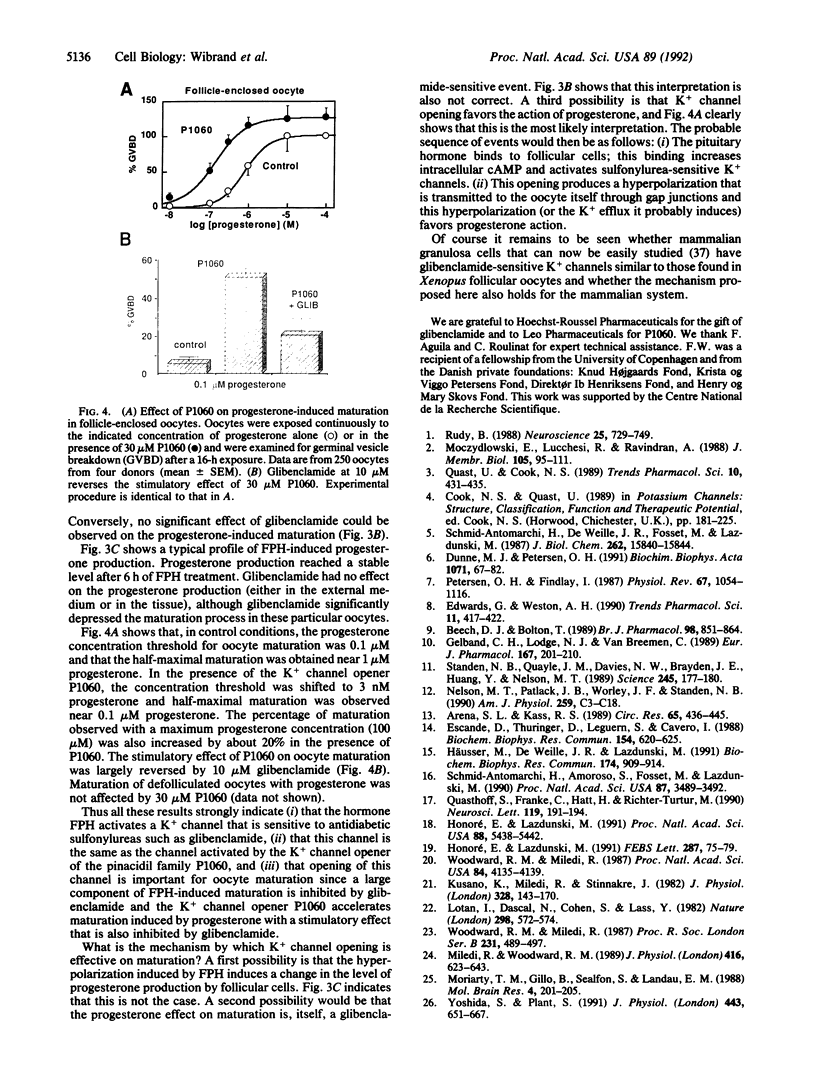
Abstract
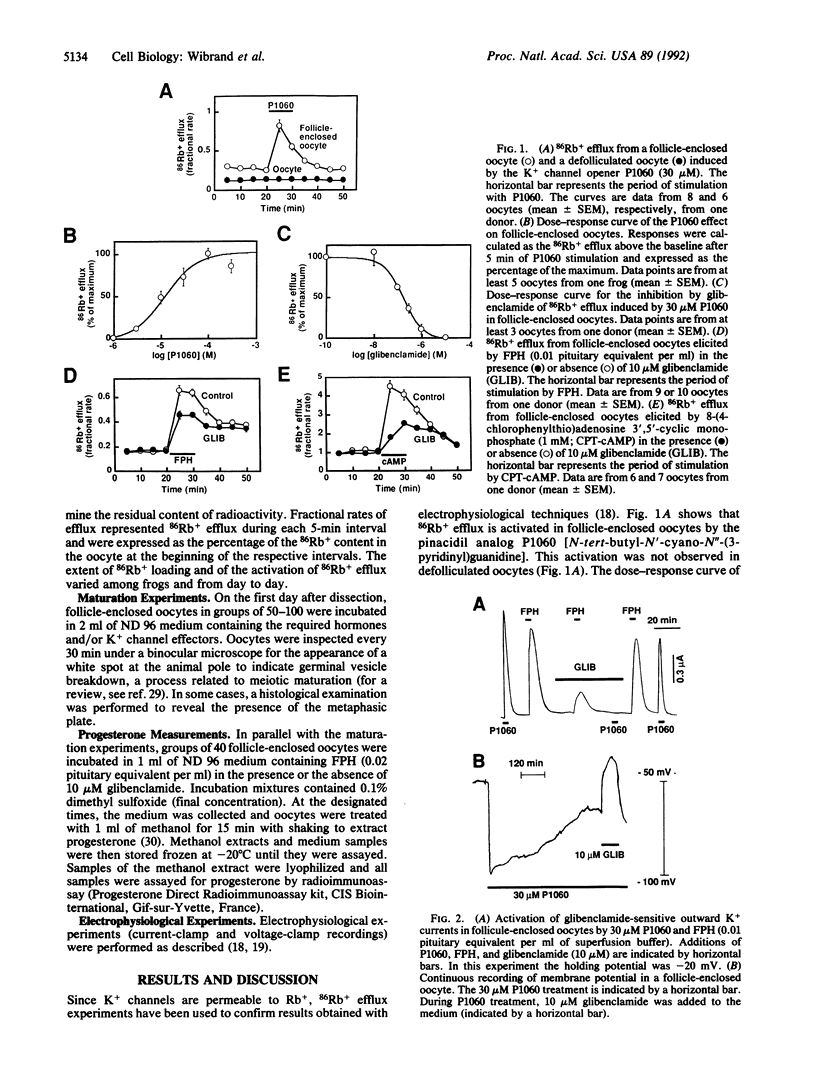
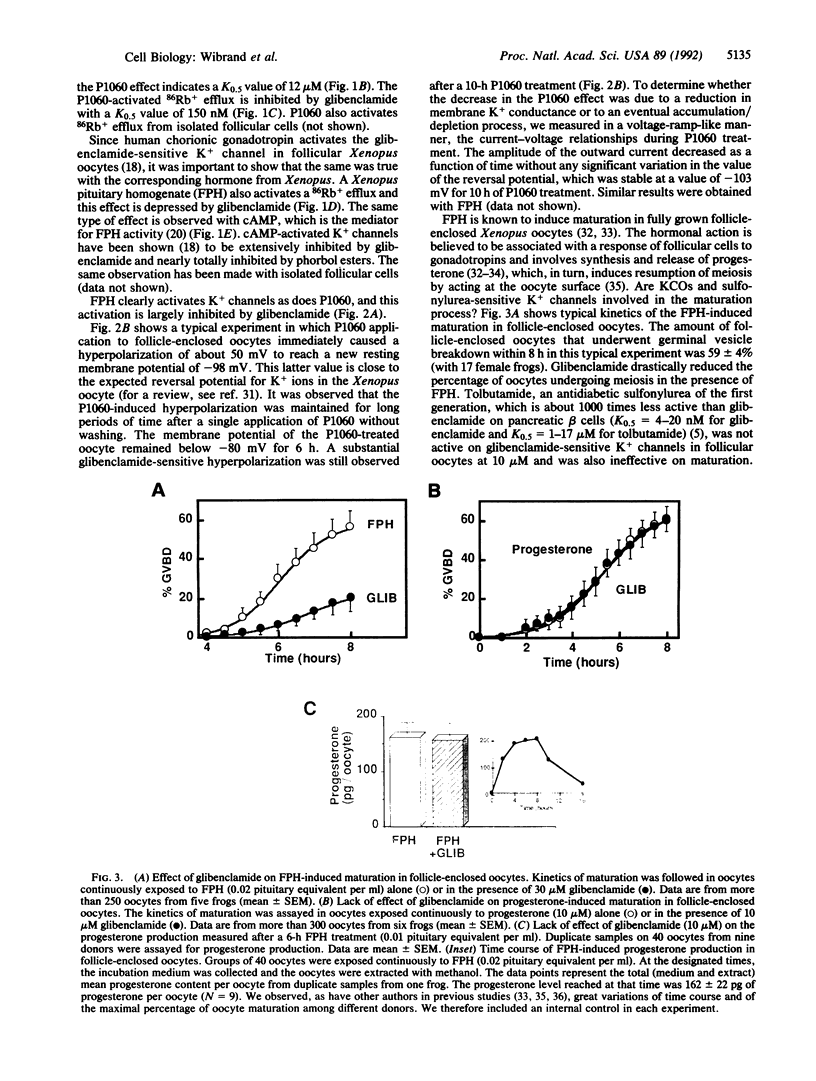
The vasorelaxing K+ channel opener P1060 (a pinacidil analog), gonadotropins, and cAMP were shown to activate a glibenclamide-sensitive 86Rb+ efflux from fully grown follicle-enclosed Xenopus oocytes. Glibenclamide-sensitive K+ channels are located in follicular cells. Glibenclamide (i) depressed the gonadotropin- but not the progesterone-induced maturation and (ii) did not significantly modify progesterone production in oocytes exposed to Xenopus gonadotropin. In follicle-enclosed oocytes, the opener P1060 very significantly enhanced the oocyte sensitivity to progesterone. This increased sensitivity to the hormone induced by the K+ channel opener was reversed by glibenclamide. Thus these results suggest that the opening of glibenclamide-sensitive K+ channels in follicular cells by gonadotropins (and other activators of this channel) induces a hyperpolarization in the oocyte that greatly facilitates maturation by increasing the oocyte sensitivity to progesterone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arena J. P., Kass R. S. Enhancement of potassium-sensitive current in heart cells by pinacidil. Evidence for modulation of the ATP-sensitive potassium channel. Circ Res. 1989 Aug;65(2):436–445. doi: 10.1161/01.res.65.2.436. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978 Oct 19;275(5681):593–598. doi: 10.1038/275593a0. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Potassium selective ion channels in insulin-secreting cells: physiology, pharmacology and their role in stimulus-secretion coupling. Biochim Biophys Acta. 1991 Mar 7;1071(1):67–82. doi: 10.1016/0304-4157(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Structure-activity relationships of K+ channel openers. Trends Pharmacol Sci. 1990 Oct;11(10):417–422. doi: 10.1016/0165-6147(90)90149-3. [DOI] [PubMed] [Google Scholar]
