Abstract
Purpose
Signal transduction pathways influence lens growth, but little is known about the role(s) of the class 1A phosphoinositide 3-kinases (PI3Ks). To further investigate how signaling regulates lens growth, we generated and characterized mice in which the p110α and p110β catalytic subunits of PI3K were conditionally deleted in the mouse lens.
Methods
Floxed alleles of the catalytic subunits of PI3K were conditionally deleted in the lens by using MLR10-cre transgenic mice. Lenses of age-matched animals were dissected and photographed. Postnatal lenses were fixed, paraffin embedded, sectioned, and stained with hematoxylin-eosin. Cell proliferation was quantified by labeling S-phase cells in intact lenses with 5-ethynyl-2′-deoxyuridine. Protein kinase B (AKT) activation was examined by Western blotting.
Results
Lens-specific deletion of p110α resulted in a significant reduction of eye and lens size, without compromising lens clarity. Conditional knockout of p110β had no effect on lens size or clarity, and deletion of both the p110α and p110β subunits resulted in a phenotype that resembled the p110α single-knockout phenotype. Levels of activated AKT were decreased more in p110α- than in p110β-deficient lenses. A significant reduction in proliferating cells in the germinative zone was observed on postnatal day 0 in p110α knockout mice, which was temporally correlated with decreased lens volume.
Conclusions
These data suggest that the class 1A PI3K signaling pathway plays an important role in the regulation of lens size by influencing the extent and spatial location of cell proliferation in the perinatal period.
Keywords: cell proliferation, knockout animals, lens epithelium, phosphoinositide 3-kinase
Class I phosphoinositide 3-kinases (PI3Ks) are lipid kinases acting downstream of cell surface receptors to phosphorylate the 3′-hydroxyl group of phosphatidylinositol-(4,5)P2. The phosphatidylinositol-(3,4,5)P3 (PIP3) that is generated activates additional signaling pathways to regulate cell growth, proliferation, motility, and survival.1 PI3Ks are grouped into different classes based on substrate specificity and sequence homology.2 The well-characterized class IA enzymes are heterodimers composed of an 85-kDa regulatory subunit and a 110-kDa catalytic subunit. Catalytic subunits p110α and p110β are widely expressed, and both of these isoforms respond to input from receptor tyrosine kinases, whereas p110β also responds to input from G protein-coupled receptors.3 Both of the isoforms generate PIP3, whose most prominent biological function is activation of the AKT signaling pathway.4–7 Somatic mutations of p110α are frequently linked to human cancer, underscoring the importance of this signaling cascade in the regulation of cell proliferation and survival.1 In addition, the interplay between PI3K and AKT is central to the regulation of many cellular processes in a wide variety of tissues, including the ocular lens.8–10
The lens is composed of a monolayer of epithelial cells that cover the anterior surface and fiber cells that differentiate from epithelial precursors, filling the lens core.11 Cell proliferation drives lens growth and occurs predominantly in a ring of epithelial cells near the lens equator known as the germinative zone.12–14 It has been well documented that lens growth and development are strongly influenced by growth factor signaling. One of the best examples is that of the fibroblast growth factors and fibroblast growth factor receptors (FGFRs) that regulate lens induction, epithelial cell proliferation, and fiber differentiation.15–17 FGFRs are receptor tyrosine kinases that stimulate the mitogen-activated protein kinase (MAPK, or Ras-Raf-Mek-ErK) or the PI3K- AKT intracellular signaling pathways to regulate proliferation, differentiation, and cell survival.8,18,19 The roles of various components of the MAPK pathway have been extensively studied in the lens, often by genetic dissection, using transgenic mice.20–26 In contrast, exploration of the role(s) played by individual components of the PI3K-AKT branch of the intracellular signaling pathway has trailed behind. Two recent studies examined the lens-specific deletion of PTEN, the lipid phosphatase that negatively regulates AKT activity and antagonizes PI3K signaling by dephosphorylation of PIP3.27,28 In both cases, loss of PTEN resulted in significantly elevated levels of phosphorylated AKT, which had different consequences depending upon the developmental timing of tissue-specific deletion.29,30 When PTEN was deleted at the lens placode stage, elevated AKT rescued the cell death phenotype caused by knockout of the FGFR2 alone.30 In contrast, when PTEN was deleted at the lens vesicle stage, elevated AKT inhibited Na+/K+-ATPase activity, ultimately leading to lens cataract and rupture.29
To date, the roles of different catalytic subunits of class 1A PI3Ks have not been extensively studied in the lens. Complete knockout of the catalytic subunits p110α and p110β in mice resulted in embryonic lethality.31,32 To distinguish between different sources of stimulation for PI3K activity and elucidate nonredundant biological roles of these two PI3Ks in the lens, we generated lens-specific conditional knockouts of both of the enzymes alone or in combination. Loss of p110α but not p110β resulted in significantly reduced eye and lens growth that stemmed from an acute reduction in magnitude and altered spatial organization of epithelial cell proliferation on postnatal day 0 (P0). These findings show that the PI3K signaling pathway plays an important role in the regulation of lens size by influencing the extent and spatial location of cell proliferation in the perinatal period.
Methods
Generation of Knockout Mice
To generate lens-specific knockouts of the PI3K catalytic subunits p110αflox/flox and p110βflox/flox, animals (in a mixed C57BL/6/129Sv genetic background)33 were interbred with MLR10-Cre mice (in a FVB/N genetic background).34 Genotypes were verified by PCR of tail DNA, and littermate controls were used for all experiments. All animal experiments used male and female mice and conformed to the ARVO statement for the use of animals in ophthalmic and vision research and were approved by the Stony Brook University IACUC.
