Abstract
Senile cataract is a clouding of the lens in the aging eye leading to a decrease in vision. Symptoms may include faded colors, blurry vision, halos around light, trouble with bright lights, and trouble seeing at night. This may result in trouble driving, reading, or recognizing faces. Cataracts are the cause of half of blindness and 33% of visual impairment worldwide. Cataracts result from the deposition of aggregated proteins in the eye lens and lens fiber cells plasma membrane damage which causes clouding of the lens, light scattering, and obstruction of vision. ROS induced damage in the lens cell may consist of oxidation of proteins, DNA damage and/or lipid peroxidation, all of which have been implicated in cataractogenesis. The inner eye pressure (also called intraocular pressure or IOP) rises because the correct amount of fluid can't drain out of the eye. With primary open-angle glaucoma, the entrances to the drainage canals are clear and should be working correctly. The clogging problem occurs further inside the drainage canals, similar to a clogged pipe below the drain in a sink.
The excessive oxidative damage is a major factor of the ocular diseases because the mitochondrial respiratory chain in mitochondria of the vital cells is a significant source of the damaging reactive oxygen species superoxide and hydrogen peroxide. However, despite the clinical importance of mitochondrial oxidative damage, antioxidants have been of limited therapeutic success. This may be because the antioxidants are not selectively taken up by mitochondria, but instead are dispersed throughout the body, ocular tissues and fluids' moieties.
This work is an attempt to integrate how mitochondrial reactive oxygen species (ROS) are altered in the aging eye, along with those protective and repair therapeutic systems believed to regulate ROS levels in ocular tissues and how damage to these systems contributes to age-onset eye disease and cataract formation.
Mitochondria-targeted antioxidants might be used to effectively prevent ROS-induced oxidation of lipids and proteins in the inner mitochondrial membrane in vivo. The authors developed and patented the new ophthalmic compositions including N-acetylcarnosine acting as a prodrug of naturally targeted to mitochondria l-carnosine endowed with pluripotent antioxidant activities, combined with mitochondria-targeted rechargeable antioxidant (either MitoVit E, Mito Q or SkQs) as a potent medicine to treat ocular diseases. Such specificity is explained by the fact that developed compositions might be used to effectively prevent ROS-induced oxidation of lipids and proteins in the inner mitochondrial membrane in vivo and outside mitochondria in the cellular and tissue structures of the lens and eye compartments.
Mitochondrial targeting of compounds with universal types of antioxidant activity represents a promising approach for treating a number of ROS-related ocular diseases of the aging eye and can be implicated in the management of cataracts and primary open-angle glaucoma.
Keywords: Aging eye, Age-related cataracts, Eye lens and aqueous humor, Membrane derangement, Mitochondria and lens epithelial cells, Reactive oxygen species, Lipid peroxidation and phospholipid hydroperoxides, Ophthalmic compositions of N-acetylcarnosine and mitochondria-targeted antioxidants
Highlights
-
•
This work considers mitochondrial reactive oxygen species (ROS) effects in the aging eye
-
•
The excessive oxidative damage is a major factor of the eye diseases (cataract, primary open-angle glaucoma)
-
•
The conventional antioxidants are not selectively taken up by mitochondria of the cells
-
•
The author developed the new ophthalmic compositions of mitochondria-targeted antioxidants
-
•
The formulation comprises N-acetylcarnosine combined with mitochondria-targeted rechargeable antioxidant
-
•
Mitochondrial targeting of antioxidants represents a promising therapeutic approach
-
•
A number of ROS-related ocular diseases of the aging eye can be treated
1. Introduction to oxidative stress
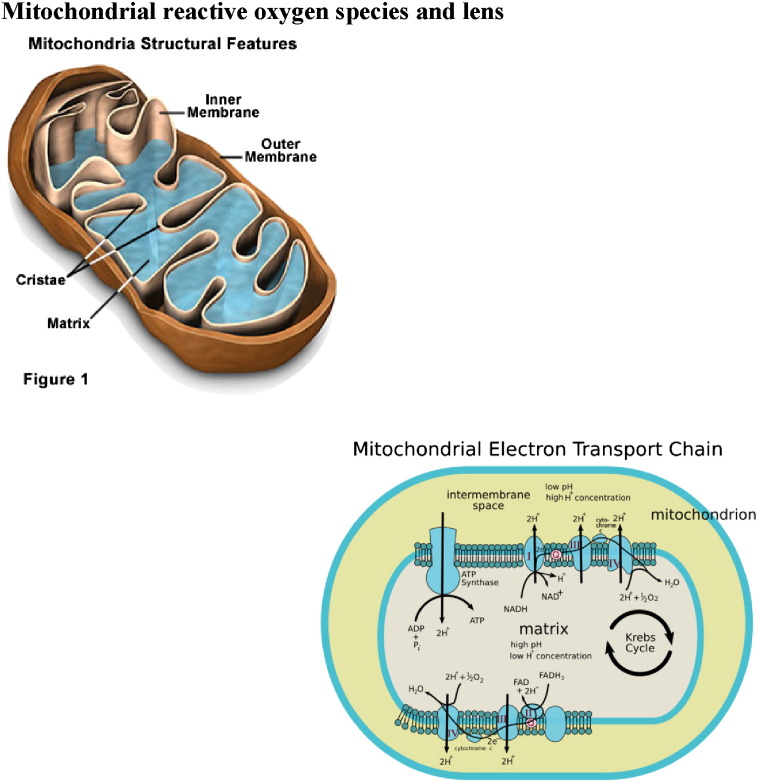
Partially reduced metabolites of molecular oxygen (O2) are referred to as “reactive oxygen species” (ROS) due to their higher reactivities relative to molecular O2. ROS are generated intracellularly through a variety of processes, for example, as by-products of normal aerobic metabolism and as second messengers in various signal transduction pathways. ROS can also be derived from exogenous sources, either being taken up directly by cells from the extracellular milieu, or produced as a consequence of the cell's exposure to some environmental insult. Reactive oxygen species (ROS) are generated as by-products of cellular metabolism, primarily in the mitochondria. Although ROS are essential participants in cell signaling and regulation, when their cellular production overwhelms the intrinsic antioxidant capacity, damage to cellular macromolecules such as DNA, proteins, and lipids ensues. Such a state of “oxidative stress” is thought to contribute to the pathogenesis of a number of age-related sight threatening eye diseases. Oxidation of phospholipids and accumulation of their oxygenated intermediates have long been considered as participants of two possibly interrelated processes: cell/tissue damage and danger signaling [1]. However, direct experimental evidence supporting these views is scarce, mostly due to technological difficulties in quantitative assessments of peroxidized phospholipids. The traditional point of view is that oxidative stress causes – through yet to identified mechanisms – random free radical peroxidation of phospholipids whereby the rule of preferable oxidation of most polyunsaturated fatty acids dominates over the types of phospholipids based on the specificity of their polar head-groups. In contrast, peroxidation pathways catalyzed by specific catalysts – such as cytochrome c in apoptotic cells – should non-randomly oxidize those classes of phospholipids that are localized in the immediate proximity to the catalytic sites. Among those of particular interest in the context of peroxidation reactions are cardiolipins (CLs), a critical phospholipid component of cellular membranes from bacteria to mammals. The importance of CL peroxidation in mammals is also emphasized by its critical role in the function of numerous mitochondrial enzymes involved in electron transport chain function and other proteins involved in energetic metabolism which include cytochrome c (cyt c) oxidase [2], creatine kinase [3], ATP synthase [4] and the mitochondrial ADP carrier [5] among others. (See Scheme 1.)
Scheme 1.
The production of reactive oxygen species (ROS) in mitochondria.
1.1. Oxidation-induced hallmark of molecular damages in human cataracts
Cataract formation, the opacification of the eye lens, is one of the leading causes of human blindness worldwide, accounting for 47.8% of all causes of blindness [6], [7]. Cataracts result from the deposition of aggregated proteins in the eye lens and lens fiber cells plasma membrane damage which causes clouding of the lens, light scattering, and obstruction of vision.
Although great advances have been made in surgical treatment, the incidence of cataracts in developing countries is so high that it overwhelms the capacity of surgical programs. Age is by far the biggest risk factor for cataract, and it is sometimes assumed that cataract is simply an amplification of this aging process. This appears not to be the case, since the lens changes associated with aging and cataract are distinct.
This review article is an attempt to integrate how mitochondrial ROS are altered in the aging eye, along with those protective and repair therapeutic systems developed by Innovative Vision Products, Inc. (IVP) (Delaware, USA) during recent years believed to regulate ROS levels in ocular tissues and how damage to these systems contributes to age-onset eye disease and, specifically, cataract formation.
Oxidative stress occurs when the level of prooxidants exceeds the level of antioxidants in cells resulting in oxidation of cellular components and consequent loss of cellular function [8]. In the anterior segment of the eye, oxidative stress has been linked to lens cataract and glaucoma while in the posterior segment of the eye oxidative stress has been associated with macular degeneration [8]. The aging eye appears to be at considerable risk from oxidative stress. In eye tissues, mitochondria are an important endogenous source of ROS. One might predict that over time, all ocular structures, from the tear film to the retina, undergo oxidative stress, and therefore, the antioxidant defenses of each tissue assume the role of a safeguard against degenerative ocular pathologies. The ocular surface and cornea protect the other ocular tissues and are significantly exposed to oxidative stress of environmental origin. Overwhelming of antioxidant defenses in these tissues clinically manifests as pathologies including pterygium, corneal dystrophies, and endothelial Fuch's dystrophy. The crystalline lens is highly susceptible to oxidative damage in aging because its cells and their intracellular proteins are not turned over or replaced, thus providing the basis for cataractogenesis. The trabecular meshwork, which is the anterior chamber tissue devoted to aqueous humor drainage, has a particular susceptibility to mitochondrial oxidative injury that affects its endothelium and leads to an intraocular pressure increase that marks the beginning of glaucoma. Photo-oxidative stress can cause acute or chronic retinal damage. The pathogenesis of age-related macular degeneration involves oxidative stress and death of the retinal pigment epithelium followed by death of the overlying photoreceptors [9]. This review outlines specifically the potential role of mitochondrial function and redox balance in age-related eye diseases (age-related cataract and glaucoma), and detail how the carnosine dipeptide repair system, its ophthalmic prodrug N-acetylcarnosine lubricant eye drops and other redox systems play key roles in the function and maintenance of the aging eye.
Oxidation is the hallmark of age-related nuclear (ARN) cataract (reviewed in Refs. [10], [11], [12], [13]). ROS induced damage in the lens cell may consist of oxidation of proteins, DNA damage and/or lipid peroxidation, all of which have been implicated in cataractogenesis (reviewed in Ref. [14]). Loss of protein sulfhydryl groups, and the oxidation of methionine residues, are progressive and increase as the cataract worsens until > 90% of cysteine and half the methionine residues are oxidized in the most advanced form. By contrast, there may be no significant oxidation of proteins in the center of the lens with advancing age, even past age 80. The key factor in preventing oxidation seems to be the concentration of nuclear glutathione (GSH). The phospholipid composition of adult human lens membranes differs dramatically from that of any other mammalian membrane [15]. The relative and absolute amount of sphingolipids, including dihydrosphingomyelin and sphingomyelin, increased with age, whereas glycerolipids, including phosphatidylcholine and two phosphatidylethanolamine-related phospholipids, decreased. These changes were exacerbated by the presence of cataract and were substantial, greater than the changes in lipid levels reported in any organ in association with any disease. The changes in the amount of lipids with age and cataract support the idea that glycerolipids are selectively oxidized over lipids with fewer double bonds, such as sphingolipids [16]. As a result of the elevation of sphingolipid levels with species, age, and cataract, lipid hydrocarbon chain order, or stiffness, increases.
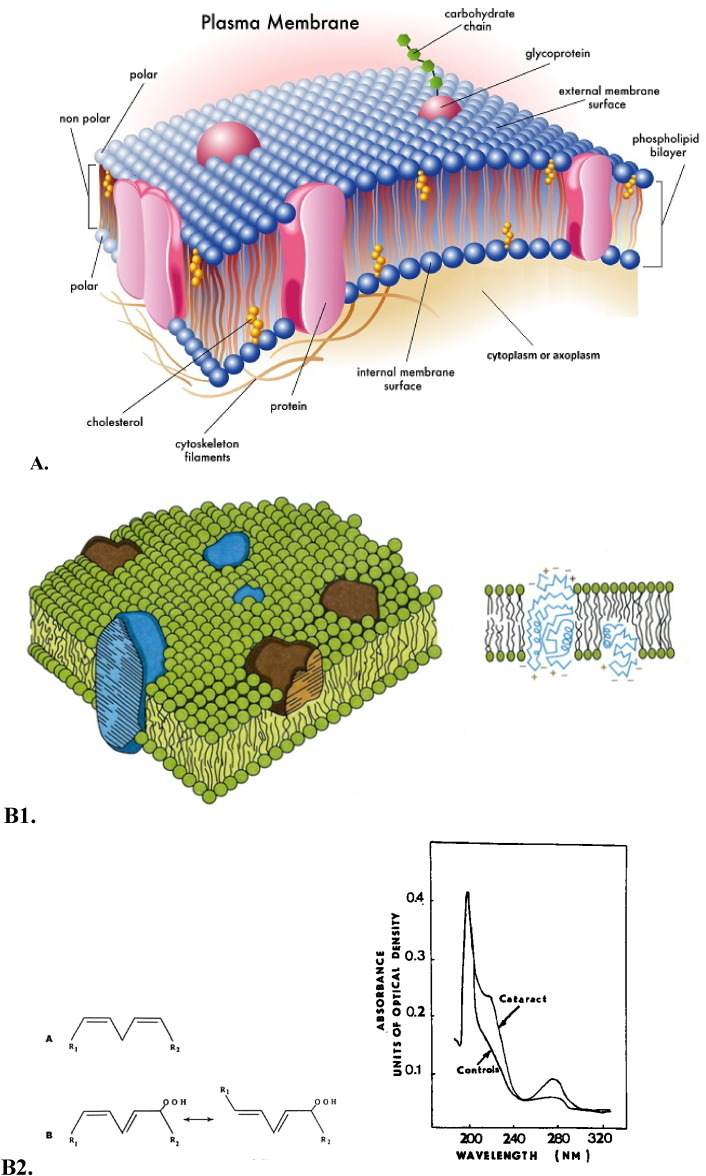
Increased membrane stiffness may increase light-scattering, reduce calcium pump activity, alter protein–lipid interactions, and perhaps slow fiber cell elongation (Fig. 1) [16].
Fig. 1.
-
A.Structure of the cell plasma membrane
Parts of the membrane
-
1)Phospholipid bilayer — two layers of phospholipids
-
a)head is polar … attracts water
-
b)tail … hates water doesn't want to be near it
-
a)
-
2)Proteins
-
a)To allow stuff in and out
-
b)Also for cell “ID tags” — recognition proteins …
-
a)
-
3)Carbohydrate chains
-
a)Attracted to proteins that work as “ID tags” … they have a pattern … similar to antlers
-
a)
-
4)Cholesterol
-
a)Stabilizes the membrane
-
a)
-
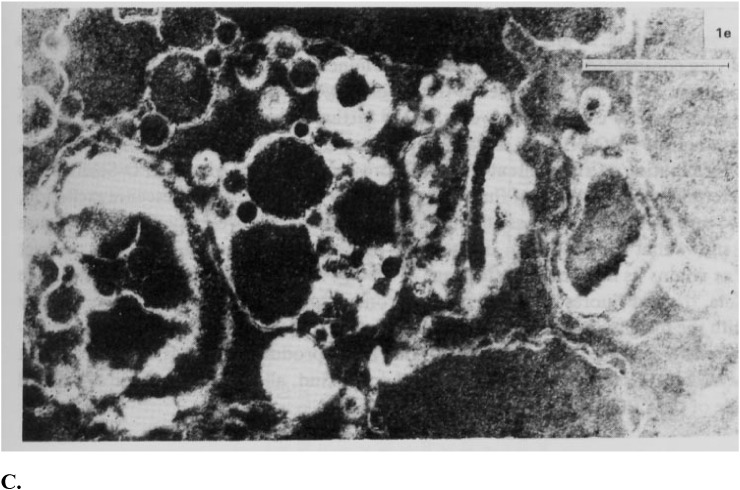
B1.Accumulation of phospholipid hydroperoxides, the secondary molecular lipid peroxidation products, in the lens fiber cells plasma membranes (B1,B2,C).
