Abstract
Treatment of ErbB2-overexpressing BT474 and MDA-MB-453 breast cancer cells with 1 to 10 μmol/L betulinic acid inhibited cell growth, induced apoptosis, downregulated specificity protein (Sp) transcription factors Sp1, Sp3, and Sp4, and decreased expression of ErbB2. Individual or combined knockdown of Sp1, Sp3, Sp4 by RNA interference also decreased expression of ErbB2 and this response was because of repression of YY1, an Sp-regulated gene. Betulinic acid–dependent repression of Sp1, Sp3, Sp4, and Sp-regulated genes was due, in part, to induction of the Sp repressor ZBTB10 and downregulation of microRNA-27a (miR-27a), which constitutively inhibits ZBTB10 expression, and we show for the first time that the effects of betulinic acid on the miR-27a:ZBTB10-Sp transcription factor axis were cannabinoid 1 (CB1) and CB2 receptor–dependent, thus identifying a new cellular target for this anticancer agent.
Introduction
Betulinic acid is a naturally occurring triterpenoid found in bark extracts, and betulinic acid and synthetic analogues exhibit a broad spectrum of pharmacologic properties including antiviral, antibacterial, anti-inflammatory, antimalarial, and anticancer activities (1, 2). Betulinic acid also inhibits growth of multiple tumors, and the large difference between the doses required for tumor growth inhibition and toxic side-effects in animal models has generated interest in clinical development of this compound for cancer chemotherapy (2, 3). The overall effectiveness of betulinic acid as an anticancer drug has been linked to the mitochondriotoxicity of betulinic acid and induction of reactive oxygen species (ROS; refs. 4–6). Research in this laboratory has shown that betulinic acid inhibits growth and induces apoptosis in prostate, bladder, and colon cancer cells and tumors, and this is accompanied by downregulation of specificity protein (Sp) transcription factors Sp1, Sp3, Sp4, and Sp-regulated genes (6–8). Similar effects have been observed for several anticancer drugs including curcumin, arsenic trioxide, nonsteroidal anti-inflammatory drugs, and triterpenoids such as celastrol, 2-cyano-1,12-dioxooleana-1,9-dien-28-oic acid (CDDO) and 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oic acid (CDODA), and their corresponding esters (6–15). The importance of betulinic acid and other agents that target Sp proteins is because of (i) the overexpression of Sp1, Sp3, and Sp4 in tumor versus nontumor tissue and (ii) the critical roles for Sp-regulated genes in mediating cancer cell growth [EGF receptor (EGFR), hepatocyte growth factor receptor (c-MET), and cyclin D1], survival (bcl-2 and survivin), angiogenesis [vascular endothelial growth factor (VEGF) and VEGF receptors 1 and 2 (VEGFR1 and VEGFR2)], and inflammation (p65NFκB; refs. 8–12, 16–18).
This study investigated the anticancer activity of betulinic acid in BT474 and MDA-MB-453 breast cancer cells, which overexpress ErbB2, an important oncogenic growth factor receptor that is not an Sp-regulated gene. Betulinic acid–inhibited cell and tumor growth, downregulated Sp1, Sp3, and Sp4 and, surprisingly, decreased ErbB2 expression; however, this effect was because of down-regulation YY-1, an Sp-regulated gene that activates ErbB2 expression (19, 20). The mechanism of Sp down-regulation by betulinic acid was because of disruption of microRNA-27a (miR-27a):ZBTB10, which was cannabinoid (CB) receptor dependent, and betulinic acid directly bound to both CB1 and CB2 receptors. This represents a novel mechanism of action of betulinic acid and highlights the clinical potential of betulinic acid and related compounds that downregulate Sp transcription factors as a new class of mechanism-based agents for treating ErbB2-overexpressing breast tumors.
Materials and Methods
Chemicals, antibodies, plasmids, and reagents
Betulinic acid and lactacystin were purchased from Sigma-Aldrich. AM251, AM630, capsazepine, and WIN-55,212-2 were purchased from Tocris Bioscience. CAY10401 was purchased from Cayman Chemical. [3H]CP-55,940 (144 Ci/mmol) was purchased from Perkin Elmer. Antibodies against ErbB2 (C-18), p-ErbB2 (Try 1248)-R, Sp1 (PEP2), Sp3 (D-20), Sp4 (V-20), Akt (H-136), p-Akt (Ser473), MAPK (C-14), p-MAPK (E-4), β-actin (C4), AP2α (C-18 and 3B5), YY1 (H-10), CB1 (H-150), and CB2 (H-60) were obtained from Santa Cruz Biotechnology. Antibodies against cleaved PARP (D214), surviving, and fatty acid amide hydrolase (FAAH; L14B8) were purchased from Cell Signaling Technology. The YY1 promoter plasmids (YY1 p-1729-luc and YY1 p-277-Luc) were kindly provided by Dr Ed Seto (University of South Florida, Tampa, FL, USA). The ZBTB10 expression vector and the 3′-untranslated region (UTR)-luc construct and other reagents have previously been described (7, 21).
Cell lines
The MDA-MB-453 and BT474 cells were purchased from the American Type Culture Collection. Cells were initially grown and multiple aliquots were frozen and stored at −80 °C for future use. Cells were purchased more than 6 months ago and were not further tested or authenticated by the authors. Cell lines were cultured with 10% FBS in Dulbecco’s Modified Eagles’ Media and were maintained at 37°C in the presence of 5% CO2.
Cell proliferation assay
Cells (2–3 × 104 per well) were plated in 12-well plates and allowed to attach for 24 hours. Then cells were treated with either vehicle (dimethyl sulfoxide, DMSO) or different concentrations of betulinic acid for up to 4 days. Fresh medium and compounds were added every 48 hours, and cells were then trypsinized and counted at the indicated time points using a Coulter Z1 cell counter. Each experiment was done in triplicate, and results are expressed as means ± SE for each set of experiments.
Western blotting and TUNEL assays
Cells were rinsed with PBS and collected by scraping cells from the culture plate in 200 μL of high-salt buffer (50 mmol/L of HEPES, 0.5 mol/L of NaCl, 1.5 mmol/L of MgCl2, 1 mmol/L of EGFTA, 10 glycerol, and 1% Triton X-100) and 10 μL/mL of protease inhibitor mixtures (Sigma-Aldrich). The cell lysates were incubated on ice for 1 hour with intermittent vortex mixing and then centrifuged at 40,000 × g for 10 minutes at 4 °C. Equal amounts of protein were separated on SDS-polyacrylamide gels and processed as previously described (8, 9, 11, 12). Cells were plated in Lab-Tek II Chamber Slide System (Nalge Nunc International) and allowed to attach for 24 hours, and the effects of betulinic acid on the terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) assay were determined as described (14).
