Abstract
Circadian clock desynchronization has been implicated in the pathophysiology of cardiovascular disease and related risk factors (eg, obesity, diabetes). Thus, we examined the extent to which circadian desynchronization exacerbates ischemic stroke outcomes and whether its detrimental effects on stroke severity and functional impairments are further modified by biological sex. Circadian entrainment of activity rhythms in all male and female rats was observed during exposure to a fixed light-dark (LD) 12:12 cycle but was severely disrupted when this LD cycle was routinely shifted (12 h advance/5 d) for approximately 7 weeks. In contrast to the regular estrous cycles in fixed LD animals, cyclicity was abolished and persistent estrus was evident in all shifted LD females. The disruption of estrous cyclicity in shifted LD females was associated with a significant increase in serum estradiol levels relative to that observed in fixed LD controls. Circadian rhythm disruption exacerbated stroke outcomes in both shifted LD male and female rats and further amplified sex differences in stroke impairments. In males, but not females, circadian disruption after exposure to the shifted LD cycle was marked by high rates of mortality. In surviving females, circadian desynchronization after exposure to shifted LD cycles produced significant increases in stroke-induced infarct volume and sensorimotor deficits with corresponding decreases in serum IGF-1 levels. These results suggest that circadian rhythm disruption associated with shift work schedules or the irregular nature of our everyday work and/or social environments may interact with other nonmodifiable risk factors such as biological sex to modulate the pathological effects of stroke.
In mammals, the generation and photoentrainment of circadian rhythms is mediated by a hierarchical organization of cell-autonomous clocks distributed throughout the body. In the suprachiasmatic nuclei of the anterior hypothalamus, the ensemble of cellular clocks functions as a master pacemaker in this hierarchical network, whereas clocks located in other brain regions and peripheral tissues provide for the local coordination of tissue- or cell-specific processes in time (1). The circadian timekeeping function of suprachiasmatic nuclei and peripheral clocks is stable under “normal” conditions. However, circadian rhythm disturbances or desynchronization are known to occur in response to shift work, jet lag and even workplace or social influences that commonly impose highly irregular schedules on our sleep-wake patterns, mealtimes, and other health-related processes.
Pathologies and environmental disturbances in the regulation of circadian rhythms have been linked to human health disorders, including vascular disease or related risk factors. Basic research studies have typically used 2 different approaches, genetic mutations in core clock genes and environmental manipulations simulating shift work-type schedules, to illustrate the effects of circadian rhythm disturbances on cardiovascular physiology. Global deletion of the clock gene Bmal1 has been shown to abolish the daily rhythm in blood pressure and induce constant hypotension (2). Furthermore, altered circadian rhythms in Clock mutant mice are accompanied by notable risk factors for cardiovascular disease including obesity and metabolic syndrome (3, 4). In a similar fashion, chronic modulation of circadian rhythms through shift work-like reversing of the light-dark (LD) cycle accelerates the development of coronary pathology and congestive heart failure in cardiomyopathic hamsters (5). Clinical studies provide further evidence implicating circadian rhythm desynchronization, due to shift work or misalignment with abnormal external cycles, as a contributing factor for vascular disease in general and stroke severity/recovery in particular. The alteration of circadian rhythms in subjects maintained on a 28-hour day is associated with hyperglycemia, hyperinsulinemia, and elevated arterial blood pressure (6), all of which substantially increase the risk for cardio- and cerebrovascular disease.
At present, little is known about how circadian disturbances affect stroke severity and how this variable interacts with other nonmodifiable risk factors such as age and biological sex to modulate the pathological effects of stroke. Stroke is an inflammatory disease and poststroke disruption of the blood brain barrier as well as activation of systemic and brain resident immune cells are key factors in the pathophysiology of stroke. Hence, the immune system may be an important target for the pathological effects of circadian disruption on stroke. This possibility is indirectly supported by recent studies demonstrating that global disruption of circadian rhythms in mice with targeted clock gene mutations increases the infiltration and activation of proinflammatory M1 macrophages and exacerbates macrophage inflammatory responses and tissue inflammation (7). Using a middle cerebral artery occlusion (MCAo)-induced model of ischemic stroke, the objective of our experiments was to examine the extent to which circadian rhythm desynchronization exacerbates stroke severity and whether this pathological impact is modified by biological sex.
Materials and Methods
Animals
Adult (5–7 mo old) female and male Sprague Dawley rats were purchased from Harlan Laboratories. All animals were housed individually in cages equipped with running wheels to provide for continuous analysis of wheel-running activity.
