Abstract
OBJECTIVES:
To describe the demographic and clinical characteristics of pediatric multiple sclerosis (MS) in the United States.
METHODS:
This prospective observational study included children and adolescents with MS. Cases were evaluated across 9 geographically diverse sites as part of the US Network of Pediatric MS Centers.
RESULTS:
A total of 490 children and adolescents (324 girls, 166 boys) were enrolled; 28% developed symptoms before 12 years of age. The proportion of girls increased with age from 58% (<12 years) to 70% (≥12 years). Race and ethnicity as self-identified were: white, 67%; African American, 21%; and non-Hispanic, 70%. Most (94%) of the cases were born in the United States, and 39% had 1 or both foreign-born parents. Fifty-five percent of cases had a monofocal presentation; 31% had a prodrome (most frequently infectious), most often among those aged <12 years (P < .001). Children aged <12 years presented more commonly with encephalopathy and coordination problems (P < .001). Sensory symptoms were more frequently reported by older children (ie, those aged ≥12 years) (P < .001); 78% of girls had MS onset postmenarche. The initial Expanded Disability Status Scale score for the group was <3.0, and the annualized relapse rate was 0.647 for the first 2 years. Interval from symptom onset to diagnosis and from diagnosis to initiation of disease-modifying therapy was longer among those <12 years of age.
CONCLUSIONS:
Pediatric MS in the United States is characterized by racial and ethnic diversity, a high proportion of children with foreign-born parents, and differences in clinical features and timing of treatment among those <12 years of age compared with older children.
What’s Known on This Subject:
Pediatric multiple sclerosis has an incidence of ≤0.64 per 10 000 and is less common among those aged <12 years. At onset, it has an almost exclusively relapsing-remitting course.
What This Study Adds:
In the United States, pediatric multiple sclerosis is characterized by racial/ethnic diversity, a high proportion of children with foreign-born parents, and differences in clinical features among those <12 years of age compared with older children.
Multiple sclerosis (MS) is a central nervous system autoimmune inflammatory demyelinating disease. Generally considered a disease of young and middle-aged adults, an estimated 2.7% to 5.4% of all patients with MS experience their first attack before 18 years of age.1–8 Initial descriptions of childhood-onset MS appeared in case reports and small case series.9–12 Greater awareness and interest led to increased publications, including larger case series, reports from single institutions, multicenter studies, and national and population-based surveys.1–8,13–17 Pediatric-onset MS (pediatric MS) was compared with adult-onset MS in a few publications.5,7,17,18 However, because pediatric MS is a comparatively rare disorder (frequency of 0.2 to 0.64/100 000),7,19–22 most investigations have been limited by relatively small sample sizes, retrospective data, or both. Studies with >150 subjects are few (and only 1 had 300 subjects).16,17 In addition, most comprised homogeneous populations in terms of race and ethnicity.
It is unclear if findings from these studies are generalizable to larger, more diverse populations and if there are differences between children with disease onset before 12 years of age compared with those with onset at age ≥12 years. The aim of the present multicenter study was to better define the demographic and clinical characteristics of pediatric MS in the United States, with an emphasis on contrasting features of younger versus older children. This extremely large sample provides an opportunity to examine subsets of patients according to age of onset as well as the influence of more specific demographic (ie, country of origin of parents) and clinical (ie, presence of encephalopathy, possible hormonal influences, timing of menarche in relation to MS onset) features, in addition to more customary MS parameters (ie, initial MS symptoms, relapse frequency).
Methods
Data Acquisition
This analysis included longitudinally collected data from the US Network of Pediatric MS Centers, Pediatric MS and Other Demyelinating Diseases, database.
Participating centers included Children’s Hospital of Alabama, Birmingham, Alabama; Boston Children’s Hospital and Massachusetts General Hospital for Children, Boston, Massachusetts; Loma Linda University Medical Center, Loma Linda, California; University of California at San Francisco, San Francisco, California; Mayo Clinic, Rochester, Minnesota; Stony Brook Children’s Hospital, State University of New York at Stony Brook, Stony Brook, New York; University of Buffalo, Buffalo, New York; and Texas Children’s Hospital, Houston, Texas.
Data from all clinic visits occurring from May 2011 through February 2015 were prospectively collected and entered into a detailed registry by using structured case-report forms. The data included demographic characteristics, family and medical history, Expanded Disability Status Scale (EDSS) scores, relapse history, laboratory findings, and MS treatment record. Clinical and demographic information were obtained by direct patient and parent/guardian interviews and examinations, and review of medical records. Data were entered into a Web-based electronic data capture system housed at the University of Utah Data Coordinating and Analysis Center.23
Participants
The US Network of Pediatric MS Centers, Pediatric MS and Other Demyelinating Diseases, database includes patients evaluated at 1 of the participating clinical centers who have suspected acquired demyelinating disease. Only patients with first symptoms occurring before age 18 years and a recorded diagnosis of MS were selected for analysis. All enrolled met criteria for pediatric MS (2007 operational definitions of the International Pediatric Multiple Sclerosis Study Group).24
Standard Protocol Approvals, Registrations, and Patient Consents
Approval was obtained from the institutional review board at each participating institution. Participants and 1 of their parents signed assent and consent forms, as required by each center’s institutional review board, before enrollment. Race and ethnicity were self-identified according to the National Institutes of Health guidelines.
