Abstract
The NCI Bladder Cancer Task Force convened a Clinical Trials Planning Meeting (CTPM) Workshop focused on Novel Therapeutics for Non-Muscle Invasive Bladder Cancer (NMIBC). Meeting attendees included a broad and multi-disciplinary group of clinical and research stakeholders and included leaders from NCI, FDA, National Clinical Trials Network (NCTN), advocacy and the pharmaceutical and biotech industry. The meeting goals and objectives were to: 1) create a collaborative environment in which the greater bladder research community can pursue future optimally designed novel clinical trials focused on the theme of molecular targeted and immune-based therapies in NMIBC; 2) frame the clinical and translational questions that are of highest priority; and 3) develop two clinical trial designs focusing on immunotherapy and molecular targeted therapy. Despite successful development and implementation of large Phase II and Phase III trials in bladder and upper urinary tract cancers, there are no active and accruing trials in the NMIBC space within the NCTN. Disappointingly, there has been only one new FDA approved drug (Valrubicin) in any bladder cancer disease state since 1998. Although genomic-based data for bladder cancer are increasingly available, translating these discoveries into practice changing treatment is still to come. Recently, major efforts in defining the genomic characteristics of NMIBC have been achieved. Aligned with these data is the growing number of targeted therapy agents approved and/or in development in other organ site cancers and the multiple similarities of bladder cancer with molecular subtypes in these other cancers. Additionally, although bladder cancer is one of the more immunogenic tumors, some tumors have the ability to attenuate or eliminate host immune responses. Two trial concepts emerged from the meeting including a window of opportunity trial (Phase 0) testing an FGFR3 inhibitor and a second multi-arm multi-stage trial testing combinations of BCG or radiotherapy and immunomodulatory agents in patients who recur after induction BCG (BCG failure).
Keywords: Non-muscle invasive bladder cancer, trial design, targeted therapy, immunotherapy, radiation therapy
ABBREVIATIONS
- 5FU
5-fluorouracil
- ALC
absolute lymphocyte count
- AUA
American Urological Association
- BCG
Bacille-Calmette Guerin
- BRAF
B-RAF proto-oncogene, serine/threonine kinase
- CBI
checkpoint blockade immunotherapy
- CIRB
National Cancer Institute Central Institutional Review Board
- CIS
carcinoma in situ
- CLIA
Clinical Laboratory Improvement Amendments of 1988
- CR
complete response
- CTLA-4
cytotoxic T-lymphocyte–associated antigen 4
- CTPM
Clinical Trials Planning Meeting
- CTSU
Cancer Trials Support Unit
- DFS
disease-free survival
- DNA
deoxyribonucleic acid
- DSS
disease-specific survival
- EAU
European Association of Urology
- EBRT
external beam radiation therapy
- ECOG-
ECOG-ACRIN Cancer Research
- ACRIN
Group (Eastern Cooperative Oncology Group and American College of Radiology Imaging Network)
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial-to-mesenchymal transition
- ERBB2
Erb-B2 receptor tyrosine kinase 2 gene
- ERCC2
excision repair cross-complementation group 2
- FDA
U.S. Food and Drug Administration
- FFPE
formalin-fixed, paraffin-embedded
- FGFR3
fibroblast growth factor receptor 3
- FNIH
Foundation for the National Institutes of Health
- GM-CSF
granulocyte macrophage colony-stimulating factor
- GU
genitourinary
- HGF
hepatocyte growth factor
- HSP90
heat shock protein 90
- IBCG
International Bladder Cancer Group
- IFN-γ
interferon gamma
- IHC
immunohistochemistry
- LG
low grade
- MAP
mitogen-activated protein
- MDSC
myeloid derived suppressor cells
- MHC
major histocompatibility complex
- MIBC
muscle invasive bladder cancer
- MMC
mitomycin-C
- mRNA
messenger ribonucleic acid
- NCI
National Cancer Institute
- NCI-
National Cancer Institute’s Coordinating
- CCCT
Center for Clinical Trials
- NCI-
NCI Molecular Analysis for Therapy
- MATCH
Choice
- NCTN
National Clinical Trials Network
- NMI
non-muscle invasive
- NMIBC
non-muscle invasive bladder cancer
- NMIUC
non-muscle invasive urothelial cancer
- NRG1
neuregulin-1
- NSCLC
non-small cell lung cancer
- PCR
polymerase chain reaction
- PD
programmed cell death protein
- PD-L1
programmed cell death-ligand1 protein
- PI
Principal Investigator
- PIK3CA
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene
- RAF
rapidly accelerated fibrosarcoma proto-oncogene
- RCC
renal cell carcinoma
- RNA
ribonucleic acid
- RT
radiation therapy
- STAMPEDE
Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy: A Multi-Stage Multi-Arm Randomised Controlled Trial
- SUO
Society for Urologic Oncology
- SWOG
Southwest Oncology Group
- SYNPO2
synaptopodin 2 gene
- TCC
transitional cell carcinoma
- TCGA
The Cancer Genome Atlas project
- TGFα
transforming growth factor alpha
- TIL
tumor infiltrating cells
- TKI
tyrosine kinase inhibitor
- TMA
tissue microarray
- TNF
tumor necrosis factor
- TUR
transurethral resection
- TURBT
transurethral resection of bladder tumor
- UC
urothelial cancer
- VEGF
vascular endothelial growth factor
- WHO/ISUP
World Health Organization/International Society of Urological Pathology
SESSION: INTRODUCTION AND OVERVIEW
STATE-OF-THE-ART AND CHALLENGES OF NON-MUSCLE-INVASIVE BLADDER CANCER (NMIBC)
Seth P. Lerner, M.D., FACS
The Bladder Cancer Task Force and the National Cancer Institute (NCI) organized a 2-day Clinical Trials Planning Meeting (CTPM) Workshop focused on Novel Therapeutics for Non-Muscle Invasive Bladder Cancer (NMIBC). This meeting brought together the multi-disciplinary clinical and research community including leaders from NCI, Food and Drug Administration (FDA), National Clinical Trials Network (NCTN), and the pharmaceutical and biotech industry. Our expressed goals were to develop therapeutic strategies around the theme of molecular targeted and immunotherapy based therapies in NMIBC.
The Task Force was constituted in 2010 with the mission to evaluate and facilitate development and implementation of large Phase II and Phase III trials in bladder and upper tract cancers (Table 1). Despite this success there are no active and accruing trials in the NMIBC space within the NCTN. Furthermore there has been only one new FDA approved drug (Valrubicin) in any bladder cancer disease state since 1998.
Table 1.
Bladder Task Force activity since 2010
| Current Active Trials |
| • RTOG-0926 – Chemo XRT for T1HG |
| • S1314 – COXEN neoadjuvant chemotherapy prior to radical cystectomy |
| • NRG-GU001 – Adjuvant XRT post RC for patients at high risk for local pelvic recurrence |
| • ECOG EA8141 – Phase II trial of neoadjuvant chemotherapy for upper tract urothelial carcinoma |
| • NRG-GU-TS001 – MRE11 retrospective validation |
| Concepts Reviewed and in Development |
| • SWOG 1602 (PRIME) |
| • MATCH-UP (Alliance) – NCI declined to move forward |
| • Adjuvant Pembrolizumab (Alliance) |
| • Chemo/XRT MIBC - MRE-11 prospective validation |
| • SWOG 1605 - Anti-PD-L1 for BCG unresponsive disease |
BCG (Tice and TheraCys) was approved for treatment of patients with CIS and high-grade papillary NMIBC in 1989 and 1990, respectively. There have been well-documented North American and global shortages due to disruptions in manufacturing and supply that have had a considerable adverse impact on our patients. This is an additional driver of innovation and drug development that is the subject of this important meeting.
We do however have a good understanding of the biology and related natural history of NMIBC. Risk stratification schemes and a revised grading system simplify assessment of probabilities of recurrence and progression and drive treatment decision making. Quality metrics for transurethral resection (TUR) and re-TUR in the case of high-grade T1 disease have been established and incorporated into guidelines. Urine cytology has a high positive predictive value for high-grade cancer, especially carcinoma in situ, and there are several FDA-approved voided urine biomarkers and novel detection system (e.g. fluorescence cystoscopy) that can be used as an adjunct to white light cystoscopy for detection of bladder cancer.
There is a robust track record of clinical trials in NMIBC that provide high level evidence supporting the current standard of care. Bacille-Calmette Guerin (BCG) is approved for treatment of high-grade papillary disease (Ta, T1) and carcinoma in situ (CIS). A 6–week induction course is superior to TURBT alone (Level 1A) and is associated with an initial complete response in 50-70% of patients. A second induction course may achieve a complete response in an additional 10-22% of patients (Level 3) and patients who relapse > 1 year following an initial complete response (CR) can be effectively treated with re-induction with BCG. However, patients with CIS who recur after 2 course of BCG should not be treated with a third course, as durable CR rates are low (Level 2B). Maintenance therapy with 3 weekly instillations following induction therapy at months 3,6 and every 6 months to 3 years is associated with improved RFS and PFS compared to induction therapy alone and is the current standard of care for high-risk disease (SWOG, 8507; Level 1B). The recent EORTC 30962 trial provided additional data to support the use of 3 years of maintenance with high-risk disease. BCG is also superior to intravesical chemotherapy with mitomycin (Level 1A) and epirubicin (Level 1B), but only when maintenance therapy is used.
The FDA and the urologic oncology community have provided guidance regarding clarification of disease states post BCG treatment for inclusion in registration trials in NMIBC [1–4]. BCG failure is defined as patients with recurrent or persistent disease following the first induction therapy. BCG unresponsive is defined as patients who recur after a minimum treatment of induction plus one course of maintenance therapy or patients who never achieve a CR within 6 months of the last BCG treatment. Patients who recur with T1HG after induction only are also considered unresponsive. The standard of care for these patients is radical cystectomy and so there is a large unmet need for drug development in this space. Valrubicin is the only approved drug for patients with CIS who are BCG-refractory and refuse or are determined to be too high a medical risk for cystectomy. The future is bright however, as there is intense interest from a broad array of pharmaceutical companies and there are novel drug delivery systems being deployed as well (Tables 2, 3).
Table 2.
Current clinical trial landscape
| Trial/Sponsor | Drug | Target/Design | Phase | Status |
| S0337 | Gemcitabine | Peri-op single dose | III | Completed |
| NCT00974818 | MMC vs. Gem | Closed early? | ||
| NCT00461591 NCT00598806 | Apaziquone | Peri-op single dose | III | Closed |
| RTOG-0926 | Chemo/XRT | T1 | II | Open |
| NCT01732107 | Dovitinib (FGFR3) | BCG refractory | II | Closed |
| Cold Genysis | CG 0070 | Rep competent ADV GMCSF | III | Open? |
| NCT02009332 | Rapamycin (mTOR) | BCG refractory | I/II | Open |
| NCT01259063 | Everolimus/Gem | BCG refractory/CIS | I/II | Open |
| NCT02197897 | Tamoxifen | ER – TaLG marker lesion | II | Open |
| NCT02010203 Heat Biologics | HS 410 (vaccine) | BCG + HS 410 (BCG naϊve) | I/II | Open |
| Viventia | Vicinium | High risk | I/II | Ph II planned |
| FKD | AD-IFN | BCG refractory | II | Completed Ph III planned |
| BioCancell | BC 819 (H19/DTA) | BCG failure/refractory | II | Completed Ph III planned |
| NCT02015104 | PANVAC+BCG vs. BCG | BCG failure | II | Open |
| Telesta Therapeutics | MCNA | Failure/Unresponsive | III | Completed |
| Altor Bioscience | ALT-803 (IL15) | BCG nϊve | I/II | Completed |
Table 3.
Novel drug delivery systems
| • Adenoviral mediated |
| • PEI/DNA plasmid |
| • Liposomal complex |
| • Nanoparticles |
| • Implantable osmotoic pump |
| • Conjugated antibody/payload |
| • Bacterial minicells |
| • Heat |
| • Iontophoresis |
| • Muco-adhesive molecules |
In the past 3 years there has been a convergence of output from diverse sources delineating the genomic landscape of urothelial bladder cancer, culminating with the recent publication of the “marker” paper from The Cancer Genome Atlas project in muscle invasive disease [5]. Maggie Knowles and others have sought to define the genomic characteristics of NMIBC along pathways that distinguish low grade from high-grade disease [6]. The Lund group has provided additional clarification describing an immunohistochemical classifier for high-grade T1 disease stratifying into urobasal and GU squamous cell like subtypes [7]. Aligned with these data is the growing number of targeted therapy agents approved and/or in development in other organ site cancers and the multiple similarities of bladder cancer with molecular subtypes in these other cancers. Additionally, although bladder cancer is one of the more immunogenic tumors seen in man, some tumors have the ability to attenuate or eliminate host immune responses.
Supplemental material is provided to share the Goals and Meeting Agenda.
LESSONS FROM ADVANCED DISEASE TRIALS APPLICABLE TO NMIBC
Dean F. Bajorin, M.D.
The Clinical Trial Planning Meeting for non-muscle invasive bladder cancer is a unique opportunity provided by the National Cancer Institute to bring together leaders in the field including cooperative group investigators, academia, industry, patient advocacy, and officials from FDA and NCI to spur the development of state of the art trials in non-muscle invasive bladder cancer. We are familiar with four major disease states seen in urothelial cancer ranging from non-muscle invasive bladder cancer to muscle invasive bladder cancer to metastatic disease treated with first-line therapy to the metastatic disease treated with salvage or second line therapy. We know from past experience in many malignancies that novel drugs active in the chemotherapy refractory metastatic disease setting can be escalated to earlier stage disease. Such is the case for bladder cancer in that novel treatment modalities identified as active in the chemotherapy refractory disease state have good rationale for clinical studies seeking to advance treatment in patients with non-muscle invasive bladder cancer.
We are fortunate in that bladder cancer is in the midst of a perfect storm, i.e., the emergence of comprehensive molecular characterization of urothelial carcinoma coupled with major advances in both targeted therapy and immunotherapy. The Cancer Genome Atlas Project (TCGA) for muscle invasive bladder cancer was published this past year and was highly informative with regard to both tumor biology and potential clinical interventions [6]. That study reported a large number of potentially actionable mutations in multiple pathways previously exploited in other diseases. For example, 93% of patients’ tumors demonstrated alterations in the p53/RB1 pathway. These included alterations in CDKN2A, ATM, RB1, and E2F3. Similarly, 72% of tumors in this study had alterations in the RTK/RAS/PI(3)K pathway. This latter pathway is particularly opportune based on reported responses seen with tyrosine kinase inhibitors to TSC 1 and FGFR 3, respectively. In a phase 2 study of everolimus in previously treated patients, this mT0RC1 inhibitor resulted in a complete response in a patient with disease refractory to gemcitabine and cisplatin. Whole genome sequencing performed in this patient demonstrated both TSC1 and NF2 mutations identified as cooperative and resulting in exquisite sensitivity to this agent. Additionally, other studies have reported responses to inhibitors of FGFR3 and PIK3CA inhibitors. We know now that many tumors harbor multiple alterations within the same pathway and it may be unknown whether an alteration is a driver or passenger mutation. Thus, sequential biopsies will be critical to dissect mechanisms of sensitivity and resistance in any proposed trial.
The other emerging treatment opportunity is the use of immunotherapy agents active in the programmed cell death (PD) pathway responsible for suppressing antitumor immunity. At the time of this symposium, the first report of a drug active in advanced bladder cancer has been published. This initial study explored MPDL3280A in the treatment of patients whose cancer had progressed despite chemotherapy. Significant responses were observed – initial results demonstrated that responses were greater in patients whose tumors expressed PD-L1 staining in tumor infiltrating cells. The initial study reported a response rate of 43% in tumors with high expression of PD-L1 and 11% in tumors with little or no expression of PD-L1. Most remarkable in the study was the duration of responses, some extending well beyond one year. Prolonged response to any chemotherapy agent in this disease state is distinctly unusual and rarely reported in the literature making this a very promising intervention to exploit across all disease states.
Investigators studying urothelial cancer can benefit from other studies of immunotherapy in different malignancies, specifically melanoma and non-small cell lung cancer. These studies demonstrate that other molecular markers of response besides PD–L1 may exist. For example, studies in both melanoma and non-small cell lung cancer demonstrate that a high mutation rate in these tumors may be prognostic. Interestingly, urothelial cancer has a very high somatic mutation rate similar that seen in melanoma and non-small cell lung cancer. In the case of non-small cell lung cancer, a distinct molecular smoking signature associated with durable benefit has been reported. Conversely, neoantigens identified in melanoma appear to have similarities to antigens expressed in infectious diseases. Both of these studies are early reports of companion diagnostics, approaches that may help us distinguish prospectively those patients who may benefit from immunotherapy in urothelial cancer.
Urothelial cancer is poised for innovative and potentially effective therapy in the advanced disease setting which may be clinically applied to the non-muscle invasive bladder cancer disease state. Additionally, bladder cancers are accessible for biopsy that will allow us to interrogate these tumors to make further advances both in urothelial cancer as well as other malignancies. Our challenge for this symposium is to create the platform for studying these novel drugs in non-muscle invasive bladder cancer. Both immunotherapy drugs and novel tyrosine kinase inhibitors are given systemically rather than by an intravesical route. Therefore, expertise is needed in both administration and management of unique toxicities and clinical trials will require enhanced collaborations between urologists and medical oncologists. These first-generation trials will need to extend our biological understanding of these new drugs in urothelial cancer and thus will require enhanced collaborations between physicians and translational scientists to establish sustainable research platforms for subsequent trials.