Experiments used a chronic LD cycle shift paradigm that has been shown to be effective in desynchronizing circadian rhythms and in inducing pathological changes in cardiovascular physiology (5). After baseline acclimation under LD 12:12 conditions (lights-on at 6 am) for about 2 weeks, animals were randomly divided into 2 groups (n = 10/treatment group) and exposed for 8 weeks either to this “fixed” LD 12:12 cycle or to a “shifted” LD 12:12 cycle. In the shifted LD 12:12 cycle, lights-on was advanced by 12 hours every 5 days for approximately 7 weeks. At the conclusion of this treatment period, animals in both groups were exposed to the same LD 12:12 schedule (lights-on at 6 am) and subjected to experimental ischemic stroke surgery at the same relative time during the circadian cycle (ie, midday or inactive phase). Sensorimotor testing was performed 2 days before and 5 days after MCAo surgery at Zeitgeber time 5 (ie, 5 h after lights-on) to assess functional deficits. At 5 days after MCAo, terminal blood samples were collected and brains were processed for histological analysis of infarct volume. This analysis was separately performed on male rats (n = 20) and on independent cohorts of females (n = 40). All animal procedures used in this study were conducted in compliance with Animal Use Protocol 2014–0021 as reviewed and approved by the Institutional Animal Care and Use Committee at Texas A&M University.
Analysis of wheel-running activity
Wheel-running activity was continuously recorded, stored in 10-minute bins, graphically depicted in actograms and analyzed using ClockLab data collection and analysis software (ActiMetrics). Entrainment and qualitative parameters of the activity rhythm were measured over the same interval for all animals. The onset of activity for a given cycle was identified as the first bin during which an animal attained 10% of peak running-wheel revolutions (ie, intensity). Activity duration (α) was determined by measuring the time interval between the daily activity onsets and offsets. In addition, χ2 periodogram analysis was used to determine the amplitude of the rhythm in wheel-running activity.
Estrous cycle determination
The effects of experimental LD cycle manipulations on estrous cyclicity were tested using separate groups of fixed (n = 8) and shifted (n = 8) LD female rats. Before and immediately after the conclusion of LD manipulations when animals in both groups were exposed to the same LD 12:12 schedule (lights-on at 6 am), estrous cyclicity was assessed by obtaining daily vaginal smears (10–11 am) over a 14- to 20-day period. Smears, obtained with a cotton swab, were placed on a slide and later examined under a microscope (×10 objective; Nikon Instruments) and staged according to commonly accepted criteria (8).
Middle cerebral artery occlusion
Animals were subjected to stereotaxic surgery to occlude the left middle cerebral artery using endothelin-1 (ET-1) as described previously (9, 10). Briefly, animals were anesthetized (ketamine/xylazine) and placed in a stereotaxic apparatus (Kopf). A midline incision was made on the scalp and a craniotomy was performed on the left side with a small drill using the following coordinates (relative to bregma): +0.9 mm anteroposterior, +3.4 mm mediolateral, −8.5 mm dorsoventral. ET-1 (3 μL of 0.5 μg/μL; American Peptide Co Inc) was injected at a rate of 0.25 μL per 30 seconds onto the middle cerebral artery. To minimize backflow, the syringe was maintained in place for 3 minutes after ET-1 administration. Rats were maintained on heating pads during the procedure and then placed under heating lamps during recovery. Poststroke survival was carefully recorded at 24-hour intervals. All surviving animals were sacrificed at day 5 after MCAo. At termination, the brain was rapidly removed and processed for triphenyl tetrazolium chloride (TTC) staining to assess infarct volume.
Infarct volume
Brain sections (2 mm) between −2.00 mm and +4.00 mm from bregma were incubated in a 2% TTC solution at 37°C for 20 minutes. Stained sections were photographed using a Nikon E950 digital camera attached to a dissecting microscope. Digitized images were used to estimate infarct volume using the Quantity One software package (Bio-Rad). Three separate slices were used for analysis, and only the superior face of each slice, which is clearly stained by TTC, was analyzed. The area of the infarct was quantified in all slices and compared with measurement of the total area of the contralateral hemisphere. In each case, infarct volume was determined using the following algorithm: the infarct area of 2 adjacent slices was averaged and then multiplied by the thickness of the slice, and values were added across all slices. A similar approach was used to determine the volume of the nonoccluded hemisphere. Then, volume of the infarct was expressed as a percentage of the contralateral (nonoccluded) hemisphere. To ensure reliable and unbiased estimation of the infarct zone, all images were first coded and then digitally converted to black and white. All volumetric traces were performed by an investigator blind to the codes.
Behavioral assays
Motor impairment after MCAo was assessed using the vibrissae-evoked forelimb placement task as well as the adhesive tape test, using procedures reported by Selvamani and Sohrabji (10) and Balden et al (11). The vibrissae-elicited forelimb placement test was used both before and after MCAo surgery. Animals were subjected to same-side and cross-midline placing trials elicited by stimulating the ipsi- and contralesional vibrissae. During the same-side forelimb placing trials, the animal was gently held such that all 4 limbs were free to move. The animal's ipsilesional vibrissae were brushed against the edge of a table to elicit a forelimb placing response, which typically involved the forelimb ipsilateral to the stimulated vibrissae. Ten trials were performed before the same task was repeated for the contralesional vibrissae. In the cross-midline placing trials, the animal was held gently by the upper body such that the ipsilesional vibrissae lie perpendicular to the tabletop and the forelimb on that side is gently restrained as the vibrissae was brushed on the top of the table to evoke a response from the contralateral limb and vice versa. Between each trial, the animal was allowed to rest all 4 limbs briefly on the tabletop to help relax its muscles. Trials in which the animal seemed to struggle or make premature forelimb movements were not counted. Scoring during the trials was done by an experimenter blind to the animal's treatment group, and was based on a 4-point scale (0–3), with a score of 3 representing brisk forward and upward movement that ended in the paw pads making a flat, full contact with the tabletop.
