Abstract
We employed field potential recordings in extended in vitro brain slices form Sprague-Dawley rats containing the piriform and entorhinal cortices (PC and EC, respectively) to identify the characteristics of epileptiform discharges and concomitant high-frequency oscillations (HFOs, ripples: 80–200 Hz, fast ripples: 250–500 Hz) during bath application of 4-aminopyridine (4AP, 50 μM). Ictal-like discharges occurred in PC and EC either synchronously or independently of each other; synchronous ictal discharges always emerged from a synchronous “fast” interictal background whereas asynchronous ictal discharges were preceded by a “slow” interictal event. In addition, asynchronous ictal discharges had longer duration and interval of occurrence than synchronous ictal discharges, and contained a higher proportion of ripples and fast ripples. Cutting the connections between PC and EC made synchronicity disappear and increased ictal discharges duration in the EC but failed in changing HFO occurrence in both areas. Finally, antagonizing ionotropic glutamatergic receptors abolished ictal activity in all experiments, increased the duration and rate of occurrence of interictal discharges occurring in PC-EC interconnected slices while it did not influence the slow asynchronous interictal discharges in both areas. Our results identify some novel in vitro interactions between olfactory (PC) and limbic (EC) structures that presumably contribute to in vivo ictogenesis as well.
Keywords: 4-aminopyridine, entorhinal cortex, epilepsy, high-frequency oscillations, piriform cortex
INTRODUCTION
Neuronal synchronization, which represents the coordinated activity of neuronal networks in one or more areas of the brain, supports physiological states such as cognitive functions or sleep, and it is mirrored by identifiable electroencephalography (EEG) patterns (Buzsáki and Draguhn, 2004; Niedermeyer and Lopes da Silva, 2005). Excessive neuronal synchronization is, however, the hallmark of epileptic discharges recorded in the EEG of both patients and animal models, and includes interictal and ictal activity along with high-frequency oscillations (HFOs, 80–500 Hz) (Avoli and de Curtis, 2011; Jefferys et al., 2012). Cellular, pharmacological and molecular studies performed over the last few decades have led to remarkable progresses in identifying the mechanisms underlying epileptiform synchronization (Noebels et al., 2012), and much of this evidence was obtained by employing in vitro brain slices comprising the hippocampus proper and parahippocampal structures such as the entorhinal cortex (EC) or the amygdala (Avoli and de Curtis, 2011; Avoli, 2014); these areas belong to the limbic system that is closely involved in the pathophysiogenesis of mesial temporal lobe epilepsy (Gloor, 1997).
Epileptiform synchronization during 4-aminopyridine (4AP) treatment has also been studied in the in vitro isolated guinea-pig brain. Epileptiform activity in this preparation is characterized by peculiar electrographic patterns that depend on whether recordings are obtained from areas of the limbic (i.e., EC or hippocampus) or of the olfactory system such as the piriform cortex (PC) (Carriero et al., 2010; Uva et al., 2013). The PC is a seizure–prone region (Piredda and Gale, 1985; Hoffman and Haberly, 1991) that sends projections to limbic structures and it is thus involved in generating limbic seizures (Demir et al., 1998, 2001). Specifically, it was shown in the in vitro isolated guinea-pig brain that the PC generates runs of fast activity along with relatively short-lasting ictal discharges that propagate to EC while prolonged ictal discharges originate from the hippocampus or EC, and do not spread to PC. However, two subsequent in vitro studies (Panuccio et al., 2012; Herrington et al., 2014), performed in coronal, sagittal or horizontal rat brain slices, have shown that during 4AP treatment the PC generates interictal and ictal discharges with features that are undistinguishable from those previously recorded from limbic neuronal structures such as the EC, the perirhinal cortex or the amygdala (Avoli and de Curtis, 2011). These findings have led us to further analyze with field potential recordings the patterns of interictal and ictal discharge as well as the HFOs occurring in adult rat horizontal brain slice preparation that included both PC and EC neuronal networks during 4AP application.
EXPERIMENTAL PROCEDURES
Brain slice preparation and maintenance
Male, adult Sprague-Dawley rats (250–275 g) were decapitated under isoflurane anesthesia according to the procedures established by the Canadian Council of Animal Care. The brain was quickly removed and placed in cold (1–3 °C), oxygenated artificial cerebrospinal fluid (ACSF) with the following composition (mM): 124 NaCl, 2 KCl, 2 CaCl2, 2 MgSO4, 1.25 KH2PO4, 26 NaHCO3, 10 D-glucose. Horizontal brain slices (450 μm thick) containing PC and EC were cut from this brain block using a vibratome. Slices were then transferred to an interface tissue chamber where they were superfused with ACSF and humidified gas (95% O2, 5% CO2) at a temperature of 31–32 °C and a pH of 7.4. 4AP (50 μM) was bath applied. Chemicals were acquired from Sigma–Aldrich Canada (Oakville, Ontario, Canada). Surgical separation of EC and PC from the hippocampus proper (Fig. 4A) or cutting of the Schaffer collaterals were accomplished under visual control with a razor blade that was mounted on a micromanipulator (Barbarosie and Avoli, 1997).