Our charge to the participants of the clinical trial planning meeting is to spur the development of two separate trial concepts to be exploited in the cooperative group mechanisms. The proposed trials will seek to test two hypotheses: 1) Molecularly targeted therapy has clinical benefit in non-muscle invasive bladder cancer; and, 2) Novel immunotherapy has clinical benefit in non-muscle invasive bladder cancer. Throughout the planning meeting, clinicians, scientists, government agencies, pharmaceutical experts, and patient advocates convened to plan how best to test each hypothesis within the cooperative group clinical trial mechanism.
INTEGRATING RADIATION FOR NMIBC: CURRENT STATUS AND FUTURE OPPORTUNITIES
Jason A. Efstathiou, M.D., D.Phil.
Management of high-risk NMIBC, especially when recurrent after BCG, is complicated by poor long-term response rates to alternative intravesical biologic/chemotherapeutic agents and regimens. Although radical cystectomy remains standard, national practice patterns suggest that many patients are not getting cystectomy when indicated, likely due to age, comorbidities and/or desire to avoid such surgery [8]. Further compounding this dilemma is that a substantial proportion (~45%) of T1 tumors are actually upstaged at time of surgery [9] suggesting that more definitive local therapy may indeed be indicated.
Combined modality therapy using chemoradiation has long been utilized as a curative therapy for MIBC [10]. Although radiation-based therapy is not standard in NMIBC, it may offer an alternative for selected patients who are otherwise unfit or unwilling to undergo cystectomy. In T1 bladder cancer, early studies using radiation therapy (RT) alone demonstrated response rates of 48-69% for unifocal tumors [11, 12]. A subsequent randomized trial of RT alone versus conservative treatment for high-grade T1 tumors showed no difference in terms of recurrence (local failure rates ~70% in both arms) or survival; however, this study was limited due to long accrual and lack of control over TUR extent and time to adjuvant therapy [13].
The experience of using combined chemoradiation following an attempt at maximal TUR as an alternative primary treatment for high-risk NMIBC is more encouraging. The University of Erlangen [14] reported on their institutional experience of 141 patients. They demonstrated a complete response rate of 88%; progression rates of 19% and 30% (13%/29% for high-grade T1 tumors) at 5- and 10-years, respectively; and disease-specific survival (DSS) rates of 82% and 73% (89%/79% for complete responders and 80%/71% for high-grade T1 tumors) at 5- and 10-years. Over 80% of survivors preserved their native bladder, and ~70% were ‘delighted’ or ‘pleased’ with their urinary function. Of note, this was an experience of alternative primary treatment (i.e. not limited to salvage for BCG refractory disease). The Massachusetts General Hospital reported on an experience of 17 patients undergoing TURBT and chemoradiation following T2 recurrence after failing BCG for non-invasive disease [15]. With 7-years follow-up, only 1 patient required cystectomy, 10 (59%) were free of any bladder recurrence, and DSS was 70%. To further inform the role of chemoradiation in NMIBC, there is currently an ongoing prospective cooperative group protocol RTOG-0926 evaluating 61.2 Gy plus concurrent cisplatin or 5FU/MMC after maximal TURBT for patients with high-risk T1 bladder cancer who recur following BCG [16].
Although at this time there is limited data in bladder cancer, RT is known to have multiple immune-mediated effects making the combination of RT and immunotherapy a promising avenue for enhanced response that warrants further investigation. RT helps liberate antigen and mature dendritic cells for effective T-cell priming, and the addition of immunotherapeutic agents (such as checkpoint inhibitors - anti-PDL1, anti-PD-1, anti-CTLA-4) could enhance the immunostimulatory effects of RT and thus improve tumor control [17]. One study demonstrated that the addition of GM-CSF to local RT directed at metastatic sites of different solid tumors produced objective abscopal responses in 27-28% of patients [18]. Current data from pre-clinical models support concurrent administration of RT plus immunotherapy and hypofractionated regimens, though abscopal effects have been observed even with single large RT doses such as 8Gy x1 [17, 18].
In summary, radiation combined with chemotherapy or immune checkpoint inhibitors holds promise for improved tumor control and enhanced immunologic response, and may help fill the need for alternative potentially curative therapies in selected patients with recurrent high-risk NMIBC. Biomarkers of radiation response may further identify subgroups of patients best served by such approaches.
OPPORTUNITIES FOR EMBEDDING BIOMARKERS IN PROSPECTIVE TRIALS OF NON-MUSCLE INVASIVE BLADDER CANCER (NMIBC)
Lisa McShane, Ph.D.
There are numerous opportunities for development of biomarkers or biomarker signatures to optimize care for patients with NMIBC (Table 4). Important roles that biomarkers could play include prognostic indicators, predictive (treatment selection) indicators, and monitoring indicators for use during or after initial treatment [19]. These biomarkers could potentially be measured in tumor tissue, normal bladder epithelium, urine, or blood. Biomarker development should focus not merely on identification of biomarkers with statistically significant associations with clinical outcomes but should provide information that can be acted upon clinically and lead to better outcome for patients [20].
Table 4.
Priority opportunities for biomarker-based tests to have clinical impact in non-muscle invasive bladder cancer
| Disease state | Specimen type | Biomarker clinical use | Example clinical study |
| Stage 0a (Ta), low risk | Tumor or normal bladder epithelium | Prognostic biomarkers to identify patients at high risk of recurrence or progression | Establish whether patients indicated by biomarkers to be high risk benefit from intravesical therapy following tumor resection |
| Stage 0a (Ta), high risk | Tumor or normal bladder epithelium | Predictive biomarkers to select optimal therapy | Establish whether biomarkers can optimally select among treatment options including intravesical immunotherapy or chemotherapy |
| Stage 0is (CIS) | Tumor or normal bladder epithelium | Predictive biomarkers to select optimal therapy | Establish whether biomarkers can optimally select among different intravesical therapy options, including various immunotherapies or chemotherapies as well as frequency and duration of therapy |
| Blood or urine | Intra-treatment monitoring to detect response or non-response | Establish whether switching therapy based on biomarkers measured during therapy that indicate lack of response leads to a lower rate of non-response or progression than not acting on the biomarkers | |
| Stage I (T1) | Tumor or normal bladder epithelium | Predictive biomarkers to select optimal therapy | Establish whether biomarkers can optimally select among treatment options including cystectomy (full or partial), or various intravesical immunotherapies or chemotherapies |
| All stages | Blood or urine | Post-treatment surveillance | Establish whether biomarkers measurable in blood or urine can replace or reduce frequency of surveillance cystoscopies without increasing rate of poor outcomes associated with recurrent or progressive disease |
Clinically useful biomarkers provide actionable information that is not already available from standard clinico-pathologic indicators, or they provide information comparable to standard indicators less invasively or with lower cost or greater convenience. The anatomic accessibility of the bladder epithelium allows for biomarkers to be readily measured in tumor tissue or in morphologically normal appearing bladder epithelium during biopsy. Biomarkers are needed for low-grade Ta disease to identify which patients have the highest likelihood of disease recurrence and might benefit from receiving intravesical therapy following tumor resection. For high grade stage Ta and stage CIS bladder cancers likely to be treated with intravesical therapy, predictive biomarkers are needed to select optimal therapeutic agents and frequency and duration of therapy to increase chances of disease eradication, lower chances of adverse effects of treatment, and decrease the need for subsequent cystectomy. An important therapeutic decision for patients with stage T1 disease is whether to have full or partial cystectomy or to first consider intravesical immunotherapy or chemotherapy. The possibility to detect tumor cells or secreted biomarkers in urine or blood also presents opportunities to identify biomarkers for non-invasive early monitoring of robustness of response to therapy or for early detection of disease recurrence or progression. Collectively, these opportunities make non-muscle invasive bladder cancer fertile ground for biomarker development and validation.
SESSION I: KEY GENETIC TARGETS AND RELEVANT PATHWAYS FOR INTERVENTION IN BLADDER CANCER
Session Co-Chairs: David Kwiatkowski, MD, PhD, Colin Dinney, MD, Jonathan Rosenberg, MD
OVERVIEW OF MOLECULAR ALTERATIONS IN BLADDER CANCER - MIBC VS. NMIBC
David Kwiatkowski, MD, PhD
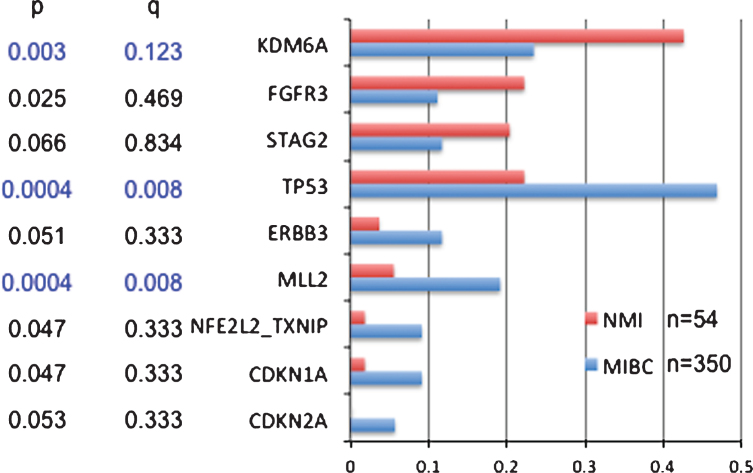
Several recent comprehensive genome-wide analyses have identified multiple genes and pathways involved in bladder cancer development [5, 21–24]. These studies have revealed that bladder cancer has a high mutation rate largely due to APOBEC-mediated mutagenesis. Over 30 genes are commonly mutated in invasive bladder cancer including those in the cell cycle, chromatin regulatory, PI3K-mTOR, Ras, receptor tyrosine kinase, and transcription regulatory pathways. Focal genomic amplifications and deletions are also common in bladder cancer and target these same pathways. Previous studies have shown that FGFR3 mutations are more common in NMIBC, while TP53 mutations are more common in muscle-invasive (MIBC) bladder cancer. Compilation of data from four studies employing whole exome sequencing [5, 21, 22, 24] including a total of 350 MIBC cases and 54 NMIBC cases indicates that both KDM6A and FGFR3 mutations are significantly more common in NMIBC than MIBC (42% vs. 23%, and 22% vs. 11%, respectively), while TP53 and MLL2 are significantly more frequent in MIBC than NMIBC (47% vs. 22%, and 22% vs. 4%, respectively) (Fig. 1). Mutations in several other genes also appear to be more common in MIBC but did not reach statistical significance: ERBB3, CDKN1A, CDKN2A, NFE2L2, TXNIP. Other studies have identified STAG2 mutations as occurring more often in NMIBC vs. MIBC.
Fig. 1.
Comparison of mutation frequencies in non-muscle invasive (NMI) vs. muscle invasive bladder cancer (MIBC). Mutation frequencies are shown for genes for which there were some evidence for a difference in mutation frequency between NMI and MIBC. P is conventional p values by Fisher exact text; q is values after correction by FDR for multiple comparisons.
These findings confirm the common model that NMIBC and MIBC develop through distinct molecular pathways, and suggest that only a fraction of MIBC progresses from precursor NMIBC tumors.
FGFR3 AND FUSION PARTNERS AS POTENTIAL TARGETS – EXAMPLES OF HIGHLY TARGETABLE MUTATIONS
Margaret Knowles, BSC, PhD
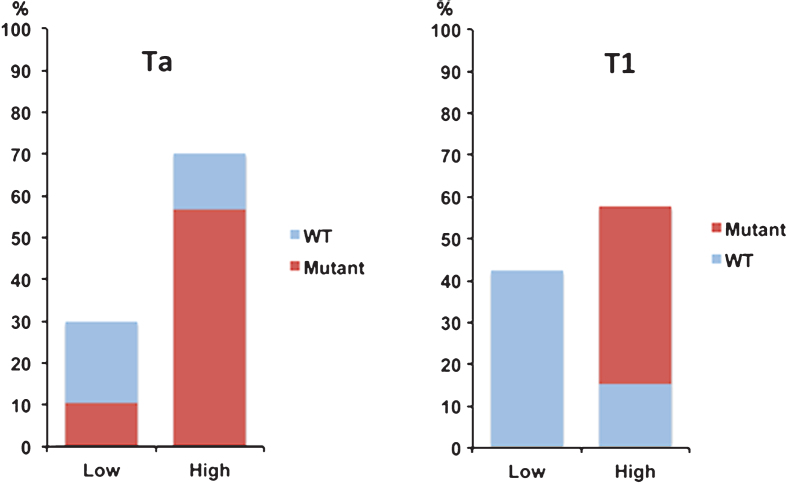
FGFR3 has been implicated in NMIBC for more than a decade. Sixty to 70% of stage Ta tumors and approximately 40% of stage T1 tumors have activating point mutations (most commonly S249C) that induce ligand-independent signaling. In addition, some tumors without point mutation (10-15%) show up-regulated FGFR3 protein expression [25] (Fig. 2) [26]. Oncogenic FGFR3 fusion proteins have been identified in approximately 2–4% of bladder cancers overall. Their grade/stage distribution remains unclear, but it is notable that the fusions we have found in bladder cancer cell lines (RT4, RT112, LUCC2, SW780) are in lines derived from grade 1 or 2 tumors or tumors described as “papillary” [27]. Taken together, FGFR3 is implicated in >80% of stage Ta and >50% of stage T1 tumors.
Fig. 2.
Relationship of FGFR3 mutation and expression in non-muscle invasive bladder cancer. Expression of FGFR3 protein (low or high) detected by immunohistochemistry in relation to presence or absence of FGFR3 point mutations (mutant vs. WT) in stage Ta and T1 bladder tumors (Data from Tomlinson, 2007 [26].)
The reported consequences of FGFR3 activation in normal urothelial cells are activation of the RAS-MAPK pathway and PLCγ but not the phosphatidylinositol 3-kinase pathway. FGFR3 has been examined as a potential therapeutic target by shRNA knockdown and treatment with small molecule inhibitors and antibodies using in vitro assays and in vivo xenograft assays. Although normal urothelial cells express low levels of FGFR3, they are not sensitive to the small molecule inhibitors tested (PD173074, AZD4547, TKI-258). Tumor cell lines with point mutation and detectable FGFR3 protein expression show variable responses (e.g. IC50 for PD173074 from 10-1000nM). Three cell lines with FGFR3 fusions (RT4, RT112 and SW7800) show high sensitivity (IC50 5-50nM). In these sensitive cell lines, cell cycle arrest rather than apoptosis is induced. Similarly, in xenograft assays, FGFR1/3 selective small molecules induce a cytostatic rather than a cytotoxic response, with tumor escape following cessation of treatment [28].
Potential resistance mechanisms have been examined in several studies. RT112 (FGFR3 fusion-containing) can be rescued from the inhibitory effects of PD173074 by NRG1 and EGF, and from BGJ398 by HGF, NRG1, TGFα and EGF. EGFR knockdown was found by RNAi screening to increase sensitivity to PD173074 in FGFR3-dependent cell lines. Conversely, FGFR3 provided escape from EGFR inhibition in EGFR-dependent cell lines, and combined inhibition of EGFR and FGFR3 had synergistic effect [29]. This cross talk between EGFR and FGFR3 signaling has also been demonstrated by the high sensitivity of RT112 to HSP90 inhibition, which caused down regulation of both of these client proteins. Importantly, HSP90 inhibition induced apoptosis rather than cell cycle arrest [30].
TARGETABLE ALTERATIONS IN NMIBC
William Kim, M.D.
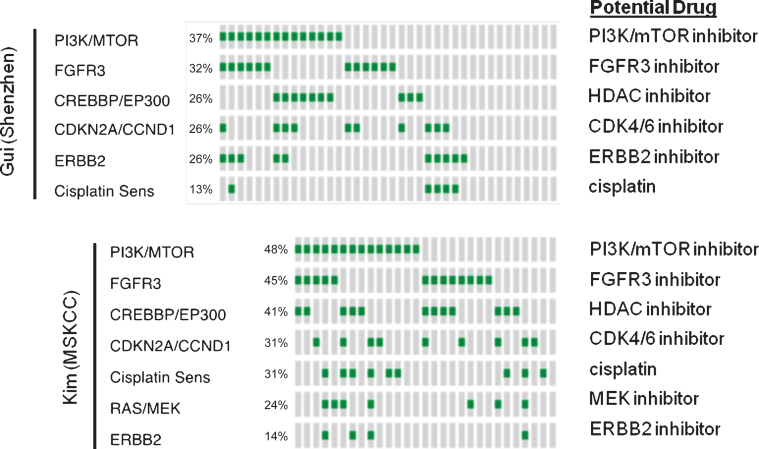
Recent publications have comprehensively characterized the landscape of genomic alterations in high-grade, muscle-invasive bladder cancer (MIBC) and found that these tumors have a high prevalence of alterations that are potentially treatable by targeted therapy. Past reports have also examined copy number alterations and mutations in non-muscle invasive bladder cancer. We collated the three largest datasets that contained high-grade, NMIBC to make the following observations [31–33]. 1) Very few CIS samples have been profiled. 2) Comparison of the mutational frequency between NMIBC and MIBC showed that a number of genes (most notably FGFR3) are more frequently mutated in high-grade, NMIBC (Fig. 3). 3) The majority of NMIBC, like MIBC, harbor alterations in pathways that are potentially treatable with targeted therapy and a small fraction of tumors may be amenable to combination therapy. While these results suggest the potential for the use of targeted therapy in HG, NMIBC, unresolved issues remain including the unknown degree of intratumoral heterogeneity and how to best prioritize competing mutations. Finally, recent research suggests that mutations in the DNA damage repair pathway may predict for response to cisplatin based chemotherapy in MIBC. Intriguingly, mutations in these genes appear to be present in NMIBC as well suggesting a potential utility for intravesical cytotoxic chemotherapy.
Fig. 3.
Pathway alterations in HG, NMIBC and potential drugs targeting specific alterations.
DEFINING THE ACTIONABLE GENOME
David Solit, M.D.
