Abstract
Hypophosphatasia (HPP) is a rare, inherited metabolic bone disease resulting from mutations in the gene encoding tissue non-specific alkaline phosphatase. The biochemical hallmark and key diagnostic indicator is low alkaline phosphatase activity, which leads to a variety of clinical manifestations across all ages. The diagnosis is easily missed in adults, who frequently present with nonspecific clinical manifestations such as fractures, osteomalacia, and pain. Here, the pathway to diagnosis and disease course is described in an adult patient presenting with pain. Low serum alkaline phosphatase activity went unnoticed for 2 years until osteomalacia was suspected, during which time he experienced multiple fractures and progressing pain. Currently, accumulated morbidity has rendered the patient unable to work, and treatment is focused on pain management. This case highlights the importance of low alkaline phosphatase in the differential diagnosis of patients with musculoskeletal pain.
Abbreviations: HPP, hypophosphatasia; PEA, phosphoethanolamine; PLP, pyridoxal-5′-phosphate; PPi, inorganic pyrophosphate; TNSALP, tissue-nonspecific isoenzyme of alkaline phosphatase
Keywords: Alkaline phosphatase, Costochondritis, Fractures, Hypophosphatasia, Pain
Highlights
-
•
Diagnosis of hypophosphatasia may be missed in adults with nonspecific features such as fractures, osteomalacia and pain
-
•
The biochemical hallmark and key diagnostic indicator is low alkaline phosphatase activity
-
•
A case is described of an adult whose diagnosis was initially missed, despite low alkaline phosphatase levels for 2 years
-
•
Low alkaline phosphatase levels are important in the differential diagnosis of patients with musculoskeletal pain
1. Introduction
Hypophosphatasia (HPP) is a rare, inherited metabolic bone disease characterized by poor bone mineralization, leading to HPP-related rickets in children and osteomalacia in adults (Rockman-Greenberg, 2013, Whyte, 2013). HPP is caused by loss-of-function mutation(s) in the gene (ALPL) that encodes the tissue-nonspecific isoenzyme of alkaline phosphatase (TNSALP). Approximately 300 pathogenic mutations in the ALPL gene have been identified to date (Mornet, 2015). Inheritance may be either autosomal recessive or autosomal dominant (Rockman-Greenberg, 2013, Mornet, 2015). Reduced TNSALP activity leads to accumulation of its substrates, pyridoxal-5′-phosphate (PLP), phosphoethanolamine (PEA), and inorganic pyrophosphate (PPi). The excess of PPi, a potent inhibitor of bone mineralization, leads to the mineralization defects in bone and teeth characteristic of HPP (Whyte, 2013).
Adult-onset HPP typically manifests during middle age, although some adults recall earlier signs of HPP such as early tooth loss or childhood rickets (Whyte, 2013). Regardless of age at onset of HPP, adults may present with osteopenia, osteomalacia, recurring and/or poorly healing fractures, pseudofractures, bone pain, joint pain caused by chrondrocalcinosis or calcific periarthritis (Whyte, 2013, Coe et al., 1986), and/or proximal muscle weakness (Whyte, 2013, Coe et al., 1986, Berkseth et al., 2013). As many of these symptoms are consistent with diagnosis of other diseases such as osteoporosis, osteoarthritis, and fibromyalgia, the diagnosis of HPP in adults can be challenging and easily missed (Mornet et al., 2014). Although recombinant TNSALP approved for treatment of HPP (asfotase alfa, Alexion Pharmaceuticals, Inc.) currently exists only in Japan, Canada, and the European Union, correct diagnosis of HPP is important as treatments for other disorders may have adverse effects for patients with HPP; for example, treatment with bisphosphonates (structural analogs of PPi) for a presumed diagnosis of osteoporosis in adults may increase fracture risk in patients with HPP (Sutton et al., 2012).
Herein, I describe the pathway to diagnosis, unique features, and the course and current management of a case of adult-onset HPP.
