Abstract
The folate receptor (FR) is over-expressed on the vascular side of cancerous cells including those of the breast, ovaries, testes, and cervix. We hypothesized that a folate-conjugated immunoglobulin (F-IgG) would bind to the FR that is over-expressed on melanoma tumor cells to target these cells for lysis by natural killer (NK) cells. Folate receptor expression was confirmed in the Mel-39 (human melanoma) cell line by flow cytometry and immunoblot analysis, using KB (human oral epithelial) and F01 (human melanoma) as a positive and negative control, respectively. FR-positive and negative cell lines were treated with F-IgG or control immunoglobulin G (C-IgG) in the presence or absence of cytokines in order to determine NK cell ability to lyse FR-positive cell lines. NK cell activation was significantly upregulated and lysis of Mel 39 tumor cells enhanced following treatment with F-IgG, as compared to C-IgG at all effector:target (E:T) ratios (p<0.01). This trend was further enhanced by NK cell stimulation with the activating cytokine interleukin-12 (IL-12). NK cell production of cytokines such as interferon-gamma (IFN-γ), macrophage inflammatory protein 1 alpha (MIP-1α), and regulated on activation normal T-cell expressed and secreted (RANTES) were also significantly increased in response to co-stimulation with IL-12 stimulation and F-IgG-coated Mel 39 target cells, as compared to controls (p<0.01). In contrast, F-IgG did not bind to the FR-negative cell line F01 and had no significant effect on NK cell lysis or cytokine production. This research indicates the potential use of F-IgG for its ability to induce an immune response from NK cells against FR-positive melanoma tumor cells which can be further enhanced by the addition of cytokines.
Keywords: Folate, Natural Killer Cell, Interferon-gamma, Immunoglobulin-G, IL-12
Introduction
Melanoma accounts for the vast majority of skin cancer associated deaths and its incidence is increasing. Patient outcomes are largely dependent on the stage of disease at the time of initial presentation, as over 80% of melanoma patients that present with localized disease can be effectively treated with surgery alone [1]. However, in patients with metastatic melanoma, long term survival remains at less than 10% [2]. Traditional regimens including dacarbazine, temozolomide, high-dose interleukin-2 (IL-2), and paclitaxel with or without cisplatin or carboplatin have demonstrated only modest response rates (<20%) [3]. More recently, novel immunotherapeutic and targeted approaches have been developed and used in the treatment of metastatic melanoma, including ipilimumab, a monoclonal antibody directed against CTLA-4, and vemurafenib, a specific inhibitor of signaling by mutated BRAF [3], but both agents possess unique limitations. Phase III trials with ipilimumab showed an overall response rate of less than 20% and revealed a serious potential for autoimmune toxicity, with immune-related events occurring in 60% of patients [4]. Vemurafenib has shown more impressive response rates (40–50%) in patients with melanoma, but its use is limited to patients with a V600E mutation in the BRAF oncogene [5]. More recently, antibodies that neutralize components of the PD-1/PD-L1 T cell inhibitory pathway have become available and have shown great promise, especially in combination with other immune checkpoint blockades. A phase I trial of nivolumab and ipilimumab confirmed an objective response of 61% in patients with advanced stage metastatic melanoma that received the combination versus a response of 11% in patients that received ipilimumab alone [6, 7]. Targeted combination therapies, including BRAF and MEK inhibiters that are approved for the treatment of patients with advanced melanoma carrying the BRAF V600E mutation, result in a high rate of initial tumor responses with significant survival advantages versus single agents [8–10]. However, drug resistance and tumor recurrences are still problematic. These regimens highlight the importance of developing novel therapeutics for the treatment of melanomas that would have relatively low off target effects.
One potential target for alternative therapeutics in melanoma is the folate receptor (FR). The natural ligand for the FR is folate, a water soluble B vitamin that functions as a co-factor in various cellular metabolic processes, such as nucleic acid synthesis and protein biosynthesis. However, the common dietary source of folate is achieved through supplemented foods, where the vitamin is present in its oxidized form, known as folic acid [11, 12]. Folic acid is thought to increase cancer cell motility, invasion, metastases and overall tumor progression [13, 14]. FR expression is normally low and high level expression is limited to specific tissues, such as the choroid plexus, epididymis and kidney. In normal polar epithelial cells, the FR is predominantly located on the non-vascular side of the cell and is thus largely inaccessible to circulating folate [15]. Upon malignant transformation, cell polarity is altered whereby the FR becomes accessible to circulating folate or folate conjugates. Importantly, the FR is over-expressed in malignant cells, such as cancers of the cervix, breast, testes, uterus, lung, ependymal brain tumors, in blasts of myeloid leukemias, and melanoma [16–20]. Estimates suggest that roughly one-third of all cancers may exhibit the upregulation of the FR, with metastatic and later-stage cancers associated with increased FR over-expression compared to the FR expression of non-malignant cells, making the FR an advantageous therapeutic target [21]. High expression of the FR on the surface of tumor cells makes it an attractive therapeutic target. Once a folate conjugate is bound, some of it may be taken up by endocytosis while a fraction may stay engaged with the FR and remain on the cell surface [15]. These parameters are thought to create a favorable toxicity profile for folate-conjugated anti-tumor compounds [17, 22, 23].