Bladder cancer is a genomically heterogeneous disease. Recent studies have identified frequent mutations in several targetable kinases including FGFR3, ERRB2, PIK3CA and others [34]. As each of these genes is mutated in only a minority of patients, novel clinical trial designs are needed to efficiently test inhibitors of these driver oncogenes in patients with advanced bladder cancer.
Basket studies are trials in which eligibility is based not upon site of tumor origin but rather the presence or absence of a particular genetic mutation. For example, in the recently reported vemurafenib basket study, patients with multiple non-melanoma cancers whose tumor harbored a codon 600 BRAF mutation were eligible [35]. This study confirmed that BRAF was a targetable oncogene in some, but not all, non-melanoma cancers. The basket design was shown to be particularly efficient for testing this agent in rare tumor types. Given the genomic heterogeneity of bladder cancers, a collection of basket studies (a tent protocol) could be the optimal way to test genotype-phenotype correlations. In such a design, patients could be screened using a central reference laboratory or locally in a CLIA-regulated laboratory. Patients with specific mutations would then be eligible for genotype matched basket studies that are testing novel agents or combinations in particular mutant populations. One limitation of this design is that a compelling clinical strategy may not be available for all mutational subtypes. For example, in patients with bladder cancer whose tumors are RB1 mutant, a promising targeted approach has yet to be identified.
INTRINSIC BASAL AND LUMINAL SUBTYPES OF BLADDER CANCER: IMPLICATIONS FOR IMMUNOTHERAPY
David McConkey, Ph.D.
Recent high profile clinical trial results indicate that immunotherapy with immune checkpoint inhibitors is clinically active in advanced, cisplatin-refractory muscle-invasive bladder cancers (MIBCs)[36]. Immunotherapy, in the form of intravesical BCG, is also highly active in high-grade NMIBCs and remains the frontline therapy for them. Therefore, there is a surge of new interest in determining whether immune checkpoint blockade could also be used to improve the clinical outcomes of patients with NMIBCs. Translational studies, mostly performed in other solid malignancies (i.e., melanoma, NSCLC), are beginning to identify the biological determinants that dictate response to these agents. Although there is not a strict correlation between checkpoint biomarker expression and response, tumors that are enriched with immune checkpoint biomarkers (CTLA4, PD1, PDL1) and/or display a strong T cell infiltrate at baseline appear to be more likely to respond [37, 38]. The biological determinants of immune checkpoint biomarker expression and T cell infiltration are still under investigation but include overall mutational burden [39], epithelial-to-mesenchymal transition (EMT) [40], and tumor-specific signal transduction pathways (i.e., active β-catenin) [41].
Several groups recently demonstrated that MIBCs can be assigned to intrinsic basal and luminal subtypes that are similar to the ones observed in breast cancer [5, 42, 43]. The subtypes respond differently to conventional chemotherapy [42] and are enriched with mutations and other biomarkers that suggest that they will also respond differently to targeted therapies [44]. In particular, there is a fraction of “mesenchymal” (“claudin-low”) basal MIBCs that is highly enriched with T and B lymphocyte gene expression signatures, including immune checkpoint biomarkers including PD-L1 [44]. Conversely, a large fraction of luminal MIBCs, corresponding to TCGA’s “papillary” subtype (cluster I) that is also characterized by enrichment with FGFR3 mutations and fusions, expresses particularly low levels of these same biomarkers [44], and results from a completed Phase 2 study of atezolizumab in patients with cisplatin-refractory advanced disease confirmed that these papillary luminal tumors were resistant to PD-L1 blockade [124]. Using whole genome mRNA expression profiling, we have found that essentially all NMIBCs express similarly low levels of lymphocyte and immune checkpoint biomarkers at baseline, although preliminary comparisons of matched tumors before and after BCG immunotherapy (n = 5) demonstrated consistent increases in CTLA4 expression post-therapy. Further investigation is required to define the biological determinants of lymphocyte infiltration, immune checkpoint biomarker expression, and sensitivity to immune checkpoint blockade across the spectrum of bladder cancer disease states. It is anticipated that this information will be crucial to informing clinical trial design and in interpreting their outcomes.
PATHOLOGICAL ASPECTS AND TISSUE ACQUISITION FOR NEXT GENERATION TRIALS IN NMIBC
Hikmat Al-Ahmadie, M.D.
Tissue analysis provides vital information in the workup of patient with bladder cancer. One important aspect of histopathological tissue evaluation is to provide accurate diagnosis, which includes establishing the presence of tumor (or its absence), assigning a grade/classification and providing the pathologic stage by assessing the depth of tumor invasion when present. These aspects of the pathologic evaluation are generally achieved by applying criteria proposed by the World Health Organization/International Society of Urological Pathology (WHO/ISUP) that are widely used [45]. In order to satisfactorily achieve these goals, adequate tissue sampling is required which includes that presence of adequate amount of tumor to assign grade and properly classify the tumor but also adequately deep tissue to properly provide tumor stage. The latter point requires the presence of portions of the muscularis propria (detrusor muscle) in the sample, particularly in invasive tumors, in order to be able to rule out its involvement by cancer.
Other important aspects of the pathologic evaluation of bladder tissue include the application of immunohistochemical staining for potential clinically relevant biomarkers or the application of next generation sequencing assays on tumor tissue. These techniques are becoming increasingly available and some have been approved for use in certified clinical settings [46]. This was made possible particularly as the tissue requirement for such analyses are becoming increasingly flexible, requiring smaller quantities of nucleic acid and being suitable for the more abundant formalin fixed paraffin embedded tissue. These technologies are very powerful and sophisticated and can provide vital information about the tumors genetic makeup that can be potentially used for diagnostic, prognostic and therapeutic purposes.
Needless to say that the samples should be of good enough quality to enable the pathologist to provide this necessary information whether it is for diagnosis purposes or for use in any correlative studies that require tissue evaluation. This includes having adequate amount of tumor tissue and also tissue that is devoid of significant thermal or autolytic/ischemic artifacts.
INTEGRATIVE CLINICAL SEQUENCING AND ANALYSIS APPROACHES FOR NMIBC
Eliezer Van Allen, M.D.
Systematic approaches to define the genomic landscape of clinically relevant NMIBC genomics may differentiate risk of recurrence and identify new treatment strategies for this disease. Furthermore, given tumor heterogeneity, approaches that utilize genome-wide data generation may yield insights into NMIBC genomics.
As proof of principle towards these approaches in bladder cancer, we performed whole exome sequencing on 50 patients with muscle invasive bladder cancer (MIBC), half of whom had complete responses to neoadjuvant cisplatin-based chemotherapy, the other half with persistent muscle invasive disease. Through mutation identification and case/control genomics algorithm development, we discovered and experimentally validated that somatic ERCC2 mutations correlated with cisplatin response in MIBC [24]. With this approach as a model, we therefore propose similar studies in key clinical nodes along the clinical pathway for NMIBC [6]. These include deep molecular characterization of NMIBC patients who do or do not progress through whole exome (and potentially transcriptome) sequencing followed by case/control genomic analyses.
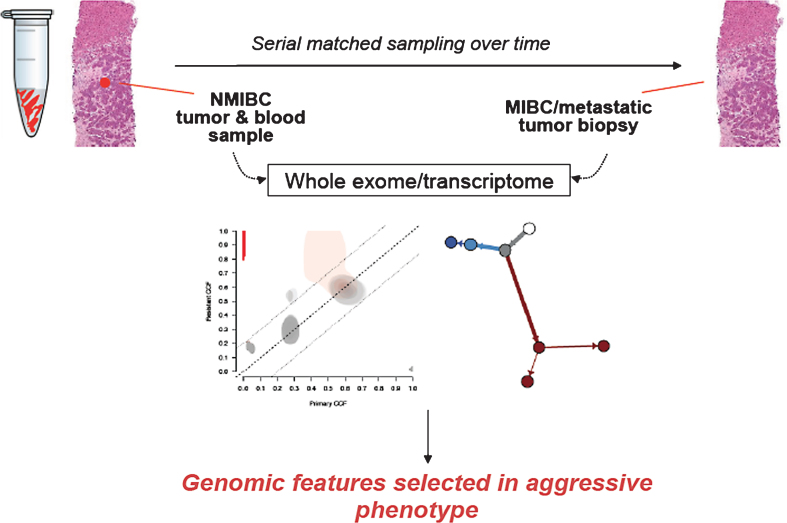
Similarly, detailed examination of longitudinally acquired NMIBC samples for study of tumor evolution may identify genomic events that trigger progression of disease (Fig. 4). We have developed and applied genomics approaches for studying such patients longitudinally in other tumor types, such as in BRAF-mutant melanoma and acquired RAF inhibitor resistance [47], thereby discovering new genomic mechanisms of acquired resistance that may inform subsequent treatment avenues. A similar strategy for NMIBC patients towards identifying such features in patient-matched samples may enable discovery of molecular switches for NMIBC progression. Ultimately, this approach may identify high-risk genomic features and, potentially, new therapeutic avenues for this disease.
Fig. 4.
Schematic for longitudinal tumor sampling of NMIBC to MIBC progression. This approach may inform genomic features of high risk disease and identify new therapeutic targets.
IDENTIFYING TARGETS THROUGH GENOME SCALE EPIGENETIC ANALYSIS
Luigi Marchionni, M.D., Ph.D.
Epigenetics is the study of heritable gene function changes that cannot be explained by changes in DNA sequence. DNA methylation, histone modifications, chromatin remodeling, and RNA-based gene regulation are the most studied epigenetic mechanisms. Altered DNA methylation represents the most common epigenetic alteration in bladder cancer and CpG island hypermethylation of tumor-suppressor gene promoters is associated with transcriptional inactivation and may occur early in carcinogenesis, making it clinically valuable for early diagnosis and risk stratification. DNA methylation can be studied using either a candidate gene or a genome-wide approach, using a number of techniques available to enrich for DNA methylation prior to sequencing, microarray, or PCR analysis.
The vast majority of studies of DNA methylation in non-muscle invasive bladder cancer (NMIBC) have used a gene candidate approach [48, 49]. These studies have, therefore, mostly focused on known tumor suppressor genes (e.g., CDKN2A (p16-INK4A and p14-ARF APC, CDH1, BRCA1, WT1, TP53), or on candidate suppressor genes (e.g., TMP3, RASSF1A, CDH13, Wnt-signaling antagonists, DAPK1, Laminins, GSPT1, RUNX3, SPINT2), which have shown associations with various clinical phenotypes in muscle-invasive bladder cancer or in other tumor types (Table 5). Only a few recent studies have used a genome-wide approach reporting multi-gene signatures (as described in a comprehensive review [48, 49]).
Table 5.
DNA methylation markers in bladder cancer associated with progression or recurrence in NMIBC
| Genes | Progression | Recurrence | Study |
| APAF1 | NA | 0.05 | Christoph et al, Int J Cancer, 2006 |
| CDH13 | 0.00 | 0.01 | Lin et al, Int Urol Nephrol, 2012 |
| CDKN2A | NA | 0.05 | Lin et al, Urol Oncol, 2010 |
| DAPK1 | NA | 0.001 | Tada et al, Cancer Res, 2002 |
| DAPK1 | NA | 0.04 | Christoph et al, Int J Cancer, 2006 |
| IGFBP3 | NA | 0.02 | Christoph et al, Int J Cancer, 2006 |
| RASSF1A | 0.004 | NA | Kim et al, Clin Genitourin Cancer, 2012 |
| RASSF1A | 0.04 | NA | Catto et al, J Clin Oncol, 2005 |
| RASSF1A, CDH1, APC, TNFSR25, EDNRB | 0.05 | NA | Yates et al, Clin Cancer Res, 2007 |
| RUNX3 | 0.01 | 0.02 | Kim et al, Cancer Res, 2005 |
| RUNX3 | 0.006 | 0.04 | Yan et al, J Surg Oncol, 2012 |
| RUNX3 | 0.013 | NA | Kim et al, J Urol, 2008 |
| SYMPO2 | 0.05 | NA | Cebrian et al, Cancer Res, 2008 |
| SYMPO2 | 0.03 | 0.01 | Alvarez-Mugica et al, J Urol, 2010 |
| TBX2, TBX3, GATA2, ZIC4 | 0.003 | NA | Kandimalla et al, Eur Urol, 2012 |
| TBX4 | 0.05 | NA | Reinert et al, Clin Cancer Res, 2011 |
| TIMP3 | NA | 0.036 | Friedrich et al, Eur J Cancer, 2005 |
| TIMP3 | 0.01 | NA | Hoque et al, JNCI, 2006 |
Most importantly DNA hypermethylation has been shown to carry prognostic information in NMIBC, with a variety of selected genes associated with disease recurrence, progression, or both (Table 5). Furthermore, one gene – synaptopodin 2 (SYNPO2) – has also been associated with resistance to BCG treatment in different cohorts of patients with T1G3 bladder cancer. Distinctive methylation patterns have been shown to differentiate between FGFR3-mutant and wild-type tumors. Finally, a few reports have also looked at histone methylation in NMIBC, and global H3K9 and H3K27 methylation has been shown to increase with progression from normal urothelium to NMIBC and MIBC. Furthermore, within NMIBC histone methylation levels correlate with increasing stage and grade.
In conclusion, DNA methylation is the most studied epigenetic modification in bladder cancer. Unique methylation patterns distinguish between NMIBC and MIBC, as well as between FGFR3-mutant and wild-type tumors. Epigenetic modifications can be used as biomarkers for bladder cancer detection, prognostication, and therapeutic benefit prediction. Indeed, the analysis of methylated genes in urine represents an ideal tool for follow-up after TURBT, and DNA methylation and histone modifications might represent a potential target for future combination therapies in NMIUC.
INTRAVESICAL TARGETED GENE THERAPY FOR LOW-GRADE (LG) NON MUSCLE-INVASIVE BLADDER CANCER (NMIBC)
Colin Dinney, M.D.
Several targetable “driver” mutations characterize low grade (LG) NMIBC. Leading the way are activating mutations and translocations of FGFR3, which are present in up to 70% of these tumors, while mutations in KDM6A, PIK3CA and HRAS are also relatively common. Multiple alterations are present in at least 25% of tumors, providing the opportunity to study their role in drug resistance [31, 50]. Intravesical targeted gene therapy employing shRNA or full-length gene constructs is a promising approach to drug delivery that provides direct contact between the vector carrying the therapeutic gene and the tumor. Reliable gene transfer to the urothelium has been achieved and all targets are potentially “druggable” by this approach, even if the exact mechanism of action of the target gene is unknown [51]. Furthermore, while several of the available targeted agents have toxicity that precludes clinical development in LG NMIBC, intravesical adenoviral and retroviral mediated gene therapy has been devoid of dose-limiting toxicity. Moreover tyrosine kinase inhibitors (TKIs) or antibodies are not formulated for intravesical delivery and effective systemic delivery to the urothelium has not yet been demonstrated.
Adenoviral mediated interferon-α gene therapy with the excipient Syn3 has demonstrated efficacy in the “BCG Unresponsive” patient population [52], and the delivery of interferon-α in next generation lentiviruses promises to enhance gene delivery while minimizing random integration of viral DNA into the host genome. Interferon-α is also as a potent activator of PDL-1 and other immune modulators, so that adenoviral mediated interferon-α gene transfer could also serve as an adjuvant to prime the immune system. Collaborations between academia and pharma are poised to explore intravesical adenoviral and lentiviral shFGFR3 and interferon-α gene therapy in immunocompetent animal models with the intent of rapid translation into clinical trials.
OPEN DISCUSSION – TARGETS AND PATHWAYS FOR INTERVENTION
Moderator: Jonathan Rosenberg, M.D.
Following the presentation of pathways and targets, a robust discussion ensued reviewing potential drug targets. Currently, all therapies approved for treatment of NMIBC are delivered intravesically. This ensures high levels of drug are administered topically to the superficial tumors. The participants felt FGFR3 was the most relevant target given the high prevalence of activating mutations in this patient population. However, intravesical delivery of the current versions of FGFR3 inhibitors is unlikely to lead to sustained kinase inhibition of FGFR3 necessary for anti-tumor activity. Discussion focused on the need for less toxic agents for systemic administration for NMIBC, and potential ways to deliver a targeted agent intravesically.
SESSION II: CHECKPOINT BLOCKADE BIOLOGY, IDENTIFIED MECHANISMS AND THERAPIES RELEVANT TO BLADDER CANCER, AND CHALLENGES IN THE NON-MUSCLE-INVASIVE DISEASE SETTING
Session Co-Chairs: Noah M. Hahn, M.D., Robert Svatek, M.D.
INTRODUCTION
Noah M. Hahn, M.D.
Simultaneous to the rapid articulation of specific targetable driver genetic alterations in bladder cancer through efforts such as the TCGA project, equally accelerated investigations of the molecular biology and candidate targets has occurred in the field of immuno-oncology [5]. One of the earliest effective immunotherapies, intravesical BCG, has been embraced as a standard of care for NMIBC for over three decades [53, 54]. While effective at decreasing or delaying NMIBC relapses, the majority of patients treated with BCG do eventually develop recurrences. For BCG-unresponsive patients, cystectomy remains the standard of care in patients medically fit for surgery. While approaches such as high-dose interleukin-2 have demonstrated the ability to cure a small subset of metastatic melanoma and renal cell carcinoma patients, the intensity and morbidity associated with these initial immunotherapy efforts limited their applicability to larger populations, particularly patient groups such as NMIBC in which concurrent medical comorbidities are common [55–57].
