Abstract
Introduction
Cardiovascular disease is the leading cause of mortality worldwide. Current surgical treatments for cardiovascular disease include vascular bypass grafting and replacement with autologous blood vessels or synthetic vascular grafts. However, there is a call for better alternative biological grafts.
Areas covered
Tissue-engineered vascular grafts (TEVGs) are promising novel alternatives to replace diseased vessels. However, obtaining enough functional and clinically usable vascular cells for fabrication of TEVGs remains a major challenge. New findings in adult stem cells and recent advances in pluripotent stem cells have opened a new avenue for stem cell-based vascular engineering. In this review, recent advances on stem cell sourcing for TEVGs including the use of adult stem cells and pluripotent stem cells, and advantages, disadvantages, and possible future implementations of different types of stem cells will be discussed. In addition, current strategies used during the fabrication of TEVGs will be highlighted.
Expert opinion
The application of patient-specific TEVGs constructed with vascular cells derived from immune-compatible stem cells possesses huge clinical potential. Advances in lineage-specific differentiation approaches and innovative vascular engineering strategies will promote the vascular regeneration field from bench to bedside.
Keywords: Adult stem cell, Pluripotent stem cell, Tissue-engineered vascular graft, Smooth muscle cell, Endothelial cell, Fibroblast
1. INTRODUCTION
1.1 Clinical background of cardiovascular disease and limitations of current treatments
Cardiovascular disease remains the leading cause of death in the world, accounting for 17.3 million deaths per year worldwide and 31.3% of all deaths, or about one in every three deaths, in the United States.1, 2 Vascular bypass graft and vascular replacement with autologous blood vessels or synthetic vascular grafts are the main treatments for coronary heart disease, aortic aneurysm, dissection, and peripheral vascular disease.3 For example, in 2010 in the US alone, there are about 1.4 million arterial bypass operations and a total of 397,000 coronary artery bypass procedures performed annually.1 However, patients with cardiovascular disease often lack optimal autologous vessels for grafting due to preexisting diseases. Although synthetic vascular grafts made of polymeric materials, including polyethylene terephthalate and expanded polytetrafluoroehylene, have been successfully applied to replace some diseased blood vessels, they can lead to many complications including acute thrombosis caused by the lack of functional endothelium coverage, restenosis resulting from chronic inflammatory responses, calcium deposition, and increased susceptibility to infection,4 especially in the case of small diameter grafts with an inner luminal diameter less than 6 mm.5, 6 Coating the intimal surface with heparin and other anticoagulant materials has been attempted, but these approaches need improvement7, 8 Moreover, in the pediatric population, children require multiple operations as they grow due to the lack of growth potential in current materials.9 Therefore, the current vascular grafts and conduits available for the treatment of cardiovascular disease are far from ideal.
1.2 Milestone works in the development of tissue-engineered vascular grafts (TEVGs)
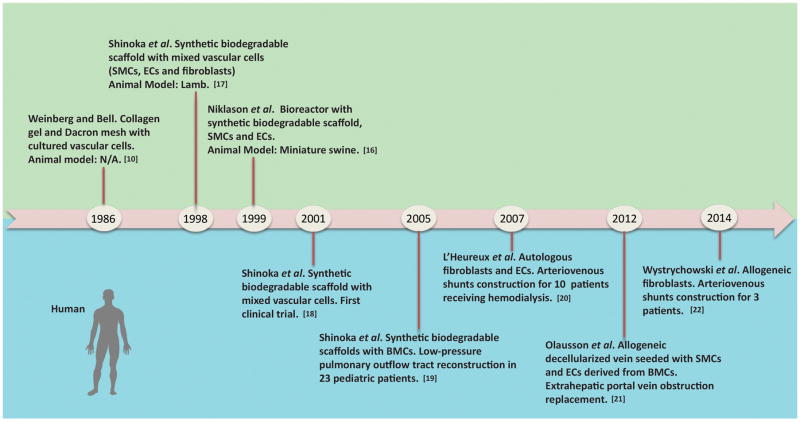
The shortage of autologous vessel sources and the disadvantages of current synthetic vascular grafts has driven the development of TEVGs, which mimic the structure and function of native blood vessels. Milestone developments of TEVGs are summarized in Figure 1. In 1986, Weinberg and Bell first tried to construct a completely biological blood vessel by seeding cultured vascular cells such as smooth muscle cells (SMCs), endothelial cells (ECs) and fibroblasts into three-dimensional collagen gel.10 However, the lack of proper mechanical properties forced them to use Dacron mesh in the media layer as structural reinforcement. Many efforts were made thereafter to improve the functionality of biological blood vessels,11–13 but the lack of proper mechanical properties, inadequate suture strength, limited ability to manipulate cell seeding, and susceptibility to rapid degradation by immune response undermined the possible advantages of completely biological scaffolds.14–16 In 1998, Shinoka et al first reported in situ work to construct living pulmonary artery conduits by seeding an isolated and expanded vascular cell mixture into synthetic biodegradable polyglactin/poly(glycolic acid) (PGA) tubular scaffolds and facilitating vascular remodeling in an ovine pulmonary artery replacement model.17 In 1999, Niklason et al developed an in vitro construction technique to produce small diameter arteries through the creation of a pulsatile perfusion bioreactor for TEVGS culture and modeling under simulated physiological mechanical stimuli.16 In 2001, Shinoka et al reported the first clinical application of TEVGs, after their early work in large animals, by in situ reconstructing a pulmonary artery with the patient’s vascular cells and a pre-designed biodegradable scaffold for a four-year old girl with congenital cardiac defect.18 Short-term observation showed no evidence of graft occlusion or aneurysmal changes. Encouraged by the first success, the Shinoka group reported the successful reconstruction of low-pressure pulmonary outflow tracts with autologous bone marrow cell-seeded biodegradable scaffolds in twenty-three pediatric patients diagnosed with cyanotic congenital defects in 2005.19 In 2007, the L'Heureux group reported the preliminary use of cell-sheet engineered TEVGs in the adult arterial system as arterial-venous shunts for hemodialysis access for six patients.20 In 2012 and 2014, clinical trials of TEVGs constructed from allogenic fibroblasts for extra-hepatic portal vein obstruction replacement and hemodialysis access showed that engineered grafts can be used off-the-shelf.21, 22 In this hemodialysis access application, the TEVGs were implanted and patent for up to eleven months with no evidence of immune response. Although challenges still exist, there is no doubt that the milestone works mentioned above represent significant advances in the clinical application of TEVGs to promote the translation of basic research from the bench to the bedside.
Figure 1. Historic development of tissue-engineered vascular grafts.
Green area above the timeline refers to studies on animals, while blue area below the timeline refers to clinical trials on human. SMCs: smooth muscle cells; ECs: endothelial cells; BMCs: bone marrow cells.