In order to eradicate FR-expressing cancer cells, it would be efficacious to engage cells of the innate immune system, such as natural killer (NK) cells. NK cells are bone marrow-derived lymphocytes that have the ability to lyse malignant cells with altered expression of major histocompaitbility agents. They have high levels of cellular adhesion molecules, express killer cell immunoglobulin receptors (KIRs), and constitutively possess receptors for stimulatory cytokines (e.g. IL-12, IL-15, IL-18). NK cells secrete several anti-tumor cytokines (e.g. IFN-γ, TNF-α, and MIP-1α) and have the ability to lyse malignant cells using cytolytic granules that contain perforin and granzymes. NK cells also express the FcγRIIIa receptor, an activating receptor that recognizes the constant (Fc) region of IgG. The FcγRIIIa receptor is critical in mediating antibody-dependent cellular cytotoxicity (ADCC) against antibody (Ab)-coated targets [24, 25].
Our group has synthesized a folate-conjugated immunoglobulin (F-IgG) that is able to bind specifically to FR-bearing tumor cell lines. As a critical first step in the development of a novel melanoma therapeutic, the present study was designed to determine the ability of the F-IgG construct to mediate ADCC and cytokine production by NK cells in FR positive melanoma cell lines. We have previously found that IL-12 can enhance monoclonal antibody therapy and wished to characterize the anti-tumor effects of the F-IgG conjugate in combination with this NK cell-activating cytokine [26].
Materials and Methods
F-IgG synthesis
The technique for conjugating folate to macromolecules has been previously described [27, 28]. Folate was reacted with N, N’-dicyclohexylcarbodiimide (DCC) and N-hydroxysuccinimide (NHS) in DMSO at stoichiometric molar ratios of 1:1:1 for 3 hrs at room temperature. The resultant product was filtered to remove precipitate. The activated folate (f-NHS) was then reacted with IgG (Equitech-Bio Inc., Kerrville, TX) in pH 8.0 PBS buffer solution at a molar ratio of 12:1 for 2 hrs at room temperature. Following the 2 hr incubation, the sample was passed through a PD-10 column equilibrated in PBS (pH 8.0) to remove unreacted f-NHS. For the preparation of FITC-labeled F-IgG (F-IgG-FITC) or IgG (IgG-FITC), activated f-NHS was reacted with IgG and FITC in pH 8.0 PBS buffer solution at a molar ratio of 12:1:5 for 2 hrs at room temperature. The folate content of the construct was determined by UV spectrometry at 371 nm and the antibody concentration was determined by the BCA protein assay method. Conjugation reactions performed at IgG-to-folate-NHS ratios of 12:1 and 100:1 yielded preparations containing an average molar ratio of 2.6 folate molecules per IgG molecule and 9.0 folates per IgG, respectively. Since excessive folate conjugation of IgG might adversely affect its effector functions, a lower conjugation level of 3 folates per IgG was chosen for further study. Unmodified human IgG molecules were used as controls (C-IgG). Conjugates were stored at 4°C in the dark prior to use. The molecular weight of the F-IgG construct is approximately 151 kDa. Our construct demonstrated similar binding and intracellular distribution characteristics in FR-positive cells as compared to previously published studies using radiolabeled-conjugate constructs [29, 30]. Binding assays from studies conducted by Henriksen et al. estimated a binding affinity of a folate-conjugated immunoglobulin to have a KD of 10−9 to 10−10 M, which is comparable to the reported high affinity binding of folic acid to the FRα (KD ~ 10−9 M) [12]. Our group has previously shown that FR binding of F-IgG is evident as early as 30 minutes post treatment, and following uptake into the cell, was retained on the cell surface for up to 24 hours in vitro [31]. Moreover, in vivo, F-IgG was present at the tumor site for up to 72 hours post treatment [31].
Cell lines
The FR-positive human cell lines KB (human nasopharyngeal epidermal carcinoma, a gift from Dr. Philip S. Low, Purdue University, West Lafayette, Indiana), Mel-39 (human melanoma, ATCC), as well as the FR-negative cell line F01 (human melanoma) were propagated in folate-free RPMI 1640, supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotic-antimycotic (Invitrogen, Grand Island, NY).