The adhesive tape test was also performed both before and after surgery. Two pieces of adhesive-backed foam tape (1 × ½”) were used as a tactile stimuli attached to the palmar surface of the paw of each forelimb. For each forelimb, the latency time to remove each stimulus (tape) from the forelimbs was recorded during 3 trials per day for each forepaw. Animals were allowed to rest for 1 minute between sessions, and each test session had a maximum time limit of 120 seconds.
Estradiol assay
Serum estradiol levels in terminal blood samples were measured using a commercial enzyme immunoassay kit (Cayman) as per manufacturer's protocol. Serum and standard samples (50 μL/well) were assayed in duplicate. Assay sensitivity ranged from 6.6 to 4000 pg/mL. Plates were read at 420 nm in a plate reader (BioTek), and sample measurements were interpolated from the standard curve. The intraassay coefficient of variation was 7.2%.
IGF-1 ELISA
Concentrations of circulating insulin-like growth factor (IGF)-1 in plasma were determined by ELISA (R&D Systems), according to manufacturer's instructions. Briefly, standards, controls, and aliquots of serum samples were loaded into a 96-well plate precoated with antibodies specific for IGF-1 and incubated at room temperature for 2 hours. With intervening washes, plates were sequentially incubated with 100 μL of conjugate for 2 hours, and 100 μL of substrate solution for 30 minutes. The color reaction was stopped by an equal volume of stop solution and read at 450 nm (540-nm reference wavelength) in a microplate reader (Bio-Tek). Standard curves were established from optical densities of wells containing known dilutions of standard (31.2–2000 pg/mL) using KC3 software (Bio-Tek), and sample measurements were interpolated from standard curves. For homogenized tissues, the results of these assays were normalized for individual sample protein content. The intraassay coefficient of variation was 4.3%.
Statistical analysis
Kaplan-Meier survival plots were used to assess poststroke mortality in males and females. Quantitative measurements of daily wheel-running activity were analyzed by two-way ANOVA for sex (male, female) and LD treatment (fixed, shifted) differences. Based on survival data, infarct volume and sensorimotor behavior were assessed further only in female subjects for statistical differences among treatment groups. For behavioral tests, a paired Student's t test was initially used within each group to compare pre- and poststroke values. For infarct volume and behavioral data, group differences were evaluated using a pooled Student's t test. In each case, group differences were considered significant at P < .05.
Results
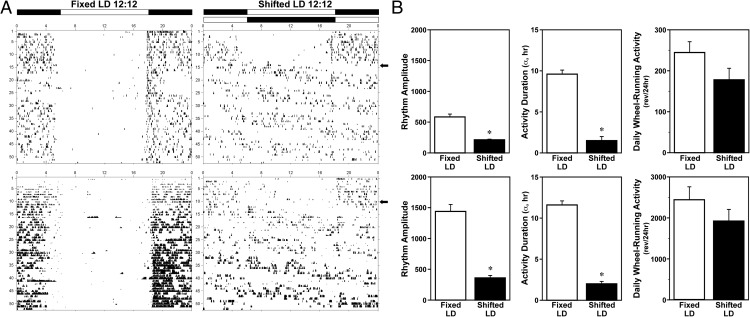
During baseline exposure to LD 12:12 lighting conditions, entrainment of the circadian rhythm of wheel-running activity was observed in all animals. Throughout the 8-week experimental analysis, all male and female rats in the fixed LD treatment group maintained stable entrainment to LD 12:12 (Figure 1A) such that their daily onsets of activity consistently occurred around 5–15 minutes after lights-off (6 pm). In contrast, male and female rats exposed to shifted LD cycles were distinguished by desynchronized patterns of wheel-running behavior in which the phase angle between the onset of activity and lights-off was highly variable from day to day. In shifted LD male and female rats, both the amplitude of the activity rhythm and the duration of wheel-running activity were significantly (P < .05) decreased in comparison with fixed LD animals of the same sex (Figure 1B). Despite these alterations in the entrainment and qualitative parameters of the activity rhythm, exposure to shifted LD cycles had no significant effect on the total amount of daily wheel-running behavior relative to that observed in fixed LD controls (Figure 1B) (main effect of LD cycle; F1,35 = 0.33). Similar to the sex differences reported previously (12, 13), daily activity levels (wheel revolutions/24 h) were significantly (main effect of sex; F1,35 = 32.03; P < .05) and approximately 10-fold greater in female subjects than in their male counterparts within each treatment group.