Real-time PCR analysis of mRNAs and miRNAs
Total RNA was isolated using the RNeasy Protect Mini kit (Qiagen) and RNA was reverse transcribed using Transcription System (Promega) according to the manufacturer’s protocols using primers that were previously described (8, 9, 11, 12).
DNA and siRNA transfection
Cells were plated in 12-well plates at 1 × 105 per well and cultured as described. After growth for 16 to 20 hours, transfections were carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol as previously described (14, 21). siRNAs for Sp1 (Sp1-1: SASI_Hs02_00363664; Sp1–2: SASI_Hs01_00070994), Sp3 (5′-GCGGCAGGUGGAGCCUUCACUTT-3′), Sp4 (5′-GCAGUGACACAUUAGUGAGCTT-3′), YY1 (YY1-1: SASI_Hs01_00155071, YY1-2: SASI_Hs01_-155072), CB1 (SASI_Hs01_00106167), and CB2 (SASI_Hs01_00041077) were purchased from Sigma-Aldrich. iLamin (5′-CUGGACUUCCAGAAGAACATT-3′), miR-27a mimic, as-miR-27a, and siRNA for FAAH were purchased from Dharmacon RNA Technologies; 100 nmol/L siRNAs were used in this study.
Luciferase assay
Transfected cells were lysed with 100 μL of 1 × reporter lysis buffer as described (10–12), and 30 μL of cell extract were used for luciferase and β-gal assays. Lumicount Luminometer (Packard Instruments) was used to quantitate luciferase and β-gal activities, and the luciferase activities were normalized to β-gal activity.
Animals, xenograft study, and immunohistochemistry
Female ovariectomized athymic nu/nu mice (5–7-week-old) were purchased from the Harlan Laboratories. Under anesthetic conditions, BT474 cells (1 × 106) were implanted with Matrigel (BD Biosciences) subcutaneously into the flank of each mouse. Ten days later, mice were randomized into 2 groups of 6 mice/group and dosed by oral gavage with corn oil or 20 mg/kg of betulinic acid every other day for 28 days (14 doses). The mice were weighed, tumor sizes were measured at the indicated time with calipers, and immunostaining was determined as described (8, 9, 11, 12).
Competitive receptor binding
Crude mouse brain or CHO-hCB2 cell homogenates for CB receptor binding were prepared essentially as described and stored at −80 °C (22). Increasing concentrations of betulinic acid (0.1–100 μmol/L) were incubated with 0.1 nmol/L of the nonselective CB1/CB2 agonist [3H]CP-55,940 in a final volume of 1 mL of binding buffer (50 mmol/L Tris, 0.05% bovine serum albumin, 5 mmol/L of MgCl2, pH 7.4) as described previously (23). Each binding assay contained 100 or 25 μg of membrane protein prepared from mouse brain or CHO-hCB2 cells, respectively. Reactions were incubated for 90 minutes at room temperature, and nonspecific binding was determined for each concentration of betulinic acid examined and was defined as binding observed in the presence of 1 mmol/L of the nonselective CB1/CB2 ligand WIN-55,212-2. Reactions were terminated by rapid vacuum filtration through Whatman GF/B glass fiber filters and the bound radioactivity was determined as described (22, 23). Specific binding was expressed as total binding minus nonspecific binding determined for each concentration of betulinic acid examined, and is graphed for each data point as a percentage of specific binding occurring in the absence of any competitor. Analysis of the binding data was conducted using the nonlinear regression (Curve Fit) function of GraphPad Prism® v5.0b to determine the concentration of the drug that displaced 50% of [3H]CP-55,940 (IC50). A measure of affinity (Ki) was derived from the IC50 values using the Cheng–Prusoff equation (24).
Results
Betulinic acid inhibits growth, induces apoptosis, and downregulates Sp1, Sp3, and Sp4 in BT474 and MDA-MB-453 cells
BT474 and MDA-MB-453 cells overexpress ErbB2, and 1–10 μmol/L betulinic acid (Fig. 1A) inhibited proliferation of both BT474 and MDA-MB-453 cells. The overall decrease in cell number was both concentration-and time (2 or 4 days)-dependent, and MDA-MB-453 cells were less responsive to betulinic acid than BT474 cells (Fig. 1B). The growth inhibitory effects of betulinic acid were accompanied by induction of cleaved PARP, a marker of apoptosis, and decreased expression of survivin, an inhibitor of apoptosis, was also observed (Fig. 1C). Induction of apoptosis was also observed in a TUNEL assay in which betulinic acid increased TUNEL staining in both cell lines (Fig. 1D).
Figure 1.
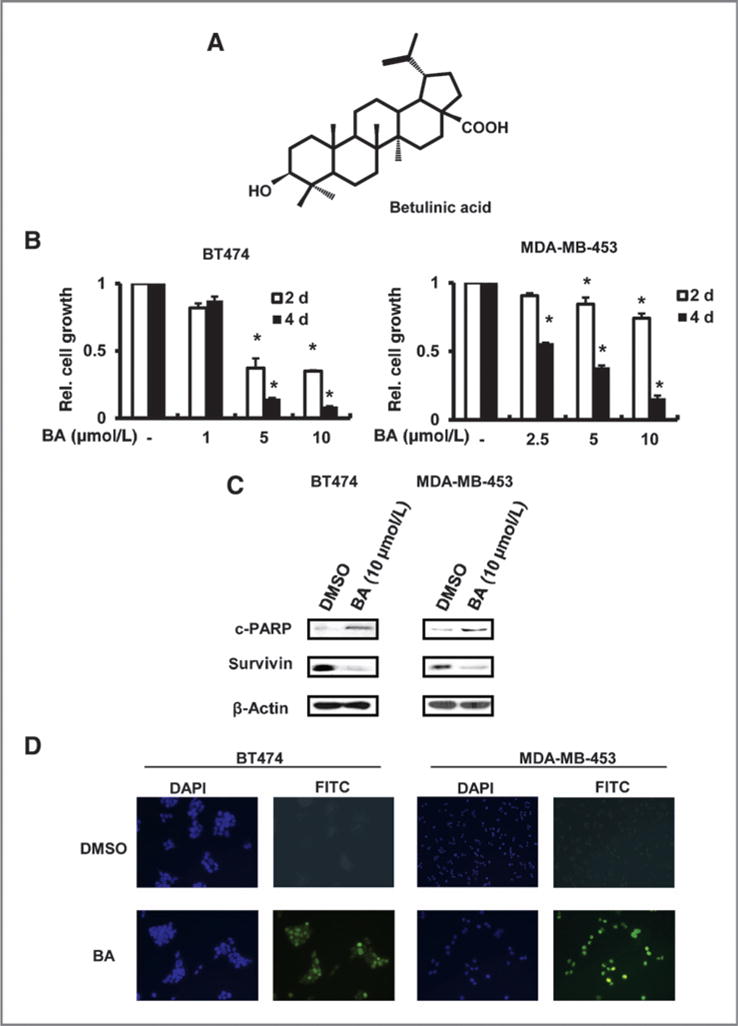
Effects of betulinic acid (BA) on cell proliferation and apoptosis. A, structure of BA. B, BA-mediated inhibition of BT474 and MDA-MB-453 cell growth. Cells were treated with different concentrations of BA for up to 4 days and the number of cells in each treatment group was determined as described in Materials and Methods. *, significant (P < 0.05) growth inhibition is indicated. Results are expressed as means ± SE for at least 3 replicate determinations for each treatment group. C, effects of BA on cleaved (c) PARP and survivin. Cells were treated with 10 μmol/L BA for 48 hours and whole-cell lysates were analyzed by Western blotting as described in Materials and Methods. D, BA induces apoptosis in cancer cells. Cells were treated with DMSO or 10 μmol/L BA for 24 hours and analyzed with a TUNEL assay as described in Materials and Methods. DAPI, 4′,6—diamidino— 2—phenylindole; FITC, fluorescein isothiocyanate.