Isolation of human NK cells
NK cells were isolated directly from fresh peripheral blood leukopacks (American Red Cross, Columbus, OH) by 30-minute incubation with RossetteSep cocktail (Stem Cell Technologies, Vancouver, BC) prior to Ficoll Hypaque (Sigma) density gradient centrifugation. Human NK cells were cultured in folate-free RPMI 1640 (purchased from The Cleveland Clinic Media Preparation Service, Cleveland, OH) supplemented with 10% heat-inactivated pooled human AB serum (HAB; C-Six Diagnostics, Germantown, WI) and 1% antibiotic-antimycotic.
Reverse transcription polymerase chain reaction (RT-PCR) for FR-α expression
Total RNA from Mel 39, KB, and F01 cells was extracted in TRIZOL® Reagent using the RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. RNA was converted to complementary DNA by reverse transcription and used as a template for RT-PCR using FR-α (forward primer, TGGGTGGCTGTAGTAGGGGAG; reverse primer, CAGGGGCACGTTCAGTACC) and FR-β primers (forward primer, ACCAATGCAGTCC-CTGGAAGAAGA; reverse primer, AGCTGGGCACTTGTTAACTCCTGA) [32]. The PCR conditions consisted of 95°C for 30s followed by 30 cycles of 95°C for 30s, 58°C for 30s, and 72°C for 40s.
Cytotoxicity assays
Purified human NK cells were plated in 96-well V-bottom plates in 10% HAB containing folate-free RPMI 1640 medium supplemented with IL-12 (10 ng/mL) and incubated overnight at 37°C. F-IgG or C-IgG-treated (100 ug/mL) 51Cr-labeled target cells were added to NK cells at different effector:target (E:T) ratios. Following a 4-hour incubation at 37°C, supernatants were harvested for quantification of chromium release. Percentage of lysis was determined as previously described [33].
In vitro co-culture assay
The FR-positive cell lines, Mel 39 and KB, or the FR-negative cell line, F01, were cultured in the wells of a 96-well flat-bottom culture plate overnight at 37°C, as previously described [12, 34]. The culture supernatant was aspirated the following day and wells were treated with 100 µg/mL F-IgG or C-IgG for 1 hr at 37°C. After washing off unbound F-IgG or C-IgG, purified NK cells were then added at 2 × 105 cells per well in 200 µL of folate free RPMI containing 10% HAB medium and 10 ng/mL IL-12. Control conditions consisted of NK cells plus tumor cells treated with medium alone, F-IgG or C-IgG alone, or cytokine alone. Culture supernatants were harvested after 48 hours and analyzed for IFN-γ, MIP-1α, and RANTES content by enzyme-linked immunosorbent assay (ELISA). The lower detection limit for all ELISAs was ≤ 30 pg/mL. All results shown are the mean of triplicate wells ± SE.
Flow cytometry
The expression of CD69 on the cell surface of NK cells was determined by flow cytometry. Purified NK cells were cultured for 48 hours with Mel39, KB, or F01 tumor cells in the same manner described above for 48 hours. Following incubation with antibody-coated tumor cells, NK cells were collected from the co-culture plate and incubated on ice for 30 mins in flow buffer (5% FBS in PBS) with anti-CD56-APC, a marker for NK cells, and anti-CD69-PE-Cy-7 (BD Biosciences). Cells were then washed and fixed in 1% formalin. Non-specific staining by an isotype control Ab was employed to determine the percent positive population. Activated NK cells were determined to be CD56+/CD69+.
Bioinformatics search
The cancer microarray database and web-based data-mining platform Oncomine was used to gather information on the gene expression of folate receptor-α (FOLR1) in a subset of melanoma patients [35]. Data analysis was performed as fold change comparing normal skin tissues with cutaneous melanoma. Following the expression analysis of FOLR1 from several databases, log-transformed median centered raw data were downloaded from Oncomine Platform.
Statistics
These experiments mainly tested whether there were synergistic effects of F-IgG and IL-12 on NK cell mediated ADCC and cytokine production. A student’s t-test and an analysis of variance (ANOVA) were utilized for two-way and multiple comparisons, respectively.