Statistical Analysis
Demographic characteristics and clinical features of the study population were described by using counts and relative frequencies for categorical variables and means ± SDs or medians and interquartile ranges for numeric variables. Characteristics were compared between age groups by using Fisher’s exact tests or Wilcoxon rank-sum tests. Due to varying rates of unknown data, we report the number of patients with available data for each description.
Disease onset was defined as the date of first clinical attack, signs, or symptoms. Clinical features reported at disease onset are described, including symptoms, localization, and cerebrospinal fluid (CSF) profile when available. The number of patients with an elevated immunoglobulin G index and/or presence of oligoclonal bands at any time between the first symptoms and last clinic visit is also described. Clinical features were compared between age groups by using Fisher’s exact test and the Wilcoxon rank-sum test.
Clinical Assessment
Data were collected by using uniform definitions of MS relapses24; EDSS scores were measured in accordance with the Neurostatus Definitions and Scoring.25
Because of variation in the timing between disease onset and the first visit to the network clinical center, we summarized EDSS scores at 2 years’ postonset in addition to EDSS scores from the first visit to the clinical center. EDSS scores were compared between age groups (<12 years versus ≥12 years at onset) by using Wilcoxon rank-sum tests.
For patients presenting to a center within 3 years of MS onset, relapse rates were calculated as the number of relapses in the sample divided by the number of follow-up patient-years. Separate rates were also calculated for the first 2 years after onset and for relapses occurring >2 years after onset. All reported attacks (relapses), not including the first attack, are included in calculations. Rates were compared between age groups by using negative binomial regression models.
We described the frequency of disease-modifying therapy (DMT) use overall and according to age groups, and estimated the median and 25th and 75th percentiles of the time interval between MS diagnosis and initiation of DMT by using survivor functions. Patients who had not begun DMT at their most recent visit were considered censored. Time to DMT was compared between age groups by using a log-rank test.
Analyses were conducted by using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Demographic Features
Age and Sex at Disease Onset
A total of 490 children and adolescents (324 girls, 166 boys) with MS met study criteria. Table 1 presents the demographic characteristics of the cohort.
TABLE 1.
Demographic Characteristics of Pediatric MS Patients
| Characteristic | Overall (N = 490) |
|---|---|
| Age at time of first event, mean ± SD [minimum–maximum], y | 13.2 ± 3.87 [1.6–18.0] |
| Male: mean ± SD [minimum–maximum], y | 12.8 ± 4.16 [1.8–17.9] |
| Female: mean ± SD [minimum–maximum], y | 13.4 ± 3.70 [1.6–18.0] |
| Age <10 y at first event | 84 (17.1) |
| Age <11 y at first event | 107 (21.8) |
| Age <12 y at first event | 139 (28.4) |
| Sex | |
| Male | 166 (33.9) |
| Female | 324 (66.1) |
| Racea | |
| White | 303 (67.0) |
| Black/African American | 93 (20.6) |
| American Indian/Alaskan Native | 8 (1.8) |
| Asian | 17 (3.8) |
| Native Hawaiian or Pacific Islander | 1 (0.2) |
| Multiracial | 30 (6.6) |
| Ethnicityb | |
| Hispanic or Latino | 139 (30.2) |
| Not Hispanic or Latino | 321 (69.8) |
Data are presented as N (%) unless indicated otherwise. Patients with unknown race (n = 38) or ethnicity (n = 30) are not included in percentage calculations.
