Abstract
Background: Physical function is integral to healthy aging, in particular as a core component of mobility and independent living in older adults, and is a strong predictor of mortality. Limited research has examined the role of diet, which may be an important strategy to prevent or delay a decline in physical function with aging.
Objective: We prospectively examined the association between the Alternative Healthy Eating Index-2010 (AHEI-2010), a measure of diet quality, with incident impairment in physical function among 54,762 women from the Nurses' Health Study.
Methods: Physical function was measured by the Medical Outcomes Short Form-36 (SF-36) physical function scale and was administered every 4 y from 1992 to 2008. Cumulative average diet was assessed using food frequency questionnaires, administered approximately every 4 y. We used multivariable Cox proportional hazards models to estimate the HRs of incident impairment of physical function.
Results: Participants in higher quintiles of the AHEI-2010, indicating a healthier diet, were less likely to have incident physical impairment than were participants in lower quintiles (P-trend < 0.001). The multivariable-adjusted HR of physical impairment for those in the top compared with those in the bottom quintile of the AHEI-2010 was 0.87 (95% CI: 0.84, 0.90). For individual AHEI-2010 components, higher intake of vegetables (P-trend = 0.003) and fruits (P-trend = 0.02); lower intake of sugar-sweetened beverages (P-trend < 0.001), trans fats (P-trend = 0.03), and sodium (P-trend < 0.001); and moderate alcohol intake (P-trend < 0.001) were each significantly associated with reduced rates of incident physical impairment. Among top contributors to the food components of the AHEI-2010, the strongest relations were found for increased intake of oranges, orange juice, apples and pears, romaine or leaf lettuce, and walnuts. However, associations with each component and with specific foods were generally weaker than the overall score, indicating that overall diet pattern is more important than individual parts.
Conclusions: In this large cohort of older women, a healthier diet was associated with a lower risk of developing impairments in physical function.
Keywords: diet quality, physical function, aging, Nurses’ Health Study, longitudinal cohort study, epidemiology
Introduction
In the United States, the proportion of the population 65 y or older is expected to reach nearly 20% by the year 2030 (1). Physical function is increasingly recognized as key to healthy aging, in particular as a core component of mobility and independent living in older adults. Prior research has demonstrated that poor physical function is related to hospitalization (2), long-term nursing home care (3, 4), and increased mortality (4, 5) among older adults. It is thus critical to identify modifiable factors that might prevent or delay physical function decline.
The Alternative Healthy Eating Index 2010 (AHEI-2010)9 was created as an update to the Alternative Healthy Eating Index and incorporates foods and nutrients predictive of chronic disease risk (6). The AHEI-2010 emphasizes the intake of whole compared with refined grains and distinguishes proteins based on individual health impacts (e.g., nuts, legumes, fish, and red and processed meats are considered separately) (6). Higher adherence to the AHEI-2010 has been associated with better lipid and inflammatory profile and decreased risk of clinical vascular disease (6). These factors have all been previously related to physical function (7–9). Prior studies have also indicated that low intake of some micronutrients may be associated with reduced physical performance, indicating that diet may play an important role in the prevention of impairment in physical function (10).
However, to our knowledge, there has been one long-term prospective study on the relation between diet quality and physical function. Thus, we used data from 54,762 participants from the Nurses’ Health Study to examine the association between the AHEI-2010 and incident impairment in physical function over 18 y of follow-up.
Methods
Study population.
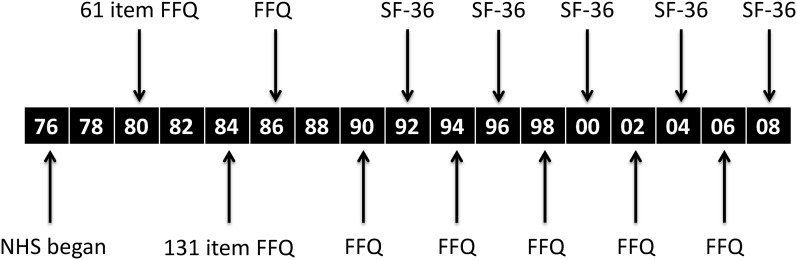
The Nurses’ Health Study began in 1976, when female registered nurses, aged 30–55 y, completed a mailed questionnaire on their health and lifestyle. Follow-up questionnaires have been mailed to participants every 2 y thereafter, and follow-up remains complete for >90%. Beginning in 1980, a FFQ was included, which was repeated in 1984, 1986, and every 4 y thereafter (11). In 1992, 1996, 2000, 2004, and 2008, the Medical Outcomes Study Short Form-36 (SF-36) was administered, a 36-item questionnaire that evaluates 8 health concepts, including physical functioning. The study was approved by the Institutional Review Board of Brigham & Women’s Hospital.
Diet assessment.
On the FFQ, participants report the average frequency of food consumption during the previous year, by specified units or standard portion sizes, by use of 9 possible responses ranging from “never or less than once/mo” to “6 or more times/d.” The FFQ in the Nurses’ Health Study has been validated carefully against repeated 7-d diet records, and reproducibility of the dietary questionnaires has been documented (12, 13).