Results
The FR is expressed on melanoma tumor cell lines
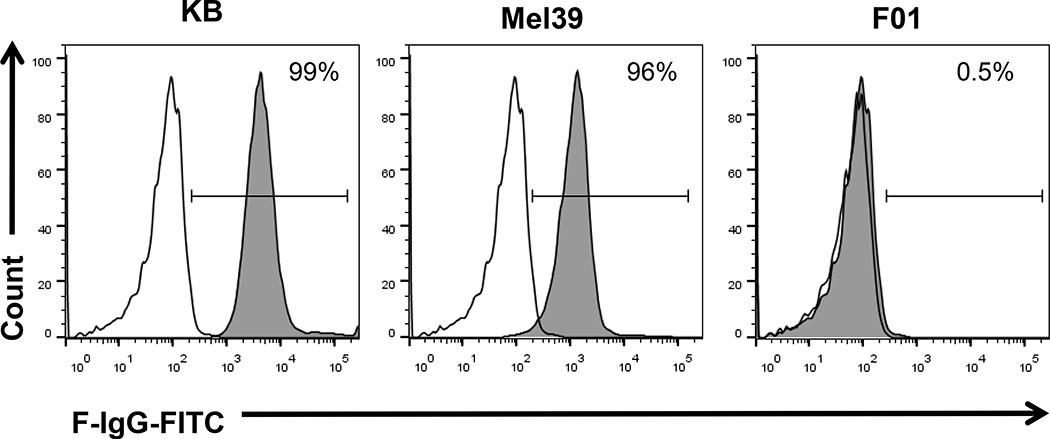
The KB, Mel-39 and F01 tumor cell lines were analyzed for folate receptor-α (FR-α) expression by RT-PCR. Both cell lines expressed the FR-α transcript, whereas it was not detected in the FR-α-negative F01 cell line (Fig. 1A). FR protein content was confirmed in the KB and Mel-39 cell lines, as demonstrated by immunoblot analysis (Fig. 1B). Surface expression of the FR was confirmed in the KB and Mel-39 cell lines, but not in the F01 cell line, as demonstrated by flow cytometry using a F-IgG-FITC labeled conjugate (Fig. 2). The FR was not expressed by NK cells (data not shown).
Figure 1. The folate receptor is expressed in KB and Mel-39 melanoma tumor cells but not in F01 melanoma tumor cells.
(A) The FR-positive KB (human oral epithelial) and Mel-39 (melanoma) and FR-negative F01 (melanoma) tumor cell lines were tested for FR-α expression by RT-PCR. (B) Folate receptor expression was confirmed by immunoblot analysis in the KB and Mel-39 tumor cell lines. The membrane was re-probed for β actin to confirm equal loading.
Figure 2. F-IgG binds to melanoma tumor cells.
F-IgG binding to the FR on the surface of KB and Mel39 tumor cells was evaluated by flow cytometry using a 200 nM F-IgG-FITC conjugate (F-IgG-FITC = shaded histogram) or a FITC-labeled control Ab (C-IgG = unfilled histogram).
FR-α expression is upregulated in patients with cutaneous melanoma versus normal skin tissues
An Oncomine® search of several publically available data sets revealed that FR-α (FOLR1) expression was 22.3-fold upregulated in cutaneous melanoma versus normal skin tissue controls (Fig. 3, p=1.804E-17). Box plot depicts log2 transformed mean-centered intensity FOLR1 gene expression of normal controls (n=26) vs. patients with cutaneous melanoma (n=64) [36–38].
Figure 3. FOLR1 gene expression is upregulated in cutaneous melanomas versus normal skin tissue.
An Oncomine search of three publicly available datasets was conducted to evaluate folate receptor-α (FOLR1) gene expression in cutaneous melanoma lesions versus normal skin tissues [36–38]. Gene expression is shown in box plots, representing the median and interquartile range, with I bars showing the range for each group. The asterisk (*) represents an outlier in the cutaneous melanoma data set as determined by SYSTAT software. Data set n=normal controls/cutaneous melanoma, p value; Talantov et al. n=7/45, p=1.426E-09 [36]; Haqq et al. n=3/5, p=0.0176 [37]; Riker et al. n=14/14, p=1.161E-15 [38].
NK cells are activated by F-IgG coated FR-positive melanoma tumor cells
NK cells can interact with antibody-coated target cells due to the fact that they constitutively express an activating, low-affinity receptor for the Fc portion of IgG, known as FcγRIIIa, or CD16. In order to assess NK cell activation, 48 hour co-culture experiments were conducted where CD69 upregulation was assessed on NK cells via flow cytometry after exposure to F-IgG-coated tumor cells. CD69 upregulation on NK cells is highly associated with augmented cytotoxicity and a productive anti-tumor response [39]. There was a significant increase in CD69 expression on NK cells in response to F-IgG-coated KB (Fig. 4A, p<0.001) and Mel-39 (Fig. 4B, p<0.001) tumor cells, but not the FR negative F01 cell line (Fig. 4C).
Figure 4. NK cells are activated in response to F-IgG-coated melanoma tumor cells.
