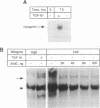
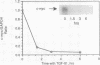
Abstract
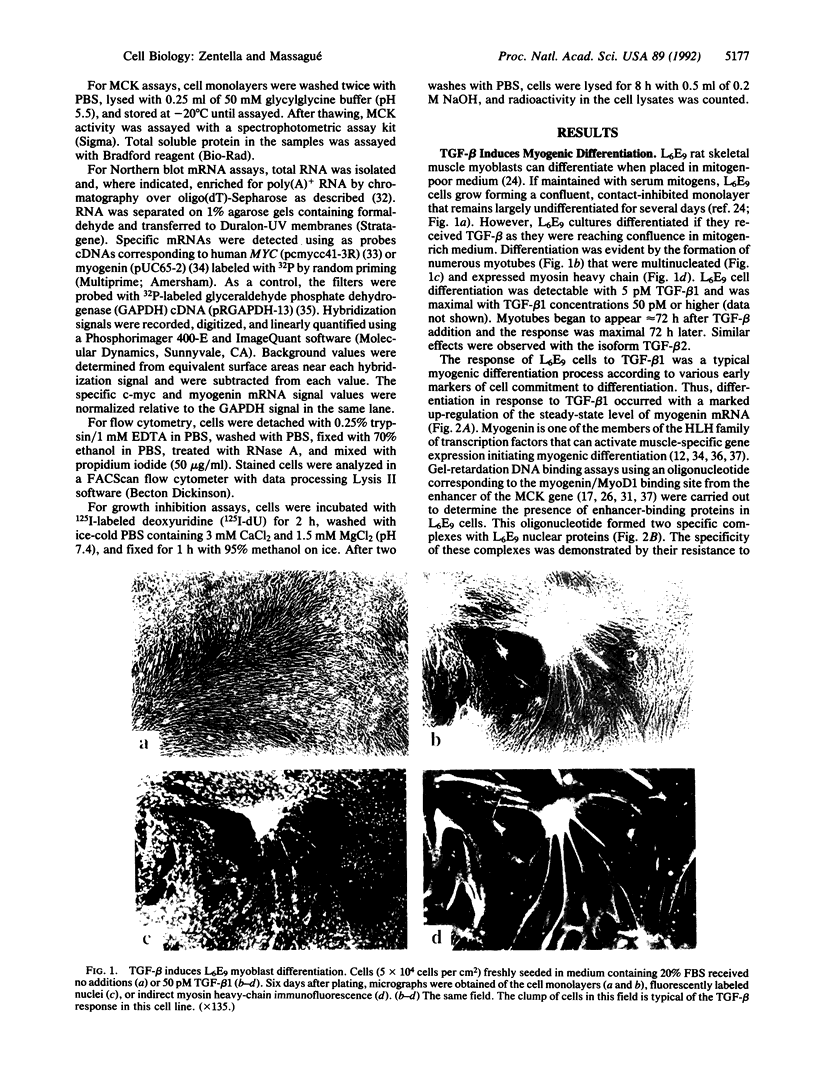
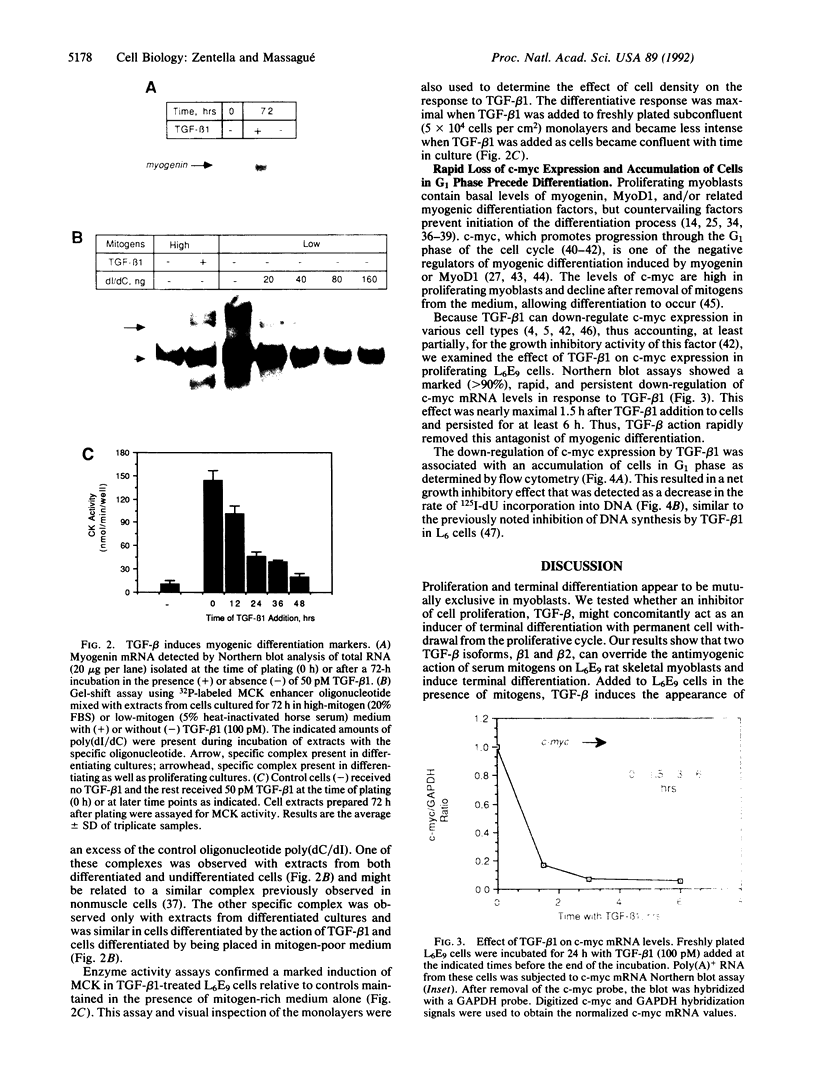
Transforming growth factor beta 1 (TGF-beta 1) added to L6E9 rat skeletal myoblasts in mitogen-rich medium induces a rapid decrease in c-myc expression and delays progression through the G1 phase of the cell cycle. This growth inhibitory response is followed by cell commitment to terminal differentiation with elevated expression of myogenin muscle determination genes, induction of muscle-specific proteins, and formation of multinucleated myotubes. These results suggest that TGF-beta 1 may act as a physiological inducer of myogenic differentiation in mitogen-rich environments, and its otherwise reversible growth inhibitory effect may become permanent if coupled to induction of terminal differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J., Lehnert S. A., Faissner A., Duffie E. TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development. 1990 Apr;108(4):645–656. doi: 10.1242/dev.108.4.645. [DOI] [PubMed] [Google Scholar]
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S., Weinmann R., Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991 Jun 14;65(6):1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Brennan T. J., Edmondson D. G., Li L., Olson E. N. Transforming growth factor beta represses the actions of myogenin through a mechanism independent of DNA binding. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3822–3826. doi: 10.1073/pnas.88.9.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., Kaelin W. G., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. doi: 10.1016/0092-8674(91)90559-h. [DOI] [PubMed] [Google Scholar]
- Clegg C. H., Linkhart T. A., Olwin B. B., Hauschka S. D. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987 Aug;105(2):949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeo-Jones D., Huang P. S., Jones R. E., Haskell K. M., Vuocolo G. A., Hanobik M. G., Huber H. E., Oliff A. Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature. 1991 Jul 18;352(6332):251–254. doi: 10.1038/352251a0. [DOI] [PubMed] [Google Scholar]