Criteria and methods for scoring of the AHEI-2010 have been previously described in detail (6); the AHEI-2010 was developed to incorporate data from food frequency questionnaires (6). Briefly, the AHEI-2010 consists of 11 components: 6 components for which higher intakes are better (vegetables, fruit, whole grains, nuts and legumes, long-chain omega-3 FAs, and PUFAs); 1 component for which moderate intake is better (alcohol: 2.5 or more drinks/d is assigned 0 points, nondrinkers are assigned 2.5 points, and 0.5–1.5 drinks/d is assigned 10 points); and 4 components for which lower intake is better (sugar-sweetened beverages and fruit juice, red and processed meats, trans fats, and sodium). Each component is given a minimal score of 0 to indicate “worst” level of intake and a maximum score of 10 to indicate “best” level of intake, with intermediate values scored proportionally. The best levels of intake were determined a priori and based on a combination of the current dietary guidelines and the scientific literature regarding the dietary factor and chronic disease risk. All of the component scores are summed to obtain the total AHEI-2010 score, with a range from 0 (nonadherence) to 110 (perfect adherence). For these analyses, to reduce measurement error and to represent long-term dietary intake, the cumulative mean of all AHEI-2010 scores from 1980 to the start of a given follow-up period was calculated at each 4-y follow-up cycle. Thus, because baseline physical function in this analysis was in 1992, at the first follow-up cycle, we averaged all dietary assessments from 1980 through 1990 (see Figure 1); at each subsequent follow-up cycle, the diet data for another year were incorporated into the cumulative mean.
FIGURE 1.
Timeline for data collection in the NHS. Black boxes represent years: 1976, 1990, 2000, 2008, etc. NHS, Nurses’ Health Study; SF-36, Medical Outcomes Short Form-36.
Physical function.
Information on physical function was collected by use of the SF-36 questionnaire, a widely used and validated instrument (14). The physical function score (PFS) is a consistent and reliable predictor of morbidity and mortality in a variety of populations (5, 15, 16). The PFS was administered to participants starting in 1992 and every 4 y thereafter and is comprised of 10 questions regarding physical limitations in performing the following activities: bathing/dressing yourself, walking one block, walking several blocks, walking more than one mile, bending/kneeling, climbing stairs, lifting groceries, moderate activities, and vigorous activities. Each question has the same 3 response choices; each answer of “Yes, limited a lot” is assigned 1 point, an answer of “Yes, limited a little” is assigned 2 points, and an answer of “No, not limited at all” is assigned 3 points. A raw score is calculated from the set of 10 questions and ranges from a minimum of 10 points to a maximum of 30 points. The raw score is then transformed to a 100-point scale. A PFS score of 100 is considered highest physical function, and a score of 80 or less is considered substantial physical impairment (17); this cutoff point has been used in other epidemiologic studies (17, 18). At the end of each follow-up cycle, incident cases of impairment were defined as a PFS decreasing to 80 or below. As an additional way to test the face validity of the PFS scoring in our cohort, we found that only 10% of participants who scored above 80 on the PFS reported adverse physical impact on their ability to perform their work or other daily activities; in contrast, 40% of even those who scored between 70 and 80 also reported limitations in daily activities caused by physical health. Thus, multiple lines of evidence support this cutoff point.
Statistical analysis.
Women were excluded from this analysis if they did not complete the FFQ at analytic baseline or had an unreasonably high (>3,500 kcal/d) or low (<500 kcal/d) caloric intake. Additionally, women with prevalent physical impairment (PFS ≤ 80) in 1992, or women who were missing information on either the AHEI-2010 or PFS score at baseline were excluded from this analysis. The final baseline population included 54,762 women in 1992.
To evaluate the association between quintiles of the AHEI-2010 score and incident impairment in physical function, we used age-adjusted and multivariable-adjusted Cox proportional hazards models. Sociodemographic, lifestyle, and health-related covariates were obtained from the questionnaires and updated at each 4-y time period in the analysis. Multivariable-adjusted models included primary, a priori risk factors for physical function impairment: BMI (continuous), total caloric intake (quintiles), physical activity [<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27 metabolic equivalents of task (METs)/wk], SF-36 Mental Health Index score (continuous), smoking status (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, or current ≥25 cigarettes/d), history of hypertension (yes or no), high cholesterol (yes or no), myocardial infarction (yes or no), stroke (yes or no), and type 2 diabetes (yes or no). There were few missing data, but missing data on BMI, physical activity, and smoking status were accounted for by carrying forward data from the previous questionnaire cycle or creating a missing indicator variable. In the Cox proportional hazards models examining the individual components of the AHEI-2010 score, we adjusted for the same potential confounders and also included, simultaneously in the model, the AHEI-2010 score without the component of interest. Because physical activity is so highly related to physical function, we modeled physical activity as a continuous variable and a categorical variable and also conducted models with and without physical activity and with and without updating physical activity at each time point.
