Abstract
Background
Chronic spontaneous urticaria (CSU) negatively impacts patient quality of life and productivity and is associated with considerable indirect costs to society.
Objective
The aim of this study was to assess the cost utility of add-on omalizumab treatment compared with standard of care (SOC) in moderate or severe CSU patients with inadequate response to SOC, from the UK societal perspective.
Methods
A Markov model was developed, consisting of health states based on Urticaria Activity Score over 7 days (UAS7) and additional states for relapse, spontaneous remission and death. Model cycle length was 4 weeks, and total model time horizon was 20 years in the base case. The model considered early discontinuation of non-responders (response: UAS7 ≤6) and retreatment upon relapse (relapse: UAS7 ≥16) for responders. Clinical and cost inputs were derived from omalizumab trials and published sources, and cost utility was expressed as incremental cost-effectiveness ratios (ICERs). Scenario analyses included no early discontinuation of non-responders and an altered definition of response (UAS7 <16).
Results
With a deterministic ICER of £3183 in the base case, omalizumab was associated with increased costs and benefits relative to SOC. Probabilistic sensitivity analysis supported this result. Productivity inputs were key model drivers, and individual scenarios without early discontinuation of non-responders and adjusted response definitions had little impact on results. ICERs were generally robust to changes in key model parameters and inputs.
Conclusions
In this, the first economic evaluation of omalizumab in CSU from a UK societal perspective, omalizumab consistently represented a treatment option with societal benefit for CSU in the UK across a range of scenarios.
Electronic supplementary material
The online version of this article (doi:10.1007/s40273-016-0412-1) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Chronic spontaneous urticaria (CSU) is a dermatological disease associated with a detrimental impact on patient quality of life and considerable societal burden. Omalizumab currently represents the only licensed treatment option for patients with inadequate response to H1 antihistamines and is used as an add-on therapy to standard of care (SOC) in clinical practice. |
| A cost-utility analysis from the UK societal perspective found omalizumab as add-on therapy to SOC to be associated with increased quality-adjusted life-years and increased costs relative to continued SOC alone. The incremental cost-effectiveness ratio for omalizumab was low (£3183 per QALY gained). |
| Although this finding was robust to a number of sensitivity analyses, further research is needed to establish accurate estimates of CSU remission and efficacy of omalizumab retreatment. |
Introduction
Chronic urticaria (CU) is a dermatological disease characterised by the rapid appearance of itchy hives, angioedema, or both, lasting for 6 weeks or more [1]. Approximately 0.5–1 % of the general population suffer from CU and over 60 % of cases are classed as chronic spontaneous (previously termed idiopathic) urticaria (CSU), in which no obvious triggers can be identified [2, 3]. The average duration of CSU is generally up to 5 years, although more severe cases can last considerably longer [2, 4].
CSU most frequently affects patients between 20 and 40 years of age and, when uncontrolled by medication, can have an underestimated impact on patient health-related quality of life (HRQoL) [2, 3, 5, 6]. In addition to the physical discomfort caused by CSU, the unpredictability of attacks, disruption of sleep quality and cosmetic disfigurement can reduce patient productivity [2, 3, 7, 8]. There are considerable indirect costs to society associated with CSU and a recent study demonstrated the impact of CSU on both absenteeism and presenteeism in employment and education [9, 10].
The current international guidelines on the definition, diagnosis and management of CU recommend second-generation non-sedating H1 antihistamines as the first-line treatment [1]; however, over half of patients have an inadequate response to licensed dose H1 antihistamines [11]. If symptoms persist, guidelines recommend increasing H1 antihistamine dosage up to four times the licensed dose [1]. Finally, if symptoms persist in the following 1–4 weeks, the guidelines recommend the addition of omalizumab, ciclosporin A or montelukast [a leukotriene receptor antagonist (LTRA)] as add-on therapy to the increased dose of H1 antihistamines [1].
Omalizumab is a humanised anti-immunoglobulin (Ig) E monoclonal antibody approved by the European Medicines Agency in 2014 as an add-on therapy for the treatment of CSU in adult and adolescent (≥12 years) patients with inadequate response to H1 antihistamines. In 2015, omalizumab underwent appraisal by the National Institute for Health and Care Excellence (NICE) and the Scottish Medicines Consortium (SMC) for patients with an inadequate response to standard of care (SOC) treatment, consisting of updosed H1 antihistamines, H2 antihistamines and/or LTRAs.
In this study, we assess the cost utility of omalizumab compared with continued SOC in patients with moderate or severe CSU with an inadequate response to SOC, from the broad UK societal perspective. The only other economic evaluation conducted specifically in CSU is a trial-based cost-effectiveness study from the French societal perspective that also emphasises the significant impact of CSU on patient HRQoL [12]. To the authors’ knowledge at the time of submitting this research for publication, the research presented here represents the first cost-utility analysis conducted for CSU and the only economic evaluation based on a decision analytic model for this indication.
Methods
Patient Population
The base-case analysis considered the population recruited to the GLACIAL phase III randomised controlled trial (RCT)—patients with moderate-to-severe CSU inadequately controlled on SOC [13]. This patient population is in line with published guidance and UK clinician feedback on the most appropriate position for omalizumab in the treatment pathway [1, 14, 15]. A scenario analysis based on two other phase III RCTs (ASTERIA I, ASTERIA II) evaluated omalizumab in its broader, licensed population of patients with an inadequate response to licensed doses of H1 antihistamines.
Comparator
Continued SOC was the comparator for this economic evaluation, and was reflected by the placebo arms of the relevant omalizumab trials. In the base-case analysis, this was the placebo arm of the GLACIAL trial (in which patients received updosed H1 antihistamines, H2 antihistamines and/or LTRAs) [13]. This comparator was considered appropriate based on clinical feedback suggesting that, prior to approval of omalizumab, CSU patients inadequately controlled on SOC may nonetheless continue to be treated with this regimen due to an absence of alternative licensed options. The SOC comparator in the scenario analysis considering omalizumab in its broader licensed population was different, being represented by the placebo arms of the ASTERIA I and ASTERIA II trials (in which patients received licensed doses of H1 antihistamines) [16, 17].
Perspective
The analysis was performed from the societal perspective, which included direct costs within the healthcare system [National Health Service (NHS) and Personal Social Services (PSS)] and indirect costs falling outside this (i.e. productivity costs). A scenario analysis considered the cost utility of omalizumab from the NHS/PSS perspective.
Time Horizon and Discounting
The starting age of patients in the model was 43 years, corresponding to the mean age of patients in the GLACIAL trial [13]. The base-case model was run over a 20-year time horizon as it was anticipated that the majority of patients would have entered spontaneous remission by 20 years following symptom onset. In determining maximum potential disease duration from the literature, a systematic literature review (see electronic supplementary material) found no primary studies providing estimates of remission probabilities beyond 10 years; however, the assumed time horizon was based on information from a review article by Beltrani which provided estimates up to 25 years [4]. Both costs and health effects were discounted at a rate of 3.5 % [18, 19].