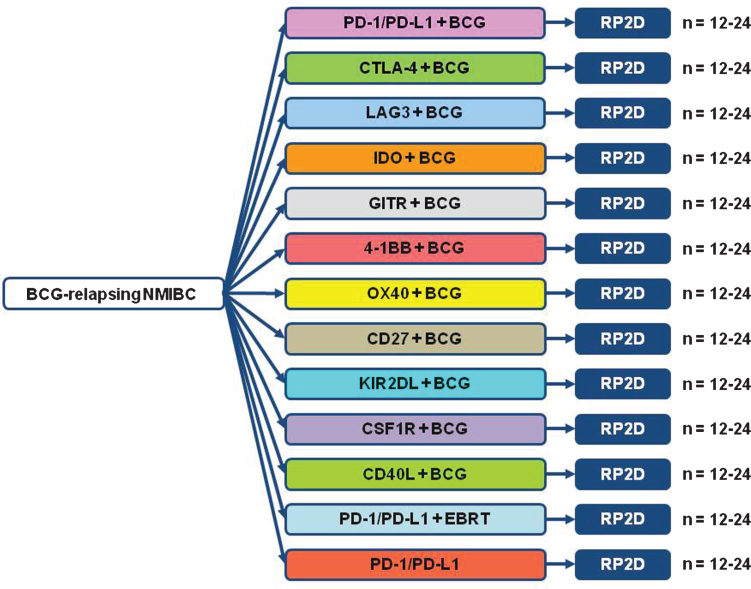
In recent years though, the development and demonstration of profound efficacy with considerably more favorable side effect profiles of modern immunotherapy agents has quickly positioned immunotherapy as a critical approach to the therapy of multiple malignancies. Specifically, novel immune checkpoint inhibitors have been developed which block the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), programmed death-1 (PD-1) and programmed death-1 protein ligand (PD-L1) mediated signaling [58, 59]. In normal physiology, the CTLA-4/PD-1/PD-L1 pathways function to dampen inflammatory immune responses as a checkpoint balance preventing unregulated destructive inflammation. In recently reported trials treating patients with metastatic urothelial cancer (UC) who have progressed after platinum based chemotherapy, response rates doubling that seen traditionally with cytotoxic chemotherapy have been reported independently for agents targeting both PD-1 and PD-L1 with grade 3-4 toxicity rates of only 8-15% [36, 60]. In small studies in NMIBC patients at least 35% of patients demonstrated moderate or marked PD-1 expression with an association noted between PD-1 expression and prior BCG therapy [61]. Additional studies have demonstrated marked expression of PD-L1 among 69% of post-BCG relapsed UC tumors compared to 19% of BCG-naϊve tumors from the same patients [62]. Collectively, the data implicates the PD-1/PD-L1 pathway as a potential key resistance mechanism to traditional BCG therapy. Agents targeting the CTLA-4/PD-1/PD-L1 pathways are now FDA approved for the treatment of metastatic melanoma and non-small cell lung cancer with approval in renal cell carcinoma expected soon. In addition, the safety and early efficacy of other checkpoint inhibitor and agonist targets (LAG3, IDO1, GITR, 4-1BB, OX40, CD27, KIR2DL, CSF1R, CD40) is currently being evaluated in ongoing phase I trials. Collectively, the existing data and ongoing development efforts of additional checkpoint inhibitors and agonists make modern immunotherapy agents an attractive therapeutic approach to investigate in NMIBC patients. The focus of CTPM session 2 was to leverage current immunotherapy clinical and translational data from bladder and other malignancies to produce optimal clinical trial designs to evaluate modern immunotherapy approaches in NMIBC patients.
CHALLENGES OF THE NMIBC SPACE – ENDPOINTS, DRUG DELIVERY AND ACCRUAL
Prof. Dr. J. A. Witjes
Currently, there are still (too) many challenges or unmet needs in NMIBC therapy. Unfortunately, there are almost no new clinical developments since decades, probably because awareness (public, pharma, funding) is limited and studies are challenging. The initial TUR is not radical in 20–30% of patients, and current adjuvant intravesical therapy reduces recurrence rates by no more than 50%. Guideline recommendations are in part conflicting [63], and this might be one of the reasons that compliance to guidelines is far from optimal. An additional current problem is the shortage of the most effective intravesical agent: BCG. Trial endpoints predominantly are tumor recurrence and progression. The impact on progression of currently used drug is controversial and probably (very) limited [64]. Still, progression is an important endpoint with a life changing therapeutic alternative (radical cystectomy) and with a chance to develop lethal disease.
A potential new method to study drug efficacy is a marker lesion study. The concept is that all but one bladder tumors is removed. The patient should have a history of low grade NMIBC since recurrences usually also are low grade. After 6 intravesical instillations, for example, the effect on the marker lesion is evaluated after another few weeks. A complete response (CR) on cystoscopic evaluation appears a pathological CR in >97%, making control biopsies in CR patients unnecessary. Twenty-three such studies with >1200 patients have been done and reviewed [65]. Typically the marker lesion shows a CR in 30% to 50% of patients. For BCG these figures are 32%-61%, and the highest response rate was reported for Apaziquone (67%). In these >1200 patients progression was seen in 7 patients, however, these were initially all high risk. No progression in intermediate risk patients was seen. Patients with a CR also have a higher recurrence free survival, so the ablative effect seems to correlate with prophylactic effect.
In conclusion, there are still many unmet needs, and studies are challenging [66]. A potential alternative might be marker lesion studies, which are safe with a clear and rapid endpoint (3 months CR). For new drugs this means a short study duration with a limited number of patients. Last, but certainly not least, patients experience a limited burden and have a longer recurrence free survival when they experience a CR.
BIOLOGY/MECHANISMS OF CHECKPOINT INHIBITORS
Arlene Sharpe, M.D., Ph.D.
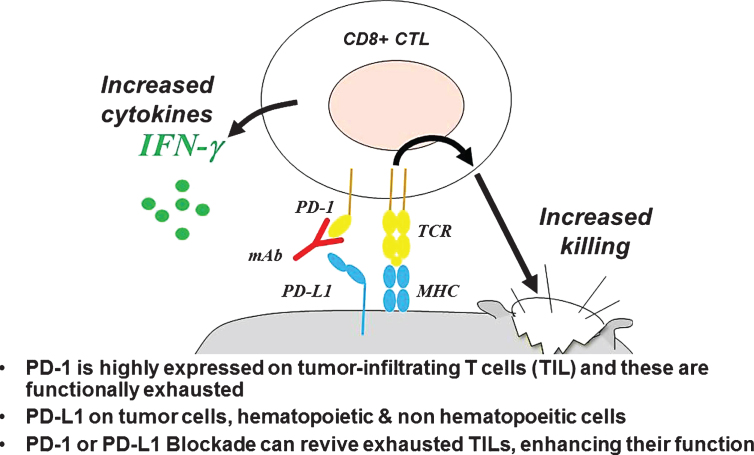
It has long been known that tumors, including bladder cancer, are infiltrated with CD8 (killer) T cells and that T cell infiltration correlates in many cases with improved outcome [67]. What was less clear, however, were the mechanisms by which cancers continued to progress, despite dense T cell infiltration. More recent data show that multiple inhibitory pathways dampen an anti-tumor T cell response; and the interactions between immune checkpoint molecules on tumor-infiltrating lymphocytes and their ligands on tumor cells or intra-tumoral myeloid cells represent druggable targets for re-invigorating an anti-tumor response [68]. Among the multiple immune checkpoints under study, that mediated by the interaction between PD-1 and its ligands PD-L1 and/or PD-L2 has risen to the forefront of clinical investigation; as antibody-mediated blockade of either PD-1 or PD-L1 induces objective responses in a number of tumor types [69]. PD-1 (Programmed Death 1) is expressed on activated T cells, as well as on T cells that have become functionally exhausted due to chronic antigen exposure, and is highly expressed on CD8 tumor infiltrating cells (TIL) in most tumor types (Fig. 5). The signal transmitted when PD-L1 or PD-L2 binds to PD-1 inhibits many aspects of T cell function, including cytokine production and perhaps most distressingly, the ability of CD8 T cells to lyse their tumor targets. While PD-1 is broadly expressed on tumor infiltrating lymphocytes, expression of the PD-1 ligands appears to be more tightly controlled, and probably evolved to protect normal tissues from CD8-mediated attack during inflammation or wound healing. In that regard, PD-L1 expression can be strongly up-regulated by pro-inflammatory cytokines secreted from T cells, most notably interferon gamma. Indeed, in some tumor types, PD-L1 expression on tumor cells seems to be strongly co-localized with infiltrating CD8 T cells [70]. These data also lead to the concept that tumors with clear CD8 infiltration and PD-L1 expression should be more likely to respond to antibody-mediated blockade of either PD-1 or PD-L1, a concept that is fairly well-supported in UBC [36], although there is a significant fraction of PD-L1 ‘negative’ patients that show evidence of clinical responses. In addition the PD-1/PD-L1 axis, there are a number of additional immune checkpoints that can be expressed on tumor infiltrating lymphocytes; these include LAG-3, TIM-3, TIGIT and several others. Pre-clinical and clinical data suggest that checkpoint mediated inhibition of T cell function likely involves multiple non-overlapping pathways [71], suggesting that, for maximal clinical efficacy, multiple immune checkpoints may need to be blocked in parallel, as recently shown in melanoma [72], kidney cancer [73] and several other tumor types.
Fig. 5.
Mechanism of Action of PD-1 or PD-L1 Blockade.
MONO VS. DUAL THERAPY FROM OTHER CANCERS
Charles Drake, M.D., Ph.D.
Although PD-1/PD-L1 blockade has clear activity in multiple tumor types; as discussed above it has become increasingly clear that multiple immune mechanisms serve to dampen anti-tumor T cell responses in patients [74]. Clinically, this is important because the all-comers response rate to PD-1/PD-L1 blockade is in the 20-25% range in several tumor types, and for PD-L1 blockade it is likely in the 15% range in urinary bladder cancer (Hoffman-Censtis, unpublished). Thus, combining PD-1/PD-L1 blockade with additional modalities may be important in increasing both the long term response rate as well as the frequency with which responses occur. The most compelling clinical data regarding combination immunotherapy come from trials in which PD-1 blockade is combined with CTLA-4 blockade; this combination was recently FDA-approved in melanoma [72], and has shown impressive activity in renal cell carcinoma (RCC) and lung cancer as well. Mechanistically, synergy between PD-1/PD-L1 blockade and CTLA-4 blockade likely occurs because CTLA-4 is highly expressed on the regulatory T cells that infiltrate tumors [75], so these two may agents target CD8 TIL and CD4 tumor infiltrating regulatory T cells, respectively. Although generally tolerable, combined CTLA-4/PD-1 blockade is associated with a high rate of Grade III and IV immune related adverse events; these events necessitate intervention with corticosteroids and occasionally with drugs that block tumor necrosis factor (TNF) [76]. While such combinations are clearly warranted in advanced cancer patients, their application to patients with NMIBC may be harder to justify. Other immune/immune combinations may prove more tolerable, such as the combined blockade of PD-1 and the immune checkpoint mediated by the interaction between LAG-3 and Class II MHC [77], an anti-LAG-3 antibody is now in a Phase I combination trial (NCT01968109).
Because PD-1/PD-L1 blockade is generally well-tolerated, and has a reasonable response rate, this intervention is often proposed as a backbone for combination regimens. In addition to immune checkpoint molecules, there are also a series of molecules expressed on either T cells or on other immune cells in the tumor microenvironment that promote immune activation (rather than repression). Examples include OX40 (expressed on T cells), 41BB (also expressed on T cells) and CD40, which is expressed on antigen-presenting cells and B cells. To target these pathways, agonist antibodies have been developed, and are in Phase I trials either alone or in combination with PD-1 blockade in several tumor types [78]. To date, however, the combination of an immune checkpoint and an immune agonist has not been comprehensively tested in either NMIBC or in muscle invasive or metastatic urothelial cancer. A second potential combination strategy involves the pairing of PD-1/PD-L1 blocking agents with conventional therapies like chemotherapy or radiation therapy (discussed below). Combining immunotherapy with chemotherapy can be challenging; most preclinical studies support the notion that only certain chemotherapy agents have immune-enhancing effects and that dose and sequencing may be critical [79]. With a few notable exceptions [80], those lessons have largely been ignored in the clinic, where immune checkpoint blockade is often blindly added to conventional, full-dose chemotherapy. A final combination of note involves co-administering immune checkpoint blocking reagents with anti-angiogenic therapies; this combination is based on data showing that tumor-infiltrating blood vessels are disordered and represent a challenge to T cell egress. Normalizing the tumor vasculature with anti-VEGF targeted antibodies may facilitate tumor infiltration with T cells, and be synergistic with immune checkpoint blockade. Preliminary data suggest activity for the combination of anti-VEGF (Bevicizumab) and anti-PD-L1 (Atezolizumab) in RCC, with an interesting Phase II trial in (NCT01984242) now fully accrued. So, taken together, both preclinical and clinical data support the notion that combined immune checkpoint blockade may be required to induce durable and/or complete responses in the majority of NMIBC patients, but also highlight the fact that the ever expanding number of potential combinations means that clinical evaluation could prove challenging.
RADIATION INDUCED IMMUNE RESPONSES
Andrew Sharabi, M.D., Ph.D.
Recently, checkpoint blockade immunotherapy (CBI) has been reported to have notable activity in metastatic bladder cancer and is establishing itself as a fourth pillar of cancer care. A current focus is understanding how to best incorporate immunotherapy into definitive and palliative treatment regimens involving surgery, chemotherapy, and radiation. Interestingly, there is now an established body of pre-clinical literature and emerging clinical data demonstrating that radiation can modify immune responses [81]. Given the known efficacy of radiation in muscle invasive bladder cancer, this raises the question of whether radiation combined with immunotherapy could be used in earlier stages of disease including the non-muscle invasive setting.
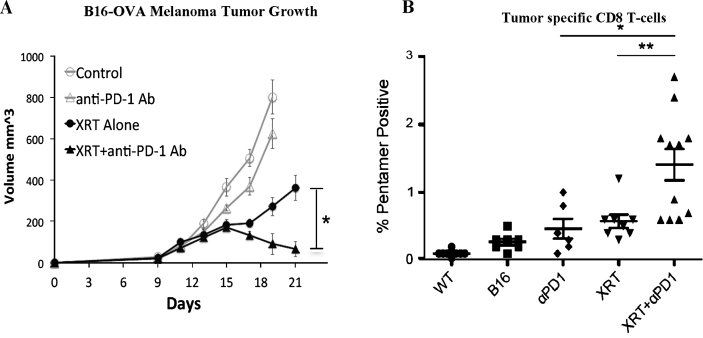
Our group has been investigating the effects of combining stereotactic radiation with anti-PD-1 checkpoint blockade immunotherapy in a number of pre-clinical models [82]. We have identified that radiation can synergize with immunotherapy specifically by causing inflammatory cell death and release of tumor antigens which are cross-presented in the draining lymph node [82]. This increase in antigen presentation drives the activation and proliferation of tumor specific cytotoxic T-cells. At the same time we have demonstrated that radiation modulates the tumor microenvironment and enhances T-cell infiltration into tumors while simultaneously causing the tumor cells to become more susceptible to immune mediated cell death [82]. These data suggest that radiation may be an ideal modality to combine with immunotherapy (Fig. 6). Given these findings we have proposed that a low dose of radiation (8Gyx1 or 6Gyx3) could be combined with checkpoint blockade immunotherapy in BCG unresponsive non-muscle invasive bladder cancer. The aim of this experimental treatment arm would be to evaluate whether radiation combined with anti-PD-1 immunotherapy improves local tumor control and development of systemic anti-tumor immune responses compared to anti-PD-1 immunotherapy alone. There are limited treatment options in this setting and this data would shed light on a potential novel therapeutic modality for non-muscle invasive bladder cancer.
Fig. 6.
Radiation combined with anti-PD-1 immunotherapy improves local tumor control (A) and development of systemic anti-tumor immune response (B)[82].
INTEGRATING PATHOLOGY INTO NMIBC DRUG DEVELOPMENT: COMPANION DIAGNOSTICS, NEOANTIGENS, AND T-CELL REARRANGEMENTS
Margaret Callahan, M.D., Ph.D.
There is a significant unmet need for companion diagnostic tests that help select patients that are most likely, or unlikely, to benefit from immunotherapy. As of the date of this meeting in March of 2015, a spectrum of potential biomarkers have been explored, primarily in small, single-institution, retrospective studies, however, no validated biomarker is in clinical use. In this session, we outlined the rationale, clinically desirable characteristics, and preliminary research on biomarkers in this area.
Immunotherapy has been an area of significant excitement and interest, especially with the growing appreciation for the activity of PD-1 and PD-L1 blockade in the diversity of cancer types, including bladder cancer. However, in most clinical scenarios, only a minority of patients benefit from immunotherapy treatment and responses are sometimes slow to develop, underscoring the utility that a predictive biomarker could play in guiding treatment choices. An ideal biomarker for this field would be present prior to initiation of treatment, or early enough in treatment to shape clinical decision-making. Moreover, this ideal biomarker would predict clinically relevant outcomes (response, survival, toxicity) with specificity and sensitivity. Lastly, this hypothetical biomarker would be an assay that would be feasible to perform on clinical samples (rapid results, without undue complexity, reproducible and robust).
Strategies for correlative analyses of human samples in patients treated with immunotherapy has comprised investigation of peripheral blood cells, serum or plasma, tumor microenvironment and other tissues. Tools have included flow cytometry, DNA and RNA sequence analysis, serology, immunohistochemistry and others. We reviewed data on peripheral blood correlates that have been investigated in patients with melanoma that have been treated with CTLA-4 blocking antibodies, including absolute lymphocyte count (ALC), myeloid derived suppressor cells (MDSC) and changes in phenotypic markers on peripheral T cells like ki67 [83–89]. We also briefly reviewed recent publications on the role that T cell receptor diversity may play in patients treated with PD-1 or CTLA-4 blocking antibodies [38, 90, 91]. We reviewed the abundant data of PD-L1 expression in the tumor microenvironment and its link to favorable clinical outcomes for patient treated with PD-1 or PD-L1 blocking antibodies across a diversity of tumor types [59, 92, 93]. It was noted, however, that most studies found that at least some patients whose tumors tested negative for PD-L1 had responses to these agents, and therefore, the specificity of this assay may not be sufficient for selection of patients. Lastly, we discussed emerging data on how the burden of random tumor mutations may influence the immune system and responses to immunotherapy [94–96].