Racial distribution for age <18 years according to 2012 US Census estimates: 73% white; 15% black/African American; 1.6% Native American/Alaskan Native; 4.8% Asian; 0.3% Native Hawaiian or Pacific Islander; and 4.8% multiracial.26
Ethnic distribution according to the 2010 US Census: 16% Hispanic or Latino; 84% non-Hispanic or Latino.27
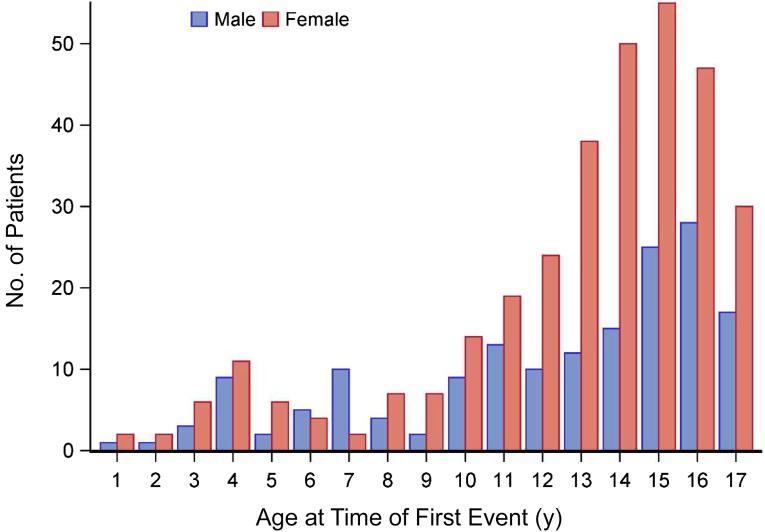
The age of disease onset for both boys and girls is presented in Fig 1. Girls had a slightly higher average age at onset. Symptom onset occurred before age 12 years for 139 patients (28%) and at age ≥12 years for 351 (72%) patients. Overall, the ratio of female-to-male subjects was 1.95 to 1. The highest frequency for first events for both sexes occurred in adolescence, with peaks at age 15 years for girls and age 16 years for boys. However, from age 8 years onward, female subjects exceeded male subjects in each year. The sex difference in onset of disease was most prominent in patients from age 12 to 17 years.
FIGURE 1.
Age distribution, according to sex, of pediatric-onset MS patients at the time of first event.
Onset of MS symptoms occurred before menarche for 22% of girls; 78% of girls had MS symptom onset postmenarche. The mean ± SD age of menarche was 11.6 ± 1.4 years (range, 7–15 years) in girls with MS symptom onset postmenarche. In contrast, in girls with MS symptom onset before menarche, the mean age of menarche was 12.2 ± 1.3 years (range, 10–15 years).
In girls with MS symptom onset postmenarche, 10% reported menarche at the same age as MS onset; 26% reported a menarchal age of 0 to 1 years before MS symptom onset, and 44% reported a menarchal age within 0 to 2 years of MS symptom onset.
Race and Ethnicity
Race and ethnicity were self-defined by 92% and 94% of cases, respectively. As shown in Table 1, most individuals identified as either white (67%) or African American (21%). Almost 70% self-identified as non-Hispanic/non-Latino. Race and ethnicity did not differ according to sex.
Ancestry
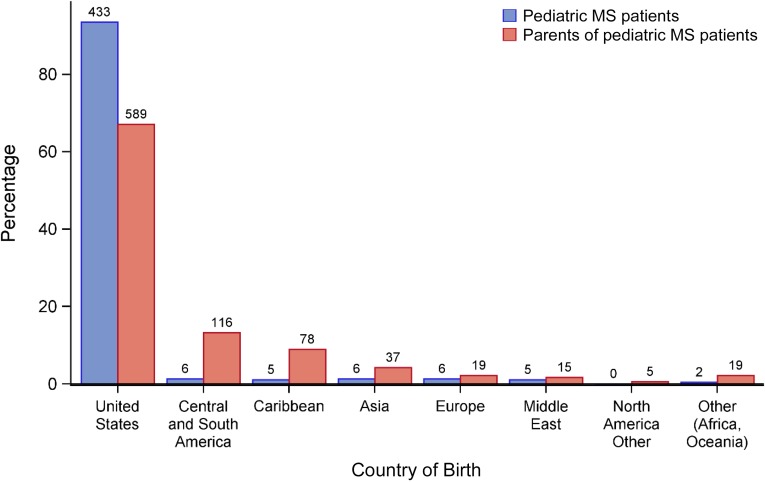
Ninety-four percent of the patients were born within the United States. For the 30 children who were not, 6 were born in Central or South America, 6 in Asia, 6 in Europe, 5 in the Caribbean, 5 in the Middle East, and 2 in Africa or Oceania (Fig 2).
FIGURE 2.
Country of birth among pediatric-onset MS patients and their parents. Patients (n = 27) and parents (n = 102) with missing birth country data are excluded. Countries are categorized as follows: Central and South America: Argentina, Brazil, Chile, Colombia, Ecuador, El Salvador, French Guiana, Guatemala, Guyana, Mexico, Nicaragua, Panama, Peru, Suriname, and Venezuela; Caribbean: Antigua, Bahamas, Barbados, Dominican Republic, Grenada, Haiti, Jamaica, Puerto Rico, Virgin Islands, and West Indies; Asia: Afghanistan, China, Hong Kong, India, Pakistan, Philippines, South Korea, Taiwan, Vietnam, and Asia otherwise unknown; Europe: Albania, Bosnia, England, United Kingdom, Germany, Italy, Netherlands, Portugal, Russia, and Serbia; Middle East: Iran, Israel, Jordan, Saudi Arabia, United Arab Emirates, and Yemen; North America other: Bermuda and Canada; Other (Africa, Oceania): Cape Verde, Egypt, Fiji, Liberia, Libya, Nigeria, Tunisia, and Outside the United States otherwise unknown.