Model Structure
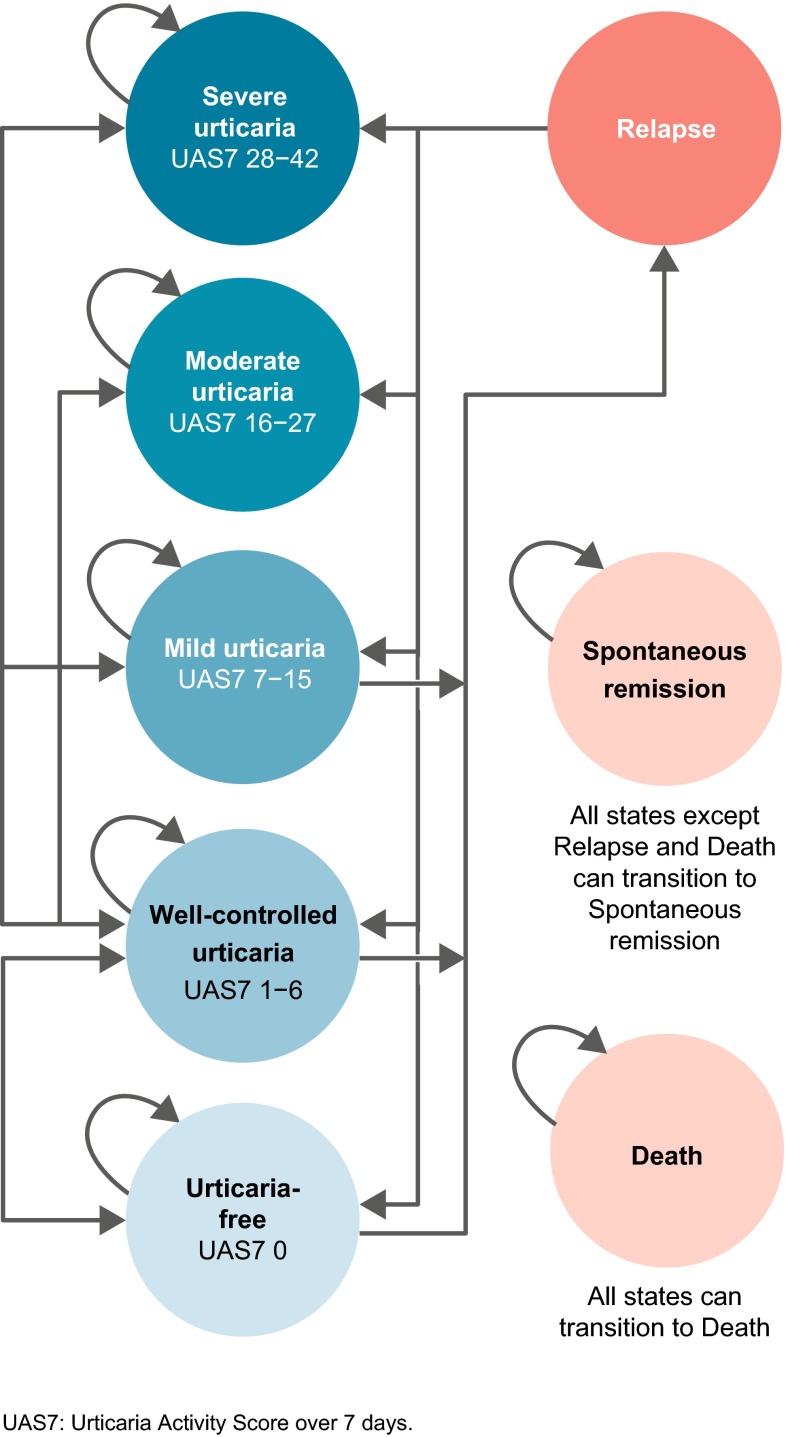
A de novo Markov model consisting of eight states that captured the major characteristics of CSU was constructed in Microsoft® Excel 2010 (Microsoft Corporation, Redmond, WA, USA). In all three phase III trials of omalizumab, disease activity was measured using the Urticaria Activity Score (UAS), a patient daily diary capturing severity of itch and number of hives. This score was calculated over 7 days (UAS7), giving a score from 0 (urticaria-free) to 42 (maximum itch and number of hives). Five model health states were defined on the basis of UAS7 disease activity: ‘severe urticaria’ (UAS7 = 28–42), ‘moderate urticaria’ (UAS7 = 16–27), ‘mild urticaria’ (UAS7 = 7–15), ‘well-controlled urticaria’ (UAS7 = 1–6) and ‘urticaria-free’ (UAS7 = 0). UAS7 is a recommended outcome for assessing disease activity and is highly correlated with HRQoL measures in CSU [20–22]. Furthermore, previous research has demonstrated that these UAS7 score ranges represent an efficient way to describe CSU health states [23]. The three additional model states consisted of a ‘relapse’ state, a ‘spontaneous remission’ state and an absorbing ‘death’ state (Fig. 1).
Fig. 1.
Diagram of the Markov model structure
The model applied SOC as background therapy in both model arms. In the omalizumab arm, patients received omalizumab as add-on therapy; no additional therapy was applied in the SOC arm, reflecting the phase III study designs [13, 16, 17]. Model cycle length was 4 weeks, consistent with the administration schedule of omalizumab.
During the initial treatment period of the model, patients could move from their beginning ‘severe urticaria’ and ‘moderate urticaria’ states to any of the five UAS7 health states every cycle, depending on treatment response. A half-cycle correction was not applied as it was considered uninformative due to the short cycle length and the unpredictability surrounding patient transitions between health states.
Response Assessment
At the week 16 assessment point, patients receiving omalizumab could be classed as either non-responders or responders (response defined as UAS7 ≤6). Non-responders at week 16 (those in ‘severe urticaria’, ‘moderate urticaria’ or ‘mild urticaria’ health states) stopped receiving omalizumab and subsequently received background SOC treatment only for the remainder of the time horizon. Responders at week 16 (‘well-controlled’ or ‘urticaria-free’ health states) continued receiving omalizumab until week 24. Between weeks 16 and 24, the proportion of responders to non-responders was held constant at the week 16 level; responders were redistributed among the response health states over this 8-week period according to data from the GLACIAL trial. Therefore, week 16 responders were assumed to maintain a state of response until at least the end of the 24-week treatment course. Patients in the SOC comparator arm were not assessed for response at 16 weeks and were instead treated with 24-week courses of background medication throughout the model time horizon.