DEFINITIONS AND ENDPOINTS IN THE NMIBC ARENA: PERSPECTIVE OF CLINICAL TRIALS
Ashish Kamat, M.D., MBBS, FACS
There is a significant unmet need for new therapies in NMIBC. Studies in this area have lagged behind due to lack of consensus on trial endpoints and appropriate control arms which handicaps regulatory bodies, investigators and results in confusion due to perceived difficulties related to these factors. In recent years, the International Bladder Cancer Group (IBCG), American Urological Association (AUA), FDA, European Association of Urology (EAU) and others to have tried to address these issues and propose trial designs to support the development of new therapies for NMIBC [63, 97]. The purpose of this discussion was to expand upon this work and provide recommendations on appropriate clinical trial designs in NMIBC based on evidence-based literature, current clinical practice guidelines and expert consensus [98].
It must be first recognized that the design of clinical trials in NMIBC should be such that they provide the most clinically relevant data for the specific risk category of interest (low, intermediate or high). Thus, the risk classification is of paramount importance. To summarize: Low Risk Category is those tumors that are solitary, primary, Ta low grade tumors that are < 3 cm in size; the High Risk Category include any T1 or high grade Ta, including CIS and Intermediate Risk tumors include everything else (i.e. recurrent/multiple TaLG tumors) [99].
Next, the patient population must be stratified based on prior exposure to immunotherapy with BCG. For trials examining the BCG-failure population, the BCG induction and maintenance schedule administered prior to failure and the type of failure (BCG unresponsive, refractory, relapsing, or intolerant) should be clearly outlined to make comparisons across trials feasible [100]. While BCG failure has been broadly defined as any recurrence or progression during therapy, this term is quite heterogeneous, and comparing salvage therapies in this population, hindered by the lack of standard definitions, inconsistent methods of reporting results does not provide meaningful data. The timing of therapy assessment is also important – it must be remembered that we need to wait until the 6-month evaluation time point to identify high-risk NMIBC as truly BCG refractory since an additional 25-67% who do not respond to an initial induction course, will respond to a second course of BCG. Also, it must be emphasized that recurrence of tumor after inadequate BCG does not carry the same prognostic implication to the patient as it does when tumor recurs after adequate BCG therapy. The term ‘BCG unresponsive’ which essentially includes ‘BCG refractory’ and ‘BCG relapsing’ (within 6 months of last BCG exposure) patients is a new term meant to denote a subgroup of patients at highest risk of recurrence and progression for whom additional BCG therapy is not a feasible option [101]. These patients can be considered for single arm studies. However, it is imperative that all subjects enrolled in trials of novel therapeutics after adequate BCG (defined as induction BCG with 6 weekly instillations and at least 1 maintenance course) be informed that treatments other than cystectomy in this population are considered oncologically inferior at present.
In general, randomized superiority trial designs are recommended for most risk levels. Since non-inferiority trials often require a large sample size, they should be used sparingly. Placebo control is considered unethical for all intermediate- and high-risk strata; therefore, control arms should comprise the current guideline-recommended standard of care for the respective risk level. Realistic efficacy thresholds should be set to ensure that novel therapies receive due review by regulatory bodies – for example, in patients with low risk disease, an absolute reduction of 6% in the percent of patients with recurrence at 2 years would be a reasonable magnitude of effect for a clinical trial to be considered ‘positive’ since this would be actually a relative risk reduction of over 40% due to low incident events. One the other hand, for patients in the BCG unresponsive category, where much more is at stake and event rates are higher, a clinically meaningful initial CR rate (for CIS) or recurrence-free rate (for papillary tumors) of at least 50% at 6 months, 30% at 12 months and 25% at 18 months is recommended. This is in agreement with the report from the AUA/FDA workshop where it was suggested that the efficacy be set such that the lower bound 95% CI excludes 20% (albeit at a longer time duration) [2].
OPEN DISCUSSION – CHECKPOINT BLOCKADE AND IMMUNOTHERAPY INTERVENTIONS
Moderator: Noah M. Hahn, M.D.
Following the aforementioned presentations by leading experts in the fields of immunotherapy and NMIBC clinical trial designs, a robust discussion ensued with engaging dialogue amongst urology, oncology, radiation oncology, pathology, immuno-oncology, and biostatistics investigators in attendance. Points of deeper discussion included:
-
1.
Analysis of optimal NMIBC populations for multi-institution academic and industry clinical trial collaborations.
-
2.
Review of the new definitions of BCG-unresponsive vs. BCG-relapsing NMIBC populations and their impact on accrual expectations.
-
3.
Appropriate clinical trial endpoints in NMIBC populations particularly papillary (Ta/T1) compared to CIS-only disease.
-
4.
Investigator comfort level with enrolling NMIBC patients to immunotherapy clinical trials given the currently available toxicity data from metastatic cancer patients.
-
5.
Presentation of candidate clinical trial concepts for feedback from the group.
REGULATORY SESSION
REGISTRATION OF THERAPY FOR NON-MUSCLE INVASIVE BLADDER CANCER
Jonathan Jarow, M.D.
The following comments are thoughts about the design of clinical trials with a regulatory intent for the development of products used to treat NMIBC. As a general rule, randomized clinical trials using a time-to-event endpoint are strongly preferred for oncology indications. The comparator may be placebo, when ethical and/or feasible, or an active control for a superiority trial. Non-inferiority trial design is acceptable against an approved agent as long as a meaningful inferiority margin can be identified and assay sensitivity is assured. The lack of contemporary placebo-controlled trials of BCG makes it difficult to determine inferiority margins for a time-to-event, recurrence-free survival, non-inferiority trial against BCG. Trials that include patients with pure papillary disease should use recurrence-free survival as the primary endpoint. Events include any recurrence within the bladder or prostatic urethra. Upper tract disease is not normally counted as events in the primary analysis for intravesical therapies but should be counted as events for trials of systemic therapies. A sensitivity analysis will be performed including/excluding these events. Disease-free survival is the preferred primary endpoint for trials that include a mix of patients with papillary disease with or without CIS. Events are dated to the time of recurrence and time zero for failure to achieve a complete response for those patients with CIS at enrollment.
Patients with high-risk disease following treatment with BCG have been the preferred population to study new therapies for NMIBC. For a variety of practical reasons, it has been extremely hard for commercial sponsors to successfully conduct a randomized controlled trial in this population. FDA encourages the use of the IBCG and Society for Urologic Oncology (SUO) definition for the “BCG-unresponsive” population for the enrollment criteria for these trials [3, 102]. Patients with BCG-unresponsive CIS go on to cystectomy in the absence of complete response. Thus, a complete response rate of sufficient magnitude and duration is clinically meaningful in this patient population. A single-arm trial is acceptable in this setting because the natural history of this disease is well characterized and we would not expect spontaneous resolution. Nevertheless, there are alternative approaches for testing a new drug in a randomized control trial either as an add-on therapy to BCG (against placebo) or in a lower risk stratum against either placebo or another active agent.
SESSION III: CLINICAL TRIAL DESIGNS, TUMOR ACQUISITION, EMBEDDED COMPANION DIAGNOSTICS, AND TRIAL REQUIREMENTS AND INFRASTRUCTURE CHALLENGES UNIQUE TO CLINICAL TRIALS IN NMIBC
Session Co-Chairs: Michael O’Donnell, M.D., Susan Groshen, Ph.D., Donna Hansel, M.D., Ph.D.
INTRODUCTION
Michael O’Donnell, M.D.
When it comes to clinical trials in general and NMIBC clinical trials in particular, the devil is in the details. Beginning with patient considerations, even the most well designed clinical trial will fail if recruitment is undermined by a perceived burdensome protocol or unclear patient-oriented benefit/risk ratio. Given the extremes of risk between untreated low-grade disease and heavily pretreated patients with high-grade disease, for instance, numerical abundance in the former may be offset by convenience of conventional therapy while the rare but motivated patient in the latter clamoring for novel treatment my be dissuaded by the worry of progression or need to travel long distances. Careful attention to the nuances in each subcategory of NMIBC is thus essential to success.
Clinical trial endpoints also vary with the population chosen. Thus, use of complete response rate is only valid for CIS or marker lesions where disease is unequivocally present at study onset. Disease-free survival (DFS) on the other hand may be necessary for patients with resected papillary patients at risk for recurrence. Trials including mixtures of CIS and papillary patients would therefore be troublesome for finding a common endpoint. Furthermore, how and when does one define the clinical meaningfulness of the endpoints selected? Even endpoints of progression and survival are problematic given dropout, subjective decisions for cystectomy and competing causes for death, for instance. Furthermore, the whole traditional cumbersome Phase I, II, and III trial architecture for getting drug approval is becoming archaic in the emerging demand for bringing new agents to market sooner and proliferation of multiple potential candidates.
Even bladder tissue histology and cytology are not without major considerations, as inter- and intra-observer variability make even referee pathologic verification problematic. Once tissue acquisition for correlative studies is required, a whole new set of issues arises including harvest (frozen or paraffin), transport, storage and even reproducible analysis. In certain cases, such as CIS, there may not even be sufficient tissue for anything but the most basic of studies. This may be especially relevant for new checkpoint inhibitors that have an associated companion diagnostic for patient selection. Similarly, liquid biospecimens, such as urine and blood, present new challenges not only in logistics and infrastructure but also in quality control. Furthermore, specimen prioritization for competing correlative studies becomes a matter in and of itself.
The sections that follow will delve into the nuances in all of these logistical matters in clinical trials of NMIBC. While not all the answers are available, at least a clearer understanding of the difficulties and challenges will be provided.
PATIENT ADVOCATE PERSPECTIVES ON CLINICAL TRIAL DESIGN
Diane Zipursky Quale, J.D.
Patient recruitment and retention are significant challenges to the success of all types of clinical trials. The primary motivation for a patient to participate in a trial is personal: What’s in it for me? How will participation in the trial improve my prognosis? To improve recruitment for NMIBC, efforts must be directed at dispelling the myth that clinical trials are only for patients with advanced disease who have no other treatment options. A trial that is designed to prevent recurrence or progression of disease represents a tangible benefit to the patient. However, if the trial requires additional cystoscopies beyond the standard of care, or involves systemic chemotherapy, which is not part of the standard of care, the benefit to participation may be greatly offset. A marker lesion trial presents significant recruitment challenges, as most patients are anxious to have their tumors removed as soon as possible. To attract a patient to this type of trial, the physician must minimize any possible risk of progression of the disease during the time the marker lesion remains in place.
Patients will travel for a perceived benefit to clinical trial participation, but important considerations are distance, number of visits required and length of visit, and whether the trial center can offer any resources to assist with travel time and expenses. Well designed and thoughtful educational and awareness tools are essential to ensuring rapid and full clinical trial enrollment. Patient advocacy organizations such as the Bladder Cancer Advocacy Network can assist in providing patient-focused input on educational materials for specific clinical trials, and can also provide outreach to the bladder cancer patient community through educational forums and social media to raise the awareness and understanding of clinical trials [103].
CLINICAL TRIAL DESIGNS AND ENDPOINTS FOR NMIBC TRIALS
Catherine M. Tangen, Dr.P.H.
The Southwest Oncology Group (SWOG) has experience enrolling NMIBC patients on their clinical trials. SWOG successfully completed accrual to a non-intergroup randomized phase III trial of newly diagnosed or recurrent Grade 1-2, Ta or T1 transitional cell carcinoma (S0337, NCT00445601), averaging 7 patients per month. In the BCG refractory population, SWOG completed a phase II trial averaging two patients per month (S0353)[104]. With respect to endpoint experience, SWOG’s prior phase III randomized BCG maintenance trial (S8507)[100] explored the composite endpoint of worsening-free survival which was defined as the first evidence of: biopsy proven ≥ T2 disease (17% of events), initiation of system chemotherapy or radiation (3%), cystectomy (9%), or death due to any cause (74% of events) (Fig. 7). If we had restricted death to only bladder cancer deaths, only one-third of deaths would have been counted as related to bladder cancer. Obtaining complete and reliable subsequent treatment information and cause-of-death for patients enrolled in a large intergroup trial many years after randomization can be challenging.
Fig. 7.
Time to Worsening Event Stratified by Maintenance BCG.
Targeted (“bucket” or “basket” design trials[105] can be conducted in the discovery phase where a number of sub-studies are placed under one umbrella with a common platform for evaluating tissue marker status. A patient is assigned to a sub-study and single-arm targeted agent based on his/her biomarker status, and the primary objective is an initial assessment of clinical activity in the specific population. For these trials, reliable marker-specific historical data are needed in order to pre-specify the level of response activity that would be of interest to pursue in subsequent studies. In contrast, a confirmatory targeted design will include a mix of randomized sub-studies. Patients are randomized between a standard and targeted treatment where the standard arm may be the same across the sub-studies, or study specific. As in the discovery targeted design, patients are assigned to a sub-study based on marker status. The sub-study can be a randomized phase II or a phase II/III trial where arms that show lack of activity at a planned interim analysis are dropped. If there is a lack of evidence about marker specificity for a treatment, allow all-comers to enroll and stratify on marker status and increase the sample size as needed to allow for some assessment of the marker by treatment interaction. If the goal is to screen a number of regimens that are not necessarily marker-targeted, then using a selection design [106, 107] or randomized phase II/III [108, 109] with multiple arms may be a good strategy.
THE PATHOLOGIST PERSPECTIVE IN TISSUE ACQUISITION AND ANALYSIS
Donna Hansel, M.D., Ph.D.
The central role that pathologists play in successful clinical trials design, implementation and correlative study completion cannot be overemphasized. As pathology has moved toward subspecialization in most major medical institutions in the United States, the detail of pathological diagnoses has also expanded in line with medical treatment parameters. Several studies have now demonstrated significant diagnostic changes in urologic pathology specimens when re-reviewed by a subspecialist [110], highlighting the critical need for advanced training. Subtyping of cancers to identify those that harbor distinct molecular and/or morphological features associated with behavior or response to drug therapy is also becoming mainstream practice, with many sites expanding in-house genomic test panels. The combination of accurate diagnostic reads and availability of molecular testing are two crucial parameters in the design and successful implementation of emerging clinical trials. In the field of bladder cancer, rapidly changing subclassifications of in situ and invasive disease, limitations in diagnosis by specimen type, and quantification of tumor volume can all impact enrollment criteria.
In conjunction with the clinical parameters associated with pathology review, most pathology departments are engaged in biorepository efforts that directly impact the collection of tissue and the application of correlative biomarkers associated with clinical trials efforts. Several standard guidelines around best practices have emerged and are publicly available [111]. Biobanking of tissue, urine and blood are routinely performed, with quality metrics that include percent tumor nuclei, percent necrosis, and morphology of harvested lesion. In NMIBC clinical trials, many specimens will include transurethral resection (TUR) specimens that are routinely fully submitted for formalin-fixed, paraffin-embedded (FFPE) material. However, consented protocols in which a portion is submitted for frozen storage with concurrent diagnostic frozen slide preparation can expand the use of this tissue without compromising patient care. The volume and type of materials available, whether FFPE or frozen, significantly impacts the type of correlative studies that can be performed as part of a clinical trial. In NMIBC studies, the majority of specimens will include FFPE biopsy and TUR material, with a more limited portion of TUR material frozen for larger cancer specimens. Use of FFPE material has been routinely used for histological and immunohistochemical analysis, with more recent inclusion of this material for whole exome sequencing. Whereas RNA-based analysis on FFPE material has improved, many studies still prefer frozen tissue for these applications. Given that the correlative endpoints of clinical trials are decided during the design phase of clinical trials, involvement of pathologists to discuss the volume of materials available and the types of testing possible, can significantly improve the successful completion of correlative studies.
PDL1 AS A BIOMARKER IN CLINICAL TRIALS OF CHECKPOINT INHIBITORS IN BLADDER CANCER AND OTHER SOLID TUMORS
Andrea B. Apolo, M.D.
Immunotherapy with immune checkpoint inhibitors is now an established treatment modality in cancer therapy. Agents are in rapid development by pharmaceutical companies (Merck, Bristol-Myers Squibb, Merck Serono, Metaimmune/AstraZeneca, Macrogenics, and Genentech) in multiple tumor types, including bladder cancer. Many bladder cancer clinical trials are in development or are ongoing in all disease states (non-muscle-invasive, muscle-invasive, and advanced refractory metastatic disease in the first-line cisplatin-ineligible and second-line setting and beyond). This has created a growing demand for predictive biomarkers to assist in selecting patients who would most benefit from these therapies. PD-L1 expression by immunohistochemistry (IHC) on tumors or in tumor-infiltrating immune cells is being developed as a companion assay. Many clinical trials have demonstrated that patients positive for PD-L1 expression have a higher radiologic response to anti-PD-1 or PD-L1 therapy [36, 37, 59, 112–117] compared to those who are PD-L1-negative. Response to PD-1/PD-L1 inhibitors is not limited to patients with PD-L1-positive tumors or tumor-infiltrating immune cells. Patients who do not express PD-L1 may still have radiologic tumor shrinkage and durable responses, although to a lesser degree. Therefore, PD-L1 expression status cannot be used as a biomarker for patient selection for treatment with PD-1/PD-L1 inhibitors, except in the context of a clinical trial. However, PD-L1 status seems to be less relevant in combination studies [118]. This is a very important factor since many clinical trials of PD-1/PD-L1 inhibitors currently in development are in combination with chemotherapy, radiation, tyrosine kinase inhibitors, and other immunotherapies. The hypothesis is that many of these agents have immunomodulatory properties, such as inhibiting tumor-induced immunosuppressive mechanisms and increasing direct or indirect stimulatory effects on immune effector molecules [118–121], which may prime the tumor and enhance the immune system. Studies have shown that PD-L1 expression on tumor-infiltrating immune cells and tumor cells can increase in patients treated with PD-1/PD-L1 inhibitors [37], supporting the theory that PD-L1 tumor status is dynamic. Therefore, pretreatment PD-L1 status may not accurately reflect the tumor microenvironment and potential immune response to these therapies.