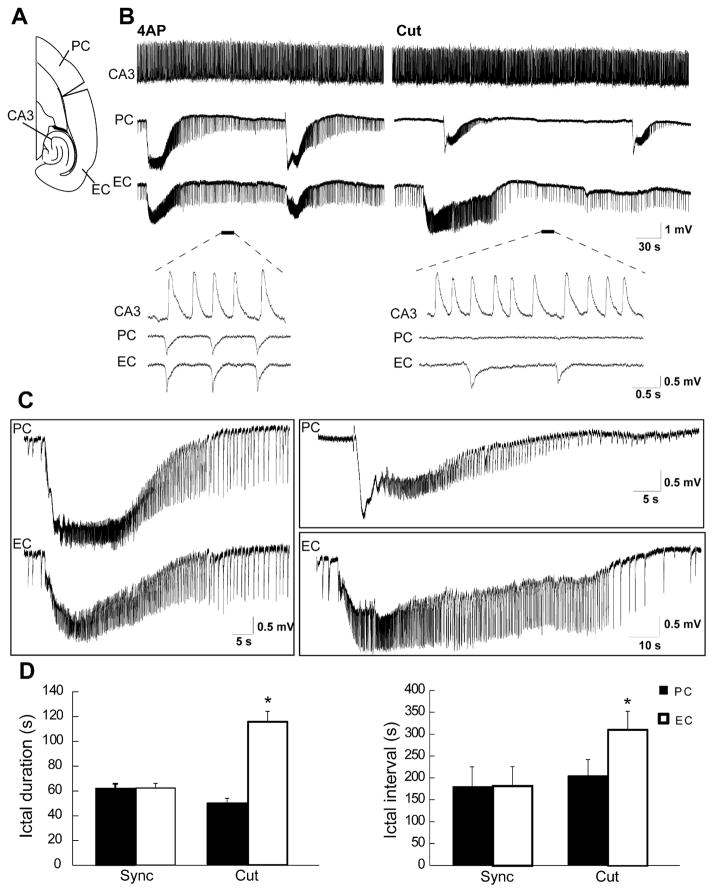
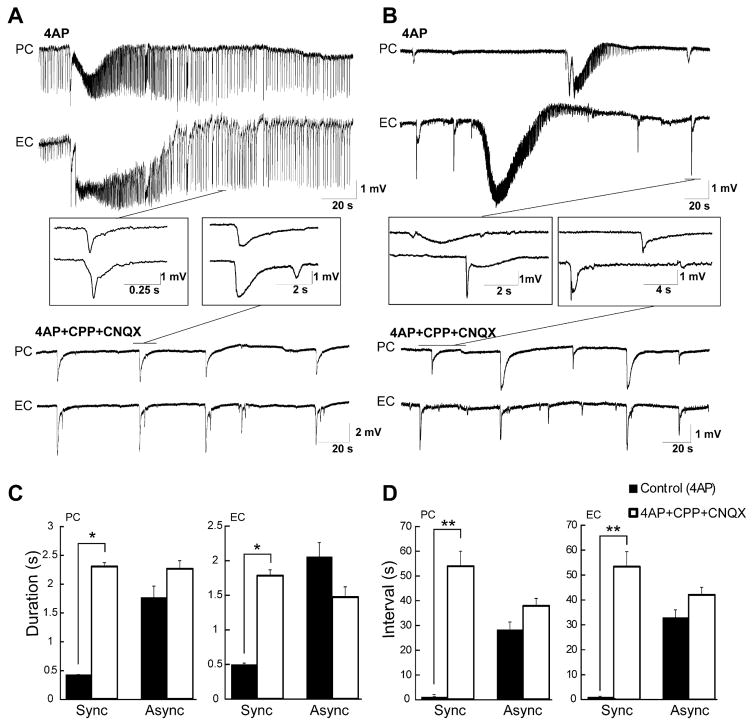
Fig. 4.
(A) Schematic drawing of a horizontal slice containing CA3, PC and EC showing the cut between PC and EC. (B) Field potential recordings obtained from these three areas during application of 4AP before and after removing the connection between PC and EC. Note that before the cut, interictal and ictal discharges recorded in this experiment are synchronous in the PC and EC while independent interictal activity is recorded from the CA3 area. (C) Enlarged portion of the ictal discharges recorded before and after removing the connection between PC and EC shown in B. (D) Bar graphs showing the average duration and interval of ictal discharges recorded from the PC and EC before and after the cut. Note that the cut did not change the duration and interval of occurrence of ictal discharges in PC while increasing them in the EC. *p<0.001.
Electrophysiological recordings
Field potential recordings were obtained with ACSF-filled, glass pipettes (1B150F-4; World Precision Instruments, Sarasota, FL, USA; tip diameter <10 μm, resistance 5–10 MΩ) that were connected to high-impedance amplifiers. The recording electrodes were positioned in the posterior PC and in the deep layers of the lateral EC (Fig. 1A). Field potential signals were sampled at 5 KHz and were fed to a computer interface (Digidata 1322A, Molecular Devices, Palo Alto, CA, USA), acquired and stored using the PCLAMP 9.2 software (Molecular Devices). Subsequent data analyses were performed with CLAMPFIT 9.2 (Molecular Devices).
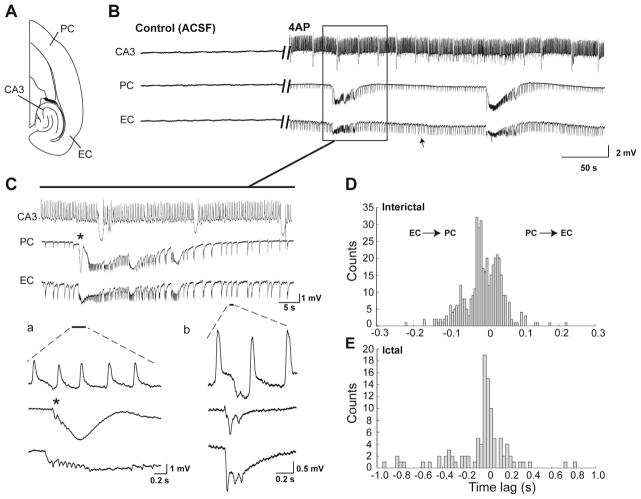
Fig. 1.