2. Case report
The patient was a 53 year-old, 6 ft tall, 215 lb Caucasian man who was referred to rheumatology in September 2011 for intractable muscle and joint pain. He reported severe diffuse pain at his first rheumatology visit. He had no personal or known family history of metabolic bone disease or dental disease. In August 2011, his primary care physician diagnosed fibromyalgia, based on chronic pain.
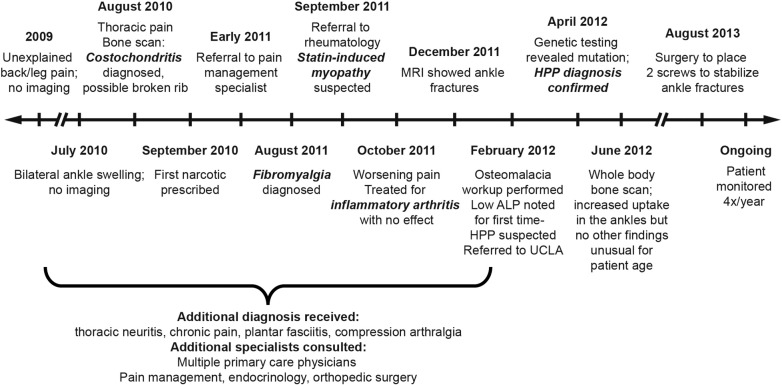
The patient first presented in 2009 (Fig. 1) with unexplained back and leg pain. Prior history included hypertension, hypercholesterolemia, and occasional insomnia, but was otherwise unremarkable. In July 2010, he experienced unexplained bilateral swelling of his ankles. In August 2010, he experienced non-traumatic thoracic pain; the first imaging in the patient's chart was a triple phase bone scan that revealed costochondritis and a possible broken rib. A follow-up MRI of the area in November 2010 did not identify a fracture. Pain medications, including narcotics, were prescribed without effect, and in early 2011, the patient was referred to a pain management specialist. By the time of his referral to rheumatology in September 2011, the patient had received varying diagnoses, including costochondritis, thoracic neuritis, chronic pain, plantar fasciitis, compression arthralgia, and fibromyalgia.
Fig. 1.
Timeline illustrating the patient's journey to correct diagnosis of adult-onset HPP.
Rheumatology workup, which included a complete serology panel with muscle enzymes, an autoimmune panel, and a full inflammatory marker panel, returned no abnormal findings. Despite normal levels of liver enzymes and creatine phosphokinase, statin-induced myopathy was considered because his simvastatin dose had recently been increased to 80 mg/day. Simvastatin was discontinued, and corticosteroids and muscle relaxants prescribed. The patient reported a self-estimated 60–70% improvement in symptoms shortly thereafter, but presented again in October 2011 with worsening pain, especially in his ankles. Radiographs of the ankles revealed possible small fractures, prompting an MRI in December 2011, which confirmed bilateral fractures of the medial malleoli and a non-displaced fracture in the left ankle with a torn tendon and joint effusion. The atypical non-traumatic nature of these fractures prompted a workup for osteomalacia in February 2012. Possible diagnoses included HPP, Wilson's disease, and celiac disease. Serum magnesium, phosphorus, copper, zinc, parathyroid hormone, calcium, and celiac markers were within normal limits. Alkaline phosphatase was low on multiple determinations at 27–32 IU/L (normal range: 40–115 IU/L). Upon chart review, serum alkaline phosphatase activity was consistently low, with values below the normal range in December 2011, April 2011, September 2010, and June 2010 (earliest available). Pyridoxal 5′-phosphate (vitamin B6) was 54 μg/L (normal range: 5–50 μg/L). Vitamin D was insufficient (14 ng/ml, normal range: 30–100 ng/ml) and was normalized with supplementation. A diagnosis of HPP was indicated based on laboratory and clinical evidence. The patient was then referred to the University of California, Los Angeles, for genetic testing, which revealed a heterozygous mutation (c.500C > T) on exon 6 of the ALPL gene (Connective Tissue Gene Tests, Allentown, PA, USA), thereby supporting the diagnosis of HPP.
