Abstract
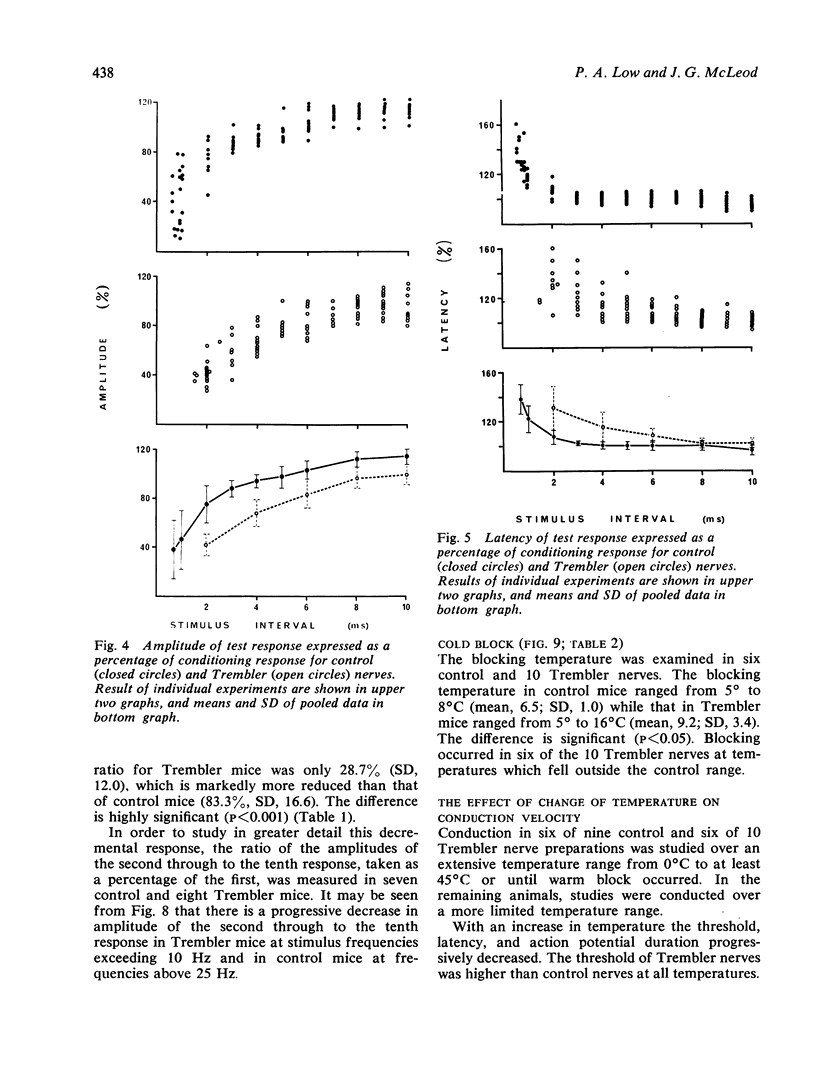
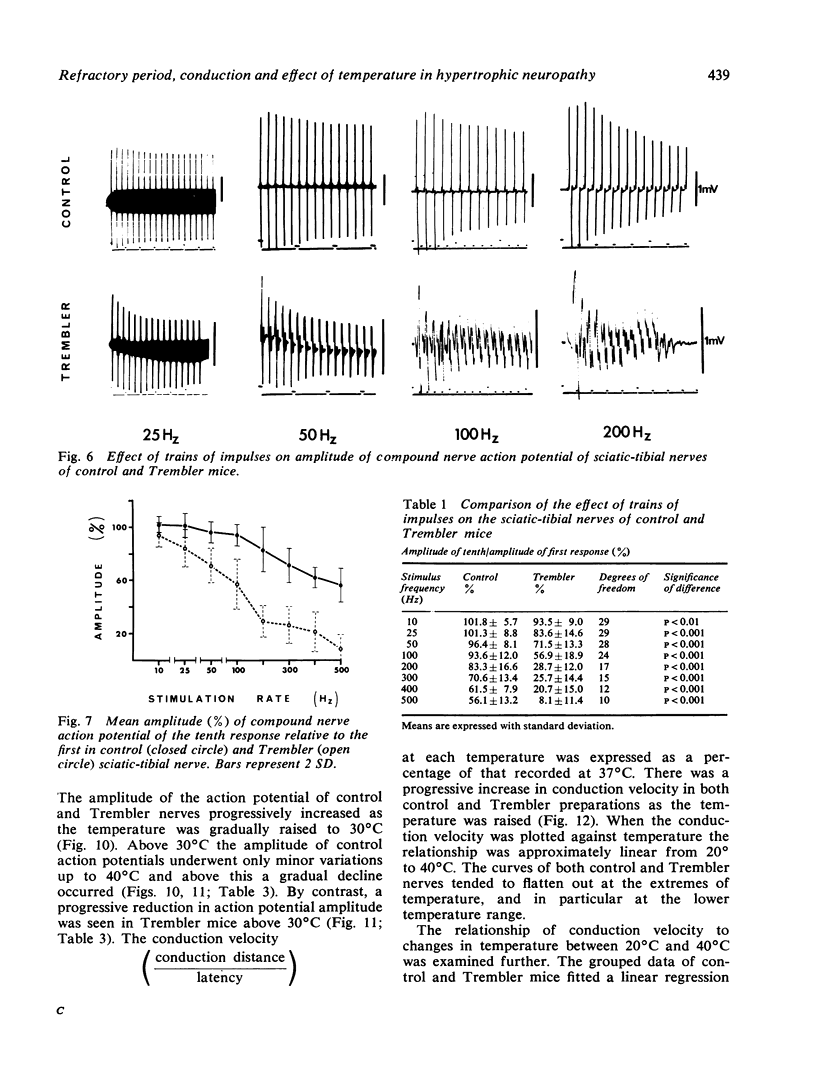
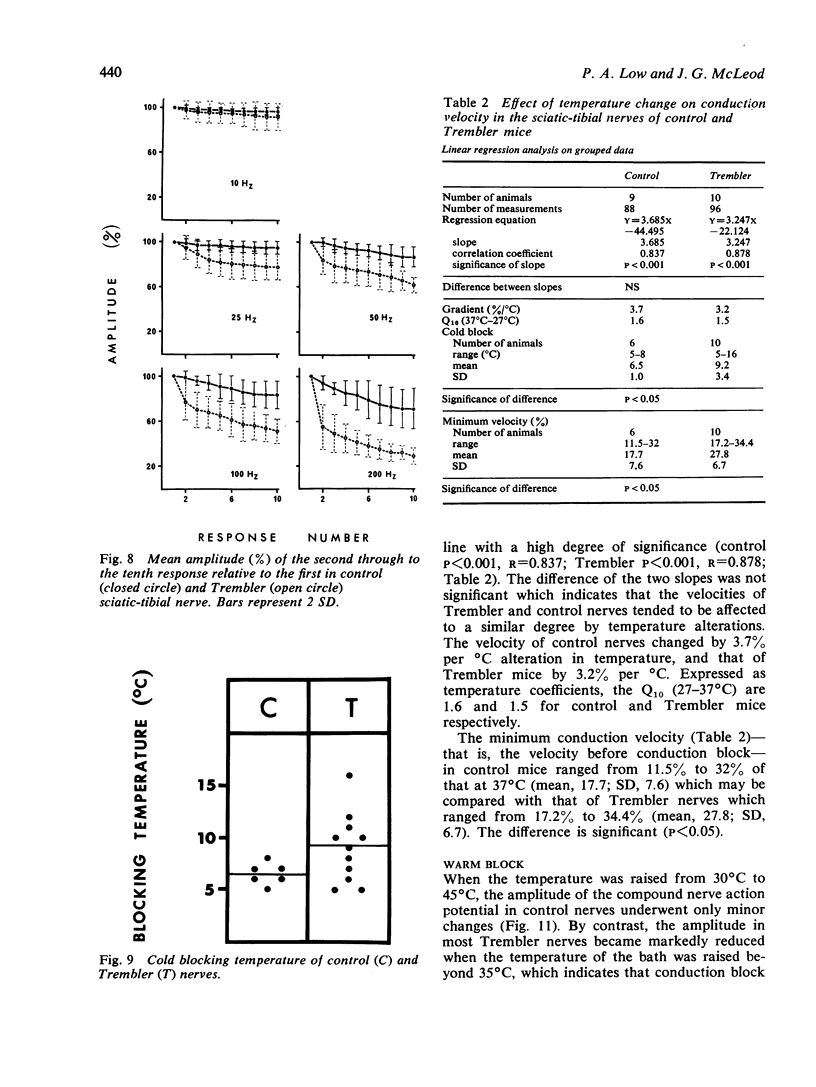
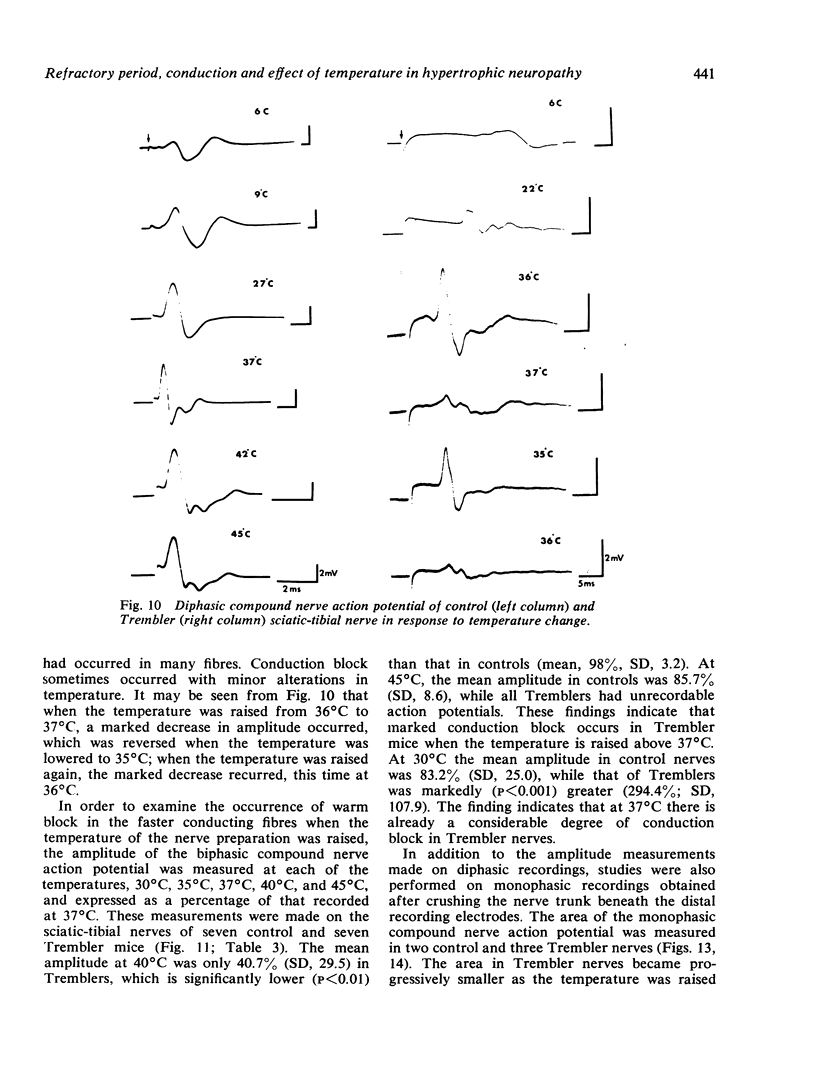
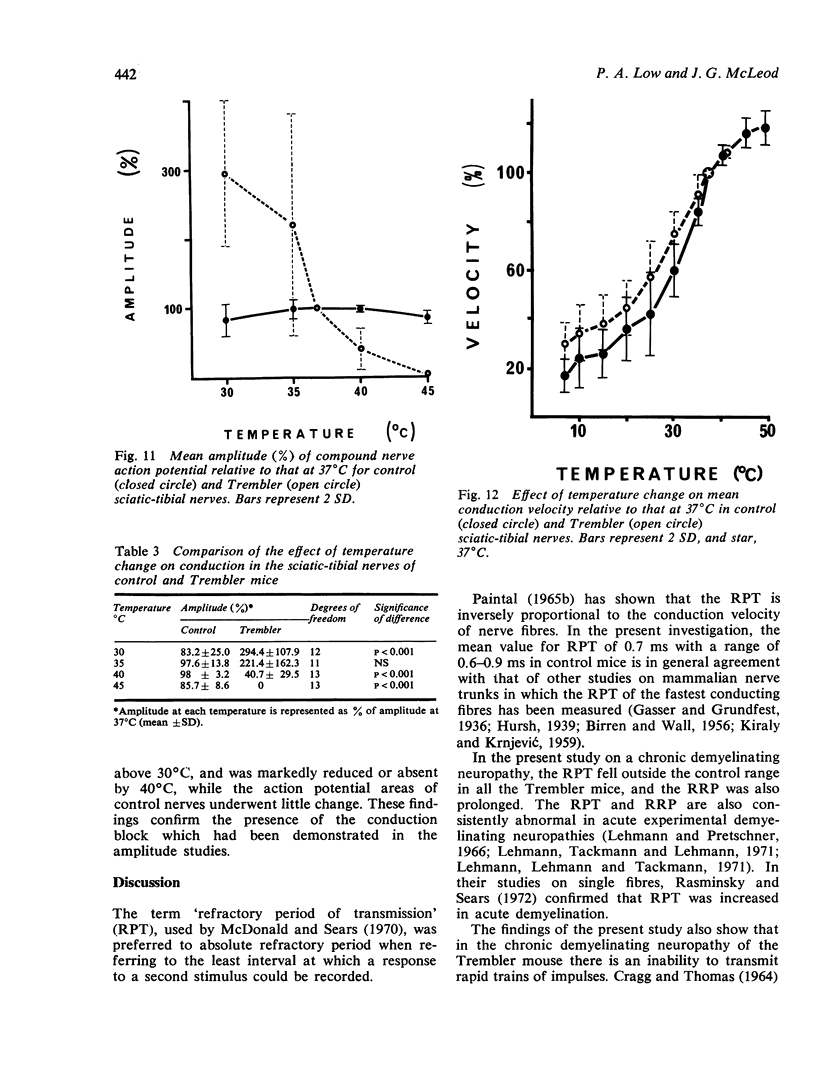
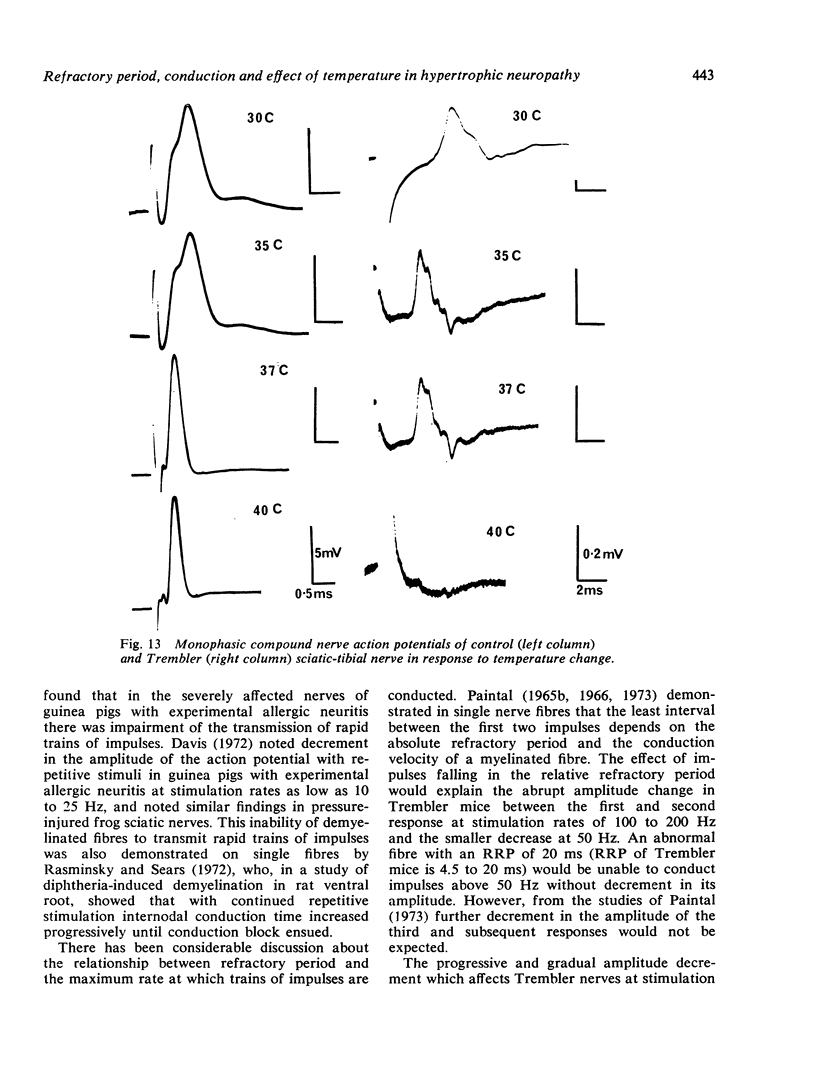
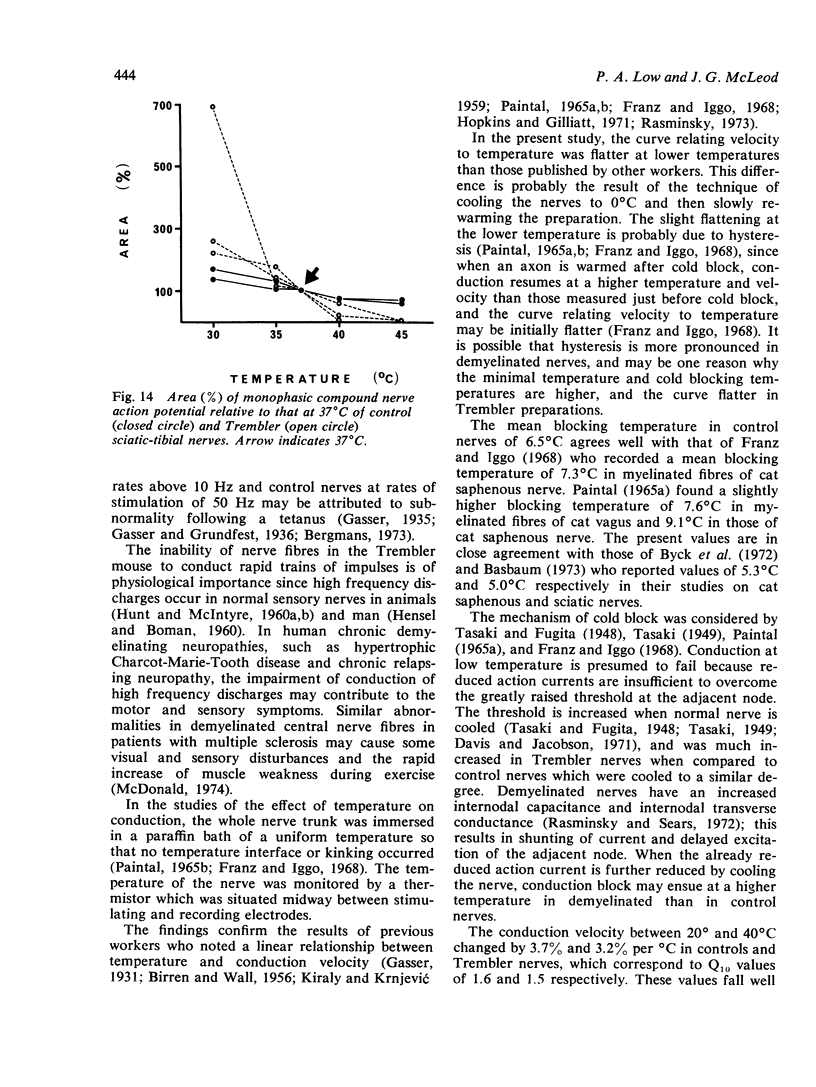
The refractory period, the ability to transmit trains of impulses, and the effect of temperature on conduction, have been studied in the sciatic-tibial nerve trunks of Trembler mice, which suffer from a dominantly inherited hypertrophic neuropathy. Both the refractory period of transmission and the relative refractory period were increased in Trembler mice when compared with controls. The nerve trunks of Trembler mice were unable to conduct rapid trains of impulses, and conduction block occurred at rates of stimulation as low as 25 Hz. Cold block occurred at temperatures significantly higher in Trembler nerves than in controls. The conduction velocity increased in an approximately linear fashion in both Trembler and control nerves when the temperature was raised from 20 degrees C to 40 degrees C, and the slopes were not significantly different. The Q10 (27 degrees C-37 degrees C) was 1.5 and 1.6 for control and Trembler nerves respectively. Conduction block was regularly observed in Trembler nerves when the temperature was raised within