred percent survival ranged from 1.5 × 106 to 3 × 106 CFU per ml for all cultures.
FIG. 2.
Survival of E. coli O157:H7 mutants in pH 3.5 apple cider. Cells were grown overnight in LB MES (pH 5.5) and diluted 1:1,000 into Thrifty Maid apple cider with a measured pH of 3.5. The cultures were incubated at 25°C for the times indicated. Survival was measured by diluting the cultures and plating samples onto LB agar. One-hundred percent survival ranged from 1.5 × 106 to 3 × 106 CFU per ml for all cultures. Strains tested include wild-type (EK274), rpoS (EK275), gadC (EF484), and adiA (EF489).
Mechanism of E. coli O157:H7 AR in the bovine gastrointestinal tract.
We speculate that E. coli O157:H7 encounters a more severe acid stress in the gastrointestinal tract of cattle; certainly the pH of the abomasum (pH 2 to 2.5) is lower than that of the apple cider tested (pH 3.5). One reason that E. coli may have multiple systems of AR is that individual systems may cope with different acid stress environments. Thus, E. coli may use one system to protect itself in apple cider and another to survive acid stress in acidic portions of the gastrointestinal tract. To test this hypothesis, quantitative bacteriology was utilized to examine survival of the E. coli O157:H7 gadC (EF484) and adiA (EF489) mutants in the calf intestine. As presented in Materials and Methods, equal numbers of the mutant and wild-type strains were coinoculated into four calves and the fecal quantities of the strains (differentially marked with antibiotic resistance) were monitored for 16 days.
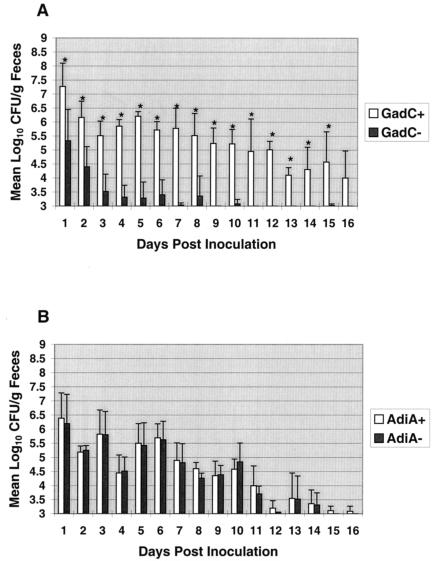
As observed previously with this model, the wild-type strain was shed in large numbers within 24 h of inoculation (14, 39) and continued to be excreted in numbers high enough to be enumerated (≥103 CFU/g of feces) for the 16-day postinoculation period (Fig. 3). In contrast, the gadC mutant was shed in significantly lower quantities than the wild-type strain from days 1 to 15 (P < 0.05). This shedding phenotype resembles that of an rpoS mutant, which also showed reduced shedding compared to that of the wild type, as was reported previously (39).
FIG. 3.
Shedding levels over time of E. coli O157:H7 wild-type (□) and GadC− (▪) (A) or wild-type (□) and AdiA− (▪) strains (B). Calves were inoculated with 1010 total CFU containing equal numbers of wild-type and mutant strains on day 0. Fecal samples were cultured daily beginning 1 day postinoculation. Colony counts are displayed as the means for four calves. Bars indicate standard deviation of mean log10 CFU/gram values. Asterisks indicate significant difference in shedding between wild-type and mutant strains at P < 0.05 using repeated measures of analysis of variance. Specimens containing <103 CFU/g of feces were below the level needed for accurate enumeration.
In contrast to the shedding phenotype displayed by the gadC mutant, the adiA mutant was shed in amounts comparable to those of the wild type, with no significant differences (P > 0.05) observed between the two strains during the 16-day experimental period (Fig. 3). The shedding phenotype of the adiA mutant resembled that of the gadA mutant, which also showed no reduction in shedding compared to that of the wild type (39).
