Abstract
BACKGROUND:
Formula-fed infants may be at greater risk for overfeeding and rapid weight gain. Different size bottles are used for feeding infants, although little is known about whether bottle size is related to weight gain in bottle-fed infants.
METHODS:
Data from the Greenlight Intervention Study, a cluster randomized trial to prevent childhood obesity at 4 pediatric resident clinics, were used to analyze the exposure to regular (<6 oz) or large (≥6 oz) bottle size at the 2-month visit on changes in weight, weight-for-age z score (WAZ), and weight-for-length z score (WLZ) at the 6-month visit. Using multivariable regression, we adjusted for potential confounders (birth weight, gender, age, weight measures at 2 months, parent race/ethnicity, education, household income and size, time between 2- and 6-month visits, and first child status).
RESULTS:
Forty-five percent (n = 386; 41% black, 35% Hispanic, 23% white, 2% other) of infants at the 2-month visit were exclusively formula-fed, and 44% used large (≥6 oz) bottles. Infants whose parents fed with large bottles had 0.21 kg (95% confidence interval [CI]: 0.05 to 0.37) more weight change, 0.24 U (95% CI: 0.07 to 0.41) more change in WAZ, and 0.31 U (95% CI: 0.08 to 0.54) more change in WLZ during this period than infants fed with regular bottles.
CONCLUSIONS:
Using a large bottle in early infancy independently contributed to greater weight gain and change in WLZ at the 6-month visit. Although growth in infancy is complex, bottle size may be a modifiable risk factor for rapid infant weight gain and later obesity among exclusively formula-fed infants.
What’s Known on This Subject:
Formula-fed infants grow more rapidly and may have greater risk for obesity; whether this outcome is related to the content of formula, to the bottle, or to residual confounding is unknown.
What This Study Adds:
We explored the relationship between bottle size and weight gain in formula-fed infants. The findings suggest that bottle size may have an independent effect on growth rates.
Developing effective preventive interventions for obesity and its comorbidities requires understanding modifiable risk factors for obesity in early life. Rapid infant weight gain, generally defined as a growth trajectory that crosses at least 1 percentile (≥0.67 SD),1–3 is a risk factor for later obesity,2,4–8 as well as metabolic,9 respiratory,10 and cardiovascular11–14 disease. Although growth trajectories in infancy are determined by using multiple factors, nutrition plays an essential role. In developed countries, infants fed primarily formula appear to have greater adiposity in late infancy and early childhood than children who were exclusively breastfed,15–18 and formula-fed infants are at greater risk for obesity later in life.19 The relationship between nutrition source and adiposity could be related to the formula itself,20 to behaviors such as feeding on a schedule (which is more common in bottle-fed infants),21 or to the introduction of complementary foods, parental education, or other socioeconomic factors.
Bottle-fed infants have less control over feeding volumes and also do not exhibit a diurnal pattern of intake compared with breast-fed infants, which may contribute to discordance between satiety mechanisms and actual intake.22 Because it has been hypothesized that the first few months of life are a critical period for the development of satiety responsiveness,23 it is important to understand what mechanisms influence intake during this early period.
Environmental components of feeding (eg, size of the bowl, plate, or glass) are known to be strongly associated with both portion sizes and intake24–26 and are routinely used by the food industry to market novel products. Although most research on these container sizes has focused on adults and older children, the size of bottles used to feed infants may introduce similar environmental influences on intake. A wide variety of bottle sizes are used during infancy, and we previously reported that larger bottles are associated with more reported daily intake of formula.27
To determine whether bottle size affects infant growth trajectories among exclusively formula-fed infants, we investigated the relationship between bottle size used at the 2-month visit and changes in weight by the 6-month visit. This approach was chosen because the first 6 months of life is the period most likely to be influenced by bottle size given the relative lack of complementary foods and the period of most rapid weight gain. We hypothesized that infants fed from a larger bottle at the 2-month visit would have a larger increase in weight-for-age z scores (WAZ) and weight-for-length z scores (WLZ) between the 2- and 6-month visits compared with infants fed from smaller bottles.