the physiological range. The abnormalities are related to the pathological changes of chronic demyelination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers M. M., Anderson R. M. Onion bulb neuropathy in the trembler mouse: a model of hypertrophic interstitial neuropathy (Dejerine-Sottas) in man. Acta Neuropathol. 1973 Jun 26;25(1):54–70. doi: 10.1007/BF00686858. [DOI] [PubMed] [Google Scholar]
- BIRREN J. E., WALL P. D. Age changes in conduction velocity, refractory period, number of fibers, connective tissue space and blood vessels in sciatic nerve of rats. J Comp Neurol. 1956 Feb;104(1):1–16. doi: 10.1002/cne.901040102. [DOI] [PubMed] [Google Scholar]
- Basbaum C. B. Induced hypothermia in peripheral nerve: electron microscopic and electrophysiological observations. J Neurocytol. 1973 Jun;2(2):171–187. doi: 10.1007/BF01474719. [DOI] [PubMed] [Google Scholar]
- Byck R., Goldfarb J., Schaumburg H. H., Sharpless S. K. Reversible differential block of saphenous nerve by cold. J Physiol. 1972 Apr;222(1):17–26. doi: 10.1113/jphysiol.1972.sp009785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. CHANGES IN NERVE CONDUCTION IN EXPERIMENTAL ALLERGIC NEURITIS. J Neurol Neurosurg Psychiatry. 1964 Apr;27:106–115. doi: 10.1136/jnnp.27.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. A. Axonal conduction studies based on some considerations of temperature effects in multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1970 Mar;28(3):281–286. doi: 10.1016/0013-4694(70)90164-1. [DOI] [PubMed] [Google Scholar]