Figure 1. Effects of shifted LD cycles on circadian entrainment and other properties of the rhythm in wheel-running activity.
A, Representative records of wheel-running activity in adult male (top panel) and female (bottom panel) rats that were maintained in a fixed LD 12:12 cycle (left) or exposed to a shifted (12 h/5 d) LD 12:12 cycle (right). Actograms are plotted over a 24-hour period. The open and closed bars at the top, respectively, signify the timing of the light and dark phase in the fixed and shifted LD 12:12 cycles. B, Rhythm amplitude, activity duration (α), and total daily wheel-running activity of adult male (top) and female (bottom) rats exposed to fixed or shifted LD cycles. Bars depict average wheel revolutions per 24 hours (±SEM). *, P < .05.
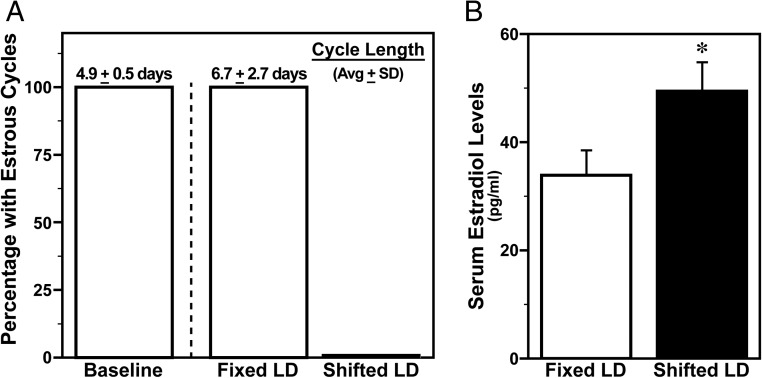
During baseline analysis before experimental LD manipulations, regular estrous cycles with a length of 4–5 days were observed in all female rats. After exposure to experimental lighting conditions for 40 days, all females on the fixed LD cycle exhibited normal estrous cycles albeit of longer duration (∼7 d), whereas cyclicity was completely abolished and smears were indicative of persistent estrus in all shifted LD animals (Figure 2A). In conjunction with this loss of estrous cyclicity and the sustained condition of persistent estrus, serum estradiol levels in shifted LD females were significantly higher (P < .05) than those found in fixed LD controls. Serum estradiol levels in blood samples collected 5 days after MCAo ranged from 18 to 98 pg/mL in shifted LD females and from 6 to 82 pg/mL in the fixed LD group.
Figure 2. Shifted LD cycles abolish estrous cyclicity and increase serum estradiol levels.
A, Determination of estrous cyclicity and cycle length in adult female rats exposed to fixed or shifted LD 12:12 cycles. Bars depict baseline determinations in all animals before experimental lighting conditions (left) and posttreatment analyses after exposure to fixed or shifted LD cycles for 2 months. The average (±SD) estrous cycle length is shown above the corresponding bar. B, Serum estradiol levels in female rats exposed to fixed or shifted LD cycles. Bars depict average estradiol levels (±SEM) in terminal blood obtained 5 days after MCAo surgery. *, P < .05.
Sex differences were clearly evident in the effects of circadian desynchronization on stroke-induced pathological outcomes. Kaplan-Meier survival plots indicate that in males, but not females, exposure to shifted LD cycles is associated with a significant increase (P < .005) in mortality and decrease in survival time relative to fixed LD controls (Figure 3). Among female subjects, the rate of mortality in response to MCAo-induced stroke was low (20%) and identical in fixed and shifted LD animals. In addition, there were no group differences in survival time, with all mortalities in control and experimental females occurring on day 2 after surgery. By comparison, male rats exposed to shifted LD cycles were distinguished by short survival times (1 d) and extremely high rates of mortality (70%).
Figure 3. Shifted LD cycles dramatically increase mortality after MCAo-induced stroke in adult male but not female rats.
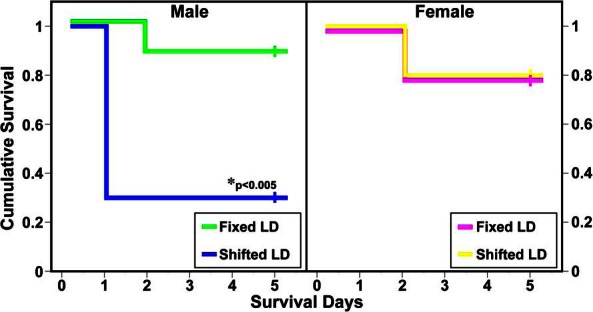
Kaplan-Meier survival plots after MCAo-induced stroke in adult male (left panel) and female (right panel) rats exposed to fixed or shifted LD 12:12 cycles. The individual plots depict the conditional probability of survival over postsurgery days 0–5. In males, but not females, exposure to shifted LD cycles had significant effects in increasing mortality (P < .005) and decreasing survival time relative to that found in fixed LD controls.