We also conducted analyses in which we investigated the relation between the top 5 contributors to the food component groups based on caloric intake in the study population: fruits, vegetables, nuts/legumes, red/processed meats, or sugar-sweetened beverages. The specific foods were categorized into servings of “never or <1/mo,” “1–3 times/mo,” “1/wk,” or “≥2/wk.” These are the response categories provided on the FFQ, with the top categories of intake collapsed into one category because of smaller numbers. For the nuts/legumes component (but not for primary analyses of nuts), we began follow-up in 1998 because more detailed information on specific types of nuts eaten was collected on the 1998 FFQ, permitting specific analyses of different nut types. Tests for trend across quintiles of the total AHEI-2010 score and score components were calculated by treating the categories as an ordinal variable in the proportional hazards models and assigning the median value for that category.
To assess possible sources of bias, especially caused by the possibility that women with early signs of physical function decline may change their diet, we conducted several secondary analyses. First, we conducted analyses in which we excluded participants with a borderline PFS (>80–85 points) at the start of each follow-up period. In another analysis, we imposed a 6-y lag period between diet assessment and physical function assessment. In additional research to consider diet at midlife, we also investigated the association between diet score at baseline and subsequent physical function. Because vascular factors could be potential intermediates, we also constructed multivariable models without these factors. To further consider vascular factors, we constructed models among those with and without hypertension at baseline and among those with and without high cholesterol at baseline. Lastly, we conducted analyses to examine effect modification by age by separately examining women <60, 60–66, and >66 y of age at analytic baseline in 1992. All analyses were conducted in SAS version 9.3 (SAS Institute).
Results
Characteristics of the study population.
Characteristics of women according to quintiles of the AHEI-2010 at baseline in 1992 are presented in Table 1. In these descriptive results, there were few apparent differences in health and lifestyle characteristics of women across AHEI-2010 categories. However, 8.6% of women in the highest quintile (i.e., healthiest diet) of AHEI-2010 were current smokers, 16.1% had master’s or doctoral degrees, and mean METs/wk were 27.8; in the lowest AHEI quintile, 19.8% of women were current smokers, 6.4% had master’s or doctoral degrees, and mean METs/wk were 15.9.
TABLE 1.
Age-standardized baseline characteristics in 1992 of women in the Nurses’ Health Study by quintile of the AHEI-20101
| AHEI-2010 |
|||||
| Quintile 1 (median = 38,n = 9748) | Quintile 2 (median = 45,n = 10,707) | Quintile 3 (median = 50,n = 10,874) | Quintile 4 (median = 56,n = 11,535) | Quintile 5 (median = 64,n = 11,898) | |
| Age,2 y | 53.9 ± 6.9 | 55.0 ± 7.0 | 55.8 ± 7.0 | 56.6 ± 6.9 | 57.8 ± 6.8 |
| BMI, kg/m2 | 25.3 ± 4.7 | 25.4 ± 4.6 | 25.3 ± 4.6 | 25.2 ± 4.6 | 24.7 ± 4.3 |
| Physical activity, METs/wk | 15.9 ± 19.2 | 18.7 ± 22.2 | 20.8 ± 23.7 | 23.2 ± 24.8 | 27.8 ± 29.4 |
| SF-36 Mental Health Index | 76.9 ± 14.2 | 77.7 ± 13.7 | 78.2 ± 13.4 | 78.5 ± 13.2 | 79.0 ± 13.1 |
| Smoking, % | |||||
| Never | 48.6 | 47.9 | 46.4 | 43.9 | 40.7 |
| Past | 31.7 | 36.8 | 39.5 | 44.3 | 50.7 |
| Current | |||||
| 1–14 cigarettes/d | 7.0 | 6.2 | 6.1 | 6.0 | 4.7 |
| 15–24 cigarettes/d | 8.6 | 6.6 | 5.7 | 4.4 | 3.1 |
| ≥25 cigarettes/d | 4.2 | 2.5 | 2.3 | 1.4 | 0.8 |
| Alcohol intake (g/d), % | |||||
| 0 | 52.4 | 41.6 | 35.4 | 30.6 | 24.6 |
| 1–14 | 34.9 | 48.0 | 54.8 | 60.1 | 67.5 |
| ≥15 | 12.7 | 10.4 | 9.9 | 9.3 | 7.9 |
| Education, % | |||||
| Registered nurse | 76.2 | 72.3 | 68.8 | 64.2 | 59.0 |
| Bachelor’s | 17.4 | 19.3 | 21.3 | 22.5 | 24.9 |
| Master’s/doctoral | 6.4 | 8.4 | 9.9 | 13.3 | 16.1 |
| Hypertension, % | 28.5 | 28.3 | 28.9 | 27.8 | 26.3 |
| High cholesterol, % | 40.5 | 42.2 | 42.8 | 42.4 | 42.4 |
| Myocardial infarction, % | 0.7 | 0.7 | 0.7 | 0.8 | 0.5 |
| Stroke, % | 0.5 | 0.6 | 0.6 | 0.6 | 0.5 |
| Type 2 diabetes, % | 3.8 | 3.7 | 3.6 | 3.5 | 3.2 |
| Total energy intake, kcal/d | 1825 ± 511 | 1770 ± 515 | 1742 ± 515 | 1712 ± 515 | 1699 ± 511 |
| Baseline PFS score | 93.9 ± 5.3 | 94.2 ± 5.3 | 94.5 ± 5.2 | 94.7 ± 5.1 | 95.0 ± 5.1 |
Values are means ± SDs or percentages. Values of polytomous variables may not sum to 100% because of rounding. AHEI-2010, Alternative Healthy Eating Index-2010; MET, metabolic equivalent of task; PFS, Physical Function Score; SF-36, Medical Outcomes Short Form-36.