- Davis F. A. Impairment of repetitive impulse conduction in experimentally demyelinated and pressure-injured nerves. J Neurol Neurosurg Psychiatry. 1972 Aug;35(4):537–544. doi: 10.1136/jnnp.35.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. A., Jacobson S. Altered thermal sensitivity in injured and demyelinated nerve. A possible model of temperature effects in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1971 Oct;34(5):551–561. doi: 10.1136/jnnp.34.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck P. J. Histologic measurements and fine structure of biopsied sural nerve: normal, and in peroneal muscular atrophy, hypertrophic neuropathy, and congenital sensory neuropathy. Mayo Clin Proc. 1966 Nov;41(11):742–774. [PubMed] [Google Scholar]
- Franz D. N., Iggo A. Conduction failure in myelinated and non-myelinated axons at low temperatures. J Physiol. 1968 Dec;199(2):319–345. doi: 10.1113/jphysiol.1968.sp008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASSER H. S. Unmedullated fibers originating in dorsal root ganglia. J Gen Physiol. 1950 Jul 20;33(6):651–690. doi: 10.1085/jgp.33.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENSEL H., BOMAN K. K. Afferent impulses in cutaneous sensory nerves in human subjects. J Neurophysiol. 1960 Sep;23:564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- HODES R. Linear relationship between fiber diameter and velocity of conduction in giant axon of squid. J Neurophysiol. 1953 Mar;16(2):145–154. doi: 10.1152/jn.1953.16.2.145. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. An analysis of fibre diameter and receptor characteristics of myelinated cutaneous afferent fibres in cat. J Physiol. 1960 Aug;153:99–112. doi: 10.1113/jphysiol.1960.sp006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. Characteristics of responses from receptors from the flexor longus digitorum muscle and the adjoining interosseous region of the cat. J Physiol. 1960 Aug;153:74–87. doi: 10.1113/jphysiol.1960.sp006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. P., Gilliatt R. W. Motor and sensory nerve conduction velocity in the baboon: normal values and changes during acrylamide neuropathy. J Neurol Neurosurg Psychiatry. 1971 Aug;34(4):415–426. doi: 10.1136/jnnp.34.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizar P., Kuno M., Miyata Y. Electrophysiological properties of spinal motoneurones of normal and dystrophic mice. J Physiol. 