At 24 weeks, all patients still receiving omalizumab (i.e. responders) were assumed to stop treatment with omalizumab and receive only background SOC. These prior responders were then retreated upon relapse. This treatment schedule reflected the unpredictable nature of CSU, which may spontaneously resolve over time; in clinical practice omalizumab would be administered for 24 weeks and then withdrawn to monitor for potential spontaneous remission (remaining symptom-free with no active treatment).
Relapse and Retreatment
The model incorporated relapse, defined as UAS7 ≥16 in the base-case analysis, as per inclusion criteria used in phase III trials of omalizumab [13, 16, 17]. Patients in the ‘mild urticaria’ (UAS7 = 7–15), ‘well-controlled urticaria’ (UAS7 = 1–6) and ‘urticaria-free’ (UAS7 = 0) health states were at risk of relapse from week 24, when active treatment with omalizumab was stopped and only SOC was continued in all patients. Although classed as non-responders, patients in the ‘mild urticaria’ state were modelled to be at risk of relapse because they had derived some benefit and it could not be assumed that this would be maintained upon discontinuation of omalizumab.
Upon relapse, prior responders to omalizumab (UAS7 ≤6 at 16 weeks) were assumed to be retreated with a 24-week course of omalizumab; prior non-responders were not retreated and stayed on SOC for the model time horizon. Patients undergoing retreatment were assumed to respond in the same way as in their initial 24-week treatment course. This was based on a retrospective analysis, which showed that 25 CU patients had the same response rate and adverse event rate upon retreatment with omalizumab as during their first course [24]. Thus, for patients who continued to exhibit a pattern of response to omalizumab followed by relapse, omalizumab treatment was modelled as intermittent 24-week courses, with length of treatment break varying depending on time to relapse. Patients in the SOC arm who relapsed after an initial response continued to receive SOC and were subject to the same probability of response as on initial treatment. As patients in the model are continuously on SOC, the model reflected this through a structural assumption of repeated 24-week SOC treatment cycles without treatment breaks.
Spontaneous Remission State
In addition to relapse, patients were also subject to a treatment-independent probability of entering a ‘spontaneous remission’ health state (defined as UAS7 = 0). Patients experiencing spontaneous remission were modelled to remain disease-free and in a ‘spontaneous remission’ health state for the duration of the model time horizon or until death.
Discontinuation of Omalizumab
The model accounted for discontinuation of omalizumab due to lack of efficacy, adverse events or physician/patient choice. Patients discontinuing omalizumab were treated with background SOC only, and it was assumed that they would not be retreated with omalizumab throughout the model time horizon.
Clinical and Data Inputs
The distribution of patients across health states for the first six model cycles was determined directly from GLACIAL patient-level data. Three datasets using different imputation methods were available for the GLACIAL trial: the base-case analysis was based on observed data; baseline observation carried forward (BOCF) and last observation carried forward (LOCF) datasets were explored in scenario analyses. Health-state distributions for each model arm over the 16-week period up to the assessment point are provided in Table 1 (data previously unpublished).
Table 1.
Distribution of patients across health states per 4-week cycle over the 16-week period up to response assessment (GLACIAL population, observed)
| Baseline | Week 4 | Week 8 | Week 12 | Week 16 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OMA | SOC | OMA | SOC | OMA | SOC | OMA | SOC | OMA | SOC | |
| n | 252 | 83 | 196 | 74 | 219 | 72 | 216 | 67 | 212 | 64 |
| ‘Severe urticaria’ (UAS7 = 28–42) (%) | 71.0 | 61.4 | 20.9 | 29.7 | 18.3 | 30.6 | 11.1 | 28.4 | 9.4 | 26.6 |
| ‘Moderate urticaria’ (UAS7 = 16–27) (%) | 29.0 | 38.6 | 20.1 | 40.5 | 13.7 | 31.9 | 13.9 | 29.9 | 10.4 | 26.6 |
| ‘Mild urticaria’ (UAS7 = 7–15) (%) | – | – | 17.5 | 27.0 | 13.2 | 27.8 | 11.6 | 26.9 | 9.9 | 29.7 |
| ‘Well-controlled urticaria’ (UAS7 = 1–6) (%) | – | – | 25.2 | 2.7 | 21.5 | 6.9 | 24.1 | 9.0 | 21.7 | 12.5 |
| ‘Urticaria-free’ (UAS7 = 0) (%) | – | – | 16.2 | 0.0 | 33.3 | 2.8 | 39.4 | 6.0 | 48.6 | 4.7 |
Data previously unpublished
OMA omalizumab, SOC standard of care, UAS7 Urticaria Activity Score over 7 days
Relapse Probabilities
Relapse probabilities for patients receiving SOC (those in the SOC arm or those who had discontinued omalizumab) were determined for each health state from patient-level data sourced from the 16-week follow-up period (weeks 24–40) of the GLACIAL trial (cumulative relapse proportions are presented in the electronic supplementary material; data previously unpublished). Patients entering spontaneous remission during this time were excluded from the denominator of the relapse probability calculation. In the base case, a linear extrapolation of cumulative relapse probabilities was used to calculate relapse rates for model cycles beyond 40 weeks; under this extrapolation, all responders had relapsed by 16 months. Exponential extrapolation was explored as a scenario analysis. Under this extrapolation assumption, some patients were not predicted to have relapsed at 16 months after omalizumab discontinuation. These patients were forced to transition to the relapse state based on the longest duration of symptom absence with omalizumab use in CSU reported in the literature [24].
Probabilities of Spontaneous Remission
Following a systematic literature review of the natural history of CSU (see the electronic supplementary material), a study by Nebiolo et al.1 was selected to model probabilities of spontaneous remission in the base case, and the data were best fitted by a log-logistic distribution [26, 27]. This study was considered the most appropriate source based on the accuracy of its definition of the patient population, large patient population, the prospective study design, long follow-up (5 years) and frequent follow-up times.
Discontinuations and Losses to Follow-Up
Discontinuations were modelled for the omalizumab arm only as patients were assumed to continually be on background SOC treatment unless they had entered remission. Discontinuation risks were estimated from GLACIAL patient-level data and the model assumed no retreatment of discontinued patients upon relapse. In the base-case analysis, patients lost to follow-up in the GLACIAL study were assumed to transition to the ‘moderate urticaria’ health state, regardless of prior health state or treatment arm; this assumption was based on the UAS ≥16 inclusion criterion of the GLACIAL trial [13]. Model inputs for the probabilities of discontinuation and loss to follow-up are provided in the electronic supplementary material. No CSU-related mortality was assumed; all-cause natural mortality was incorporated using annual mortality rates for each age group from the UK Office for National Statistics life tables [28].