Value is not age adjusted.
AHEI-2010 and risk of physical function impairment.
In age-adjusted models (Table 2), the HR of incident impairment in physical function was 0.71 (95% CI: 0.69, 0.73; P-trend < 0.001) comparing women in the highest quintile of AHEI-2010 score with those the lowest quintile. After controlling for numerous potential confounders, this HR was attenuated but remained significant (HR: 0.87; 95% CI: 0.84, 0.90; P-trend < 0.001). The results were similar after excluding participants with a borderline PFS at the start of each cycle; imposing a 6-y lag between assessment of diet and of physical function; removing vascular factors from multivariable-adjusted models; stratifying by hypertension or high cholesterol at baseline; modeling physical activity in different ways; and stratifying by age (results not shown). In analyses in which we examined only baseline/mid-life AHEI-2010 (i.e., we did not update diet at each cycle), we found a similar reduced risk of impairment (e.g., for top compared with bottom quintile of AHEI-2010 in 1990, HR: 0.86; 95% CI: 0.83, 0.89). In addition, the correlation between diet score at baseline and diet score in 2002 was 0.52, indicating that diet patterns remained fairly consistent over time.
TABLE 2.
HRs (95% CIs) of incident physical impairment, measured by the Physical Function scale of the SF-36, in women in the Nurses’ Health Study by quintile of the overall AHEI-2010 score and AHEI-2010 score components1
| Quintiles of AHEI-2010 score |
||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | |
| Overall AHEI-2010 score | ||||||
| Median | 39.9 | 46.8 | 51.9 | 57.3 | 65.2 | |
| Person-years | 41,377 | 45,493 | 47,158 | 50,108 | 53,704 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 0.94 (0.91, 0.98) | 0.89 (0.86, 0.92) | 0.82 (0.79, 0.85) | 0.71 (0.69, 0.73) | <0.001 |
| Multivariable-adjusted HR2 (95% CI) | 1.0 (Ref) | 0.96 (0.93, 0.99) | 0.93 (0.90, 0.96) | 0.90 (0.87, 0.93) | 0.87 (0.84, 0.90) | <0.001 |
| Natural categories of AHEI-2010 score components | ||||||
| Vegetables | ||||||
| Median, servings/d | 1.63 | 2.42 | 3.09 | 3.90 | 5.34 | |
| Person-years | 42,434 | 46,635 | 48,751 | 49,172 | 50,848 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 0.98 (0.95, 1.02) | 0.96 (0.93, 1.00) | 0.94 (0.91, 0.97) | 0.87 (0.84, 0.90) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 0.99 (0.96, 1.03) | 0.98 (0.95, 1.01) | 0.98 (0.95, 1.02) | 0.95 (0.91, 0.98) | 0.003 |
| Fruits | ||||||
| Median, servings/d | 0.54 | 1.01 | 1.43 | 1.92 | 2.81 | |
| Person-years | 44,459 | 47,792 | 48,913 | 48,876 | 47,800 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 0.93 (0.90, 0.96) | 0.90 (0.87, 0.93) | 0.87 (0.84, 0.90) | 0.79 (0.77, 0.82) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 0.95 (0.92, 0.98) | 0.96 (0.92, 0.99) | 0.95 (0.92, 0.98) | 0.94 (0.91, 0.98) | 0.02 |
| Nuts and legumes | ||||||
| Median, servings/d | 0.07 | 0.15 | 0.25 | 0.38 | 0.68 | |
| Person-years | 43,529 | 46,267 | 48,023 | 49,324 | 50,697 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 1.04 (1.00, 1.07) | 1.03 (0.99, 1.06) | 1.02 (0.99, 1.05) | 0.97 (0.93, 1.00) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 1.02 (0.98, 1.05) | 1.01 (0.97, 1.04) | 1.03 (0.99, 1.06) | 1.06 (1.02, 1.10) | <0.001 |
| Red and processed meats | ||||||
| Median, servings/d | 0 | 0.45 | 0.77 | 1.10 | 1.61 | |
| Person-years | 46,287 | 48,844 | 49,857 | 48,752 | 44,100 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 0.94 (0.91, 0.97) | 1.03 (0.99, 1.06) | 1.11 (1.07, 1.14) | 1.20 (1.16, 1.24) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 0.90 (0.87, 0.93) | 0.92 (0.89, 0.95) | 0.94 (0.91, 0.97) | 0.97 (0.94, 1.00) | 0.4 |
| Sugar-sweetened beverages | ||||||
| Median, servings/d | 0.18 | 0.55 | 0.93 | 1.29 | 2.05 | |
| Person-years | 47,908 | 48,423 | 47,191 | 48,126 | 46,192 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 1.06 (1.03, 1.10) | 1.03 (0.99, 1.06) | 1.01 (0.98, 1.04) | 1.04 (1.00, 1.07) | 0.5 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 1.04 (1.00, 1.07) | 1.01 (0.98, 1.05) | 1.03 (0.99, 1.06) | 1.08 (1.04, 1.12) | <0.001 |
| Alcohol | ||||||