1975 Jun;248(1):231–246. doi: 10.1113/jphysiol.1975.sp010971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960 Jul;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRALY J. K., KRNJEVIC K. Some retrograde changes in function of nerves after peripheral section. Q J Exp Physiol Cogn Med Sci. 1959 Jul;44:244–257. doi: 10.1113/expphysiol.1959.sp001397. [DOI] [PubMed] [Google Scholar]
- Koles Z. J., Rasminsky M. A computer simulation of conduction in demyelinated nerve fibres. J Physiol. 1972 Dec;227(2):351–364. doi: 10.1113/jphysiol.1972.sp010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H. J., Lehmann G., Tackmann W. Refraktärperiode und Ubermittlung von Serienimpulsen im N. tibialis des Meerschweinchens bei experimenteller allergischer Neuritis. Z Neurol. 1971 Apr 28;199(1):67–85. [PubMed] [Google Scholar]
- Lehmann H. J., Pretschner D. P. Experimentelle Untersuchungen zum Engpasssyndrom peripherer Nerven. Dtsch Z Nervenheilkd. 1966 May 6;188(4):308–330. [PubMed] [Google Scholar]
- Lehmann H. J., Tackmann W. Die Ubermittlung frequenter Impulsserien in demyelinisierten und in degenerierenden Nervenfasern. Arch Psychiatr Nervenkr (1970) 1970;213(3):215–227. doi: 10.1007/BF00342658. [DOI] [PubMed] [Google Scholar]
- Lehmann H. J., Tackmann W., Lehmann G. Funktionsänderung markhaltiger Nervenfasern im N. tibialis des Meerschweinchens bei postdiphtherischer Polyneuritis. Z Neurol. 1971 Apr 28;199(1):86–104. [PubMed] [Google Scholar]