(A) Schematic drawing of a horizontal slice containing CA3, PC and EC. (B) Field potential recordings obtained from these three areas during normal ACSF and application of 4AP. Note that the interictal and ictal discharges recorded in this experiment are synchronous in the PC and EC while independent interictal activity is recorded from the CA3 area. (C) Enlarged portion of the recordings shown in (B) with traces that are further expanded at the start of an ictal discharge and during interictal activity. (D and E) Histograms showing the distribution of delays between PC and EC for interictal (D) and ictal (E) discharges. Events occurring in the PC were used as temporal reference (i.e., time 0). Note that interictal and ictal discharges could initiate in PC or EC in any given experiment.
Detection of high-frequency oscillatory events
Time-periods containing ictal and interictal discharges recorded from the PC and EC were extracted. To identify oscillations in each frequency range (80–200 Hz and 250–500 Hz), a multi-parametric algorithm was employed using routines based on standardized functions (Matlab Signal Processing Toolbox). Raw field potential recordings were first band-pass filtered in the 80–200-Hz and in the 250–500-Hz frequency range using a Finite Impulse Response (FIR) filter. Zero-phase digital filtering was used to avoid phase distortion. A 10-s artifact-free period was selected as a reference for signal normalization. In this way, field potential recordings were normalized using their own reference period (Salami et al., 2012).
To be considered as HFOs, oscillatory events in each frequency range had to show at least four consecutive cycles, having amplitude of 3 SD above the mean of the reference period. The time lag between two consecutive cycles had to be between 5 and 12.5 ms for ripples (80–200 Hz) and between 2 and 4 ms for fast ripples (250–500 Hz). Ripples were kept for analysis only if they were visible in the 80–200-Hz range, whereas fast ripples were kept only if they were visible in the 250–500-Hz range. Oscillatory events containing overlapping ripples and fast ripples were excluded from the analysis (Bénar et al., 2010).
Statistical analysis
We used CLAMPFIT 9.2 (Molecular Devices) for off-line analysis of the duration and interval of occurrence of ictal and interictal discharges. The duration of both interictal and ictal discharges were defined as the time between the first deflection of the discharge from baseline to its return to baseline. The interval of occurrence of both interictal and ictal discharges was defined as the time between the onsets of two consecutive discharges. Since the kurtosis and skewness measures showed that values were not normally distributed, we transformed the raw data to Z-scores and performed One-way ANOVAs followed by Tukey post hoc tests to identify differences between experimental conditions regarding the rate of occurrence and duration of interictal and ictal discharges. Similar statistical tests were used to compare rates of interictal discharges associated to ripples and fast ripples and rates of ripples and fast ripples during ictal discharges.
When analyzing the dynamics of HFO occurrence, in order to account for differences in duration, ictal discharges were first transformed into a time scale from 0 (start of the ictal event) to 100 (end of the ictal event). The ictal period was then divided into three equal parts and rates of ripples and fast ripples in each region (PC and EC) were compared using non-parametric Wilcoxon signed rank tests followed by Bonferroni–Holm corrections for multiple comparisons. This allowed us to evaluate if ripples or fast ripples predominated at specific moments of the ictal event in each region. Statistical tests were performed in Matlab R2013a (Matworks, Natick, MA, USA) and the level of significance was set at p<0.05. Results are expressed as mean±standard error of mean (mean ±SEM) and n indicates the number of slices used for analysis.
RESULTS
Epileptiform activity induced by 4AP in piriform and entorhinal cortices
Both interictal (arrows in Fig. 1B) and ictal (solid line in Fig. 1B) discharges occurred during 4AP application in the PC and EC of “intact” brain slices (n=47 experiments). Simultaneous field potential recordings from PC and EC revealed that in 19 of 47 brain slices interictal and ictal discharges occurred synchronously in both structures (Fig. 1B). Interictal discharges in these synchronous experiments recurred every 1.5±0.06 s (mean±SEM) and lasted 0.74±0.02 s. These interictal discharges were shorter and more frequent than interictal discharges recorded in the asynchronous experiments (see below); in addition, they were often characterized by polyspike features. Ictal discharges recorded from these slices were characterized by sudden onset that emerged from the pattern of continuous interictal discharge. The duration of these ictal discharges was 66.8±4.5 s, and they recurred every 117.9±12.4 s (Fig. 1C). In any given experiment, interictal and ictal discharges could initiate from either PC or EC as indicated by the time lag histograms shown in Fig. 1D, E.