Of note, 39% of the patients with pediatric MS were second-generation Americans, defined as persons with 1 or both parents foreign-born.25 Of the 289 parents born outside of the United States, 116 were born in Central or South America, 78 in the Caribbean, 37 in Asia, 19 in Europe, 15 in the Middle East, 5 in North America, and 19 in Africa or Oceania (Fig 2).
Family History of MS and Other Autoimmune Diseases
MS in family members was reported at the time of the first clinic visit. Five percent of the cohort had a family member with MS, including siblings (1%), parents (3%), and grandparents (2%). Other autoimmune diseases in the family were reported by 35% of patients, most frequently among grandparents (21%) and least among siblings (5%). Thyroid diseases (including Hashimoto’s and Grave’s disease, 14%), rheumatoid arthritis (11%), inflammatory bowel disorders (5%), and systemic lupus erythematosus (2%) were the most frequent conditions (Supplemental Table 3).
Clinical Features According to Age of Onset
Features of First Attack
As shown in Table 2, many features differed between those aged <12 years (younger age group) compared with those aged ≥12 years. Antecedents were more frequent among the younger group (P < .001) with previous infection the most common. Encephalopathy, constitutional symptoms, and coordination problems were more frequent among the younger children while sensory symptoms were more frequently reported by older children. The only anatomic localization that differed between age groups was the higher proportion of older children with a spinal cord localization (P < .001).
TABLE 2.
Clinical Characteristics of Patients With Pediatric-Onset MS
| Characteristic | Age <12 Years (n = 139) | Age ≥12 Years (n = 351) | Overall (N = 490) | P |
|---|---|---|---|---|
| Clinical antecedent | ||||
| Any 1 or more clinical antecedent | 49/105 (47%) | 76/300 (25%) | 125/405 (31%) | <.001a |
| Infection | 36/105 (34%) | 48/300 (16%) | 84/405 (21%) | <.001a |
| Trauma | 7/105 (7%) | 9/300 (3%) | 16/405 (4%) | .14a |
| Vaccination | 5/105 (5%) | 7/300 (2%) | 12/405 (3%) | .31a |
| Other (eg, emotional stressor, rash) | 10/105 (10%) | 15/300 (5%) | 25 /405 (6%) | .10a |
| First event symptoms | ||||
| Encephalopathy | 17/108 (16%) | 6/295 (2%) | 23/403 (6%) | <.001a |
| Monofocal | 61/109 (56%) | 163/295 (55%) | 224/404 (55%) | .91a |
| Vision symptom | 50/105 (48%) | 110/282 (39%) | 160/387 (41%) | .13a |
| Motor symptom | 60/108 (56%) | 133/294 (45%) | 193/402 (48%) | .07a |
| Sensory symptom | 20/99 (20%) | 159/294 (54%) | 179/393 (46%) | <.001a |
| Coordination symptom | 54/107 (50%) | 91/288 (32%) | 145/395 (37%) | <.001a |
| Bowel bladder symptom | 14/108 (13%) | 22/293 (8%) | 36/401 (9%) | .11a |
| Constitutional symptom | 45/107 (42%) | 85/292 (29%) | 130/399 (33%) | .02a |
| CSF profile | ||||
| First event total white blood cell count, N | 74 | 182 | 256 | .82b |
| Mean ± SD cells/uL | 20.2 ± 35.73 | 13.6 ± 17.96 | 15.5 ± 24.57 | |
| First event percent lymphocytes, N | 54 | 143 | 197 | <.001b |
| Mean ± SD % | 73.1 ± 23.28 | 85.6 ± 20.88 | 82.2 ± 22.22 | |
| First event percent neutrophils, N | 24 | 34 | 58 | .001b |
| Mean ± SD % | 16.0 ± 14.24 | 4.5 ± 7.86 | 9.3 ± 12.26 | |
| Any elevated immunoglobulin G index | 28/71 (39%) | 105/147 (71%) | 133/218 (61%) | <.001a |
| Any positive test result for oligoclonal bands | 47/92 (51%) | 168/217 (77%) | 215/309 (70%) | <.001a |
| Clinical course | ||||
| Total EDSS from first visit, N | 138 | 348 | 486 | .16b |
| Mean ± SD | 1.7 ± 1.41 | 1.5 ± 1.33 | 1.6 ± 1.35 | |
| Total EDSS 2-y post onset, N | 38 | 151 | 189 | .49b |
| Mean ± SD | 1.1 ± 0.96 | 1.3 ± 1.16 | 1.2 ± 1.13 | |
| ARR overall | .87c | |||
| Patients: events/patient-years | 68: 105/191 | 254: 339/613 | 322:444/804 | |
| Rate (SE) | 0.550 (0.054) | 0.553 (0.030) | 0.552 (0.026) | |
| ARR in the 2 y after disease onset | .71c | |||
| Patients: events/patient-years | 68: 72/107 | 254: 245/383 | 322: 317/490 | |
| Rate (SE) | 0.673 (0.079) | 0.640 (0.041) | 0.647 (0.036) | |
| ARR after 2 y from disease onset | .96c | |||
| Patients: events/patient-years | 39: 33/84 | 129: 94/230 | 168: 127/314 | |
| Rate (SE) | 0.393 (0.068) | 0.409 (0.042) | 0.404 (0.036) |
For categorical characteristics, the number of patients with the characteristic and the number of patients with available data are shown as a fraction, along with the calculated percentage. For continuous measures, the number of patients with available data is shown along with the mean ± SD. For relapse rates, the number of patients, the total number of events, and the total number of patient-years of follow-up are shown along with the estimated annualized relapse rate (ARR) and SE.