-
B2:UV absorption spectra of lipid extracts (methanol/heptane: 5:1, v/v) from aqueous humor samples obtained from human eyes: normal (control, lower curve) and mature cataract (upper curve).
-
C:Electron microphotograph of the midzonal area of the human lens; ripe nuclear cataract. Bar = 1 μm.
At the stage of ripe nuclear cataract, which is biochemically characterized by large high molecular aggregate formation in the human lens, an ultrastructural characteristic of the membrane lesion is distinct with the lenticular fiber plasma membrane twisted fragments becoming a central mass of amorphous electron-gray debris and globules of different size.
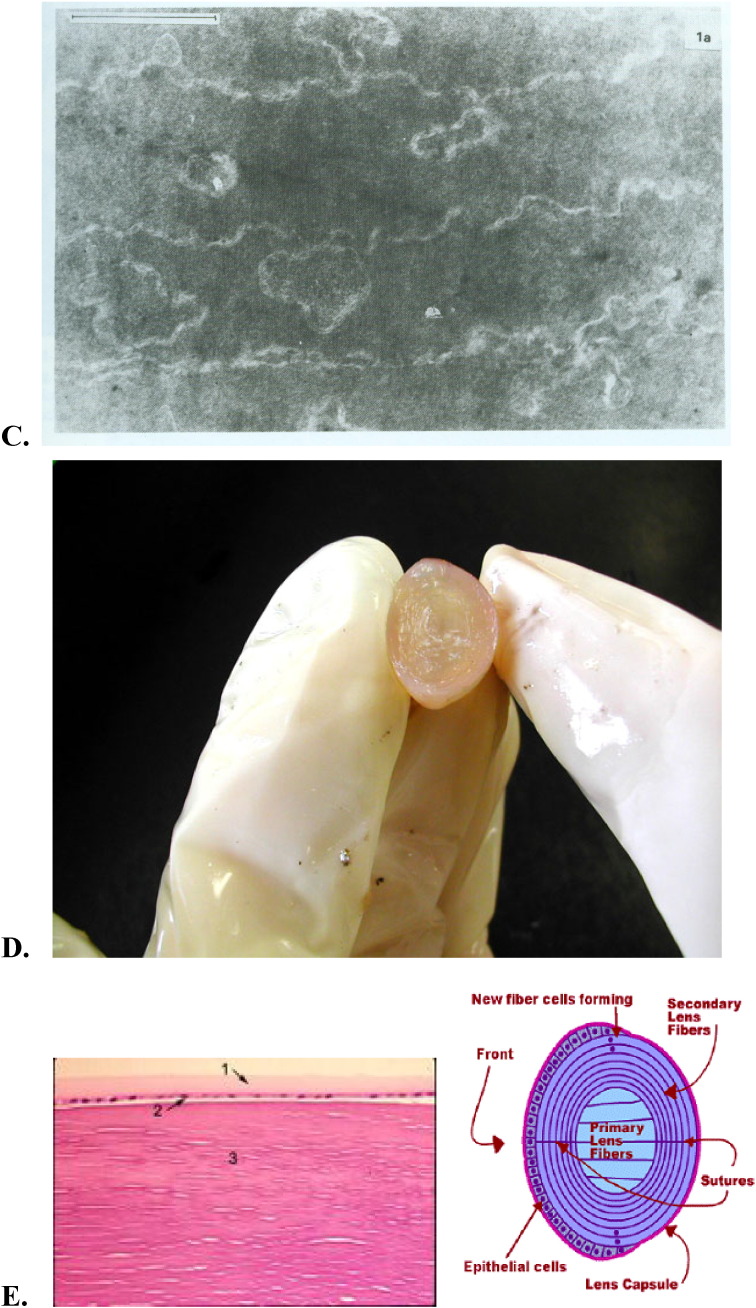
The mammalian lens can be divided into an elongating compartment in the posterior lens and a proliferation compartment in the epithelium of the anterior lens. Cell division in the lens is restricted to the proliferating compartment of the epithelial cells [17], [18]. This proliferation compartment in the lens epithelium can be further divided in to three subcompartments, related to the anatomy of the anterior lens and a rate of cell division. Lens epithelial cells found in the proximity of the ciliary body (equator) show the fastest mitotic rate and are referred to as the equatorial zone. Epithelial cells located in the proximity of the iris show slower mitotic rates and are referred to as the intermediate zone. Finally, epithelial cells overlying the anterior suture regions of the lens undergo little or no mitosis and are referred to as the central zone. At the lens equator, epithelial cells of the equatorial zone differentiate into fiber cells, producing an elongating compartment (Fig. 2A–E) [17], [18].
Fig. 2.
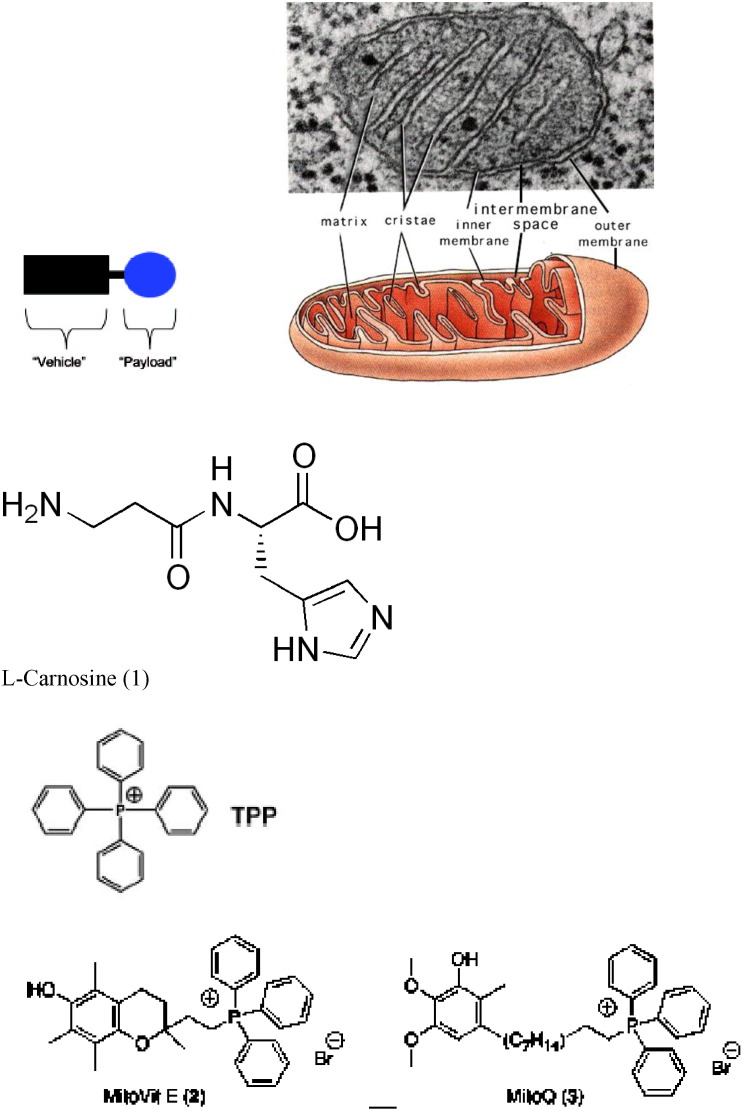
A, B, C, D, E. Schematic presentation of the crystalline lens, location within the eye and the structure (1), lens epithelial cells (2) and lens fiber cells (3–7). The lens is a transparent organ located behind the cornea and the iris. The outer edge of the lens consists of a single layer of epithelial cells, and a membrane that covers the entire organ. Lens epithelial cells do not divide except when undergoing repair. Some epithelial cells lose their nuclei and other organelles, and become lens fiber cells. At the cellular level, the evidences of amazing lens design are clear. The lens is comprised of numerous highly elongated cells which grow like an onion (3–5). The lens fibers are anucleate, ribbon-like cells located throughout the central region of the iris (6). These cells do not have a blood supply and do not contain any nervous or connective tissue. They are full of crystallins, grown especially to attain a highly transparent lens quality (7). B, C: Within each layer, the fiber cells are arranged in parallel. The alignment of the lens fiber cells in concentric layers is essential to lens transparency. In order to maintain their arrangement, adjacent cells are interlocked by ball-and-socket joints at the cells' edges. This prevents movement of cells against each other during the lens shape changes associated with focusing. D. Lens — cut in half. The long, slender epithelial cells that grow in concentric rings to form the lens. D, E. The adult lens measures about 9 mm in diameter and is 3.5 mm thick. It is completely enveloped by the thickest basement membrane in the body, the capsule (#1 in photomicrograph), which is 10–20 μm thick of hyaline material containing type IV collagen. There is a layer of large cuboidal epithelial cells (the lens epithelium) beneath the anterior capsule (#2 in photomicrograph). In the center (#3 in photomicrograph) tightly packed cells have lost their nuclei and become packed by special transparent proteins (crystallins) to form so-called lens fibers. E, F. Schematic presentation of a mitochondria. Mitochondria are small compartments partitioned by membranes and found exclusively in complex cells. These organelles are often called the “power plants” of the cell because their main job is to make energy. Mitochondria are highly unusual — they contain their own genetic material and protein-making machinery enwrapped in a double membrane. F. Mitochondria are abundant in the lens, but only within the epithelium and differentiating fibers, mature fibers in the core of the lens lack mitochondria. It became widely accepted that the lens epithelium plays the most important role in lens metabolism [37], [38], [39], [40]. It was further proposed that ions and metabolites gain access to the fiber cells, which lack organelles, including mitochondria, via gap junctions connecting the closely apposed apical membranes of lens epithelial and superficial cortical fiber cells [40].
Oxidative stress, a major and ubiquitous stressing factor, was initially selected for investigating the cellular response to stress. Most studies investigating such cellular response have employed examination of the cell either during or shortly after exposure to stress. Several authors have employed a different approach arguing that the short-term response to stress obscures the biological changes that allow the cell to continue to thrive in its new environment [19].
Reflecting this oxidation concept, murine and human cell lines capable of surviving regular exposure to toxic levels of H2O2 or tert-butyl hydroperoxide (TBOOH) have been developed. It was found that certain fundamental long-term changes in cell biology had occurred. The peroxide-resistant cells are diploid rather than aneuploid, show fundamental changes in the cytoskeletal cellular structure, suggesting less rigid more flexible cells, express a new lower molecular mass of p53, a key stress protein responder involved in adaptation, and finally have an immunochemical modification in alpha A-crystallin, a small heat-shock protein. Previously, it was found that there is a dramatic increase in catalase and glutathione S-transferase activity and a remarkable limited change in expression in other antioxidative genes in these cells (reviewed in Ref. [19]).
2. Oxidative stress, mitochondria and the apoptotic pathway
Tissues with high energy demands such as muscles, heart, liver, endocrine glands, brain and retina, have higher numbers of mitochondria per cell.
The lens depends on redox balance to maintain transparency and considerable evidence points to mitochondrial dysfunction and ROS imbalance in the etiology of age related cataract. Distribution of mitochondria in the lens is associated with its development [8]. The lens is composed of cells that differentiate from an anterior layer of cuboidal epithelia and migrate posteriorly to form elongated lens fiber cells that make up the lens nucleus. During this process fiber cells synthesize high levels of lens crystallins before losing their nuclei and mitochondria. Thus, the single monolayer of epithelial cells that lines the anterior of the lens, are the only lens cells that carry out aerobic metabolism and contain mitochondria aside from newly differentiated fiber cells.
The lens is especially susceptible to damage with aging since lens the cells and their cellular proteins are not turned over or replaced in this encapsulated tissue. The proteins at the center of the eye are some of the oldest in the body and obviously susceptible to age related oxidative damage. Damage to the mitochondria of the epithelial cells may result in ROS production that is thought to affect the proteins and lipid plasma cell membranes of the underlying fiber cells.
Mitochondria have a significant role in regulating apoptosis and necrosis, ROS levels, cellular signaling, control of the cell cycle, and growth and differentiation [20].
The electron transport chain generates ATP by oxidative phosphorylation, creating a proton gradient through sequential transfer of electrons donated by reducing equivalents. This complex system is made up of five multi enzyme subunits, NADH dehydrogenase comprises complex I (46 subunits), succinate dehydrogenase is complex II (4 subunits), cytochrome C reductase and cytochrome C oxidase make up complexes III and IV respectively (11 and 13 subunits). Complex V is the ATP synthase (16 subunits) that uses the proton gradient created by the first four complexes to drive phosphorylation of ADP to form the energy rich ATP [8].
In addition to the well-established role of the mitochondria in energy metabolism, regulation of cell death has recently emerged as a second major function of these organelles. This, in turn, seems to be intimately linked to their role as the major intracellular source of reactive oxygen species (ROS), which are mainly generated at complex I and III of the respiratory chain. Excessive ROS production can lead to oxidation of macromolecules and has been implicated in mtDNA mutations, aging, and cell death [21].
Increasingly, mitochondria are thought to play a regulatory role in cell death partly due to its role as a source of ROS and due to the release of cytochrome C and other pro-apoptotic factors that activate caspases and trigger apoptosis. Cytochrome C is a small globular heme containing electron carrier in the electron transport chain of the mitochondria. Its primary role in the electron transport chain is a crucial one, shuttling electrons from complex III (ubiquinol:cytochrome c reductase) to complex IV (cytochrome oxidase), however, its release from the mitochondria to the cytosol is the initiating factor for the internal apoptotic pathway. The release of cytochrome C is a two step process, initiated by release of the hemoprotein from its binding to cardiolipin at the inner mitochondrial membrane [21]; this results in a pool of free cytochrome C in the intermembrane space. Subsequent permeabilization of the outer mitochondrial membrane releases cytochrome C into the cytosol where it binds apoptotic peptidase activating factor 1 (APAF1).