Further analysis of surviving females revealed that circadian desynchronization during exposure to shifted LD cycles also amplified the extent of stroke-induced brain injury and functional impairment. Based on TTC staining in coronal sections obtained 5 days after ET-1-induced MCAo, all surviving females in both LD paradigms exhibited evidence of cortical and striatal infarction. However, females exposed to shifted LD cycles had significantly larger (P < .05) infarct volumes compared with fixed LD controls (Figure 4). At 5 days after MCAo, the size of stroke-induced infarction in shifted LD females was increased by approximately 2-fold relative to the stroke volume observed in the fixed LD group.
Figure 4. Shifted LD cycles amplify the size of MCAo-induced infarction in adult female rats.
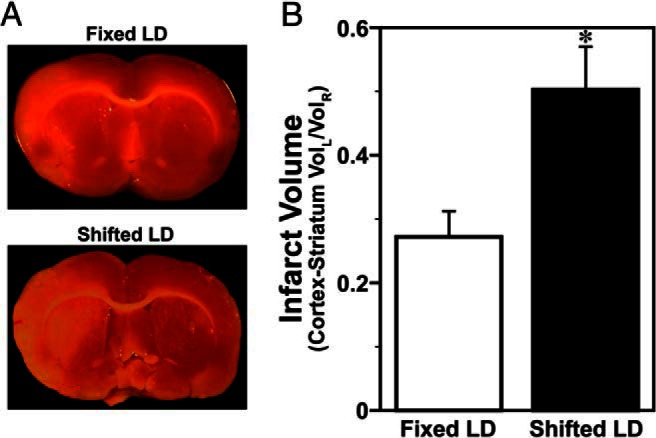
TTC staining was used to quantify MCAo-induced infarct volume in adult female rats exposed to fixed (n = 18) or shifted (n = 18) LD 12:12 cycles. A, Representative TTC-stained sections illustrating cortical and striatal lesions (unstained) in the left hemisphere. B, Bars depict infarct volumes (cortex and striatum) normalized to the contralateral hemisphere. *, P < .05.
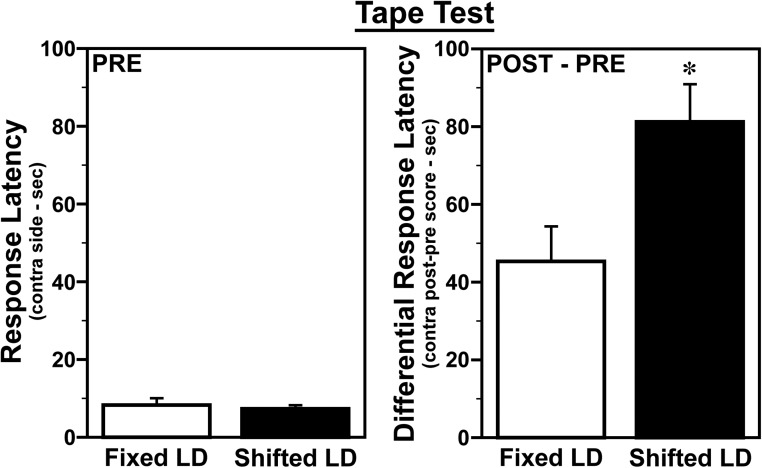
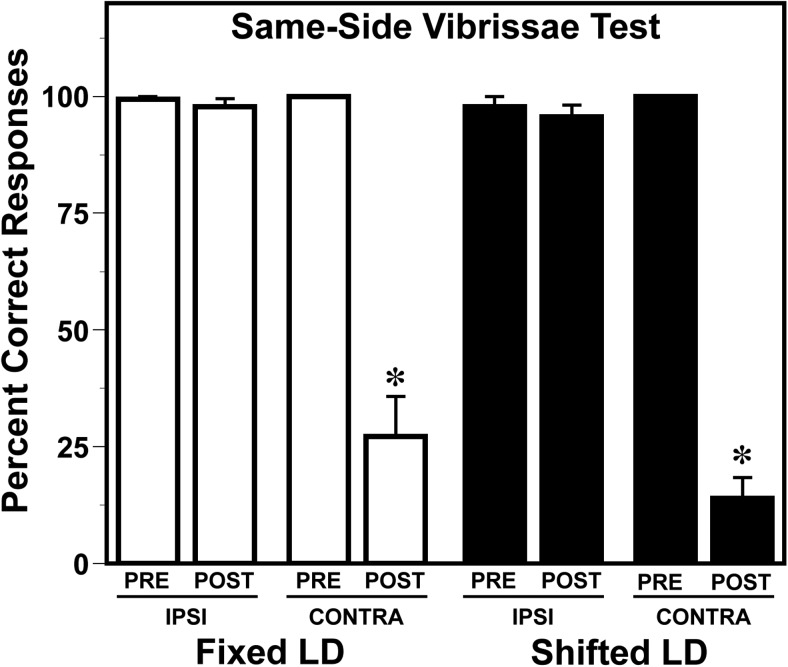
Functional deficits associated with MCAo-induced infarction were assessed using 2 independent tests of sensorimotor performance. MCAo reduced accuracy on the vibrissae-evoked paw placement task. Performance on this task, which requires a reaching movement with the front paw in response to stimulation of the vibrissae, was accurate for both paws in all animals before stroke (Figure 5). After stroke, performance in all animals was unchanged with the ipsilesional paw, but was significantly impaired (P < .05) with the contralesional paw relative to prestroke standards, typical of MCAo-induced infarction. However, the extent of this impairment with the contralesional paw was similar in both fixed LD and shifted LD groups. Analysis of performance on the adhesive tape test, which is commonly used in MCAo stroke models as a more sensitive indicator of sensorimotor deficits (14), resolved treatment group differences in stroke-induced functional impairment. Consistent with the effects of circadian desynchronization on infarct volume, MCAo-induced impairment of sensorimotor function on the adhesive removal test was more severe in shifted LD females than fixed LD controls. No significant pre- and poststroke differences between the fixed and shifted LD groups were observed in the average latency for tape removal from the ipsilesional paw. In both groups, the latency for tape removal was increased in the contralesional paw after stroke (Figure 6), but the differences between pre- and poststroke times in shifted LD females were about 2-fold and significantly greater (P < .05) than those observed in fixed LD controls.