The patient is seen every 3 months to monitor his condition. A whole body bone scan in June 2012 identified increased uptake in the ankles consistent with fracture sites but no other findings unusual for the patient's age. In August 2013, he underwent orthopedic surgery to have two screws placed to stabilize the ankle fractures. He has declined both placement of stabilizer rods and experimental teriparatide treatment. Orthopedists have expressed discomfort with further surgical treatment given the fragility of his bones. As of March 2015, the patient had progressing pain and rib fractures. He fatigues easily and prefers appointments before noon. He regularly wraps his legs for support, and uses a cane or walker to assist ambulation. Decreased mobility has rendered the patient unable to work. Pain is poorly controlled with a 100 μg fentanyl patch/72 h and 20 mg short-acting oxycodone every 4 h. Vitamin D supplementation is provided as needed to maintain serum concentrations within normal ranges. The patient's serum concentration of vitamin D was 48 ng/ml in April 2012 with supplementation, after being 14 ng/ml in March 2012 (normal range: 30–100 ng/ml). Current treatment is focused on pain management due to limited treatment options.
3. Discussion
HPP may present with nonspecific musculoskeletal manifestations, particularly in adults (Berkseth et al., 2013). The patient described here initially presented with pain, and sought help from a variety of medical specialists, including pain management, endocrinology, and orthopedic surgery, before referral to rheumatology. During his 2-year search for the cause of his symptoms, the patient experienced progressive diffuse pain and multiple fractures, which ultimately prompted a workup for osteomalacia and the finding of low serum alkaline phosphatase activity. This case illustrates the difficulty adult patients with HPP may have in obtaining an accurate diagnosis (Girschick et al., 2007, Whyte et al., 2013, Sorensen and Flodgaard, 1975), and highlights the importance of determining and recognizing the clinical significance of low alkaline phosphatase activity level as part of differential diagnosis in patients with musculoskeletal complaints, including undefined pain.
The clinical significance of low serum alkaline phosphatase is underappreciated and may be overlooked (McKiernan et al., 2014). In the present case, the finding of low serum alkaline phosphatase activity, which should prompt consideration of HPP, was repeatedly missed. It was not until osteomalacia was suspected, due to the non-traumatic ankle fractures, that consistently low values of serum alkaline phosphatase were noted in the patient's record, dating back to June 2010 (earliest available, after the initial complaint of leg and back pain). As alkaline phosphatase levels can increase during fracture healing, this finding raised suspicion for HPP and led to examination of PLP levels, a referral for genetic testing, and, ultimately, the correct diagnosis of HPP.
Pain is a frequent complaint by patients with HPP (Whyte, 2013, Berkseth et al., 2013), and may result from ectopic calcification in the joints (Berkseth et al., 2013, Guañabens et al., 2014, Metab et al., 2012), inflammation (Beck et al., 2009, Girschick et al., 1999), fractures, and/or pseudofractures (Whyte, 2013, Harper, 1989, Fallon et al., 1984, Whyte et al., 1982). In the present case, there was no indication of ectopic calcification by X-ray or MRI, and neither initial nor continued complaints of generalized severe musculoskeletal pain could be completely attributed to his fractures. Although nonsteroidal anti-inflammatory drugs have been reported to improve pain in some HPP patients (Girschick et al., 1999), they did not provide relief in this patient. Treatment remains focused primarily on pain management with narcotics.
HPP may result from autosomal recessive or autosomal dominant inheritance. The location of the gene mutation influences the level of residual enzymatic activity of the resulting TNSALP protein (Mornet, 2013). In the present case, a single c.500C > T mutation was found on exon 6 of ALPL. This mutation has been determined to result in no residual alkaline phosphatase activity in an in vitro site-directed mutagenesis assay, and it has been identified previously in a single patient with infantile HPP, as part of a compound heterozygous genotype with a c.571G > A mutation (Mornet, 2015). The c.571G > A mutation has been documented in several patients, and to date, appears only to result in symptomatic HPP when part of a compound heterozygote genotype (Henthorn et al., 1992). Thus, the present patient may express autosomal dominant inheritance, consistent with other reports of patients with single mutations and adult onset of HPP symptoms (Whyte, 2012, Fauvert et al., 2009). Although the patient reported no family history of HPP or skeletal or dental disease, it would be of interest to screen close family members for the mutation and possible symptoms. The patient's chart did not state whether he had siblings or children, which would only have been noted if they had relevant history.