Mitochondria-generated ROS play an important role in the release of cytochrome c and other pro-apoptotic proteins, which can trigger caspase activation and apoptosis. Cytochrome c release occurs by a two-step process that is initiated by the dissociation of the hemoprotein from its binding to cardiolipin, which anchors it to the inner mitochondrial membrane. Oxidation of cardiolipin reduces cytochrome c binding and results in an increased level of “free” cytochrome c in the intermembrane space. A central step in the mechanism of naturally-occurring or radiation-induced apoptosis is damage to the mitochondria through the action of free radicals and reactive intermediates of peroxidase complexes of cytochrome c on a mitochondria-specific phospholipid, cardiolipin [22], [23], [24]. Relocation of cardiolipin from the inner into the outer mitochondrial membrane, formation of the peroxidase cytochrome c/cardiolipin complex, peroxidation of cardiolipin, detachment of cytochrome c from the membrane, mitochondrial permeabilization and release of cytochrome c, along with other pro-apoptosis factors, from mitochondria into the cytochromeosol designate a point of no return in the process of apoptosis [22], [25], [26]. A likely essential role of cardiolipin peroxidation products in mitochondrial permeabilization is supported by the ability of oxidized cardiolipin to release Smac/Diablo from mitochondria isolated from cytochrome c-deficient cells [22]. Superoxide radicals and their dismutation product, H2O2, produced spontaneously or via superoxide dismutase-catalyzed reactions, are essential for feeding the peroxidase cycle of cardiolipin oxidation [22], [26]. For this reason, elimination of intracellular ROS, particularly its major source, mitochondrial ROS, by antioxidants may be effective in protecting cells against apoptosis. Conversely, mitochondrial antioxidant enzymes protect from apoptosis. Hence, there is accumulating evidence supporting a direct link between mitochondria, oxidative stress and cell death [21]. It is suggested that mitochondrial-induced ROS production promotes cytochrome c release from mitochondria by a two-steps process, consisting of the dissociation of this protein from cardiolipin, followed by permeabilization of the outer membrane, probably by interaction with voltage-dependent anion channel (VDAC). The published data may help clarify the molecular mechanism underlying the release of cytochrome c from the mitochondria to the cytosol and the role of ROS and cardiolipin in this release [27].
Oxidative stress has long been recognized as an important mediator of apoptosis in lens epithelial cells and also plays an important role in the pathogenesis of cataracts [28], [29], [30], [31]. Apoptosis is a physiologic process of cell death that plays a critical role in a variety of biologic systems, which has been identified as providing an important molecular basis for both the initiation and progression of cataracts [32], [33]. The lens epithelial cells differentiate into lens fiber cells through a process, which utilizes the same regulators as those in apoptosis at multiple signaling steps. In addition, introduction of exogenous wild-type or mutant genes or knock-out of the endogenous genes leads to apoptosis of the lens epithelial cells followed by absence of the ocular lens or formation of abnormal lens. Finally, both in vitro and in vivo studies have shown that treatment of adult lens with stress factors induces apoptosis of lens epithelial cells, which is followed by cataractogenesis [32]. The results provide evidence for the involvement of an oxidative process in the apoptosis elicited by TGF-beta(2) (transforming growth factor beta(2) (TGF-beta(2)), a growth regulator of human lens epithelial cells (HLECs)) in HLECs [31].
The lens exists in an environment that is rich in endogenous sources of reactive oxygen species (ROS) and phospholipid hydroperoxides, which are produced by the high local oxygen concentration, the chronic exposure to light, and the pathogenic activities of lens epithelial cells (Fig. 2A–E) [34]. Lipid peroxidation (LPO) was about three times higher after growth in a hyperoxic atmosphere compared with cells grown in a normoxic atmosphere. The lack of change in the relative amount of sphingomyelin and the decrease in phosphatidylcholine coupled with the increase in lysophosphatidylcholine support the idea that similar mechanisms may be responsible for the lipid compositional changes in both lens epithelial and fiber cells. It is postulated that lipases eliminate oxidized unsaturated glycerolipids, leaving a membrane increasingly composed of more ordered and more saturated sphingolipids [34]. Oxidative stress leads to changes in membrane composition that are consistent with those seen with age in human epithelial cells. Oxidation-induced epithelial phospholipid change is an area of research that has gone virtually unexplored in the human lens and could be relevant to all cell types and may be important to lens clarity [34]. These results support the free radical theory of aging and reinforce the importance of mitochondria as a source of these radicals. Since H2O2 is a relatively stable and readily diffusible molecule a number of systems exist to limit its damaging potential. These include catalase, glutathione peroxidase and the peroxiredoxins. Mitochondrial localization of catalase appears to be restricted to heart mitochondria, however, overexpression of catalase targeted to mitochondria in mice extended lifespan and delayed cataract formation [35] indicating the importance of eliminating the toxic effects of H2O2 in lens cells. Peroxiredoxins (Prxs) are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels which mediate signal transduction in mammalian cells. Prxs can be regulated by changes to phosphorylation, redox and possibly oligomerization states. Prxs are divided into three classes: typical 2-Cys Prxs; atypical 2-Cys Prxs; and 1-Cys Prxs. All Prxs share the same basic catalytic mechanism, in which an active-site cysteine (the peroxidatic cysteine) is oxidized to a sulfenic acid by the peroxide substrate. The recycling of the sulfenic acid back to a thiol is what distinguishes the three enzyme classes [36]. Peroxiredoxins use redox active cysteines to reduce and detoxify H2O2, peroxynitrite and a wide range of organic hydroperoxides [36]. The peroxiredoxin III enzyme was found to be redox sensitive in lens cells and is localized to human lens and mitochondria of lens epithelial cells [37].
These data demonstrate that PRDX3 is present throughout the lens and localized to the mitochondria in lens epithelial cells. PRDX3 was specifically induced by low levels of H2O2 in human lens epithelial cells and rat lenses suggesting that induction of PRDX3 is an acute response of the lens to increased H2O2 levels. These data provide evidence for an important role for PRDX3 in lens H2O2-detoxification, mitochondrial maintenance, and possibly cataract formation [37].
Although multiple physiologic defenses exist to protect the lens from the toxic effects of light and oxidative damage, mounting evidence suggests that chronic exposure to oxidative stress over the long-term may damage the lens and predispose it to cataract development [38], [39].
3. The relation of mitochondria to age-onset eye disease
Maintaining the redox balance within the mitochondria is critical for cellular homeostasis since the mitochondria house the energy producing systems of the cell and it is widely recognized that damage to the mitochondria plays a key role in aging and age-related disorders. Production of reactive oxygen species (ROS) species such as the hydroxyl radical (•OH), singlet oxygen (1O2), hydrogen peroxide (H2O2) and peroxynitrite (OONO−) is finely balanced with sophisticated antioxidant and repair systems located in this complex organelle. Loss of these antioxidant and scavenging systems leads to protein and lipid membrane oxidations that are hallmarks of many ocular diseases including cataract, glaucoma and retinal degeneration [8].
Mitochondrial protection and repair is mediated by the reducing agents, primary antioxidants and chaperones, antioxidant enzymes and specific protein repair systems. In the mitochondria all of the systems work in concert to protect against ROS-induced damage [8].
Reducing systems in the mitochondria employ electron donors such as glutathione (GSH), thioredoxin (Trx), NADPH, NADH, FADH2 and certain amino acids. Reducing equivalents act as important electron donors in order to maintain the redox status of a number of essential proteins and aid antioxidant enzyme systems. In the eye, GSH is a primary protectant of lens, cornea, and retina against ROS induced damage [8], [40]. The eye lens in particular contains high levels of reduced GSH, in cataractous lenses GSH has been shown to be depleted by up to 60% [31] while GSH levels have been shown to decrease with age particularly in the nucleus of the lens [8], [41].
A critical mitochondrial protective and repair system for the eye and other tissues employs repair enzymes that operate on protein sulfhydryl groups sensitive to oxidative stress. These moieties can easily conjugate with nonprotein thiols (S-thiolation) to form protein-thiol mixed disulfides. A number of systems exist to combat and/or repair this type of oxidative damage. The glutathione (GSH) system, consisting of reduced GSH, oxidized glutathione (GSSG) and a number of related enzymes, is the main redox control system of the cell. The GSH system detoxifies H2O2, dehydroascorbic acid and lipid peroxides and maintains protein thiols in a reduced state. Glutathione peroxidase reduces H2O2 to water with the concomitant oxidation of GSH to GSSG; GSH is maintained in its reduced form by the enzyme glutathione reductase in an NADPH dependent reaction [8]. A reduction in glutathione peroxidase and glutathione reductase activities has been observed in cataractous lenses, but this may be a consequence of lens injury rather than the cause [40]. Glutathione and the related enzymes belong to the defense system protecting the eye against chemical and oxidative stress. This review focuses on GSH and two key enzymes, glutathione reductase and glucose-6-phosphate dehydrogenase in lens, cornea, and retina. Lens contains a high concentration of reduced glutathione, which maintains the thiol groups in the reduced form [40]. These contribute to lens complete transparency as well as to the transparent and refractive properties of the mammalian cornea, which are essential for proper image formation on the retina. In cornea, glutathione also plays an important role in maintaining normal hydration level, and in protecting cellular membrane integrity. In retina, glutathione is distributed in the different types of retinal cells. Intracellular enzyme, glutathione reductase, involved in reducing the oxidized glutathione has been found at highest activity in human and primate lenses, as compared to other species. Besides the enzymes directly involved in maintaining the normal redox status of the cell, glucose-6-phosphate dehydrogenase which catalyzes the first reaction of the pentose phosphate pathway, plays a key role in protection of the eye against reactive oxygen species [40]. Another vital system acting on free and mixed disulfides in the lens is the thioltransferase system which uses reduced GSH for reduction of protein thiols to prevent disulfide bond formation and potentially protein aggregation. Mitochondrial thioltransferase or glutaredoxin 2 has been shown to protect against disruption of the mitochondrial transmembrane potential during oxidative stress in lens epithelial cells [42]. Thioltransferase (or Grx) belongs to the oxidoreductase family and is known to regulate redox homeostasis in cells.
Mitochondrial Grx2 is a recent discovery, but its function is largely unknown. Grx2 has a novel function as a peroxidase, accepting electrons both from GSH and thioredoxin reductase (TT) [43]. This unique property may play a role in protecting the mitochondria from oxidative damage. Xing and Lou [44] showed that overexpression of thioltransferase in HLE-B3 cells enhanced protection against H2O2 induced oxidative stress. These data indicate a new physiological function of TTase, which involves in the reactivation of the oxidatively inactivated enzymes through dethiolation; thus this redox-regulating enzyme can protect the human lens epithelial cells and maybe other cell types by preventing them from permanent oxidative damage [44]. The cited authors also showed that cellular thioltransferase activity was inhibited by addition of cadmium to the medium [44].
Thioredoxin 2 (Trx-2) is a small redox protein containing the thioredoxin active site Trp-Cys-Gly-Pro-Cys that is localized to the mitochondria by a mitochondrial leader sequence and encoded by a nuclear gene (Trx-2). Trx-2 plays an important role in cell viability and the regulation of apoptosis in vitro. The mitochondrial form of thioredoxin, thioredoxin 2 (Txn2), plays an important role in redox control and protection against ROS-induced mitochondrial damage. The mitochondrial thioredoxin system consists of thioredoxin 2 (Trx2) and thioredoxin reductase 2 (TrxR2) and also works to maintain mitochondrial proteins in their reduced state. Mitochondrial Trx 2 haploinsufficiency (a single functional copy of a gene, where insufficient product is produced) in mice was shown to reduce ATP production and increase ROS production [45], [46]. In addition, absence of Trx2 causes early embryonic lethality in mice [46].
Thioredoxin, like GSH, must also be maintained in its reduced state, the enzyme thioredoxin reductase reduces thioredoxin in a NADPH dependent reaction. NADPH, the source of which is the pentose phosphate pathway (PPP), also acts as a reducing agent for glutathione reductase, which maintains GSH in its reduced state. Under conditions of oxidative stress the PPP can increase NADPH production making it of key importance in cellular redox control [47]. NADH and FADH are formed during glycolysis, fatty acid oxidation and the citric acid cycle and are funneled into the electron transport chain of the mitochondria to act as electron donors. NADH transfers electrons to complex I of the electron transport chain while FADH2 transfers to complex II during the oxidation of succinate to fumarate.
Damage to the mitochondria of the lens epithelial cells may result in ROS production that is thought to affect the proteins of the underlying fiber cells [8]. The central retina mediates high acuity vision, and its progressive dysfunction due to macular degeneration is the leading cause of visual disability among adults in industrialized societies [48]. The retina is the most oxygen consuming tissue in the body with consumption level around 50% higher than the brain or kidneys [48]. In the retina, mitochondria are found throughout but the highest number of mitochondria per cell is found in the photoreceptors (reviewed in Ref. [8]).
Antioxidant enzymes present in the mitochondria work in concert with the reducing and protein repair systems and many have been shown to be crucial for protection against ROS mediated damage and cell death in the eye. Among the critical antioxidant enzymes that protect the cells against oxidative stress are superoxide dismutases: CuZnSOD (SOD 1) and MnSOD (SOD 2). The latter is also implicated in apoptosis [8], [49], [50], [51], [52]. MnSOD (SOD 2) in the mitochondrial matrix converts O2−• generated by the electron transport chain to hydrogen peroxide. Up and down-regulation of SOD 2 has been shown to be important for protection of lens epithelial cells against oxidative stress [8], [49]. Gene knockouts serve as useful experimental models to investigate the role of antioxidant enzymes in protection against oxidative stress in the lens. In the absence of gene knockout animals for Mn-containing superoxide dismutase (MnSOD), the effect of this enzyme on oxidative stress was investigated in a human lens epithelial cell line (SRA 01/04) in which the enzyme was up- or downregulated by transfection with sense and antisense expression vectors for MnSOD. These findings demonstrate the protective effect of MnSOD in antioxidant defense of cultured lens epithelial cells [49]. CuZnSOD (SOD 1) is present in the intermembrane space in some cells types, where it also converts O2−• to H2O2 permitting further diffusion into the cytosol [50]. Overexpression of SOD 1 in whole lens has been shown to prevent H2O2-mediated damage in the lens and thus was proposed to help prevent cataract, although this overexpression was not mitochondrial specific [51]. Reddy et al. [52] found that in SOD 2 deficient lens epithelial cells challenged with superoxide there were dramatic mitochondrial changes, cytochrome C leakage, caspase 3 activation and increased apoptotic death. The functional role of Sod2 in apoptosis was examined in cultured human lens epithelial cells [52]. Cells with higher enzyme levels were more resistant to the cytotoxic effects of H2O2, O2−• and UV-B radiation. Furthermore, SOD2-deficient cells showed dramatic mitochondrial damage, cytochrome C leakage, caspase 3 activation and increased apoptotic cell death when they were challenged with O2−•.
Thus, mitochondrial enzyme (SOD2) deficiency plays an important role in the initiation of apoptosis in the lens epithelium [52].
There are distinct mechanisms that execute apoptosis according to various different apoptotic stimuli, and these are classified into the mitochondria-dependent pathway (intrinsic pathway) and the death receptor-dependent pathway (extrinsic pathway). Previous studies have demonstrated the capacity of antioxidant protection of the mitochondria-dependent pathway associated with lens opacification in cultured lenses [7], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]. Mitochondrial damage results in the release of cytochrome c from the impaired mitochondria into the cytoplasm, which contributes to programmed cell death [57].
4. The role of molecular chaperone proteins in ocular tissues and for mitochondrial function
Mitochondria contribute significantly to the cellular production of reactive oxygen species (ROS). The deleterious effects of increased ROS levels have been implicated in a wide variety of pathological reactions. Apart from a direct detoxification of ROS molecules, protein quality control mechanisms are thought to protect protein functions in the presence of elevated ROS levels. The reactivities of molecular chaperones and proteases removes damaged polypeptides, maintaining enzyme activities, thereby contributing to cellular survival both under normal and stress conditions [58].
In addition to free radical scavengers chaperones are believed to be essential for mitochondrial function and to play important roles in eye disease. The mitochondrion contains two specific molecular chaperones, Hsp 70 and Hsp 60 [8]. While Hsp 60 appears to be particularly involved in protecting against protein misfolding, the house-keeping functions of the Hsp70 chaperones include transport of proteins between cellular compartments, degradation of unstable and misfolded proteins, prevention and dissolution of protein complexes, folding and refolding of proteins, uncoating of clathrin coated vesicles, and control of regulatory proteins [59]. The human heat shock protein 70 (Hsp70) family contains at least eight homologous chaperone proteins.