- Denis N., Blanc S., Leibovitch M. P., Nicolaiew N., Dautry F., Raymondjean M., Kruh J., Kitzis A. c-myc oncogene expression inhibits the initiation of myogenic differentiation. Exp Cell Res. 1987 Sep;172(1):212–217. doi: 10.1016/0014-4827(87)90107-8. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Tomek R., Woods C., Bhambi B. Cardiac fibroblasts are predisposed to convert into myocyte phenotype: specific effect of transforming growth factor beta. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):795–799. doi: 10.1073/pnas.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini J. R., Ewton D. Z., Magri K. A. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Roberts A. B., Ewton D. Z., Falen S. L., Flanders K. C., Sporn M. B. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem. 1986 Dec 15;261(35):16509–16513. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F., Lombardi L., Inghirami G., Sternas L., Cechova K., Dalla-Favera R. Negative autoregulation of c-myc gene expression is inactivated in transformed cells. EMBO J. 1990 Dec;9(12):3913–3922. doi: 10.1002/j.1460-2075.1990.tb07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haub O., Goldfarb M. Expression of the fibroblast growth factor-5 gene in the mouse embryo. Development. 1991 Jun;112(2):397–406. doi: 10.1242/dev.112.2.397. [DOI] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J., Massagué J. Cell adhesion to collagen and decreased myogenic gene expression implicated in the control of myogenesis by transforming growth factor beta. J Biol Chem. 1990 Jun 25;265(18):10181–10184. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph-Silverstein J., Consigli S. A., Lyser K. M., Ver Pault C. Basic fibroblast growth factor in the chick embryo: immunolocalization to striated muscle cells and their precursors. J Cell Biol. 1989 Jun;108(6):2459–2466. doi: 10.1083/jcb.108.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Pallas D. C., DeCaprio J. A., Kaye F. J., Livingston D. M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991 Feb 8;64(3):521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991 Jul 26;66(2):305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Miner J. H., Wold B. J. c-myc inhibition of MyoD and myogenin-initiated myogenic differentiation. Mol Cell Biol. 1991 May;11(5):2842–2851. doi: 10.1128/mcb.11.5.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Yoon K., Prince C. W., Butler W. T., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J Biol Chem. 1988 Sep 25;263(27):13916–13921. [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Pampusch M. S., Hembree J. R., Hathaway M. R., Dayton W. R. Effect of transforming growth factor beta on proliferation of L6 and embryonic porcine myogenic cells. J Cell Physiol. 1990 Jun;143(3):524–528. doi: 10.1002/jcp.1041430317. [DOI] [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Holt J. T., Stein R. W., Moses H. L. Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci U S A. 1990 May;87(10):3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. D., Dagle J. M., Walder J. A., Weeks D. L., Runyan R. B. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J., Rodgers C. c-myc antisense transcripts accelerate differentiation and inhibit G1 progression in murine erythroleukemia cells. Mol Cell Biol. 1988 Sep;8(9):3683–3695. doi: 10.1128/mcb.8.9.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D. M., Stempien S. A., Thompson A. Y., Seyedin S. M. Transforming growth factor-beta modulates the expression of osteoblast and chondroblast phenotypes in vitro. J Cell Physiol. 1988 Mar;134(3):337–346. doi: 10.1002/jcp.1041340304. [DOI] [PubMed] [Google Scholar]
- Sejersen T., Sümegi J., Ringertz N. R. Density-dependent arrest of DNA replication is accompanied by decreased levels of c-myc mRNA in myogenic but not in differentiation-defective myoblasts. J Cell Physiol. 1985 Dec;125(3):465–470. doi: 10.1002/jcp.1041250315. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Tucker R. F., Moses H. L. Type beta transforming growth factor/growth inhibitor stimulates entry of monolayer cultures of AKR-2B cells into S phase after a prolonged prereplicative interval. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4147–4151. doi: 10.1073/pnas.82.12.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Weintraub H. MyoD and the regulation of myogenesis by helix-loop-helix proteins. J Clin Invest. 1991 Apr;87(4):1133–1138. doi: 10.1172/JCI115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Vaidya T. B., Rhodes S. J., Taparowsky E. J., Konieczny S. F. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989 Aug;9(8):3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom R., Lee W. Translation of c-myc mRNA is required for its post-transcriptional regulation during myogenesis. J Biol Chem. 1990 Nov 5;265(31):19015–19021. [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Developmental changes preceding cell fusion during muscle differentiation in vitro. Exp Cell Res. 1971 May;66(1):33–48. doi: 10.1016/s0014-4827(71)80008-3. [DOI] [PubMed] [Google Scholar]
- Zentella A., Weis F. M., Ralph D. A., Laiho M., Massagué J. Early gene responses to transforming growth factor-beta in cells lacking growth-suppressive RB function. Mol Cell Biol. 1991 Oct;11(10):4952–4958. doi: 10.1128/mcb.11.10.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]