Fisher’s exact test for homogeneity.
Wilcoxon rank-sum test for homogeneity.
Likelihood ratio test from a negative binomial regression model; Poisson rates and SEs. Patients presenting >3 years’ post-onset are not included in analyses of annual relapse rate.
The first documented CSF profile exhibited an elevated cell count in both age groups, with no significant difference, but the percentage of neutrophils was significantly higher and the lymphocyte counts significantly lower in the younger group. Rather striking group differences were noted for greater frequency of oligoclonal bands and elevation of the immunoglobulin G index among older versus younger children (Table 2).
Disease severity features were similar between older and younger children at presentation and at 2-year follow-up. Younger children in our cohort, however, experienced a longer time interval between onset of symptoms and diagnosis of MS (median [interquartile range] in patients aged <12 years: 155 days [0–677 days]; median [interquartile range] in patients aged ≥12 years: 61 days [0–299 days]). Due to our inclusion criterion of MS diagnosis before our analysis, and the possibility that patients with longer time intervals might not be among our sample due to the study design, no statistical comparisons were conducted between age groups.
Treatment
Overall, 75% of the cohort received DMT at some time during the study (Supplemental Table 4). The interval between diagnosis and start of DMT was delayed among the younger children relative to the older children. Among those <12 years of age, median time from diagnosis of MS to initiation of DMT was 178 days vs 78 days among patients ≥12 years of age (log-rank test, P = .002).
Discussion
This large, multicenter, observational investigation evaluated demographic and clinical features of childhood-onset MS across multiple diverse geographic regions in the United States and had a wider range of ethnic and racial groups than previously studied. The large sample size (the largest reported to date) enabled us to confidently compare disease features among younger versus older children. Several distinctive features of childhood-onset MS in the United States were elucidated.
We noted that 17% of patients seen at our centers had a young age of onset (ie, <10 years), a considerably higher proportion than reported from the only other study of >300 children with MS (a multicenter investigation conducted in France and Belgium) in which just 7.6% had disease onset before age 10 years and only 18% by age 12 years.17 Among our youngest cases, there was an almost equal sex ratio (girls only slightly outnumbered boys). Female preponderance increased with age, particularly in adolescence, in agreement with other reports. A novel finding from our database is that 44% of girls with postmenarchal MS onset reported a menarchal age within 0 to 2 years after age at MS symptom onset. Consistent with 2 recent reports of smaller cohorts, the observed temporal link between onset of menarche and MS in our sample supports the potential role of sex hormones in the complex immunologic process occurring in the onset of MS in adolescent girls.28,29
Clinical features that distinguished younger versus older MS cases included the following: younger children were more likely to report a prodromal event before the first MS attack, present with encephalopathy, and have more motor and coordination problems. In contrast, older children had more sensory symptoms suggesting spinal cord involvement (similar to findings recently reported from Germany comparing 47 children aged <11 years with those >14 years of age,30 and more consistent with findings reported for adult-onset MS).31 Younger versus older children had a slightly different CSF profile interpreted as manifesting a more prominent role in the innate immune response (confirming our previous observations in a smaller cohort).32 Although the frequency of DMT treatment was similar between younger and older children, there was a delay among younger children in reaching an MS diagnosis and after diagnosis in starting DMT.