The decreased shedding exhibited by the gadC mutant indicated that this AR determinant is critical to E. coli O157:H7 survival through the calf gastrointestinal tract. To confirm the overall role of AR in shedding, four calves were inoculated with equal numbers of stationary-phase (and therefore acid-resistant) wild-type strain (EK274) and logarithmic-phase (and therefore AS) gadA mutant (EF501). The gadA mutant was chosen for these experiments because previous work had shown it to have both wild-type AR at pH 2.5 and a wild-type shedding phenotype (39). In addition, because the pRR10 insertion confers ampicillin resistance on its recipient, the gadA mutant could be easily distinguished from the wild-type strain in fecal culture.
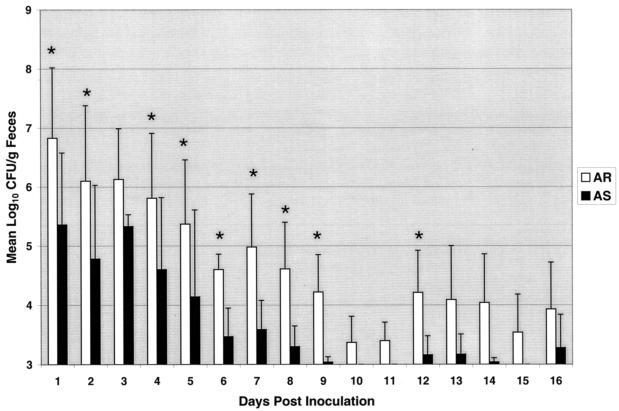
The shedding results from the calves that received the AR-AS mixture of E. coli O157:H7 showed that, especially during the first 9 days of shedding, the acid-resistant strain was shed in significantly greater amounts than was the AS strain (P < 0.05; Fig. 4). After day 9, the AS cells continued to shed at lower numbers than the AR cells. However, with the exception of day 12, statistical significance was unattainable due to the low numbers of cells being shed.
FIG. 4.
Shedding levels over time of E. coli O157:H7 acid-resistant (□) and AS (▪) strains. Calves were inoculated with 1010 total CFU containing an equal mixture of stationary-phase-grown EK274 cells (AR) and logarithmic-grown EF501 cells (AS) on day 0. Fecal samples were cultured daily beginning 1 day postinoculation. Colony counts are displayed as the means of four calves. Bars indicate standard deviation of mean log10 CFU/gram values. Asterisks indicate significant difference between shedding of acid-resistant and AS strains at P < 0.05 using repeated measures analysis of variance. Specimens containing <103 CFU/g of feces were below the level needed for accurate enumeration.
Finally, to address possible unforeseen effects on shedding manifested by the gadA mutant when grown under conditions designed to render E. coli O157:H7 AS, we reversed the growth conditions of the two strains and inoculated two calves with equal numbers of the AS wild-type strain (EK274) and the acid-resistant gadA mutant strain (EF501). The results confirmed previous findings in that greater numbers of the acid-resistant-cultured gadA strain were shed than the AS-cultured wild-type strain (data not shown).
DISCUSSION
The purpose of the present study was to examine how E. coli O157:H7 protects itself when exposed to two very different acidic environments relevant to food safety. Apple cider was chosen for one of these environments, because it is naturally acidic (∼pH 3.5) and has been the source for human outbreaks of E. coli O157:H7 disease (5). Cattle were chosen as the other environment, because they serve as reservoir hosts for E. coli O157:H7, which must traverse the low pH (2.0 to 2.5) of the abomasum to reach the colon. By unraveling the AR mechanisms used by E. coli O157:H7 to survive in these and other acidic environments, it may be possible to design strategies targeting this survival mechanism as a means of pathogen reduction.