2. STEM CELL SOURCES FOR REGENERATING TEVGS
The fabrication of functional and clinically translatable SMC-based TEVGs involves three key components: obtaining numerous functional SMCs, fabricating optimal scaffolds for cell seeding and vascular tissue development, and facilitating the integration and remodeling of cell-scaffold constructs under various biochemical and biomechanical factors in vitro, in vivo and in situ.23, 24 All three aspects are complementary and essential in the development of functional TEVGs with a hierarchy of organized structure. In addition, selection of an optimal animal model for the evaluation of TEVGs is crucial, which has been comprehensively reviewed elsewhere.25
As this review is focused on SMC-based vascular grafts, it is worth noting that TEVGs have also been successfully constructed using extracellular matrix (ECM)-embedded fibroblasts in a bioreactor. 26, 27 The construction of TEVGs with different cell types and ECM components has been reviewed elsewhere. 28 Some groups have reported that properly-designed acellular biodegradable synthetic grafts can regenerate short blood vessels in small animals by recruiting host vascular cells and improving vascular remodeling.29–31 Since this strategy bypasses the in vitro cell culture step, it is more cost-effective and easier to control the batch quality. However, the potential of acellular grafts in regenerating longer blood vessels in large animals has not been exclusively demonstrated, largely due to limited trans-anastomotic host cell migration over an extended distance.32 Clinical evidence accumulated from the practice of implanting non-degradable synthetic grafts in humans over the past sixty years shows that trans-anastomotic vascular cell ingrowth only occurs in the immediate peri-anastomotic region. No more than 1 to 2cm migration distance is achieved even after years of implantation.32, 33 Given that many human peripheral bypass grafts are longer than 40 cm, it remains a great challenge for acellular biodegradable grafts to be translated into wide clinical use.
While many efforts have been made in the development of biomaterials and optimization of the microenvironment for SMC-based vascular tissue formation,24, 34 a vascular cell source remains a bottleneck problem for cell-based vascular therapy. Efforts to construct TEVGs using mature vascular SMCs isolated from explanted donor vascular segments have been extensively reported, especially at the early stage of TEVGs’ development.35–37 Thus, it is not surprising that the first clinical trial of TEVGs was carried out with autologous vascular cells. Unfortunately, mature vascular cells isolated from donor vessels are inadequate in quantity and suffer from limited proliferation potentials, reduction of collagen matrix production, and rapid decline of cell functions during extensive in vitro expansion. With potent proliferative capacity and differentiation potential into various subtypes of vascular cells, stem cells are becoming a promising cell source in vascular tissue engineering and regenerative medicine. Along with other researchers in the vascular regeneration field, we have successfully differentiated pluripotent stem cells such as mouse embryonic stem cells (ESCs), mouse induced pluripotent stem cells (iPSCs), and human iPSCs, into SMCs, and demonstrated the feasibility of harnessing pluripotent stem cells to serve as an advanced, unlimited cell source for vascular engineering.38–40 However, the application of pluripotent stem cell-derived SMCs to the construction of TEVGs for clinical use still encounters challenges. Heterogeneous cell populations derived from pluripotent stem cells may lead to undesired tissue formation. Here, recent advances on stem cell sourcing for TEVGs including the use of adult stem cells and pluripotent stem cells, and advantages, disadvantages, and possible future implementations of different types of stem cells are reviewed. In addition, current strategies used during the fabrication of TEVGs are highlighted.
2.1. Adult stem cells
Adult stem cells are undifferentiated cells found in many tissues in the human body and capable of differentiating into specialized cell types.41 In fact, the application of adult stem cells as clinical therapies has existed for decades.42 Recent advances indicate that adult stem cells, particularly mesenchymal stem cells (MSCs), have the capacity to differentiate into SMCs and ECs and, therefore, may be used in vascular regenerative medicine. In this section, we will discuss the use of bone marrow-derived stem cells and other adult stem cells derived from a variety of tissues with resident MSC-like cells.
2.1.1. Bone marrow-derived stem cells
Bone marrow is a physiological active tissue wherein hematopoietic stem cells and MSCs sustain hematopoiesis. Bone marrow stem cells have many advantages such as simple isolation from bone marrow aspirates,43 abundant stem cell quantity,19, 44 and lack of an immune response due to the absence of major histocompatibility complex class II.45 These characteristics of bone marrow-derived stem cells are advantageous for use in regenerative medicine. In particular, bone marrow mononuclear cells and bone marrow mesenchymal stem cells can be used to construct TEVGs.
2.1.1.1. Bone marrow mononuclear cells (BM-MNCs)
The first evidence that BM-MNCs contribute to the formation of vascular tissue through differentiation into SMCs and ECs was shown in the in situ regeneration study by Matsumura et al in 2003.46 This study investigated how implanted adult stem cells interact in an in vivo microenvironment during vascular tissue formation. In this work, canine BM-MNCs were pre-labeled with green fluorescence and seeded into poly(L-lactide) (PLLA) mesh enforced poly(ε-caprolactone and L-lactide) (PCL/LA) copolymer tubular scaffolds that were 8 mm in diameter, 2 cm in length and 0.6 mm in thickness. The cell-seeded tubular scaffolds were implanted into inferior vena cavas of original respective BM-MNCs isolated dogs. Post-implantation immunohistochemistry observation showed that the blood vessels were composed of bone marrow-differentiated SMCs and ECs and remained patent for up to two years. This pioneering work demonstrated that BM-MNCs contribute to the construction of TEVGs. In the clinical trial and series work conducted by the Shinoka group,19, 47, 48 autologous BM-MNCs were isolated and seeded into PLLA or PGA mesh enforced PCL/LA copolymer scaffolds. Cell-seeded-scaffold constructs were implanted as an extra cardiac cavopulmonary conduit in pediatric patients with single ventricle pathology. Middle and late term observations revealed that there was no graft-related mortality, no evidence of aneurysm formation, graft rupture, graft infection, or ectopic calcification.19,43 This clinical trial demonstrates that TEVGs can be successfully applied in a low-pressure pulmonary system in pediatric patients. It also shows that an in situ regeneration strategy has many advantages such as bypassing extensive in vitro cell culture and freedom from exposure to xenogeneic serum. However, the precise mechanisms underlying how seeded human bone marrow-derived cells proliferate, differentiate, and arrange themselves to contribute to a new tissue remain elusive, which requires advanced technologies to track the fate of donor cells, including genetic labeling and in vivo imaging.
Without an acellular control and cell lineage tracing observation, debates arise on if the seeded human BM-MNCs truly contribute to the formation of vascular tissue through in situ differentiation into SMCs and ECs. A recent study carried out in rodents found that seeded human BM-MNCs were replaced by mouse monocytes after one week of implantation, and then the scaffolds were repopulated by mouse originated SMCs and ECs.49 This study showed that human components are not maintained in developing vascular tissue. Instead, seeded human cells increased early host monocyte recruitment and improved vascular remodeling through an inflammation-mediated process. In corroboration with these findings, other studies have raised questions on the ability of BM-MNCs to differentiate into functional cells in ischemic disease therapies.50–52 Cell fusion may account for the co-stain of fluorescent cell membrane labeling with differentiated vascular cell markers.53–55 However, it remains unclear if studies on rodents can fully represent works on large animal models because host cell replacement and replenishment may occur much faster and more dramatically in small animals with shorter life span. Another concern is that BM-MNCs can differentiate into a variety of mature cells and are not lineage-specific for SMCs and ECs. To this end, it is important to distinguish the differentiated SMCs in synthetic or contractile phenotype from myofibroblasts or activated fibroblasts with similar marker expression patterns.
Another strategy is to seed BM-MNCs into a scaffold and differentiate the cells in vitro into mature SMCs and ECs before implantation. Cho et al first reported the utilization of BM-MNCs in generating small-diameter blood vessels with internal diameter of 3 mm.56 BM-MNCs fractions of SMC α-actin- and smooth muscle heavy chain-positive cells were isolated and cultured in Medium 199 containing 10% fetal bovine serum, and BM-MNCs fractions of vWF/CD31-positive cells were cultured in medium supplemented with vascular endothelial growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor and ascorbic acid for 3 weeks prior to be seeded into decellularized arteries. Results showed that cell seeding and differentiation significantly improved the patency of vascular grafts in a canine carotid artery replacement model. The donor cells pre-labeled with a fluorescent dye were detectable in the retrieved vascular grafts. Furthermore, they found that the post-operative administration of granulocyte colony-stimulating factor enhanced in vivo endothelialization and reduced intimal hyperplasia.57 In addition, Brennan et al found that TEVGs constructed with BM-MNCs demonstrated growth potential in a juvenile animal model. 58 It was found that all grafts explanted at 6 months were patent, and the grafts increased in volume as measured by difference in pixel summation in magnetic resonance angiography at 1 month and 6 months.