Adverse Events
In the GLACIAL study, no meaningful differences in adverse event rates between omalizumab and SOC were observed [13]. Despite this, several adverse events were conservatively included to capture any potential impact on cost utility of omalizumab versus SOC. Adverse events occurring with ≥1 % frequency in any treatment arm within pooled data from the GLACIAL, ASTERIA I and ASTERIA II trials, and occurring ≥2 % more frequently in the omalizumab 300 mg arm than the SOC arm in this pooled analysis, were included. Derivation of 4-week risks of individual adverse events is provided in the electronic supplementary material.
Health Outcomes
Health outcomes were measured in quality-adjusted life-years (QALYs), with utilities for each of the five health states (Table 2) based on a published mixed-effects regression model constructed from pooled EQ-5D data across the GLACIAL, ASTERIA I and ASTERIA II trials (details in the electronic supplementary material) [29]. The model also incorporated disutilities associated with individual adverse events; these disutilities were small in magnitude (see electronic supplementary material).
Table 2.
Health-state utility inputs
| Variable | Value (SD; distribution) |
|---|---|
| ‘Severe urticaria’ (UAS7 = 28–42) | 0.712 (0.31; Beta) |
| ‘Moderate urticaria’ (UAS7 = 16–27) | 0.782 (0.26; Beta) |
| ‘Mild urticaria’ (UAS7 = 7–15) | 0.845 (0.24; Beta) |
| ‘Well-controlled urticaria’ (UAS7 = 1–6) | 0.859 (0.24; Beta) |
| ‘Urticaria-free’ (UAS7 = 0) | 0.897 (0.25; Beta) |
Costs
Direct healthcare costs incorporated within the model included drug, administration and health state costs (Table 3; further breakdown is provided in the electronic supplementary material). Costs of adverse events (see electronic supplementary material) were applied for the duration of each 4-week model cycle. This is a conservative assumption as the adverse events included would likely resolve in less than 4 weeks.
Table 3.
Direct healthcare cost inputs
| Variable | Value (SD), £ | Source |
|---|---|---|
| Drug and administration costs | ||
| Omalizumab 300 mg cost per dosea | 512.30 (NA) | BNF July 2014 |
| H1 antihistamine cost per day | 0.21 (0.04) | BNF July 2014 |
| H2 antihistamine cost per day | 0.33 (0.07) | BNF July 2014 |
| LTRA cost per day | 0.36 (0.07) | BNF July 2014 |
| Omalizumab cost per administration | 14.21 (2.85) | PSSRU 2013 (10 min of day-ward nurse time), inflated to 2014 |
| Omalizumab cost of monitoring for administration 1–3 (per administration)b | 42.64 (8.53) | PSSRU 2013 (day-ward nurse time costs), inflated to 2014. |
| Omalizumab cost of monitoring for administration 4b | 21.32 (4.26) | |
| Health state | Mean cost (SD), γ distributionc, £ | Source | ||
|---|---|---|---|---|
| Outpatient visits | A&E/hospital visits | Laboratory costs | ||
| Annual direct healthcare cost per year by health state | ||||
| ‘Severe urticaria’ (UAS7 = 28–42) | 356.97 (282.83) | 12.20 (37.20) | 93.64 (93.74) | ASSURE-CSU Routine/emergency visit costs were sourced from NHS Reference Costs 2012–2013 (inflated to May 2014) and laboratory tests were sourced from NIHR Industry Costing Template April 2013 (inflated to May 2014) |
| ‘Moderate urticaria’ (UAS7 = 16–27) | 341.82 (183.60) | 8.97 (33.28) | 69.69 (68.27) | |
| ‘Mild urticaria’ (UAS7 = 7–15) | 302.79 (260.12) | 13.66 (41.15) | 71.49 (68.85) | |
| ‘Well-controlled urticaria’ (UAS7 = 1–6) | 254.57 (172.69) | 35.87 (55.56) | 61.12 (75.38) | |
| ‘Urticaria-free’ (UAS7 = 0) | 0.00 (NA) | 0.00 (NA) | 0.00 (NA) | Assumption: no patients with UAS7 = 0 were enrolled in the ASSURE-CSU study |
| Cost of identifying a relapse | 97.80 (19.56) | NHS Reference Cost Schedule 2012/2013 (inflated to 2014) | ||
A&E accident and emergency, BNF British National Formulary, LTRA leukotriene receptor antagonist, NA not applicable, NHS National Health Service, NICE National Institute for Health and Care Excellence, NIHR National Institute for Health Research, PSSRU Personal Social Services Research Unit, SD standard deviation, UAS7 Urticaria Activity Score over 7 days
aA confidential simple discount patient access scheme is currently available in the UK for omalizumab but the publically available list price was used for this analysis
bMonitoring requirements for omalizumab were assumed to be 2 h for the first three doses and 1 h for the fourth dose in a treatment course. It was assumed no monitoring was required for the fifth and sixth doses in a treatment course. Fifteen minutes of nurse time per hour of monitoring was assumed. These assumptions are consistent with those applied in the NICE appraisals of omalizumab in severe persistent asthma and CSU [25, 40]
cAll health-state costs were associated with a γ distribution, with the exception of cost of identifying a relapse, which was normally distributed
The cost of relapse was taken from the NHS National Schedule of Reference Costs 2012–13 (inflated to May 2014), weighted based on single professional and multi-professional non-admitted face-to-face follow-up outpatient appointments across Allergy, Clinical Immunology and Dermatology specialties [30]. Health-state costs were based on prior 12-month resource utilisation observed for patients in each current health state in the ASSURE-CSU study, a non-interventional burden-of-illness study conducted across seven countries (data applied from UK centres only) [9, 31, 32].
The model considered the societal perspective by incorporating productivity costs based on data from the same ASSURE-CSU study (Table 4). This study reported on the proportion of patients in employment (51.35 %) and the average number of days of absenteeism and presenteeism by health state [9, 10]. Costs associated with absenteeism and presenteeism were based on the human capital approach and were calculated from average weekly earnings data sourced from the Office for National Statistics (£478.00) [33]. A 160-hour working month was assumed.
Table 4.