Methods
Sample
An analysis of longitudinal data was performed from the Greenlight Intervention Study, a cluster randomized trial of an obesity prevention intervention during the first 2 years of life. Methods of the Greenlight study have been published previously28; briefly, parent–infant dyads were enrolled at the 2-month preventive visit at 4 clinic sites from December 2009 through June 2014. To be included in the study, infants were between 6 and 16 weeks of age, born at ≥34 weeks’ gestation weighing >1500 g, and had weight-for-recumbent length at the third percentile or higher (based on World Health Organization growth standards),29 and were generally healthy. Caregivers were excluded only if they had a significant neurologic or mental illness or had uncorrected visual acuity problems. A literacy- and numeracy-sensitive intervention targeting obesity prevention and based on social cognitive theory was delivered at 2 sites; the 2 active control sites implemented an injury prevention curriculum designed by the American Academy of Pediatrics.30 The intervention did not specifically address the size of the bottle.
Measures
The study included responses from a questionnaire of diet and physical activity at the 2-month visit and measurements of weight and recumbent length at both the 2- and 6-month visits. This questionnaire assessed feeding behaviors, content of feedings, and other information considered important in obesity risk, and it was administered in-person at the 2-month visit. Clinic staff trained to accurately measure infant’s weight and recumbent length31 entered this information into the electronic medical record at each well-child visit.
To assess the relationship between bottle size and growth independent of milk type, we included only parents who responded “formula only” to the question: “What type of milk does your child drink now?” at the 2-month visit. Our main predictor was bottle size used at this visit, which was directly verified and recorded by study personnel after an affirmative response to the question: “Do you have one of the bottles with you that you use to feed [child’s first name] formula?” If the parents did not have a bottle with them (only 2% of sample), they were asked to choose (in person) from 3 bottles presented to them (4, 6, or 8 oz) to represent the one “most like the one they usually used to feed” their infant. For analyses, an a priori decision was made to dichotomize bottle size at 6 oz based on what represents age-appropriate volume. In our previous analysis,27 we chose the same cut point, which showed significant differences in reported formula intake. In this study, we therefore refer to “large” bottles as those ≥6 oz.
The main study outcome was change in WLZ, which is a common surrogate for adiposity in this age group. Other outcomes were change in WAZ and change in weight between the 2- and 6-month visits. We calculated z scores based on World Health Organization gender-specific growth curves. Covariates were considered that might confound the relationship between bottle size and growth between the 2- and 6-month visits, including infant gender, race/ethnicity, birth weight, age at the 2-month visit, time elapsed between 2- and 6-month visits, household size, household annual income, level of completed education by the primary caregiver, and whether the infant received assistance through the Special Supplemental Nutrition Program for Women, Infants, and Children. Considering that growth may have been affected by perception of weight or new information gained from the intervention, we also adjusted for study site and 2-month measures of weight, WAZ, and WLZ.
Analysis
Each of the aforementioned covariates was first compared according to exposure to either small or large bottle sizes. The statistical significance between groups was then tested by using Pearson’s χ2 tests and unadjusted logistic regression models. We then compared unadjusted relationships between bottle size and change in weight, WAZ, and WLZ between the 2- and 6-month visits by using ordinary least squares (OLS) regression. Finally, 3 models of OLS regression were examined, with changes in weight, WAZ, and WLZ as outcomes. All 3 outcomes were normally distributed and, thus, no transformations were required for OLS. Covariates were included that were either clinically or statistically significant in the relationship between bottle size and weight changes. We adjusted for the child’s gender, age, and whether they were a first child, as well as the parent’s race/ethnicity, education, household income, and household size. We also adjusted for birth weight and the relevant 2-month visit measure (weight, WAZ, or WLZ), time between the 2- and 6-month visits, and study site. All analyses were performed by using Stata version 13 (Stata Corp, College Station, TX).