In addition to having an opportunity to study younger versus older children with MS, our pool of patients drawn from multiple geographic areas of the United States offered an opportunity to evaluate the self-reported racial, ethnic, and heritage characteristics. In contrast to the predominantly white and principally of Northern European heritage noted for adult-onset MS in the United States, our pediatric cohort had a relatively lower proportion of white and a higher proportion of African-American and Hispanic/Latino cases corroborating preliminary observations in smaller samples from our group and others.7,20,33 In addition, we found a surprisingly high proportion of second-generation Americans (United States native-born children whose parents were foreign-born). Although parent-origin countries were diverse, Mexico, Central American, and Caribbean countries predominated. Similar trends were noted in another North American study. A pediatric MS sample from Canada found that patients were less likely to report European ancestry and more likely to report Asian, Middle Eastern, or Caribbean ancestry relative to the adult-onset MS Canadian sample.34 Reasons for the current differences between the pediatric and adult-onset MS population in the United States are not totally clear. The increase in racial/ethnic diversity may simply reflect the current changing and increasing diversity of the population of US children (ie, a mirroring the change and shift in the ethnic distribution of the general population), manifesting the increased wave of immigrants with a concomitant increased proportion of second-generation children.26,27 The broader racial/ethnic diversity in pediatric MS versus adult-onset MS populations may also reflect the increased migration from low MS incident areas to the higher MS North American incident area. This ethnic and racial diversity is complex, as emphasized by our preliminary report using a genetic ancestry marker.35 A combination of genetic and environmental influences both independently and interactively may help explain those findings.
There are several limitations to our study, including potential source of selection bias, as most but not all centers are located in large urban areas (areas often are composed of a more racially and ethnically diverse population than more rural areas), which could have led to an overrepresentation of immigrants and minority groups.26,27 It is possible that sicker children with active disease were preferentially referred to the centers. However, the sample’s lower relapse rate than reported in other studies36 does not support the conclusion that sicker patients were overrepresented. Conversely, incomplete patient recall could have led to a lower reported relapse rate. The possibility of incomplete memory bias was addressed by limiting the analyses to only those patients evaluated at the centers within 3 years of symptom onset. The early and widespread initiation of DMT in the patients could also have accounted for a lower than expected relapse rate.
The strengths of the study are its longitudinal and multicenter design, use of common data capture with enrollment of a large ethnically and racially diverse sample allowing analysis of subgroups based on age of onset.
Conclusions
Our findings suggest that a more diverse heritage, racial, and ethnic composition among those with pediatric-onset MS should be followed up with studies of genetic markers. The observation that the younger patient group exhibits clinical features distinct from the older children, and had a longer time to diagnosis and time to begin DMT, deserves attention. Further recognition of clinical features in younger MS patients should help close the gap in diagnosis and treatment relative to their older counterparts.
Acknowledgments
Participating centers (with collaborators) are listed here in alphabetical order: Children’s Hospital of Alabama (J. Ness, Y. Harris), Boston Children’s Hospital (M. Gorman, L. Benson), Loma Linda University Medical Center (G. Aaen), Massachusetts General Hospital for Children (T. Chitnis), Mayo Clinic (J. Tillema, M. Rodriguez), State University of New York at Stony Brook (A. Belman, L. Krupp), Texas Children’s Hospital (T. Lotze), University of Buffalo (B. Weinstock-Guttman), University of California at San Francisco (E. Waubant, J. Graves), and University of Utah Data Coordinating and Analysis Center (T. C. Casper, C. Olsen, J. Rose, S. Roalstad, T. Hunt).
Glossary
- CSF
cerebrospinal fluid
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- MS
multiple sclerosis
Footnotes