Betulinic acid inhibits LNCaP prostate cancer cell growth and this is due, in part, to activation of proteasome-dependent degradation of Sp1, Sp3, and Sp4 proteins (7). Treatment of BT474 and MDA-MB-453 cells with 10 mmol/L betulinic acid for 48 hours decreased expression of Sp1, Sp3, Sp4, and survivin (an Sp-regulated gene) proteins (Fig. 2A) and mRNA (Fig. 2B) in both cell lines, and BT474 cells were more responsive than MDA-MB-453 cells. The proteasome inhibitor MG132 alone was cytotoxic to BT474 and MDA-MB-453 cells (data not shown), whereas lactacystin was not toxic. Treatment of BT474 and MDA-MB-453 cells with 10 μmol/L betulinic acid alone or in combination with 1 μmol/L lactacystin for 48 hours showed that lactacystin did not affect betulinic acid–induced downregulation of Sp proteins (Fig. 2A) and this contrasted to results for betulinic acid in prostate cancer cells (7).
Figure 2.
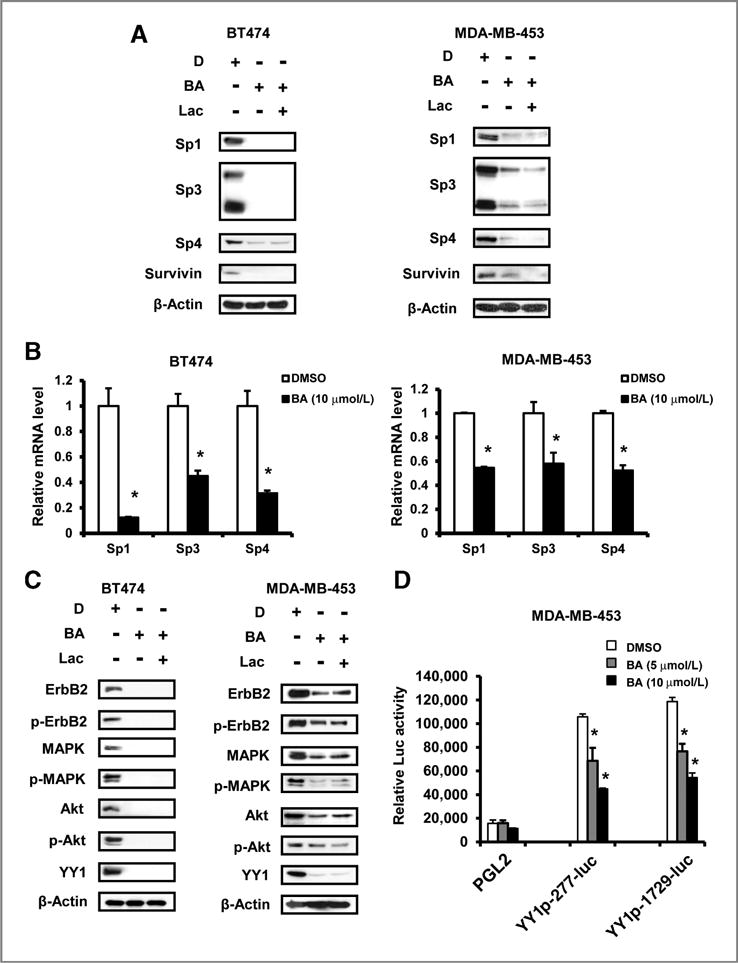
Effects of betulinic acid (BA) on Sp1, Sp3, Sp4, YY1, ErbB2, and ErbB2-dependent proteins. A, BA decreases Sp protein and survivin levels in BT474 and MDA-MB-453 cells. Cells were treated with DMSO (D), 10 μmol/L BA alone or in combination with 1 μmol/L lactacystin (Lac) for 48 hours, and whole-cell lysates were analyzed by Western blotting as described in Materials and Methods. B, BA decreases mRNA levels of Sp proteins. Cells were treated with 10 μmol/L BA for 16 hours, and mRNA levels were determined as described in Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (P < 0.05) decreases are indicated (*). C, BA decreases protein levels of ErbB2 and ErbB2-dependent proteins. Cells were treated with DMSO (D), 10 μmol/L BA alone or in combination with 1 μmol/L lactacystin for 48 hours, and whole-cell lysates were analyzed by Western blotting as described in Materials and Methods. D, BA decreases YY1 promoter activity. MDA-MB-453 cells were transfected with empty vector (PGL2), the YY1 p-277-luc, or the YY1 p-1729-luc construct. Cells were then treated with 5 or 10 μmol/L BA for 24 hours. Luciferase activity was determined as described in Materials and Methods. Results are means ± SE for 3 separate determinations and significant (P < 0.05) induction of luciferase activity by BA is indicated (*).