Upon questioning, patients presenting with HPP in adulthood may recall symptoms of HPP in childhood, such as early tooth loss or rickets (Whyte, 2013). In a retrospective study at the Mayo Clinic, 9% of patients diagnosed with HPP as adults recalled earlier symptoms (Berkseth et al., 2013). Despite the presence of a previously reported inactivating mutation (Mornet, 2015), the current patient did not recall any prior symptoms, and he is of normal stature with no periodontal disease. The cause of the sudden onset of his pain and fractures in his 50s is unclear. Epigenetic regulation and dietary mineral intake may influence alkaline phosphatase activity (Whyte, 2013, Mornet, 2013), while bisphosphonate treatment may have precipitated symptoms in other patients with adult-onset HPP (Sutton et al., 2012, Cundy et al., 2015). However, the majority of patients with adult-onset HPP also lack a clear precipitating cause, and the factors contributing to a shift from a patient being an asymptomatic carrier to exhibiting active disease require further investigation.
The course of HPP is highly variable and not well characterized in adults. In the present case, a rapid accumulation of morbidities followed the initial onset of symptoms in 2009, leading to progressive disability, which ultimately rendered the patient unable to work. This progression is remarkable but not unique (Weinstein and Whyte, 1981a, Weinstein and Whyte, 1981b, Anderton, 1979, Schalin-Jäntti et al., 2010), although several of the cases with similar adult trajectories report onset of symptoms prior to adulthood. Together, these cases illustrate the potential severity of HPP with risk of deterioration and decreased quality of life in adult patients with HPP regardless of age at onset, and the need for careful monitoring and management of patients with HPP.
Asfotase alfa, a recombinant human TNSALP enzyme-replacement therapy, is undergoing development as a medical treatment for HPP (Whyte et al., 2012), and was recently approved by the Japanese, Canadian, and European Union health authorities. Current treatment is typically focused on symptom management, including medications for pain and surgical fixation to aid fracture healing (Coe et al., 1986), as described for this patient. Attempted treatment of HPP with teriparatide, a bone anabolic agent (Hodsman et al., 2005), has resulted in some patients reporting improvement in pain and fracture healing over 1–3 years of follow-up (Doshi et al., 2009, Silva et al., 2012, Camacho et al., 2008, Whyte et al., 2007), while others experienced only transient benefits (Schalin-Jäntti et al., 2010, Camacho et al., 2008, Gagnon et al., 2010) or are unable to tolerate the treatment due to increased pain (Laroche, 2012). These reports show, at most, minimal and transient effects of teriparatide on alkaline phosphatase and substrate levels, which do not attain normal ranges even when some clinical benefit is observed (Schalin-Jäntti et al., 2010, Doshi et al., 2009, Camacho et al., 2008, Whyte et al., 2007, Gagnon et al., 2010). Effects of teriparatide may depend on the level of residual alkaline phosphatase activity associated with a given genotype (Whyte, 2013). The patient described herein declined teriparatide treatment based on his independent assessment of the benefit:risk of teriparatide treatment for HPP. In managing patients with HPP, it is critical to avoid use of bisphosphonates due to their structural similarity with PPi (Drake et al., 2008). As elevation of PPi is the primary mechanism underlying mineralization deficits seen in HPP, bisphosphonates further impair bone mineralization in patients with HPP (Sutton et al., 2012), and result in exacerbation of disease (Cundy et al., 2015). Caution is also needed when considering supplemental vitamin D, as vitamin D supplementation in patients with HPP may result in hypercalcemia (Berkseth et al., 2013, Eisenberg and Pimstone, 1967).