Indirect costs (absenteeism and presenteeism)
| Health state | Number of days absent per 4-week cycle | Mean (SD) cost of absenteeism per 4-week cycle, £ | Number of days impaired work (presenteeism) per 4-week cycle | Mean (SD) cost of impaired work (presenteeism) per 4-week cycle, £ | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Distribution (alpha, beta) | Mean (SE) | Distribution (alpha, beta) | |||
| ‘Severe urticaria’ (UAS7 = 28–42) | 2.89 (1.94) | Gamma (2.22, 1.30) | 300.30 (637.12) | 8.80 (1.67) | Gamma (27.92, 0.32) | 913.10 (546.43) |
| ‘Moderate urticaria’ (UAS7 = 16–27) | 2.94 (1.32) | Gamma (4.93, 0.59) | 304.60 (531.09) | 7.57 (1.83) | Gamma (17.12, 0.44) | 785.60 (710.43) |
| ‘Mild urticaria’ (UAS7 = 7–15) | 0.07 (0.07) | Gamma (1.00, 0.07) | 7.20 (20.38) | 5.50 (1.68) | Gamma (10.72, 0.51) | 570.70 (492.96) |
| ‘Well-controlled urticaria’ (UAS7 = 1–6) | 0.00 | Undefined | 0.00 | 0.00 | Undefined | 0.00 |
Assumed that 51.35 % of CSU patients are employed [PSA (alpha, beta): Beta distribution (38, 36 %)]. Weekly average earnings assumed to be £478.00 (Office for National Statistics, May 2014), and monthly working hours assumed to be 160. The ASSURE-CSU study collected data on symptomatic patients only. The model assumed no impact on absenteeism and presenteeism in the ‘urticaria-free’ state based on the results observed for the ‘well-controlled urticaria’ health state
PSA probabilistic sensitivity analysis, SD standard deviation, SE standard error, UAS7 Urticaria Activity Score over 7 days
All costs were inflated to 2014 values where necessary, using the UK Consumer Price Index for Outpatient Services (May 2014) [34].
Model Outcomes
The model calculated total discounted costs and total discounted QALYs for the two treatment arms. Results of the cost-utility analysis were expressed as incremental cost-effectiveness ratios (ICERs).
Scenario Analyses and Sensitivity Analyses
The following scenario analyses explored uncertainty of structural assumptions within the model:
Altered definition of response.
Altered probability of response on retreatment.
Use of datasets based on different imputation methods (BOCF and LOCF).
Alternative extrapolation for relapse assumptions.
Alternative sources of spontaneous remission data [4, 35, 36].
An alternative assumption of CSU patients who received omalizumab not requiring background medications aside from the licensed dose of H1 antihistamines.
Consideration of a narrower NHS/PSS perspective.
Shorter (10-year) time horizon.
Cost utility of omalizumab in CSU patients with an inadequate response to H1 antihistamines, matching the licensed indication of omalizumab (using pooled data from the ASTERIA studies).
Exploration of alternative stopping rules for omalizumab.
One-way sensitivity analysis (OWSA) was conducted to identify key drivers of model results, and probabilistic sensitivity analysis (PSA) was performed (1000 iterations) to assess the impact of combined uncertainty in model parameters. Details of how parameters varied in OWSA are provided in the electronic supplementary material. Parameters and distributions for each input in the PSA are provided in Tables 1, 2, 3 and 4. Correlation was maintained through use of a Dirichlet distribution for patient health-state distribution, a single random number for utilities and a single random variable for cumulative relapse risks over time.
Model Validation
The model structure was validated through iterative discussions with clinical experts from the UK and Germany and a UK professor of health economics, some of whom are authors on this paper. The model was further validated for technical accuracy through extensive internal and external review. Full details of model validation and methods for gathering expert feedback are summarised in the electronic supplementary material.
Results
Base-Case Results
In the base-case analysis, omalizumab was associated with increased cost but also increased benefit (QALYs) compared with SOC, with a deterministic ICER of £3183 per QALY (Table 5). Table 6 presents a breakdown of the costs associated with the omalizumab and SOC arms in the analysis.
Table 5.
Deterministic and mean probabilistic ICERs for omalizumab versus SOC—base-case analysis
| Omalizumab | SOC | Incremental cost, £ | Incremental QALYs | ICER (£ per QALY) | Probability of omalizumab being cost-effective (at stated WTP threshold) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cost, £ | QALYs | Cost, £ | QALYs | £20,000 | £30,000 | ||||
| Deterministic | 36,372 | 12.2 | 35,729 | 12.0 | 643 | 0.202 | 3183 | – | – |
| Probabilistic | 36,500 | 12.2 | 35,812 | 12.0 | 688 | 0.2 | 3566 | 95.4 % | 100 % |
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year, SOC standard of care, WTP willingness to pay
Table 6.
Breakdown of costs accrued in the omalizumab and SOC arms
| Type of cost | Omalizumab, cost (£) | SOC, cost (£) | Incremental cost (£) | Proportion of total absolute increment (%) |
|---|---|---|---|---|
| Technology costs | 9323 | 2061 | 7262 | 32.6 |
| Administration costs | 204 | 0 | 204 | 0.9 |
| Monitoring costs | 390 | 0 | 390 | 1.8 |
| Adverse event costs | 17 | 14 | 3 | 0.0 |
| Other direct healthcare costs | 12,440 | 4926 | 7513 | 33.8 |
| Indirect healthcare costs | 23,932 | 30,803 | −6871 | 30.9 |
| Total | 36,372 | 35,729 | 643 | 100 |
SOC standard of care
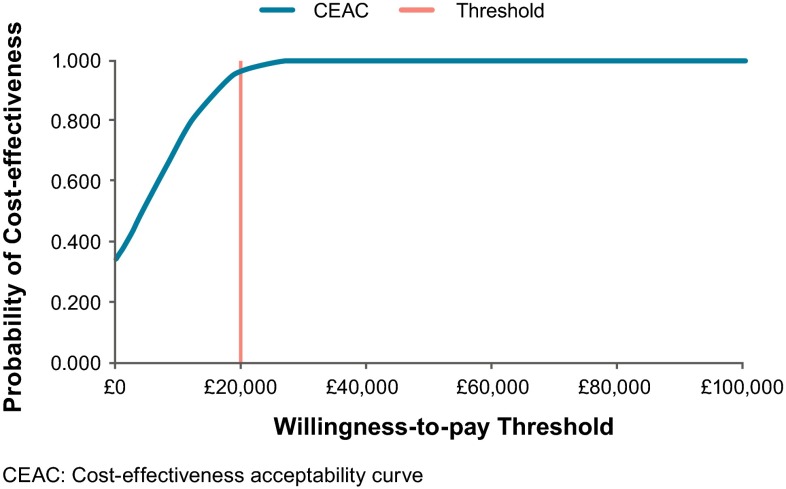
Results of the probabilistic model were consistent with those of the deterministic model, with a mean probabilistic ICER of £3395 per QALY. The scatterplot of probabilistic results is presented in Fig. 2, and the probability of cost effectiveness of omalizumab at different willingness-to-pay (WTP) thresholds is presented in the cost-effectiveness acceptability curve (Fig. 3).
Fig. 2.
Scatterplot of probabilistic sensitivity analysis
Fig. 3.