The role played by the rpoS-encoded global stress response regulator σS in the AR of E. coli O157:H7 and in the ability of this organism to survive in cattle has been previously examined (39). An rpoS mutant of E. coli O157:H7 lacks AR system 1 and is susceptible to other stresses present in the gastrointestinal tract. This mutant was shed from experimentally inoculated mice and calves in significantly lower numbers than its wild-type parent (39). While low gastric pH was the reason for reduced shedding of the rpoS mutant from mice, the same conclusion could not be drawn from the calf studies because neutralization of the acid in the calf abomasum was not possible.
Because gadC and adiA affect no known stress responses other than AR, testing of these mutants was expected to more definitively characterize a role for AR in surviving the gastrointestinal tract and acidic foods. In the present work, deletion of gadC resulted in elimination of the glutamate-dependent AR system 2, while deletion of adiA abolished the arginine-dependent AR system 3 (Table 2). The results clearly show that eliminating AR system 1 reduced fecal shedding as well as survival in apple cider. In contrast, removing AR system 2 specifically affected fecal shedding.
One might question why previous results indicated a gadA mutant was unaffected with respect to fecal shedding and AR (39), while the present work clearly showed that a gadC mutant was shed at very low levels and was AS. The reason for this is that there is only one gene encoding the glutamate:γ-amino butyric acid antiporter (GadC), but there are two isozymic forms of glutamate decarboxylase (gadA and gadB). As long as one of the decarboxylase genes is active and the GadC antiporter is present, the cells will retain glutamate-dependent AR (9).
In contrast to expectations, results with the adiA mutant indicated that the arginine-dependent AR system 3 is neither required for survival in apple cider nor necessary for survival in calves (Fig. 2 and 3). The reason for this may be that sufficient arginine is not available in either the stomach or apple cider environments. Alternatively, in the apple cider pH 3.5 environment the intracellular pH may not be optimum for arginine decarboxylase activity.
One possible reason why neither the glutamate nor arginine system facilitated survival in pH 3.5 apple cider is that the intracellular pH of pH 3.5-stressed cells may not be low enough to activate glutamate or arginine decarboxylase (45). The pH optima of these E. coli enzymes are pH 4 and 5, respectively. Another possibility is that apple cider might contain concentrations of glutamate and arginine too low for AR 2 and AR 3 to be active, because the activity of these two AR systems is amino acid dependent. This is a less likely hypothesis for the following reasons. A recent report presented the concentration of glutamate in apple juice to be between 0.4 and 1.2 mM (13). Our work has shown that a minimum glutamate concentration of approximately 0.2 mM is needed for in vitro survival at pH 2.5 (data not shown). Thus, it appears that apple juice (and presumably apple cider) contains plenty of glutamate to supply an active glutamate decarboxylase system.
The finding that glutamate-dependent AR system 2 is not required for survival of E. coli O157:H7 in apple cider is interesting in light of the findings of Cotter et al., who reported on the role of an analogous GAD system found in another food-borne pathogen, Listeria monocytogenes (13). These researchers found that a glutamate decarboxylase double mutant (gadAB) of L. monocytogenes was more rapidly killed than the wild-type strain in apple cider. Our results indicated that it is the rpoS-dependent AR system 1, and not the glutamate-dependent AR system 2, that is critical for survival of E. coli O157:H7 in apple cider. As noted above, possible explanations for this apparent contradiction may be a difference in the pH optima of the Listeria versus E. coli glutamate decarboxylases, a difference in the internal pH of the two organisms at external pH 3.5, or a difference in the organic acid concentration of apple juice versus apple cider. It was previously shown that the glutamate-dependent AR system will protect cells at higher external pH values if the organic acid concentration is elevated (29).
Perhaps the most remarkable finding of the study was that although gadC is not required for E. coli O157:H7 survival in apple cider, it is required for survival in calves (Fig. 3). In contrast to the acid-resistant wild-type E. coli O157:H7, the AS gadC mutant is killed in greater numbers during passage through the calf gastrointestinal tract. This difference in survival in these two acidic environments (apple cider versus calf gastrointestinal tract) could be due to the lower pH (2.0 to 2.5) in the bovine abomasum or, more likely, to the distinction between the organic acid found in apple cider and the inorganic acid of the calf abomasum. A mutant carrying a deletion in another AR system 2 gene, gadA, was shown previously to retain AR and to be shed normally in calves (39). This caveat may be explained by the fact that the glutamate decarboxylase system is redundant in E. coli, with enzyme molecules being produced by two unlinked genes, gadA and gadB. Thus, enough decarboxylase is produced in the gadA mutant by its intact gadB gene to retain its AR phenotype.