Growth Analysis and Lens Photography
Age-matched littermate animals between 1 and 24 weeks old were weighed. After they were euthanized by CO2 inhalation, their eyes were dissected, weighed, and transferred to a Petri dish containing 37°C Tyrode solution on a warm stage. Lenses were dissected and photographed using a model SZX16 dissecting microscope equipped with a digital camera (Olympus, Waltham, MA, USA). Lens diameters were measured and used to calculate lens volume, assuming a spherical shape.35
Western Blotting
Lenses were dissected from eyes and transferred to Tyrode solution. The lens capsule was then peeled away from the fiber cell mass by using fine forceps. For Western blotting, capsules were transferred to 3× sample buffer, separated by SDS-PAGE, and transferred to nitrocellulose membranes. Blots were probed using rabbit monoclonal antibodies against PI3K p110α or p110β; mouse monoclonal antibodies against serine 473 phospho-Akt (Cell Signaling Technology, Danvers, MA, USA); or rabbit polyclonal antibodies against total AKT1/2/3 (Santa Cruz Biotechnology, Dallas, TX, USA). Peroxidase-conjugated goat anti-rabbit (Jackson ImmunoResearch Labs, West Grove, PA, USA), or sheep anti-mouse (GE Healthcare, Pittsburgh, PA, USA) secondary antibodies were used prior to enhanced chemoluminescence detection. Band densities from independent blots were quantified using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).36
Histology
Mouse eyes were dissected and fixed in a 4% formaldehyde solution in PBS for 16 to 24 hours at room temperature. Fixed eyes were rinsed with PBS, dehydrated using an ethanol series, and embedded in paraffin. Sections of 2 to 3 μm were cut on a diamond knife, deparaffinized, and stained with hematoxylin-eosin. Histological sections were viewed using a model BX51 microscope and photographed with a DP72 digital camera (Olympus).
5-Ethynyl-2′-Deoxyuridine Staining
Pregnant females or neonatal pups were injected with 50 μg/g 5-ethynyl-2′-deoxyuridine (EdU; Click-iT; Thermo Fisher Scientific, Waltham, MA, USA). Embryonic lenses were dissected 4 hours afterward, and postnatal lenses were dissected 2 hours after EdU injection, and were then photographed and fixed in a 4% formaldehyde in PBS for 1 hour. Lens permeabilization and EdU staining were performed according to the manufacturer's instructions and published protocols.37 Fluorescing z-stack images were obtained using an Axiovert 200M microscope (Zeiss, Thornwood, NY, USA), and flattened. Image postprocessing and statistical analysis were performed using ImageJ or MATLAB software (MathWorks, Portola Valley, CA, USA). Images were converted to grayscale by using the rgb2gray function of MATLAB, and background noise hue was removed. For whole-lens analysis, a computational mask was created by thresholding the fluorescing region, and the total amount of fluorescence signal was quantified for each lens. For line-scan analysis, six lenses of each genotype at each age were measured across the lens diameter with the plot profile function in ImageJ software. Diameters were normalized within genotypes at each age, and mean ± standard error (SE) values were plotted.
Results
Loss of p110α but not p110β reduced lens and eye size. Male and female mice with lens-specific knockout of either the p110α or the p110β catalytic subunit of PI3K exhibited normal overall growth (Fig. 1A). However, p110α knockout animals had noticeably smaller eyes throughout life than their control littermates. Dissecting and weighing the eyes showed that at all postnatal ages tested, p110α knockout eyes were ∼20% smaller by wet weight than wild-type eyes (P < 0.05, Student's t-test) (Fig. 1B). This difference was accompanied by a ∼25% reduction in lens volume in p110α knockout animals (P < 0.05). Deletion of p110β did not affect lens (or eye) size, and double deletion of p110α and p110β did not cause reductions greater than that of knockout of p110α alone (Fig. 1C). These data suggested that the activity of p110α but not p110β is required by the lens to achieve normal ocular growth.
Figure 1.