Endoplasmatic reticulum and mitochondria have their specific Hsp70 proteins, whereas the remaining six family members reside mainly in the cytosol and nucleus. These important proteins may provide a means of protecting damaged protein in the aging eye and may play a role in the pathogenesis of a number of maculopathies where protein aggregation appears to be the cause [8]. In addition to the large heat shock proteins, small heat shock proteins are also believed to play key roles. The small heat shock protein α-crystallin, which is the major protein of the mammalian lens, is an aggregate assembled from two polypeptides (αA and αB). Each polypeptide has a molecular weight around 20,000 kDa and these structural proteins are known to act as molecular chaperones [60]. Alpha A-crystallin (alpha A) and alpha B-crystallin (alpha B) are among the predominant proteins of the vertebrate eye lens. In vitro, the alpha-crystallins, which are isolated together as a high molecular mass aggregate, exhibit a number of properties, the most interesting of which is their ability to function as molecular chaperones for other proteins. Both proteins appear to have extensive nonlens roles [61]. Brady et al. [60] showed that in αA crystallin knockout mice the lenses were smaller than wild type and developed opacification in the nucleus of the lens that became widespread with age. Alpha A is essential for maintaining lens transparency, possibly by ensuring that alpha B or proteins closely associated with this small heat shock protein remain soluble [62]. In the retina, studies on RPE from wild type and αA and αB knockout mice showed that both proteins were localized to the mitochondria and that a lack of alpha crystallins rendered RPE more susceptible to apoptosis following oxidative stress [63]. Lack of alpha-crystallins renders RPE cells more susceptible to apoptosis from oxidative stress. Mitochondrial alpha-crystallins may play an important role in the protection from increased susceptibility of RPE in oxidative stress [63]. It may be that a loss of crystallin chaperone activity during oxidative stress may lead to RPE death and contribute to the pathogenesis of macular degeneration.
5. Reactive oxygen species and the mitochondria of vertebrate lenses
The key function of mitochondria is energy production through oxidative phosphorylation and lipid oxidation (Fig. 2F) [64]. The process takes place in the cellular structures of ocular tissues within the mitochondrial inner membrane and includes five multi-subunit enzyme complexes. Several other metabolic functions are performed by mitochondria including urea production, heme and non-heme iron biogenesis, steroid biogenesis, intracellular Ca2 + homeostasis, and interaction with the endoplasmic reticulum.
For many of these mitochondrial functions, there is only a partial understanding of the components involved, with even less information on mechanisms and regulation [65], [66].
Growth of the ocular lens is directed by the division and differentiation of a single layer of epithelial cells located at the equatorial region. It is conceivable that this region of the lens capsule presents a special microenvironment modulated by molecular cues emanating from the surrounding tissues [61]. General knowledge of the structure of mitochondria has paralleled the development of techniques for the preparation of fixed biological samples for electron microscopy. Early electron microscopy studies of mitochondria of vertebrate lenses showed an absence of mitochondria in lens fiber cells and the presence of very few, short mitochondria in the epithelial cells [61].
It became widely accepted that the lens epithelium plays the most important role in lens metabolism [67], [68], [69]. It was further proposed that ions and metabolites gain access to the fiber cells, which lack organelles, including mitochondria, via gap junctions connecting the closely apposed apical membranes of lens epithelial and superficial cortical fiber cells [69]. The permeability results suggest that the lens cells are capable of metabolic cooperation, mediated by an extensive gap junction network [69]. The hypothesis that normal fiber cell metabolism is maintained by epithelial cells has prompted numerous studies of the role of epithelial cell dysfunction and its role in lens damage.
In recent years the basic understanding of mitochondrial morphology and distribution has been enhanced by advances in confocal microscopy that permits imaging of living cells with the aid of fluorescent dye technology. Recent studies using specific fluorescent dyes and confocal microscopy of live chick [70], rat [56], and bovine lenses [71], [17] show that both the epithelial cells and the superficial cortical fiber cells of the lens contain numerous metabolically active mitochondria, suggesting that the superficial cortical fiber cells play a much more active role in lens metabolism than previously suspected. In order to elucidate the correlation between lens optical function and metabolic function, in vitro bovine lens optical quality and mitochondrial integrity was measured following treatment with carbonyl cyanide m-chlorophenylhydrazone (the mitochondrial depolarizing agent, CCCP). The results of this study indicate that lens optical function and mitochondrial integrity are closely correlated [71]. There appears to be little or no information regarding mitochondrial movement in the lens. The recent study, using confocal scanning laser microscopy, reports the dynamic movement of the mitochondria specific dye tetramethylrhodamine ethyl ester (TMRE) in the epithelium and superficial cortex of whole live bovine lenses [72]. The observed movement of TMRE across a mitochondrial network could represent change in the distribution of potential across the inner membrane, presumably allowing energy transmission across the cell from regions of low to regions of high ATP demand [72].
The recent recognition of mitochondria as an arbiter of the life and death of cells has drawn awareness to the need to develop antioxidants and other cytoprotective agents targeted to mitochondria. Physiologically, mitochondria perform a variety of key cellular vital regulatory processes, including ATP production, reactive oxygen species (ROS) generation and detoxification, and apoptosis [66].
The mitochondrial electron-transport chain is the main source of ROS during normal metabolism [68], [69]. The rate of ROS production from mitochondria is increased in a variety of pathologic conditions including hypoxia, aging, and chemical inhibition of mitochondrial respiration (reviewed in Ref. [73], [74], [75]).
Key to many oxidative stress conditions are alterations in the efficiency of mitochondrial respiration resulting in superoxide (O2−•) production [8]. Superoxide production precedes subsequent reactions that form potentially more dangerous reactive oxygen species (ROS) species such as the hydroxyl radical (OH•), hydrogen peroxide H2O2 and peroxynitrite (OONO−). The major source of ROS in the mitochondria, and in the cell overall, is leakage of electrons from complexes I and III of the electron transport chain. It is estimated that 0.2–2% of oxygen taken up by cells is converted to ROS, through mitochondrial superoxide generation, by the mitochondria [8]. Generation of superoxide at complexes I and III has been shown to occur at both the matrix side of the inner mitochondrial membrane and the cytosolic side of the membrane [8]. While exogenous sources of ROS such as UV light, visible light, ionizing radiation, chemotherapeutics, and environmental toxins may contribute to the oxidative milieu, mitochondria are perhaps the most significant contribution to ROS production affecting the aging process. In addition to producing ROS, mitochondria are also a target for ROS which in turn reduces mitochondrial efficiency and leads to the generation of more ROS in a vicious self-destructive cycle. Consequently, the mitochondria have evolved a number of antioxidant and key repair systems to limit the damaging potential of free oxygen radicals and to repair damaged proteins [8].
Complex I and complex III of the electron-transport chain are the major sites for ROS production [76], [77], [78]. The highest rate of superoxide (O2−•) generation in mitochondria not inhibited with any toxin is observed in complex I during the reverse transfer of electrons from succinate to NAD+ at the maximum proton potential (i.e. in the absence of ADP). This rate equal to 1 nmol O2/mg protein per minute is nearly fivefold higher than the rate of O2−• production in complex III under the same conditions [74]. It is 10% of the respiration rate without ADP (in state 4) and about 1.5% of the maximal respiration rate in the presence of ADP (in state 3). Even if this rate is lowered because of partial inhibition by NADH [74], a rather large amount of generated superoxide may be dangerous for the cell due to the high toxicity of O2−• and products formed from O2−•. In fact, we consider the mitochondria a potential generator of strong toxins and oxidants, which can easily kill the cells. This catastrophe will be caused not only by the direct toxic action of ROS, but as a consequence of triggering apoptosis and necrosis which are induced by ROS [79].
Complex I inhibition by rotenone can increase ROS generation in submitochondrial particles [77], [78], [80]. The oxidation of either complex I or complex II substrates in the presence of complex III inhibition with antimycin A increases ROS [80], [81], [82]. However, the major site of production of reactive oxygen species in mitochondria oxidizing complex I substrates remains unclear. For comparison, in the pathologic condition of myocardial ischemia, administration of rotenone decreases the production of ROS [83] and ameliorates damage to mitochondria during ischemia in the isolated rat heart [75]. These data suggest that rotenone inhibition of complex I decreases rather than increases ROS production by mitochondria during ischemia [75]. In summary, oxidation and reduction reactions clearly play a significant role in pathogenesis of eye disease, including onset of cataracts. Multiple mitochondrial antioxidant and repair systems work in concert to maintain transparency in the lens and retinal function.
6. Oxidative stress-induced damages in glaucoma
Glaucoma is the main cause of irreversible blindness worldwide. This disease is characterized by apoptosis of retinal ganglion cells (RGC) and visual field loss that seems to be related to elevated intraocular pressure (IOP). Several lines of evidences have implicated the crucial role of mitochondrial dysfunction in the pathogenesis of glaucoma. Increased mitochondrial oxidative stress in RGC may underlie or contribute to susceptibility of RGC to apoptosis.
The molecular basis of POAG is mostly unknown. Several theories of its pathogenesis have been proposed, including mechanic and ischemic ones (reviewed in ref. [84]). The etiology of these conditions is thought to fit with the ‘free radical theory’ of aging which postulates that aging and age-related diseases result from the accumulation of cellular damage from ROS. Oxidation–reduction mechanisms have special importance in the eye. Oxidative damage can result in a number of molecular changes that contribute to the development of glaucoma, cataract, and other eye diseases [85], [86]. If the free radical theory of aging is applied to the eye, an altered antioxidant/oxidant balance should be evident for age-related ocular diseases, such as age-related macular degeneration, cataract, and glaucoma [87].
The studies investigating the relation between POAG, oxidant stress, and antioxidant systems were carried out at the tissue and cellular levels. ROS are generated as by-products of cellular metabolism, primarily in the mitochondria. Although ROS are essential participants in cell signaling and regulation, when their cellular production overwhelms the intrinsic antioxidant capacity, damage to cellular macromolecules such as DNA, proteins, and lipids ensues. Such a state of “oxidative stress” is thought to contribute to the pathogenesis of a number of neurodegenerative diseases. Growing evidence supports the involvement of oxidative stress as a common component of glaucomatous neurodegeneration in different subcellular compartments of retinal ganglion cells (RGCs) (Fig. 3). Besides the evidence of direct cytotoxic consequences leading to RGC death, it also seems highly possible that ROS are involved in signaling RGC death by acting as a second messenger and/or modulating protein function by redox modifications of downstream effectors through enzymatic oxidation of specific amino acid residues. Different studies provide cumulating evidence, which supports the association of ROS with different aspects of the neurodegenerative process. Oxidative protein modifications during glaucomatous neurodegeneration increase neuronal susceptibility to damage and also lead to glial dysfunction. Oxidative stress-induced dysfunction of glial cells may contribute to spreading neuronal damage by secondary degeneration. Oxidative stress also promotes the accumulation of advanced glycation end products in glaucomatous tissues. In addition, oxidative stress takes part in the activation of immune response during glaucomatous neurodegeneration, as ROS stimulate the antigen presenting ability of glial cells and also function as co-stimulatory molecules during antigen presentation [88]. The trabecular meshwork (TM) plays an important role in primary open-angle glaucomas. Indeed, the TM is the ocular tissue structure, through which the aqueous humor flows from the anterior chamber to Schlemm's canal and collecting channels. Until recently, the TM, which is constituted by endothelial-like cells, was described as a kind of passive filter. The cells delineating the structures of the collagen framework of the TM are endowed with a cytoskeleton, and are thus able to change their shape. These cells also have the ability to secrete the extracellular matrix, which expresses proteins, such as fibronectin, thrombospondin and cytokines, and are capable of phagocytosis and autophagy. The cytoskeleton is attached to the nuclear membrane and can, in millionths of a second, send signals to the nucleus in order to alter the expression of genes in an attempt to adapt to biomechanical insult. Oxidative stress, as happens in aging, has a deleterious exaggerating effect on the TM, leading eventually to cell decay, tissue malfunction, subclinical inflammation, changes in the extracellular matrix and cytoskeleton, altered motility, reduced outflow facility and (ultimately) increased intraocular pressure (IOP). TM failure in primary open-angle glaucoma is the most relevant factor in the cascade of events characteristic to glaucoma disease triggering apoptosis in the inner retinal layers, including ganglion cells [89], [90], [91].
Fig. 3.

Primary open-angle glaucoma. The glaucomas are a group of chronic progressive optic neuropathies that have in common characteristic morphologic changes at the optic nerve and retinal nerve fiber layer.
Factors associated with the glaucomas
-
•Unphysiologic intraocular pressure
-
•Aqueous outflow restrictions
-
•Abnormal ocular perfusion
-
•Abnormal rates of apoptosis
-
•Other factors
Glaucomatous optic atrophy
-
•Changes in coloration
-
•Enlargement of the optic cup
-
•Wipeout of the neuroretinal rim
-
•Fallout of the retinal nerve fiber layer.
7. Targeting mitochondria during therapeutic treatment of cataracts and cellular structures of tissues in anterior eye chamber
The major function of mitochondria in human cells is to provide ATP by oxidative phosphorylation. However, mitochondria have many other roles including the modulation of intracellular calcium concentration and the regulation of apoptotic cell death. Furthermore, the mitochondrial respiratory chain is a major source of damaging free radicals [92]. Therefore, strategies to prevent mitochondrial damage or to manipulate mitochondrial function in ophthalmic clinically useful ways may provide new therapies for a range of human disorders, including age-related cataracts. Accordingly, mitochondria are a potentially important target for drug delivery and there are several technologies developed to deliver bioactive molecules selectively to mitochondria within cells.
The above sections of the article demonstrate that mitochondrial oxidative damage contributes to a range of ocular sight threatening degenerative diseases, including cataracts. Consequently, the selective inhibition of mitochondrial oxidative damage is a promising therapeutic strategy. One way to do this is to invent antioxidants that are selectively accumulated into mitochondria within ophthalmic patients, including those disabled with age-related cataracts. In addition, because mitochondria play a role in many critical cell processes, mitochondrial targeting has the potential to protect, repair or kill the cells selectively, and may become a key tool in the development of gene therapy for mitochondrial DNA diseases [93].