Results
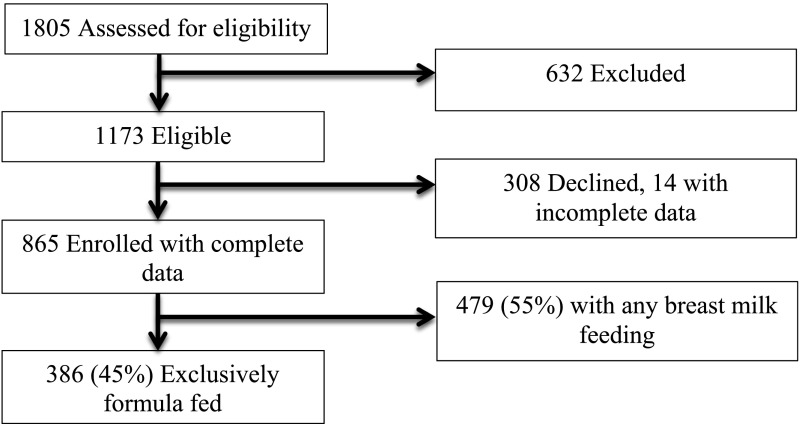
A total of 1805 parent–infant dyads were assessed for eligibility for enrollment in the Greenlight study. Of these, 632 potential participants were excluded, most commonly because the parent had plans to move or did not plan to attend all visits through 2 years. Of the 1173 eligible dyads, 865 were enrolled, and 386 (45% of enrollees) parents reported feeding only formula at the 2-month visit (Fig 1). Most of the 386 infants eligible for the analyses were of racial/ethnic minority groups, including 41% black and 35% Hispanic participants, from households earning less than $20 000 per year (62%), and with parents having less than or equal to a high school diploma (63%) (Table 1). Most of the primary caregivers were mothers, and the majority of dyads (86%) received assistance from the Special Supplemental Nutrition Program for Women, Infants, and Children. Fifty-three percent of the formula-fed infants were female, and mean ± SD age at the 2-month visit was 9.3 ± 1.8 weeks. At the 6-month visit, 298 of 386 bottle-fed infants had complete information on weight and length, and these infants were included in the analyses.
FIGURE 1.
Enrollment, eligibility, and study sample.
TABLE 1.
Sociodemographic Characteristics and Bivariate Analyses According to Bottle Size
| Variable | Overall (N = 386) | Small Bottle (n = 208)a | Large Bottle (n = 171)a |
|---|---|---|---|
| Race/ethnicity* | |||
| Black | 156 (41) | 73 (35) | 80 (47) |
| Hispanic | 133 (35) | 87 (42) | 45 (26) |
| White | 87 (23) | 45 (22) | 41 (24) |
| Other | 8 (2) | 2 (1) | 4 (2) |
| Annual income | |||
| <$10 000 | 126 (35) | 63 (32) | 61 (38) |
| $10 000–$19 999 | 97 (27) | 52 (26) | 43 (27) |
| $20 000–$39 999 | 97 (27) | 58 (29) | 37 (23) |
| $40 000–$59 999 | 28 (8) | 16 (8) | 11 (7) |
| ≥$60 000 | 16 (4) | 8 (4) | 8 (5) |
| Education level | |||
| Less than high school | 96 (25) | 59 (29) | 36 (21) |
| High school graduate | 146 (38) | 71 (34) | 73 (43) |
| Some college | 101(26) | 55 (27) | 44 (26) |
| College graduate | 39(10) | 21 (10) | 16 (9) |
| WIC enrollment | 324 (86) | 178 (86) | 146 (86) |
| Child, female* | 204 (53) | 121 (60) | 81 (40) |
| Child, only child | 152 (40) | 81 (39) | 69 (41) |
| Age at 2 mo, wk* | 9.3 ± 1.8 | 9.1 ± 1.8 | 9.6 ± 1.8 |
| Time between 2- and 6-mo visits, wk | 20 ± 3 | 20 ± 3 | 19 ± 3 |
Data are presented as n (%) or mean ± SD. WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
n = 379 for bottle size due to missing data.