Purified NK cells were co-cultured with C-IgG or F-IgG-coated tumor cells for 48 hours. NK cell activation was assessed via flow cytometric analysis with Mel39 (A), KB (B) and F01 (C) tumor cells. Dual positive CD56+/CD69+ cells represent activated NK cells. Each graph depicts the results from one representative donor ± SD. Mean fluorescent intensity (MFI) determined for anti-CD69-PeCy7 positivity within the anti-CD56-APC positive group. Three normal donors were tested per cell line. The asterisk (*) denotes p<0.001 versus all conditions shown.
F-IgG treatment of FR-positive melanoma tumor results in their lysis by NK cells and is further enhanced by IL-12
The effect of F-IgG on NK cell ADCC of FR-positive tumor targets was examined using Mel-39 (Fig. 5A) and KB (Fig. 5B) tumor cells as targets. Target cells were labeled with 51Cr and treated with either C-IgG or F-IgG before use in a standard 4-hr ADCC assay with healthy donor human NK cells serving as effectors. There was a significant enhancement of NK cell-mediated ADCC with F-IgG coating of targets at all effector:target (E:T) ratios in every donor tested, as compared to C-IgG (Fig. 5, p<0.001). F-IgG treatment did not enhance the lysis of the FR-negative cell line F01 (data not shown). Treatment of NK cells with 10 ng/mL of IL-12 (a prototypic NK-activating cytokine) led to significant enhancement of F-IgG-mediated cell cytotoxicity of both Mel-39 and KB tumor cells (p<0.001). The combination of F-IgG and IL-12 resulted in significantly higher tumor cell lysis than either F-IgG or cytokine treatment alone across all E:T ratios.
Figure 5. F-IgG treatment of FR-bearing melanoma tumor cells promotes NK cell mediated ADCC and is enhanced by IL-12.
Purified human NK cells were incubated overnight in medium alone or in medium supplemented with 10 ng/ml IL-12. The lytic activity of IL-21-activated NK cells was then assessed in a standard 4 hr chromium release assay using F-IgG-coated FR-positive KB (A) or Mel39 (B–D) cancer cells as targets. The percentage of lysis was calculated as previously described. Each graph depicts the results from one representative donor ± SD. Four normal donors were tested per cell line. The asterisk (*) denotes p<0.001 versus all conditions shown.
IL-12 enhances F-IgG-mediated NK cell production of IFN-γ, MIP-1α and RANTES
Our group has previously demonstrated that co-stimulation of NK cells via the IL-12R and FcγRIIIa leads to synergistic production of IFN-γ due to co-localization of these receptors at cell membrane lipid rafts and subsequent enhancement of signal transduction [34]. We therefore hypothesized that NK cell activating cytokines would enhance NK cell production of IFN-γ following exposure to F-IgG-coated KB tumor cells. An in vitro assay was performed in which purified NK cells were co-cultured with F-IgG-coated FR-positive tumor cells. KB and Mel-39 (FR-positive) and F01 (FR-negative) cells were treated with F-IgG or C-IgG and plated with purified NK cells in the presence or absence of IL-12 (10 ng/mL). After 48 hrs of co-culture, supernatants were harvested and assayed for IFN-γ content. As was observed in the ADCC experiments, F-IgG treatment not only resulted in a significant increase in NK cell IFN-γ secretion, but also demonstrated a highly significant interaction with IL-12 (p<0.001) (Fig. 6A). The levels of MIP-1α and RANTES were also measured via ELISA, as co-activation of the IL-12R and FcγRIIIa on NK cells can stimulate the secretion of these chemokines, in addition to IFN-γ, and have been shown to play a role in coordinating the adaptive immune response [40]. NK cell production of MIP1-α (Fig. 6B) and RANTES (Fig 6C) was significantly enhanced following treatment with F-IgG coated tumor cells and IL-12, as compared to control conditions (p<0.001).
Figure 6. Human NK cells secrete high levels of IFN-γ, MIP-1α and RANTES in response to F-IgG-coated melanoma tumor cells and IL-12.
The FR-positive KB and Mel39 and FR-negative F01 tumor cell lines were cultured with human NK cells in an in vitro co-culture assay. Control conditions consisted of tumor cells and NK cells cultured with C-IgG alone, F-IgG alone, or antibody-coated tumor cells in the presence of IL-12. Culture supernatants were harvested at 48 hrs and analyzed for (A) IFN-γ, (B) MIP-1α and (C) RANTES levels by ELISA. Each graph depicts the results from one representative donor ± SD. Three normal donors were tested per cell line. The asterisk (*) denotes p<0.001 versus all conditions shown.