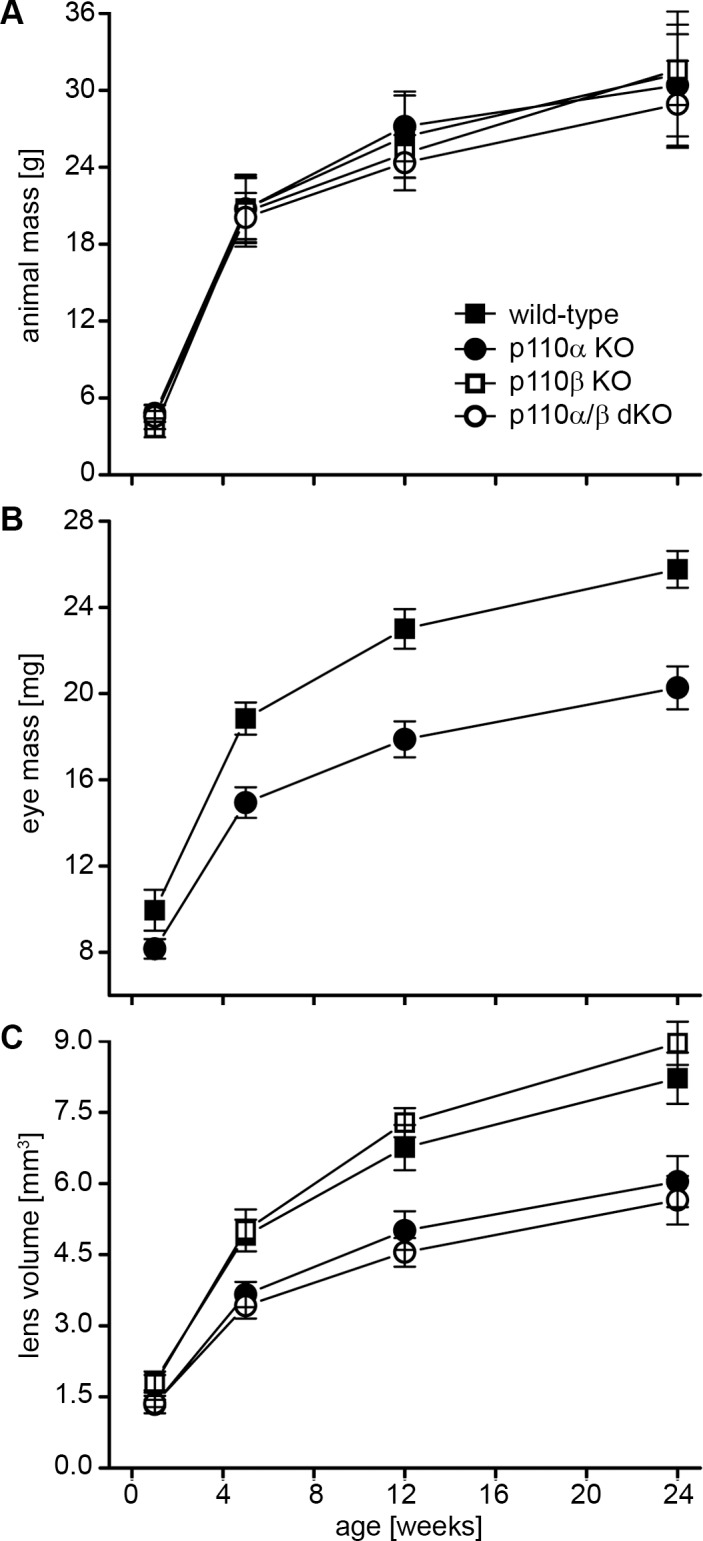
Deletion of p110α significantly reduced sizes of eyes and lenses. (A). There were no significant differences in overall body sizes among wild-type, p110α KO, p110β KO, and p110α/p110β double-KO animals up to 24 weeks of age (P > 0.05). (B) In contrast, eye mass was reduced 18% at 1 week and 22% at 12 weeks in p110α KO animals compared to that in wild-type littermates (P < 0.05). (C) Plotting lens volume versus age for all knockouts showed that p110α KO lenses were 27% smaller at 1 week and 26% smaller at 12 weeks than those of wild-type animals (P < 0.05). p110β KO lenses were similar to those of wild-type mice at all ages examined, and the size of p110α/p110β double-KO lenses resembled those of the p110α single-KOs. Data are mean ± SD; n = 11–46 animals of each genotype at each time point.
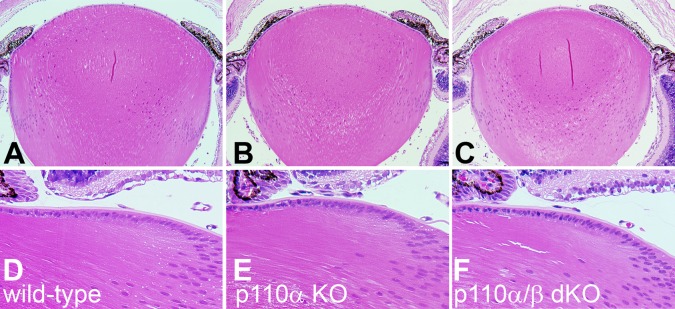
Loss of PI3K did not affect lens clarity or structure. Although loss of p110α reduced lens and eye size, conditional deletion of p110α or p110β alone or in combination did not disturb lens clarity. All lenses from PI3K single- and double-knockout animals between 1 and 24 weeks of age were transparent and free of cataract (Fig. 2). This was corroborated by histological analysis of PI3K single- and double-knockout lenses. Eyes from 1-week-old mice were dissected, fixed, sectioned, and stained with hematoxylin-eosin. Sagittal sections through the central region of wild-type lenses appeared normal (Fig. 3A), as did sections from p110α knockout (Fig. 3B) and p110α and p110β double-knockout lenses (Fig. 3C). Higher-magnification views of sagittal sections from mice of all genotypes showed the normal differentiation of lens fibers from equatorial epithelial cells (Fig. 3D–F). Thus, deletion of p110α and/or p110β from the lens failed to disrupt lens morphology or transparency.
Figure 2.