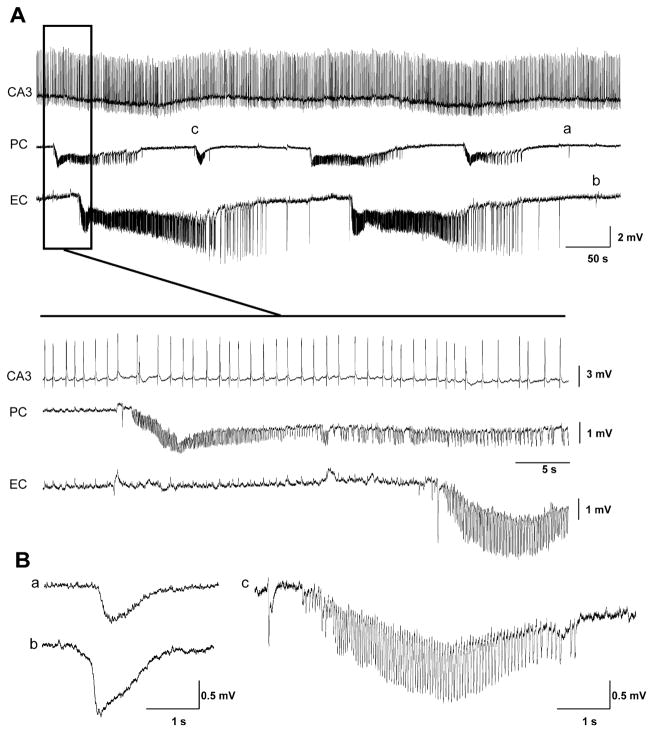
In the remaining experiments (n=28 slices), epileptiform activity occurred independently in PC and EC (Fig. 2A). Interictal discharges were characterized by slow spikes in both areas (Fig. 2Ba and b), occurred every 9.2±2.0 s and lasted 1.2±0.1 s in the PC whereas they occurred every 21.3±1.44 s and had duration of 2.5±0.13 s in the EC. Ictal discharges recorded from PC and EC in these experiments also had different durations and intervals of occurrence. In the PC, ictal discharges occurred every 112.8±12 s and lasted 72.3±7.6 s while in the EC they recurred every 234.6±25.3 s and lasted 125.9±8 s. In the PC, 79% (87 out of 110 events) of the ictal discharges recorded in synchronous and asynchronous experiments initiated with a slow, negative going, event (asterisk in Fig. 1C); a similar event was identified in only 3 out of 91 of ictal discharges in the EC. Runs of fast activity at 10–15 Hz, similar to those reported in the in vitro isolated guinea-pig brain (Carriero et al., 2010), were only occasionally recorded from the PC in these experiments (Fig. 2Cc).
Fig. 2.
(A) Field potential recordings obtained from CA3, PC and EC during application of 4AP in a brain slice that generated asynchronous slow interictal and ictal discharges in the PC and EC. Note that the interictal activity which is recorded from the CA3 area did not propagate to the PC and EC. Note that ictal discharges are asynchronous in PC and EC. (B) Expanded samples of slow interictal discharges recorded in PC and EC (a and b, respectively) as well as of a fast run in PC (c).
Simultaneous recordings from the PC, EC and CA3 always showed that interictal activity generated by CA3 networks (occurring at intervals of 1.92±0.38 s) could not be recorded in PC and EC (n=16 synchronous and 21 asynchronous experiments). Hence, we presumed that the hippocampus proper was functionally disconnected from the PC and EC. To further test the validity of this assumption we surgically cut in 10 experiments the Schaffer collaterals (Barbarosie and Avoli, 1997) to further ensure that CA3-driven output activity did not influence the excitability of PC and EC networks. The characteristics of interictal and ictal discharges generated by PC and EC following the cut in these experiments (n=3 and 7 slices generating synchronous and asynchronous epileptiform activity, respectively) were similar to those obtained from the 37 slices in which both PC and EC appeared to be functionally disconnected from the hippocampus (data not shown).
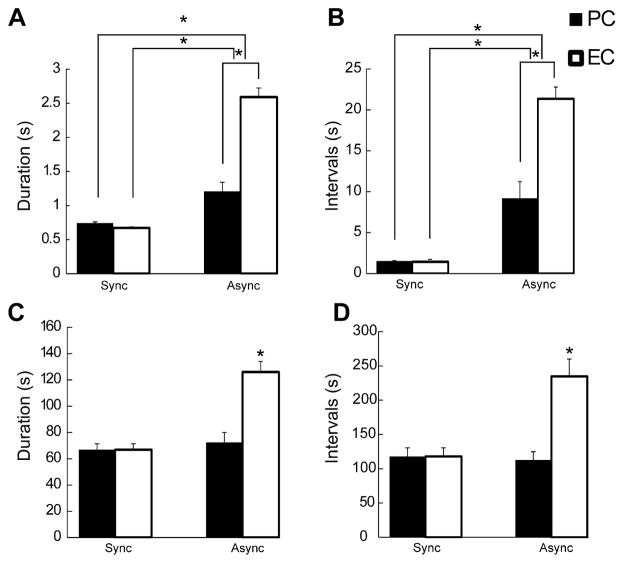
As illustrated in Fig. 3A, the duration of slow interictal discharges in brain slices generating asynchronous epileptiform activity was significantly (p<0.0001) longer than that seen with fast spiking interictal discharges in the synchronous experiments; in addition, in asynchronous experiments, the duration of interictal discharges was longer in EC than in PC (p<0.0001). Also, intervals of occurrence of the slow interictal discharges were significantly (p<0.0001) higher as compared with those of fast spiking synchronous interictal discharges (Fig. 3B). Finally, we found in those slices generating asynchronous epileptiform activity that the interval of occurrence of the slow interictal was significantly (p<0.0001) higher in the EC when compared to the PC (Fig. 3B).
Fig. 3.
(A and B) Bar graphs showing the average duration (A) and interval of occurrence (B) of interictal discharges recorded from the PC and EC in 47 experiments. Note that duration and interval of occurrence of interictal discharges are significantly longer in the EC as compared to PC in asynchronous experiments. Note also that duration and interval of occurrence of interictal discharges are significantly higher in asynchronous experiment when compared to synchronous experiments in both regions. (C and D) Bar graphs showing the average duration (C) and interval of occurrence (D) of ictal discharges recorded from the PC and EC. Note that duration and interval of occurrence of ictal discharges are significantly longer in the EC as compared to PC in asynchronous experiment and EC in synchronous experiments. *p<0.0001.
Next we compared the duration and interval of occurrence of ictal discharges in PC and EC that were recorded from slices generating synchronous or asynchronous epileptiform discharges. The duration of ictal discharges was similar in synchronous experiments whereas in asynchronous experiments duration of ictal discharges was higher in the EC (Fig. 3C, p < 0.0001); in addition, the duration of the synchronous and asynchronous ictal discharges recorded from the PC was not different but synchronous ictal discharges generated by the EC were significantly shorter when compared to those occurring asynchronously (Fig. 3C, p < 0.0001). Also, the intervals of occurrence of ictal discharges in the EC in the asynchronous experiments were significantly (p <0.0001) higher than in synchronous experiment (Fig. 3D, p <0.0001). Finally, ictal discharges in EC had a longer interval of occurrence than those recorded in PC in the asynchronous experiments (Fig. 3D).