2.1.1.2. Bone marrow mesenchymal stem cells (BM-MSCs)
In addition to the direct application of BM-MNCs, BM-MSCs are a special multipotent stromal fraction of bone marrow stem cells that can be cultured and expanded in vitro prior to scaffold seeding. The derived cells are capable of differentiating into a variety of mesodermal cell types including SMCs, osteoblasts, chondrocytes, and adipocytes.59–63 BM-MSCs are isolated by plating the mononuclear cell fraction on a cell culture surface to remove the non-adherent cells by positive or negative surface marker sorting. Although the current research lacks specific markers for BM-MSCs, a few characteristics have been established. BM-MSCs are plastic-adherent. When maintained in standard culture conditions, the cells express surface markers including CD105, CD73 and CD90, and are negative for CD45, CD34, CD14, CD11b, CD79α, CD19 and HLA-DR. The cells can be differentiated into osteoblasts, adipocytes, and chondrocytes in vitro with standardized differentiation procedures.64 Though BM-MSCs have been widely studied, a disadvantage of the cells is clear as they account for only about 0.01% of the total mononuclear cell population, and both the percentage and differentiation capacity decrease with increased age.62, 63, 65
BM-MSCs have been used as SMC sources in fabricating TEVGs. In 2007, an in situ regeneration study by Hashi et al66 was successful in directly seeding BM-MSCs into electrospun PLLA/PCL scaffold to regenerate TEVGs. Then, BM-MSC seeded scaffolds were implanted in a rat common carotid artery replacement model. The results showed acellular control grafts had significant intimal thickening while BM-MSC seeded grafts resulted in well-organized layers of SMC and ECs and long-term patency. However, most of the seeded human BM-MSCs disappeared after seven days of implantation, and the majority of cells in the regenerated tissues were from the host. The results indicated that BM-MSCs mainly exerted their function through an antithrombogenic effect. In large animal models, Zhang et al seeded canine BM-MSCs into biodegradable three-layered tubular scaffolds with inner and outer layers made of poly(lactic glycolic acid) (PLGA) and an elastic polyurethane middle layer.67 After seven days of in vitro culture, the constructs were implanted into a canine abdominal aorta replacement model. The cell-seeded grafts remained fully patent without any signs of dilation or obstruction after three months post-implantation. In another study, the Andreadis group further purified smooth muscle progenitor cells from differentiated bone marrow-derived cell mixture with smooth muscle α actin genetic labeling and fluorescence-activated cell sorting.68 TEVGs were successfully built from bone marrow-derived smooth muscle progenitor cells and bone marrow-derived endothelial cells and implanted in an ovine jugular vein replacement model. The TEVGs demonstrated extensive remodeling capability and a high amount of collagen and elastin synthesis from bone marrow-derived smooth muscle progenitor cells as compared with terminally-differentiated SMCs.
Similar to BM-MNCs, the application of BM-MSCs has been investigated with an in vitro regeneration strategy as well. By using nonwoven PGA scaffolds and a biomimetic perfusion system, Gong et al showed that human BM-MSCs can serve as a promising SMC source for small diameter vessel engineering through the optimization of various biochemical and biophysical factors including matrix proteins, growth factors, and mechanical forces that modulate SMC proliferation and differentiation.69 Zhao et al isolated ovine BM-MSCs and differentiated the cells into SMCs in medium supplemented with 5% fetal bovine serum, insulin and transforming growth factor-β1, and into ECs in medium supplemented with 5% fetal bovine serum, vascular endothelial growth factor, basic fibroblast growth factor and ascorbic acid before seeding the cells into decellularized blood vessel grafts.70 After one week of maturation in vitro, burst pressure and suture strength were measured and the results showed mechanical properties comparable to native arteries. Then the cell-seeded grafts were implanted in an ovine model and the grafts were demonstrated to be mechanically stable and patent over five months while the unseeded controls quickly occluded in two weeks.
2.1.2. Adult stem cells derived from other tissues with resident MSC-like cells
Although bone marrow was the original tissue and main location containing adult stem cells, many other tissue sources including adipose, hair follicles, and neonatal and infant thymus have been explored.71, 72 Several studies demonstrated the successful differentiation of adipose-derived stem cells into SMCs73–76 and ECs.77–80 In one study, adipose-derived stem cells treated with transforming growth factor-β1 and bone morphogenetic protein 4 were differentiated into SMCs. Then the derived SMCs were seeded into a nonwoven PGA scaffold to fabricate vascular wall tissue.81 In another study, adipose-derived stem cells were differentiated into contractile SMCs under treatment with sphingosylphosphorylcholine and transforming growth factor-β1. The derived cells can attach to decellularized vein grafts.75 Since adipose-derived stem cells are readily-available and easy to obtain, more attention is focused on this cell type.82
Other studies have shown hair follicle-derived stem cells have similar proliferation and differentiation potential as compared to BM-MSCs83 and could be driven to differentiate into functional contractile SMCs.61, 84 Furthermore, Liu et al showed that miR-18b inhibited transforming growth factor-β1-induced differentiation of hair follicle stem cells into SMCs by targeting SMAD2, which revealed one molecular mechanism underlying SMC lineage differentiation.85
Recently, autologous amniotic fluid cells have been used as another cell source for cardiovascular tissue engineering applications. These cells were differentiated into several mesodermal derivatives, which were then used for the in vitro fabrication of small- and large-diameter TEVGs and cardiovascular patches.86 The study exhibited feasible extraction, identification, and application of this cell source in cardiovascular tissue engineering.
2.1.3. Brief summary of adult stem cells
Adult stem cells are promising cell sources for vascular tissue engineering with advantages such as being readily attainable from various tissues, minimal or lack of in vitro cell culture time, ability to differentiate into SMCs and ECs, and most importantly, lack of risk of tumorigenesis.87, 88 However, cellular senescence and decreased differentiation capability associated with increasing donor age remain one of the largest barriers for their application in elderly patients.89–91 For instance, a recent report Krawiec et al showed that adipose-derived MSCs from elderly patients may not be suitable for autologous TEVGs due to inadequate SMCs migration and differentiation.92 More research on basic physiological changes of adult stem cells during in vitro culturing and vascular remodeling is needed.93
2.2. Pluripotent stem cells
Pluripotency refers to the ability to develop into all three germ layers: ectoderm, mesoderm, and endoderm. Embryonic stem cells (ESCs) were the first established pluripotent stem cells with unlimited growth and self-renewal abilities. Mouse ESCs were isolated from the inner cell mass of blastocyst and expanded in vitro by Martin et al in 1981.94 But not until almost twenty years later, the first human ESCs culture was established by the Thomson group.95 Similar to ESCs, induced pluripotent stem cells (iPSCs) reprogrammed from somatic cellular populations with four canonical reprogramming factors including OCT4, SOX2, KLF4, and cMyc have the ability to differentiate into almost all cell types.96–99 Both ESCs and iPSCs have been differentiated into SMCs and used for generation of TEVGs (Table 1). In addition, human iPSCs derived from patients provide an excellent human cell-based tool for cardiovascular disease modeling and drug screening.100–102
Table 1.