P < .05.
Mean birth weight, weight at the 2-month visit, and weight at the 6-month visit were 3.2 ± 0.6, 5.3 ± 0.8, and 8.0 ± 1.0 kg, respectively (Table 2). The mean birth WLZ was –0.52 ± 1.1 U, increasing to 0.27 ± 1.1 U at the 2-month visit. Mean WAZ at the 2 month visit was –0.31 ± 0.96 U. The time interval between the 2- and 6-month visits ranged from 12 to 30 weeks, with a mean interval of 19.5 ± 3.1 weeks. Over this interval, infants gained a mean of 2.7 ± 0.7 kg, with a mean increase in WAZ of 0.44 ± 0.7 U. There was no change in WLZ (mean change, –0.004 ± 1.1).
TABLE 2.
Anthropomorphic Characteristics and Bivariate Analyses According to Bottle Size
| Variable | Overall (N = 386) | Small Bottle (n = 208)a | Large Bottle (n = 171)a |
|---|---|---|---|
| Birth weight | 3.2 ± 0.6 | 3.2 ± 0.5 | 3.3 ± 0.6 |
| Birth WLZ | −0.52 ± 1.1 | −0.6 ± 1.2 | −0.5 ± 1.1 |
| Weight at 2 mo, kg* | 5.3 ± 0.8 | 5.1 ± 0.7 | 5.4 ± 0.8 |
| WAZ at 2 mo | −0.31 ± 0.96 | −0.38 ± 1.1 | −0.20 ± 1.2 |
| WLZ at 2 mo | 0.27 ± 1.1 | 0.19 ± 1.1 | 0.36 ± 1.2 |
| Weight at 6 mo, kg* | 8.0 ± 1.0 | 7.8 ± 0.9 | 8.2 ± 1.0 |
| WAZ at 6 mo* | 0.11 ± 0.99 | −0.07 ± 0.91 | 0.37 ± 1.02 |
| WLZ at 6 mo* | 0.24 ± 1.04 | 0.11 ± 1.05 | 0.44 ± 1.00 |
Data are presented as mean ± SD.
n = 379 for bottle size due to missing data.
P < .05.
At the 2-month visit, parents used bottle sizes that ranged from 2 to 10 oz; 55% of parents reported using a “small” bottle (<6 oz) and 45% used a “large” bottle (≥6 oz) (Table 1). Hispanic parents were one-half as likely as white parents to use a large bottle (odds ratio [OR]: 0.57 [95% confidence interval (CI): 0.33 to 0.99]). Infants using larger bottles were more likely to be male (OR: 1.54 [95% CI: 1.02 to 2.32]) and older, with 15% higher odds of larger bottle use with each week older (OR: 1.15 [95% CI: 1.03 to 1.29]). Infants weighing more at the 2-month visit had higher odds of using a larger bottle (OR: 1.56 [95% CI: 1.19 to 2.05]), although there were no significant differences between parents using larger bottles and the infant’s birth weight, WLZ at birth, or WLZ at the 2-month visit. Furthermore, there were no significant relationships between bottle size and time between the 2- and 6-month visits or whether the infant was an only child.
According to the unadjusted OLS regression, use of a larger bottle at the 2-month visit predicted 0.16 kg more weight gain (95% CI: 0.01 to 0.32; P = .043) and an additional 0.18 U WAZ change (95% CI: 0.01 to 0.34; P = .034) between the 2-month and the 6-month visits (Table 3). Infants using larger bottles also gained an additional 0.26 U of WLZ over the period (95% CI: –0.004 to 0.52; P = .05), although this finding was not statistically significant. When adjusting for the appropriate growth parameter at the 2-month visit, birth weight, time between visits, study site, and other socioeconomic covariates, the relationships between bottle size and weight change, WAZ change, and WLZ change were statistically significant. Weight change and WAZ change was 0.21 kg (95% CI: 0.05 to 0.37; P = .01) and 0.24 U (95% CI: 0.07 to 0.41; P = .006) greater among infants using a larger bottle, respectively. WLZ increased by 0.31 U more in infants using a larger bottle (95% CI: 0.08 to 0.54; P = .01).