Lesioning piriform–entorhinal cortex connectivity replicates the patterns of asynchronous epileptiform activity
The synchronicity of the epileptiform discharges recorded from PC and EC in 19 out of 47 experiments presumably reflected preservation of connections between these two structures. Therefore, in five experiments in which synchronous epileptiform discharges occurred under control conditions, we surgically disconnected the PC from EC (Fig. 4A). Such procedure led to the independent occurrence of ictal discharges in both areas (Fig. 4B). After the cut, the duration of the ictal discharges in the PC did not change while those occurring in the EC always increased in length (n=5; Fig. 4C, p<0.001). In addition, the interval of occurrence of the ictal discharges in PC did not change after the cut while it increased in EC (n=5, p<0.001). Interestingly, cutting the connections between PC and EC abolished interictal discharges in the PC in all experiments but did not change the duration and rate of occurrence of interictal discharges in the EC (Fig. 4B). In these experiments as well interictal activity generated by CA3 networks occurred at intervals of 1.23±0.05 s and did not propagate to the EC or PC (n=5; Fig. 4B). Durations and intervals of occurrence of discharges recorded in PC and EC under control conditions and following the cut are summarized in Fig. 4D.
Isolated interictal activity recorded during ionotropic glutamatergic receptor antagonism
Previous studies (cf., Avoli and de Curtis, 2011) have shown that the slow interictal discharges recorded in the presence of 4AP continue to occur during blockade of ionotropic glutamatergic transmission while ictal discharges are readily abolished. Hence, we compared the effects induced by concomitant application of the glutamatergic receptor antagonists CPP and CNQX on the epileptiform activity recorded from brain slices that generated either synchronous or asynchronous epileptiform activity. As illustrated in Fig. 5A, B, this pharmacological procedure abolished ictal discharges in both experimental groups but influenced interictal discharges depending on the type of brain slice. Those generating asynchronous, slow interictal activity continued to do so in both PC and EC at similar frequency and duration (n=8). In contrast, interictal discharges occurring synchronously were increased in duration (p<0.001) and were reduced in the rate of occurrence (p<0.0001) in both PC and EC (n=7). Thus, during application of glutamatergic receptor antagonists both types of brain slices could generate similar interictal spikes although they continued to be characterized by either synchronous or asynchronous features according to what was seen under control conditions. These data are summarized in Fig. 5C, D.
Fig. 5.
(A) Concomitant application of CNQX and CPP blocks ictal discharges that occur synchronously during synchronous interictal discharges. It also increases the duration and the interval of occurrence of the synchronous interictal discharges. (B) The same pharmacological procedure abolishes asynchronous ictal discharges while slow interictal discharges continue to occur with characteristics similar to those seen under control (4AP) conditions. (C and D) Quantification of the effects induced by CNQX+CPP on the two types of interictal discharge. Note that the duration and the interval of occurrence of the slow interictal discharges recorded from asynchronous experiments under control conditions are longer than synchronous interictal discharges observed in synchronous experiments. *p<0.001, *p<0.0001.
HFOs in piriform and entorhinal cortices during 4AP application
Finally, we analyzed the occurrence of HFOs in both ripple (80–200 Hz) and fast ripple (250–500 Hz) frequency range that were associated to interictal and ictal discharges recorded from the PC and EC during 4AP application. Ripples and fast ripples were observed during both synchronous and asynchronous interictal discharges in the PC and EC (Fig. 6A, B). As illustrated in Fig. 6C, the rate of occurrence of ripples and fast ripples related to frequently occurring synchronous interictal discharges were not different in PC and EC; however in both structures, the rate of occurrence of fast ripples was significantly higher than that of ripples during the slow interictal discharges (Fig. 6D, p<0.01). In addition, ripples and fast ripples occurred in both PC and EC more often during slow interictal discharges (30% of 95 events, n=10 slices) than during synchronous (fast) interictal discharges (10% of 557 events, n=6 slices); this difference, however, did not reach statistical significance. In both regions, HFO occurrence outside of interictal discharges was negligible.
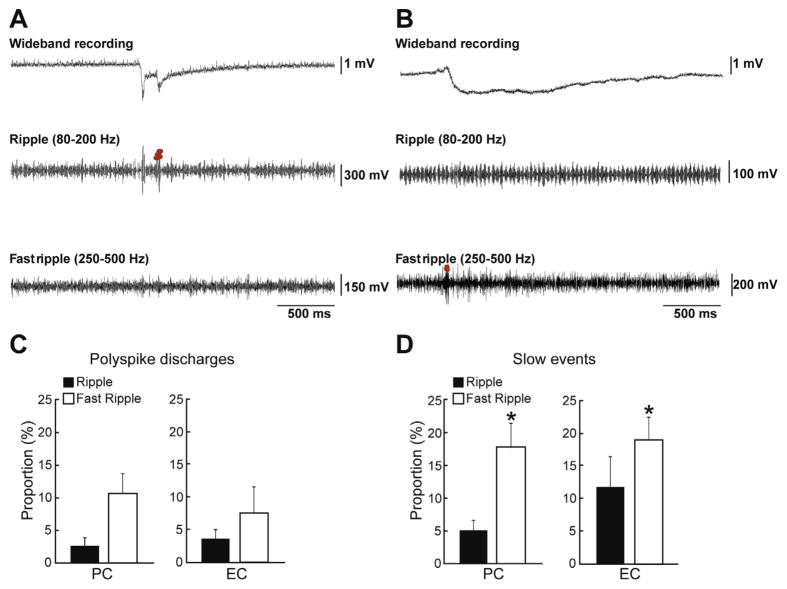
Fig. 6.