Betulinic acid–induced downregulation of YY1, ErbB2, and ErbB2-regulated genes is due to decreased Sp1, Sp3, and Sp4 expression
ErbB2 plays a major role in the proliferation of BT474 and MDA-MB-453 cells. Betulinic acid alone decreased ErbB2, p-ErbB2, and downstream kinases mitogen—activated protein kinase (MAPK), p-MAPK, Akt, and p-Akt expression (Fig. 2C), and these effects were not reversed after coincubation with the proteasome inhibitor lactacystin. Betulinic acid–mediated downregulation of MAPK and Akt total proteins has previously been observed in bladder cancer cells (8), and results of Sp knockdown suggest that these effects are Sp-independent and are currently being investigated. YY1 is a key upstream regulator of ErbB2 in breast cancer cells (19), and betulinic acid decreased expression of YY1 in both cell lines in the presence or absence of lactacystin (Fig. 2C). Because of the YY1 promoter contains multiple GC-rich Sp binding sites (20), we investigated the effects of betulinic acid on YY1 promoter activity and, in MDA-MB-453 cells transfected with GC-rich YY1 p-277-Luc or p-1729-luc constructs, treatment with betulinic acid for 24 hours decreased luciferase activity (Fig. 2D). Supplementary Fig. S1A and S1B shows that transfection of siRNAs against Sp1 (iSp1), Sp3 (iSp3), Sp4 (iSp4), and their combination (iSp1/3/4) resulted in specific knockdown of the target Sp proteins and also decreased expression of YY1 and ErbB2 proteins. iSp1-1 and iSp1-2 were targeted against Sp1 but did not affect Sp3 or Sp4 expression (data not shown). In a second set of experiments in MDA-MB-453 cells (Supplementary Fig. S1B), the siRNAs for Sp1 and Sp4 were highly specific; however, iSp3 also decreased expression of Sp3 and Sp4 proteins. iSp1, iSp4, and iSp1/3/4 decreased levels of both YY1 and ErbB2, whereas Sp3 knockdown had minimal effects on either protein. Previous RNA interference studies showed that knockdown of YY1 decreases expression of ErbB2 (19, 20), and we also observed that YY1 knockdown decreased ErbB2 levels in both cell lines (Supplementary Fig. S2).
Role of CB receptors
Betulinic acid–induced downregulation of Sp transcription factors was proteasome-independent (Fig. 2) and was not reversed by ROS inhibitors (data not shown) as previously reported for other compounds (11, 12, 14, 15). Preliminary studies in other cancer cell lines show that CBs decrease Sp proteins (data not shown); therefore, the effects of CB1 and CB2 receptor antagonists AM251 and AM630, respectively, and capsazepine (vanilloid receptor antagonist) on betulinic acid–mediated repression of Sp1, Sp3, and Sp4 and survivin were also investigated. The vanilloid receptor antagonist was included because this receptor also binds some CBs (25). The CB receptors are expressed in BT474 and MDA-MB-453 cells, and cotreatment with betulinic acid and either AM251 or AM630 attenuated the effects of betulinic acid–induced down-regulation of Sp1, Sp3, Sp4, and survivin, whereas capsazepine inhibited the effects of betulinic acid only in MDA-MB-453 cells (Fig. 3A and B). Figure 3B also shows that the antagonists alone had minimal effects on ErbB2, Sp1, Sp3, and YY1. The CB1 and CB2 receptor antagonists inhibited betulinic acid–mediated downregulation of ErbB2, p-ErbB2, p-MAPK, p-Akt, and YY1 in BT474 and MDA-MB-453 cells, whereas capsazepine was active as an inhibitor only in the latter cell line (Fig. 3C and D). Expression of AP2 was highly variable in both cell lines and was not further investigated. These results indicated that the CB1 and CB2 receptors mediated betulinic acid–induced effects on Sp1, Sp3 and Sp4, ErbB2, and YY1 in both cell lines. In contrast, the effects of betulinic acid on MAPK and Akt (total and phospho proteins) were CB receptor-independent and also Sp-independent in bladder cancer cells (8) and are currently being investigated.
Figure 3.
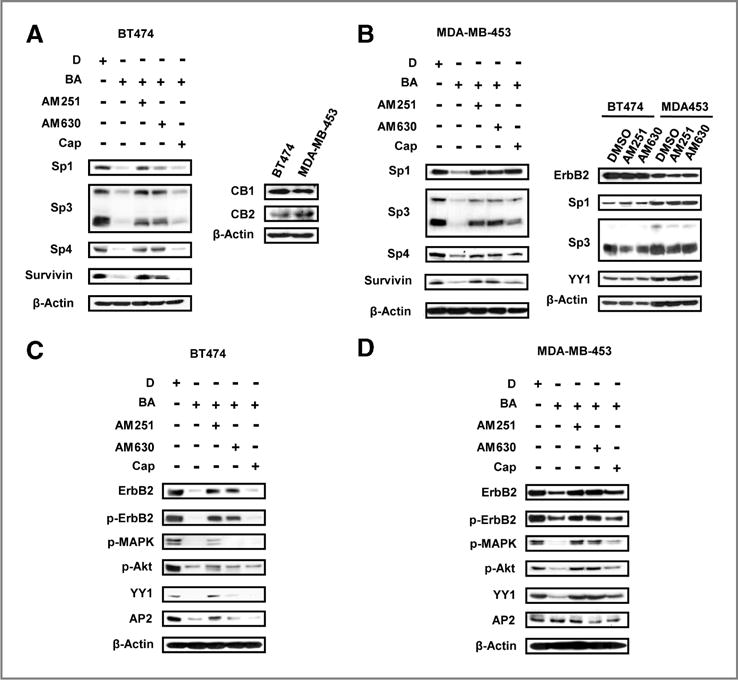
Effects of cannabinoid and vanilloid receptor antagonists on betulinic acid (BA)-induced responses. Effects of AM251, AM630, and capsazepine (Cap) on BA-mediated repression of Sps and survivin proteins in BT474 (A) and MDA-MB-453 (B) cells. Effects of AM251, AM630, and capsazepine on BA-mediated downregulation of ErbB2 and ErbB2-regulated kinases in BT474 (C) and MDA-MB-453 (D) cells and expression of CB receptors (D). Cells were pretreated with or without 6 μmol/L AM251, 6 μmol/L AM630, or 2 μmol/L capsazepine for 1 hour, and then DMSO (D) or 10 μmol/L BA were added to the medium for 48 hours, and whole-cell lysates were analyzed by Western blotting as described in Materials and Methods.