Discussion
In the current study, it was demonstrated that a F-IgG construct can bind to the FR of melanoma tumor cells. F-IgG-treatment of FR-positive melanoma tumor cells led to enhanced NK cell CD69 expression and NK cell-mediated ADCC. Pre-treatment of NK cells with IL-12 significantly enhanced ADCC. Likewise, the addition of IL-12 to the F-IgG stimulus resulted in robust production of IFN-γ, MIP-1α and RANTES by NK cells, significantly exceeding levels observed with either stimulus alone. These results support the investigation of F-IgG and IL-12 in the context of FR-positive melanoma tumor models and as a potential cancer therapeutic. To our knowledge, this is the first evidence of an immunotherapeutic approach designed to exploit the expression of FR in melanoma tumor cells.
The FR functions to concentrate exogenous ligands into the cell cytosol by endocytosis. These endocytic vesicles rapidly become acidified, allowing the FR to release folate into the cell compartment to be used as one of the basic components of cell metabolism and DNA synthesis and repair. It is thought that the over expression of FR confers a growth advantage by modulating folate uptake from serum, but direct evidence for this mechanism has yet to be provided [41]. FRα, the most widely studied FR isoform, has restricted expression in normal cells, but is highly expressed in various nonmucinous tumors of epithelial origin. Cell culture studies show that expression of the FRα gene (FOLR1) is regulated by extracellular folate depletion, increased homocytstein accumulation, steroid hormone concentrations, interaction with specific transcription factors and cytosolic proteins, and, possibly, epigenetic mutations [12]. The blockade of folate uptake has been shown to decrease tumor cell proliferation, migration, invasion, and tumor burden [12–14, 42]. Therefore, receptor expression is maintained in the metastatic setting and represents a potential therapeutic target.
The increased expression of the FR on the surface of malignant tumor cells and the high affinity for unidirectional folate transport makes exploitation of the FR an advantageous therapeutic target. In melanoma cells, gene expression of folate receptor alpha (FOLR1), -beta (FOLR2), and -gamma (FOLR3) are dysregulated as compared to normal skin tissues, with a combined 22-fold increase in FOLR1 mRNA levels in cutaneous malignant melanoma [36–38]. This discovery suggests that a FR-directed therapy could selectively target malignant cells and avoid healthy tissues. The FR has previously been utilized for the investigational delivery of cytotoxic drugs, radiopharmaceuticals, nanoparticles, liposomes, and immunotherapy in other tumor settings [17, 43]. Recently, there has been evidence that a folate-conjugated agent could be involved in the induction of autophagic cell death in melanoma cells. A folate-conjugated methyl-β-cyclodextrin showed cytotoxic effects against FRα-positive melanoma tumor cells through FR-α-mediated endocytosis by eliciting the formation of autophagosomes [44]. Furthermore, encapsulation of vincristine into folic acid-conjugated PEGylated liposomes overcame multidrug resistance to chemotherapy in KBv200 cells [45]. A bispecific monoclonal antibody directed to the CD3 molecule on T lymphocytes and to the folate receptor on ovarian carcinoma cells showed clinical efficacy, as 3 of 19 patients showed a complete response, three had a partial response, and seven had stable disease [46]. These data, along with the present study, support the development of FR-directed therapeutic immunoconjugates.
Targeting tumors with antibodies constitutes an attractive therapeutic strategy, since these molecules are highly specific for their target and can function through various mechanisms against cancer cells, such as signaling to induce tumor cell growth arrest, engendering apoptosis, and promoting immune cell-mediated cytotoxicity and complement activation. Antibodies coupled to toxins, radionuclides or cytokines are expected to specifically deliver those toxic payloads to cancer cells. Antibodies may also act as immunogens or engage Fc receptors on APCs such as DCs to promote antigen presentation and induction of adaptive immune responses against cancer cells. Several efforts to exploit mAb therapy in the context of melanoma have been made aiming to enhance T cell activation, target VEGF, or highlight the expression of high-molecular-weight melanoma-associated antigen [47–50]. The present approach uses the affinity of folate for the FR to decorate the cancer cell with IgG molecules.
We have shown that NK cell recognition of F-IgG-coated FR-positive melanoma tumor cells leads to an increase in cytoxocitiy and cytokine production. NK cells exert their effector functions via multiple mechanisms, either via the direct cytolysis of transformed cells by the release of perforin and granzyme B through antibody-dependent cytoxicity or by the secretion of cytokines and chemokines that can influence the host’s immune response. We have previously shown that the dual engagement of NK cell CD16 and cytokine receptors by cytokines such as IL-2, IL-12, or IL-21 results in synergistic ADCC and IFN-γ production in solid tumors [34, 51–54]. Here, robust and synergistic increases in ADCC and the levels of IFN-γ, MIP-1α, and RANTES were observed in response to F-IgG and IL-12 over either stimulus alone in melanoma tumor cells. Thus, the co-administration of IL-12 and the F-IgG construct could be expected to promote an NK-dependent anti-tumor response in the clinical setting and offers promise in the situations where other targeted agents have failed. Given the recent success of immune checkpoint inhibitors as a systemic therapeutic agent for the treatment of metastatic melanoma, the use of anti-PD-1/PD-L1 or anti-CTLA4 therapy could potentially enhance the immunologic activity of F-IgG. Additionally, the fusion of cytokines to antibodies is a promising strategy that would limit the toxicities observed with systemic administration of immunostimulatory cytokines. A F-IgG:IL-12 fusion is under consideration.