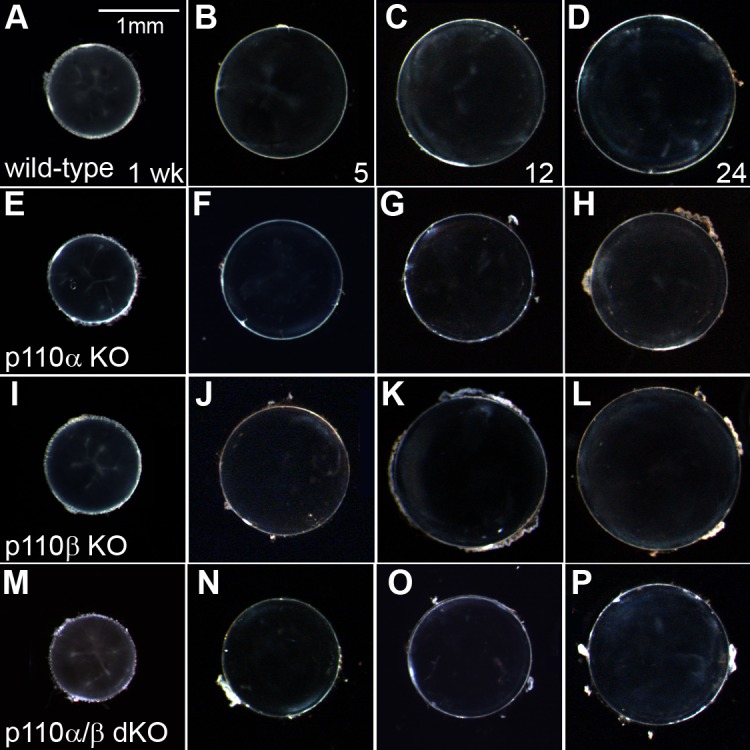
Loss of PI3K did not affect lens clarity. (A–D) Dissected wild-type lenses remained transparent for 1 to 24 weeks. (E–H) p110α KO. (I–L) p110β KO, and (M–P) p110α/p110β double-KO lenses also remained clear and free of cataracts throughout the time period examined, although p110α KO and p110α/p110β double-KO lenses were noticeably smaller than those of wild-type or p110β KO mice. Scale bar: (A): 1 mm.
Figure 3.
PI3K knockout lenses lacked histological abnormalities. (A) On P7, sagittal sections through the central region of wild-type lenses appeared normal (200×). (B) p110α KO and (C) p110α/p110β double-KO lenses also lacked any structural anomalies, except they were smaller than those of wild-type mice. (D–F) Higher magnification (400×) views showed normal differentiation of lens fibers from equatorial epithelial cells in all three genotypes of lenses.
Phosphorylated AKT levels were differentially reduced in p110α and p110β knockout lenses. To determine how PI3K signaling was affected in p110α and p110β knockout lenses, epithelial cell extracts were probed using Western blot analysis. Wild-type epithelial cells expressed both the p110α (Fig. 4A) and the p110β (Fig. 4B) catalytic subunits of PI3K, which were absent in cells from the respective conditional knockout mice. Levels of phosphorylated AKT, the principal downstream effector of PI3K signaling, were reduced in both p110α and p110β knockout epithelial cells compared to those of wild-type cells, and total AKT levels were not affected by p110α or p110β deletion. Quantitation of band densities (Fig. 4C) showed that, compared to wild-type lenses, the phospho-AKT-to-total AKT ratios were reduced 63% in the p110α knockouts and 30% in the p110β knockouts (P < 0.05). These data implied that the loss of PI3K catalytic activity resulted in differentially reduced levels of phospho-AKT, which in the case of the p110α subunit, produced a reduction of ∼25% in lens volume.
Figure 4.
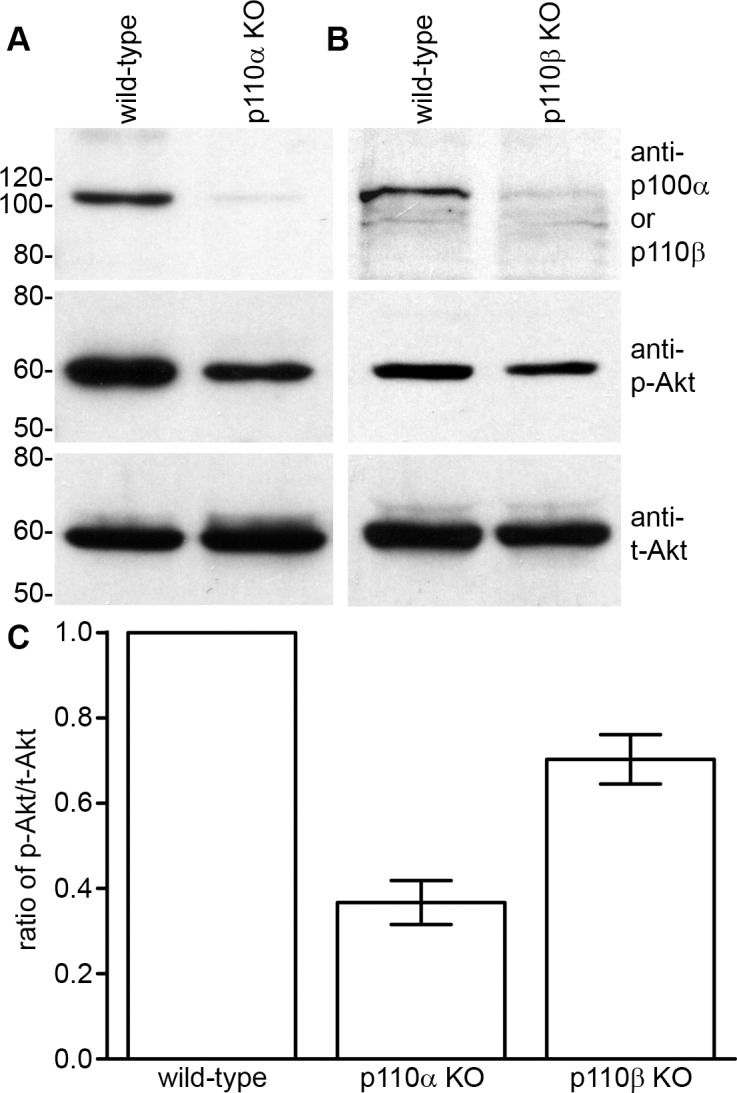
AKT signaling was reduced in PI3K KO lenses. (A) Wild-type lens epithelial cells expressed p110α, which was absent in KO mice. (B) Wild-type lenses also expressed p110β, which was lacking in lenses from KO mice. Levels of phosphorylated AKT were reduced in both p110α and p110β KO epithelial cells. Total AKT levels were similar in both wild-type and PI3K KO animals. (C) Independent blots (n = 3) were quantitated and plotted. Phospho-AKT-to-total AKT ratios were reduced 63% in p110α and 30% in p110β KO epithelial cells (P < 0.05). Data are mean ± SE and are derived from 2-week-old mice.