The work presented here also illustrated that in the absence of mutations affecting AR, parental cells grown to be acid resistant (stationary phase) survived in the calf gastrointestinal tract better than parental cells grown to be AS (log phase) (Fig. 4). The reason for this is that the rpoS-dependent stress response systems (AR system 1) and the glutamate-dependent AR system 2 specific for acid survival are not active in exponentially growing cells (39). This is consistent with stationary-phase cells having greater resistance to acid pH.
This is the first report that AR systems in E. coli O157:H7 are differentially utilized based on the type of acidic environment encountered. The fact that the σS-regulated AR system 1 is required for E. coli O157:H7 survival in both apple cider and cattle highlights the important role played by RpoS in the AR response of this pathogen. Future studies aimed at targeting this global regulatory protein in E. coli O157:H7-colonized calves are presently under way.
Acknowledgments
J.W.F. was supported by grants 97-02329 and 2000-02590 from the National Research Initiative Competitive Grants Program of the U.S. Department of Agriculture and grant RO1-GM61147 from the National Institutes of Health. S.B.P., J.C.W., and F.J.D. were supported by the Alabama Agricultural Experiment Station and the College of Veterinary Medicine, Auburn University.
We thank Scott Richardson, Bob Strong, Dustin Hamilton, and Misako Hwang for providing excellent technical assistance.
REFERENCES
- 1.Anonymous. 1995. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami. Morb. Mortal. Wkly. Rep. 44:157-160. [PubMed] [Google Scholar]
- 2.Arnold, K. W., and C. W. Kaspar. 1995. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:2037-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger, E. A., K. E. Redding, T. Plumb, L. C. Childs, S.-Y. Meng, and G. N. Bennett. 1989. Construction of lac fusions to the inducible arginine and lysine decarboxylase genes of Escherichia coli K-12. Mol. Microbiol. 3:609-620. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin, M. M., and A. R. Datta. 1995. Acid tolerance of enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 61:1669-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barrett, J. E. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 6.Bopp, D. J., B. D. Sauders, A. L. Waring, J. Ackelsberg, N. Dumas, E. Braun-Howland, D. Dziewulski, B. J. Wallace, M. Kelly, T. Halse, K. A. Musser, P. F. Smith, D. L. Morse, and R. J. Limberger. 2003. Detection, isolation, and molecular subtyping of Escherichia coli O157:H7 and Campylobacter jejuni associated with a large waterborne outbreak. J. Clin. Microbiol. 41:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan, R. L., and S. G. Edelson. 1999. pH-dependent stationary-phase acid resistance response of enterohemorrhagic Escherichia coli in the presence of various acidulants. J. Food Prot. 62:211-218. [DOI] [PubMed] [Google Scholar]
- 8.Byrnes, J. J., and J. L. Moake. 1986. Thrombotic thrombocytopenic purpura and the haemolytic-uraemic syndrome: evolving concepts of pathogenesis and therapy. Clin. Haematol. 15:413-442. [PubMed] [Google Scholar]
- 9.Castanie-Cornet, M.-P., T. A. Penfound, D. Smith, J. F. Elliott, and J. W. Foster. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Prevention and Control. 1999. Public health dispatch: outbreak of Escherichia coli O157:H7 and Campylobacter among attendees of the Washington County Fair-New York, 1999. Morb. Mortal. Wkly. Rep. 48:803-804. [PubMed] [Google Scholar]
- 11.Cheville, A. M., K. W. Arnold, C. Buchrieser, C.-M. Cheng, and C. W. Kaspar. 1996. rpoS regulation of acid, heat, and salt tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 62:1822-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleary, T. G. 1988. Cytotoxin producing Escherichia coli and the hemolytic uremic syndrome. Pediatr. Clin. N. Am. 35:458-501. [DOI] [PubMed] [Google Scholar]