The patient presented herein represents one example of the broad spectrum of presentations associated with HPP, including pain, recurrent, non-traumatic, and non-healing fractures, progressive loss of mobility, and diminished quality of life. This case illustrates the challenges of recognizing, diagnosing, and treating HPP, and highlights the need to consider serum alkaline phosphatase activity levels in patients presenting with nonspecific pain.
Authors' roles
NB managed the patient, collected and interpreted the data, drafted, revised, and approved the final manuscript for submission, and takes responsibility for the integrity of the content.
Role of the funding source
Alexion Pharmaceuticals Inc. provided funding for editorial and writing assistance of the manuscript.
Conflict of interest
None.
Acknowledgments
The author thanks John Adams, MD for helpful consultations on the case. Editorial and writing support were provided by Alexion Pharmaceuticals and Fishawack Communications GmbH.
References
- Anderton J.M. Orthopaedic problems in adult hypophosphatasia: a report of two cases. J. Bone Joint Surg. (Br.) 1979;61(1):82–84. doi: 10.1302/0301-620X.61B1.422640. (Feb) [DOI] [PubMed] [Google Scholar]
- Beck C., Morbach H., Richl P., Stenzel M., Girschick H.J. How can calcium pyrophosphate crystals induce inflammation in hypophosphatasia or chronic inflammatory joint diseases? Rheumatol. Int. 2009;29(3):229–238. doi: 10.1007/s00296-008-0710-9. (Jan) [DOI] [PubMed] [Google Scholar]
- Berkseth K.E., Tebben P.J., Drake M.T., Hefferan T.E., Jewison D.E., Wermers R.A. Bone. Vol. 54. Elsevier Inc.; 2013. Clinical spectrum of hypophosphatasia diagnosed in adults; pp. 21–27. (May) [DOI] [PubMed] [Google Scholar]
- Camacho P.M., Painter S., Kadanoff R. Treatment of adult hypophosphatasia with teriparatide. Endocr. Pract. 2008;14(2):204–208. doi: 10.4158/EP.14.2.204. (Mar) [DOI] [PubMed] [Google Scholar]
- Coe J.D., Murphy W.A., Whyte M.P. Management of femoral fractures and pseudofractures in adult hypophosphatasia. J. Bone Joint Surg. Am. 1986;68(7):981–990. (Sep) [PubMed] [Google Scholar]
- Cundy T., Michigami T., Tachikawa K., Dray M., Collins J.F., Paschalis E.P., Gamsjaeger S., Roschger A., Fratzl-Zelman N., Roschger P., Klaushofer K. Reversible deterioration in hypophosphatasia caused by renal failure with bisphosphonate treatment. J. Bone Miner. Res. 2015;4:1–29. doi: 10.1002/jbmr.2495. (Mar) [DOI] [PubMed] [Google Scholar]
- Doshi K.B., Hamrahian A.H., Licata A.A. Teriparatide treatment in adult hypophosphatasia in a patient exposed to bisphosphonate: a case report. Clin. Cases Miner. Bone Metab. 2009;6(3):266–269. (Sep) [PMC free article] [PubMed] [Google Scholar]
- Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83(9):1032–1045. doi: 10.4065/83.9.1032. (Sep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Pimstone B. Hypophosphatasia in an adult. A case report. Clin. Orthop. Relat. Res. 1967;52:199–212. doi: 10.1097/00003086-196700520-00016. [DOI] [PubMed] [Google Scholar]
- Fallon M.D., Teitelbaum S.L., Weinstein R.S., Goldfischer S., Brown D.M., Whyte M.P. Hypophosphatasia: clinicopathologic comparison of the infantile, childhood, and adult forms. Medicine (Baltimore) 1984;63(1):12–24. (Jan) [PubMed] [Google Scholar]