On the basis of these results, the direct binding of betulinic acid to the CB receptors was investigated in a competitive binding assay using [3H]CP-55,940 as the radioligand. Preliminary studies showed that high concentrations of betulinic acid (>10−5 mol/L) enhanced binding of [3H]CP-55,940; however, this was because of a concentration-dependent increase in both total and nonspecific binding (data not shown). Therefore, the [3H]CP-55,940 specific binding to the mCB1 and hCB2 receptor was determined by subtracting the nonspecific binding from the total binding as outlined in the Experimental Procedures (Fig. 4A). Betulinic acid competitively bound to both receptors, and the Ki values over 5 separate determinations (Fig. 4B) were 36. ± 4.1 and 41. ± 12.1 μmol/L for mCB1 and hCB2 receptors, respectively. As a positive control, Fig. 4B shows the competitive displacement curves using the CB WIN-55,212-2, which binds both receptors with anticipated low nanometers affinity. These results show that betulinic acid directly binds the CB receptors. We also show that knockdown of CB1 or CB2 receptors by RNA interference partially reversed betulinic acid–induced downregulation of Sp1, Sp3, and Sp4 (Fig. 4C), confirming a role for both receptors in mediating the effects of betulinic acid. A potential indirect effect of betulinic acid on CB-induced downregulation of Sp proteins could be due to inhibition of FAAH, which could increase endocannabinoid levels (26); however, knockdown of FAAH by RNA interference (in MDA-MB-453 cells) or inhibition of FAAH by the specific FAAH inhibitor CAY10401 (in BT474 cells) did not affect levels of Sp1, Sp3, or Sp4 proteins (Fig. 4D), indicating that this pathway is not involved in downregulation of Sp proteins.
Figure 4.
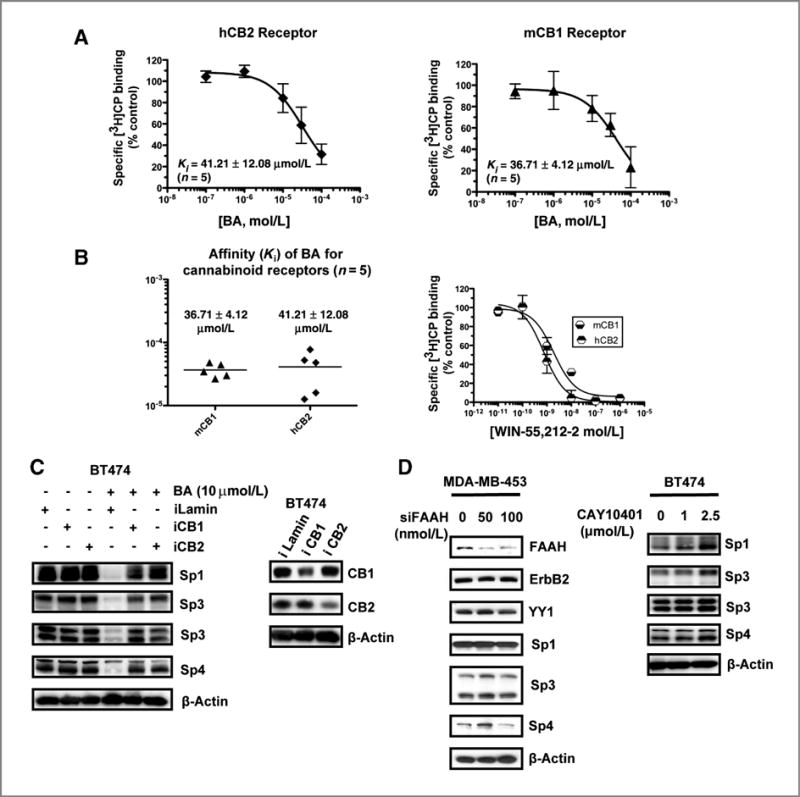
Betulinic acid (BA) is a CB receptor agonist. Specific binding of BA to CB receptors (A) and binding affinities (B). The specific binding and binding affinities of BA to CB1 and CB2 receptors were determined as described in Materials and Methods. C, knockdown of CB receptors by RNA interference. BT474 cells were transfected with iLamin (control) or iCB1 receptor or iCB2 receptor (oligonucleotides), and whole-cell lysates were analyzed by Western blotting as described in Materials and Methods. D, effects of FAAH knockdown or CAY10401 on Sp proteins. MDA-MB-453 cells were transfected with siFAAH or BT474 cells were treated with DMSO or CAY10401 for 24 hours, and whole-cell lysates were analyzed by Western blotting as described in Materials and Methods.
Betulinic acid disrupts miR-27a regulation of ZBTB10 and inhibits tumor growth
Proteasome-independent downregulation of Sp1, Sp3, and Sp4 by betulinic acid and other anticancer agents has been linked to downregulation of miR-27a and induction of the transcriptional repressor ZBTB10 (6, 10, 15). Treatment of BT474 and MDA-MB-453 cells with 5 or 10 μmol/L betulinic acid resulted in significant downregulation of miR-27a in both cell lines, and cotreatment with AM251 or AM630 inhibited this response (Fig. 5A), which was most pronounced in BT474 cells. Downregulation of miR-27a in cells treated with betulinic acid was accompanied by induction of ZBTB10 mRNA levels in both cell lines, and cotreatment with AM251 or AM630 inhibited the induction response (Fig. 5B). A >6-fold induction of ZBTB10 was observed in BT474 cells, whereas ZBTB10 was induced >2.5-fold in MDA-MB-453 cells treated with 5 or 10 μmol/L betulinic acid for 24 hours. The effects of antisense-miR-27a (as-miR-27a) and ZBTB10 overexpression on levels of Sp1, Sp3, Sp4, YY1, and ErbB2 proteins were also determined in BT474 and MDA-MB-453 cells (Fig. 5C and D), and both treatments decreased expression of Sp and Sp-regulated gene products. The effects of a miR-27a mimic and as-miR-27a on luciferase activity in BT474 and MDA-MB-453 cells transfected with ZBTB10 (UTR)-luc construct containing a miR-27a binding site resulted in decreased (miR-27a mimic) and increased (as-miR-27a) luciferase activity. In contrast, the mimic or antisense oligonucleotide did not affect luciferase activity in cells transfected with a construct [ZBTB10 (mUTR)-luc] containing a mutation in the miR-27a binding sites, confirming interactions of miR-27a with the target sequence in the 3′UTR of ZBTB10.
Figure 5.