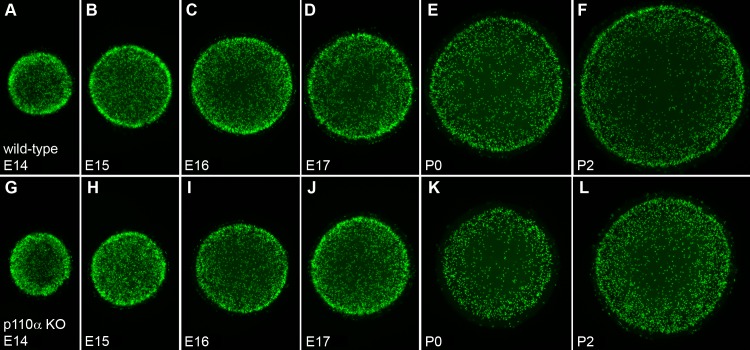
The pattern of mitosis in p110α knockout lenses was altered on P0. The smaller lenses observed in p110α knockout mice could have resulted from abnormal epithelial cell mitosis in the embryonic or postnatal period, as has been observed for other mouse knockout models with microphthalmia,38,39 or was due to an increased level of apoptosis. TUNEL staining, or immunostaining for cleaved caspase 3, showed that any apoptosis was very difficult to detect in both the wild-type and the p110α-deficient lenses, with no increase observed in knockout animals (data not shown). To examine whether loss of p110α influenced the magnitude or pattern of mitosis, lenses were labeled with EdU between embryonic day 14 (E14) and P2. From E14 through E17, both the wild-type (Fig. 5A–D) and the knockout (Fig. 5G–J) lenses displayed similar patterns of robust EdU labeling, with the highest level of fluorescence in the germinative zone near the lens equator as previously reported.40,41 On P0, wild-type lenses continued to show the greatest level of proliferation at the equator (Fig. 5E), whereas the p110α knockout lenses lacked the ring of increased labeling at the equator and showed a more homogeneous pattern of EdU incorporation (Fig. 5K). By P2, both the wild-type (Fig. 5F) and the knockout (Fig. 5L) lenses once again displayed the greatest levels of proliferation in the equatorial germinative zone. To quantify this transient change in mitotic pattern, line scans were performed using EdU-labeled images from wild-type and p110α knockout lenses on P0 (Fig. 6A) and P2 (Fig. 6B), and the mean (±SE) values were plotted against the position along the lens diameter. On P0, wild-type lenses had clear peaks of fluorescence intensity near the equator. In contrast, p110α knockout lenses lacked the equatorial peaks and had maximum fluorescence values 41% lower than those in wild-type lenses (P < 0.05) lenses. On P2, both the wild-type and the knockout lenses displayed clear peaks of EdU signal in the equatorial germinative zone, and the mean value of maximum fluorescence in p110α-deficient lenses was only 12% lower than those in wild-type lenses (P > 0.05). These data suggested that the significant reduction in lens size following p110α deletion could be caused by a transient loss of proliferating epithelial cells in the germinative zone on P0.
Figure 5.
Distribution of proliferating cells is altered in p110α KO lenses. (A–F) Wild-type lenses displayed a characteristic pattern of EdU labeling between E14 and P2, with the greatest proliferation observed in the circumferential germinative zone near the lens equator. (G–J) Between E14 and E17, p110α KO lenses had a pattern of proliferation similar to that of wild-type littermates. (K) On P0, EdU staining in the circumferential germinative zone was greatly reduced in p110α KO mice. (L) On P2, the characteristic pattern of EdU labeling was restored in p110α KO lenses.
Figure 6.
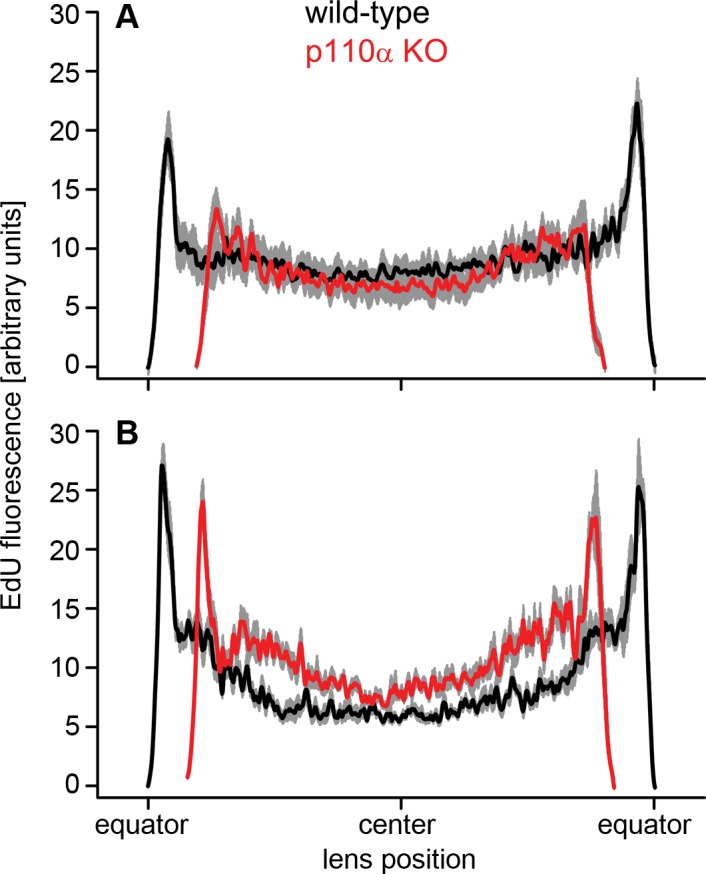
Quantification of germinative zone staining on P0 and P2. (A) On P0, p110α knockout lenses (red line) had peak fluorescent intensity values near the equator that were 41% lower than those in wild-type (black line [P < 0.05]). (B) By P2, there were clear peaks of EdU labeling near the equator of p110α-deficient lenses, and the maximum fluorescence was only 12% lower than that in wild-type (P > 0.05). Data are mean ± SE (gray lines) and aligned at the center of the lens. The diameters of p110α KO lenses were 11% to 12% smaller on P0 and P2. n = 6 lenses of each genotype at each time point.
