Abstract
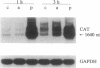
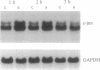
Chronic incubation of cultured renal tubular epithelial cells in acid medium causes an increase in Na/H antiporter activity that persists after removal from acid, is dependent on protein synthesis, and is associated with an increase in Na/H antiporter mRNA. Chronic activation of protein kinase C has similar effects in these cells. The present studies examined the role of protein kinase C in the effect of acid incubation. Incubation of MCT cells in acid for 24 h caused a 50% increase in Na/H antiporter activity. This was prevented by inhibition of protein kinase C, either with sphingosine or by protein kinase C downregulation. Pertussis toxin pretreatment did not prevent the increase in antiporter activity. Acid incubation caused an increase in transcription factor AP-1 activity, as shown by an increase in expression from a reporter gene containing six tandem AP-1 binding sites. This was associated with transient increases in c-fos and c-jun mRNAs. This response is typical of that for gene activation by protein kinase C. These studies demonstrate that acid activation of the Na/H antiporter requires protein kinase C and is associated with c-fos and c-jun expression and increased AP-1 activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiba T., Rocco V. K., Warnock D. G. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest. 1987 Aug;80(2):308–315. doi: 10.1172/JCI113074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Bartel D. P., Sheng M., Lau L. F., Greenberg M. E. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989 Mar;3(3):304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu R., Imagawa M., Imbra R. J., Bockoven J. R., Karin M. Multiple cis- and trans-acting elements mediate the transcriptional response to phorbol esters. Nature. 1987 Oct 15;329(6140):648–651. doi: 10.1038/329648a0. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Klahr S., Hammerman M. R. Metabolic acidosis and parathyroidectomy increase Na+-H+ exchange in brush border vesicles. Am J Physiol. 1983 Aug;245(2):F217–F222. doi: 10.1152/ajprenal.1983.245.2.F217. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Curran T., Gordon M. B., Rubino K. L., Sambucetti L. C. Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2(1):79–84. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty J. G., Agarwal N., Reilly R. F., Adelberg E. A., Slayman C. W. Pharmacologically different Na/H antiporters on the apical and basolateral surfaces of cultured porcine kidney cells (LLC-PK1). Proc Natl Acad Sci U S A. 1988 Sep;85(18):6797–6801. doi: 10.1073/pnas.85.18.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Greenberg C. S., Bell R. M. Sphingosine inhibition of agonist-dependent secretion and activation of human platelets implies that protein kinase C is a necessary and common event of the signal transduction pathways. J Biol Chem. 1987 Oct 5;262(28):13620–13626. [PubMed] [Google Scholar]
- Hata A., Akita Y., Konno Y., Suzuki K., Ohno S. Direct evidence that the kinase activity of protein kinase C is involved in transcriptional activation through a TPA-responsive element. FEBS Lett. 1989 Jul 31;252(1-2):144–146. doi: 10.1016/0014-5793(89)80907-x. [DOI] [PubMed] [Google Scholar]
- Horie S., Moe O., Miller R. T., Alpern R. J. Long-term activation of protein kinase c causes chronic Na/H antiporter stimulation in cultured proximal tubule cells. J Clin Invest. 1992 Feb;89(2):365–372. doi: 10.1172/JCI115594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie S., Moe O., Tejedor A., Alpern R. J. Preincubation in acid medium increases Na/H antiporter activity in cultured renal proximal tubule cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4742–4745. doi: 10.1073/pnas.87.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Cunha-Melo J. R., Beaven M. A., Huang K. P. Differential down-regulation of protein kinase C isozymes. J Biol Chem. 1989 Mar 5;264(7):4238–4243. [PubMed] [Google Scholar]
- Iwaki K., Sukhatme V. P., Shubeita H. E., Chien K. R. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an alpha 1-mediated response. J Biol Chem. 1990 Aug 15;265(23):13809–13817. [PubMed] [Google Scholar]
- Kharbanda S. M., Sherman M. L., Kufe D. W. Transcriptional regulation of c-jun gene expression by arabinofuranosylcytosine in human myeloid leukemia cells. J Clin Invest. 1990 Nov;86(5):1517–1523. doi: 10.1172/JCI114870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange activity in renal brush border membrane vesicles in response to metabolic acidosis: The role of glucocorticoids. Proc Natl Acad Sci U S A. 1984 Jan;81(2):630–634. doi: 10.1073/pnas.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOTSPEICH W. D. RENAL HYPERTROPHY IN METABOLIC ACIDOSIS AND ITS RELATION TO AMMONIA EXCRETION. Am J Physiol. 1965 Jun;208:1135–1142. doi: 10.1152/ajplegacy.1965.208.6.1135. [DOI] [PubMed] [Google Scholar]
- Lowe J. H., Huang C. L., Ives H. E. Sphingosine differentially inhibits activation of the Na+/H+ exchanger by phorbol esters and growth factors. J Biol Chem. 1990 May 5;265(13):7188–7194. [PubMed] [Google Scholar]
- Lucibello F. C., Lowag C., Neuberg M., Müller R. trans-repression of the mouse c-fos promoter: a novel mechanism of Fos-mediated trans-regulation. Cell. 1989 Dec 22;59(6):999–1007. doi: 10.1016/0092-8674(89)90756-3. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. T., Counillon L., Pages G., Lifton R. P., Sardet C., Pouysségur J. Structure of the 5'-flanking regulatory region and gene for the human growth factor-activatable Na/H exchanger NHE-1. J Biol Chem. 1991 Jun 15;266(17):10813–10819. [PubMed] [Google Scholar]
- Moe O. W., Miller R. T., Horie S., Cano A., Preisig P. A., Alpern R. J. Differential regulation of Na/H antiporter by acid in renal epithelial cells and fibroblasts. J Clin Invest. 1991 Nov;88(5):1703–1708. doi: 10.1172/JCI115487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara L., Sistonen L., Bos T. J., Vogt P. K., Keski-Oja J., Alitalo K. Enhanced jun gene expression is an early genomic response to transforming growth factor beta stimulation. Mol Cell Biol. 1989 Mar;9(3):1255–1262. doi: 10.1128/mcb.9.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig P. A., Alpern R. J. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest. 1988 Oct;82(4):1445–1453. doi: 10.1172/JCI113750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. N., de Roux N., Sardet C., Pouysségur J., Berk B. C. Na+/H+ antiporter gene expression during monocytic differentiation of HL60 cells. J Biol Chem. 1991 Jul 25;266(21):13485–13488. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Ryder K., Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Sherman M. L., Stone R. M., Datta R., Bernstein S. H., Kufe D. W. Transcriptional and post-transcriptional regulation of c-jun expression during monocytic differentiation of human myeloid leukemic cells. J Biol Chem. 1990 Feb 25;265(6):3320–3323. [PubMed] [Google Scholar]
- Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991 Feb 15;251(4995):804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Tsai C. J., Ives H. E., Alpern R. J., Yee V. J., Warnock D. G., Rector F. C., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush border vesicles from rabbits with metabolic acidosis. Am J Physiol. 1984 Aug;247(2 Pt 2):F339–F343. doi: 10.1152/ajprenal.1984.247.2.F339. [DOI] [PubMed] [Google Scholar]