| Median, drinks/d | 0 | 0.04 | 0.14 | 0.45 | 1.27 | |
| Person-years | 51,988 | 31,210 | 49,156 | 53,398 | 52,088 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 1.04 (1.00, 1.08) | 0.93 (0.90, 0.96) | 0.88 (0.85, 0.90) | 0.87 (0.84, 0.90) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 0.94 (0.90, 0.97) | 0.91 (0.88, 0.94) | 0.90 (0.87, 0.93) | 0.92 (0.89, 0.95) | 0.02 |
| Whole grains | ||||||
| Median, g/d | 5.3 | 10.9 | 16.4 | 23.0 | 34.9 | |
| Person-years | 41,021 | 47,176 | 49,132 | 50,258 | 50,253 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 1.03 (0.99, 1.06) | 0.99 (0.96, 1.02) | 0.96 (0.92, 0.99) | 0.88 (0.85, 0.91) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 1.00 (0.97, 1.04) | 1.00 (0.96, 1.03) | 1.01 (0.97, 1.04) | 1.03 (1.00, 1.07) | 0.04 |
| trans fats | ||||||
| Median, % of energy | 0.009 | 0.012 | 0.014 | 0.017 | 0.021 | |
| Person-years | 52,234 | 49,654 | 48,139 | 43,019 | 41,794 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 1.14 (1.10, 1.17) | 1.23 (1.19, 1.27) | 1.30 (1.26, 1.34) | 1.28 (1.24, 1.32) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 0.98 (0.95, 1.01) | 1.01 (0.98, 1.04) | 1.02 (0.98, 1.05) | 1.02 (0.98, 1.06) | 0.03 |
| ω-3 FAs | ||||||
| Median, mg/d | 75.5 | 130 | 190 | 270 | 422.5 | |
| Person-years | 44,211 | 45,915 | 49,173 | 49,490 | 49,051 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 1.04 (1.01, 1.07) | 1.01 (0.98, 1.05) | 0.98 (0.95, 1.01) | 0.95 (0.92, 0.98) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 1.01 (0.97, 1.04) | 0.98 (0.95, 1.01) | 0.98 (0.94, 1.01) | 0.98 (0.95, 1.02) | 0.2 |
| PUFAs | ||||||
| Median, % of energy | 4.35 | 5.14 | 5.73 | 6.37 | 7.41 | |
| Person-years | 46,411 | 48,190 | 49,007 | 48,323 | 45,909 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 1.06 (1.02, 1.09) | 1.11 (1.08, 1.15) | 1.12 (1.08, 1.16) | 1.12 (1.08, 1.16) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 0.97 (0.94, 1.00) | 0.99 (0.96, 1.03) | 0.98 (0.94, 1.01) | 1.01 (0.97, 1.04) | 0.6 |
| Sodium | ||||||
| Median, mg/d | 1357 | 1772 | 2102 | 2479 | 3113 | |
| Person-years | 47,444 | 49,024 | 48,730 | 48,135 | 44,507 | |
| Age-adjusted HR (95% CI) | 1.0 (Ref) | 1.08 (1.04, 1.11) | 1.14 (1.10, 1.18) | 1.16 (1.12, 1.20) | 1.26 (1.21, 1.30) | <0.001 |
| Multivariable-adjusted HR3 (95% CI) | 1.0 (Ref) | 1.04 (1.01, 1.08) | 1.09 (1.05, 1.13) | 1.10 (1.05, 1.14) | 1.15 (1.10, 1.19) | <0.001 |
AHEI-2010, Alternative Healthy Eating Index-2010; MET, metabolic equivalent of task; Q, quintile; Ref, reference; SF-36, Medical Outcomes Short Form-36.
Models adjusted for BMI (continuous), total caloric intake (quintiles), physical activity (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27 METs/wk), SF-36 Mental Health Index (continuous), smoking (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, or current ≥25 cigarettes/d), hypertension (yes or no), high cholesterol (yes or no), myocardial infarction (yes or no), stroke (yes or no), and type 2 diabetes (yes or no).
Models adjusted for BMI (continuous), total caloric intake (quintiles), physical activity (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27 METs/wk), SF-36 Mental Health Index (continuous), smoking (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, or current ≥25 cigarettes/d), hypertension (yes or no), high cholesterol (yes or no), myocardial infarction (yes or no), stroke (yes or no), type 2 diabetes (yes or no), and the AHEI-2010 score without the component of interest.
AHEI-2010 score components, food contributors, and physical function impairment.
To ascertain whether specific individual components of the AHEI-2010 varied in importance, we examined the association between each AHEI-2010 component and risk of incident impairment in physical function (Table 2). In general, we found modest associations between each individual component and physical function, suggesting that overall dietary pattern is more important than its components. Only for sodium intake did we find HRs similar in magnitude to the overall HR of AHEI-2010 score; comparing extreme quintiles of sodium as mg/d, the HR was 1.15 (95% CI: 1.10, 1.19; P-trend < 0.001).