Another major issue in categorizing patients by PD-L1 status is the large variability in assays and interpretation of PD-L1 IHC staining (Table 6) [122]. Of the many PD-L1 antibody assays, some measure PD-L1 in the tumor, some measure PD-L1 in immune-infiltrating cells, and some measure both. Nor do the assays use common cutoffs for positivity. This lack of standardization among assays under development, along with a lack of comparative data among assays, is among the limitations of using PD-L1 IHC status to categorize patients.
Table 6.
Selected assays for immunohistochemistry assessment of PD-L1 status (reprinted with permission from Apolo AB, 2016, Eur Urol Focus 1:269-271, Elsevier Ltd.) [122].
| Source | Monoclonal antibody | Clone | Automated | Cells evaluated | Staining location | Positive Cutoff |
| Genentech/Rochea [37] | Rabbit | SP142 | Yes | Tumor cell and tumor-infiltrating immune cells | Membrane | IHC 0b <1% IHC1b ≥1% to ≤5% IHC2 ≥5% to ≤10% IHC3 ≥10% |
| Merck | Murine | 22C3 | Yes | Tumor cell and tumor-infiltrating immune cells | Membrane | ≥1% |
| Bristol-Myers Squibb [118] | Rabbit | 28-8 | Yes | Tumor cell | Membrane | ≥5% |
| Hopkins | Murine | 5H1 | No | Tumor cell | Membrane | ≥5% |
a Commercially available b IHC 0/1 are considered negative
IHC = immunohistochemistry
TOXICITY OF IMMUNE CHECKPOINT INHIBITORS
The toxicity profile for PD-1/PD-L1 inhibitors is favorable compared to standard cytotoxic treatments. Grade ≥ 3 toxicities (mainly in the skin, liver, and kidneys) range from 5% to 55% in solid tumor clinical trials and 4% to 16% in the two bladder cancer trials reported to date [36, 123, 124]. Furthermore, these agents may produce rare but serious immune-mediated toxicities such as rhabdomyolysis, neuromyopathy, and toxic encephalopathy [123]. Patients with non-muscle-invasive bladder cancer who have a good prognosis will be less tolerant of these toxicities than patients with advanced incurable metastatic bladder cancer.
THE CORRELATIVES CHALLENGES –E.G., EMBEDDED STUDIES OF MECHANISMS OF RESPONSE/RESISTANCE; VALIDATING GENETIC TARGETS
Joaquim Bellmunt, M.D., Ph.D.
The overarching principle of precision medicine in urothelial cancer treatment is the coupling of molecular, genomic, and clinic-pathologic prognostic factors with therapeutics to optimize the effective anticancer strategies.
With the advent of the new checkpoint inhibitors that are changing the treatment landscape in bladder cancer, more creative and innovative strategies are clearly required. Trial strategies with incorporation of predictive biomarkers of response related to the tumor itself and to the tumor microenvironment need to be fully understood. New technological and computational analyses are now being implemented to uncover the predictive biomarkers of response and resistance in clinical trials of targeted therapies and immunotherapy.
Many centers are now routinely molecularly characterizing bladder cancer with either whole genome or whole exome sequencing or targeted sequencing of mutational hotspots for patients referred for genomically driven clinical trials. However, limitations are the low frequency of relatively rare targetable genomic alterations. Trial enrollment for a single tumor and molecular subtype may be challenging. This has led to the development of umbrella and basket trials implemented now in bladder cancer. Limitations of personalized treatments in genomically driven trials are the intratumoral heterogeneity and the limitations on obtaining multiple tumor biopsies at sequential time points. Cell-free DNA now in its infancy, may help to adequately provide timely information on the mechanisms of response and resistance.
One of the benefits of incorporating biomarker enrichment strategies earlier in clinical trials is that it allows for the continuous refinement of technologies. For the checkpoint inhibitor trials, programmed death-ligand 1 (PD-L1) expression has emerged as a potential predictive biomarker for PD-1-directed therapy. While PD-L1 staining on immune and tumor cells correlates with response, the positive predictive value of these biomarkers is insufficient for routine clinical use. Several studies have presented now additional hypotheses to predict which patients respond, including neoantigen prediction and increase in mutational load. Also there are proposed response modifiers to PD-1/PD-L1 inhibitors in urothelial carcinoma including: Tumor-induced PD-L1 or immunostimulatory neoantigen production, germline immune factors including immune microenvironmental properties that can induce PD-1/PD-L1 in monocytes, or increased activity in solid tumors through new tumor antigens or tumor molecular subgroups that have significantly increased expression of immune genes. Exploring all these newly described potential mechanisms in bladder cancer clinical trials is a must.
Many patients who demonstrate clinical benefit with immunotherapy or targeted agents subsequently become resistant to therapy. Exploring the mechanisms of response and resistance to these agents is an important area of investigation with sequential biomarker studies. In urothelial cancer trials with targeted and immunotherapeutic agents, the continued interrogation of both tumor and tumor microenvironment, including the underlying germ line components, will further accelerate the impact of the novel antitumor agents in bladder cancer patients.
BIOSPECIMEN ACQUISITION, QC AND LOGISTICS
Nilsa Ramirez, M.D.
Overseeing the collection, processing, banking and distribution of biospecimens obtained from consented patients enrolled in SWOG-sponsored clinical trials is the responsibility of the SWOG Biospecimen Bank. This resource also assists investigators in numerous aspects of clinical trial development, including cost estimates for various projects (e.g., R01, pilot projects), histology services (e.g., tissue microarray (TMA) creation, IHC stains), nucleic acid extractions, virtual microscopy, and informatics.
When an investigator (named as a co-investigator of a correlative science study integrated into the clinical trial design itself), requests biospecimens for a pre-approved study, the Bank works with the SWOG Statistical and Data Management Center so the selected biospecimens are distributed to the investigator following the instructions noted in the protocol. There is no fee for this specific Bank service (covered by the SWOG U24 grant), unless highly specialized, expensive, or onerous biospecimen processing is required; in those cases, the work should be supported by the trial or correlative science study budget, which is determined prior to the start of the study. In some cases, biospecimens that are remaining after the completion of trial-associated correlative science studies (legacy biospecimens) may be available for secondary use in other correlative science studies. A scientific merit review process is required for research proposals before legacy biospecimen access is granted. Investigators within SWOG as well as external investigators are welcome to submit proposals for consideration. At this time the application process instructions are available at the SWOG website (http://www.swog.org/Visitors/TranslationalMed.asp) under “Collaborative Use of Specimens for Translational Medicine Research”. In this setting, a fee for service is required for biospecimen processing (including quality assurance) and distribution, as well as any additional testing (e.g., nucleic acid extractions, TMA creation). In the near future a new mechanism will be instituted in response to the NCTN Group reorganization and research community feedback. The new NCTN Core Correlative Science Committee, in conjunction with the NCTN Biospecimen ‘Front Door Service’ and biospecimen Navigator tool, will improve the efficiency and transparency of the biospecimen request process for the entire cancer research community. The NCTN Front Door staff will guide investigators through biospecimen query, application, and regulatory filing procedures. The web-based Navigator tool will allow investigators to independently query for Group Bank biospecimens that meet their criteria, and track their request through the review and approval process. Numerous other NCI supported bioresources are also available to assist investigators; for more information, visit the NCI specimen resource locator (https://specimens.cancer.gov/tissue/default.htm).
HOW NCTN GROUPS WORK TOGETHER – DIFFERENT PARADIGM
Jeffrey Abrams, M.D., Bhupinder Mann, MBBS, Abdul Tawab Amiri, Ph.D.
The NCI, for several decades, supported a system for conducting clinical trials in cancer through a number of national cooperative groups. The system served us well, and clinical trials led to advances in treatment of cancer. Nevertheless, improvements in efficiency were necessary as the resources were rapidly becoming limited, and with increasing use of molecular techniques, each disease was being redefined as consisting of molecularly distinct subsets. Reorganization of the cooperative group system into the National Clinical Trials Network (NCTN) since 2014 now offers the investigators several new platforms for conducting clinical and correlative science research. One example of the reorganization is the increasingly central role of the Cancer Trials Support Unit (CTSU). CTSU worked with the network groups to implement Medidata Rave as a single data management tool, and all NCTN sites can now participate in each other’s phase 3 and selected phase 2 studies. In addition, the institutional review board procedures have been streamlined by increasing participation in the NCI Central IRB (CIRB) for all NCTN phase 3 and selected phase 2 trials, and the time required for IRB approval has been dramatically reduced by its use. Finally, the NCTN Biospecimen Navigator tool provides a comprehensive inventory of NCTN banked biospecimens and provides a single, unified gateway for requests to use specimens collected on NCTN trials for correlative research. Navigator makes it possible to look across the NCTN to see what biospecimens and resources are available for innovative research projects.
Regarding the NCTN groups, the NCI has modified the incentives to stimulate more collaboration. Network groups are no longer required to lead a trial in each disease area in which they work [125]. Rather, they can succeed in peer review by leading some trials but also by participating actively in trials led by other network groups. Investigators are encouraged to collaborate across groups both via accrual and by adding interesting correlative science objectives to individual trials, irrespective of the group leading the trial. In fact, if groups don’t collaborate well, they will be penalized at peer review. We expect that this boost in collaboration among the NCTN participants will allow the network to enroll faster to its trials, and find answers faster for patients. The NCI Molecular Analysis for Therapy Choice (NCI-MATCH) clinical trial is a terrific example of collaboration in the new system, with 15-20 specific sub-protocols, each one having a Principal Investigator (PI) who is not necessarily a member of the lead group (ECOG-ACRIN Cancer Research Group) conducting the trial, but who can come from any of the network groups. Thus, we are already having much more integration of group investigators on this single protocol.
With rapid developments in molecular biology, particularly in gene sequencing technology, ever increasing resources are becoming necessary, and this is occurring in an era of constrained budgets for NCI. Leveraging resources from non-governmental entities to help NCI conduct its clinical trials is becoming more critical. This was very clear in designing Lung-MAP. We created a public-private partnership to conduct this trial. The genomic sequencing for molecular alterations required for every patient entering this trial necessitated additional funds. So, we set up a collaboration that includes Friends of the Cancer Research (an advocacy organization), Foundation for the National Institutes of Health (the foundation used by the government to bring funds to NCI from outside organizations), and we had participation from the FDA in helping to design this trial. These partnerships are not without their own challenges as it does require that network groups adapt to these partnerships which require modification of customary network group procedures.
In closing, bladder cancer offers some similarities to squamous cell lung carcinoma as it may require screening of large numbers of tumors to find those with a specific actionable gene alteration, given the relatively low (10–15%) frequency of these alterations. Resources for screening large numbers of patients are necessary for successful conduct of such trials and need to be thought about at the early stages of developing the study. Hopefully, several new tools and platforms now operational in the NCTN will facilitate the conduct of these necessary yet complex trial designs.
CONSENSUS DISCUSSION TO PRIORITIZE NCTN GROUP TRIALS
MOLECULARLY TARGETED CLINICAL TRIAL IN PATIENTS WITH NMIBC
Jonathan Rosenberg, M.D. and Eugene K. Cha, M.D.
The molecular target demonstrating the most promise for intervention at this time in NMIBC is the fibroblast growth factor receptor 3 (FGFR3). This gene is frequently mutated in this disease state, although the frequency of mutation declines with increasing grade and stage. FGFR3 activation appears to be an early event, and non-muscle-invasive tumors are highly enriched for activating mutations and fusions. These FGFR3 alterations occur in 50-70% of non-muscle-invasive bladder cancer (NMIBC), and are more prevalent in tumors of low grade and low stage [30–33, 36]. FGFR3 mutant tumors recur at the same frequency as FGFR3 wild-type tumors, but have a lower rate of progression to muscle invasion, with one study demonstrating a 5 year progression free survival of 91% in FGFR3 mutant patients compared to 74% in FGFR3 wild-type patients [36]. The FGFR3 mutation status in bladder tumor recurrences is often concordant with the primary tumor [37]. The high recurrence rate of NMIBC leads to significant morbidity and health care spending due to the need for frequent cystoscopic monitoring and operative intervention. Therefore, an effective oral agent to reduce the recurrence of NMIBC would be of high value.
Testing FGFR3 targeted agents in NMIBC requires careful balancing of risks and benefits when considering trial designs. These agents are currently being investigated as systemic agents, and do not currently have intravesical formulations. Therefore, the potential toxicity of the treatment must be balanced with the risk of the disease under treatment. In addition, systemic toxicity is observed with these drugs (e.g. hyperphosphatemia, keratopathy, fatigue), although they are readily reversible upon cessation of the study drug. Studies to demonstrate anti-tumor activity are required to determine whether systemic administration of FGFR3 inhibitors can lead to anticancer effects on tumors in the lining of the bladder. These pilot studies would provide the rationale for larger “adjuvant” or post-TUR studies which are time-to-event driven studies.
One study design using a “marker lesion” was heavily discussed at the meeting. This design requires that a small (≤1cm) non-invasive appearing tumor is left in place while systemic therapy is administered. The use of a marker lesion within the bladder has precedent in multiple phase II studies of NMIBC [6, 44–49]. These studies used the marker lesion to determine the ablative effect of therapy. These pilot studies can demonstrate preliminary evidence of anti-cancer activity that would justify larger trials. However, these studies have some additional, albeit low, risk to patients in that a small tumor is left in place for a period of time. To minimize this risk, any remaining tumor is removed after 2-3 months of treatment. This time period has been demonstrated to be safe, though very rare incidences of progression have been reported.
An alternative approach is the “window of opportunity” trial, which takes advantage of the interval between identification of recurrent tumor on office cystoscopy, and the operative TUR, usually performed several weeks later. The investigational agent is given for a defined, relatively short, period of time, and the anti-tumor activity is evaluated at transurethral resection. This approach requires mapping of the tumors present prior to initiation of therapy, and then re-evaluation at TUR after a defined period of time. This approach also allows assessment of anti-tumor ablative activity of an experimental agent. To be safe, this approach requires that only a limited therapeutic course is administered, as the entire tumor(s) is left in place. Defining success short of complete response is controversial, as measurement of tumor in situ in the bladder is quite difficult, and so shrinkage but not disappearance would be difficult to accurately quantify.
Discussion focused on the acceptability of the marker lesion concept to patients and physicians. While the marker lesion design as been shown to be safe, there is a theoretical risk of progression during the interval of treatment (shown to be <1%). Therefore, a majority of the participants felt that a window-of-opportunity study would be a more acceptable approach. Concepts are under development to test FGFR3 inhibitors using this study design.
IMMUNOLOGY-BASED CLINICAL TRIAL IN PATIENTS WITH NMIBC
Noah M. Hahn, M.D.
Given the wide array of immune checkpoint inhibitor and agonist therapies currently being tested for safety and initial efficacy in multiple ongoing clinical trials, a broad multi-arm phase Ib trial design to be led by ECOG-ACRIN was proposed in patients with NMIBC who have recurred after induction BCG (BCG failure) patients to test the safety of modern immune therapies as monotherapy, in combination with intravesical BCG, and in combination with external beam radiation therapy as depicted in the schema in Fig. 8. The study schema shown is meant to represent all rational immune therapy targets suitable for combination with BCG for which phase I clinical trial safety data exists or is expected in the next 6-12 months. It is anticipated that the eventual number of trial arms will be less than is shown in the schema. The exact number of arms will be dictated by sponsor interest and safety data as it emerges. A short phase I lead-in design is anticipated in each arm. The immunotherapy agent of interest will not be dose reduced. If dose limiting toxicity is encountered amongst the first 6 patients in a study arm, an additional 6 patients may be enrolled at one-third dose BCG. It is anticipated that a cap will be placed on the number of Ta/T1 patients within each arm to ensure an adequate number of CIS-only patients in each arm such that confidence interval estimates of the 6-month relapse free survival rate within NMIBC patient subsets will be of value in making decisions about subsequent phase II/III registration trial designs (i.e. Ta/T1 compared to CIS-only). Lastly, the study is envisioned as utilizing a flexible randomization strategy similar to the recently reported STAMPEDE prostate cancer trial (https://clinicaltrials.gov/show/NCT00268476) in which patients will be randomized between arms open to accrual [126]. In such a design, arms can be added in or taken out throughout the life cycle of the trial based on emerging safety data or new target identification.
Fig. 8.
Preliminary schema proposed for a broad multi-arm phase Ib trial in NMIBC patients who have recurred after induction BCG to test immune therapies in combination with intravesical BCG and with external beam radiation therapy.
After a summary of the day 1 immuno-oncology discussion points and a thorough deliberation of the proposed clinical trial concepts from day 1, a consensus was reached on the following three themes:
-
1.
While there are expected to be several single-arm industry-sponsored trials in the BCG-unresponsive NMIBC population making competition for patient accrual fierce, the collective ability of intergroup investigators to make major translational contributions to the field in this population is immense. Therefore, a trial design in BCG-unresponsive patients with a PD-1 or PD-L1 checkpoint inhibitor should be pursued and supported by intergroup investigators.
-
2.