(A and B) Examples of a synchronous interictal discharge (A) and of a slow interictal discharge (B) that co-occurred with a ripple or a fast ripple in EC. (C and D) Bar graphs showing the proportion of synchronous interictal discharge (C) and slow interictal (D) discharges co-occurring with ripples and fast ripples in PC and EC. Data were obtained by analyzing 557 synchronous interictal discharge and 95 slow interictal discharges in 6 and 10 brain slices, respectively. Note that the proportions of interictal discharges co-occurring with fast ripples are higher than proportions of interictal discharges co-occurring with ripples for the slow interictal discharges in both regions. *p<0.01.
HFOs were also identified in both PC and EC during synchronous (28 events, n=7 slices) and asynchronous (51 events, n=11 slices) ictal discharges. Asynchronous ictal discharges generated by PC and EC networks were characterized by a higher proportion of ripples (Fig. 7A, B) and fast ripples (Fig. 7A, B) than synchronous ictal discharges (p<0.0001). In addition, when comparing the rates of ripples and fast ripples between PC and EC, we found that ripple rates occurring during synchronous ictal discharges in PC were not different from those in EC but fast ripples rates were significantly higher in PC than in EC (Table 1, p<0.001). Finally, during asynchronous ictal discharges, rates of ripples and fast ripples were also significantly higher in PC than in EC (Table 1, p<0.0001). Cutting the connection between PC and EC did not change the occurrence of ripples and fast ripples during the ictal discharges in both structures (Fig. 7A, B).
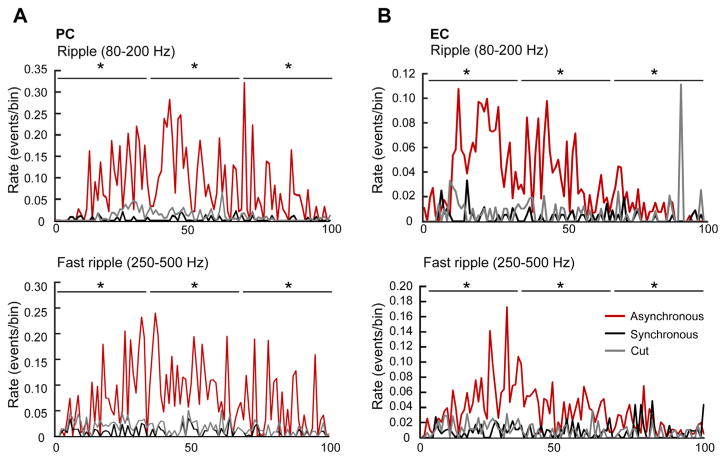
Fig. 7.
Rate of occurrence of ripples and fast ripples during synchronous and asynchronous ictal discharges in the PC (A) and in the EC (B). The duration of ictal discharges was normalized from 0 (start of the ictal discharge) to 100 (end of the ictal discharge) to account for different durations. Data were obtained by analyzing 28 synchronous ictal discharges recorded from seven brain slices as well as 31 and 20 asynchronous ictal discharges from PC (n=10 brain slices) and EC (n=8 brain slices), respectively. Note that in both PC and EC, asynchronous ictal discharges show more ripples and fast ripples compared to synchronous ictal discharges. *p<0.0001.
Table 1.
Average rate of occurrence of HFOs in synchronous and asynchronous ictal discharges in PC and EC
| PC | EC | |
|---|---|---|
| Synchronous ictals (n=7) | ||
| Ripples | 0.0031±0.0005 | 0.0064±0.001 |
| Fast ripples | 0.0084±0.0009 | 0.0048±0.0007* |
| Asynchronous ictals (n=11) | ||
| Ripples | 0.078±0.008 | 0.031±0.003** |
| Fast ripples | 0.074±0.006 | 0.03±0.003** |
p<0.001.
p<0.0001.
DISCUSSION
The main findings of our study can be summarized as follows. First, 4AP application induced interictal and ictal discharges occurring in PC and EC either synchronously or asynchronously. Second, the duration of both interictal and ictal discharges was longer in the asynchronous experiments. Third, removing the connection between PC and EC in the synchronous experiments changed the interval of occurrence and the duration of ictal discharges in EC while in PC it abolished interictal discharges. Fourth, blockade of glutamatergic transmission changed the duration and interval of occurrence of synchronous interictal discharges while it did not affect the duration and interval of occurrence of the slow asynchronous interictal discharges. Fifth, the rate of occurrence of fast ripples was higher than that of ripples during the slow interictal discharges in both PC and EC. Finally, rates of occurrence of ripples and fast ripples were higher during asynchronous ictal discharges in both PC and EC.
Epileptiform activity induced by 4AP in the PC and EC
Consistent with previous studies from our laboratory (Panuccio et al., 2012; Herrington et al., 2014), we have found that 4AP induces interictal and ictal discharges in the PC that are similar to those recorded from other limbic structures such as the EC or the amygdala (Avoli and de Curtis, 2011). These findings diverge from those obtained in the guinea-pig whole-brain in vitro preparation where fast runs and ictal discharges could originate from PC and propagate to the lateral EC connected to the olfactory cortex, but not in the medial EC (Carriero et al., 2010). In those experiments, removing the connection between PC and EC led to disappearance of both fast runs and ictal discharges in the lateral part of the EC while they continued to occur in PC (Carriero et al., 2010). In contrast, in our experiments we could only seldom record fast runs in the PC and cutting the connection between PC and EC resulted in asynchronous epileptiform activity in PC and EC. This diversity may result from different degrees of connectivity that presumably exist in these two in vitro preparations. However, connectivity per se might not be the sole contributor to these results. For instance, inhibitory circuitry in the PC has a functional gradient with the strength of interneuron-to-pyramidal cell contact increasing along the rostro-caudal axis or from the anterior subregion toward the posterior (Luna and Pettit, 2010). In line with these views, preliminary findings obtained from the PC in horizontal and coronal slices of guinea-pig brain indicate that 4AP application can induce ictal activity similar to what was described here (Uva, Cickladze and de Curtis, unpublished observations) thus excluding species-related differences.