- Escande D., Thuringer D., Leguern S., Cavero I. The potassium channel opener cromakalim (BRL 34915) activates ATP-dependent K+ channels in isolated cardiac myocytes. Biochem Biophys Res Commun. 1988 Jul 29;154(2):620–625. doi: 10.1016/0006-291x(88)90184-2. [DOI] [PubMed] [Google Scholar]
- Fortune J. E., Concannon P. W., Hansel W. Ovarian progesterone levels during in vitro oocyte maturation and ovulation in Xenopus laevis. Biol Reprod. 1975 Dec;13(5):561–567. doi: 10.1095/biolreprod13.5.561. [DOI] [PubMed] [Google Scholar]
- Fortune J. E. Steroid production by Xenopus ovarian follicles at different developmental stages. Dev Biol. 1983 Oct;99(2):502–509. doi: 10.1016/0012-1606(83)90299-3. [DOI] [PubMed] [Google Scholar]
- Gelband C. H., Lodge N. J., Van Breemen C. A Ca2+-activated K+ channel from rabbit aorta: modulation by cromakalim. Eur J Pharmacol. 1989 Aug 22;167(2):201–210. doi: 10.1016/0014-2999(89)90580-3. [DOI] [PubMed] [Google Scholar]
- Honoré E., Lazdunski M. Hormone-regulated K+ channels in follicle-enclosed oocytes are activated by vasorelaxing K+ channel openers and blocked by antidiabetic sulfonylureas. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5438–5442. doi: 10.1073/pnas.88.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Lazdunski M. Two different types of channels are targets for potassium channel openers in Xenopus oocytes. FEBS Lett. 1991 Aug 5;287(1-2):75–79. doi: 10.1016/0014-5793(91)80019-y. [DOI] [PubMed] [Google Scholar]