- Fauvert D., Brun-Heath I., Lia-Baldini A.-S., Bellazi L., Taillandier A., Serre J.-L., de Mazancourt P., Mornet E. Mild forms of hypophosphatasia mostly result from dominant negative effect of severe alleles or from compound heterozygosity for severe and moderate alleles. BMC Med. Genet. 2009;10:51. doi: 10.1186/1471-2350-10-51. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon C., Sims N.A., Mumm S., McAuley S.A., Jung C., Poulton I.J., Ng K.W., Ebeling P.R. Lack of sustained response to teriparatide in a patient with adult hypophosphatasia. J. Clin. Endocrinol. Metab. 2010;95(3):1007–1012. doi: 10.1210/jc.2009-1965. (Mar) [DOI] [PubMed] [Google Scholar]
- Girschick H.J., Mornet E., Beer M., Warmuth-Metz M., Schneider P. Chronic multifocal non-bacterial osteomyelitis in hypophosphatasia mimicking malignancy. BMC Pediatr. 2007;7:3. doi: 10.1186/1471-2431-7-3. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girschick H.J., Seyberth H.W., Huppertz H.I. Treatment of childhood hypophosphatasia with nonsteroidal antiinflammatory drugs. Bone. 1999;25(5):603–607. doi: 10.1016/s8756-3282(99)00203-3. (Nov) [DOI] [PubMed] [Google Scholar]
- Guañabens N., Mumm S., Möller I., González-Roca E., Peris P., Demertzis J.L., Whyte M.P. Calcific periarthritis as the only clinical manifestation of hypophosphatasia in middle-aged sisters. J. Bone Miner. Res. 2014;29(4):929–934. doi: 10.1002/jbmr.2110. (Apr) [DOI] [PubMed] [Google Scholar]
- Harper M.C. Metabolic bone disease presenting as multiple recurrent metatarsal fractures: a case report. Foot Ankle. 1989;9(4):207–209. doi: 10.1177/107110078900900412. (Feb 1) [DOI] [PubMed] [Google Scholar]
- Henthorn P.S., Raducha M., Fedde K.N., Lafferty M.A., Whyte M.P. Different missense mutations at the tissue-nonspecific alkaline phosphatase gene locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc. Natl. Acad. Sci. U. S. A. 1992;89(20):9924–9928. doi: 10.1073/pnas.89.20.9924. (Oct 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodsman A.B., Bauer D.C., Dempster D.W., Dian L., Hanley D.A., Harris S.T., Kendler D.L., McClung M.R., Miller P.D., Olszynski W.P., Orwoll E., Yuen C.K. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr. Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- Laroche M. Failure of teriparatide in treatment of bone complications of adult hypophosphatasia. Calcif. Tissue Int. 2012;90(3):250. doi: 10.1007/s00223-011-9562-5. (Mar) [DOI] [PubMed] [Google Scholar]
- McKiernan F.E., Berg R.L., Fuehrer J. Clinical and radiographic findings in adults with persistent hypophosphatasemia. J. Bone Miner. Res. 2014;29(7):1651–1660. doi: 10.1002/jbmr.2178. (Jul) [DOI] [PubMed] [Google Scholar]
- Metab J.B.M., Fukushi K.I.J., Fujiwara T., Iida K.-I., Fukushi J.-I., Oda Y., Iwamoto Y. Adult hypophosphatasia with painful periarticular calcification treated with surgical resection. J. Bone Miner. Metab. 2012;30(6):722–725. doi: 10.1007/s00774-011-0338-9. (Nov) [DOI] [PubMed] [Google Scholar]
- Mornet E., Hofmann C., Bloch-Zupan A., Girschick H., Le Merrer M. Clinical utility gene card for: hypophosphatasia — update 2013. Eur. J. Hum. Genet. 2014;22(4):1–6. doi: 10.1038/ejhg.2013.177. (Apr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet E. Genetics of hypophosphatasia. Clin. Rev. Bone Miner. Metab. 2013;11(2):71–77. (May 24) [Google Scholar]