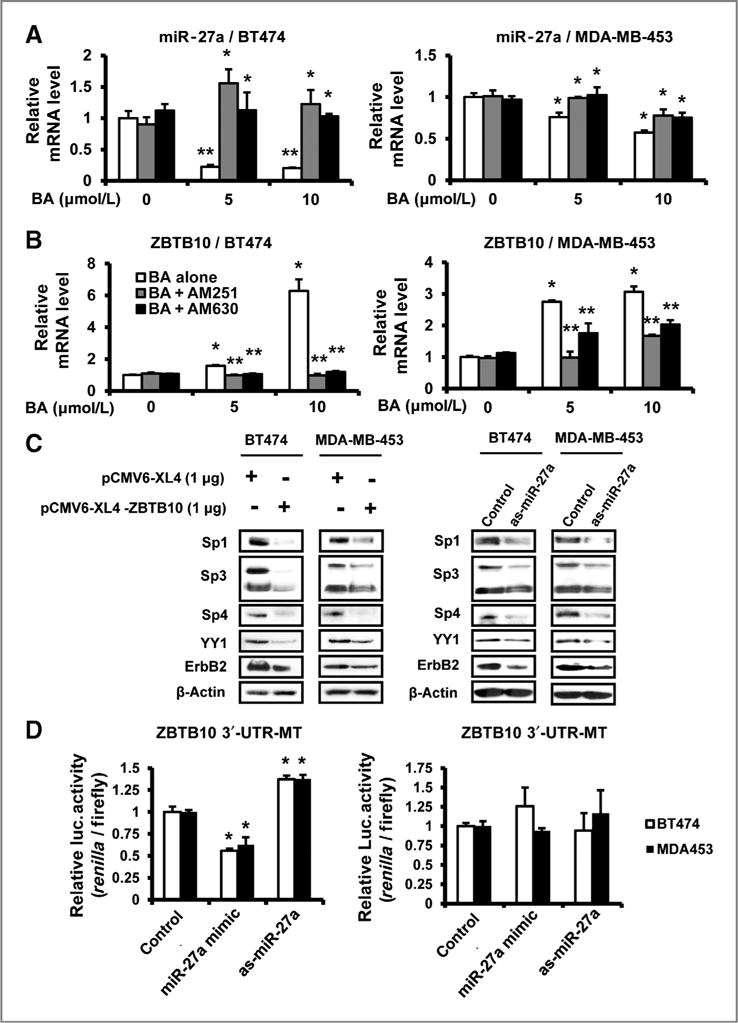
Effects of betulinic acid (BA) on miR-27a and ZBTB10, and the role of cannabinoid receptors on BA-mediated effects. A, downregulation of miR-27a. Cells were pretreated with or without 6 μmol/L AM251 or 6 μmol/L AM630 for 1 hour, DMSO or 5 or 10 μmol/L BA were added to the medium for 24 hours, and miR-27a levels were determined as described in Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (P < 0.05) inhibition of miR-27a (**) and inhibition by the antagonists are indicated (*). B, induction of ZBTB10. Cells were treated and processed as described in A, and significant (P < 0.05) induction by BA (*) and inhibition by the antagonists (**) are indicated. C, effects of ZBTB10 overexpression and antisense miR-27a on Sp protein levels, YY1, and ErbB2 proteins. Cells were transfected with 1 μg pCMV6-XL4-ZBTB10 plasmid or empty vector, 50 nmol/L antisense miR-27a (as-miR-27a), or control, and whole-cell lysates were analyzed by Western blotting as described in Materials and Methods. D, effects of miR-27a mimic or as-miR-27a on luciferase activity in ZBTB10 3′UTR-luc construct transfected cells. MiR-27a mimic (50 nmol/L) or as-miR-27a were transfected into BT474 and MDA-MB-453 cells as described in Materials and Methods, and a dual luciferase reporter assay was conducted according to the manufacturer’s instructions. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (P < 0.05) decreases or induction are indicated (*).
The in vivo effects of betulinic acid on tumor growth were also investigated in athymic nude mice bearing BT474 cells as xenografts. Betulinic acid was administered over a period of 28 days at a dose of 20 mg/kg/d. Tumor volumes and tumor weight were significantly inhibited, and betulinic acid decreased expression of Sp1, Sp3, and Sp4 proteins in tumors (Fig. 6A–C). Figure 6D illustrates that immunostaining of ErbB2 and Sp1 proteins were decreased in fixed tumor tissue from betulinic acid–treated mice compared with control (corn oil)-treated animals, and these in vivo data complement the results of in vitro studies.
Figure 6.
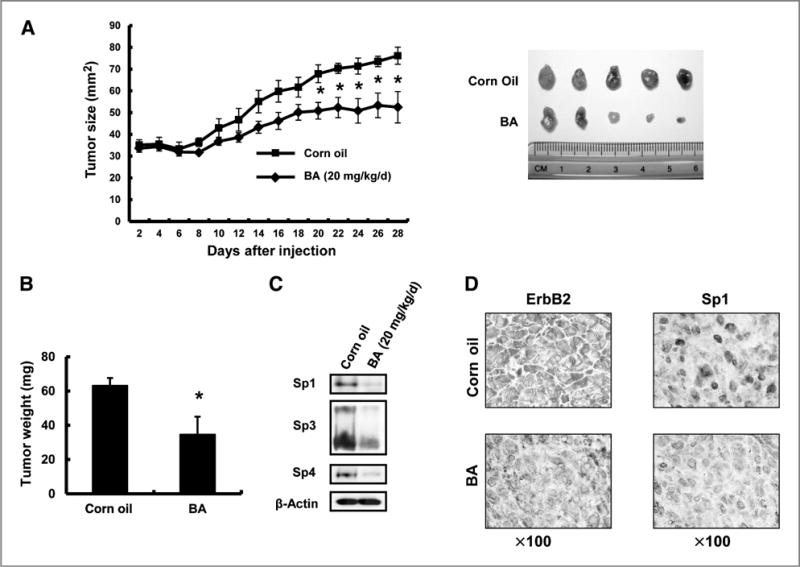
Betulinic acid (BA) inhibits tumor growth in BT474 xenografts. Inhibition of tumor size (A) and weight (B). Athymic nude mice bearing BT474 cells as xenografts were treated with BA (20 mg/kg/d), and tumor sizes and weights were determined as described in Materials and Methods. Significantly (P < 0.05) decreased tumor sizes and weights are indicated (*). C, BA decreases expression of Sp1, Sp3, and Sp4 proteins in tumors. Whole-cell lysates from corn oil and BA-treated tumors were analyzed by Western blotting as described in Materials and Methods. D, immunostaining for ErbB2 and Sp1. Fixed tumor tissue from corn oil-and BA-treated mice were stained with ErbB2 and Sp1 antibodies as described in Materials and Methods.
Discussion
Endocrine therapies with antiestrogens and aromatase inhibitors have been successful for treating patients with early stage ER-positive breast cancer (27–29), whereas patients with ER-negative or ErbB2-overexpressing tumors must undergo more aggressive treatment and their overall prognosis and survival are much lower than patients with early stage breast cancer (30–32). The development of the monoclonal antibody Herceptin that binds the extracellular domain of ErbB2 has provided significant treatment benefits for patients with these aggressive ErbB2-overexpressing tumors (33, 34). Although Herceptin has been successfully used alone and in combination therapy, there is concern regarding cardiotoxic side-effects of this antibody, and development of other agents including tyrosine kinase inhibitors is being actively pursued for treatment of tumors that overexpress ErbB2 and other growth factor receptors (35).