- Low P. A. Hereditary hypertrophic neuropathy in the trembler mouse. Part 2. Histopathological studies: electron microscopy. J Neurol Sci. 1976 Dec;30(2-3):343–368. doi: 10.1016/0022-510x(76)90139-8. [DOI] [PubMed] [Google Scholar]
- Low P. A., McLeod J. G. Hereditary demyelinating neuropathy in the trembler mouse. J Neurol Sci. 1975 Dec;26(4):565–574. doi: 10.1016/0022-510x(75)90057-x. [DOI] [PubMed] [Google Scholar]
- McDonald W. I. Pathophysiology in multiple sclerosis. Brain. 1974 Mar;97(1):179–196. doi: 10.1093/brain/97.1.179. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Sears T. A. The effects of experimental demyelination on conduction in the central nervous system. Brain. 1970;93(3):583–598. doi: 10.1093/brain/93.3.583. [DOI] [PubMed] [Google Scholar]
- NELSON D. A., McDOWELL F. The effects of induced hyperthermia on patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 1959 May;22(2):113–116. doi: 10.1136/jnnp.22.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. Block of conduction in mammalian myelinated nerve fibres by low temperatures. J Physiol. 1965 Sep;180(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. Effects of temperature on conduction in single vagal and saphenous myelinated nerve fibres of the cat. J Physiol. 1965 Sep;180(1):20–49. [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. The influence of diameter of medullated nerve fibres of cats on the rising and falling phases of the spike and its recovery. J Physiol. 1966 Jun;184(4):791–811. doi: 10.1113/jphysiol.1966.sp007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasminsky M., Sears T. A. Internodal conduction in undissected demyelinated nerve fibres. J Physiol. 1972 Dec;227(2):323–350. doi: 10.1113/jphysiol.1972.sp010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasminsky M. The effects of temperature on conduction in demyelinated single nerve fibers. Arch Neurol. 1973 May;28(5):287–292. doi: 10.1001/archneur.1973.00490230023001. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Koles Z. J. Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. Am J Physiol. 1970 Nov;219(5):1256–1258. doi: 10.1152/ajplegacy.1970.219.5.1256. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G. Responses in human A and C fibres to repeated electrical intradermal stimulation. J Neurol Neurosurg Psychiatry. 1974 Jun;37(6):653–664. doi: 10.1136/jnnp.37.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON C. W. Effect of lowering of body temperature on the symptoms and signs of multiple sclerosis. N Engl J Med. 1959 Dec 17;261:1253–1259. doi: 10.1056/NEJM195912172612501. [DOI] [PubMed] [Google Scholar]