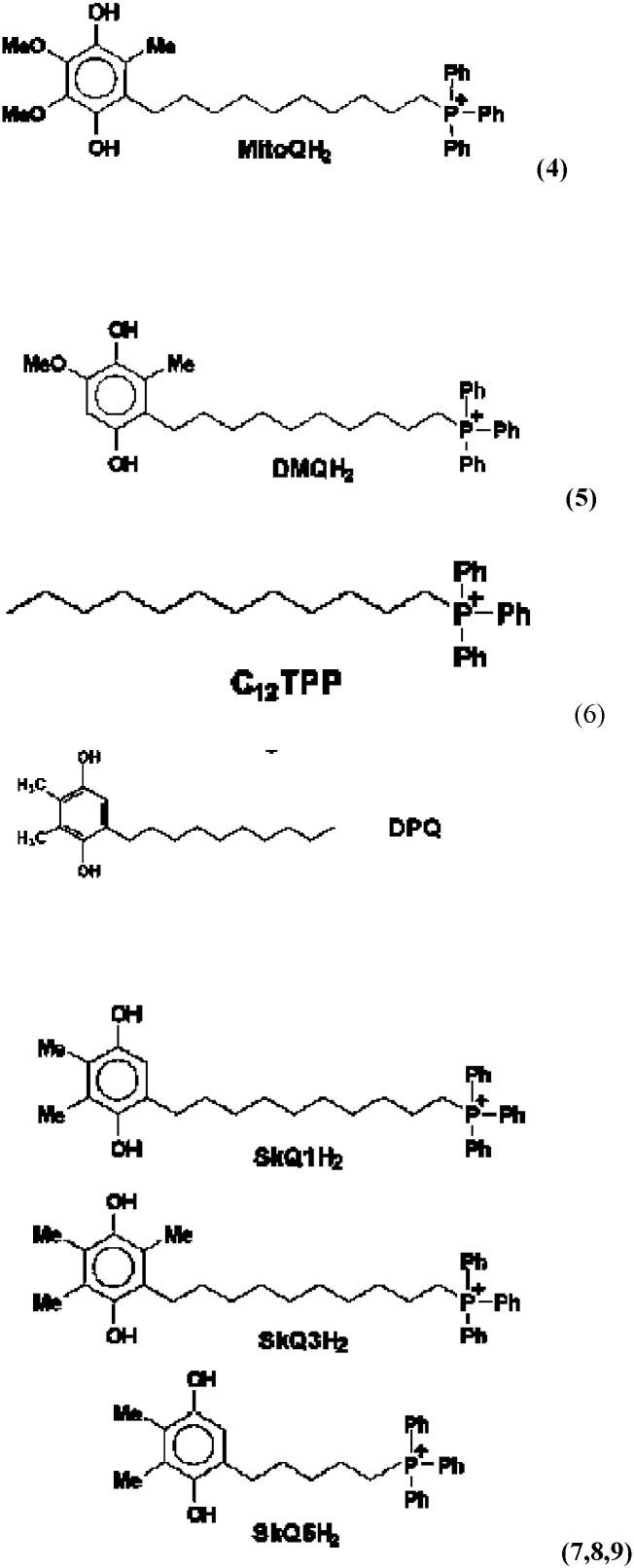
In the past ten years, the search for new protective remedies against damage caused by excessive free radical formation in mitochondria has accelerated. Similar to our body's and organ's own natural defenses against ROS, research has been primarily focused on molecules combining antioxidant utilities with recycling capacities [94], [95]. Incomplete scavenging of ROS and RNS particularly affects the mitochondrial lipid cardiolipin (CL), triggers the release of mitochondrial cytochrome c, and activates the intrinsic death pathway [95]. Due to the active redox environment and the excess of NADH and ATP at the inner mitochondrial membrane, a broad range of agents including electron acceptors, electron donors, and hydride acceptors can be used to influence the biochemical pathways. The key to therapeutic value is to enrich selective redox modulators at the target sites [95]. Large doses of antioxidants proved ineffective at preventing oxidative damage in animal disease models, presumably because the antioxidants and proteins such as manganese superoxide dismutase (MnSOD) cannot penetrate cell membranes effectively and therefore do not reach the relevant sites of ROS and RNS generation. One solution to this general problem is to attach a molecule with antioxidant properties onto a vehicle that can penetrate both the cellular and outer mitochondrial membranes and thereby deliver the “payload” to a site where it can scavenge ROS and ameliorate oxidative damage (Fig. 4). Since the mitochondrial membrane spans across a negative potential, most agents have a positively charged moiety that takes advantage of electrostatic forces in locating its target [95]. Alternative chemistry-based approaches to targeting mitochondria include the use of proteins and peptides, as well as the attachment of payloads to lipophilic cationic compounds, sulfonylureas, anthracyclines, and other agents with proven or hypothetical affinities for mitochondria. Manganese superoxide dismutase (MnSOD), SS tetrapeptides with 2′,6′-dimethyltyrosine (Dmt) residues, rhodamine, triphenylphosphonium salts, nonopioid analgesics, adriamycin, and diverse electron-rich aromatics and stilbenes were used to influence mitochondrial biochemistry and the biology of aging. Some general structural principles for effective therapeutic agents are now emerging. Among these are the presence of basic or positively charged functional groups, hydrophobic substructures, and, most promising for future selective strategies, classes of compounds that are actively shuttled into mitochondria, bind to mitochondria-specific proteins, or show preferential affinity to mitochondria-specific lipids [95].
Fig. 4.
Concept of targeting mitochondria with functional agents (1–9) that use a vehicle to deliver an ROS scavenging payload into mitochondria (for details, see Section 7 in the text).
Mitochondrial function can be manipulated selectively by targeting bioactive compounds to mitochondria in living cells. Current targeting mechanisms involve harnessing either the mitochondrial membrane potential or the mitochondrial protein-import machinery to take up molecules linked to lipophilic cations or mitochondrial signal peptides, respectively. One solution to this general problem is to attach a molecule with antioxidant properties onto a vehicle that can penetrate both the cellular and outer mitochondrial membranes and thereby deliver the “payload” to a site where it can scavenge ROS and ameliorate oxidative damage (Fig. 3). Since the mitochondrial membrane spans across a negative potential, most agents have a positively charged moiety that takes advantage of electrostatic forces in locating its target. The use of lipophilic cations as selective targeting agents has been explored to capitalize on this physiological phenomenon. Research included the series of papers published in 1969–1970, where mitochondria-addressed penetrating synthetic cations were described and the idea to use these cations as “electric locomotives” targeting non-charged compounds to mitochondria was put forward [96], [97]. Such mitochondria-targeted antioxidants have been developed by conjugating the lipophilic triphenylphosphonium cation to an antioxidant moiety, such as ubiquinol or alpha-tocopherol. Furthermore, because of their positive charge they are accumulated several-hundredfold within mitochondria driven by the membrane potential, enhancing the protection of mitochondria from oxidative damage [98]. These compounds pass easily through all biological membranes, including the ocular-blood barrier, and thus reach those ocular tissues most affected by mitochondrial oxidative damage. Murphy and coworkers initiated the practical realization of the theoretical concept of mitochondria-targeted antioxidants [92], [93], [97], [98], [99]. They synthesized and tested just a few of mitochondria-targeted antioxidants conjugated to the lipophilic alkyltriphenylphosphonium cations.
Based on the published studies, the ubiquinone moiety linked to triphenylphosphonium cation by C10 aliphatic chain, MitoQ (Fig. 3), seemed to be one of the promising therapeutic modalities for the treatment of eye diseases [98], [99], [100]. In 2006, an attempt was undertaken in Skulachev's group to replace the ubiquinone moiety in MitoQ by plastoquinone. As a result, a series of mitochondria-targeted antioxidants named SkQ has been synthesized [79], [101]. It was reported [102], [103], [104] that the reactivity of the “tailless” plastoquinol analogs to the peroxyl radicals was higher than that of natural ubiquinols. The technique based on monitoring oxygen consumption was applied to study 12 alkyl- and methoxy-substituted p-hydroquinones (QH(2)) as a chain-breaking antioxidant during the oxidation of styrene and methyl linoleate (ML) in bulk as well as ML oxidation in micellar solution of sodium dodecyl sulfate (SDS) at 37 °C. The antioxidant activities of QH(2) were characterized by two parameters: the rate constant k(1) for reaction of QH(2) with the peroxy radical LO(2)*:QH(2) + LO(2)* → QH* + LOOH and the stoichiometric factor of inhibition, f, which shows how many kinetic chains may be terminated by one molecule of QH(2) [103]. The features of QH(2) as an antioxidant in aqueous environment are suggested to associate with the reactivity of semiquinone (Q*(−)). Q*(−) reacts readily with molecular oxygen with formation of superoxide (O(2)*(−)); further reactions of O(2)*(−) result in fast depleting QH(2) and chain propagation. The addition of SOD results in purging a reaction mixture from O(2)*(−) and, as a corollary, in depressing undesirable reactions with the participation of O(2)*(−). With all the oxidation models, QH(2) were found to be very reactive to LO(2)* [103]. All the tested QH(2) displayed a pronounced antioxidant activity. The oxidized forms of the same compounds did not inhibit ML peroxidation. The value of k(1) for SkQH(2) far exceeded k(1) for MitoQH(2). For the biologically active geroprotectors SkQ1H(2), the k(1) value found to be as high as 2.2 × 10(5) M(−) (1)s(−) (1), whereas for MitoQH(2), it was 0.58 × 10(5) M(−) (1)s(−) (1). The kinetic behavior of QH(2) suggested that SkQ1H(2) can rather easily diffuse through lipid–water microheterogeneous systems [105].
Mitochondria-targeted cationic plastoquinone derivative SkQ1 (10-(6′-plastoquinonyl) decyltriphenylphosphonium) has been investigated as a potential tool for treating a number of ROS-related ocular diseases [106]. In OXYS rats suffering from a ROS-induced progeria, very small amounts of SkQ1 (50 nmol/kg per day) added to food were found to prevent development of age-induced cataract and retinopathies of the eye, lipid peroxidation and protein carbonylation in skeletal muscles, as well as a decrease in bone mineralization. Instillation of drops of 250 nM SkQ1 reversed cataract and retinopathies in 3–12-month-old (but not in 24-month-old) OXYS rats [106]. In ex vivo studies of cultivated posterior retina sector, it was found that 20 nM SkQ1 strongly decreased macrophagal transformation of the retinal pigmented epithelial cells, an effect which might explain some of the above SkQ1 activities. It is concluded that low concentrations of SkQ1 are promising in treating retinopathies, cataract, uveitis, glaucoma, and some other ocular diseases [106].
The concentration and distribution of oxygen within the lens is of considerable interest clinically. In age-related cataracts, cytosolic and membrane components are subjected to free-radical induced oxidation extensively. The identity of the oxidant(s) responsible has not been established unequivocally. Because oxidative damage plays a key role in cataract pathology, it has been suggested that possible hypoxic damage would have to be carefully controlled in the lens. It is important to note that about half of the lens is made up of differentiating fibers with what is considered a normal сomplement of organelles [107], [87]. Therefore the maintenance of healthy mitochondria is critical to prevent lens damage during hypoxia. Oxidized phospholipids play an important role in execution of the mitochondrial stage of apoptosis and clearance of apoptotic cells. During the lipid peroxidation (LPO) reaction, lipid hydroperoxides are formed as primary products. Several lines of evidence suggest that lipid hydroperoxides can trigger cell death in many cell types, which may be mediated by mitochondria dysfunction pathway. With extended hyperoxic insult, the oxidants overwhelm the antioxidant defense system and eventually cell death ensues [108]. ROS generation correlated inversely with mitochondrial membrane potential and the amount of cardiolipin, factors likely to contribute to loss of cell viability [108].
The critical role of mitochondria in programmed cell death leads to the design of mitochondriotropic agents as a strategy in regulating apoptosis. According to the new IVP mitochondrion concept, mitochondria do not exist stably as distinct, individual, autonomous organelles [108], [109], [110], [111], [112]. Rather, mitochondria form a network within cells; their continuous fusion and fission is a highly dynamic process, adapting to the role the mitochondrion actually has in the cell. Increasing results confirm the role of mitochondrial fission and fragmentation in most forms of apoptosis, even as a cause. This suggests that fragmented mitochondria are in a “bad” condition, under oxidative stress. To overcome this problem, we developed natural forms of mitochondria-targeted antioxidants (Fig. 3 (1)), non-hydrolyzed carnosine associated with the ophthalmic carrier typified for topical use (N-acetylcarnosine lubricant eye drops) (major proposed administration way), ocular injection (intra-vitreal, subconjunctival, parabulbar) or even oral administration (synergistic administration with chaperones and reduced glutathione synthesis booster) [109], [110], [111], [112].
The effect of carnosine on self-organization of mitochondrial assemblies was studied in rat liver homogenate of quiescent and excited animals. It was shown in separate electron microscopy experiments with serial slices that under our conditions of preparation of homogenate, blocks of native mitochondrial–reticular network in the cell, assemblies of mitochondria, are kept. Carnosine was shown to prevent dissociation of assemblies during storage. Its effect is maximal for more dissociated assemblies from excited animals with decreased ability for self-organization. Prevention of disassembly of organelles by carnosine can serve as one of the mechanisms of carnosine-induced diminishing of muscle fatigue under prolonged work [113]. l-Carnosine prevented both 12-O-tetradecanoylphorbol-13-acetate (TPA)- and H2O2-induced DNA fragmentation, the loss of mitochondrial membrane potentials and blocked the release of cytochrome c into cytosol. Subsequently, the cleavages of poly (ADP-ribose) polymerase were significantly reduced in l-carnosine-treated cells. However, western blotting analysis revealed that p53 protein level did not change for 12 h after TPA- and H2O2-treatment.
Therefore, these results suggested that l-carnosine, an antioxidant, protected both H2O2- and TPA-induced apoptosis through mitochondrial pathways [114]. Carnosine addition to mitochondria or its accumulation in mitochondria under hypoxia is associated with activation of alpha-ketoglutarate oxidation and its formation through transamination [115].
Recently, phospholipid peroxidation products gained a reputation as key regulatory molecules and participants in oxidative signaling pathways. Oxidation of two anionic phospholipids – cardiolipin (CL) in mitochondria and phosphatidylserine (PS) in extramitochondrial compartments – is important signaling event, particularly during the execution of programmed cell death and clearance of apoptotic cells. Quantitative analysis of CL and PS oxidation products is central to understanding their molecular mechanisms of action [116], [117]. Furthermore, specific characteristics of CL in mitochondria – its asymmetric transmembrane distribution and mechanisms of collapse, the regulation of its synthesis, remodeling, and fatty acid composition – are given significant consideration [117], [118]. Cytochrome c (cyt c) acts as a CL-specific peroxidase very early in apoptosis. At this stage, the hostile events are still secluded within the mitochondria and do not reach the cytosolic targets. CL oxidation process is required for the release of pro-apoptotic factors into the cytosol. Manipulation of cyt c interactions with CL, inhibition of peroxidase activity, and prevention of CL peroxidation are prime targets for the discovery of anti-apoptotic drugs acting before the “point-of-no-return” in the fulfillment of the cell death program. During apoptosis, a mitochondria-specific phospholipid, cardiolipin (CL), interacts with cytochrome c (cyt c) to form a peroxidase complex that catalyzes CL oxidation; this process plays a pivotal role in the mitochondrial stage of the execution of the cell death program. Several works were focused on redox mechanisms and essential structural features of cyt c's conversion into a CL-specific peroxidase that represent an interesting and may be still unique example of a functionally significant ligand change in hemoproteins [116], [117].
Recently, it was demonstrated that peroxidase cyt c/CL complexes can utilize free fatty acid hydroperoxides (FFA-OOH) at exceptionally high rates that are approximately 3 orders of magnitude higher than for H2O2 [116].