Reduced cell division in p110α knockout lenses on P0 was temporally correlated with decreased lens volume. To quantify global changes in proliferation between E14 and P2, total EdU fluorescence was plotted for wild-type and p110α knockout mice (Fig. 7A). Between E14 and E16, levels of EdU fluorescence were slightly lower in the p110α-deficient lenses; however, on P0, total EdU fluorescence in knockout lenses was maximally reduced by 38% compared to that in wild-type. On P2, EdU fluorescence levels had partially recovered in knockout lenses to a level that was 22% less than that in wild-type lenses. To relate cell proliferation to organ growth, lens volumes were calculated over the same time period (Fig. 7B). Similar to the EdU fluorescence data, volumes of p110α-deficient lenses were slightly less than those of wild-type lenses between E14 and E17. The greatest differences in volumes were observed at P0, where p110α knockout lenses were 32% smaller than wild-type lenses (P < 0.05), which correlated with the maximum reduction in EdU fluorescence also observed at this time point. At P2, p110α-deficient lenses remained 29% smaller than wild-type lenses, consistent with the reduction in lens volume of ∼25% observed throughout life in the conditional knockout animals (Fig.1C). These data implied that a discrete perinatal event, where the population of germinative zone epithelial cells failed to proliferate on P0, was a major contributor to a permanently reduced lens size in adult p110α knockout mice.
Figure 7.
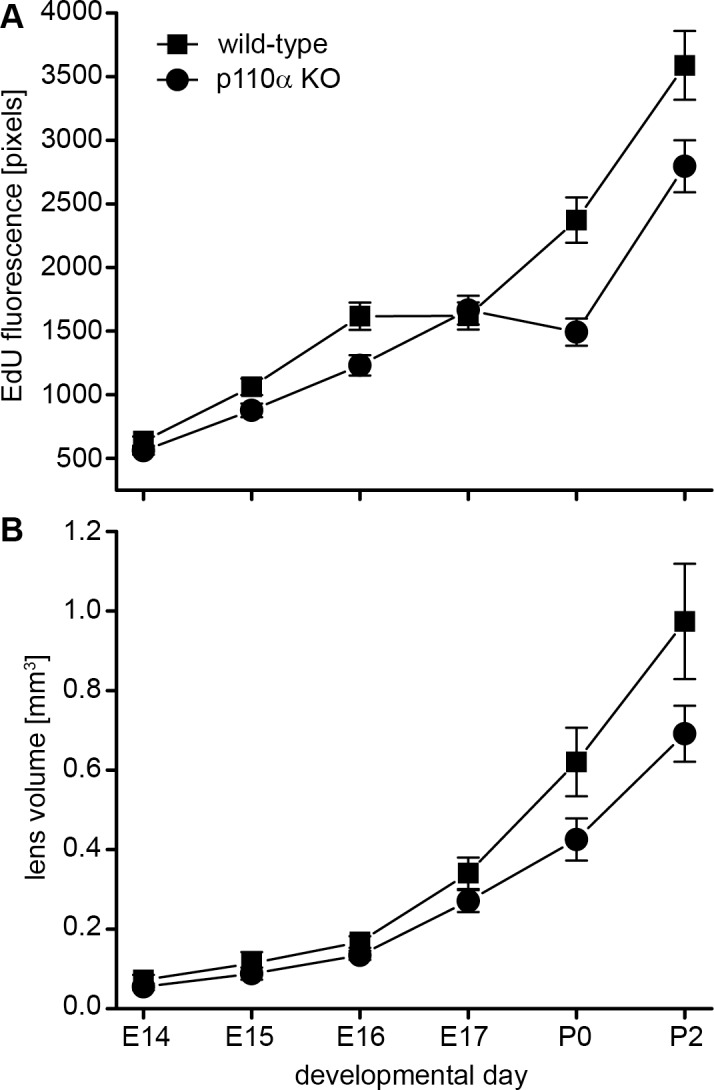
Failure of germinative zone epithelial cells to proliferate on P0 in p110α knockout mice reduced lens volume. (A) Between E14 and E17, total EdU fluorescence levels for wild-type were similar to those for p110α KO lenses. On P0, EdU fluorescence was reduced 38% in p110α animals compared to that in wild-type mice. By P2, EdU fluorescence levels had partially recovered in p110α KO lenses, to a level 22% less than that in wild-type mice. (B) Calculation of lens volumes showed the greatest differences were observed on P0, when p110α KO lenses were 32% smaller than those of wild-type mice (P < 0.05). Data are mean ± SD. n = 8–23 lenses of each genotype at each time point.
