Summary
Delivery of proteins therapeutics to the CNS has proven to be a challenge to due to the presence of the blood-brain barrier (BBB), which prevents the passage of most proteins and large molecules from invading the neuronal space. Recently, we, and others, have developed technologies to circumvent this barrier by targeting receptors on the surface of the endothelial cells of the BBB to facilitate transport of therapeutic proteins. We describe here one such approach for targeting the LDLR by fusion of 38 amino acids from the ApoB protein to a therapeutic protein.
Keywords: Blood-brain barrier, transytosis, LDLR, ApoB, ApoE, protein transport, CNS delivery, therapeutics
1. Introduction
The blood-brain barrier (BBB) controls the passage of substances from the blood into the central nervous system. Thus, a major challenge for the delivery of protein therapeutics following intra-venous delivery is the transport of these large proteins to the CNS. The BBB is composed of tight junction forming endothelial cells, pericytes and astrocytes. This combination functions to allow only small molecules and directed transport by receptor-mediated trancytosis from the blood to the CNS.
The receptors expressed on the endothelial cells of the BBB function to capture proteins in the blood, endocytose the protein/ receptor complex and trancytose the complex to the neuronal side of the cells where the protein is released. Starzyk et al were the first to show that targeting a receptor on the blood-brain barrier could transport a “cargo” protein to the neuronal side of the BBB [1]. An antibody developed against the transferrin receptor expressed on the blood-brain barrier was able to transport methotrexate to the CNS. This same approach has been used to target the transport of proteins and peptides across the BBB efficiently [2,3]. Other well-characterized BBB receptors include: low-density lipoprotein receptor, insulin receptor and insulin growth factor receptor [4].
The low-density lipoprotein receptor family is a group of cell surface receptors that bind lipoprotein complexes for internalization to the lysosomes. The family comprises approximately ten different receptors with the most common examples being low-density lipoprotein receptor (LDLR), low-density lipoprotein related receptor (LRP), very-low density lipoprotein receptor (VLDL), megalin and apolipoprotein E receptor. The receptors are expressed in a tissue specific manner and primarily bind apolipoprotein complexes. The apolipoprotein, of which the two most prominent members are apolipoprotein B (ApoB) and apolipoprotein E (ApoE), function to bind lipids in the blood stream and target them for lysosomal degradation. Binding of the apolipoproteins to the receptor results in endocytosis and transport to the lysosome where the low pH compartment facilitates the release of the protein complex. The LDL receptor is then recycled to the cell surface. At the blood brain barrier, the LDL receptor binds lipoproteins resulting in endocytosis. Following endocytosis by the endothelial cells, a portion of the LDL receptor is shuttled to the apical side of the BBB where presumably, the apolipoprotein is released to be taken up by neurons and/ or astrocytes (reviewed in [5,6]).
We and others have identified receptor binding domains from Apolipoprotein B (38 amino acids) [7] and Apolipoprotein E (19 amino acids) [8,7] that can be readily fused to target proteins including antibodies. Addition of the LDLR binding domain significantly increased brain penetration of the cargo protein but also presented an unique cellular uptake and clearance mechanism in the brain for antibodies targeted to the CNS [9].
The ApoB sequence facilitates binding to and transport with the LDL receptor at the blood-brain barrier [7]. The LDLR receptor is also highly expressed in the liver and the spleen as well as blood circulating macrophages [5]. Single-dose administration of the ApoB-cargo protein results in significant accumulation in the liver as would be expected [7]. Previous investigation of LDLR targeted recombinant proteins has shown uptake in spleen and muscle [7]. In contrast, the lung does not express the LDLR, and we have not observed recombinant protein accumulation in this organ.
In this chapter we will describe the methods for cloning the ApoB LDLR binding domain to a cargo protein for transport to the CNS across the blood-brain barrier. The recombinant protein can then be expressed by a viral vector from a depot organ such as the liver [10,11,7] or as be delivered by i.v. or i.p. injection as a recombinant protein [12].