A trial design of combined immune checkpoint blockade (i.e. anti-CTLA-4 therapy plus anti-PD-1 or anti-PD-L1 therapy) in combination with intravesical BCG posed too high of a risk for serious adverse events in a BCG-relapsing NMIBC population and should not be pursued at this time.
-
3.
An early phase trial design incorporating modern immunotherapy agents as monotherapy, in singular combinations with intravesical BCG, and in combination with external beam radiation therapy received widespread support and should be pursued.
As a result of the session 2 discussions, both a concept by led by SWOG of anti-PD-L1 therapy in BCG-unresponsive and a multi-arm concept of modern immunotherapy agents as monotherapy, in combination with BCG, or in combination with external beam radiation therapy (EBRT) led by ECOG-ACRIN in BCG-relapsing patients were endorsed by the group to proceed forward for development as intergroup clinical trials.
FUNDING SOURCE
This work is an outcome of the Clinical Trials Planning Meeting entirely supported by the National Cancer Institute’s Coordinating Center for Clinical Trials (NCI-CCCT) under the leadership of the NCI’s Genitourinary Cancers’ Steering Committee.
Author Conflicts of Interest and Financial disclosures
| Seth P. Lerner, M.D., FACS | Clinical trial support from Endo, FKD, Viventia; Consulting for Biocancell, Telesta, Theracoat, Vaxiion; Scientific/Advisory Committee member for Ferring, Nucleix, OncoGeneX, Sitka, Taris |
| Dean F. Bajorin, M.D. | Consulting for Roche/Genentech, Eli Lilly, Novartis, and Merck and UroGen; Research support for clinical trials from Novartis, Merck, Roche/Genentech, Novartis |
| Colin P. Dinney, M.D. | Consulting for Novartis Pharmaceuticals Corp. and Schering-Plough Pharmaceuticals; Scientific/Advisory Committee member for Sitka Biopharma, FKD Therapies, and University of Michigan Comprehensive Cancer Center |
| Noah M. Hahn, M.D. | Research support to institution from AstraZeneca/MedImmune, Bristol Myers-Squibb, Heat Biologics, Novartis, Merck, Mirati, OncoGenex, and Roche/Genentech. Relationships not relevant to the content of the article. |
| Michael O’Donnell, M.D. | Consulting/Advisory board for Biocancell, Telesta, Viventia, Medical Enterprises. Research support for clinical trials from Abbot Molecular, Roche/Genentech, and Photocure; Equity ownership/stock options and consultant for Theralase |
| Jonathan Rosenberg, M.D. | Consulting for Roche/Genentech, Eli Lilly, Sanofi, and Agensys; Research support for clinical trials from Agensys, Mirati, Novartis, and Roche/Genentech; Stock from Merck and Illumina |
| Margaret Callahan, M.D., Ph.D. | Institutional research funding from Bristol-Myers Squibb. Relationship not relevant to the content of the article. |
| Charles Drake, M.D., Ph.D. | Patents: Bristol-Myers Squibb and Janssen; Consultant: Agenus, Bristol-Myers Squibb, Compugen, Dendreon, Medimmune, NexImmune, ImmunExcite, Janssen, Eli Lilly, Merck, Novartis, Pierre Fabre, Potenza Therapeutics, Roche/Genentech, Vesuvius. |
| Ashish Kamat, M.D., MBBS, FACS | Recipients for grant/research support from FKD Industries, Photocure, Heat Biologics, Merck, Pacific Edge, and Photocure; Patent Pending. |
| William Kim, M.D. | Research Funding from Merck; Stock from Bristol Myers Squibb. Relationships not relevant to the content of the article. |
| David McConkey, Ph.D. | Grant support- Astra-Zeneca; Stock options- Apocell, Inc. Neither relationship is relevant to the content of the article. |
| Andrew Sharabi, M.D., Ph.D. | Consulting for StemImmune |
| Arlene H. Sharpe, M.D., Ph.D. | Patents from Boehringer-Ingelheim, EMD Serono, Astrazeneca, Roche, and Novartis; Licensing fees from Pfizer; Research grants from Novartis and Roche |
| David Solit, M.D. | Pfizer and Loxo Oncology; relationships not relevant to the meeting or content of the article. |
| Eliezer Van Allen, M.D. | Consulting for Third Rock Ventures |
ACKNOWLEDGMENTS
This work is an outcome of the Clinical Trials Planning Meeting entirely supported by the National Cancer Institute’s Coordinating Center for Clinical Trials (NCI-CCCT) under the leadership of the NCI’s Genitourinary Cancers’ Steering Committee.
REFERENCES
- [1]. Jarow J, Kluetz PG, Lerner SP, Liu K, Sridhara R, Bajorin D, et al. Reply by the authors. Urology 2014;84(2):495–6. doi: 10.1016/j.urology.2014.04.004. PubMed PMID: 25065996. [DOI] [PubMed] [Google Scholar]
- [2]. Jarow JP, Lerner SP, Kluetz PG, Liu K, Sridhara R, Bajorin D, et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: report of a Food and Drug Administration and American Urological Association public workshop. Urology 2014;83(2):262–4. doi: 10.1016/j.urology.2013.10.030. PubMed PMID: 24332121. [DOI] [PubMed] [Google Scholar]
- [3]. Lerner SP, Dinney C, Kamat A, Bivalacqua TJ, Nielsen M, O’Donnell M, et al. Clarification of Bladder Cancer Disease States Following Treatment of Patients with Intravesical BCG. Bl Cancer 2015;1(1):29–30. doi: 10.3233/BLC-159002. PubMed PMID: 26807434; PubMed Central PMCID: PMC4720147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Lerner SP, Jarow J, Maher VE, Tang S, Ibrahim A, Kim G, et al. Development of Systemic and Topical Drugs to Treat Non-muscle Invasive Bladder Cancer. Bl Cancer 2015;1(2):133–6. doi: 10.3233/BLC-150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507(7492):315–22. doi: 10.1038/nature12965. PubMed PMID: 24476821; PubMed Central PMCID: PMC3962515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nature Reviews Cancer 2015;15(1):25–41. doi: 10.1038/nrc3817. PubMed PMID: 25533674. [DOI] [PubMed] [Google Scholar]
- [7]. Patschan O, Sjodahl G, Chebil G, Lovgren K, Lauss M, Gudjonsson S, et al. A Molecular Pathologic Framework for Risk Stratification of Stage T1 Urothelial Carcinoma. European Urology 2015;68(5):824–32; discussion 35-6. doi: 10.1016/j.eururo.2015.02.021. PubMed PMID: 25770486. [DOI] [PubMed] [Google Scholar]
- [8]. Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Lin CC, Zietman AL, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. European Urology 2013;63(5):823–9. doi: 10.1016/j.eururo.2012.11.015. PubMed PMID: 23200811. [DOI] [PubMed] [Google Scholar]
- [9]. Gray PJ, Lin CC, Jemal A, Shipley WU, Fedewa SA, Kibel AS, et al. Clinical-pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the national cancer data base. International Journal of Radiation Oncology, Biology, Physics 2014;88(5):1048–56. doi: 10.1016/j.ijrobp.2014.01.001. PubMed PMID: 24661658. [DOI] [PubMed] [Google Scholar]
- [10]. Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. Journal of Clinical Oncology 2014;32(34):3801–9. doi: 10.1200/JCO.2014.57.5548. PubMed PMID: 25366678; PubMed Central PMCID: Pmc4239302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Gospodarowicz MK, Rider WD, Keen CW, Connolly JG, Jewett MA, Cummings BJ, et al. Bladder cancer: long-term follow-up results of patients treated with radical radiation. Clinical oncology (Royal College of Radiologists (Great Britain)) 1991;3(3):155–61. PubMed PMID: 1906339. [DOI] [PubMed] [Google Scholar]
- [12]. Quilty PM, Duncan W. Treatment of superficial (T1) tumours of the bladder by radical radiotherapy. British Journal of Urology 1986;58(2):147–52. PubMed PMID: 3083903. [DOI] [PubMed] [Google Scholar]
- [13]. Harland SJ, Kynaston H, Grigor K, Wallace DM, Beacock C, Kockelbergh R, et al. A randomized trial of radical radiotherapy for the management of pT1G3 NXM0 transitional cell carcinoma of the bladder. The Journal of Urology 2007;178(3 Pt 1):807–13; discussion 13. doi: 10.1016/j.juro.2007.05.024. PubMed PMID: 17631326. [DOI] [PubMed] [Google Scholar]
- [14]. Weiss C, Wolze C, Engehausen DG, Ott OJ, Krause FS, Schrott KM, et al. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer: an alternative to intravesical therapy or early cystectomy? Journal of Clinical Oncology 2006;24(15):2318–24. doi: 10.1200/JCO.2006.05.8149. PubMed PMID: 16710030. [DOI] [PubMed] [Google Scholar]
- [15]. Wo JY, Shipley WU, Dahl DM, Coen JJ, Heney NM, Kaufman DS, et al. The results of concurrent chemo-radiotherapy for recurrence after treatment with bacillus Calmette-Guerin for non-muscle-invasive bladder cancer: is immediate cystectomy always necessary? BJU International 2009;104(2):179–83. doi: 10.1111/j.1464-410X.2008.08299.x. PubMed PMID: 19154448. [DOI] [PubMed] [Google Scholar]
- [16]. Shipley WU, Dahl DM, Michaelson MD, Wu CL, Parker W, Winter K. Protocol RTOG-0926: A phase II protocol for patients with stage T1 bladder cancer to evaluate selective bladder preserving treatment by radiation therapy concurrent with radiosensitizing chemotherapy following a thorough transurethral surgical re-staging. Philadelphia, Pennsylvania: Radiation Therapy Oncology Group; 2009. [updated May 8, 2015; cited 2016 March]. Available from: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0926. [Google Scholar]
- [17]. Buchwald ZS, Efstathiou JA. Immunotherapy and radiation - A new combined treatment approach for bladder cancer? Bl Cancer 2015;1(1):15–27. doi: 10.3233/BLC-150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. The Lancet Oncology 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6. PubMed PMID: 26095785. [DOI] [PubMed] [Google Scholar]
- [19]. McShane LM. Statistical challenges in the development and evaluation of marker-based clinical tests. BMC Medicine 2012;10:52. doi: 10.1186/1741-7015-10-52. PubMed PMID: 22642713; PubMed Central PMCID: PMC3379945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. McShane LM, Polley MY. Development of omics-based clinical tests for prognosis and therapy selection: the challenge of achieving statistical robustness and clinical utility. Clinical Trials (London, England) 2013;10(5):653–65. doi: 10.1177/1740774513499458. PubMed PMID: 24000377; PubMed Central PMCID: PMC4410005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Balbas-Martinez C, Sagrera A, Carrillo-de-Santa-Pau E, Earl J, Marquez M, Vazquez M, et al. Recurrent inactivation of STAG2 in bladder cancer is not associated with aneuploidy. Nature Genetics 2013;45(12):1464–9. doi: 10.1038/ng.2799. PubMed PMID: 24121791; PubMed Central PMCID: PMC3840052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Guo G, Sun X, Chen C, Wu S, Huang P, Li Z, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nature Genetics 2013;45(12):1459–63. doi: 10.1038/ng.2798. PubMed PMID: 24121792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Kim J, Akbani R, Creighton CJ, Lerner SP, Weinstein JN, Getz G, et al. Invasive Bladder Cancer: Genomic Insights and Therapeutic Promise. Clinical Cancer Research 2015;21(20):4514–24. doi: 10.1158/1078-0432.CCR-14-1215. PubMed PMID: 26473186; PubMed Central PMCID: PMC4610178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Van Allen EM, Mouw KW, Kim P, Iyer G, Wagle N, Al-Ahmadie H, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discovery 2014;4(10):1140–53. doi: 10.1158/2159-8290.CD-14-0623. PubMed PMID: 25096233; PubMed Central PMCID: PMC4238969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. di Martino E, Tomlinson DC, Knowles MA. A Decade of FGF Receptor Research in Bladder Cancer: Past, Present, and Future Challenges. Advances in Urology 2012;2012:429213. doi: 10.1155/2012/429213. PubMed PMID: 22899908; PubMed Central PMCID: PMC3415141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. The Journal of Pathology 2007;213(1):91–8. doi: 10.1002/path.2207. PubMed PMID: 17668422; PubMed Central PMCID: PMC2443273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Williams SV, Hurst CD, Knowles MA. Oncogenic FGFR3 gene fusions in bladder cancer. Human Molecular Genetics 2013;22(4):795–803. doi: 10.1093/hmg/dds486. PubMed PMID: 23175443; PubMed Central PMCID: PMC3554204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Lamont FR, Tomlinson DC, Cooper PA, Shnyder SD, Chester JD, Knowles MA. Small molecule FGF receptor inhibitors block FGFR-dependent urothelial carcinoma growth in vitro and in vivo . British Journal of Cancer 2011;104(1):75–82. doi: 10.1038/sj.bjc.6606016. PubMed PMID: 21119661; PubMed Central PMCID: PMC3039817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Herrera-Abreu MT, Pearson A, Campbell J, Shnyder SD, Knowles MA, Ashworth A, et al. Parallel RNA interference screens identify EGFR activation as an escape mechanism in FGFR3-mutant cancer. Cancer Discovery 2013;3(9):1058–71. doi: 10.1158/2159-8290.CD-12-0569. PubMed PMID: 23744832; PubMed Central PMCID: PMC3770512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Acquaviva J, He S, Zhang C, Jimenez JP, Nagai M, Sang J, et al. FGFR3 translocations in bladder cancer: differential sensitivity to HSP90 inhibition based on drug metabolism. Molecular Cancer Research: MCR 2014;12(7):1042–54. doi: 10.1158/1541-7786. MCR-14-0004. PubMed PMID: 24784839. [DOI] [PubMed] [Google Scholar]
- [31]. Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nature Genetics 2011;43(9):875–8. doi: 10.1038/ng.907. PubMed PMID: 21822268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Kim PH, Cha EK, Sfakianos JP, Iyer G, Zabor EC, Scott SN, et al. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. European Urology 2015;67(2):198–201. doi: 10.1016/j.eururo.2014.06.050. PubMed PMID: 25092538; PubMed Central PMCID: PMC4312739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Sjodahl G, Lauss M, Gudjonsson S, Liedberg F, Hallden C, Chebil G, et al. A systematic study of gene mutations in urothelial carcinoma; inactivating mutations in TSC2 and PIK3R1. PloS one 2011;6(4):e18583. doi: 10.1371/journal.pone.0018583. PubMed PMID: 21533174; PubMed Central PMCID: PMC3077383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Iyer G, Al-Ahmadie H, Schultz N, Hanrahan AJ, Ostrovnaya I, Balar AV, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. Journal of Clinical Oncology 2013;31(25):3133–40. doi: 10.1200/JCO.2012.46.5740. PubMed PMID: 23897969; PubMed Central PMCID: PMC3753703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. The New England Journal of Medicine 2015;373(8):726–36. doi: 10.1056/NEJMoa1502309. PubMed PMID: 26287849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515(7528):558–62. doi: 10.1038/nature13904. PubMed PMID: 25428503. [DOI] [PubMed] [Google Scholar]
- [37]. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515(7528):563–7. doi: 10.1038/nature14011. PubMed PMID: 25428504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515(7528):568–71. doi: 10.1038/nature13954. PubMed PMID: 25428505; PubMed Central PMCID: PMC4246418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Champiat S, Ferte C, Lebel-Binay S, Eggermont A, Soria JC. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology 2014;3(1):e27817. doi: 10.4161/onci.27817. PubMed PMID: 24605269; PubMed Central PMCID: PMC3937193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nature Communications 2014;51:5241. doi: 10.1038/ncomms6241. PubMed PMID: 25348003; PubMed Central PMCID: PMC4212319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015;523(7559):231–5. doi: 10.1038/nature14404. PubMed PMID: 25970248. [DOI] [PubMed] [Google Scholar]
- [42]. Choi W, Porten S, Kim S, Willis D, Plimac ER, Hoffman-Censit J, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25(2):152–65. doi: 10.1016/j.ccr.2014.01.009. PubMed PMID: 24525232; PubMed Central PMCID: PMC4011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proceedings of the National Academy of Sciences of the United States of America 2014;111(8):3110–5. doi: 10.1073/pnas.1318376111. PubMed PMID: 24520177; PubMed Central PMCID: PMC3939870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. McConkey DJ, Choi W, Ochoa A, Siefker-Radtke A, Czerniak B, Dinney CP. Therapeutic opportunities in the intrinsic subtypes of muscle-invasive bladder cancer. Hematology/Oncology Clinics of North America 2015;29(2):377–94, x-xi. doi: 10.1016/j.hoc.2014.11.003. PubMed PMID: 25836941. [DOI] [PubMed] [Google Scholar]
- [45]. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. The American Journal of Surgical Pathology 1998;22(12):1435–48. PubMed PMID: 9850170. [DOI] [PubMed] [Google Scholar]
- [46]. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of Molecular Diagnostics: JMD 2015;17(3):251–64. doi: 10.1016/j.jmoldx.2014.12.006. PubMed PMID: 25801821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Van Allen EM, Wagle N, Sucker A, Treac DJ , , Johannessen CM , Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discovery 2014;4(1):94–109. doi: 10.1158/2159-8290.CD-13-0617. PubMed PMID: 24265153; PubMed Central PMCID: PMC3947264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Kandimalla R, van Tilborg AA, Zwarthoff EC. DNA methylation-based biomarkers in bladder cancer. Nature Reviews Urology 2013;10(6):327–35. doi: 10.1038/nrurol.2013.89. PubMed PMID: 23628807. [DOI] [PubMed] [Google Scholar]
- [49]. Sanchez-Carbayo M. Hypermethylation in bladder cancer: biological pathways and translational applications. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine 2012;33(2):347–61. doi: 10.1007/s13277-011-0310-2. PubMed PMID: 22274923. [DOI] [PubMed] [Google Scholar]