TABLE 3.
Unadjusted and Adjusted Multivariate Linear Regression
| Variable | Unadjusted, (95% CI) | Adjusteda, (95% CI) |
|---|---|---|
| Weight change, kg | 0.16 (0.01 to 0.32)* | 0.21 (0.05 to 0.37)* |
| WAZ change | 0.18 (0.01 to 0.34)* | 0.24 (0.07 to 0.41)* |
| WLZ change | 0.26 (–0.004 to 0.52) | 0.31 (0.08 to 0.54)* |
Adjusted for child gender, child age, birth weight, appropriate 2-month visit measure (weight, WAZ, or WLZ), parent race/ethnicity, parent education, household income, household size, time between 2- and 6-month visits, first child status, and study site.
P < .05.
Discussion
In a large, multisite sample of diverse, low-income, formula-fed infants, we found that infants fed from a larger bottle at the 2-month preventive visit demonstrated significantly greater weight change (0.21 kg), WAZ change (0.24 U), and WLZ change (0.31 U) after adjusting for potentially confounding factors. Our analysis found that bottle size had a significant relationship to growth rate in the short period of time between the 2- and 6-month visits among exclusively formula-fed infants, and suggests that the mode of feeding may have an important influence on intake. This amount change (0.31) in WLZ, the most common clinical adiposity measure at this age, suggests an effect on early weight gain, although the significance of this effect between the 2- and 6-month visits is unclear.
A recent meta-analysis found that there is a positive, stepwise relationship in the change in weight SD score (z score) in the first year of life with childhood obesity.2 With a 1 U increase in weight z score, there was a twofold increased risk for obesity, and with >1.33 U increase, there was a fourfold increased risk of childhood obesity. In this context, our observation of a 0.3 U change in infants using larger bottles is modest. However, this difference was reported in a relatively short period of time; its effects should be investigated over a longer period of infancy. Prospective studies have shown that change in WAZ ≥0.5 U between 2 and 4 months of life increases the odds of overweight at 18 to 24 months nearly fourfold. This period conveyed significantly more risk than weight gain between 0 and 2 months, and between 4 and 6 months, suggesting that 2 to 4 months may be an especially critical period for differences in weight trajectory on later obesity.32 Ultimately, whether these differences in weight and weight-for-length persist to influence BMI later in childhood needs to be determined.
The hypothesis that the mode of feeding (ie, the bottle) rather than the milk type is responsible for differences in weight gain between formula-fed and breast-fed infants is supported by longitudinal research showing that infants fed only human milk by bottle gain more weight than breastfed infants.33 The directionality of this relationship is not completely understood, and it is possible that parents may choose to feed more by bottle if the infant is growing particularly fast. Regardless, any discordance between the infant’s needs and the volume of intake provided might alter developing satiety responsiveness via neuroendocrine pathways and nutritional programming.23,34–36
Another mechanism by which growth may be affected, external to the child’s needs, is through parental feeding behaviors. Specific feeding behaviors, such as encouraging emptying of the bottle, are linked to encouraging children to “clean their plates” when older,37 demonstrating the ongoing external influences on food intake. The relationship between early parental feeding beliefs and behaviors, infant feeding behaviors, and later obesity risk should continue to be studied with valid and reliable measures38 in longitudinal studies.39 However, if a simple external influence (eg, bottle size) can be adjusted, this method may improve concordance between an infant’s nutritional needs and intake and attenuate rapid infant weight gain.