Interictal and ictal discharges induced by 4AP in our study could occur in the PC and EC either synchronously or asynchronously. Synchronous ictal discharges had similar durations in these two regions, whereas the asynchronous ictal activity was characterized by structure-specific differences with interval of occurrence and duration that were longer in the EC than in PC. Moreover, the duration of asynchronous ictal discharges occurring in the EC was longer than what was observed when ictal events occurred synchronously. These findings indicate that interconnectivity between these two areas reduces the duration of ictal discharges rather than increasing it. Indeed, this evidence may suggest that interactions between brain structures hamper ictogenesis, a view that is in line with previous findings obtained in combined hippocampus-EC brain slices in which ictal discharges could not be recorded from EC networks when they were connected with the hippocampus (Barbarosie and Avoli, 1997).
Connections between PC and EC are well characterized with the lateral EC receiving inputs directly from the olfactory bulb, and indirectly through PC (Wouterlood et al., 1985). It has also been reported that the PC is a seizure–prone region and it is involved in the propagation of epileptiform activity to the EC and to the other structures of the limbic system (Piredda and Gale, 1985; Hoffman and Haberly, 1991). Removing the connection between PC and EC in the synchronous experiments led to the disappearance of synchronicity between these two regions while ictal discharges continued to occur in both PC and EC, implying that the presence of synchronous and asynchronous epileptiform discharge in different experiments reflects different degrees of connectivity between PC and EC. This finding may result from the slicing procedure. In addition, the duration of ictal discharges in PC remained as before the cut while this procedure increased ictal discharge length in EC, implying that EC networks generate stronger ictal activity when isolated. Therefore, not only propagated epileptiform discharges originating from the PC are not needed for the generation of ictal discharges in the EC but, once more, the preservation of connections between these two areas reduces ictal discharge duration in EC.
We have also found that synchronous ictal discharges emerge from synchronous interictal discharges whereas asynchronous ictal discharges are shortly preceded (and thus presumably initiated) by isolated slow interictal spikes. These findings replicate those identified in the EC (Avoli et al., 2013) and may suggest a different involvement of GABAergic and glutamatergic networks in the two types of brain slices (i.e., connected and not connected).
Previous studies performed in the 4AP in vitro model of epileptiform synchronization have shown that synchronous interictal discharges are mainly contributed by ionotropic glutamatergic receptor mechanisms, whereas slow interictal spikes (such as those recorded here from the PC and EC in the asynchronous experiments) continue to occur during blockade of glutamatergic transmission with amplitude and rates that are similar to those seen under application of 4AP only (Lopantsev and Avoli, 1998; Avoli et al., 2013). Here, we could confirm this evidence in the EC and identify similar patterns in the PC. Interestingly, during blockade of glutamatergic ionotropic receptors, the duration and the rate of occurrence of interictal discharges in both areas increased thus becoming similar to those seen with asynchronous interictal events under control conditions.
Occurrence of HFOs in synchronous and asynchronous epileptiform activity
We have found that fast ripples were more frequently observed than ripples during slow interictal discharges whereas the rate of occurrence of ripples and fast ripples was not significantly different in the synchronous interictal discharges. It has been proposed that ripples reflect inhibitory postsynaptic potentials (IPSPs) generated by principal neurons entrained by a network of synchronously active interneurons (Buzsaki et al., 1992; Ylinen et al., 1995), while fast ripples would not strictly depend on GABA receptor signaling (Dzhala and Staley, 2003; Engel et al., 2009; Bragin et al., 2011). Thus, the preferential occurrence of fast ripples during slow interictal discharges could be caused by the concomitant occurrence of synchronous IPSPs and EPSPs.
We have also found that asynchronous ictal discharges, which appeared to be triggered by slow interictal discharges (cf., Avoli et al., 2013), contained a higher proportion of ripples and fast ripples compared to synchronous ictal discharges. These results replicate in part what was previously reported by us, namely that ictal discharges triggered by slow interictal events during the application of 4AP are mostly associated to ripples (Panuccio et al., 2012) and that they contain a higher proportion of ripples compared to sudden onset ictal discharges (Avoli et al., 2013). It should also be emphasized that the asynchronous ictal discharges recorded here are similar to low-voltage fast-onset (LVF) seizures that occur in epileptic patients (Velasco et al., 2000), in animal models of epilepsy (Bragin et al., 1999; Lévesque et al., 2012) as well as following acute systemic injection of 4AP (Lévesque et al., 2013). Spontaneous LVF seizures recorded in vivo also contain a higher proportion of ripples (Lévesque et al., 2013) compared to fast ripples, suggesting that ictal discharges triggered by slow interictal discharges may reflect the involvement of GABAergic networks.
Removing the connection between PC and EC did not change the rate of occurrence of ripples and fast ripples during epileptiform activity. This finding supports the view that ripples and fast ripples are local oscillatory field potentials reflecting synchronized firing of interneurons and principal cells that are organized in small, discrete neuronal clusters (Bragin et al., 2010).