Figure 5. Shifted LD cycles exacerbate MCAo-induced impairment on the adhesive tape test in adult female rats.
Sensorimotor performance in female rats exposed to fixed or shifted LD cycles was assessed 2 days before (PRE) and 5 days after (POST) MCAo surgery using the adhesive tape test. Individual panels depict prestroke response latency for tape removal (left panel) and differences between post- and prestroke latency times (right panel) on the contralesional (right) side. Bars represent average values (±SEM). *, P < .05.
Figure 6. MCAo-induced impairment on the vibrissae-evoked forelimb placement task is similar in adult female rats exposed to fixed or shifted LD cycles.
Sensorimotor performance in female rats exposed to fixed or shifted LD cycles was examined 2 days before (PRE) and 5 days after (POST) MCAo surgery using the vibrissae evoked forelimb placement task. Bars depict percent correct responses for the ipsilesional (IPSI) and contralesional (CONTRA) paw before and after stroke. *, P < .05.
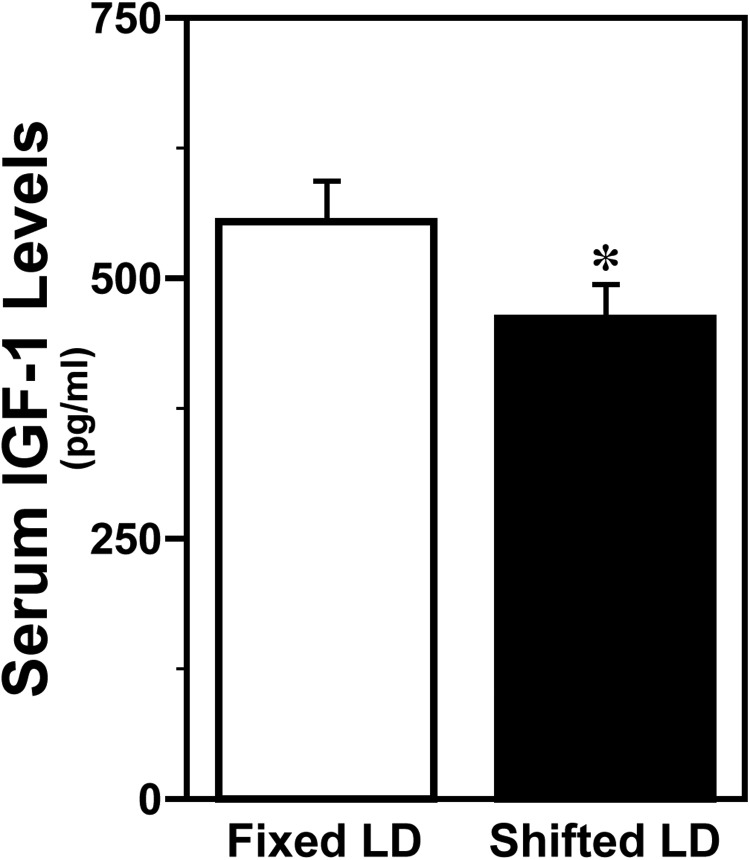
Because IGF-1 is an intermediary factor that acts synergistically with estrogen to modulate neuroprotective responses to stroke (9, 10), circulating levels of IGF-1 were also analyzed in all animals poststroke. IGF-1 was ubiquitously detected in serum from both treatment groups, but circulating levels of this growth factor were significantly decreased (P < .05) in shifted LD females (Figure 7). Specifically, exposure to shifted LD cycles reduced serum IGF-1 levels by 17% in comparison with those observed in fixed LD controls.