Betulinic acid inhibited growth and induced apoptosis in BT474 and MDA-MB-453 breast cancer cell lines (Fig. 1) and this was accompanied by downregulation of Sp1, Sp3, and Sp4 protein and mRNA levels (Fig. 2). Moreover, betulinic acid also inhibited tumor growth and downregulated Sp1, Sp3, and Sp4 in tumors from athymic nude mice bearing BT474 cells as xenografts (Fig. 6). In BT474 and MDA-MB-453 breast cancer cells, betulinic acid decreased expression of ErbB2, p-ErbB2, and downstream ErbB2-dependent kinases p-MAPK/MAPK and p-Akt/Akt (Fig. 2C and D), suggesting that ErbB2 downregulation may also be due repression of Sp proteins. However, unlike the EGFR that is an Sp-regulated gene (8), ErbB2 expression is dependent on other transcription factors including YY1, which contains multiple GC-rich promoter sites that bind Sp proteins (20). Betulinic acid decreased YY1 protein expression in BT474 and MDA-MB-453 cells (Fig. 2C and D) and, not surprisingly, knockdown of Sp proteins (individually and combined) decreased expression of YY1 and ErbB2 (Supplementary Fig. S1). Moreover, YY1 knockdown by RNA interference also decreased ErbB2 (Supplementary Fig. S2), showing that betulinic acid–mediated downregulation of ErbB2 is linked to decreased expression of Sp1, Sp3, Sp4, and Sp-regulated YY1.
The mechanisms of betulinic acid–induced down-rgulation of Sp1, Sp3, and Sp4 were dependent on tumortype and cell context. Betulinic acid induced proteasome-dependent downregulation in LNCaP prostate and SW480 colon cancer cells (6, 7), whereas in RKO colon cancer cells, this response was primarily ROS-dependent (7) and was because of repression of miR-27a and induction of ZBTB10, a transcriptional repressor that binds GC-rich promoter sites and downregulates Sp transcription factors and Sp-regulated genes (7, 10, 12, 15, 21). However, even in RKO cells, the CB receptor antagonists partially blocked the effects of betulinic acid on Sp proteins (Supplementary Fig. S3). Betulinic acid–induced repression of Sp1, Sp3, and Sp4 was proteasome-independent and not affected by lactacystin (Fig. 2A), and similar results were observed for ROS inhibitors such as glutathione (data not shown). Results summarized in Fig. 5 show that betulinic acid also decreased miR-27a and induced ZBTB10 in BT474 and MDA-MB-453 cells, suggesting that the critical downstream effects of betulinic acid on the miR-27a: ZBTB10 complex are similar to those observed in previous studies with the synthetic triterpenoids CDDO-Me and CDODA-Me (10, 12). CDDO-Me–mediated downregulation of miR-27 in pancreatic cancer cells was dependent on upstream disruption of mitochondria and induction of ROS; however, in contrast to these results, betulinic acid did not induce ROS in BT474 and MDA-MB-453 cells and the antioxidant glutathione did not modulate effects of betulinic acid on Sp1, Sp3, and Sp4 (data not shown).
Ongoing studies in this laboratory show that, like betulinic acid, CBs downregulate Sp1, Sp3, and Sp4 in cancer cell lines (data not shown), and we show for the first time that betulinic acid binds directly to CB1 and CB2 receptors (Fig. 4A). The competitive binding assay was slightly modified to determine total and nonspecific binding at all concentrations of betulinic acid, and Ki values were 36.7 and 41.2 μmol/L for the CB1 and CB2 receptors (Fig. 4B). The CB receptor antagonists AM251 and AM630 inhibited betulinic acid–induced miR-27a (downregulation), ZBTB10 (induction), Sp1, Sp3, Sp4, YY1, and ErbB2 (downregulation), and downregulation of Sp proteins by betulinic acid were also blocked by knockdown of CB1 and CB2 receptors by RNA interference (Fig. 4C). These results show that the effects of betulinic acid on BT474 and MDA-MB-453 cells on Sp transcription factors and ErbB2 are mediated through activation of the CB1 and CB2 receptors, which subsequently modulate the miR-27a: ZBTB10-Sp axis. CB1 and CB2 receptors are expressed in both cell lines and in human breast cancer cells and tumors, and one study showed a correlation between CB2 receptor and ErbB2 expression in human mammary tumors (36).
In summary, this study shows that betulinic acid inhibits ErbB2-overexpressing breast cancer cell and tumor growth, and this is accompanied by a cascade of events involving activation of the CB1 and CB2 receptors, resulting in modulation of the miR-27a:ZBTB10-Sp transcription factor axis and downregulation of the Sp-dependent gene YY1 and the YY1-dependent gene ErbB2. This CB receptor–dependent pathway significantly contributes to the effects of this compound as an inhibitor of ErbB2-overexpressing breast cancer cell and tumor growth and is consistent with the well-known anticancer activities of CBs (37, 38). Moreover, ongoing studies with CBs also show that they target Sp transcription factors (unpublished observations). Current studies are focused on the mechanistic link between activation of the CB receptors and modulation of miR-27a:ZBTB10 and the efficacies and mechanism of action of other agents that repress Sp transcription factors. We are also investigating the effects of betulinic acid as an inducer of the newly identified Sp-repressor ZBTB4, which is regulated by miR-17-92 cluster miRs (39). These data show a novel pathway for targeting ErbB2 and identify a new therapeutic approach for treating patients with breast cancer that overexpress this oncogene.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by DOD Breast Cancer Research Award (BC095260), NIH (R01-CA136571), and Texas AgriLife.