Although intervening to encourage healthy behaviors is a common component of obesity prevention and intervention trials, we have failed to identify an effective intervention to prevent obesity. Adjusting an external influence, such as bottle size, could provide a simple intervention that is not burdensome or expensive.40 The z score changes of the magnitude we found over a relatively short period of time likely reflect an independent influence of bottle size on volumes of formula given to infants.
Although our study results may provide an important insight into why formula feeding is related to obesity risk, it has important limitations. First, we did not directly measure intake or bottle-emptying behaviors, nor did we assess for bottle size changes over time or the range of bottle sizes a family may be using. It is possible that families use different sizes of bottles or use smaller bottles but offer >1 bottle over a given feeding period. However, we believe that our direct observation of the bottle size used at the 2-month visit is a reproducible and feasible way to assess patterns of intake in the clinical setting. Although the Greenlight intervention did not include bottle size reduction, it is possible that other components of the intervention affected infant growth, but we found no significant differences in bivariate analyses, and including intervention in the adjusted model did not change the outcomes. Diet and activity factors, such as introduction of complementary foods, likely influence weight gain and have been reported to differ by race and ethnicity in this sample.41 We have no information on growth in the study participants from birth to the 2-month preventive visit, although we did use birth weight from the health record, which we believe is equally reliable for all participants. Another limitation is the quality of the measurement of length; length is difficult to measure during infancy and is therefore potentially unreliable. For this reason, our personnel received additional measurement training with the use of a standardized module.31 We also assessed growth by using multiple parameters (weight-for-age and weight-for-length), as there is no standard, reliable measure of adiposity that clearly predicts obesity risk and can be easily measured in the office setting. Finally, the clinical relevance of the changes we found remains unclear and should be studied in the context of known and proposed risk factors for obesity that can be detected and modified in infancy.
Conclusions
Infants in low-income populations experience both higher rates of exclusive infant formula-feeding41 and higher risk for later-onset obesity.42 The results of this study suggest that early-childhood obesity interventions should target reduced bottle size in early infancy. We found that using a larger bottle in early infancy predicted significantly greater adiposity at the 6-month visit among formula-fed infants. Given the complexity of infant growth, future research should consider influences such as feeding practices and should include rigorous measurement of intake and body composition. Nearly all parents use a bottle to feed their infant at some point during their infancy, and further efforts to more completely understand the mechanisms linking bottle-feeding, development of satiety responsiveness, and obesity risk may also inform obesity prevention interventions.
Glossary
- CI
confidence interval
- OLS
ordinary least squares
- OR
odds ratio
- WAZ
weight-for-age z score
- WLZ
weight-for-length z score
Footnotes
Dr Wood conceptualized the study and performed the analyses, drafted the initial manuscript, and participated in revisions; Dr Skinner conceptualized and performed the analyses, and participated in revisions; Dr Yin conceptualized and designed the parent study and data collection instruments at 4 sites, supervised data collection at the New York University site, and critically reviewed the analysis plan and manuscript; Dr Rothman conceptualized and designed the parent study and data collection instruments at 4 sites, supervised data collection at the Vanderbilt University site, and critically reviewed the analysis plan and manuscript; Drs Sanders and Delamater conceptualized and designed the parent study and data collection instruments at 4 sites, helped supervise data collection at the University of Miami site, and critically reviewed the analysis plan and manuscript; Dr Perrin helped conceptualize the study, conceptualized and designed the parent study and data collection instruments at 4 sites, supervised data collection at the University of North Carolina site, participated in revisions, and critically reviewed the analysis plan and manuscript; and all authors approved the manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01040897).