- 13.Cotter, P. D., K. O'Reilly, and C. Hill. 2001. Role of the glutamate decarboxylase acid resistance system in the survival of Listeria monocytogenes LO28 in low pH foods. J. Food Prot. 64:1362-1368. [DOI] [PubMed] [Google Scholar]
- 14.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings, J. H. 1981. Short chain fatty acids in the human colon. Gut 22:763-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng, Y., J. H. Ryu, and L. R. Beuchat. 1999. Tolerance of acid-adapted and non-adapted Escherichia coli O157:H7 cells to reduced pH as affected by type of acidulant. J. Appl. Microbiol. 86:203-210. [DOI] [PubMed] [Google Scholar]
- 17.Gong, S., H. Richard, and J. W. Foster. 2003. YjdE (AdiC) is the arginine:agmatine antiporter essential for arginine-dependent acid resistance in Escherichia coli. J. Bacteriol. 185:4402-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorden, J., and P. L. C. Small. 1993. Acid resistance in enteric bacteria. Infect. Immun. 61:364-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin, P. M., B. P. Bell, P. R. Cieslak, J. Tuttle, T. J. Barrett, M. P. Doyle, A. M. McNamara, A. M. Shefer, and J. G. Wells. 1994. Large outbreak of Escherichia coli O157:H7 infections in the western United States: the big picture, p. 7-12. In M. A. Karmali and A. G. Goglio (ed.), Recent advances in verocytotoxin-producing Escherichia coli infections. Elsevier Science Publishing, New York, N.Y.
- 20.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 21.Hancock, D. D., T. E. Besser, M. L. Kinsel, M. L. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington state. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hersh, B. M., F. T. Farooq, D. N. Barstad, D. L. Blankenshorn, and J. L. Slonczewski. 1996. A glutamate-dependent acid resistance gene in Escherichia coli. J. Bacteriol. 178:3978-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilborn, E. D., P. A. Mshar, T. R. Fiorentino, Z. F. Dembek, T. J. Barrett, R. T. Howard, and M. L. Cartter. 2000. An outbreak of Escherichia coli O157:H7 infections and haemolytic uraemic syndrome associated with consumption of unpasteurized apple cider. Epidemiol. Infect. 124:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hovde, C. J., P. R. Austin, K. A. Cloud, C. J. Williams, and C. W. Hunt. 1999. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl. Environ. Microbiol. 65:3233-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan, K. N., L. Oxford, and C. P. O'Byrne. 1999. Survival of low-pH stress by Escherichia coli O157:H7: correlation between alterations in the cell envelope and increased acid tolerance. Appl. Environ. Microbiol. 65:3048-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine, M. M. 1987. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J. Infect. Dis. 155:377-389. [DOI] [PubMed] [Google Scholar]
- 27.Leyer, G. J., L.-L. Wang, and E. A. Johnson. 1995. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl. Environ. Microbiol. 61:3752-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, J., I. S. Lee, J. Frey, J. L. Slonczewski, and J. W. Foster. 1995. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 177:4097-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, J., M. P. Smith, K. C. Chapin, H. S. Baik, G. N. Bennett, and J. W. Foster. 1996. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 62:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, Z., S. Gong, H. Richard, D. L. Tucker, T. Conway, and J. W. Foster. 2003. GadE (YhiE) activates glutamate decarboxylase-dependent acid resistance in Escherichia coli K-12. Mol. Microbiol. 49:1309-1320. [DOI] [PubMed] [Google Scholar]