Discussion
We generated and characterized mice with lens-specific deletions of the p110α and p110β catalytic subunits of PI3K. Loss of p110β partially reduced levels of phosphorylated AKT in mouse lenses but failed to noticeably alter lens growth, clarity, or development. Knockout of p110α more substantially reduced phosphorylated AKT levels, in addition to producing a discrete growth defect on P0 that resulted in reduced lens size throughout life. This same phenotype was observed whether p110α alone was deleted or was deleted in combination with p110β, and was caused by a transient reduction in the number of proliferating lens epithelial cells present in the germinative zone during the first day of life.
The process of rodent lens growth undergoes a transition at approximately P0, the time where we observed a significant decrease in dividing epithelial cells in p110α knockout lenses. During the preceding embryonic period, the lens diameter increases at a constant linear rate, and the lens volume grows as a smooth exponential.42,43 In the early postnatal period, the growth of the lens becomes oscillatory in a manner that corresponds to the timing of the epithelial cell cycle, and that can be mimicked in organ culture by the pulsatile administration of growth factors.44,45 Our data showed that, on E17 and P2, the germinative zone was clearly present in p110α knockout and wild-type lenses but transiently vanished in p110α-deficient mice at approximately P0. This suggests that both the embryonic constant growth and the postnatal pulsatile growth mechanisms produce maximum cell division in the germinative zone and that this does not require the activity of p110α. In contrast, the transitional period between these two growth mechanisms was sensitive to the loss of p110α activity but not p110β. The precise mechanism whereby PI3K activity is required for the preservation of normal germinative zone proliferation around P0 is currently not known.
Significant reductions in lens size have been linked to discrete changes in epithelial cell proliferation in other mouse lens knockout models. Deletion of Cx50 or its functional replacement with Cx46 resulted in mouse lenses that were significantly smaller than those of wild-type mice and specifically failed to achieve a postnatal pulse of epithelial cell proliferation between P2 and P3.35,38,39,46 Interestingly, Cx50 has the greatest level of functional activity in epithelial cells in the early postnatal period when lens mitosis switches to the pulsatile growth pattern, and Cx50 conductance can be upregulated by p110α activity.39,47 Thus, one possibility is the absence of p110α results in a delay in the upregulation of Cx50 gap junction channels in epithelial cells at P0, producing a transient decrease in germinative zone proliferation. The availability of a lens-specific p110α knockout mouse model will allow future testing of this hypothesis through interbreeding with Cx50-deficient mice to test for functional epistasis.
Acknowledgments
Supported by US National Institutes of Health Grant EY013163 (TWW).
Disclosure: C. Sellitto, None; L. Li, None; E. Vaghefi, None; P.J. Donaldson, None; R.Z. Lin, None; T.W. White, None
References
- 1. Ilic N,, Roberts TM. Comparing the roles of the p110alpha and p110beta isoforms of PI3K in signaling and cancer. Curr Top Microbiol Immunol. 2010; 347: 55–77. [DOI] [PubMed] [Google Scholar]
- 2. Domin J,, Waterfield MD. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997; 410: 91–95. [DOI] [PubMed] [Google Scholar]
- 3. Vanhaesebroeck B,, Guillermet-Guibert J,, Graupera M,, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010; 11: 329–341. [DOI] [PubMed] [Google Scholar]
- 4. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002; 296: 1655–1657. [DOI] [PubMed] [Google Scholar]
- 5. Engelman JA,, Luo J,, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006; 7: 606–619. [DOI] [PubMed] [Google Scholar]
- 6. Fruman DA,, Meyers RE,, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998; 67: 481–507. [DOI] [PubMed] [Google Scholar]
- 7. Hawkins PT,, Anderson KE,, Davidson K,, Stephens LR. Signalling through class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006; 34: 647–662. [DOI] [PubMed] [Google Scholar]
- 8. Martinez G,, de Iongh RU. The lens epithelium in ocular health and disease. Int J Biochem Cell Biol. 2010; 42: 1945–1963. [DOI] [PubMed] [Google Scholar]
- 9. Teo ZL,, McQueen-Miscamble L,, Turner K,, et al. Integrin linked kinase (ILK) is required for lens epithelial cell survival, proliferation and differentiation. Exp Eye Res. 2014; 121: 130–142. [DOI] [PubMed] [Google Scholar]
- 10. Weber GF,, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci. 2006; 47: 4490–4499. [DOI] [PubMed] [Google Scholar]
- 11. Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981; 19: 134–153. [DOI] [PubMed] [Google Scholar]
- 12. McAvoy JW,, Chamberlain CG,, de Iongh RU,, Hales AM,, Lovicu FJ. Lens development. Eye (Lond). 1999; 13 (Pt 3b): 425–437. [DOI] [PubMed] [Google Scholar]
- 13. McAvoy JW. Cell division cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J Embryol Exp Morphol. 1978; 45: 271–281. [PubMed] [Google Scholar]
- 14. Sikic H,, Shi Y,, Lubura S,, Bassnett S. A stochastic model of eye lens growth. J Theor Biol. 2015; 376: 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lovicu FJ,, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001; 128: 5075–5084. [DOI] [PubMed] [Google Scholar]