Accordingly, the new concepts in drug discovery based on the design of mitochondria-targeted inhibitors of cyt c/CL peroxidase and CL peroxidation with antiapoptotic effects have appeared. Therefore, mitochondria-targeted disruptors and inhibitors of cyt c/CL peroxidase complexes and suppression of CL peroxidation represent new strategies in anti-apoptotic drug discovery [119]. We have originally discovered that both carnosine and carcinine (10–25 mM) are capable of inhibiting the catalysis of linoleic acid and phosphatidylcholine liposomal peroxidation (LPO) by the O2−•-dependent iron-ascorbate and lipid-peroxyl-radical-generating linoleic acid 13-monohydroperoxide (LOOH)-activated hemoglobin systems, as measured by thiobarbituric-acid-reactive substance. Carcinine and carnosine are good scavengers of OH• radicals, as detected by iron-dependent radical damage to the sugar deoxyribose. This suggests that carnosine and carcinine are able to scavenge free radicals or donate hydrogen ions. The iodometric, conjugated diene and t.l.c. assessments of lipid hydroperoxides (13-monohydroperoxide linoleic acid and phosphatidylcholine hydroperoxide) showed their efficient reduction and deactivation by carnosine and carcinine (10–25 mM) in the liberated and bound-to-artificial-bilayer states. This suggests that the peroxidase activity exceeded that susceptible to direct reduction with glutathione peroxidase. Imidazole, solutions of beta-alanine, or their mixtures with peptide moieties did not show antioxidant potential [120]. Due to the combination of weak metal chelating (abolished by EDTA), OH• and lipid peroxyl radicals scavenging, reducing activities to liberated fatty acid and phospholipid hydroperoxides, carnosine and carcinine appear to be physiological antioxidants able to efficiently protect the lipid phase of biological membranes and aqueous environments and act as the anti-apoptotic natural drug compounds. Removal of excessive mitochondrial reactive oxygen species by electron scavengers and antioxidants is a promising therapeutic strategy to reduce the detrimental effects of UV radiation and other cataractogenic factors exposure. Local mitochondrial interaction is mediated by superoxide (O2−•) diffusion and the O2−•-dependent activation of an inner membrane anion channel (IMAC). In contrast to other mitochondria-targeted antioxidants studied by Mitchel and Skulachev's groups, it is important that l-carnosine can scavenge superoxide anion radical released from mitochondria in the outside compartments of the cells. Even at low concentrations, dipeptide carnosine forms a charge-transfer complex (Car … O2−•, lambda max = 265 nm) with the superoxide radical which changes the reactivity of O2−•. The absorbance band of the complex was shifted towards lower energy as compared to superoxide radical lambda max = 255 nm). The interaction of carnosine with OH-radicals proceeding at very high rate and resulting in the formation of a stable product suggested another type of dipeptide activity [121].
The IVP patented concept includes the integrated lens and ocular tissues mitochondrial targeting with triphenyl-phosphonium (TPP)-conjugated hydrophobic compounds combined with l-carnosine ophthalmic prodrug N-acetylcarnosine (Fig. 4), represents a promising approach for the development of novel eye and specifically, cataract universal antioxidant protectors [109], [110].
8. Conclusion
In addition to the well-established role of the mitochondria in energy metabolism, regulation of cell death has recently emerged as a second major function of these organelles. This, in turn, seems to be intimately linked to their role as the major intracellular source of reactive oxygen species (ROS) which are mainly, generated at complex I and III of the respiratory chain. Excessive ROS production can lead to oxidation of macromolecules and has been implicated in mtDNA mutations, aging, and cell death. Although mitochondrial dysfunction can cause ATP depletion and necrosis, these organelles are also involved in the regulation of apoptotic cell death by mechanisms, which have been conserved through evolution [122].
A great deal of research indicates that dysfunctional mitochondria are the primary site of ROS [122], [123], [124]. More than 95% of O2−• produced during normal metabolism is generated by the electron transport chain in the inner mitochondrial membrane. Ironically, mitochondria are also the major target of ROS [122]. Mitochondrial DNA may be more susceptible to oxidative damage than to nuclear DNA [125] because of its proximity to electron transport chains, lack of protective histones, and insufficient repair mechanisms [126]. Such damage to the mitochondrial DNA may contribute to the age-associated decline in mitochondrial function. Furthermore, mitochondrial DNA encodes several subunits of different respiratory complexes [127]. The essential role of mitochondrial oxidative phosphorylation in cellular energy production, the generation of reactive oxygen species, and the initiation of apoptosis has suggested a number of novel mechanisms for mitochondrial pathology in sight-threatening eye diseases, including age-related cataract formation. ROS-induced DNA damage may lead to the production of more ROS, thus perpetuating a vicious cycle. Following publication of these data, it was suggested that penetrating cations can be used as “molecular electric locomotives” to target uncharged substances attached to these cations specifically to mitochondria [97], [128].
As reported in the preceding studies (reviewed in Refs. [106], [129]), very small amounts of mitochondria-targeted plastoquinone derivatives (SkQs) show pronounced antioxidant effects on model membranes, isolated mitochondria, and cell cultures. In the latter case, strong antiapoptotic and antinecrotic effects were observed in situations when cell death was induced or mediated by reactive oxygen species (ROS) (reviewed in Refs. [106], [129]). N-acetylcarnosine eye-drops have been shown to have measurable affects to prevent and partially reverse cataracts within only 3–6-month of topical ocular use [112], [124]. However, it is recommended that for maximum efficacy, that administration be continued for a period not less than 3–5 months. In addition, the drops' effectiveness is increased the sooner they are used after a cataract is detected.
Also, considering that senile cataracts are an on-going aging disorder, N-acetylcarnosine may be required on a regular basis to help maintain the eye's natural anti-oxidant defenses. Other than senile cataract, N-acetylcarnosine may have other benefits. The unique N-acetylcarnosine formula with its added and synergistic lubricants, could also provide beneficial results with the following eye-disorders [112], [124]:
-
•
Presbyopia
-
•
Open-angle primary glaucoma (in combination with beta-blockers)
-
•
Corneal disorders
-
•
Computer vision syndrome
-
•
Eye strain
-
•
Ocular inflammation
-
•
Blurred vision
-
•
Dry eye syndrome
-
•
Retinal diseases
-
•
Vitreous opacities and lesions
-
•
Complications of diabetes mellitus and other systemic diseases
-
•
Contact lens difficulties, particularly with soft contact lenses. (Not only do the lubricants in the Can-C N-acetylcarnosine eye-drop help to make wearing contact lenses more comfortable, but n-acetylcarnosine is also believed to reduce the buildup of lactic acid in the eye, thus enabling the lens to be left safely in the eye for longer).
There are numerous indications of a crucial role of ROS in the main age-related ocular pathologies, i.e. retinopathies (age-related macular degeneration [130], [131], retinitis pigmentosa [132], [133], hereditary optic neuropathy [134]), as well as glaucoma [135], [136], [137], cataract [112], [138], [139], and autoimmune uveitis [140], [141]. Polyunsaturated fatty acids in mitochondrial cardiolipin are first of all attacked by mitochondria produced ROS that are quenched by the considered natural and synthetic mitochondria-targeted antioxidants (Fig. 4) [112], [142]. Mitochondria-targeted antioxidants might be used to effectively prevent ROS-induced oxidation of lipids and proteins in the inner mitochondrial membrane in vivo [142].
This review article demonstrates that mitochondria are an important source of toxic oxygen radicals in the onset of age-related cataracts and confirms new approaches for treating cataracts using mitochondria-targeted antioxidants (Fig. 5).
Fig. 5.
Human cataract reversal within 5 months of treatment with 1% N-acetylcarnosine lubricant eye drops (carrier ophthalmic system equipped with corneal absorption promoter for natural mitochondria-targeted antioxidant l-carnosine). Upper image: The lens before treatment; lower image: after 5 months of daily treatment with 1% N-acetylcarnosine lubricant eye drops (for details, see Ref. [124]).
Mitochondria are usually either the primary source of ROS [143] or mediators of burst of ROS formation in other cellular compartments (“ROS-induced ROS release” [142], [144]). This is why it is reasonable to combine the N-acetylcarnosine lubricant eye drops acting as the ophthalmic prodrug of naturally targeted to mitochondria l-carnosine endowed with pluripotent types of antioxidant activities with mitochondria-targeted rechargeable antioxidant (see a number of compounds presented in Fig. 3) as a medicine to treat ocular diseases [109], [110], [111], [112], [120], [124].
We conclude that mitochondrial targeting of compounds with universal of antioxidant activities represents a promising approach for the development of novel therapeutic protectors of the aging eye and can be implicated in the management of cataracts.
Conflict of interest
Declaration of interest: The author reports the interest in the intellectual property of the described modalities protected with the patents. The author bears primary responsibility for accuracy of made statements and employment of the described products and for the content and writing of the paper.
Transparency Document
Transparency document.
Acknowledgments
This work was planned, organized, and supported by Innovative Vision Products, Inc. (County of New Castle, DE, USA). Innovative Vision Products Inc. is a pharmaceutical and nanotechnology development company with a focus on innovative chemical entities, drug delivery systems, and unique medical devices to target specific biomedical applications. Over the last decade IVP has developed a track record in developing these technologies to effectively address the unmet needs of specific diseased populations. The therapeutic modalities described in details in the text and the content of the current submission have been specifically patented by the author (Dr. Mark A. Babizhayev) with the list of the International PCT patent applications worldwide available and US Patents allowed and pending: WO 94/19325; WO 95/12581; WO 2004/064866 A1. Babizhayev, M.A. and Meguro, K., 2004. Combined use of carnosinase inhibitor with l-carnosines and composition. WO 2004/064866 PCT/JP2004/000351. Filed 20 January 2003 (20.01.2003).
Footnotes
The eyes are one of the most powerful tools a man can have. With one look, one can relay the most intimate message. After the connection is made, words cease to exist.
The Transparency document associated with this article can be found, in the online version.
References
- 1.Bochkov V.N., Oskolkova O.V., Birukov K.G., Levonen A.L., Binder C.J., Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson N.C. Specificity and binding affinity of phospholipids to the high-affinity cardiolipin sites of beef heart cytochrome c oxidase. Biochemistry. 1982;21:184–188. doi: 10.1021/bi00530a031. [DOI] [PubMed] [Google Scholar]
- 3.Cheneval D., Carafoli E. Identification and primary structure of the cardiolipin-binding domain of mitochondrial creatine kinase. Eur. J. Biochem./FEBS. 1988;171:1–9. doi: 10.1111/j.1432-1033.1988.tb13750.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou M., Morgner N., Barrera N.P., Politis A., Isaacson S.C., Matak-Vinkovic D., Murata T., Bernal R.A., Stock D., Robinson C.V. Mass spectrometry of intact V-type ATPases reveals bound lipids and the effects of nucleotide binding. Science. 2011;334:380–385. doi: 10.1126/science.1210148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann B., Stockl A., Schlame M., Beyer K., Klingenberg M. The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J. Biol. Chem. 1994;269:1940–1944. [PubMed] [Google Scholar]
- 6.Resnikoff S., Pascolini D., Etya'ale D., Kocur I., Pararajasegaram R., Pokharel G.P., Mariotti S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 7.Yao K., Ye P., Zhang L., Tan J., Tang X., Zhang Y. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol. Vis. Jan 31 2008;14:217–223. [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan L.A., Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye Res. Feb 2009;88(2):195–203. doi: 10.1016/j.exer.2008.05.018. (Epub 2008 Jun 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saccà S.C., Roszkowska A.M., Izzotti A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat. Res. Apr-Jun 2013;752(2):153–171. doi: 10.1016/j.mrrev.2013.01.001. (Epub 2013 Jan 19) [DOI] [PubMed] [Google Scholar]
- 10.Truscott R.J. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. May 2005;80(5):709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Spector A. Review: oxidative stress and disease. J. Ocul. Pharmacol. Ther. Apr 2000;16(2):193–201. doi: 10.1089/jop.2000.16.193. [DOI] [PubMed] [Google Scholar]
- 12.Spector A. The search for a solution to senile cataracts. Proctor lecture. Invest. Ophthalmol. Vis. Sci. Feb 1984;25(2):130–146. [PubMed] [Google Scholar]
- 13.Babizhayev M.A., Deyev A.I., Linberg L.F. Lipid peroxidation as a possible cause of cataract. Mech. Ageing Dev. 1988 Jul;44(1):69–89. doi: 10.1016/0047-6374(88)90080-2. [DOI] [PubMed] [Google Scholar]
- 14.Babizhayev M.A., Deyev A.I., Yermakova V.N., Brikman I.V., Bours J. Lipid peroxidation and cataracts: N-acetylcarnosine as a therapeutic tool to manage age-related cataracts in human and in canine eyes. Drugs R&D. 2004;5(3):125–139. doi: 10.2165/00126839-200405030-00001. [DOI] [PubMed] [Google Scholar]
- 15.Estrada R., Puppato A., Borchman D., Yappert M.C. Reevaluation of the phospholipid composition in membranes of adult human lenses by (31)P NMR and MALDI MS. Biochim. Biophys. Acta. Mar 2010;1798(3):303–311. doi: 10.1016/j.bbamem.2009.11.008. (Epub 2009 Nov 17) [DOI] [PubMed] [Google Scholar]
- 16.Huang L., Grami V., Marrero Y., Tang D., Yappert M.C., Rasi V., Borchman D. Human lens phospholipid changes with age and cataract. Invest. Ophthalmol. Vis. Sci. May 2005;46(5):1682–1689. doi: 10.1167/iovs.04-1155. [DOI] [PubMed] [Google Scholar]
- 17.McAvoy J.W. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J. Embryol. Exp. Morpholog. 1978;45:271–281. [PubMed] [Google Scholar]