Application of pluripotent stem cells in constructing tissue-engineered vascular grafts
| Cell Source | Implanted Cell Type | Scaffold | Animal Model | Implantation Site | Duration (weeks) | Reference |
|---|---|---|---|---|---|---|
| mESCs | SM22á-LacZ selected SMCs | Macroporous nanofibrous PLLA | Nude mouse | SQ | 2 | [35] |
| miPSCs | SMCs | Macroporous nanofibrous PLLA | Nude mouse | SQ | 2 | [34] |
| miPSCs | Differentiated mixture | Nonwoven PGA mesh with P(CL/LA) sealant | SCID mouse | Interior vena cava interposition | 10 | [102] |
| hESCs | MSCs | Nonwoven PGA mesh | * N/A | * N/A | 8 | [109] |
| hiPSCs | SMCs | Macroporous nanofibrous PLLA | Nude mouse | SQ | 2 | [36] |
| hiPSCs | MSCs | Nonwoven PGA mesh | * N/A | * N/A | 8 | [110] |
| hPiPSCs | SMCs, ECs | Decellularized vessel | SCID mouse | Carotid artery interposition | 3 | [107,108] |
Abbreviations: ECs: endothelial cells; hESCs, human embryonic stem cells; hiPSCs, human induced pluripotent stem cells; hPiPSCs, human partially-induced pluripotent stem cells; mESCs, mouse embryonic stem cells; miPSCs, mouse induced pluripotent stem cells; MSCs, mesenchymal stem cells; PLLA, poly(L-lactide); PGA, poly(glycolic acid); P(CL/LA), poly(å-caprolactone and L-lactide); SCID, severe combined immunodeficiency; SMCs, smooth muscle cells; SQ, subcutaneous
in vitro bioreactor culture
2.2.1. Application of pluripotent stem cell-derived SMCs and ECs
More discoveries have fostered a deeper understanding of crucial extrinsic signals and intrinsic pathways involved in SMCs linage specification and improved approaches to differentiate and purify SMCs.103–105 Based on these advancements, SMCs derived from pluripotent stem cells have been used in constructing TEVGs. We were the first to explore the potential use of SMCs differentiated from mouse iPSCs for vascular tissue engineering.38 Mouse iPSCs were treated with all-trans retinoid acid and the derived SMCs were seeded into three-dimensional macroporous nanofibrous PLLA scaffolds. Subcutaneous implantation results showed promising vascular tissue formation. Furthermore, we enriched and purified SMCs derived from mouse ESCs by developing a LacZ genetic label under the control of SM22α promoter as the positive sorting marker. We demonstrated the potential use of enriched SMCs to construct vascular tissues on three-dimensional scaffolds with better morphological and functional outcomes.39 In order to develop a highly efficient method to generate functional SMCs from human iPSCs and to construct patient-specific vascular tissues for translational study, we established human iPSCs from primary human aortic fibroblasts and differentiated human iPSCs into SMCs through an embryonic body-intermediated route. Contractile response to carbachol treatment and up-regulation of vascular collagen subtype genes and matrix metalloproteinase subtype genes under pharmacological stimulation demonstrated that the differentiated SMCs were functional. Subcutaneous implantation of the SMC-scaffold constructs in nude mice showed vascular tissue formation with robust collagenous matrix deposition and maintenance of differentiated SMC phenotype.40 In corroboration with these findings, Breuer and his colleagues investigated the use of mouse iPSCs-derived cell mixture sheets in the construction of TEVGs.106 However, insufficient induction of the pluripotent cells led to significant teratoma formation in a mouse inferior vena cava interposition implantation model. These pioneering studies underline the importance of development of efficient methodologies for complete lineage specification in the construction of TEVGs. Moreover, as vascular SMCs have distinct embryonic origins,107 embryological origin-associated disease susceptibilities were observed in different aortic segments.108 By adopting a reprogramming strategy from peripheral blood mononuclear cells,109, 110 we recently established patient-specific, integration-free iPSCs and then differentiated the cells into SMCs with distinct embryonic origins. Recently published data revealed that SMCs differentiated from human iPSCs-derived cardiovascular progenitor cell intermediates could be a more promising cell source for TEVGs because the cells exhibit a functional phenotype comparable to primary cultured human aortic SMCs without the risk of tumorigenesis.111
To reduce tumorigenesis and shorten the reprogramming time, the Xu group used a partially induced pluripotent stem cells (PiPSCs) strategy to reprogram human fibroblast cells into SMCs and ECs.112, 113 Through transient transfection with four reprogramming factors (OCT4, SOX2, KLF4 and c-MYC) for as short as four days, tumor formation was not observed in vivo and the transfected temporary cells displayed the potential to be further re-differentiated into SMCs on collagen IV-coated surface and ECs in differentiation factor-supplemented defined media. Human PiPSC-derived SMCs, together with human PiPSC-derived ECs, repopulated decellularized blood vessels and gave rise to functional TEVGs. The experimental TEVGS implantation group had a 60% survival rate at three weeks after mouse carotid artery interposition surgery as compared to a 20% survival rate of animals implanted with un-seeded controls.
2.2.2. Application of pluripotent stem cell-derived progenitor cells
Instead of directly seeding pluripotent stem cells-derived terminally-differentiated SMCs or ECs into scaffold, seeding pluripotent stem cell-derived progenitor cells may improve cellular engraftment. Recently, the Niklason group114, 115 showed that human ESCs- and iPSCs-derived mesenchymal progenitor intermediate cells were able to be seeded onto small-diameter vascular grafts in vitro in a bioreactor. However, markers representative of unwanted cartilage and bone tissues were detected in the engineered vessels, which raises concerns of lineage specification efficiency when incompletely differentiated progenitor cells are used. Moreover, the in situ vascular regeneration capability and the engraftment of pluripotent stem cells-derived progenitor cells remain to be explored.
2.2.3. Brief summary of pluripotent stem cells
The application of human ESCs is restricted by ethical concerns regarding their isolation from human embryos and the potential for immune rejection. In contrast, human iPSCs are not restricted by such limitations and have additional advantages. Human iPSCs can be reprogrammed from a patient’s own somatic cells to circumvent immune rejection and have the ability to develop into vascular cells similar to human ESCs. However, safety concerns arise from human iPSCs generation through the use of viruses, a genome-integrated method for reprogramming, and the accumulation of chromosomal abnormalities.116, 117 Recently, advanced integration-free reprogramming using non-integrated plasmid, mRNA, proteins, or small molecules118–120 facilitates the generation of human iPSCs with reduced genotoxicity.121 Abnormal cells bearing chromosomal abnormalities resulted from either extensive in vitro culturing or the original mutant somatic cells122, 123 and should be excluded prior to further tissue regeneration manipulation. In addition, genetic aberrations like copy number variations during reprogramming have been reported.116, 117, 124 These genetic and epigenetic abnormality issues should be addressed before human iPSCs are translated into clinical use.