- Häusser M. A., de Weille J. R., Lazdunski M. Activation by cromakalim of pre- and post-synaptic ATP-sensitive K+ channels in substantia nigra. Biochem Biophys Res Commun. 1991 Jan 31;174(2):909–914. doi: 10.1016/0006-291x(91)91504-6. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. W., Schuetz A. W. Intrafollicular action of estrogen in regulating pituitary-induced ovarian progesterone synthesis and oocyte maturation in Rana pipiens: temporal relationship and locus of action. Gen Comp Endocrinol. 1985 Jun;58(3):421–435. doi: 10.1016/0016-6480(85)90115-7. [DOI] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Cohen S., Lass Y. Adenosine-induced slow ionic currents in the Xenopus oocyte. Nature. 1982 Aug 5;298(5874):572–574. doi: 10.1038/298572a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Membrane currents elicited by prostaglandins, atrial natriuretic factor and oxytocin in follicle-enclosed Xenopus oocytes. J Physiol. 1989 Sep;416:623–643. doi: 10.1113/jphysiol.1989.sp017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Lucchesi K., Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988 Oct;105(2):95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Moriarty T. M., Gillo B., Sealfon S., Landau E. M. Activation of ionic currents in Xenopus oocytes by corticotropin-releasing peptides. Brain Res. 1988 Nov;464(3):201–205. doi: 10.1016/0169-328x(88)90026-5. [DOI] [PubMed] [Google Scholar]
- Mulner O., Ozon R. The roles of follicular envelopes in the initiation of Xenopus oocyte maturation. Gen Comp Endocrinol. 1981 Jul;44(3):335–343. doi: 10.1016/0016-6480(81)90010-1. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Quast U., Cook N. S. Moving together: K+ channel openers and ATP-sensitive K+ channels. Trends Pharmacol Sci. 1989 Nov;10(11):431–435. doi: 10.1016/S0165-6147(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Quasthoff S., Franke C., Hatt H., Richter-Turtur M. Two different types of potassium channels in human skeletal muscle activated by potassium channel openers. Neurosci Lett. 1990 Nov 13;119(2):191–194. doi: 10.1016/0304-3940(90)90831-s. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., Amoroso S., Fosset M., Lazdunski M. K+ channel openers activate brain sulfonylurea-sensitive K+ channels and block neurosecretion. Proc Natl Acad Sci U S A. 1990 May;87(9):3489–3492. doi: 10.1073/pnas.87.9.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Smith L. D. The induction of oocyte maturation: transmembrane signaling events and regulation of the cell cycle. Development. 1989 Dec;107(4):685–699. doi: 10.1242/dev.107.4.685. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Suh B. S., Amsterdam A. Establishment of highly steroidogenic granulosa cell lines by cotransfection with SV40 and Ha-ras oncogene: induction of steroidogenesis by cyclic adenosine 3'-5'-monophosphate and its suppression by phorbol ester. Endocrinology. 1990 Nov;127(5):2489–2500. doi: 10.1210/endo-127-5-2489. [DOI] [PubMed] [Google Scholar]
- Thibier-Fouchet C., Mulner O., Ozon R. Progesterone biosynthesis and metabolism by ovarian follicles and isolated oocytes Xenopus laevis. Biol Reprod. 1976 Apr;14(3):317–326. doi: 10.1095/biolreprod14.3.317. [DOI] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Hormonal activation of ionic currents in follicle-enclosed Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4135–4139. doi: 10.1073/pnas.84.12.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Membrane currents elicited by porcine vasoactive intestinal peptide (VIP) in follicle-enclosed Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Sep 22;231(1265):489–497. doi: 10.1098/rspb.1987.0057. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Plant S. A potassium current evoked by growth hormone-releasing hormone in follicular oocytes of Xenopus laevis. J Physiol. 1991 Nov;443:651–667. doi: 10.1113/jphysiol.1991.sp018856. [DOI] [PMC free article] [PubMed] [Google Scholar]