- 18.Bantseev V., McCanna D., Banh A., Wong W.W., Moran K.L., Dixon D.G., Trevithick J.R., Sivak J.G. Mechanisms of ocular toxicity using the in vitro bovine lens and sodium dodecyl sulfate as a chemical model. Toxicol. Sci. 2003 May;73(1):98–107. doi: 10.1093/toxsci/kfg060. Epub 2003 Apr 15. [DOI] [PubMed] [Google Scholar]
- 19.Andley U.P., Spector A. Peroxide resistance in human and mouse lens epithelial cell lines is related to long-term changes in cell biology and architecture. Free Radic. Biol. Med. 2005 Sep 15;39(6):797–810. doi: 10.1016/j.freeradbiomed.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen P.L. Mitochondrial events in the life and death of animal cells: a brief overview. J. Bioenerg. Biomembr. 1999 Aug;31(4):291–304. doi: 10.1023/a:1005453700533. [DOI] [PubMed] [Google Scholar]
- 21.Ott M., Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007 May;12(5):913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 22.Kagan V.E., Tyurin V.A., Jiang J., Tyurina Y.Y., Ritov V.B., Amoscato A.A., Osipov A.N., Belikova N.A., Kapralov A.A., Borisenko G.G. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalvez F., Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 24.Ott M., Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 25.Ott M., Robertson J.D., Gogvadze V., Zhivotovsky B., Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagan V.E., Borisenko G.G., Tyurina Y.Y., Tyurin V.A., Jiang J., Potapovich A.I., Kini V., Amoscato A.A., Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic. Biol. Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Petrosillo G., Ruggiero F.M., Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003 Dec;17(15):2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 28.Ottonello S., Foroni C., Carta A., Petrucco S., Maraini G. Oxidative stress and age-related cataract. Ophthalmologica. 2000 Jan-Feb;214(1):78–85. doi: 10.1159/000027474. [DOI] [PubMed] [Google Scholar]
- 29.Spector A., Ma W., Wang R.R. The aqueous humor is capable of generating and degrading H2O2. Invest. Ophthalmol. Vis. Sci. 1998 Jun;39(7):1188–1197. [PubMed] [Google Scholar]
- 30.Spector A. Oxidation and cataract. CIBA Found. Symp. 1984;106:48–64. doi: 10.1002/9780470720875.ch4. [DOI] [PubMed] [Google Scholar]
- 31.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995 Sep;9(12):1173–1182. Review. [PubMed] [Google Scholar]
- 32.Yan Q., Liu J.P., Li D.W. Apoptosis in lens development and pathology. Differentiation. 2006 Jun;74(5):195–211. doi: 10.1111/j.1432-0436.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 33.Yao K., Tan J., Gu W.Z., Ye P.P., Wang K.J. Reactive oxygen species mediates the apoptosis induced by transforming growth factor beta(2) in human lens epithelial cells. Biochem. Biophys. Res. Commun. 2007 Mar 2;354(1):278–283. doi: 10.1016/j.bbrc.2006.12.198. Epub 2007 Jan 10. [DOI] [PubMed] [Google Scholar]
- 34.Huang L., Estrada R., Yappert M.C., Borchman D. Oxidation-induced changes in human lens epithelial cells. 1. Phospholipids. Free Radic. Biol. Med. 2006 Nov 1;41(9):1425–1432. doi: 10.1016/j.freeradbiomed.2006.07.022. Epub 2006 Aug 4. [DOI] [PubMed] [Google Scholar]
- 35.Schriner S.E., Linford N.J., Martin G.M., Treuting P., Ogburn C.E., Emond M., Coskun P.E., Ladiges W., Wolf N., Van Remmen H., Wallace D.C., Rabinovitch P.S. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005 Jun 24;308(5730):1909–1911. doi: 10.1126/science.1106653. Epub 2005 May 5. [DOI] [PubMed] [Google Scholar]
- 36.Wood Z.A., Schröder E., Robin Harris J., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003 Jan;28(1):32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 37.Lee W., Wells T., Kantorow M. Localization and H2O2-specific induction of PRDX3 in the eye lens. Mol. Vis. 2007 Aug 27;13:1469–1474. [PubMed] [Google Scholar]
- 38.Babizhayev M.A., Deyev A.I., Chernikov A.V. Peroxide-metabolizing systems of the crystalline lens. Biochim. Biophys. Acta. 1992 Jan 16;1138(1):11–19. doi: 10.1016/0925-4439(92)90145-d. [DOI] [PubMed] [Google Scholar]
- 39.Babizhayev M.A., Costa E.B. Lipid peroxide and reactive oxygen species generating systems of the crystalline lens. Biochim. Biophys. Acta. 1994 Feb 22;1225(3):326–337. doi: 10.1016/0925-4439(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 40.Ganea E., Harding J.J. Glutathione-related enzymes and the eye. Curr. Eye Res. 2006 Jan;31(1):1–11. doi: 10.1080/02713680500477347. [DOI] [PubMed] [Google Scholar]
- 41.Bova L.M., Sweeney M.H., Jamie J.F., Truscott R.J. Major changes in human ocular UV protection with age. Invest. Ophthalmol. Vis. Sci. 2001 Jan;42(1):200–205. [PubMed] [Google Scholar]
- 42.Babizhayev M.A., Yegorov Y.E. Reactive oxygen species and the aging eye: specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation-induced maturity onset cataract — a novel platform of mitochondria-targeted antioxidants with broad therapeutic potential for redox regulation and detoxification of oxidants in eye diseases. Am. J. Ther. 2016 Jan-Feb;23(1):e98–e117. doi: 10.1097/MJT.0b013e3181ea31ff. [DOI] [PubMed] [Google Scholar]
- 43.Fernando M.R., Lechner J.M., Löfgren S., Gladyshev V.N., Lou M.F. Mitochondrial thioltransferase (glutaredoxin 2) has GSH-dependent and thioredoxin reductase-dependent peroxidase activities in vitro and in lens epithelial cells. FASEB J. 2006 Dec;20(14):2645–2647. doi: 10.1096/fj.06-5919fje. Epub 2006 Oct 25. [DOI] [PubMed] [Google Scholar]
- 44.Xing K., Lou M.F. The possible physiological function of thioltransferase in cells. FASEB J. 2003 Nov;17(14):2088–2090. doi: 10.1096/fj.02-1164fje. Epub 2003 Sep 4. [DOI] [PubMed] [Google Scholar]
- 45.Pérez V.I., Lew C.M., Cortez L.A., Webb C.R., Rodriguez M., Liu Y., Qi W., Li Y., Chaudhuri A., Van Remmen H., Richardson A., Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic. Biol. Med. 2008 Mar 1;44(5):882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. Epub 2007 Dec 7. [DOI] [PubMed] [Google Scholar]
- 46.Nonn L., Williams R.R., Erickson R.P., Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 2003 Feb;23(3):916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giblin F.J., Nies D.E., Reddy V.N. Stimulation of the hexose monophosphate shunt in rabbit lens in response to the oxidation of glutathione. Exp. Eye Res. 1981;33(3):289–298. doi: 10.1016/s0014-4835(81)80052-8. [DOI] [PubMed] [Google Scholar]
- 48.Rattner A., Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat. Rev. Neurosci. 2006 Nov;7(11):860–872. doi: 10.1038/nrn2007. Epub 2006 Oct 11. [DOI] [PubMed] [Google Scholar]
- 49.Matsui H., Lin L.R., Ho Y.S., Reddy V.N. The effect of up- and downregulation of MnSOD enzyme on oxidative stress in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2003 Aug;44(8):3467–3475. doi: 10.1167/iovs.02-0830. [DOI] [PubMed] [Google Scholar]
- 50.Ott M., Robertson J.D., Gogvadze V., Zhivotovsky B., Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. U. S. A. 2002 Feb 5;99(3):1259–1263. doi: 10.1073/pnas.241655498. Epub 2002 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin D., Barnett M., Grauer L., Robben J., Jewell A., Takemoto L., Takemoto D.J. Expression of superoxide dismutase in whole lens prevents cataract formation. Mol. Vis. 2005 Oct;11(11):853–858. [PubMed] [Google Scholar]
- 52.Reddy V.N., Kasahara E., Hiraoka M., Lin L.R., Ho Y.S. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp. Eye Res. 2004 Dec;79(6):859–868. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Kilic F., Trevithick J.R. Modelling cortical cataractogenesis. XXIX. Calpain proteolysis of lens fodrin in cataract. Biochem. Mol. Biol. Int. 1998 Aug;45(5):963–978. doi: 10.1002/iub.7510450514. [DOI] [PubMed] [Google Scholar]
- 54.Kilic F., Handelman G.J., Traber K., Tsang K., Packer L., Trevithick J.R. Modelling cortical cataractogenesis XX. In vitro effect of alpha-lipoic acid on glutathione concentrations in lens in model diabetic cataractogenesis. Biochem. Mol. Biol. Int. 1998 Oct;46(3):585–595. doi: 10.1080/15216549800204112. [DOI] [PubMed] [Google Scholar]
- 55.Kilic F., Handelman G.J., Serbinova E., Packer L., Trevithick J.R. Modelling cortical cataractogenesis 17: in vitro effect of a-lipoic acid on glucose-induced lens membrane damage, a model of diabetic cataractogenesis. Biochem. Mol. Biol. Int. 1995 Oct;37(2):361–370. [PubMed] [Google Scholar]
- 56.Bantseev V.L., Herbert K.L., Trevithick J.R., Sivak J.G. Mitochondria of rat lenses: distribution near and at the sutures. Curr. Eye Res. 1999 Dec;19(6):506–516. doi: 10.1076/ceyr.19.6.506.5279. [DOI] [PubMed] [Google Scholar]
- 57.Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997 Nov 14;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 58.Bender T., Leidhold C., Ruppert T., Franken S., Voos W. The role of protein quality control in mitochondrial protein homeostasis under oxidative stress. Proteomics. 2010 Feb 22 doi: 10.1002/pmic.200800619. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Daugaard M., Rohde M., Jäättelä M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007 Jul 31;581(19):3702–3710. doi: 10.1016/j.febslet.2007.05.039. Epub 2007 May 25. [DOI] [PubMed] [Google Scholar]
- 60.Horwitz J. α-Crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagineni C.N., Bhat S.P. Lens fiber cell differentiation and expression of crystallins in co-cultures of human fetal lens epithelial cells and fibroblasts. Exp. Eye Res. 1992 Feb;54(2):193–200. doi: 10.1016/s0014-4835(05)80208-8. [DOI] [PubMed] [Google Scholar]
- 62.Brady J.P., Garland D., Duglas-Tabor Y., Robison W.G., Jr., Groome A., Wawrousek E.F. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc. Natl. Acad. Sci. U. S. A. 1997 Feb 4;94(3):884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yaung J., Jin M., Barron E., Spee C., Wawrousek E.F., Kannan R., Hinton D.R. Alpha-crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol. Vis. 2007 Apr 4;13:566–577. [PMC free article] [PubMed] [Google Scholar]
- 64.Frey T.G., Mannella C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000;25:319–324. doi: 10.1016/s0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- 65.Wallace D.C., Brown M.D., Melov S., Graham B., Lott M. Mitochondrial biology, degenerative diseases and aging. Biofactors. 1998;7:187–190. doi: 10.1002/biof.5520070303. [DOI] [PubMed] [Google Scholar]
- 66.Tzagoloff A. Plenum Press; New York: 1982. Mitochondria. [Google Scholar]
- 67.Huang Z., Jiang J., Tyurin V.A., Zhao Q., Mnuskin A., Ren J., Belikova N.A., Feng W., Kurnikov I.V., Kagan V.E. Cardiolipin deficiency leads to decreased cardiolipin peroxidation and increased resistance of cells to apoptosis. Free Radic. Biol. Med. 2008 Jun 1;44(11):1935–1944. doi: 10.1016/j.freeradbiomed.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kinsey V.E., Reddy D.V. Studies on the crystalline lens. XI. The relative role of the epithelium and capsule in transport. Investig. Ophthalmol. 1965;34:104–116. [PubMed] [Google Scholar]
- 69.Goodenough D.A., Dick J.S., 2nd, Lyons J.E. Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J. Cell Biol. 1980;86:576–589. doi: 10.1083/jcb.86.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bassnett S., Beebe D.C. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Dev. Dyn. 1992 Jun;194(2):85–93. doi: 10.1002/aja.1001940202. [DOI] [PubMed] [Google Scholar]
- 71.Bantseev V., Cullen A.P., Trevithick J.R., Sivak J.G. Optical function and mitochondrial metabolic properties in damage and recovery of bovine lens after in vitro carbonyl cyanide m-chlorophenylhydrazone treatment. Mitochondrion. 2003 Aug;3(1):1–11. doi: 10.1016/S1567-7249(03)00059-X. [DOI] [PubMed] [Google Scholar]
- 72.Bantseev V., Sivak J.G. Confocal laser scanning microscopy imaging of dynamic TMRE movement in the mitochondria of epithelial and superficial cortical fiber cells of bovine lenses. Mol. Vis. 2005 Jul 14;11:518–523. [PubMed] [Google Scholar]
- 73.Lesnefsky E.J., Moghaddas S., Tandler B., Kerner J., Hoppel C.L. Mitochondrial dysfunction in cardiac disease: ischemia–reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001 Jun;33(6):1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 74.Wallace D.C. Mitochondrial defects in cardiomyopathy and neuromuscular disease. Am. Heart J. 2000 Feb;139(2 Pt 3):S70–S85. doi: 10.1067/mhj.2000.103934. Review. [DOI] [PubMed] [Google Scholar]
- 75.Chen Q., Vazquez E.J., Moghaddas S., Hoppel C.L., Lesnefsky E.J. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003 Sep 19;278(38):36027–36031. doi: 10.1074/jbc.M304854200. Epub 2003 Jul 2. [DOI] [PubMed] [Google Scholar]
- 76.Sugioka K., Nakano M., Totsune-Nakano H., Minakami H., Tero-Kubota S., Ikegami Y. Mechanism of O2- generation in reduction and oxidation cycle of ubiquinones in a model of mitochondrial electron transport systems. Biochim. Biophys. Acta. 1988 Dec 7;936(3):377–385. doi: 10.1016/0005-2728(88)90014-x. [DOI] [PubMed] [Google Scholar]
- 77.Turrens J.F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grivennikova V.G., Vinogradov A.D. Generation of superoxide by the mitochondrial complex I. Biochim. Biophys. Acta. 2006 May-Jun;1757(5–6):553–561. doi: 10.1016/j.bbabio.2006.03.013. Epub 2006 Apr 17. [DOI] [PubMed] [Google Scholar]
- 79.Skulachev V.P. A biochemical approach to the problem of aging: “megaproject” on membrane-penetrating ions. The first results and prospects. Biochemistry (Mosc) 2007 Dec;72(12):1385–1396. doi: 10.1134/s0006297907120139. [DOI] [PubMed] [Google Scholar]
- 80.Turrens J.F., Alexandre A., Lehninger A.L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985 Mar;237(2):408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 81.Herrero A., Barja G. Sites and mechanisms responsible for the low rate of free radical production of heart mitochondria in the long-lived pigeon. Mech. Ageing Dev. 1997 Nov;98(2):95–111. doi: 10.1016/s0047-6374(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 82.St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002 Nov 22;277(47):44784–44790. doi: 10.1074/jbc.M207217200. Epub 2002 Sep 16. [DOI] [PubMed] [Google Scholar]
- 83.Becker L.B., Vanden Hoek T.L., Shao Z.H., Li C.Q., Schumacker P.T. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am. J. Phys. 1999 Dec;277(6 Pt 2):H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 84.Quigley H.A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 1996 May;80(5):389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Augusteyn R.C. Protein modification in cataract: possible oxidative mechanisms. In: Duncan G., editor. Mechanisms of Cataract Formation in the Human Lens. Academic Press; London: 1981. pp. 71–116. [Google Scholar]
- 86.Berman E.R. Plenum Press; New York: 1991. Biochemistry of the Eye. [Google Scholar]
- 87.Webb M. Toxicological significance of metallothionein. Experientia Suppl. 1987;52:109–134. doi: 10.1007/978-3-0348-6784-9_6. [DOI] [PubMed] [Google Scholar]