2. Materials
2.1 Oligonucleotides
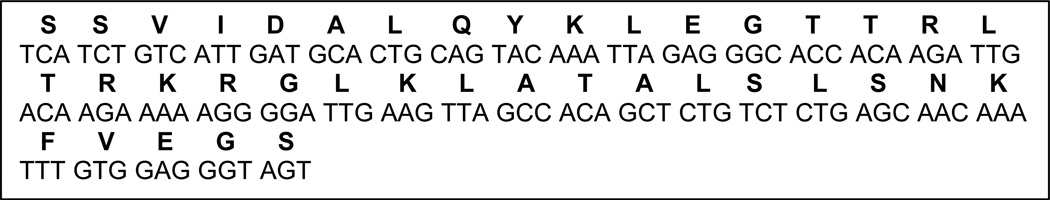
Oligonucleotides (Figure 1) can be ordered from any local vendor that is capable of synthesizing long oligonucleotides.
Order both complementary strands of the oligonucleotide including the necessary overhangs for your cloning restriction site (see Note 1), stop codon (see Note 2), and epitope tag (see Note 3).
To reduce the incorporation of errors in the long synthesis of the oligonucleotides, specify PAGE purification.
Specify 5’ phosphorylation on the oligonucleotide order. Alternatively, you can phosphorylate the oligonucleotides prior to the annealing step with T4 Kinase.
Figure 1.
ApoB LDLR binding domain oligonucleotides and amino acid sequence.
2.2 Transfection reagents
-
500ml 2XBBS
50ml 2.8M NaCl (Sigma, St. Louis, MO, USA)
50ml 500mM BES (Calbiochem, Darmstadt, Germany)
5ml 150mM Na2HPO4 (Sigma, St. Louis, MO, USA)
Adjust pH 6.95 with 1M NaOH
Filter sterilize through 0.22um filter
Store @ 4°C for 3–4 days prior to validation
-
10XCaCl2
3. Methods
3.1 Annealing oligonucleotides
Dilute oligonucleotides to 40μM in H2O.
-
Set up the following reaction in a thin-wall PCR tube.
1μl each oligonucleotide
2μl T4 ligase buffer (NEB Biolabs, Ipswich, MA, USA)
16μl H2O
Place in 95°C water bath (3L) for 2 minutes.
Turn off water bath and allow to cool to room temperature.
3.2 Cloning ApoB oligonucleotide
Dilute annealing reaction from above 1:2 with 20μl H2O.
Digest the expression plasmid containing your cargo gene with the appropriate restriction enzymes.
Run the restriction reaction on an agarose gel and isolate the vector. Clean the vector from the agarose before proceeding with the next step (Wizard SV Gel and PCR Clean Up, Promega, WI USA).
-
Set up the following reaction in 0.5ml tube.
50ng linearized vector
2μl phosphorylated annealed oligonucleotides from above reaction
1μl T4 ligase buffer
QS 9μl H2O
1μl T4 ligase (NEB Biolabs, Ipswich, MA, USA)
Incubate 16°C overnight.
-
Transform full ligation mix into chemically competent bacterial cells such as Top10.
Plate bacteria onto LB agar plates containing selection media
Be sure to include vector only control
-
The next day pick 3–5 colonies and grow up in LB broth containing selection media overnight.
Isolate the DNA from the clones
Sequence the DNA with an appropriate primer to verify the insertion of the ApoB oligonucleotide sequence
3.3 Transfecting
Fibroblast cells such as 293 (human embryonic kidney) or CHO (Chinese hamster ovary) have previously served to produce good quantities of protein.
Add 10μg of the final expression plasmid with the cloned ApoB LDLR binding domain along with H2O so the final volume including the CaCl2 of the next step equals 0.5ml for each 10cm dish of cells at 80% confluency.
Add CaCl2 at the dilution you established during validation (see Note 5).
Add 2xBBS to the solution (0.5ml for each 10cm dish).
Mix gently by inverting ~3–5 times, and incubate for 15 minutes at room temperature.
Add the DNA/ CaCl2 mix drop wise to the dish (1.0ml per 10cm dish). Spread DNA by carefully rocking the plate.
Place the cells @ 3% CO2 @ 37°C.
Change medium next morning, and incubate at 10% CO2.
Use complete media with serum or alternatively, use OptiMem (Life Technologies).
At pre-determined time the medium is collected and filtered through a 0.22μm filter.
3.4 Protein Purification
3.5 Virus Vector Production
For virus vector production, see one of the previously published protocols for details on packaging the vector construct [13].
Acknowledgments
This work supported by NIH grants AG18440, AG10435
Footnotes
Addition of unique restriction sites in the cargo gene will aid in cloning the ApoB peptide. Site directed mutagenesis could be achieved with the QuickChange Lightning Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA, USA).