Cost-effectiveness acceptability curve
One-Way Sensitivity Analyses
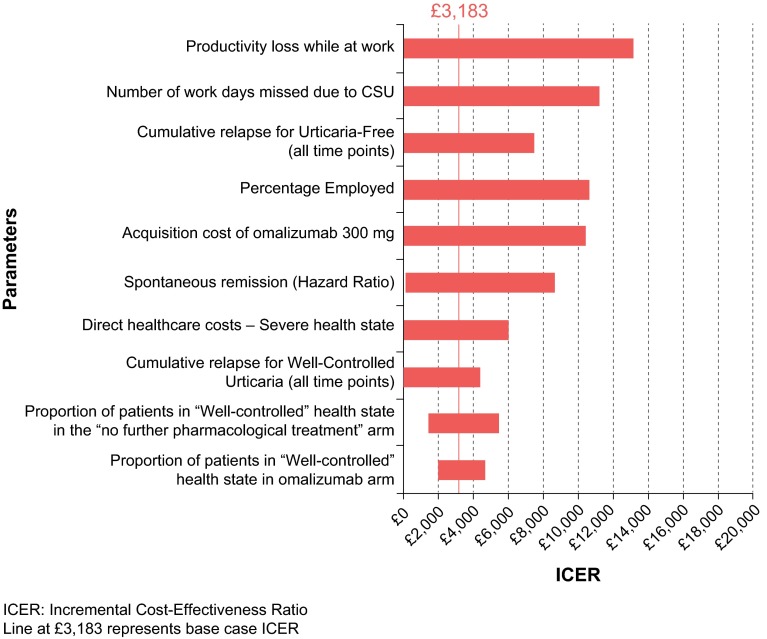
OWSA (Fig. 4) found the ICERs to be most sensitive to assumptions regarding productivity inputs, with three of the five biggest drivers of model results related to productivity (productivity loss at work, number of work days missed and percentage employed). Regardless of which input was varied, the ICERs still fell below a WTP threshold of £20,000 per QALY for all OWSAs.
Fig. 4.
Tornado diagram of one-way sensitivity analyses
Scenario Analyses
The results of scenario analyses exploring key structural and parameter assumptions in the model are presented in Table 7. Considering the narrower NHS/PSS perspective, which excludes societal costs, the incremental costs of omalizumab are substantially higher, leading to an ICER of over £35,000 per QALY. By contrast, ICERs below a £20,000 per QALY WTP threshold were consistently demonstrated when evaluating alternative sources of remission data, alternative relapse extrapolations and different assumptions around omalizumab efficacy on retreatment, among other scenarios. In addition, omalizumab was similarly found to be associated with low ICERs, indicating a net societal benefit, when used at an earlier stage of the treatment pathway in line with the full licensed indication. Exploration of alternative stopping rules for omalizumab found that the most cost effective stopping rule for omalizumab is an early stop for non-responders after four doses (16 weeks) (see Table 8).
Table 7.
Scenario analyses
| Scenario | Omalizumab | SOC | Incremental cost (£) | Incremental QALYs | ICER (£ per QALY) |
||
|---|---|---|---|---|---|---|---|
| Total cost (£) | Total QALYs | Total cost (£) | Total QALYs | ||||
| Base case | 36,372 | 12.20 | 35,729 | 12.00 | 643 | 0.202 | 3,183 |
| Response defined as UAS7≤16 | 36,904 | 12.22 | 35,729 | 12.00 | 1,174 | 0.221 | 5,304 |
| Omalizumab re-treatment efficacy | |||||||
| 5% of prior responders do not respond on re-treatment | 36,551 | 12.18 | 35,729 | 12.00 | 822 | 0.177 | 4,635 |
| Probability of response on re-treatment of prior responders is the same as for initial treatment | 37,252 | 12.11 | 35,729 | 12.00 | 1,523 | 0.108 | 14,099 |
| Imputation methods | |||||||
| BOCF | 38,215 | 12.16 | 37,302 | 11.87 | 914 | 0.293 | 3,116 |
| LOCF | 37,028 | 12.20 | 36,810 | 11.89 | 218 | 0.310 | 704 |
| Exponential relapse extrapolationb | 35,361 | 12.22 | 35,472 | 12.01 | -110 | 0.212 | Dominant |
| Alternative spontaneous remission source | |||||||
| Beltrani 2002 | 31,828 | 12.28 | 31,568 | 12.10 | 260 | 0.183 | 1,419 |
| Toubi 2004 | 26,042 | 12.41 | 25,276 | 12.26 | 766 | 0.155 | 4,936 |
| Van der Valk 2002a | 49,271 | 11.91 | 49,124 | 11.67 | 147 | 0.244 | 601 |
| Background medication sparing effect | 34,886 | 12.20 | 35,729 | 12.00 | -843 | 0.202 | Dominant |
| Narrower perspective (NHS/PSS) |
12,440 | 12.20 | 4,926 | 12.00 | 7,513 | 0.202 | 37,218 |
| Alternative time horizon (10 years) | 29,926 | 7.11 | 29,220 | 6.93 | 706 | 0.187 | 3,777 |
| Pooled ASTERIA I and ASTERIA II datac | 27,048 | 12.47 | 26,495 | 12.36 | 554 | 0.120 | 4,631 |
BOCF baseline observation carried forward; ICER incremental cost-effectiveness ratio; LOCF last observation carried forward; NHS National Health Service; PSS Personal Social Services; SOC standard of care; QALYs quality-adjusted life years; UAS7 Urticaria Activity Score over 7 days
aNumbers based on the CSU population reported in the Van der Valk publication; bExponential extrapolations do not reach 100% of patients. Patients remaining in response at 16 months post treatment discontinuation are “forced” to relapse in this scenario; cFor this scenario, data from the ASTERIA I and ASTERIA II trials was used in place of data from GLACIAL and the following parameters were altered: omalizumab treatment stopped after 3 doses; exponential extrapolation of relapse used; Beltrani used as the source of remission data; observed data (no imputation for missing data) used
Table 8.
Exploration of alternative stopping rules with omalizumab
| Scenario analysis exploring alternative stopping rules for omalizumab | Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£ per QALY) |
|---|---|---|---|---|---|
| SOC | 35,729 | – | |||
| Omalizumab with early stop for non-responders after one dose | 35,933 | 12.124 | 204 | 0.125 | 1633 |
| Omalizumab with early stop for non-responders after four doses (base case) | 36,372 | 12.201 | 438 | 0.077 | 5710 |
| Omalizumab with 6 months of treatment for all | 36,642 | 12.206 | 270 | 0.005 | 52,235 |
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-years, SOC standard of care
Discussion
The cost effectiveness of omalizumab has previously been evaluated by NICE and the SMC from an NHS/PSS perspective in the CSU patient population with an inadequate response to SOC [25]. Given that CSU affects patients of working age and has a demonstrable impact on absenteeism and work impairment, the societal perspective may be more appropriate [9, 10]. The current analysis demonstrates productivity inputs to be key model drivers, and finds that omalizumab is of net societal benefit when considering this broader perspective. This finding is robust to exploration of uncertainty through PSA, OWSA and a range of scenario analyses. While reimbursement decisions in the UK are based on a narrower perspective, these results highlight the potential impact on decision making of considering the societal perspective.