- Mornet E. The Tissue Nonspecific Alkaline Phosphatase Gene Mutations Database [Internet] 2015. http://www.sesep.uvsq.fr/03_hypo_mutations.php [updated 20015 May 06; cited 2015 May 12]. Available from:
- Rockman-Greenberg C. Hypophosphatasia. Pediatr. Endocrinol. Rev. 2013;10(Suppl. 2):380–388. (Jun) [PubMed] [Google Scholar]
- Schalin-Jäntti C., Mornet E., Lamminen A., Välimäki M.J. Parathyroid hormone treatment improves pain and fracture healing in adult hypophosphatasia. J. Clin. Endocrinol. Metab. 2010;95(12):5174–5179. doi: 10.1210/jc.2010-1168. (Dec) [DOI] [PubMed] [Google Scholar]
- Silva I., Castelão W., Mateus M., Branco J.C. Childhood hypophosphatasia with myopathy: clinical report with recent update. Acta Reumatol. Port. 2012;37(1):92–96. [PubMed] [Google Scholar]
- Sorensen E., Flodgaard H. Adult hypophosphatasia. Acta Med. Scand. 1975;197(5):357–360. doi: 10.1111/j.0954-6820.1975.tb04934.x. (May) [DOI] [PubMed] [Google Scholar]
- Sutton R., Mumm S., Coburn S., Ericson K., Whyte M. “Atypical femoral fractures” during bisphosphonate exposure in adult hypophosphatasia. J. Bone Miner. Res. 2012;27(5):987–994. doi: 10.1002/jbmr.1565. (May) [DOI] [PubMed] [Google Scholar]
- Weinstein R.S., Whyte M.P. Heterogeneity of adult hypophosphatasia. Report of severe and mild cases. Arch. Intern. Med. 1981;141(6):727–731. (May) [PubMed] [Google Scholar]
- Weinstein R.S., Whyte M.P. Fifty-year follow-up of hypophosphatasia. Arch. Intern. Med. 1981;141(12):1720–1721. doi: 10.1001/archinte.141.12.1720. (Nov) [DOI] [PubMed] [Google Scholar]
- Whyte M. In: Hypophosphatasia. Thakker R., Whyte M., Eisman J., Igarashi T., editors. Elsevier; 2013. pp. 337–360. (Genet. Bone Biol. Skelet. Dis.). [Google Scholar]
- Whyte M.P. Hypophosphatasia. In: Glorieux F., Pettifor J., Juppner H., editors. Pediatr. Bone. 2nd ed. Elsevier; 2012. pp. 771–794. [Google Scholar]
- Whyte M.P., Greenberg C.R., Salman N.J., Bober M.B., McAlister W.H., Wenkert D., Van Sickle B.J., Simmons J.H., Edgar T.S., Bauer M.L., Hamdan M.A., Bishop N., Lutz R.E., McGinn M., Craig S., Moore J.N., Taylor J.W., Cleveland R.H., Cranley W.R., Lim R., Thacher T.D., Mayhew J.E., Downs M., Millán J.L., Skrinar A.M., Crine P., Landy H. Enzyme-replacement therapy in life-threatening hypophosphatasia. N. Engl. J. Med. 2012;366:904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- Whyte M.P., Leelawattana R., Reinus W.R., Yang C., Mumm S., Novack D.V. Acute severe hypercalcemia after traumatic fractures and immobilization in hypophosphatasia complicated by chronic renal failure. J. Clin. Endocrinol. Metab. 2013;98(12):4606–4612. doi: 10.1210/jc.2013-1811. (Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte M.P., Mumm S., Deal C. Adult hypophosphatasia treated with teriparatide. J. Clin. Endocrinol. Metab. 2007;92(4):1203–1208. doi: 10.1210/jc.2006-1902. (Apr) [DOI] [PubMed] [Google Scholar]
- Whyte M.P., Murphy W.A., Fallon M.D. Adult hypophosphatasia with chondrocalcinosis and arthropathy. Variable penetrance of hypophosphatasemia in a large Oklahoma kindred. Am. J. Med. 1982;72(4):631–641. doi: 10.1016/0002-9343(82)90474-0. (Apr) [DOI] [PubMed] [Google Scholar]