3. CONCLUSION
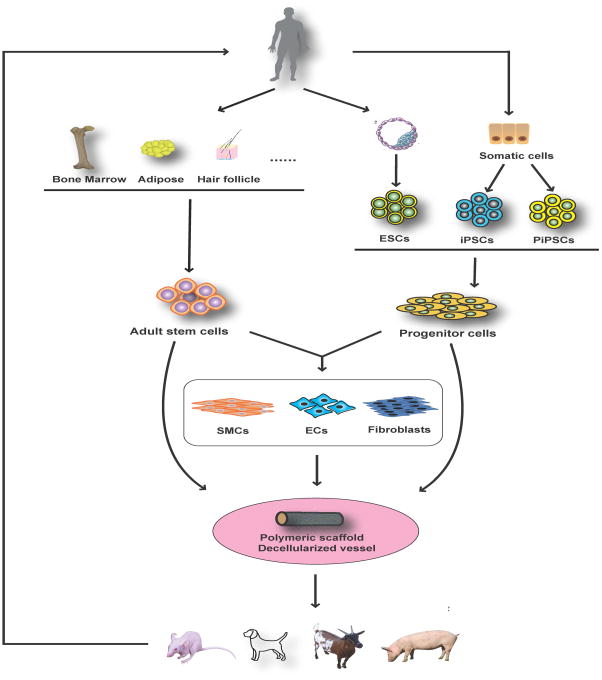
Though advances in the field of vascular regenerative medicine and the development of a variety of strategies to regenerate vascular tissues have occurred (Figure 2), the fabrication of clinically-translatable TEVGs is still at an early stage. Many challenges must be overcome to generate TEVGs with proper mechanical strength and functionality. In particular, vascular cell source, one of the key components in engineered vessels, still encounters many challenges and obstacles. Stem cells are a promising cell source, but current formulations have limitations. Adult stem cells are easy to obtain, but the viability is variable due to the donor age and health status. Human ESCs have unlimited self-renewal and differentiation capacities. However, the ethical concern regarding the sacrifice of embryos limit their therapeutic availability and utility. Human iPSCs have been proved to be an ideal cell source for TEVGs, but potential carcinogenic concerns and an unstable genome after reprogramming limit its prompt clinical application. Nevertheless, with continuous efforts to improve safety and more clinical trials studying the application of iPSCs in other fields, there is no doubt that human iPSCs will ultimately be widely used in regenerating blood vessels.
Figure 2. Current strategies applied in fabricating tissue-engineered vascular grafts.
ESCs: embryonic stem cells; iPSCs: induced pluripotent stem cells; PiPSCs: partially induced pluripotent stem cells; SMCs: smooth muscle cells; ECs: endothelial cells.
4. EXPERT OPINION
4.1. Current stage and challenges
With several decades of development and landmark studies throughout its infancy and exploratory stages, vascular regenerative medicine is advancing quickly. But it also faces challenges. Cell source is one of the crucial components in fabricating TEVGs and we summarized the advantages and disadvantages of different cell sources (Table 2). Some advantages and disadvantages to consider include accessibility to target cells, quantity of harvestable cells, exposure to xenogeneic serum in in vitro culture, functionality of derived cells, potential to become patient-specific, capability to correct genomic mutation in patients such as Loeys-Dietz Syndrome patients,125, 126 and the total time needed to fabricate TEVGs. All cell sources have their limitations. Primary mature vascular cells were the first used cell sources and significantly contributed to the establishment of the vascular tissue engineering paradigm. However, the quantity and quality of isolated vascular cells are far from clinical use, and most patients requiring vascular bypass and replacement surgery lack proper blood vessel segments for cell harvesting. Adult stem cells can be immediately used on patients after isolation. However, cell viability is correlated with patient age and health status. The use of embryonic stem cells is restricted by ethical concerns and is not currently applicable in the clinical setting. iPSCs are a newly established, widely investigated technology in a variety of regenerative medicine fields. With advantages including unlimited cell quantity, patient-specific cell sourcing, vascular lineage-specific differentiation, and ability to correct genetic mutations before scaffold seeding, iPSCs must be further researched for use in vascular tissue engineering.
Table 2.
Advantages and disadvantages of various cell sourcing in clinical utility
| Mature Vascular Cells | Adult Stem Cells | ESCs | iPSCs | |||||
|---|---|---|---|---|---|---|---|---|
| Advantage | Disadvantage | Advantage | Disadvantage | Advantage | Disadvantage | Advantage | Disadvantage | |
| Isolation methodology | Easy | - | Easy | - | - | Ethnical Concerns | - | Reprogramming Safety |
| Extracted Cells Numbers | - | Limited | - | Limited | Unlimited | - | Unlimited | - |
| Xenogeneic Serum Exposure | - | Yes | in situ: No | in vitro: Yes | No | - | No | - |
| SMC Contractile Function and ECM Secretion Ability | - | Limited | - | Limited | Unlimited | - | Unlimited | - |
| Patient-Specific | Yes | - | Yes | - | - | No | Yes | - |
| SMC Lineage Specific | Yes | - | - | No | Yes | - | Yes | - |
| Genomic Mutation Correction Capability | - | No | - | No | Yes | - | Yes | - |
| Key Steps for TEVGs Construction |
|
in situ:
in vitro:
|
|
|
||||
Abbreviations: ECM, extracellular matrix; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; SMC, smooth muscle cells; TEVG, tissue-engineered vascular grafts
Definitions: Limited, limitations due to age and health status; Unlimited, no limitations since ESCs and iPSCs have unlimited self-renewal and full differentiation capabilities.
4.2. Future of the field
With future advances in SMC differentiation methodology and knowledge on SMC physiology, lineage-specific SMCs differentiated from stem cells will be widely used and more research is needed to explore the underlying mechanisms of how scaffolds are remodeled and new vascular tissues are developed in situ. As for adult stem cell sources, recently Tang et al identified a new type of adult stem cell in the vessel wall called vascular multipotent stem cells and showed that the cells may contribute to regenerated vascular tissue after vessel injury.127 This type of vascular resident stem cell may be another source for TEVGs, although the identity of the cells is still under debate due to the limited methodology available to precisely trace the cell fate in situ.128 As for cell reprogramming, research is needed on the direct cell conversion of SMCs from other somatic cells.129 Recently, the Niklason group reported the application of decellularized TEVGs made from extracellular matrix secreted from allogeneic primary mature SMCs.130, 131 This study suggests that SMCs derived from other cell sources may be used in the same strategy and will open a new avenue to fabricate off-the-shelf biological grafts in the future.
In conclusion, with more researchers investigating how seeded cells coordinate with scaffolds and microenvironments, future advancements will facilitate the regeneration of vascular tissue in situ, achieve long term application of TEVGs in high-pressure adult arterial system, and elucidate the utility of TEVGS in disease modeling and drug screening.
Article Highlights Box.
Autologous mature vascular cells contribute to the foundation of vascular tissue engineering, but are greatly restricted by cell quantity and quality.
Adult stem cells demonstrate promising outcomes, but lack lineage specificity and are limited by donor age and health status.
Pluripotent stem cells, particularly human iPSCs cells, are a feasible option and possess advantages that other cell sources do not.
Advances in knowledge on SMC physiology and advances in differentiation approaches of vascular cells will promote the application of lineage-specific vascular cells.