This model was developed in close collaboration with clinicians and health economists, which supports the validity of the model design. Patient-level data from the high-quality GLACIAL RCT (as well as the ASTERIA RCTs for scenario analysis) provided the source for several model inputs and thus the model reflects observations from these trials; for example, in reflecting the observation that patients who received placebo in these trials nevertheless experienced a response [13]. Furthermore, the model structure allowed patients to ‘jump’ between UAS7 health states, which accurately reflects the patient experience of CSU as one of non-linear and unpredictable changes in disease activity.
Other strengths of the analysis include the use of a systematic literature review to determine probabilities of spontaneous remission, a UK-based primary source of productivity inputs and the use of NHS reference costs appropriate to the UK perspective [9, 27, 30]. Furthermore, health-state utility inputs were based on the well-established EQ-5D questionnaire, collected from patients within the trials and valued using an algorithm derived from preferences of the UK general public [37].
A key limitation of the analysis is the lack of available evidence to comprehensively evaluate all potential comparators with omalizumab. Treatment options are limited for patients with inadequate response to SOC, with omalizumab representing the only licensed alternative to continuation of SOC. Nonetheless, in clinical practice, ciclosporin might also be used to treat these patients [1]. Similarly, in the scenario analysis based on the ASTERIA I and ASTERIA II trials, alternative comparator treatments according to treatment guidelines could include ciclosporin or LTRAs [1]; however, a systematic literature review identified an insufficient evidence base to allow for inclusion of these comparators [38].
The analysis was unable to capture some potentially important elements of CSU and its treatment. One limitation of the model structure is a potential insensitivity to improvement in patient condition. Within the model, patients can experience a large improvement in UAS7 (e.g. moving from ‘severe’ to ‘mild’ urticaria) without achieving a response, whereas a patient experiencing a much smaller absolute improvement in UAS7 might be considered a responder due to crossing the response ‘threshold’. The model is also unable to capture HRQoL benefits that may arise from rapid onset of treatment effects in time frames shorter than the model cycle length of 4 weeks. Omalizumab has demonstrated a rapid onset of action, the benefit of which may not be captured [13, 24]. Similarly, this analysis did not account for any potential impact of omalizumab in reducing requirements for concomitant steroids and immunosuppressants alongside SOC. Real-world evidence has suggested the potential for omalizumab to reduce requirements for these therapies; if modelled, this would reduce costs in the omalizumab arm and further increase the net societal benefit of omalizumab [39, 40].
Finally, presenteeism and absenteeism inputs were based on Work Productivity and Activity Impairment (WPAI) data from UK patients in the real-world ASSURE-CSU study [32]. A strength of this source is simultaneous collection of WPAI data alongside UAS7 scores, generating robust productivity estimates by health state.The difference in mean presenteeism estimates for ‘mild urticaria’ and ‘well-controlled urticaria’ health states might be interpreted as inconsistent with the similar utility estimates for these two states. However, an analysis reducing mean presenteeism in the ‘mild urticaria’ health state to 0 did not meaningfully impact the ICER (£4758 per QALY).
Areas of Further Research
Spontaneous remission is a key feature of CSU; however, reported remission rates vary [4, 26, 35, 36]. Although this analysis attempted to account for this by exploring alternative sources of remission data, further research to understand the ‘true’ rates of spontaneous remission (and in the UK population specifically) are required. Larger, controlled studies investigating the efficacy of omalizumab upon retreatment, and further data sources for time to relapse following discontinuation of omalizumab, would be welcome as these represent key elements of the model structure.
Conclusions
Omalizumab as an add-on therapy to SOC for patients with an inadequate response to SOC represents a treatment option with societal benefit to the UK compared with continued SOC alone. This evaluation highlights the importance of considering the appropriate perspective and presents, to the authors’ knowledge, the first decision analytic model for the health economic analysis of omalizumab in this indication. Finally, this research highlights important areas for future research to further develop modelling approaches for this burdensome condition.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors acknowledge Costello Medical Consulting, UK, for writing and editorial assistance, and Dr. Oliver Rivero-Arias for independent quality control review of the economic model on which the model presented in this publication is based.
Compliance with Ethical Standards
Disclosure of potential conflicts of interest
Jonathan Graham, Doreen McBride, Donald Stull, Matthew Griffiths and Ion Agirrezabal were paid consultants of Novartis during the development of this research. Anna Halliday, Stamatia Theodora Alexopoulos and Maria-Magdalena Balp were employees of Novartis during the development of this research. Torsten Zuberbier has received consulting fees, research grants and/or honoraria from Novartis. Alan Brennan has received consulting fees from Novartis.
Funding
This research was funded by Novartis.
Footnotes
A discrepancy between the details in the text and the Kaplan–Meier curve in the Nebiolo et al. publication was identified and corrected by the Southampton Health Technology Assessments Centre as part of the NICE Technology Appraisal 339 [25]. The corrected values were used in this evaluation.
References
- 1.Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69(7):868–887. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 2.Maurer M, Weller K, Bindslev-Jensen C, Gimenez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN Task Force report. Allergy. 2011;66(3):317–330. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 3.Zuberbier T. Chronic urticaria. Curr Allergy Asthma Rep. 2012;12(4):267–272. doi: 10.1007/s11882-012-0270-7. [DOI] [PubMed] [Google Scholar]
- 4.Beltrani VS. An overview of chronic urticaria. Clin Rev Allergy Immunol. 2002;23(2):147–169. doi: 10.1385/CRIAI:23:2:147. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014;34(1):89–104. doi: 10.1016/j.iac.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Grob JJ, Revuz J, Ortonne JP, Auquier P, Lorette G. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol. 2005;152(2):289–295. doi: 10.1111/j.1365-2133.2005.06385.x. [DOI] [PubMed] [Google Scholar]
- 7.O’donnell B, Lawlor F, Simpson J, Morgan M, Greaves M. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136(2):197–201. doi: 10.1046/j.1365-2133.1997.d01-1168.x. [DOI] [PubMed] [Google Scholar]
- 8.Balp M-M, Vietri J, Tian H, Isherwood G. The impact of chronic urticaria from the patient’s perspective: a survey in five European countries. Patient. 2015;8(6):551–558. doi: 10.1007/s40271-015-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grattan C, Balp M-M, Halliday A, Abouzakouk M, Hollis K, McBride D, et al. ASSURE-CSU preliminary UK results: assessing the impact of CSU/CIU on absence from work and work productivity [abstract]. In: Presented at the 23rd World Congress of Dermatology; 8–13 Jun 2015: Vancouver.