- [50]. Kompier LC, Lurkin I, van der Aa MN, van Rhijn BW, van der Kwast TH, Zwarthoff EC. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PloS one 2010;5(11):e13821. doi: 10.1371/journal.pone.0013821. PubMed PMID: 21072204; PubMed Central PMCID: PMC2972209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Dinney CP, Fisher MB, Nava N , O’Donnel MA, Cutler D, Abraham A, et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-alpha2b formulated in Syn3 for Bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. The Journal of Urology 2013;190(3):850–6. doi: 10.1016/j.juro.2013.03.030. PubMed PMID: 23507396; PubMed Central PMCID: PMC3951790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Cheng T, Roth B, Choi W, Black PC, Dinney C, McConkey DJ. Fibroblast growth factor receptors-1 and -3 play distinct roles in the regulation of bladder cancer growth and metastasis: implications for therapeutic targeting. PloS one 2013;8(2):e57284. doi: 10.1371/journal.pone.0057284. PubMed PMID: 23468956; PubMed Central PMCID: PMC3582560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Lamm DL. Bacillus Calmette-Guerin immunotherapy for bladder cancer. The Journal of Urology 1985;134(1):40–7. PubMed PMID: 3892050. [DOI] [PubMed] [Google Scholar]
- [54]. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. The Journal of Urology 1976;116(2):180–3. PubMed PMID: 820877. [DOI] [PubMed] [Google Scholar]
- [55]. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of Clinical Oncology 1999;17(7):2105–16. PubMed PMID: 10561265. [DOI] [PubMed] [Google Scholar]
- [56]. Dash A, Galsky MD, Vickers AJ, Serio AM, Koppie TM, Dalbagni G, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006;107(3):506–13. doi: 10.1002/cncr.22031. PubMed PMID: 16773629. [DOI] [PubMed] [Google Scholar]
- [57]. Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. Journal of Clinical Oncology 2003;21(16):3127–32. doi: 10.1200/JCO.2003.02.122. PubMed PMID: 12915604; PubMed Central PMCID: PMC2275327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England Journal of Medicine 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. PubMed PMID: 20525992; PubMed Central PMCID: PMC3549297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. PubMed PMID: 22658127; PubMed Central PMCID: PMC3544539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Plimack ER, Bellmunt J, Gupta S, Berger R, Montgomery RB, Heath K, et al. Pembrolizumab (MK-3475) for advanced urothelial cancer: Updated results and biomarker analysis from KEYNOTE-012. [American Society of Clinical Oncology Annual Meeting; Meeting abstract 4502]. Journal of Clinical Oncology 2015;33(Suppl:abstr 4502. [Google Scholar]
- [61]. Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clinical Cancer Research 2008;14(15):4800–8. doi: 10.1158/1078-0432.CCR-08-0731. PubMed PMID: 18676751. [DOI] [PubMed] [Google Scholar]
- [62]. Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 2007;109(8):1499–505. doi: 10.1002/cncr.22588. PubMed PMID: 17340590. [DOI] [PubMed] [Google Scholar]
- [63]. Brausi M, Witjes JA, Lamm D, Persad R, Palou J, Colombel M, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. The Journal of Urology 2011;186(6):2158–67. doi: 10.1016/j.juro.2011.07.076. PubMed PMID: 22014799. [DOI] [PubMed] [Google Scholar]
- [64]. Malmstrom PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. European Urology 2009;56(2):247–56. doi: 10.1016/j.eururo.2009.04.038. PubMed PMID: 19409692. [DOI] [PubMed] [Google Scholar]
- [65]. Gofrit ON, Zorn KC, Shikanov S, Steinberg GD. Marker lesion experiments in bladder cancer—what have we learned? The Journal of Urology 2010;183(5):1678–84. doi: 10.1016/j.juro.2009.12.104. PubMed PMID: 20299042. [DOI] [PubMed] [Google Scholar]
- [66]. Witjes JA, Palou J, Soloway M, Lamm D, Kamat AM, Brausi M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): results of an international individual patient data survey (IPDS). BJU International 2013;112(6):742–50. doi: 10.1111/bju.12012. PubMed PMID: 23452187; PubMed Central PMCID: PMC3933735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England Journal of Medicine 2003;348(3):203–13. doi: 10.1056/NEJMoa020177. PubMed PMID: 12529460. [DOI] [PubMed] [Google Scholar]
- [68]. Freeman GJ, Sharpe AH. A new therapeutic strategy for malaria: targeting T cell exhaustion. Nature immunology 2012;13(2):113–5. doi: 10.1038/ni.2211. PubMed PMID: 22261959; PubMed Central PMCID: PMC3690321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nature reviews Clinical oncology 2014;11(1):24–37. doi: 10.1038/nrclinonc.2013.208. PubMed PMID: 24247168; PubMed Central PMCID: PMC4086654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. PubMed PMID: 22461641; PubMed Central PMCID: PMC3568523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology 2009;10(1):29–37. doi: 10.1038/ni.1679. PubMed PMID: 19043418; PubMed Central PMCID: PMC2605166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England Journal of Medicine 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. PubMed PMID: 26027431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Hammers HJ, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) [2014 ASCO Annual Meeting, meeting abstract 4504]. Journal of Clinical Oncology 2014;32(Suppl):5s (abstr 4504). [Google Scholar]
- [74]. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27(4):450–61. doi: 10.1016/j.ccell.2015.03.001. PubMed PMID: 25858804; PubMed Central PMCID: PMC4400238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunology Research 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. PubMed PMID: 24777248. [DOI] [PubMed] [Google Scholar]
- [76]. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. Journal of Clinical Oncology 2012;30(21):2691–7. doi: 10.1200/JCO.2012.41.6750. PubMed PMID: 22614989. [DOI] [PubMed] [Google Scholar]
- [77]. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer research 2012;72(4):917–27. doi: 10.1158/0008-5472.CAN-11-1620. PubMed PMID: 22186141; PubMed Central PMCID: PMC3288154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Ghasemzadeh A, Bivalacqua TJ, Hahn NM, Drake CG. New Strategies in Bladder Cancer: A Second Coming for Immunotherapy. Clinical Cancer Research 2016;22(4):793–801. doi: 10.1158/1078-0432.CCR-15-1135. PubMed PMID: 26683632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015;28(6):690–714. doi: 10.1016/j.ccell.2015.10.012. PubMed PMID: 26678337. [DOI] [PubMed] [Google Scholar]
- [80]. Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. Journal of Clinical Oncology 2009;27(35):5911–8. doi: 10.1200/JCO.2009.23.3494. PubMed PMID: 19805669; PubMed Central PMCID: PMC2793039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Sharabi AB, Tran PT, Lim M, Drake CG, Deweese TL. Stereotactic radiation therapy combined with immunotherapy: augmenting the role of radiation in local and systemic treatment. Oncology (Williston Park, NY: ) 2015;29(5):331–40. PubMed PMID: 25979541. [PMC free article] [PubMed] [Google Scholar]
- [82]. Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunology Research 2015;3(4):345–55. doi: 10.1158/2326-6066.CIR-14-0196. PubMed PMID: 25527358; PubMed Central PMCID: PMC4390444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Berman DM, Wolchok JD, Weber JS, Hamid O, O’Day SJ, Chasalow SD. Association of peripheral blood absolute lymphocyte count (ALC) and clinical activity in patients (pts) with advanced melanoma treated with ipilimumab [2009 ASCO Annual Meeting, poster abstract 3020]. Journal of Clinical Oncology 2009;27(Suppl):15s (abstr 3020). [Google Scholar]
- [84]. Callahan MK, Horak CE, Curran MA, Hollman T, Schaer DA, Yuan J, et al. Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab [2013 ASCO Annual Meeting, abstract 3003]. Journal of Clinical Oncology 2013;31(Suppl:abstr 3003. [Google Scholar]
- [85]. Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Annals in Oncology 2013;24(6):1697–703. doi: 10.1093/annonc/mdt027. PubMed PMID: 23439861. [DOI] [PubMed] [Google Scholar]
- [86]. Kitano S, Postow MA, Ziegler CG, Kuk D, Panageas KS, Cortez C, et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunology Research 2014;2(8):812–21. doi: 10.1158/2326-6066.CIR-14-0013. PubMed PMID: 24844912; PubMed Central PMCID: PMC4125466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 2010;116(7):1767–75. doi: 10.1002/cncr.24951. PubMed PMID: 20143434; PubMed Central PMCID: PMC2917065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Postow MA, Chasalow SD, Yuan J, Kuk D, Panageas KS, Cheng M, et al. Pharmacodynamic effect of ipilimumab on absolute lymphocyte count (ALC) and association with overall survival in patients with advanced melanoma [2013 ASCO Annual Meeting, poster abstract 9052]. Journal of Clinical Oncology 2013;31(Suppl:abstr 9052. [Google Scholar]
- [89]. Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. The Lancet Oncology 2010;11(2):155–64. doi: 10.1016/S1470-2045(09)70334-1. PubMed PMID: 20004617. [DOI] [PubMed] [Google Scholar]
- [90]. Robert L, Harview C, Emerson R, Wang X, Mok S, Homet B, et al. Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology 2014;3:e29244. doi: 10.4161/onci.29244. PubMed PMID: 25083336; PubMed Central PMCID: PMC4108466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clinical Cancer Research 2014;20(9):2424–32. doi: 10.1158/1078-0432.CCR-13-2648. PubMed PMID: 24583799; PubMed Central PMCID: PMC4008652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Callahan MK. Understanding the Biology Behind Responses to Immunotherapy (Section: Developmental Therapeutics – Immunotherapy). American Socieity for Clinical Oncology Annual Meeting; May; Chicago, IL: ASCO; 2014. [Google Scholar]
- [93]. Callahan MK, Ott PA, Odunsi K, Bertolini SV, Pan LS, Venhaus RR, et al. A phase 1 study to evaluate the safety and tolerability of MEDI4736, an anti–PD-L1 antibody, in combination with tremelimumab in patients with advanced solid tumors [2014 ASCO Annual Meeting, abstract TPS3120]. Journal of Clinical Oncology 2014;32(5 s (suppl)):5 s (abstr TPS3120).24190110 [Google Scholar]
- [94]. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, NY: ) 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. PubMed PMID: 25765070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Snyder A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade. The New England Journal of Medicine 2015;372 8:783. doi: 10.1056/NEJMc1415938. PubMed PMID: 25693024. [DOI] [PubMed] [Google Scholar]
- [96]. Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Advances in cancer research 1993;62:153𲀓77. PubMed PMID: 8109317. [DOI] [PubMed] [Google Scholar]
- [97]. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. European Urology 2013;64(4):639–53. doi: 10.1016/j.eururo.2013.06.003. PubMed PMID: 23827737. [DOI] [PubMed] [Google Scholar]
- [98]. Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, endpoints, and clinical trial designs for non-muscle invasive bladder cancer (NMIBC): Recommendations from the International Bladder Cancer Group (IBCG). Submitted 2015. [Google Scholar]
- [99]. Kamat AM, Witjes JA, Brausi M, Soloway M, Lamm D, Persad R, et al. Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. The Journal of Urology 2014;192(2):305–15. doi: 10.1016/j.juro.2014.02.2573. PubMed PMID: 24681333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. The Journal of Urology 2000;163(4):1124–9. PubMed PMID: 10737480. [PubMed] [Google Scholar]
- [101]. Steinberg RL, Thomas LJ, O’Donnell MA. Bacillus Calmette-Guérin (BCG) treatment failures in non-muscle invasive bladder cancer: What truly constitutes unresponsive disease. Bladder Cancer 2015;1(2):105–16. doi: 10.3233/BLC-150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Lamm D, Persad R, Brausi M, Buckley R, Witjes JA, Palou J, et al. Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. The Journal of Urology 2014;191(1):20–7. doi: 10.1016/j.juro.2013.07.102. PubMed PMID: 23973937. [DOI] [PubMed] [Google Scholar]
- [103].Institute of Medicine. Public Engagement and Clinical Trials: New Models and Disruptive Technologies - Workshop Summary. Washington, D.C: National Academies Press; 2012. Available from: www.nap.edu. [PubMed] [Google Scholar]
- [104]. Skinner EC, Goldman B, Sakr WA, Petrylak DP, Lenz HJ, Lee CT, et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guerin. The Journal of Urology 2013;190(4):1200–4. doi: 10.1016/j.juro.2013.04.031. PubMed PMID: 23597452; PubMed Central PMCID: PMC4113593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Redman MW, Allegra CJ. The Master Protocol Concept. Seminars in Oncology 2015;42(5):724–30. doi: 10.1053/j.seminoncol.2015.07.009. PubMed PMID: 26433553; PubMed Central PMCID: PMC4681517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Liu PY, Dahlberg S, Crowley J. Selection designs for pilot studies based on survival. Biometrics 1993;49(2):391–8. PubMed PMID: 8369375. [PubMed] [Google Scholar]
- [107]. Sargent DJ, Goldberg RM. A flexible design for multiple armed screening trials. Statistics in Medicine 2001;20(7):1051–60. doi: 10.1002/sim.704. PubMed PMID: 11276035. [DOI] [PubMed] [Google Scholar]
- [108]. Goldman B, LeBlanc M, Crowley J. Interim futility analysis with intermediate endpoints. Clinical Trials (London, England: ) 2008;5(1):14–22. doi: 10.1177/1740774507086648. PubMed PMID: 18283075. [DOI] [PubMed] [Google Scholar]
- [109]. Redman MW, Goldman BH, LeBlanc M, Schott A, Baker LH. Modeling the relationship between progression-free survival and overall survival: the phase II/III trial. Clinical Cancer Research 2013;19(10):2646–56. doi: 10.1158/1078-0432.CCR-12-2939. PubMed PMID: 23669424; PubMed Central PMCID: PMC4131693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Lee MC, Levin HS, Jones JS. The role of pathology review of transurethral bladder tumor resection specimens in the modern era. The Journal of Urology 2010;183(3):921–7. doi: 10.1016/j.juro.2009.11.049. PubMed PMID: 20089271. [DOI] [PubMed] [Google Scholar]
- [111]. Hansel DE, Jewell SD, editors. Developing and Organizing an Institutional Biorepository. Northfield, Illinois: College of American Pathologists; 2014. [Google Scholar]
- [112]. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England Journal of Medicine 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. PubMed PMID: 22658128; PubMed Central PMCID: PMC3563263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113]. Dummer R, Daud A, Puzanov I, Hamid O, Schadendorf D, Robert C, et al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma [Melanoma Bridge Meeting, Naples, Italy, December 2014: Meeting abstract O5]. J Transl Med 2015;13(Suppl 1):O5. doi: 10.1186/1479-5876-13-S1-O5. [Google Scholar]
- [114]. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England Journal of Medicine 2015;372(21):2018–28. doi: 10.1056/NEJMoa1501824. PubMed PMID: 25891174. [DOI] [PubMed] [Google Scholar]
- [115]. Grosso J, Horak CE, Inzunza D, Cardona DM, Simon JS, Gupta AK, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) [ASCO meeting abstract 3016]. Journal of Clinical Oncology 2013;31(Suppl:Abstr 3016. [Google Scholar]
- [116]. Hamid O, Sosman JA, Lawrence DP, Sullivan RJ, Ibrahim N, Kluger HM, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) [ASCO meeting abstract 9010]. Journal of Clinical Oncology 2013;31(Suppl:abstr 9010. [Google Scholar]
- [117]. Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. Journal of Clinical Oncology 2015;33(13):1430–7. doi: 10.1200/JCO.2014.59.0703. PubMed PMID: 25452452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England Journal of Medicine 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. PubMed PMID: 23724867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Apolo AB, Tomita Y, Lee M-J, Lee S, Frosch A, Steinberg SM, et al. Effect of cabozantinib on immunosuppressive subsets in metastatic urothelial carcinoma (ASCO Meeting Abstract 4501). Journal of Clinical Oncology 2014;32(Suppl):5s (Abstract 4501). [Google Scholar]
- [120]. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death and Differentiation 2014;21(1):15–25. doi: 10.1038/cdd.2013.67. PubMed PMID: 23787994; PubMed Central PMCID: PMC3857622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clinical Cancer Research 2005;11(9):3353–62. doi: 10.1158/1078-0432.CCR-04-2062. PubMed PMID: 15867235. [DOI] [PubMed] [Google Scholar]
- [122]. Apolo AB. PDL1: The Illusion of an Ideal Biomarker. European Urology Focus 2016;1(3):269–71. doi: 10.1016/j.euf.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Plimack ER, Gupta S, Bellmunt J, Berger R, Montgomery B, Gonzalez EJ, et al. A phase 1b study of pembrolizumab (Pembro; MK-3475) in patients with advanced urothelial tract cancer (Meeting Abstract LBA23, European Society for Medical Oncology, Madrid, Spain). Annals in Oncology 2014;25(Suppl 4):LBA23. doi: 10.1093/annonc/mdu438.24. [Google Scholar]
- [124]. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England: ) 2016. doi: 10.1016/S0140-6736(16)00561-4. PubMed PMID: 26952546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Abrams J, Conley B, Mooney M, Zwiebel J, Chen A, Welch JJ, et al. National Cancer Institute’s Precision Medicine Initiatives for the new National Clinical Trials Network. American Society of Clinical Oncology Educational Book / ASCO American Society of Clinical Oncology Meeting 2014:71–6. doi: 10.14694/EdBook_AM.2014.34.71. PubMed PMID: 24857062. [DOI] [PubMed] [Google Scholar]
- [126]. James N, Sydes M, Mason M, Clarke N, Dearnaley D, Spears M, et al. Docetaxel and/or zoledronic acid for hormone-naϊve prostate cancer: First overall survival results from STAMPEDE (NCT00268476). Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33 (2015 (suppl; abstr 5001)). [DOI] [PMC free article] [PubMed] [Google Scholar]