Dr Belman conceptualizated and designed the study in addition to the acquisition of data, analysis, interpretation of the data, and drafting and revising the manuscript; Drs Krupp, Chitnis, Ness, Gorman, and Weinstock-Guttman carried out the acquisition of data, interpretation of data, and revising the manuscript; Mr Olsen carried out the statistical analysis, interpretation of data, and revising the manuscript; Dr Waubant carried out the acquisition of data, design of the study, interpretation of data, and revising the manuscript; Dr Rodriguez carried out acquisition of the data, interpretation of data, and review of the manuscript; Drs Lotze and Aaen carried out acquisition of data and revising the manuscript; Dr Benson carried out acquisition of data; Dr Graves carried out acquisition of data, analysis and interpretation of data, and revising the manuscript; Dr Rose carried out interpretation of data and revising the manuscript; and Dr Casper carried out the design of study, data collection, analysis and interpretation of data, and revising the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: Dr Belman has received honoraria as a one-time advisory board participant for Biogen. Dr Krupp is supported by the National MS Society, the National Institutes of Health (NIH), the Robert and Lisa Lourie Foundation, and the Department of Defense; she has received honoraria, consulting payments, grant support, or royalties from Biogen, MedImmune, Novartis, Teva Neuroscience, Sanofi-Aventis, and EMD Serono. Dr Rose has received research funding from Teva Neuroscience and Biogen; he is a member of the Medical Advisory Board for the DECIDE trial, which is funded by Biogen and AbbVie. Dr Chitnis has served as a consultant for Biogen Idec, Teva Neurosciences, Novartis, and Sanofi-Aventis, and has received grant support from the NIH, National MS Society, Guthy-Jackson Charitable Foundation, CMSC and Merck-Serono, and Novartis. Dr Graves was supported by the Foundation for Consortium of Multiple Sclerosis Centers and the NIH Bridging Interdisciplinary Research Careers in Women’s Health programs (5K12HD052163) during this work; she has been a one-time advisory board participant for EMD Serono. Drs Harris and Ness are supported by the National MS Society, NIH, and Novartis. Dr Waubant is funded by the National MS Society, the NIH, and the Race to Erase MS; she has received honorarium from Roche for 1 educational lecture, and is an ad hoc volunteer consultant for Biogen and Roche. Dr Waubant also volunteers on an advisory board for a clinical trial of Novartis. Dr Tillema receives grant support from the NIH (KL2TR000136-09). Dr Weinstock-Guttman received honoraria for serving in advisory boards and educational programs from Teva Pharmaceuticals, Biogen Idec, Acorda EMD Serono, Novartis, Genzyme, and Sanofi; she also received support for research activities from the NIH, National Multiple Sclerosis Society, National Science Foundation, Department of Defense, EMD Serono, Biogen Idec, Teva Neuroscience, Novartis, Acorda, Genzyme, and the Jog for the Jake Foundation. Dr Casper has been supported by the National MS Society (HC 0165 and the NIH [R01NS071463]; he is an ad hoc consultant for Biovest International, Inc. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: All phases of this study were supported by the National Multiple Sclerosis Society, Network of Pediatric MS Centers (HC 0165; Principal Investigator, Dr Casper). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Duquette P, Murray TJ, Pleines J, et al. Multiple sclerosis in childhood: clinical profile in 125 patients. J Pediatr. 1987;111(3):359–363 [DOI] [PubMed] [Google Scholar]
- 2.Sindern E, Haas J, Stark E, Wurster U. Early onset MS under the age of 16: clinical and paraclinical features. Acta Neurol Scand. 1992;86(3):280–284 [DOI] [PubMed] [Google Scholar]
- 3.Ghezzi A, Deplano V, Faroni J, et al. Multiple sclerosis in childhood: clinical features of 149 cases. Mult Scler. 1997;3(1):43–46 [DOI] [PubMed] [Google Scholar]
- 4.Ruggieri M, Polizzi A, Pavone L, Grimaldi LM. Multiple sclerosis in children under 6 years of age. Neurology. 1999;53(3):478–484 [DOI] [PubMed] [Google Scholar]
- 5.Simone IL, Carrara D, Tortorella C, et al. Course and prognosis in early-onset MS: comparison with adult-onset forms. Neurology. 2002;59(12):1922–1928 [DOI] [PubMed] [Google Scholar]
- 6.Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D; University of British Columbia MS Clinic Neurologists . Early onset multiple sclerosis: a longitudinal study. Neurology. 2002;59(7):1006–1010 [DOI] [PubMed] [Google Scholar]
- 7.Chitnis T, Glanz B, Jaffin S, Healy B. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Mult Scler. 2009;15(5):627–631 [DOI] [PubMed] [Google Scholar]
- 8.Harding KE, Liang K, Cossburn MD, et al. Long-term outcome of paediatric-onset multiple sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2013;84(2):141–147 [DOI] [PubMed] [Google Scholar]
- 9.Low NL, Carter S. Multiple sclerosis in children. Pediatrics. 1956;18(1):24–30 [PubMed] [Google Scholar]
- 10.Gall JC Jr, Hayles AB, Siekert RG, Keith HM. Multiple sclerosis in children; a clinical study of 40 cases with onset in childhood. Pediatrics. 1958;21(5):703–709 [PubMed] [Google Scholar]
- 11.Boutin B, Esquivel E, Mayer M, Chaumet S, Ponsot G, Arthuis M. Multiple sclerosis in children: report of clinical and paraclinical features of 19 cases. Neuropediatrics. 1988;19(3):118–123 [DOI] [PubMed] [Google Scholar]
- 12.Hanefeld F, Bauer HJ, Christen HJ, Kruse B, Bruhn H, Frahm J. Multiple sclerosis in childhood: report of 15 cases. Brain Dev. 1991;13(6):410–416 [DOI] [PubMed] [Google Scholar]