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01HD059794 with Centers for Disease Control and Prevention supplements 04S1 and 04S2; NIH grant UL1TR001111; NIH/National Center for Advancing Translational Sciences grant UL1TR000445; NIH/National Center for Research Resources grants U54RR023499, UL1RR025747, and UL1TR000038; Robert Wood Johnson Foundation Physician Faculty Scholars program (Dr Yin); and Health Resources and Services Administration grant T32HP014001. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Ong KK, Loos RJ Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 2006;95(8):904–908 [DOI] [PubMed]
- 2.Druet C, Stettler N, Sharp S, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26(1):19–26 [DOI] [PubMed] [Google Scholar]
- 3.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen LG, Holst C, Michaelsen KF, Baker JL, Sorensen TI Weight and weight gain during early infancy predict childhood obesity: a case-cohort study. Int J Obes (Lond) 2012;36(10):1306–1311 [DOI] [PubMed]
- 5.Taveras EM, Rifas-Shiman SL, Sherry B, et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–998 [DOI] [PubMed] [Google Scholar]
- 6.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77(6):1374–1378 [DOI] [PubMed] [Google Scholar]
- 7.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karaolis-Danckert N, Buyken AE, Bolzenius K, Perim de Faria C, Lentze MJ, Kroke A. Rapid growth among term children whose birth weight was appropriate for gestational age has a longer lasting effect on body fat percentage than on body mass index. Am J Clin Nutr. 2006;84(6):1449–1455 [DOI] [PubMed] [Google Scholar]
- 9.Ekelund U, Ong KK, Linné Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92(1):98–103 [DOI] [PubMed] [Google Scholar]
- 10.Sonnenschein-van der Voort AM, Howe LD, Granell R, et al. Influence of childhood growth on asthma and lung function in adolescence. J Allergy Clin Immunol. 2015;135(6):1435–1443.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belfort MB, Rifas-Shiman SL, Rich-Edwards J, Kleinman KP, Gillman MW. Size at birth, infant growth, and blood pressure at three years of age. J Pediatr. 2007;151(6):670–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Shlomo Y, McCarthy A, Hughes R, Tilling K, Davies D, Smith GD. Immediate postnatal growth is associated with blood pressure in young adulthood: the Barry Caerphilly Growth Study. Hypertension. 2008;52(4):638–644 [DOI] [PubMed] [Google Scholar]
- 13.Skilton MR, Marks GB, Ayer JG, et al. Weight gain in infancy and vascular risk factors in later childhood. Pediatrics. 2013;131(6). Available at: www.pediatrics.org/cgi/content/full/131/6/e1821 [DOI] [PubMed] [Google Scholar]
- 14.Howe LD, Chaturvedi N, Lawlor DA, et al. Rapid increases in infant adiposity and overweight/obesity in childhood are associated with higher central and brachial blood pressure in early adulthood. J Hypertens. 2014;32(9):1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fomon SJ. Assessment of growth of formula-fed infants: evolutionary considerations. Pediatrics. 2004;113(2):389–393 [DOI] [PubMed] [Google Scholar]
- 16.Dewey KG. Growth characteristics of breast-fed compared to formula-fed infants. Biol Neonate. 1998;74(2):94–105 [DOI] [PubMed] [Google Scholar]
- 17.Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lönnerdal B. Growth of breast-fed and formula-fed infants from 0 to 18 months: the DARLING Study. Pediatrics. 1992;89(6 pt 1):1035–1041 [PubMed] [Google Scholar]
- 18.Nommsen-Rivers LA, Dewey KG. Growth of breastfed infants. Breastfeed Med. 2009;4(suppl 1):S45–S49 [DOI] [PubMed] [Google Scholar]
- 19.Gillman MW, Rifas-Shiman SL, Camargo CA Jr, et al. Risk of overweight among adolescents who were breastfed as infants. JAMA. 2001;285(19):2461–2467 [DOI] [PubMed] [Google Scholar]