For the 5 food groups in the AHEI-2010, we also considered the top individual contributors in our cohort (by caloric intake) to each food group (Table 3). In multivariable-adjusted models, similar to findings for AHEI-2010 components, the overall pattern appeared more important than individual foods. Among the foods examined, the strongest relations were found for greater intake of oranges, orange juice, apples and pears, romaine or leaf lettuce, and walnuts; we found a HR of 0.91 (95% CI: 0.88, 0.95) for extreme intakes of oranges, a HR of 0.86 (95% CI: 0.81, 0.92) for extreme intakes of orange juice, a HR of 0.89 (95% CI: 0.82, 0.99) for extreme intakes of apples and pears, a HR of 0.88 (95% CI: 0.83, 0.93) for extreme intakes of romaine or leaf lettuce, and a HR of 0.93 (95% CI: 0.86, 0.99) comparing those who ate ≥2 servings of walnuts/d compared with <1 serving/mo.
TABLE 3.
HRs (95% CIs) of incident physical impairment, measured by the physical function scale of the SF-36, in women in the Nurses’ Health Study by top food contributors to AHEI-2010 food components1
| Servings |
|||||
| Never or <1/mo | 1–3/mo | 1/wk | ≥2 /wk | P-trend | |
| Fruits2 | |||||
| Bananas, 1 | 1.0 (Ref) | 0.95 (0.90, 1.00) | 0.94 (0.89, 0.99) | 0.96 (0.91, 1.01) | 0.9 |
| Fresh apples or pears, 1 | 1.0 (Ref) | 0.92 (0.85, 1.01) | 0.90 (0.82, 0.98) | 0.89 (0.82, 0.97) | 0.005 |
| Raisins or grapes, 1/2 cup (28 g) | 1.0 (Ref) | 0.95 (0.90, 1.00) | 0.98 (0.92, 1.03) | 0.97 (0.91, 1.03) | 0.2 |
| Oranges, 1 | 1.0 (Ref) | 0.94 (0.90, 0.97) | 0.95 (0.91, 0.99) | 0.91 (0.88, 0.95) | <0.001 |
| Peaches or plums, 1 fresh or 1/2 cup (115 g) canned | 1.0 (Ref) | 0.93 (0.87, 0.99) | 0.95 (0.88, 1.00) | 0.96 (0.89, 1.02) | 0.06 |
| Vegetables2 | |||||
| Tomatoes, 2 slices | 1.0 (Ref) | 1.04 (0.90, 1.19) | 1.08 (0.94, 1.23) | 1.05 (0.92, 1.20) | 0.4 |
| Iceberg or head lettuce, 1 cup (75 g) | 1.0 (Ref) | 0.88 (0.81, 0.97) | 0.88 (0.81, 0.97) | 0.87 (0.80, 0.95) | 0.2 |
| Onions as a garnish or in salad, 1 slice | 1.0 (Ref) | 0.96 (0.89, 1.05) | 0.93 (0.85, 1.01) | 0.95 (0.87, 1.02) | 0.4 |
| Romaine or leaf lettuce, 1 cup (75 g) | 1.0 (Ref) | 0.91 (0.86, 0.97) | 0.92 (0.86, 0.98) | 0.88 (0.83, 0.93) | 0.002 |
| Raw carrots, 1/2 a carrot or 2–4 sticks | 1.0 (Ref) | 0.96 (0.92, 1.00) | 0.97 (0.93, 1.02) | 0.95 (0.91, 0.99) | 0.02 |
| Nuts/legumes2,3 | |||||
| Peanut butter, 1 tablespoon (16 g) | 1.0 (Ref) | 1.02 (0.98, 1.07) | 1.05 (0.99, 1.11) | 1.07 (1.02, 1.13) | 0.01 |
| Beans or lentils, 1/2 cup (90 g) | 1.0 (Ref) | 0.99 (0.95, 1.03) | 0.98 (0.93, 1.03) | 1.01 (0.95, 1.07) | 0.7 |
| Peanuts, 1 oz (28 g) | 1.0 (Ref) | 0.97 (0.93, 1.01) | 0.98 (0.92, 1.05) | 0.97 (0.90, 1.04) | 0.8 |
| Other nuts, 1 oz (28 g) | 1.0 (Ref) | 1.05 (1.01, 1.08) | 1.06 (0.99, 1.13) | 1.05 (0.99, 1.11) | 0.2 |
| Walnuts, 1 oz (28 g) | 1.0 (Ref) | 1.00 (0.96, 1.03) | 0.93 (0.86, 1.00) | 0.93 (0.86, 0.99) | 0.02 |
| Red/processed meats2 | |||||
| Beef or lamb as a main dish, 4–6 oz (112–168 g) | 1.0 (Ref) | 1.01 (0.97, 1.06) | 1.02 (0.97, 1.07) | 1.01 (0.96, 1.07) | 0.9 |
| Beef or lamb as a mixed dish | 1.0 (Ref) | 1.00 (0.96, 1.04) | 1.02 (0.97, 1.07) | 1.06 (1.01, 1.11) | 0.002 |
| Lean hamburger, 1 patty | 1.0 (Ref) | 0.98 (0.94, 1.03) | 1.02 (0.97, 1.07) | 1.04 (0.99, 1.10) | 0.008 |
| Pork as a main dish, 4–6 oz (112–168 g) | 1.0 (Ref) | 0.89 (0.84, 0.94) | 0.88 (0.83, 0.93) | 0.89 (0.82, 0.96) | 0.1 |
| Bacon, 2 slices | 1.0 (Ref) | 1.03 (1.01, 1.06) | 1.08 (1.05, 1.12) | 1.11 (1.06, 1.17) | 0.01 |
| Sugar-sweetened beverages2 | |||||
| Orange juice, small glass | 1.0 (Ref) | 0.90 (0.84, 0.95) | 0.88 (0.82, 0.94) | 0.86 (0.81, 0.92) | <0.001 |
| Other fruit juices, small glass | 1.0 (Ref) | 1.05 (1.02, 1.08) | 1.08 (1.04, 1.12) | 1.11 (1.08, 1.15) | <0.001 |
| Punch, lemonade, sports drinks, or sugared iced tea, 1 glass, bottle, or can (355 mL) | 1.0 (Ref) | 0.98 (0.93, 1.03) | 0.96 (0.90, 1.02) | 1.02 (0.96, 1.08) | 0.004 |
| Carbonated beverage with caffeine and sugar, 1 glass, bottle, or can (355 mL) | 1.0 (Ref) | 0.95 (0.93, 0.98) | 0.98 (0.93, 1.03) | 0.97 (0.93, 1.01) | 0.1 |
| Other carbonated beverages with sugar, 1 glass, bottle, or can (355 mL) | 1.0 (Ref) | 0.90 (0.86, 0.94) | 0.94 (0.88, 1.01) | 0.99 (0.93, 1.06) | 0.007 |
AHEI-2010, Alternative Healthy Eating Index-2010; MET, metabolic equivalent of task; oz, ounce; Ref, reference; SF-36, Medical Outcomes Short Form-36.