Footnotes
Financial and competing interests disclosure
The authors would like to acknowledge the financial support from University of Michigan Frankel Cardiovascular Center Inaugural Grant (BY), University of Michigan MCubed Grant (BY, YEC, and PXM), and the National Institute of Health Grant (HL114038: PXM and YEC). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of particular interest have been highlighted as:
* of special interest
** of outstanding interest
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation. 2015 Jan 27;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Laslett LJ, Alagona P, Jr, Clark BA, 3rd, Drozda JP, Jr, Saldivar F, Wilson SR, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. Journal of the American College of Cardiology. 2012 Dec 25;60(25 Suppl):S1–49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circulation research. 2006 Jan 6;98(1):25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 4.L'Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L, McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts--from research to clinical practice. Nature clinical practice Cardiovascular medicine. 2007 Jul;4(7):389–95. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 5.Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. Journal of vascular surgery. 1986 Jan;3(1):104–14. doi: 10.1067/mva.1986.avs0030104. [DOI] [PubMed] [Google Scholar]
- 6.Chard RB, Johnson DC, Nunn GR, Cartmill TB. Aorta-coronary bypass grafting with polytetrafluoroethylene conduits. Early and late outcome in eight patients. The Journal of thoracic and cardiovascular surgery. 1987 Jul;94(1):132–4. [PubMed] [Google Scholar]
- 7.Devine C, Hons B, McCollum C. Heparin-bonded Dacron or polytetrafluoroethylene for femoropopliteal bypass grafting: a multicenter trial. Journal of vascular surgery. 2001 Mar;33(3):533–9. doi: 10.1067/mva.2001.113578. [DOI] [PubMed] [Google Scholar]
- 8.Kidane A, Lantz GC, Jo S, Park K. Surface modification with PEO-containing triblock copolymer for improved biocompatibility: in vitro and ex vivo studies. Journal of biomaterials science Polymer edition. 1999;10(10):1089–105. doi: 10.1163/156856299x00702. [DOI] [PubMed] [Google Scholar]
- 9.Shinoka T, Breuer C. Tissue-engineered blood vessels in pediatric cardiac surgery. The Yale journal of biology and medicine. 2008 Dec;81(4):161–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986 Jan 24;231(4736):397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 11.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. American journal of physiology Heart and circulatory physiology. 2005 Mar;288(3):H1451–60. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 12.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004 Aug;25(17):3699–706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 13.Grassl ED, Oegema TR, Tranquillo RT. A fibrin-based arterial media equivalent. Journal of biomedical materials research Part A. 2003 Sep 1;66(3):550–61. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 14.Hoerstrup SP, Zund G, Sodian R, Schnell AM, Grunenfelder J, Turina MI. Tissue engineering of small caliber vascular grafts. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2001 Jul;20(1):164–9. doi: 10.1016/s1010-7940(01)00706-0. [DOI] [PubMed] [Google Scholar]
- 15.Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, et al. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg. 1999 Dec;68(6):2298–304. doi: 10.1016/s0003-4975(99)01055-3. [DOI] [PubMed] [Google Scholar]
- *16.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999 Apr 16;284(5413):489–93. doi: 10.1126/science.284.5413.489. The first evidence of functional artery engineered in vitro. [DOI] [PubMed] [Google Scholar]
- 17.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiov Sur. 1998 Mar;115(3):536–45. doi: 10.1016/S0022-5223(98)70315-0. [DOI] [PubMed] [Google Scholar]
- **18.Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. The New England journal of medicine. 2001 Feb 15;344(7):532–3. doi: 10.1056/NEJM200102153440717. Pioneering clinical tiral with TEVGs fabricated from autologus BM-MNCs for repair of congenital heart defect. [DOI] [PubMed] [Google Scholar]
- **19.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. The Journal of thoracic and cardiovascular surgery. 2005 Jun;129(6):1330–8. doi: 10.1016/j.jtcvs.2004.12.047. Midterm follow-up study of first clinical tiral with TEVGs fabricated from autologus BM-MNCs for repair of congenital heart defect. [DOI] [PubMed] [Google Scholar]
- 20.L'Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. The New England journal of medicine. 2007 Oct 4;357(14):1451–3. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 21.Olausson M, Patil PB, Kuna VK, Chougule P, Hernandez N, Methe K, et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet. 2012 Jul 21;380(9838):230–37. doi: 10.1016/S0140-6736(12)60633-3. [DOI] [PubMed] [Google Scholar]
- 22.Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L, L'Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. Journal of vascular surgery. 2014 Nov;60(5):1353–57. doi: 10.1016/j.jvs.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Sengupta D, Chien S. Vascular tissue engineering: from in vitro to in situ. Wiley interdisciplinary reviews Systems biology and medicine. 2014 Jan-Feb;6(1):61–76. doi: 10.1002/wsbm.1246. [DOI] [PubMed] [Google Scholar]
- 24.Bajpai VK, Andreadis ST. Stem cell sources for vascular tissue engineering and regeneration. Tissue engineering Part B, Reviews. 2012 Oct;18(5):405–25. doi: 10.1089/ten.teb.2011.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swartz DD, Andreadis ST. Animal models for vascular tissue-engineering. Curr Opin Biotech. 2013 Oct;24(5):916–25. doi: 10.1016/j.copbio.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011 Jan;32(3):714–22. doi: 10.1016/j.biomaterials.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syedain ZH, Meier LA, Lahti MT, Johnson SL, Tranquillo RT. Implantation of Completely Biological Engineered Grafts Following Decellularization into the Sheep Femoral Artery. Tissue Eng Pt A. 2014 Jun;20(11–12):1726–34. doi: 10.1089/ten.tea.2013.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaikh FM, Callanan A, Kavanagh EG, Burke PE, Grace PA, McGloughlin TM. Fibrin: a natural biodegradable scaffold in vascular tissue engineering. Cells, tissues, organs. 2008;188(4):333–46. doi: 10.1159/000139772. [DOI] [PubMed] [Google Scholar]
- 29.Wu W, Allen RA, Wang YD. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med. 2012 Jul;18(7):1148. doi: 10.1038/nm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tara S, Kurobe H, Maxfield MW, Rocco KA, Yi T, Naito Y, et al. Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft. Journal of vascular surgery. 2014 Apr 15; doi: 10.1016/j.jvs.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Kurobe H, Maxfield MW, Tara S, Rocco KA, Bagi PS, Yi T, et al. Development of small diameter nanofiber tissue engineered arterial grafts. PloS one. 2015;10(4):e0120328. doi: 10.1371/journal.pone.0120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials. 2007 Dec;28(34):5009–27. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Berger K, Wood SJ, Sauvage LR, Rao AM. Healing Of Arterial Prostheses In Man - Its Incompleteness. Annals of surgery. 1972;175(1):118. doi: 10.1097/00000658-197201000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krawiec JT, Vorp DA. Adult stem cell-based tissue engineered blood vessels: a review. Biomaterials. 2012 Apr;33(12):3388–400. doi: 10.1016/j.biomaterials.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Hu J, Sun X, Ma H, Xie C, Chen YE, Ma PX. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials. 2010 Nov;31(31):7971–7. doi: 10.1016/j.biomaterials.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou GD, et al. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials. 2008 Apr;29(10):1464–72. doi: 10.1016/j.biomaterials.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, et al. Morphologic and mechanical characteristics of engineered bovine arteries. Journal of vascular surgery. 2001 Mar;33(3):628–38. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- *38.Xie C, Hu J, Ma H, Zhang J, Chang LJ, Chen YE, et al. Three-dimensional growth of iPS cell-derived smooth muscle cells on nanofibrous scaffolds. Biomaterials. 2011 Jul;32(19):4369–75. doi: 10.1016/j.biomaterials.2011.02.049. Pioneering and fundamental works on applying pluripotent stem cells in fabrication of tissue-engineered vascular tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Hu J, Xie C, Ma H, Yang B, Ma PX, Chen YE. Construction of vascular tissues with macro-porous nano-fibrous scaffolds and smooth muscle cells enriched from differentiated embryonic stem cells. PloS one. 2012;7(4):e35580. doi: 10.1371/journal.pone.0035580. Pioneering and fundamental works on applying pluripotent stem cells in fabrication of tissue-engineered vascular tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *40.Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, et al. Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials. 2014 Oct;35(32):8960–9. doi: 10.1016/j.biomaterials.2014.07.011. Pioneering and fundamental works on applying pluripotent stem cells in fabrication of tissue-engineered vascular tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? The New England journal of medicine. 2003 Aug 7;349(6):570–82. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 42.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem cells. 2001;19(3):180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 43.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proceedings of the National Academy of Sciences of the United States of America. 2000 Mar 28;97(7):3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunology Letters. 2003;89(2–3):267–70. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 45.Caplan AI. Why are MSCs therapeutic? New data: new insight. The Journal of pathology. 2009 Jan;217(2):318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumura G, Miyagawa-Tomita S, Shin'oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003 Oct 7;108(14):1729–34. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 47.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. The Journal of thoracic and cardiovascular surgery. 2010 Feb;139(2):431–6. 36 e1–2. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 48.Patterson JT, Gilliland T, Maxfield MW, Church S, Naito Y, Shinoka T, et al. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regenerative medicine. 2012 May;7(3):409–19. doi: 10.2217/rme.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **49.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proceedings of the National Academy of Sciences of the United States of America. 2010 Mar 9;107(10):4669–74. doi: 10.1073/pnas.0911465107. Study demonstrates limited vascular differentiaiton of BM-MSCs during vascular remodeling of TEVGs in small animals and raises the debate on the role of BM-MSCs in fabricating TEVGs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziegelhoeffer T, Heil M, Voswinckel R, Fernandez B, Kostin S, Schaper W. Bone marrow derived stem cells do not differentiate into vascular cells during collateral artery growth in mice. Circulation. 2002 Nov 5;106(19):82–82. [Google Scholar]
- 51.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Nauheim B, Voswinckel R, et al. Bone marrow-derived cells do not incorporate in the adult growing vasculature. Circulation. 2003 Oct 28;108(17):222–23. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 52.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Littleevidencefordevelopmental plasticity of adult hematopoietic stem cells. Science. 2002 Sep 27;297(5590):2256–59. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 53.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002 Apr 4;416(6880):542–5. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003 Oct 30;425(6961):968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 55.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004 May;10(5):494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 56.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Annals of surgery. 2005 Mar;241(3):506–15. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho SW, Lim JE, Chu HS, Hyun HJ, Choi CY, Hwang KC, et al. Enhancement of in vivo endothelialization of tissue-engineered vascular grafts by granulocyte colony-stimulating factor. Journal of biomedical materials research Part A. 2006 Feb;76(2):252–63. doi: 10.1002/jbm.a.30535. [DOI] [PubMed] [Google Scholar]
- 58.Brennan MP, Dardik A, Hibino N, Roh JD, Nelson GN, Papademitris X, et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Annals of surgery. 2008 Sep;248(3):370–76. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 60.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. The international journal of biochemistry & cell biology. 2004 Apr;36(4):568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Liu JY, Peng HF, Gopinath S, Tian J, Andreadis ST. Derivation of Functional Smooth Muscle Cells from Multipotent Human Hair Follicle Mesenchymal Stem Cells. Tissue Eng Pt A. 2010 Aug;16(8):2553–64. doi: 10.1089/ten.tea.2009.0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbeck Arch Surg. 2011 Apr;396(4):489–97. doi: 10.1007/s00423-011-0762-2. [DOI] [PubMed] [Google Scholar]
- 63.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. Journal of cellular physiology. 2007 Nov;213(2):341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 64.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 65.Bieback K, Kern S, Kocaomer A, Ferlik K, Bugert P. Comparing mesenchymal stromal cells from different human tissues: bone marrow, adipose tissue and umbilical cord blood. Bio-medical materials and engineering. 2008;18(1 Suppl):S71–6. [PubMed] [Google Scholar]
- *66.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jul 17;104(29):11915–20. doi: 10.1073/pnas.0704581104. Study demonstreates limited vascular differentiation of BM-MSCs during vascular remodeling of TEVGs in small animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, Zhou J, Lu Q, Wei Y, Hu S. A novel small-diameter vascular graft: in vivo behavior of biodegradable three-layered tubular scaffolds. Biotechnology and bioengineering. 2008 Mar 1;99(4):1007–15. doi: 10.1002/bit.21629. [DOI] [PubMed] [Google Scholar]
- 68.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovascular research. 2007 Aug 1;75(3):618–28. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 69.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008 Jun;22(6):1635–48. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao YL, Zhang S, Zhou JY, Wang JL, Zhen MC, Liu Y, et al. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials. 2010 Jan;31(2):296–307. doi: 10.1016/j.biomaterials.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Mundada L, Johnson S, Wong J, Witt R, Ohye RG, et al. Characterization and Angiogenic Potential of Human Neonatal and Infant Thymus Mesenchymal Stromal Cells. Stem Cells Transl Med. 2015 Feb 23; doi: 10.5966/sctm.2014-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin ZB, Qian B, Yang YZ, Zhou K, Sun J, Mo XM, et al. Isolation, characterization and cardiac differentiation of human thymus tissue derived mesenchymal stromal cells. Journal of cellular biochemistry. 2014 Dec 23; doi: 10.1002/jcb.25072. [DOI] [PubMed] [Google Scholar]
- 73.Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, Heydarkhan S, Xu Y, Zuk PA, et al. Human adipose stem cells: a potential cell source for cardiovascular tissue engineering. Cells, tissues, organs. 2008;187(4):263–74. doi: 10.1159/000113407. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America. 2006 Aug 8;103(32):12167–72. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko TN, DiMuzio PJ. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. The Journal of surgical research. 2011 Jun 15;168(2):306–14. doi: 10.1016/j.jss.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y, et al. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials. 2010 Feb;31(4):621–30. doi: 10.1016/j.biomaterials.2009.09.086. [DOI] [PubMed] [Google Scholar]
- 77.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004 Feb 10;109(5):656–63. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 78.Cao Y, Sun Z, Liao LM, Meng Y, Han Q, Zhao RCH. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Bioph Res Co. 2005 Jul 1;332(2):370–79. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 79.Fischer LJ, McIlhenny S, Tulenko T, Golesorkhi N, Zhang P, Larson R, et al. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. The Journal of surgical research. 2009 Mar;152(1):157–66. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang P, Moudgill N, Hager E, Tarola N, Dimatteo C, McIlhenny S, et al. Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease. Stem Cells Dev. 2011 Jun;20(6):977–88. doi: 10.1089/scd.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang C, Yin S, Cen L, Liu QH, Liu W, Cao YL, et al. Differentiation of Adipose-Derived Stem Cells into Contractile Smooth Muscle Cells Induced by Transforming Growth Factor-beta 1 and Bone Morphogenetic Protein-4. Tissue Eng Pt A. 2010 Apr;16(4):1201–13. doi: 10.1089/ten.TEA.2009.0303. [DOI] [PubMed] [Google Scholar]
- 82.Johal KS, Lees VC, Reid AJ. Adipose-derived stem cells: selecting for translational success. Regenerative medicine. 2015 Jan;10(1):79–96. doi: 10.2217/rme.14.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoogduijn MJ, Gorjup E, Genever PG. Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev. 2006 Feb;15(1):49–60. doi: 10.1089/scd.2006.15.49. [DOI] [PubMed] [Google Scholar]
- 84.Liu JY, Peng HF, Andreadis ST. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovascular research. 2008 Jul 1;79(1):24–33. doi: 10.1093/cvr/cvn059. [DOI] [PubMed] [Google Scholar]
- 85.Liu X, Song L, Liu J, Wang S, Tan X, Bai X, et al. miR-18b inhibits TGF-beta1-induced differentiation of hair follicle stem cells into smooth muscle cells by targeting SMAD2. Biochem Biophys Res Commun. 2013 Aug 30;438(3):551–6. doi: 10.1016/j.bbrc.2013.07.090. [DOI] [PubMed] [Google Scholar]