- 20.Koletzko B, von Kries R, Closa R, et al. ; European Childhood Obesity Trial Study Group . Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89(6):1836–1845 [DOI] [PubMed] [Google Scholar]
- 21.Mihrshahi S, Battistutta D, Magarey A, Daniels LA. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright P, Fawcett J, Crow R. The development of differences in the feeding behaviour of bottle and breast fed human infants from birth to two months. Behav Processes. 1980;5(1):1–20 [DOI] [PubMed] [Google Scholar]
- 23.Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes. 2008;32(32 suppl 7):S62–S71 [DOI] [PubMed] [Google Scholar]
- 24.Wansink B, Cheney MM. Super Bowls: serving bowl size and food consumption. JAMA. 2005;293(14):1727–1728 [DOI] [PubMed] [Google Scholar]
- 25.DiSantis KI, Birch LL, Davey A, et al. Plate size and children’s appetite: effects of larger dishware on self-served portions and intake. Pediatrics. 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1451 [DOI] [PubMed] [Google Scholar]
- 26.Wansink B, van Ittersum K, Payne CR. Larger bowl size increases the amount of cereal children request, consume, and waste. J Pediatr. 2014;164(2):323–326 [DOI] [PubMed] [Google Scholar]
- 27.Wood CT, Skinner AC, Yin HS, et al. Association between bottle size and formula intake in 2-month-old infants. Acad Pediatr. 2016;16(3):254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders LM, Perrin EM, Yin HS, Bronaugh A, Rothman RL; Greenlight Study Team . “Greenlight study”: a controlled trial of low-literacy, early childhood obesity prevention. Pediatrics. 2014;133(6). Available at: www.pediatrics.org/cgi/content/full/133/6/e1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onis M; WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica. 2006;95(S450):76–85 [DOI] [PubMed] [Google Scholar]
- 30.Krassner L. TIPP usage. Pediatrics. 1984;74(5 pt 2):976–980 [PubMed] [Google Scholar]
- 31.US Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau Growth chart training. Available at: http://depts.washington.edu/growth/module5/text/page4a.htm. Accessed October 13, 2013
- 32.Young BE, Johnson SL, Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Adv Nutr. 2012;3(5):675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Magadia J, Fein SB, Grummer-Strawn LM. Risk of bottle-feeding for rapid weight gain during the first year of life. Arch Pediatr Adolesc Med. 2012;166(5):431–436 [DOI] [PubMed] [Google Scholar]
- 34.Singhal A, Farooqi IS, O’Rahilly S, Cole TJ, Fewtrell M, Lucas A. Early nutrition and leptin concentrations in later life. Am J Clin Nutr. 2002;75(6):993–999 [DOI] [PubMed] [Google Scholar]
- 35.Balonan LC, Sheng HP. Perinatal feedings adversely affect lipogenic activities but not glucose handling in adult rats. Pediatr Res. 2000;48(5):668–673 [DOI] [PubMed] [Google Scholar]
- 36.Plagemann A, Harder T, Rake A, et al. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999;11(7):541–546 [DOI] [PubMed] [Google Scholar]
- 37.Li R, Scanlon KS, May A, Rose C, Birch L. Bottle-feeding practices during early infancy and eating behaviors at 6 years of age. Pediatrics. 2014;134(suppl 1):S70–S77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson AL, Mendez MA, Borja JB, Adair LS, Zimmer CR, Bentley ME. Development and validation of the Infant Feeding Style Questionnaire. Appetite. 2009;53(2):210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agras WS, Kraemer HC, Berkowitz RI, Hammer LD. Influence of early feeding style on adiposity at 6 years of age. J Pediatr. 1990;116(5):805–809 [DOI] [PubMed] [Google Scholar]
- 40.Robinson TN, Matheson D, Desai M, et al. Family, community and clinic collaboration to treat overweight and obese children: Stanford GOALS—a randomized controlled trial of a three-year, multi-component, multi-level, multi-setting intervention. Contemp Clin Trials. 2013;36(2):421–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrin EM, Rothman RL, Sanders LM, et al. Racial and ethnic differences associated with feeding- and activity-related behaviors in infants. Pediatrics. 2014;133(4). Available at: www.pediatrics.org/cgi/content/full/133/4/e857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]