Models adjusted for BMI (continuous), total caloric intake (quintiles), physical activity (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27 METs/wk), SF-36 Mental Health Index (continuous), smoking (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, or current ≥25 cigarettes/d), hypertension (yes or no), high cholesterol (yes or no), myocardial infarction (yes or no), stroke (yes or no), type 2 diabetes (yes or no), and the AHEI-2010 score without the component of interest.
Follow-up started in 2000.
Discussion
In this large, prospective study, greater adherence to the AHEI-2010 was associated with a lower risk of developing physical impairment over 18 y of follow-up. Overall, the AHEI diet pattern appeared more strongly associated with physical function than the individual components or individual foods, although greater intake of vegetables and fruits; moderate alcohol intake; and lower intake of sugar-sweetened beverages, trans fats, and sodium were all significantly associated with modestly lower rates of incident physical impairment. Similarly, greater intakes of oranges, orange juice, apples and pears, romaine or leaf lettuce, and walnuts were associated with reduced risk of physical function impairment.
Our results are consistent with the existing, although limited, literature that supports an association between diet quality and physical function. Most prior studies have been cross-sectional with modest sample sizes. These studies have reported that better diet quality is associated with better physical function, as measured by the SF-36 combined with an in-person assessment (19) or self-reported disability (20); in 2 cross-sectional studies that also used the SF-36 PFS as in our analysis, participants with better diet quality had significantly higher mean physical function scores (21, 22). However, in cross-sectional studies, it is plausible that better physical function may lead to better diet rather than the reverse. To our knowledge, there has been only one prospective study conducted. Among 3,000 participants in a French cohort of middle-aged adults, those with best adherence to dietary guidelines had increased physical function scores as measured by the SF-36 over the 12-y follow-up period (23), consistent with our findings. The epidemiologic research is supported by biologic research demonstrating that higher adherence to the AHEI-2010 is associated with a better lipid and inflammatory profile and decreased risk of clinical vascular disease (6). These factors are all strongly related to physical function (7–9) and thus provide a clear biological rationale for the findings observed in this analysis.
One somewhat surprising overall finding was that increased intake of nuts and legumes was associated with an increased risk of physical impairment. However, after we separated this component into the top 5 food contributors, we found the association was driven by increased intake of peanut butter, with no increased risk associated with peanuts or other nuts and a significantly reduced risk of impairment with greater walnut intake. Thus, there is no clear explanation for this isolated increase with peanut butter; it could potentially be a chance finding but deserves further investigation. Moreover, evidence from the PREDIMED (Prevención con Dieta Mediterránea) randomized trial has demonstrated that a Mediterranean diet [made up of components very similar to the AHEI-2010 (6)] supplemented with mixed nuts (24) leads to reduced blood pressure (25) and LDL cholesterol levels (26) and a reduction in the incidence of type 2 diabetes (27) and cardiovascular disease (28) compared with placebo; these are all associated with diminished physical function (7–9).
Our study has numerous strengths including the prospective design with 18 y of follow-up, the multiple measures of diet and physical function, the ability to control for multiple potential confounders, and the large sample size. Potential limitations also need to be considered. Residual confounding cannot be ruled out in an observational study, and thus the results should be interpreted with caution. However, associations between diet quality and physical function remained strong and significant after adjustment for a wide array of health and lifestyle factors. Also, there is potential for measurement error in both the dietary assessment and the outcome measurement. However, both assessment instruments are validated, and cumulative averages of diet were used to reduce measurement error of the exposure. Additionally, dietary intake was collected prospectively, and thus any misreporting of diet is expected to be random and would result in bias to the null, suggesting that our results may underestimate true associations.