Figure 7. Shifted LD cycles decrease serum IGF-1 levels in adult female rats.
Serum IGF-1 levels in female rats exposed to fixed or shifted LD cycles. Bars depict average IGF-1 levels (±SEM) in terminal blood obtained 5 days after MCAo surgery. *, P < .05.
Discussion
Shift work schedules have been linked to circadian rhythm disruption and its attendant role in chronic heath disorders especially vascular disease. Among women in the Nurses Health Study, the risk of cardiovascular disease after adjustment for cigarette smoking and related health factors was higher in subjects who had worked rotating night shifts relative to those with no shift work experience (15). Corresponding results from a Danish cohort study indicate that the relative risk for cardiovascular diseases was again increased among nonday workers compared with day workers (16). Data indicating that stroke- and cardiovascular-related, but not overall, mortality are significantly increased among male shift workers in Sweden provides further evidence for the susceptibility of the vascular system to circadian rhythm disruption (17). In support of these epidemiological observations on the link between shift work and vascular disease, the present study demonstrates that periodic rotation of the LD cycle disrupts circadian entrainment of the activity rhythm and increases the severity of stroke outcomes in adult rats. Circadian rhythm disruption in response to the shifted LD cycle was accompanied by an increase in MCAo-induced mortality in males, whereas this exacerbated stroke severity in females such that infarct volume and sensorimotor deficits were increased. It is noteworthy that in females, exposure to the shifted LD cycle also abolished estrous cyclicity, resulting in constant estrus and elevated levels of circulating estradiol.
Stroke occurrence and severity are gated on a rhythmic basis. In a study of cases in the Takashima Stroke Registry with a classifiable time of onset, ischemic events were marked by significant diurnal variation, with the largest proportion occurring in the morning (40.7) and the lowest during the night (14.0) even after adjustment for age, gender, and risk factors (18). Furthermore, case fatality was higher for morning strokes as compared with other times of day. Coincident with this pattern, molecular mediators of myocardial infarction and stroke, such as plasminogen activator inhibitor, peak in the morning and may therefore contribute to the time-dependent gating of infarction (19).
Stroke risk, presentation, and long-term recovery differ in males and females; in younger demographics, more strokes occur in men than women but with advancing age (60+ y), where stroke morbidity is much higher, women have a greater risk for the disease with worse outcomes (reviewed in Ref. 20). Sex differences in response to experimental ischemic stroke are also well established in animal models. Young females sustain smaller infarcts and better cerebral blood flow than age-matched males (21–23). In the present study, circadian rhythm disruption appears to exacerbate these sex differences in stroke impairments because exposure to shifted LD cycles dramatically increased stroke-induced mortality in male but not female rats. Ovarian hormones, especially estrogen, may mediate the female advantage with regard to the lethal pathological effects of circadian rhythm disruption on stroke outcomes. This hypothesis is indirectly supported by studies demonstrating that infarct volume is increased in ovariectomized females relative to intact animals or estrogen-replaced animals (9, 24–26) and that exogenous estrogen treatment also promotes neuroprotection in response to stroke in males (27).
Although the neuroprotective effects of estrogen are germane in explaining sex differences in stroke outcomes associated with circadian rhythm disruption, other factors may play a more critical role in stroke neuroprotection in females than estrogen alone. In this regard, IGF-1 plays a key role in modulating neuroprotective responses to stroke in young females. Low levels of IGF-1 are associated with increased morbidity and mortality in ischemic heart disease and stroke (28–30). In addition, exogenous IGF-1 reduces ischemic injury (31–33), stimulates stroke-induced neurogenesis (34) and promotes neuronal survival, neuronal myelination and angiogenesis (35, 36). In the current study, increased infarct size and sensorimotor deficits in shifted LD females were accompanied by an apparent decrease in circulating levels of IGF-1. Because this finding is based on single time-point analysis during the midday coincident with the rhythmic peak in circulating levels of IGF-1 (37), the observed changes in IGF-1 levels in shifted LD females may alternatively reflect a temporal modification in the phase of the IGF-1 rhythm. Nonetheless, it is possible that these changes in the levels or rhythmic pattern of IGF-1 in the circulation, coupled with the loss of estrous cyclicity, may impose an aging phenotype in young females that is characterized by poor stroke outcomes.
The specific mechanisms by which circadian disturbances affect stroke severity and interact with other nonmodifiable risk factors to modulate the pathological effects of stroke are unknown. Stroke is an inflammatory disease and poststroke disruption of the blood brain barrier in concert with activation of systemic and brain resident immune cells have been implicated in the pathophysiology of stroke. Hence, the immune system may be an important target for the pathological effects of circadian disruption on stroke. This possibility is indirectly supported by studies demonstrating that global disruption of circadian rhythms in mice with targeted mutations in the clock genes Per1 and Per2 potentiates the activation and inflammatory responses of proinflammatory M1 macrophages, leading to increased tissue inflammation (7). It is anticipated that future applications of this model will yield novel insight into the pathophysiological changes in endocrine signals, cytokines and growth factors that link disordered circadian clocks to cerebrovascular disease.