- 86.Weber B, Kehl D, Bleul U, Behr L, Sammut S, Frese L, et al. In vitro fabrication of autologous living tissue-engineered vascular grafts based on prenatally harvested ovine amniotic fluid-derived stem cells. Journal of tissue engineering and regenerative medicine. 2013 Jul 24; doi: 10.1002/term.1781. [DOI] [PubMed] [Google Scholar]
- 87.Hibino N, Nalbandian A, Devine L, Martinez RS, McGillicuddy E, Yi T, et al. Comparison of human bone marrow mononuclear cell isolation methods for creating tissue-engineered vascular grafts: novel filter system versus traditional density centrifugation method. Tissue engineering Part C, Methods. 2011 Oct;17(10):993–8. doi: 10.1089/ten.tec.2011.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurobe H, Tara S, Maxfield MW, Rocco KA, Bagi PS, Yi T, et al. Comparison of the Biological Equivalence of Two Methods for Isolating Bone Marrow Mononuclear Cells for Fabricating Tissue-Engineered Vascular Grafts. Tissue engineering Part C, Methods. 2014 Nov 14; doi: 10.1089/ten.tec.2014.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beltrami AP, Cesselli D, Beltrami CA. At the stem of youth and health. Pharmacology & therapeutics. 2011 Jan;129(1):3–20. doi: 10.1016/j.pharmthera.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Madonna R, Renna FV, Cellini C, Cotellese R, Picardi N, Francomano F, et al. Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. European journal of clinical investigation. 2011 Feb;41(2):126–33. doi: 10.1111/j.1365-2362.2010.02384.x. [DOI] [PubMed] [Google Scholar]
- 91.Han J, Liu JY, Swartz DD, Andreadis ST. Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells. Cardiovascular research. 2010 Jul 1;87(1):147–55. doi: 10.1093/cvr/cvq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krawiec JT, Weinbaum JS, St Croix CM, Phillippi JA, Watkins SC, Rubin JP, et al. A Cautionary Tale for Autologous Vascular Tissue Engineering: Impact of Human Demographics on the Ability of Adipose-Derived Mesenchymal Stem Cells to Recruit and Differentiate into Smooth Muscle Cells. Tissue Eng Pt A. 2015 Feb 1;21(3–4):426–37. doi: 10.1089/ten.tea.2014.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem cells. 2014 Jul;32(7):1713–23. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- 94.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1981 Dec;78(12):7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 98.Okita K, Nakagawa M, Hong HJ, Ichisaka T, Yamanaka S. Generation of Mouse Induced Pluripotent Stem Cells Without Viral Vectors. Science. 2008 Nov 7;322(5903):949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 99.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007 Jul 19;448(7151):313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 100.Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells--opportunities for disease modelling and drug discovery. Nature reviews Drug discovery. 2011 Dec;10(12):915–29. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- 101.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012 Jan 19;481(7381):295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell stem cell. 2013 Jun 6;12(6):656–68. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 103.Chen YE. Vascular Cell Lineage Determination and Differentiation. Arterioscl Throm Vas. 2011 Jul;31(7):1467–68. doi: 10.1161/ATVBAHA.111.230813. [DOI] [PubMed] [Google Scholar]
- 104.Xie C, Ritchie RP, Huang H, Zhang J, Chen YE. Smooth muscle cell differentiation in vitro: models and underlying molecular mechanisms. Arteriosclerosis, thrombosis, and vascular biology. 2011 Jul;31(7):1485–94. doi: 10.1161/ATVBAHA.110.221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mack CP. Signaling Mechanisms That Regulate Smooth Muscle Cell Differentiation. Arterioscl Throm Vas. 2011 Jul;31(7):1495–505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hibino N, Duncan DR, Nalbandian A, Yi T, Qyang Y, Shinoka T, et al. Evaluation of the use of an induced puripotent stem cell sheet for the construction of tissue-engineered vascular grafts. J Thorac Cardiov Sur. 2012 Mar;143(3):696–703. doi: 10.1016/j.jtcvs.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscl Throm Vas. 2007 Jun;27(6):1248–58. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 108.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nature biotechnology. 2012 Feb;30(2):165–73. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su RJ, Baylink DJ, Neises A, Kiroyan JB, Meng X, Payne KJ, et al. Efficient generation of integration-free ips cells from human adult peripheral blood using BCL-XL together with Yamanaka factors. PloS one. 2013;8(5):e64496. doi: 10.1371/journal.pone.0064496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao N, Liang H, Huang J, Wang J, Chen Y, Chen Z, et al. Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell research. 2013 Sep;23(9):1119–32. doi: 10.1038/cr.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu J, Wang Y, Jiao J, Liu Z, Zhao C, Zhou Z, et al. Patient-specific cardiovascular progenitor cells derived from integration-free induced pluripotent stem cells for vascular tissue regeneration. Biomaterials. 2015 Sep 11;73:51–59. doi: 10.1016/j.biomaterials.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proceedings of the National Academy of Sciences of the United States of America. 2012 Aug 21;109(34):13793–98. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *113.Karamariti E, Margariti A, Winkler B, Wang XC, Hong XC, Baban D, et al. Smooth Muscle Cells Differentiated From Reprogrammed Embryonic Lung Fibroblasts Through DKK3 Signaling Are Potent for Tissue Engineering of Vascular Grafts. Circulation research. 2013 May 24;112(11):1433. doi: 10.1161/CIRCRESAHA.111.300415. PiPSCs-derived SMCs and ECs shortens fabrication time needed for TEVGs construction. [DOI] [PubMed] [Google Scholar]
- 114.Sundaram S, Echter A, Sivarapatna A, Qiu CH, Niklason L. Small-Diameter Vascular Graft Engineered Using Human Embryonic Stem Cell-Derived Mesenchymal Cells. Tissue Eng Pt A. 2014 Feb 1;20(3–4):740–50. doi: 10.1089/ten.tea.2012.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sundaram S, One J, Siewert J, Teodosescu S, Zhao LP, Dimitrievska S, et al. Tissue-Engineered Vascular Grafts Created From Human Induced Pluripotent Stem Cells. Stem Cell Transl Med. 2014 Dec;3(12):1535–43. doi: 10.5966/sctm.2014-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell stem cell. 2011 Jan 7;8(1):106–18. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011 Mar 3;471(7336):63–7. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nature methods. 2009 May;6(5):363–9. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou HY, Wu SL, Joo JY, Zhu SY, Han DW, Lin TX, et al. Generation of Induced Pluripotent Stem Cells Using Recombinant Proteins. Cell stem cell. 2009 May 8;4(5):381–84. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013 Aug 9;341(6146):651–4. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 121.Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, et al. A comparison of non-integrating reprogramming methods. Nature biotechnology. 2015 Jan;33(1):58–63. doi: 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell stem cell. 2010 Oct 8;7(4):521–31. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 123.Narva E, Autio R, Rahkonen N, Kong L, Harrison N, Kitsberg D, et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nature biotechnology. 2010 Apr;28(4):371–7. doi: 10.1038/nbt.1615. [DOI] [PubMed] [Google Scholar]
- 124.Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011 Mar 3;471(7336):58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 125.MacCarrick G, Black JH, 3rd, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genetics in medicine : official journal of the American College of Medical Genetics. 2014 Aug;16(8):576–87. doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Laer L, Dietz H, Loeys B. Loeys-Dietz Syndrome. Adv Exp Med Biol. 2014;802:95–105. doi: 10.1007/978-94-007-7893-1_7. [DOI] [PubMed] [Google Scholar]
- 127.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nature communications. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nguyen AT, Gomez D, Bell RD, Campbell JH, Clowes AW, Gabbiani G, et al. Smooth muscle cell plasticity: fact or fiction? Circulation research. 2013 Jan 4;112(1):17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell stem cell. 2015 Feb 5;16(2):119–34. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 130.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, et al. Readily available tissue-engineered vascular grafts. Science translational medicine. 2011 Feb 2;3(68):68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 131.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proceedings of the National Academy of Sciences of the United States of America. 2011 May 31;108(22):9214–19. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]