In summary, it has been shown that immunoglobulin conjugated folate (F-IgG) complexes can be utilized to specifically enhance the immune response by NK cells against FR positive melanoma tumor cells in vitro. Furthermore, it was shown that there is a large increase in FOLR1 expression in cutaneous melanoma versus normal controls. Treatment of NK cells with the immune stimulatory cytokine IL-12 significantly augmented their ability to lyse FR targeted tumor cells and secrete immune modulating cytokines. Thus, F-IgG and IL-12 can target NK cells to melanoma tumor cells by means of the FR.
Acknowledgments
This work was supported by National Institutes of Health Grants PO1 CA95426, K24 CA93670 (WEC) and T32 GM068412 (ACJ-R). CCS was an undergraduate Pelotonia Fellow.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Silva E. Adjunct primer for the use of national comprehensive cancer network guidelines for the surgical management of cutaneous malignant melanoma patients. World J Surg Oncol. 2012;10:54. doi: 10.1186/1477-7819-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 3.Dillman RO, Barth NM, VanderMolen LA, Mahdavi K, McClure SE. Should high-dose interleukin-2 still be the preferred treatment for patients with metastatic melanoma? Cancer Biother Radiopharm. 2012;27(6):337–343. doi: 10.1089/cbr.2012.1220. [DOI] [PubMed] [Google Scholar]
- 4.Specenier P. Ipilimumab in melanoma. Expert Rev Anticancer Ther. 2012;12(12):1511–1521. doi: 10.1586/era.12.132. [DOI] [PubMed] [Google Scholar]
- 5.Keating GM. Vemurafenib: in unresectable or metastatic melanoma. BioDrugs. 2012;26(5):325–334. doi: 10.2165/11209860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 11.Anger M, Friedhofer H, Fukutaki MF, Ferreira MC, Landman G. Primary cutaneous melanoma: an 18-year study. Clinics (Sao Paulo) 2010;65(3):257–263. doi: 10.1590/S1807-59322010000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119(2):243–250. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 13.Dudkowska M, Bajer S, Jaworski T, Zielinska J, Manteuffel-Cymborowska M, Grzelakowska-Sztabert B. Antifolate/folate-activated HGF/c-Met signalling pathways in mouse kidneys-the putative role of their downstream effectors in cross-talk with androgen receptor. Arch Biochem Biophys. 2009;483(1):111–119. doi: 10.1016/j.abb.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Huang GW, Zhang XM, Ren DL, J XW. Folic Acid supplementation stimulates notch signaling and cell proliferation in embryonic neural stem cells. J Clin Biochem Nutr. 2010;47(2):174–180. doi: 10.3164/jcbn.10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54(5):675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008;5(3):309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- 17.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26(1):141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann LC, Keeney GL, Lingle WL, Christianson TJ, Varghese B, Hillman D, et al. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121(5):938–942. doi: 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- 19.Dhawan D, Ramos-Vara JA, Naughton JF, Cheng L, Low PS, Rothenbuhler R, et al. Targeting folate receptors to treat invasive urinary bladder cancer. Cancer Res. 2012;73(2):875–884. doi: 10.1158/0008-5472.CAN-12-2101. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-del-Campo L, Montenegro MF, Cabezas-Herrera J, Rodríguez-López JN. The critical role of alpha-folate receptor in the resistance of melanoma to methotrexate. Pigment Cell Melanoma Res. 2009;22(5):588–600. doi: 10.1111/j.1755-148X.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Sega E, Low PS. Folate receptor-targeted immunotherapy: induction of humoral and cellular immunity against hapten-decorated cancer cells. Int J Cancer. 2005;116(5):710–719. doi: 10.1002/ijc.21126. [DOI] [PubMed] [Google Scholar]
- 22.Low PS, Antony AC. Folate receptor-targeted drugs for cancer and inflammatory diseases. Adv Drug Deliv Rev. 2004;56(8):1055–1058. doi: 10.1016/j.addr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Sabharanjak S, Mayor S. Folate receptor endocytosis and trafficking. Adv Drug Deliv Rev. 2004;56(8):1099–1109. doi: 10.1016/j.addr.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Robertson MJ, Mier JW, Logan T, Atkins M, Koon H, Koch KM, et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res. 2006;12(14 Pt 1):4265–4273. doi: 10.1158/1078-0432.CCR-06-0121. [DOI] [PubMed] [Google Scholar]
- 25.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76(3):519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 26.Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, et al. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-gamma production. J Immunol. 2011;186(6):3401–3409. doi: 10.4049/jimmunol.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leamon CP, Low PS. Cytotoxicity of momordin-folate conjugates in cultured human cells. J Biol Chem. 1992;267(35):24966–24971. [PubMed] [Google Scholar]