- 16. Garcia CM,, Yu K,, Zhao H,, et al. Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev Dyn. 2005; 233: 516–527. [DOI] [PubMed] [Google Scholar]
- 17. Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006; 17: 726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Q,, Stump R,, McAvoy JW,, Lovicu FJ. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp Eye Res. 2009; 88: 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iyengar L,, Patkunanathan B,, Lynch OT,, McAvoy JW,, Rasko JE,, Lovicu FJ. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp Eye Res. 2006; 83: 667–678. [DOI] [PubMed] [Google Scholar]
- 20. Xie L,, Overbeek PA,, Reneker LW. Ras signaling is essential for lens cell proliferation and lens growth during development. Dev Biol. 2006; 298: 403–414. [DOI] [PubMed] [Google Scholar]
- 21. Upadhya D,, Ogata M,, Reneker LW. MAPK1 is required for establishing the pattern of cell proliferation and for cell survival during lens development. Development. 2013; 140: 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gong X,, Wang X,, Han J,, Niesman I,, Huang Q,, Horwitz J. Development of cataractous macrophthalmia in mice expressing an active MEK1 in the lens. Invest Ophthalmol Vis Sci. 2001; 42: 539–548. [PubMed] [Google Scholar]
- 23. Shakespeare TI,, Sellitto C,, Li L,, et al. Interaction between Connexin50 and mitogen-activated protein kinase signaling in lens homeostasis. Mol Biol Cell. 2009; 20: 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le AC,, Musil LS. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J Cell Biol. 2001; 154: 197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le AC,, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001; 233: 394–411. [DOI] [PubMed] [Google Scholar]
- 26. Iyengar L,, Wang Q,, Rasko JE,, McAvoy JW,, Lovicu FJ. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation. 2007; 75: 662–668. [DOI] [PubMed] [Google Scholar]
- 27. Cantley LC,, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999; 96: 4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waite KA,, Eng C. Protean PTEN: form and function. Am J Hum Genet. 2002; 70: 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sellitto C,, Li L,, Gao J,, et al. AKT activation promotes PTEN hamartoma tumor syndrome-associated cataract development. J Clin Invest. 2013; 123: 5401–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chaffee BR,, Hoang TV,, Leonard MR,, et al. FGFR and PTEN signaling interact during lens development to regulate cell survival. Dev Biol. 2016; 410: 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bi L,, Okabe I,, Bernard DJ,, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002; 13: 169–172. [DOI] [PubMed] [Google Scholar]
- 32. Bi L,, Okabe I,, Bernard DJ,, Wynshaw-Boris A,, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999; 274: 10963–10968. [DOI] [PubMed] [Google Scholar]
- 33. Lu Z,, Jiang YP,, Wang W,, et al. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation. 2009; 120: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao H,, Yang Y,, Rizo CM,, Overbeek PA,, Robinson ML. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci. 2004; 45: 1930–1939. [DOI] [PubMed] [Google Scholar]
- 35. White TW,, Goodenough DA,, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998; 143: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider CA,, Rasband WS,, Eliceiri KWNIH. Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wiley LA,, Shui YB,, Beebe DC. Visualizing lens epithelial cell proliferation in whole lenses. Mol Vis. 2010; 16: 1253–1259. [PMC free article] [PubMed] [Google Scholar]
- 38. Sellitto C,, Li L,, White TW. Connexin50 is essential for normal postnatal lens cell proliferation. Invest Ophthalmol Vis Sci. 2004; 45: 3196–3202. [DOI] [PubMed] [Google Scholar]
- 39. White TW,, Gao Y,, Li L,, Sellitto C,, Srinivas M. Optimal lens epithelial cell proliferation is dependent on the connexin isoform providing gap junctional coupling. Invest Ophthalmol Vis Sci. 2007; 48: 5630–5637. [DOI] [PubMed] [Google Scholar]
- 40. Shi Y,, De Maria A,, Lubura S,, Sikic H,, Bassnett S. The penny pusher: a cellular model of lens growth. Invest Ophthalmol Vis Sci. 2015; 56: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harding CV,, Hughes WL,, Bond VP,, Schork P. Autoradiographic localization of tritiated thymidine in wholemount preparations of lens epithelium. Arch Ophthalmol. 1960; 63: 58–65. [DOI] [PubMed] [Google Scholar]
- 42. Foster FS,, Zhang M,, Duckett AS,, Cucevic V,, Pavlin CJ. In vivo imaging of embryonic development in the mouse eye by ultrasound biomicroscopy. Invest Ophthalmol Vis Sci. 2003; 44: 2361–2366. [DOI] [PubMed] [Google Scholar]
- 43. Mu J,, Slevin JC,, Qu D,, McCormick S,, Adamson SL. In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound. Reprod Biol Endocrinol. 2008; 6: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brewitt B,, Clark JI. Growth and transparency in the lens, an epithelial tissue, stimulated by pulses of PDGF. Science. 1988; 242: 777–779. [DOI] [PubMed] [Google Scholar]
- 45. Brewitt B,, Teller DC,, Clark JI. Periods of oscillatory growth in developing ocular lens correspond with cell cycle times. J Cell Physiol. 1992; 150: 586–592. [DOI] [PubMed] [Google Scholar]
- 46. White TW. Unique and redundant connexin contributions to lens development. Science. 2002; 295: 319–320. [DOI] [PubMed] [Google Scholar]
- 47. Martinez JM,, Wang HZ,, Lin RZ,, Brink PR,, White TW. Differential regulation of Connexin50 and Connexin46 by PI3K signaling. FEBS Lett. 2015; 589: 1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]