- 13.Ghezzi A, Pozzilli C, Liguori M, et al. Prospective study of multiple sclerosis with early onset. Mult Scler. 2002;8(2):115–118 [DOI] [PubMed] [Google Scholar]
- 14.Ozakbas S, Idiman E, Baklan B, Yulug B. Childhood and juvenile onset multiple sclerosis: clinical and paraclinical features. Brain Dev. 2003;25(4):233–236 [DOI] [PubMed] [Google Scholar]
- 15.Deryck O, Ketelaer P, Dubois B. Clinical characteristics and long term prognosis in early onset multiple sclerosis. J Neurol. 2006;253(6):720–723 [DOI] [PubMed] [Google Scholar]
- 16.Mikaeloff Y, Caridade G, Assi S, Suissa S, Tardieu M. Prognostic factors for early severity in a childhood multiple sclerosis cohort. Pediatrics. 2006;118(3):1133–1139 [DOI] [PubMed] [Google Scholar]
- 17.Renoux C, Vukusic S, Mikaeloff Y, et al. ; Adult Neurology Departments KIDMUS Study Group . Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356(25):2603–2613 [DOI] [PubMed] [Google Scholar]
- 18.Ashtari F, Shaygannejad V, Farajzadegan Z, Amin A Does early-onset multiple sclerosis differ from adult-onset form in Iranian people. J Res Med Sci 2010;15(2):94–99 [PMC free article] [PubMed]
- 19.Etemadifar M, Nasr-Esfahani AH, Khodabandehlou R, Maghzi AH. Childhood-onset multiple sclerosis: report of 82 patients from Isfahan, Iran. Arch Iran Med. 2007;10(2):152–156 [PubMed] [Google Scholar]
- 20.Langer-Gould A, Zhang JL, Chung J, Yeung Y, Waubant E, Yao J. Incidence of acquired CNS demyelinating syndromes in a multiethnic cohort of children. Neurology. 2011;77(12):1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ketelslegers IA, Catsman-Berrevoets CE, Neuteboom RF, et al. Incidence of acquired demyelinating syndromes of the CNS in Dutch children: a nationwide study. J Neurol. 2012;259(9):1929–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinhardt K, Weiss S, Rosenbauer J, Gärtner J, von Kries R. Multiple sclerosis in children and adolescents: incidence and clinical picture—new insights from the nationwide German surveillance (2009-2011). Eur J Neurol. 2014;21(4):654–659 [DOI] [PubMed] [Google Scholar]
- 23.Casper TC, Rose JW, Roalstad S, et al. ; US Network of Pediatric Multiple Sclerosis Centers . The US Network of Pediatric Multiple Sclerosis Centers: development, progress, and next steps. J Child Neurol. 2015;30(10):1381–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krupp LB, Banwell B, Tenembaum S; International Pediatric MS Study Group . Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16 suppl 2):S7–S12 [DOI] [PubMed] [Google Scholar]
- 25.Neurostatus Systems AG. Neurostatus scoring. Available at: https://www.neurostatus.net/scoring/index.php. Accessed May 9, 2016
- 26.United States Census Bureau. Annual Estimates of the resident population by sex, age, race, and Hispanic origin for the United States and States: April 1, 2010. to July 1, 2014: 2014 population estimates. Available at: http://factfinder.census.gov/bkmk/table/1.0/en/PEP/2014/PEPASR6H?slice=year∼est72012. Accessed May 9, 2016
- 27.Nations Foreign-Born Population Nears 37 Million [press release]. October 10th, 2010. Available at: https://www.census.gov/newsroom/releases/archives/foreignborn_population/cb10-159.html. Accessed May 9, 2016
- 28.Lulu S, Graves J, Waubant E. Menarche increases relapse risk in pediatric multiple sclerosis. Mult Scler. 2016;22(2):193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn JJ, O’Mahony J, Moshkova M, et al. Puberty in females enhances the risk of an outcome of multiple sclerosis in children and the development of central nervous system autoimmunity in mice. Mult Scler. 2015;21(6):735–748 [DOI] [PubMed] [Google Scholar]
- 30.Huppke B, Ellenberger D, Rosewich H, Friede T, Gärtner J, Huppke P. Clinical presentation of pediatric multiple sclerosis before puberty. Eur J Neurol. 2014;21(3):441–446 [DOI] [PubMed] [Google Scholar]
- 31.Farber R, Hannigan C, Alcauskas M, Krieger S. Emergency department visits before the diagnosis of MS. Mult Scler Relat Disord. 2014;3(3):350–354 [DOI] [PubMed] [Google Scholar]
- 32.Chabas D, Ness J, Belman A, et al. ; US Network of Pediatric MS Centers of Excellence . Younger children with MS have a distinct CSF inflammatory profile at disease onset. Neurology. 2010;74(5):399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boster AL, Endress CF, Hreha SA, Caon C, Perumal JS, Khan OA. Pediatric-onset multiple sclerosis in African-American black and European-origin white patients. Pediatr Neurol. 2009;40(1):31–33 [DOI] [PubMed] [Google Scholar]
- 34.Kennedy J, O’Connor P, Sadovnick AD, Perara M, Yee I, Banwell B. Age at onset of multiple sclerosis may be influenced by place of residence during childhood rather than ancestry. Neuroepidemiology. 2006;26(3):162–167 [DOI] [PubMed] [Google Scholar]
- 35.Graves JS, Barcellos LF, Shao X, et al. . Genetic predictors of relapse rate in pediatric MS. [published online ahead of print January 14, 2016]. Mult Scler. doi:10.1177/1352458515624269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66(1):54–59 [DOI] [PubMed] [Google Scholar]