- 10.Balp MM, Chambenoit O, Chiva-Razavi S, Lynde C, Sussman G, Chapman-Rothe N, et al. Work productivity and impairment among chronic spontaneous/idiopathic urticaria patients: results from the first international burden of illness study (ASSURE-CSU) [poster]. In: Presented at the 18th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research; Nov 2015: Milan.
- 11.Weller K, Viehmann K, Bräutigam M, Krause K, Siebenhaar F, Zuberbier T, et al. Management of chronic spontaneous urticaria in real life–in accordance with the guidelines? A cross-sectional physician-based survey study. J Eur Acad Dermatol Venereol. 2013;27(1):43–50. doi: 10.1111/j.1468-3083.2011.04370.x. [DOI] [PubMed] [Google Scholar]
- 12.Kapp A, Demarteau N. Cost effectiveness of levocetirizine in chronic idiopathic urticaria : a pooled analysis of two randomised controlled trials. Clin Drug Investig. 2006;26(1):1–11. doi: 10.2165/00044011-200626010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132(1):101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Grattan CE, Humphreys F. Guidelines for evaluation and management of urticaria in adults and children. Br J Dermatol. 2007;157(6):1116–1123. doi: 10.1111/j.1365-2133.2007.08283.x. [DOI] [PubMed] [Google Scholar]
- 15.Powell RJ, Leech SC, Till S, Huber PA, Nasser SM, Clark AT. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45(3):547–565. doi: 10.1111/cea.12494. [DOI] [PubMed] [Google Scholar]
- 16.Maurer M, Rosén K, Hsieh H-J, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 17.Saini SS, Bindslev-Jensen C, Maurer M, Grob J-J, Baskan EB, Bradley MS, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study [published erratum appears in J Invest Dermatol. 2015;135(3):925] J Invest Dermatol. 2015;135(1):67–75. doi: 10.1038/jid.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treasury HM. The green book: appraisal and evaluation in Central Government. London: TSO; 2003. [Google Scholar]
- 19.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. Available from: https://www.nice.org.uk/article/PMG9/chapter/1-Introduction. 2013. Accessed 9 May 2016 [PubMed]
- 20.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Gimenez-Arnau A, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64(10):1417–1426. doi: 10.1111/j.1398-9995.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 21.Mlynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63(6):777–780. doi: 10.1111/j.1398-9995.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 22.Stull DE, McBride D, Balp MM, Gnanasakthy A. Correlations between changes in the Urticaria Activity Score (UAS7) and the Dermatology Life Quality Index (DLQI) from baseline to 28 or 40 weeks: comparisons of trajectories of change in patients with chronic spontaneous/idiopathic urticaria (CSU/CIU) Value Health. 2013;16(7):A509. doi: 10.1016/j.jval.2013.08.1183. [DOI] [Google Scholar]
- 23.Stull D, McBride D, Georgiou P, Zuberbier T, Grattan C, Balp M-M. Measuring patient severity in chronic spontaneous/idiopathic urticaria (CSU/CIU) as categorical health states: efficient and informative. Allergy. 2014;69(Suppl 99):317. [Google Scholar]
- 24.Metz M, Ohanyan T, Church MK, Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci. 2014;73(1):57–62. doi: 10.1016/j.jdermsci.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence. Omalizumab for previously treated chronic spontaneous urticaria. NICE technology appraisal guidance 339 [TA339]. 8 Jun 2015.
- 26.Nebiolo F, Bergia R, Bommarito L, Bugiani M, Heffler E, Carosso A, et al. Effect of arterial hypertension on chronic urticaria duration. Ann Allergy Asthma Immunol. 2009;103(5):407–410. doi: 10.1016/S1081-1206(10)60360-2. [DOI] [PubMed] [Google Scholar]
- 27.Marsland A, Balp MM, Halliday A, Alexopoulos ST, Allen F, Buchanan-Hughes A, et al. The natural course of chronic spontaneous urticaria: a systematic review [abstract no. COP15-1728]. In: 24th European Academy of Dermatology and Venereology Congress; 7–11 Oct 2015; Copenhagen.
- 28.Office for National Statistics. Life tables. May 2014. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsregisteredinenglandandwalesseriesdrreferencetables. Accessed 9 May 2016
- 29.Hawe E, McBride D, Balp M-M, Tian H, Halliday A, Stull DE. EQ-5D utilities in chronic spontaneous/idiopathic urticaria. Pharmacoeconomics. 2016;34(5):521–527. doi: 10.1007/s40273-015-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Health Service. National schedule of reference costs 2013–14. https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014. Accessed 9 May 2016
- 31.Tian H, Chambenoit O, Chiva-Razavi S, Lynde C, Sussman G, Chapman-Rothe N, et al. Healthcare resource utilisation among chronic spontaneous/idopathic urticaria patients: findings from the first international burden of illness study (ASSURE-CSU) [poster]. In: Presented at the 18th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research; Nov 2015: Milan.
- 32.Weller K, Maurer M, Grattan C, Nakonechna A, Abuzakouk M, Berard F, et al. ASSURE-CSU: a real-world study of burden of disease in patients with symptomatic chronic spontaneous urticaria. Clin Transl Allergy. 2015;5:29. doi: 10.1186/s13601-015-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Office for National Statistics. Weekly earnings. May 2014.http://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/datasets/summaryoflabourmarketstatistics/current. Accessed 9 May 2016
- 34.Office for National Statistics. Consumer price inflation May 2014.http://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/icvj. Accessed 9 May 2016
- 35.Toubi E, Kessel A, Avshovich N, Bamberger E, Sabo E, Nusem D, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy. 2004;59(8):869–873. doi: 10.1111/j.1398-9995.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 36.van der Valk PG, Moret G, Kiemeney LA. The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br J Dermatol. 2002;146(1):110–113. doi: 10.1046/j.1365-2133.2002.04582.x. [DOI] [PubMed] [Google Scholar]
- 37.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316(7133):736–741. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell S, Balp MM, Samuel M, McBride D, Maurer M. Systematic review of treatments for chronic spontaneous urticaria with inadequate response to licensed first-line treatments. Int J Dermatol. 2015;54(9):1088–1104. doi: 10.1111/ijd.12727. [DOI] [PubMed] [Google Scholar]
- 39.Song CH, Stern S, Giruparajah M, Berlin N, Sussman GL. Long-term efficacy of fixed-dose omalizumab for patients with severe chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2013;110(2):113–117. doi: 10.1016/j.anai.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence. Omalizumab for treating severe persistent allergic asthma (review of technology appraisal guidance 133 and 201). NICE technology appraisal guidance 278 [TA278]. 24 Apr 2013.
- 41.Hawe E, Stull DE, McBride D, Balp M. Estimating utility data for patient symptom severity in chronic spontaneous urticaria [poster]. In: Presented at the 17th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research; Nov 2014: Amsterdam. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.