- 88.Nita M., Grzybowski A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734. Epub 2016 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saccà S.C., Gandolfi S., Bagnis A., Manni G., Damonte G., Traverso C.E., Izzotti A. The outflow pathway: a tissue with morphological and functional unity. J. Cell. Physiol. 2016 Jan 11 doi: 10.1002/jcp.25305. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 90.Babizhayev M.A. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundam. Clin. Pharmacol. 2012 Feb;26(1):86–117. doi: 10.1111/j.1472-8206.2011.00969.x. Epub 2011 Aug 24. [DOI] [PubMed] [Google Scholar]
- 91.Babizhayev M.A., Yegorov Y.E. Senescent phenotype of trabecular meshwork cells displays biomarkers in primary open-angle glaucoma. Curr. Mol. Med. 2011 Oct;11(7):528–552. doi: 10.2174/156652411800615126. [DOI] [PubMed] [Google Scholar]
- 92.Murphy M.P., Smith R.A. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv. Drug Deliv. Rev. 2000 Mar 30;41(2):235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 93.Murphy M.P. Selective targeting of bioactive compounds to mitochondria. Trends Biotechnol. 1997 Aug;15(8):326–330. doi: 10.1016/S0167-7799(97)01068-8. [DOI] [PubMed] [Google Scholar]
- 94.Mitchell J.B., Russo A., Kuppusamy P., Krishna M.C. Radiation, radicals, and images. Ann. N. Y. Acad. Sci. 2000;899:28–43. doi: 10.1111/j.1749-6632.2000.tb06174.x. [DOI] [PubMed] [Google Scholar]
- 95.Hoye A.T., Davoren J.E., Wipf P., Fink M.P., Kagan V.E. Targeting mitochondria. Acc. Chem. Res. 2008 Jan;41(1):87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 96.Liberman E.A., Topali V.P., Tsofina L.M., Jasaitis A.A., Skulachev V.P. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 97.Severin S.E., Skulachev V.P., Iaguzhinskiĭ L.S. Possible role of carnitine in the transport of fatty acids through the mitochondrial membrane. Biokhimiia. 1970 Nov-Dec;35(6):1250–1253. [PubMed] [Google Scholar]
- 98.Murphy M.P., Smith R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 99.Smith R.A., Kelso G.F., James A.M., Murphy M.P. Targeting coenzyme Q derivatives to mitochondria. Methods Enzymol. 2004;382:45–67. doi: 10.1016/S0076-6879(04)82003-2. [DOI] [PubMed] [Google Scholar]
- 100.Victor V.M., Rocha M. Targeting antioxidants to mitochondria: a potential new therapeutic strategy for cardiovascular diseases. Curr. Pharm. Des. 2007;13(8):845–863. doi: 10.2174/138161207780363077. [DOI] [PubMed] [Google Scholar]
- 101.Silachev D.N., Plotnikov E.Y., Zorova L.D., Pevzner I.B., Sumbatyan N.V., Korshunova G.A., Gulyaev M.V., Pirogov Y.A., Skulachev V.P., Zorov D.B. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone and Thymoquinone in a Rat Model of Brain Ischemia/Reperfusion Injury. Molecules. 2015 Aug 11;20(8):14487–14503. doi: 10.3390/molecules200814487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Loshadkin D., Roginsky V., Pliss E. Substituted p-hydroquinones as a chain-breaking antioxidant during the oxidation of styrene. In. J. Chem. Kinet. 2002;34:162–171. [Google Scholar]
- 103.Roginsky V., Barsukova T., Loshadkin D., Pliss E. Substituted p-hydroquinones as inhibitors of lipid peroxidation. Chem. Phys. Lipids. 2003 Sep;125(1):49–58. doi: 10.1016/s0009-3084(03)00068-9. [DOI] [PubMed] [Google Scholar]
- 104.Kruk J., Jemiola-Rzeminska M., Strzalka K. Plastoquinol and α-tocopherol quinol are more active than ubiquinol and α-tocopherol in inhibition of lipid peroxidation. Chem. Phys. Lipids. 1997;87:73–80. [Google Scholar]
- 105.Roginsky V.A., Tashlitsky V.N., Skulachev V.P. Chain-breaking antioxidant activity of reduced forms of mitochondria-targeted quinones, a novel type of geroprotectors. Aging (Albany NY) 2009 May 12;1(5):481–489. doi: 10.18632/aging.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neroev V.V., Archipova M.M., Bakeeva L.E., Fursova A.Z., Grigorian E.N., Grishanova A.Y., Iomdina E.N., Ivashchenko Z.N., Katargina L.A., Khoroshilova-Maslova I.P., Kilina O.V., Kolosova N.G., Kopenkin E.P., Korshunov S.S., Kovaleva N.A., Novikova Y.P., Philippov P.P., Pilipenko D.I., Robustova O.V., Saprunova V.B., Senin I.I., Skulachev M.V., Sotnikova L.F., Stefanova N.A., Tikhomirova N.K., Tsapenko I.V., Shchipanova A.I., Zinovkin R.A., Skulachev V.P. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age-related eye disease. SkQ1 returns vision to blind animals. Biochemistry (Mosc) 2008 Dec;73(12):1317–1328. doi: 10.1134/s0006297908120043. [DOI] [PubMed] [Google Scholar]
- 107.McNulty R., Wang H., Mathias R.T., Ortwerth B.J., Truscott R.J.W., Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J. Physiol. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang L., Tang D., Yappert M.C., Borchman D. Oxidation-induced changes in human lens epithelial cells 2. Mitochondria and the generation of reactive oxygen species. Free Radic. Biol. Med. 2006 Sep 15;41(6):926–936. doi: 10.1016/j.freeradbiomed.2006.05.023. Epub 2006 May 26. [DOI] [PubMed] [Google Scholar]
- 109.Babizhayev M.A., Meguro K. 2004. Combined Use of Carnosinase Inhibitor With l-Carnosines and Composition. WO 2004/064866 PCT/JP2004/000351. Filed 20 January 2003 (20.01.2003) [Google Scholar]
- 110.Babizhayev M.A. 2004. Method for Topical Treatment of Eye Disease and Composition and Device for Said Treatment. PCT Patent Application. International Publication Number WO 2004/028536 A1. International Publication Date: 8 April 2004. [Google Scholar]
- 111.Babizhayev M.A. Ophthalmic pharmacology of N-acetylcarnosine lubricant eye drops. J. Pharmacol. Toxicol. 2006;1(3):201–233. [Google Scholar]
- 112.Babizhayev M.A., Micans P., Guiotto A., Kasus-Jacobi A. N-acetylcarnosine lubricant eyedrops possess all-in-one universal antioxidant protective effects of l-carnosine in aqueous and lipid membrane environments, aldehyde scavenging, and transglycation activities inherent to cataracts: a clinical study of the new vision-saving drug N-acetylcarnosine eyedrop therapy in a database population of over 50,500 patients. Am. J. Ther. 2009 Nov-Dec;16(6):517–533. doi: 10.1097/MJT.0b013e318195e327. [DOI] [PubMed] [Google Scholar]
- 113.Zakharchenko M.V., Temnov A.V., Kondrashova M.N. Effect of carnosine on self-organization of mitochondrial assemblies in rat liver homogenate. Biochemistry (Mosc) 2003 Sep;68(9):1002–1005. doi: 10.1023/a:1026064613289. [DOI] [PubMed] [Google Scholar]
- 114.Kang K.S., Yun J.W., Lee Y.S. Protective effect of l-carnosine against 12-O-tetradecanoylphorbol-13-acetate- or hydrogen peroxide-induced apoptosis on v-myc transformed rat liver epithelial cells. Cancer Lett. 2002 Apr 8;178(1):53–62. doi: 10.1016/s0304-3835(01)00821-7. [DOI] [PubMed] [Google Scholar]
- 115.Korobov V.N., Doliba N.M. Telegus IaV. [Carnosine in adaptation to hypobaric hypoxia] Biokhimiia. 1993 May;58(5):740–744. [PubMed] [Google Scholar]
- 116.Belikova N.A., Tyurina Y.Y., Borisenko G., Tyurin V., Samhan Arias A.K., Yanamala N., Furtmüller P.G., Klein-Seetharaman J., Obinger C., Kagan V.E. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: antioxidant function in mitochondria. J. Am. Chem. Soc. 2009 Aug 19;131(32):11288–11289. doi: 10.1021/ja904343c. [DOI] [PubMed] [Google Scholar]
- 117.Kagan V.E., Bayir H.A., Belikova N.A., Kapralov O., Tyurina Y.Y., Tyurin V.A., Jiang J., Stoyanovsky D.A., Wipf P., Kochanek P.M., Greenberger J.S., Pitt B., Shvedova A.A., Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic. Biol. Med. 2009 Jun 1;46(11) doi: 10.1016/j.freeradbiomed.2009.03.004. 1439–53. Epub 2009 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tyurin V.A., Tyurina Y.Y., Jung M.Y., Tungekar M.A., Wasserloos K.J., Bayir H., Greenberger J.S., Kochanek P.M., Shvedova A.A., Pitt B., Kagan V.E. Mass-spectrometric analysis of hydroperoxy- and hydroxy-derivatives of cardiolipin and phosphatidylserine in cells and tissues induced by pro-apoptotic and pro-inflammatory stimuli. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009 Sep 15;877(26):2863–2872. doi: 10.1016/j.jchromb.2009.03.007. Epub 2009 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kagan V.E., Bayir A., Bayir H., Stoyanovsky D., Borisenko G.G., Tyurina Y.Y., Wipf P., Atkinson J., Greenberger J.S., Chapkin R.S., Belikova N.A. Mitochondria-targeted disruptors and inhibitors of cytochrome c/cardiolipin peroxidase complexes: a new strategy in anti-apoptotic drug discovery. Mol. Nutr. Food Res. 2009 Jan;53(1):104–114. doi: 10.1002/mnfr.200700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Babizhayev M.A., Seguin M.-C., Gueyne J., Evstigneeva R.P., Ageyeva E.A., Zheltukhina G.A. l-Carnosine (β-alanyl-l-histidine) and carcinine (β-alanylhistamine) act as natural antioxidants with hydroxyl-radical-scavenging and lipid peroxidase activities. Biochem. J. 1994;304:509–516. doi: 10.1042/bj3040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pavlov A.R., Revina A.A., Dupin A.M., Boldyrev A.A., Yaropolov A.I. The mechanism of interaction of carnosine with superoxide radicals in water solutions. Biochim. Biophys. Acta. 1993 Jul 11;1157(3):304–312. doi: 10.1016/0304-4165(93)90114-n. [DOI] [PubMed] [Google Scholar]
- 122.Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab. Rev. 2007;39(2–3):443–455. doi: 10.1080/03602530701468516. [DOI] [PubMed] [Google Scholar]
- 123.Jiang J., Stoyanovsky D.A., Belikova N.A., Tyurina Y.Y., Zhao Q., Tungekar M.A., Kapralova V., Huang Z., Mintz A.H., Greenberger J.S., Kagan V.E. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat. Res. 2009 Dec;172(6):706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Babizhayev M.A., Kasus-Jacobi A. State of the art clinical efficacy and safety evaluation of N-acetylcarnosine dipeptide ophthalmic prodrug. Principles for the delivery, self-bioactivation, molecular targets and interaction with a highly evolved histidyl-hydrazide structure in the treatment and therapeutic management of a group of sight-threatening eye diseases. Curr. Clin. Pharmacol. 2009 Jan;4(1):4–37. doi: 10.2174/157488409787236074. [DOI] [PubMed] [Google Scholar]
- 125.Hudson E.K., Hogue B.A., Souza-Pinto N.C., Croteau D.L., Anson R.M., Bohr V.A., Hansford R.G. Age-associated change in mitochondrial DNA damage. Free Radic. Res. 1998 Dec;29(6):573–579. doi: 10.1080/10715769800300611. [DOI] [PubMed] [Google Scholar]
- 126.Caron F., Jacq C., Rouvière-Yaniv J. Characterization of a histone-like protein extracted from yeast mitochondria. Proc. Natl. Acad. Sci. U. S. A. 1979 Sep;76(9):4265–4269. doi: 10.1073/pnas.76.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wallace D.C. Mitochondrial diseases in man and mouse. Science. 1999 Mar 5;283(5407):1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 128.Skulachev V.P. Springer-Verlag; Berlin: 1988. Membrane bioenergetics. (442 pp.) [Google Scholar]
- 129.Skulachev V.P., Antonenko Y.N., Cherepanov D.A., Chernyak B.V., Izyumov D.S., Khailova L.S., Klishin S.S., Korshunova G.A., Lyamzaev K.G., Pletjushkina O.Y., Roginsky V.A., Rokitskaya T.I., Severin F.F., Severina I.I., Simonyan R.A., Skulachev M.V., Sumbatyan N.V., Sukhanova E.I., Tashlitsky V.N., Trendeleva T.A., Vyssokikh M.Y., Zvyagilskaya R.A. Prevention of cardiolipin oxidation and fatty acid cycling as two antioxidant mechanisms of cationic derivatives of plastoquinone (SkQs) Biochim. Biophys. Acta. 2010 Jun-Jul;1797(6-7):878–889. doi: 10.1016/j.bbabio.2010.03.015. Epub 2010 Mar 20. [DOI] [PubMed] [Google Scholar]
- 130.Justilien V., Pang J.J., Renganathan K., Zhan X., Crabb J.W., Kim S.R., Sparrow J.R., Hauswirth W.W., Lewin A.S. SOD2 knockdown mouse model of early AMD. Invest. Ophthalmol. Vis. Sci. 2007 Oct;48(10):4407–4420. doi: 10.1167/iovs.07-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.King A., Gottlieb E., Brooks D.G., Murphy M.P., Dunaief J.L. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem. Photobiol. 2004 May;79(5):470–475. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- 132.Komeima K., Rogers B.S., Lu L., Campochiaro P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. U. S. A. 2006 Jul 25;103(30):11300–11305. doi: 10.1073/pnas.0604056103. Epub 2006 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Komeima K., Rogers B.S., Campochiaro P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007 Dec;213(3):809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 134.Ghelli A., Porcelli A.M., Zanna C., Martinuzzi A., Carelli V., Rugolo M. Protection against oxidant-induced apoptosis by exogenous glutathione in Leber hereditary optic neuropathy cybrids. Invest. Ophthalmol. Vis. Sci. 2008 Feb;49(2):671–676. doi: 10.1167/iovs.07-0880. [DOI] [PubMed] [Google Scholar]
- 135.McKinnon S.J. Glaucoma: ocular Alzheimer's disease? Front. Biosci. 2003 Sep 1;8:s1140–s1156. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- 136.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog. Retin. Eye Res. 2006 Sep;25(5):490–513. doi: 10.1016/j.preteyeres.2006.07.003. Epub 2006 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Babizhayev M.A., Bunin A.Y. Lipid peroxidation in open-angle glaucoma. Acta Ophthalmol. 1989 Aug;67(4):371–377. doi: 10.1111/j.1755-3768.1989.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 138.Olofsson E.M., Marklund S.L., Behndig A. Glucose-induced cataract in CuZn-SOD null lenses: an effect of nitric oxide? Free Radic. Biol. Med. 2007 Apr 1;42(7):1098–1105. doi: 10.1016/j.freeradbiomed.2007.01.012. Epub 2007 Jan 12. [DOI] [PubMed] [Google Scholar]
- 139.Lassen N., Bateman J.B., Estey T., Kuszak J.R., Nees D.W., Piatigorsky J., Duester G., Day B.J., Huang J., Hines L.M., Vasiliou V. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knock-out mice. J. Biol. Chem. 2007 Aug 31;282(35):25668–25676. doi: 10.1074/jbc.M702076200. Epub 2007 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brito B.E., Marcano J.C., Salazar E., Cano M., Baute L., Bernal G., Gonzalez L.R. Age as a determinant factor for endotoxin induced uveitis. Ocul. Immunol. Inflamm. 2006 Apr;14(2):117–124. doi: 10.1080/09273940500328503. [DOI] [PubMed] [Google Scholar]
- 141.Pararajasegaram G., Sevanian A., Rao N.A. Suppression of S antigen-induced uveitis by vitamin E supplementation. Ophthalmic Res. 1991;23(3):121–127. doi: 10.1159/000267110. [DOI] [PubMed] [Google Scholar]
- 142.Antonenko Y.N., Avetisyan A.V., Bakeeva L.E., Chernyak B.V., Chertkov V.A., Domnina L.V., Ivanova O.Y., Izyumov D.S., Khailova L.S., Klishin S.S., Korshunova G.A., Lyamzaev K.G., Muntyan M.S., Nepryakhina O.K., Pashkovskaya A.A., Pletjushkina O.Y., Pustovidko A.V., Roginsky V.A., Rokitskaya T.I., Ruuge E.K., Saprunova V.B., Severina I.I., Simonyan R.A., Skulachev I.V., Skulachev M.V., Sumbatyan N.V., Sviryaeva I.V., Tashlitsky V.N., Vassiliev J.M., Vyssokikh M.Y., Yaguzhinsky L.S., Zamyatnin A.A., Jr., Skulachev V.P. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry (Mosc) 2008 Dec;73(12):1273–1287. doi: 10.1134/s0006297908120018. [DOI] [PubMed] [Google Scholar]
- 143.Skulachev V.P., Longo V.D. Aging as a mitochondria-mediated atavistic program: can aging be switched off? Ann. N. Y. Acad. Sci. 2005 Dec;1057:145–164. doi: 10.1196/annals.1356.009. [DOI] [PubMed] [Google Scholar]
- 144.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000 Oct 2;192(7):1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.