Care should be taken to ensure that the ApoB LDLR binding domain remains in the same reading frame as the target protein. Additionally, a stop codon should be added at the end of the sequence if it is placed at the 3’ of the target protein.
Addition of an epitope tag is often desirable to differentiate endogenous protein from the targeted protein or to identify proteins with poor commercial antibodies. Addition of an epitope tag should be placed between the ApoB LDLR and the targeted protein so that the ApoB LDLR binding domain is always at the terminus of the protein.
Pre-wet the filter prior to filtering the CaCl2 solution to allow the high salt solution to pass through the membrane easier.
Check CaCl2 diluted 1:8, 1:10 and 1:12 in combination with 2XBBS in a transfection experiment with a GFP reporter such as pcDNA3-GFP (Addgene, Cambridge, MA, USA). Usually 293T’s in 10cm dishes are used for this. Using the combination that gives the best transfection results are recommended, which will also be useful for large-scale protein production later. Transfect a 10cm dish of 293T cell (70–80% confluent) with 20μg GFP reporter plasmid and check transfection efficiency next day. Should be at least 90–95% GFP+ cells. Aliquot 2XBBS store at 4°C.
A functional assay for the targeted protein is desirable to determine if addition of the ApoB LDLR targeting sequence alters or abolishes normal protein function.
The 38 amino acid ApoB LDLR binding domain is highly hydrophobic and could affect protein purification protocols. It may be advisable to consider this when designing the protein and to add a poly-histidine tag for purification purposes.
References
- 1.Friden PM, Walus LR, Musso GF, Taylor MA, Malfroy B, Starzyk RM. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc Natl Acad Sci U S A. 1991;88(11):4771–4775. doi: 10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin SU, Friden P, Moran M, Olson T, Kang YS, Pardridge WM, Morrison SL. Transferrin-antibody fusion proteins are effective in brain targeting. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(7):2820–2824. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boado RJ, Zhang Y, Zhang Y, Pardridge WM. Genetic engineering, expression, and activity of a fusion protein of a human neurotrophin and a molecular Trojan horse for delivery across the human blood-brain barrier. Biotechnology and bioengineering. 2007;97(6):1376–1386. doi: 10.1002/bit.21369. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge WM. Molecular biology of the blood-brain barrier. Mol Biotechnol. 2005;30(1):57–70. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- 5.Hussain MM, Strickland DK, Bakillah A. The mammalian low-density lipoprotein receptor family. Annu Rev Nutr. 1999;19:141–172. doi: 10.1146/annurev.nutr.19.1.141. [DOI] [PubMed] [Google Scholar]
- 6.Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev. 2001;46(1-3):247–279. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 7.Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bockenhoff A, Cramer S, Wolte P, Knieling S, Wohlenberg C, Gieselmann V, Galla HJ, Matzner U. Comparison of five Peptide vectors for improved brain delivery of the lysosomal enzyme arylsulfatase a. J Neurosci. 2014;34(9):3122–3129. doi: 10.1523/JNEUROSCI.4785-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer B, Emadi S, Desplats P, Michael S, Kosberg K, Shen J, Rockenstein E, Patrick C, Adame A, Gonzalez T, Sierks M, Masliah E. ESCRT mediated uptake and degradation of brain targeted α-synuclein-single chain antibody attenuates neuronal degeneration in vivo. 2014 doi: 10.1038/mt.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Spencer B, Emadi S, Desplats P, Eleuteri S, Michael S, Kosberg K, Shen J, Rockenstein E, Patrick C, Adame A, Gonzalez T, Sierks M, Masliah E. ESCRT-mediated uptake and degradation of brain-targeted alpha-synuclein single chain antibody attenuates neuronal degeneration in vivo. Mol Ther. 2014;22(10):1753–1767. doi: 10.1038/mt.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Spencer B, Marr RA, Gindi R, Potkar R, Michael S, Adame A, Rockenstein E, Verma IM, Masliah E. Peripheral delivery of a CNS targeted, metalo-protease reduces abeta toxicity in a mouse model of Alzheimer's disease. PLoS One. 2011;6(1):e16575. doi: 10.1371/journal.pone.0016575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer B, Verma I, Desplats P, Morvinski D, Rockenstein E, Adame A, Masliah E. A Neuroprotective Brain Penetrating Endopeptidase Fusion Protein Ameliorates Alzheimer's Disease Pathology and Restores Neurogenesis. J Biol Chem. 2014 doi: 10.1074/jbc.M114.557439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1(1):241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]