Acknowledgments
This work was supported by the American Heart Association (AHA) Grant 14GRNT18370013 (to D.J.E.) and the National Institutes of Health (NIH) Grant R01-NS074895 (to F.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ET-1
- endothelin-1
- IGF-1
- insulin-like growth factor-1
- LD
- light-dark
- MCAo
- middle cerebral artery occlusion
- TTC
- triphenyl tetrazolium chloride.
References
- 1. Bell-Pedersen D, Cassone VM, Earnest DJ, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, FitzGerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA. 2007;104:3450–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–H2337. [DOI] [PubMed] [Google Scholar]
- 6. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu H, Li H, Woo S-L, et al. Myeloid cell-specific circadian clock disruption exacerbates diet-induced inflammation and insulin resistance. J Biol Chem. 2014;289:16374–16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldman JM, Murr AS, Cooper R. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. [DOI] [PubMed] [Google Scholar]
- 9. Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2010;31:1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30:6852–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagenauer MH, King AF, Possidente B, et al. Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Horm Behav. 2011;60:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schull J, Walker J, Fitzgerald K, et al. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav. 1989;46:341–346. [DOI] [PubMed] [Google Scholar]
- 14. Zhang L, Chen J, Li Y, Zhang ZG, Chopp M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J Neurol Sci. 2000;174:141–146. [DOI] [PubMed] [Google Scholar]
- 15. Kawachi I, Colditz GA, Stampfer MJ, et al. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. [DOI] [PubMed] [Google Scholar]
- 16. Tuchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med. 2006;63:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karlsson B, Alfredsson L, Knutsson A, Andersson E, Torén K. Total mortality and cause-specific mortality of Swedish shift- and dayworkers in the pulp and paper industry in 1952–2001. Scand J Work Environ Health. 2005;31:30–35. [DOI] [PubMed] [Google Scholar]
- 18. Turin TC, Kita Y, Rumana N, et al. Is there any circadian variation consequence on acute case fatality of stroke? Takashima Stroke Registry, Japan (1990–2003). Acta Neurol Scand. 2012;125:206–212. [DOI] [PubMed] [Google Scholar]
- 19. Angleton P, Chandler WL, Schmer G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation. 1989;79:101–106. [DOI] [PubMed] [Google Scholar]
- 20. Sohrabji F, Welsh CJ, Reddy DS. Sex differences in neurological diseases. In: Shansky RM, ed. Sex Differences in the Central Nervous System. Book 4 in the Neuroscience-Net Reference Book Series. London, United Kingdom: Academic Press; 2015:297–323. [Google Scholar]
- 21. Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. [DOI] [PubMed] [Google Scholar]
- 22. Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selvamani A, Williams MH, Miranda RC, Sohrabji F. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clin Sci (Lond). 2014;127:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukuda K, Yao H, Ibayashi S, et al. Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats. Stroke. 2000;31:155–160. [DOI] [PubMed] [Google Scholar]
- 25. Simpkins JW, Rajakumar G, Zhang YQ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. [DOI] [PubMed] [Google Scholar]
- 26. Dubal DB, Kashon ML, Pettigrew LC, et al. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. [DOI] [PubMed] [Google Scholar]
- 27. Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. [DOI] [PubMed] [Google Scholar]
- 28. Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke. 1997;28:1744–1748. [DOI] [PubMed] [Google Scholar]
- 29. Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–120. [DOI] [PubMed] [Google Scholar]
- 30. Johnsen SP, Hundborg HH, Sørensen HT, et al. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. 2005;90:5937–5941. [DOI] [PubMed] [Google Scholar]
- 31. Gluckman P, Klempt N, Guan J, et al. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182:593–599. [DOI] [PubMed] [Google Scholar]
- 32. Guan J, Bennet L, George S, et al. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21:493–502. [DOI] [PubMed] [Google Scholar]
- 33. Liu XF, Fawcett JR, Hanson LR, Frey WH., II The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis. 2004;13:16–23. [DOI] [PubMed] [Google Scholar]
- 34. Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. [DOI] [PubMed] [Google Scholar]
- 35. Wang JM, Hayashi T, Zhang WR, Sakai K, Shiro Y, Abe K. Reduction of ischemic brain injury by topical application of insulin-like growth factor-I after transient middle cerebral artery occlusion in rats. Brain Res. 2000;859:381–385. [DOI] [PubMed] [Google Scholar]
- 36. Smith LE. IGF-1 and retinopathy of prematurity in the preterm infant. Biol Neonate. 2005;88:237–244. [DOI] [PubMed] [Google Scholar]
- 37. Bertani S, Carboni L, Criado A, Michielin F, Mangiarini L, Vicentini E. Circadian profile of peripheral hormone levels in Sprague-Dawley rats and in common marmosets (Callithrix jacchus). In Vivo. 2010;24:827–836. [PubMed] [Google Scholar]