Footnotes
Authors’ Contributions
Conception and design: X. Liu, K. Kim, P.L. Prather, S. Safe
Development of methodology: I. Jutooru, P. Lei, K. Kim, S. Safe
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): X. Liu, I. Jutooru, P. Lei, S.-O. Lee, L.K. Brents, P.L. Prather, S. Safe
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): X. Liu, I. Jutooru, P. Lei, K. Kim, S.-O. Lee, L.K. Brents, P.L. Prather, S. Safe
Writing, review, and/or revision of the manuscript: X. Liu, L.K. Brents, P.L. Prather, S. Safe
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): X. Liu, S. Safe
Study supervision: X. Liu, S. Safe
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
References
- 1.Alakurtti S, Makela T, Koskimies S, Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci. 2006;29:1–13. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Fulda S. Betulinic acid for cancer treatment and prevention. Int J Mol Sci. 2008;9:1096–107. doi: 10.3390/ijms9061096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1:1046–51. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 4.Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today. 2009;14:885–90. doi: 10.1016/j.drudis.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Fulda S, Scaffidi C, Susin SA, Krammer PH, Kroemer G, Peter ME, et al. Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J Biol Chem. 1998;273:33942–8. doi: 10.1074/jbc.273.51.33942. [DOI] [PubMed] [Google Scholar]
- 6.Chintharlapalli S, Papineni S, Lei P, Pathi S, Safe S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer. 2011;11:371. doi: 10.1186/1471-2407-11-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 8.Chadalapaka G, Jutooru I, Burghardt R, Safe S. Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol Cancer Res. 2010;8:739–50. doi: 10.1158/1541-7786.MCR-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R, III, Li X, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–54. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chintharlapalli S, Papineni S, Abdelrahim M, Abudayyeh A, Jutooru I, Chadalapaka G, et al. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18beta-olean-1,12-dien-30-oate in colon cancer cells. Int J Cancer. 2009;125:1965–74. doi: 10.1002/ijc.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. J Biol Chem. 2010;285:25332–44. doi: 10.1074/jbc.M109.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutooru I, Chadalapaka G, Abdelrahim M, Basha MR, Samudio I, Konopleva M, et al. Methyl 2-cyano-3,12-dioxooleana-1,9-dien-28-oate decreases specificity protein transcription factors and inhibits pancreatic tumor growth: role of microRNA-27a. Mol Pharmacol. 2010;78:226–36. doi: 10.1124/mol.110.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol. 2005;68:317–29. doi: 10.1124/mol.105.011825. [DOI] [PubMed] [Google Scholar]
- 14.Jutooru I, Chadalapaka G, Sreevalsan S, Lei P, Barhoumi R, Burghardt R, et al. Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Exp Cell Res. 2010;316:2174–88. doi: 10.1016/j.yexcr.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathi SS, Jutooru I, Chadalapaka G, Sreevalsan S, Anand S, Thatcher GR, et al. GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Mol Cancer Res. 2011;9:195–202. doi: 10.1158/1541-7786.MCR-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelrahim M, Baker CH, Abbruzzese JL, Sheikh-Hamad D, Liu S, Cho SD, et al. Regulation of vascular endothelial growth factor receptor-1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer Res. 2007;67:3286–94. doi: 10.1158/0008-5472.CAN-06-3831. [DOI] [PubMed] [Google Scholar]
- 17.Abdelrahim M, Smith R, III, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res. 2004;64:6740–9. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- 18.Higgins KJ, Abdelrahim M, Liu S, Yoon K, Safe S. Regulation of vascular endothelial growth factor receptor-2 expression in pancreatic cancer cells by Sp proteins. Biochem Biophys Res Commun. 2006;345:292–301. doi: 10.1016/j.bbrc.2006.04.111. [DOI] [PubMed] [Google Scholar]
- 19.Begon DY, Delacroix L, Vernimmen D, Jackers P, Winkler R. Yin Yang 1 cooperates with activator protein 2 to stimulate ERBB2 gene expression in mammary cancer cells. J Biol Chem. 2005;280:24428–34. doi: 10.1074/jbc.M503790200. [DOI] [PubMed] [Google Scholar]
- 20.Yao YL, Dupont BR, Ghosh S, Fang Y, Leach RJ, Seto E. Cloning, chromosomal localization and promoter analysis of the human transcription factor YY1. Nucleic Acids Res. 1998;26:3776–83. doi: 10.1093/nar/26.16.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Mertens-Talcott SU, Zhang S, Kim K, Ball J, Safe S. MicroRNA-27a indirectly regulates estrogen receptor a expression and hormone responsiveness in MCF-7 breast cancer cells. Endocrinology. 2010;151:2462–73. doi: 10.1210/en.2009-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brents LK, Medina-Bolivar F, Seely KA, Nair V, Bratton SM, Nopo-Olazabal L, et al. Natural prenylated resveratrol analogs arachidin-1 and -3 demonstrate improved glucuronidation profiles and have affinity for cannabinoid receptors. Xenobiotica. 2012;42:139–56. doi: 10.3109/00498254.2011.609570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314:868–75. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Prusoff W. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (i50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 25.Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–87. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 26.Yates ML, Barker EL. Inactivation and biotransformation of the endogenous cannabinoids anandamide and 2-arachidonoylglycerol. Mol Pharmacol. 2009;76:11–7. doi: 10.1124/mol.109.055251. [DOI] [PubMed] [Google Scholar]
- 27.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–61. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 28.Group EBCTC. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 29.Howell A, Pippen J, Elledge RM, Mauriac L, Vergote I, Jones SE, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma: a prospectively planned combined survival analysis of two multicenter trials. Cancer. 2005;104:236–9. doi: 10.1002/cncr.21163. [DOI] [PubMed] [Google Scholar]
- 30.Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, Godwin J, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008;371:29–40. doi: 10.1016/S0140-6736(08)60069-0. [DOI] [PubMed] [Google Scholar]
- 31.Buzdar AU. Advances in endocrine treatments for postmenopausal women with metastatic and early breast cancer. Oncologist. 2003;8:335–41. doi: 10.1634/theoncologist.8-4-335. [DOI] [PubMed] [Google Scholar]
- 32.Hobday TJ, Perez EA. Molecularly targeted therapies for breast cancer. Cancer Control. 2005;12:73–81. doi: 10.1177/107327480501200202. [DOI] [PubMed] [Google Scholar]
- 33.Moulder S, Hortobagyi GN. Advances in the treatment of breast cancer. Clin Pharmacol Ther. 2008;83:26–36. doi: 10.1038/sj.clpt.6100449. [DOI] [PubMed] [Google Scholar]
- 34.Amar S, Moreno-Aspitia A, Perez EA. Issues and controversies in the treatment of HER2 positive metastatic breast cancer. Breast Cancer Res Treat. 2008;109:1–7. doi: 10.1007/s10549-007-9636-2. [DOI] [PubMed] [Google Scholar]
- 35.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–8. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caffarel MM, Sarrio D, Palacios J, Guzman M, Sanchez C. Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res. 2006;66:6615–21. doi: 10.1158/0008-5472.CAN-05-4566. [DOI] [PubMed] [Google Scholar]
- 37.Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3:745–55. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 38.Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008;68:339–42. doi: 10.1158/0008-5472.CAN-07-2785. [DOI] [PubMed] [Google Scholar]
- 39.Kim K, Chadalapaka G, Lee SO, Yamada D, Sastre-Garau X, Defossez PA, et al. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31:1034–44. doi: 10.1038/onc.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