CONCLUSIONS
Our findings show that 4AP induces interictal and ictal discharges in PC and EC that can be characterized by either synchronous or asynchronous patterns. Synchronous ictal discharges consistently emerge from fast spiking interictal background while asynchronous ictal discharges are preceded by asynchronous slow interictal discharges. We have also found that removing the connection between PC and EC in those experiments in which synchronous epileptiform discharges were recorded, led to occurrence of asynchronous epileptiform discharges in both regions. Our results are remarkably different from the results obtained in the in vitro guinea-pig whole brain, which may result from different levels of connectivity between these two in vitro preparations. Answers to these questions await further studies to be performed in vivo.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR grants 8109 and 74609). We thank Ms. R. Herrington and Ms. P. Salami for helping with recording procedures and data analysis, and Dr. M. de Curtis for reading an early version of this paper.
Abbreviations
- 4AP
4-aminopyridine
- ACSF
artificial cerebrospinal fluid
- EC
entorhinal cortex
- EEG
electroencephalography
- HFOs
high-frequency oscillations
- IPSPs
inhibitory postsynaptic potentials
- LVF
low-voltage fast-onset
- PC
piriform cortex
- SEM
standard error of mean
Footnotes
CONFLICTS OF INTEREST
None of the authors has any conflict of interest to disclose.
References
- Avoli M. Mechanisms of epileptiform synchronization in cortical neuronal networks. Curr Med Chem. 2014;21:653–662. doi: 10.2174/0929867320666131119151136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Panuccio G, Herrington R, D’Antuono M, de Guzman P, Lévesque M. Two different interictal spike patterns anticipate ictal activity in vitro. Neurobiol Dis. 2013;52:168–176. doi: 10.1016/j.nbd.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal–entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Vizentin E, Mathern GW. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999;40:1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23:151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Benassi SK, Kheiri F, Engel J. Further evidence that pathological high frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011;52:45–52. doi: 10.1111/j.1528-1167.2010.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Carriero G, Uva L, Gnatkovsky V, Avoli M, de Curtis M. Independent epileptiform discharge patterns in the olfactory and limbic areas of the in vitro isolated guinea pig brain during 4-aminopyridine treatment. J Neurophysiol. 2010;103:2728–2736. doi: 10.1152/jn.00862.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir R, Haberly LB, Jackson MB. Voltage imaging of epileptiform activity in slices from rat piriform cortex: onset and propagation. J Neurophysiol. 1998;80:2727–2742. doi: 10.1152/jn.1998.80.5.2727. [DOI] [PubMed] [Google Scholar]
- Demir R, Haberly LB, Jackson MB. Epileptiform discharges with in-vivo-like features in slices of rat piriform cortex with longitudinal association fibers. J Neurophysiol. 2001;86:2445–2460. doi: 10.1152/jn.2001.86.5.2445. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J Neurosci Off J Soc Neurosci. 2003;23:7873–7880. doi: 10.1523/JNEUROSCI.23-21-07873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Gloor P. The temporal lobe and limbic system. New York: Oxford University Press; 1997. [Google Scholar]
- Herrington R, Lévesque M, Avoli M. Neurosteroids modulate epileptiform activity and associated high-frequency oscillations in the piriform cortex. Neuroscience. 2014;256:467–477. doi: 10.1016/j.neuroscience.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WH, Haberly LB. Bursting-induced epileptiform EPSPs in slices of piriform cortex are generated by deep cells. J Neurosci Off J Soc Neurosci. 1991;11:2021–2031. doi: 10.1523/JNEUROSCI.11-07-02021.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Jiruska P, de Curtis M, Avoli M. Limbic network synchronization and temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- Lévesque M, Salami P, Behr C, Avoli M. Temporal lobe epileptiform activity following systemic administration of 4-aminopyridine in rats. Epilepsia. 2013;54:596–604. doi: 10.1111/epi.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Laminar organization of epileptiform discharges in the rat entorhinal cortex in vitro. J Physiol. 1998;509:785–796. doi: 10.1111/j.1469-7793.1998.785bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna VM, Pettit DL. Asymmetric rostro-caudal inhibition in the primary olfactory cortex. Nat Neurosci. 2010;13:533–535. doi: 10.1038/nn.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva FH. Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; 2005. Electroencephalography. [Google Scholar]
- Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. Jasper’s basic mechanisms of the epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- Panuccio G, Sanchez G, Lévesque M, Salami P, de Curtis M, Avoli M. On the ictogenic properties of the piriform cortex in vitro. Epilepsia. 2012;53:459–468. doi: 10.1111/j.1528-1167.2012.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piredda S, Gale K. A crucial epileptogenic site in the deep prepiriform cortex. Nature. 1985;317:623–625. doi: 10.1038/317623a0. [DOI] [PubMed] [Google Scholar]
- Salami P, Lévesque M, Gotman J, Avoli M. A comparison between automated detection methods of high-frequency oscillations (80–500 Hz) during seizures. J Neurosci Methods. 2012;211:265–271. doi: 10.1016/j.jneumeth.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva L, Trombin F, Carriero G, Avoli M, de Curtis M. Seizure-like discharges induced by 4-aminopyridine in the olfactory system of the in vitro isolated guinea pig brain. Epilepsia. 2013;54:605–615. doi: 10.1111/epi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco AL, Wilson CL, Babb TL, Engel J., Jr Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plasticity. 2000;7:49–63. doi: 10.1155/NP.2000.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Mugnaini E, Nederlof J. Projection of olfactory bulb efferents to layer I GABAergic neurons in the entorhinal area. Combination of anterograde degeneration and immunoelectron microscopy in rat. Brain Res. 1985;343:283–296. doi: 10.1016/0006-8993(85)90746-2. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]