- 28.Li H, Lu Y, Piao L, Wu J, Yang X, Kondadasula SV, et al. Folate-immunoglobulin G as an anticancer therapeutic antibody. Bioconjug Chem. 2010;21(5):961–968. doi: 10.1021/bc900545h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henriksen G, Bruland OS, Larsen RH. Preparation and preclinical assessment of folate-conjugated, radiolabelled antibodies. Anticancer Res. 2005;25(1A):9–15. [PubMed] [Google Scholar]
- 30.Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci U S A. 1991;88(13):5572–5576. doi: 10.1073/pnas.88.13.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaime-Ramirez AC, Kondadasula S, Jones NB, Mani A, Roda J, Karpa V, et al. Anti-tumor effects of a folate-immunoglobulin conjugate are enhanced by cytokine treatment in vitro and in vivo. Cancer Res. 2011;71(8 Suppl) Abstract nr 2686. [Google Scholar]
- 32.Parihar R, Carson WE., 3rd Novel cytokines in the treatment of malignancies. Cancer Treat Res. 2005;126:353–373. doi: 10.1007/0-387-24361-5_15. [DOI] [PubMed] [Google Scholar]
- 33.Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, et al. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31(10):3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::aid-immu3016>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 34.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110(7):983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, et al. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11(20):7234–7242. doi: 10.1158/1078-0432.CCR-05-0683. [DOI] [PubMed] [Google Scholar]
- 37.Haqq C, Nosrati M, Sudilovsky D, Crothers J, Khodabakhsh D, Pulliam BL, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci U S A. 2005;102(17):6092–6097. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clausen J, Vergeiner B, Enk M, Petzer AL, Gastl G, Gunsilius E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology. 2003;207(2):85–93. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 40.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 41.Kane MA, Elwood PC, Portillo RM, Antony AC, Najfeld V, Finley A, et al. Influence on immunoreactive folate-binding proteins of extracellular folate concentration in cultured human cells. J Clin Invest. 1988;81(5):1398–1406. doi: 10.1172/JCI113469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oleinik NV, Krupenko NI, Krupenko SA. ALDH1L1 inhibits cell motility via dephosphorylation of cofilin by PP1 and PP2A. Oncogene. 2010;29(47):6233–6244. doi: 10.1038/onc.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 44.Motoyama K, Onodera R, Tanaka N, Kameyama K, Higashi T, Kariya R, et al. Evaluation of antitumor effects of folate-conjugated methyl-β-cyclodextrin in melanoma. Biol Pharm Bull. 2015;38(3):374–379. doi: 10.1248/bpb.b14-00531. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Feng L, Yang X, Wang F, Lu W. Folic acid-conjugated liposomal vincristine for multidrug resistant cancer therapy. Asian Journal of Pharmaceutical Sciences. 2013:118–127. doi: 10.1016/j.ajps.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canevari S, Stoter G, Arienti F, Bolis G, Colnaghi MI, Di Re EM, et al. Regression of advanced ovarian carcinoma by intraperitoneal treatment with autologous T lymphocytes retargeted by a bispecific monoclonal antibody. J Natl Cancer Inst. 1995;87(19):1463–1469. doi: 10.1093/jnci/87.19.1463. [DOI] [PubMed] [Google Scholar]
- 47.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23(35):8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 49.Hsu JY, Wakelee HA. Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy. BioDrugs. 2009;23(5):289–304. doi: 10.2165/11317600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 50.Taraban VY, Rowley TF, O'Brien L, Chan HT, Haswell LE, Green MH, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32(12):3617–3627. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 51.Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, et al. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-γ production. J Immunol. 2011;186(6):3401–3409. doi: 10.4049/jimmunol.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE., 3rd Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol. 2006;177(1):120–129. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- 53.Roda JM, Joshi T, Butchar JP, McAlees JW, Lehman A, Tridandapani S, et al. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13(21):6419–6428. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 54.Parihar R, Nadella P, Lewis A, Jensen R, De Hoff C, Dierksheide JE, et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res. 2004;10(15):5027–5037. doi: 10.1158/1078-0432.CCR-04-0265. [DOI] [PubMed] [Google Scholar]