In summary, we found that better diet quality as measured by the AHEI-2010 was associated with a lower risk of incident physical impairment among older women and that the overall diet quality appeared more important than individual components or foods. Given the value of physical function to healthy aging and quality of life, this may represent a particularly compelling public health rationale for persons to improve their diet.
Acknowledgments
MJS and FG designed the research; KAH and SEC conducted the research; KAH performed the statistical analyses; KAH, MJS, JNK, and FG wrote the paper; and KAH had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AHEI-2010, Alternative Healthy Eating Index-2010; MET, metabolic equivalent of task; PFS, physical function score; SF-36, Medical Outcomes Short Form-36.
References
- 1.Centers for Disease Control and Prevention. The State of Aging and Health in America 2013. Atlanta (GA): Centers for Disease Control and Prevention, US Department of Health and Human Services; 2013. [Google Scholar]
- 2.Motl RW, McAuley E. Physical activity, disability, and quality of life in older adults. Phys Med Rehabil Clin N Am 2010;21:299–308. [DOI] [PubMed] [Google Scholar]
- 3.Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, Ebrahim S. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008;371:725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 5.Cesari M, Onder G, Zamboni V, Manini T, Shorr RI, Russo A, Bernabei R, Pahor M, Landi F. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr 2008;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesari M, Onder G, Russo A, Zamboni V, Barillaro C, Ferrucci L, Pahor M, Bernabei R, Landi F. Comorbidity and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study). Gerontology 2006;52:24–32. [DOI] [PubMed] [Google Scholar]
- 8.Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev 2010;6:134–43. [DOI] [PubMed] [Google Scholar]
- 9.Akbaraly TN, Shipley MJ, Ferrie JE, Virtanen M, Lowe G, Hamer M, Kivimaki M. Long-term adherence to healthy dietary guidelines and chronic inflammation in the prospective Whitehall II study. Am J Med 2015;128:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartali B, Frongillo EA, Guralnik JM, Stipanuk MH, Allore HG, Cherubini A, Bandinelli S, Ferrucci L, Gill TM. Serum micronutrient concentrations and decline in physical function among older adults. JAMA 2008;299:308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–99. [DOI] [PubMed] [Google Scholar]
- 12.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FR. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 13.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 14.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–63. [DOI] [PubMed] [Google Scholar]
- 16.Haley SM, McHorney CA, Ware JE Jr. Evaluation of the MOS SF-36 physical functioning scale (PF-10): I. Unidimensionality and reproducibility of the Rasch item scale. J Clin Epidemiol 1994;47:671–84. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE. SF-36 Health Survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center;1993. [Google Scholar]
- 18.Lin J, Curhan GC. Kidney function decline and physical function in women. Nephrol Dial Transplant 2008;23:2827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson SM, Jameson KA, Syddall HE, Dennison EM, Cooper C, Aihie Sayer A. Clustering of lifestyle risk factors and poor physical function in older adults: the Hertfordshire cohort study. J Am Geriatr Soc 2013;61:1684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu B, Houston D, Locher JL, Zizza C. The association between Healthy Eating Index-2005 scores and disability among older Americans. Age Ageing 2012;41:365–71. [DOI] [PubMed] [Google Scholar]
- 21.Gopinath B, Russell J, Flood VM, Burlutsky G, Mitchell P. Adherence to dietary guidelines positively affects quality of life and functional status of older adults. J Acad Nutr Diet 2014;114:220–9. [DOI] [PubMed] [Google Scholar]
- 22.Milte CM, Thorpe MG, Crawford D, Ball K, McNaughton SA. Associations of diet quality with health-related quality of life in older Australian men and women. Exp Gerontol 2015;64:8–16. [DOI] [PubMed] [Google Scholar]
- 23.Germain L, Latarche C, Kesse-Guyot E, Galan P, Hercberg S, Briancon S. Does compliance with nutrition guidelines lead to healthy aging?: a quality-of-life approach. J Acad Nutr Diet 2013;113:228–240. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-González MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol M, Wärnberg J, Arós F, Ruíz-Gutiérrez V, Lamuela-Raventós RM, et al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 2012;41:377–85. [DOI] [PubMed] [Google Scholar]
- 25.Toledo E, Hu FB, Estruch R, Buli-Cosiales P, Corella D, Salas-Salvadó J, Covas MI, Arós F, Gómez-Gracia E, Fiol M, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med 2013;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damasceno NR, Sala-Vila A, Cofán M, Pérez-Heras AM, Fitó M, Ruiz-Gutiérrez V, Martínez-González MÁ, Corella D, Arós F, Estruch R, et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013;230:347–53. [DOI] [PubMed] [Google Scholar]
- 27.Salas-Salvadó J, Bulló M, Babio N, Martínez-González MÁ, Ibarrola-Jurado N, Basora J, Estruch R, Covas MI, Corella D, Arós F, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómes-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]