- 31.Ma, Z., H. Richard, D. L. Tucker, T. Conway, and J. W. Foster. 2002. Collaborative regulation of Escherichia coli glutamate-dependent acid resistance by two AraC-like regulators, GadX and GadW (YhiW). J. Bacteriol. 184:7001-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machin, S. J. 1984. Clinical annotation: thrombotic thrombocytopenic purpura. Br. J. Haematol. 56:191-197. [DOI] [PubMed] [Google Scholar]
- 33.Michino, H., K. Araki, S. Minami, S. Takaya, N. Sakai, M. Miyazaki, A. Ono, and H. Yanagawa. 1999. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 150:787-796. [DOI] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padhye, N. V., and M. P. Doyle. 1992. Escherichia coli O157:H7: epidemiology, pathogenesis, and methods of detection in food. J. Food Prot. 55:555-565. [DOI] [PubMed] [Google Scholar]
- 37.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 38.Peterson, W. L., P. A. Mackowiak, C. C. Barnett, M. Marling-Cason, and M. L. Haley. 1989. The human gastric bactericidal barrier: mechanisms of action, relative antibacterial activity and dietary influences. J. Infect. Dis. 159:979-983. [DOI] [PubMed] [Google Scholar]
- 39.Price, S. B., C. M. Cheng, C. W. Kaspar, J. C. Wright, F. J. DeGraves, T. A. Penfound, M. P. Castanie-Cornet, and J. W. Foster. 2000. Role of rpoS in acid resistance and fecal shedding of Escherichia coli O157:H7. Appl. Environ. Microbiol. 66:632-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen, M. A., J. W. C. Cray, T. A. Casey, and S. C. Whipp. 1993. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol. Lett. 114:79-84. [DOI] [PubMed] [Google Scholar]
- 41.Remis, R. S., K. L. McDonald, L. W. Riley, N. D. Puhr, J. G. Wells, B. R. Davis, P. A. Blake, and M. L. Cohen. 1984. Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7. Ann. Intern. Med. 101:728-742. [DOI] [PubMed] [Google Scholar]
- 42.Roering, A. M., J. B. Luchansky, A. M. Ihnot, S. E. Ansay, C. W. Kaspar, and S. C. Ingham. 1999. Comparative survival of Salmonella typhimurium DT104, Listeria monocytogenes, and Escherichia coli O157:H7 in preservative-free apple cider and simulated gastric fluid. Int. J. Food Microbiol. 46:263-269. [DOI] [PubMed] [Google Scholar]
- 43.Shere, J. A., K. J. Bartlett, and C. W. Kaspar. 1998. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl. Environ. Microbiol. 64:1390-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Small, P., D. Blankenhorn, D. Welty, E. Zinser, and J. L. Slonczewski. 1994. Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoS and growth pH. J. Bacteriol. 176:1729-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Small, P. L., and S. R. Waterman. 1998. Acid stress, anaerobiosis and gadCB: lessons from Lactococcus lactis and Escherichia coli. Trends Microbiol. 6:214-216. [DOI] [PubMed] [Google Scholar]
- 46.Tuttle, J., T. Gomez, M. P. Doyle, J. G. Wells, T. Zhao, R. V. Tauxe, and P. M. Griffin. 1999. Lessons from a large outbreak of Escherichia coli O157:H7 infections: insights into the infectious dose and method of widespread contamination of hamburger patties. Epidemiol. Infect. 122:185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 48.Wells, J. G., B. R. Davis, I. K. Wachsmuth, L. W. Riley, R. S. Remis, R. Sokolow, and G. K. Morris. 1983. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J. Clin. Microbiol. 18:512-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whipp, S. C., M. A. Rasmussen, and W. C. Cray, Jr. 1994. Animals as a source of Escherichia coli pathogenic for human beings. J. Am. Vet. Med. Assoc. 204:1168-1175. [PubMed] [Google Scholar]
- 50.Yukioka, H., and S. Kurita. 1997. Escherichia coli O157 infection disaster in Japan, 1996. Eur. J. Emerg. Med. 4:165. [DOI] [PubMed] [Google Scholar]