Abstract
Normal glucocorticoid secretion is critical for physiological and mental health. Glucocorticoid secretion is dynamically regulated by glucocorticoid-negative feedback; however, the mechanisms of that feedback process are poorly understood. We assessed the temporal characteristics of glucocorticoid-negative feedback in vivo using a procedure for drug infusions and serial blood collection in unanesthetized rats that produced a minimal disruption of basal ACTH plasma levels. We compared the negative feedback effectiveness present when stress onset coincides with corticosterone's (CORT) rapidly rising phase (30 sec pretreatment), high plateau phase (15 min pretreatment), or restored basal phase (60 min pretreatment) as well as effectiveness when CORT infusion occurs after the onset of stress (5 min poststress onset). CORT treatment prior to stress onset acted remarkably fast (within 30 sec) to suppress stress-induced ACTH secretion. Furthermore, fast feedback induction did not require rapid increases in CORT at the time of stress onset (hormone rate independent), and those feedback actions were relatively long lasting (≥15 min). In contrast, CORT elevation after stress onset produced limited and delayed ACTH suppression (stress state resistance). There was a parallel stress-state resistance for CORT inhibition of stress-induced Crh heteronuclear RNA in the paraventricular nucleus but not Pomc heteronuclear RNA in the anterior pituitary. CORT treatment did not suppress stress-induced prolactin secretion, suggesting that CORT feedback is restricted to the control of hypothalamic-pituitary-adrenal axis elements of a stress response. These temporal, stress-state, and system-level features of in vivo CORT feedback provide an important physiological context for ex vivo studies of molecular and cellular mechanisms of CORT-negative feedback.
Glucocorticoid insufficiency or excess contributes to widespread adverse effects on physiological and mental health (1, 2). Understanding mechanisms of glucocorticoid negative feedback is important because alteration of that feedback process may be responsible for inappropriate levels of glucocorticoid secretion. It has long been recognized that glucocorticoid-negative feedback depends on a dynamic temporal progression of cellular changes distributed across cellular components both intrinsic and extrinsic to the hypothalamus-pituitary-adrenal (HPA) axis (3–5). Nevertheless, the specific mechanistic details of this process remains to be determined. Recent ex vivo studies (eg, tissue slice and primary cell culture) have identified novel molecular, cellular and synaptic processes by which glucocorticoids produce rapid and delayed effects on CRH neuron and corticotroph activity (6–10). It is not clear, however, whether these findings are directly relevant to the in vivo systems physiology of HPA axis operation.
Dallman and colleagues (3, 11, 12) were early research pioneers in establishing much of what we know today about glucocorticoid negative feedback. They not only reestablished an appreciation of the physiological importance of glucocorticoid-negative feedback (13), but they also systematically delineated the in vivo parametric features of negative feedback (11, 14–16). The primary empirical approach of those studies was to isolate the effects of acute elevations of glucocorticoids, such as may occur with acute stress, from the other neurophysiological effects of stress by pretreating animals with acute exogenous glucocorticoids prior to HPA axis stimulation (11). This methodological approach has served as the primary paradigm for subsequent studies of glucocorticoid-negative feedback, although it may not represent the more physiologically relevant situation in which elevations of endogenous corticosterone (CORT) follow the stress-induced onset of HPA axis activity.
Early studies found that glucocorticoid suppression of subsequent stimulated HPA axis hormone release depended on two mechanistically distinct temporal domains labeled as fast and delayed CORT-negative feedback (12). Fast feedback was observed when glucocorticoids were administered less than 10 minutes prior to stimulus-induced HPA axis activity, whereas delayed feedback was observed when glucocorticoids were administered more than 45 minutes prior to HPA axis activation (12). Some early studies also observed an absence of glucocorticoid-negative feedback effects (silent period) if glucocorticoid administration occurred during this intervening time period of 10–45 minutes prior to HPA axis stimulation (11, 12, 17, 18).
Given this temporal phenomenology, Dallman and Yates (11) made some important inferences about the mechanistic requirements for the induction of fast and delayed feedback. Because fast feedback could be induced only by glucocorticoid pretreatments that occurred within 10 minutes of HPA axis activation, they concluded that fast feedback induction was hormone rate sensitive and that the expression of fast feedback was short lasting (<10 min). They reasoned that the induction of the suppressive effects required rapid increases in circulating CORT levels and that the expression of those effects rapidly dissipated once rising glucocorticoid levels approached peak plateau levels. In contrast, they reasoned that delayed feedback required glucocorticoid alteration of cellular genomic processes (transcriptional and translational) that took considerable time to develop (12, 19).
Another interesting feature of the original studies by Dallman and Yates (11), which has received little attention, is that the administration of glucocorticoid to rats within several minutes after stimulation of the HPA axis had no influence on the ongoing HPA axis response. This raises the interesting prospect that fast feedback cannot be induced if CORT increases occur after stress onset.
It is noteworthy that some ex vivo studies of glucocorticoid-negative feedback do not support a rate-sensitive requirement for glucocorticoid fast feedback induction, nor do they find that fast feedback expression is short lasting (6, 20). To the best of our knowledge, the stress-state dependence of glucocorticoid fast feedback operation has not been directly tested in ex vivo studies. Although the classic in vivo studies described above have been instrumental in revealing the critical importance of temporal domains of negative feedback, some parametric features of those studies may not be truly representative of the normal physiological situation due to technical limitations at the time (19). Perhaps most noteworthy is that those studies were performed on anesthetized animals (eg, references 11 and 21–23). Consequently, those studies were not only performed on animals in an altered state of central secretion of γ-aminobutyric acid (GABAergic) tone, but also stimulation of the axis could not use psychological stressors but instead relied on various chemical or physical stressors (eg, histamine, ether, laparotomy) (11, 21, 22). Those early studies also lacked the ability to directly measure plasma ACTH.
In light of the limitation of those classic studies, the goal of this study was to use modern methodology to reexamine the in vivo temporal requirements and restrictions (eg, rate sensitivity and silent period) of glucocorticoid-negative feedback on the HPA axis response to acute psychological stress. We used procedures that ensured that CORT treatment, and repeated blood samples were performed in a relatively stress free manner. We monitored HPA axis activity by measuring ACTH levels rather than CORT levels, which provides better temporal resolution of an ongoing HPA axis response and permits a test of the negative feedback effects of CORT on the plasma HPA axis hormone levels. CORT, the rat's endogenous glucocorticoid, may uniquely engage mineralocorticoid receptors, glucocorticoid receptors (GRs), and perhaps other membrane binding sites compared with most synthetic glucocorticoids that selectively bind GR (24). Finally, we also measured postmortem plasma prolactin hormone levels and Crh and Pomc heteronuclear RNA (hnRNA) (25, 26), which each may provide some indirect information about the site of actions for CORT-negative feedback. Although prolactin is not secreted via the HPA axis, it is acutely increased in both male and female rats in response to psychological stress (27–29). The effect or lack of effect of CORT-negative feedback on stress-induced prolactin secretion may provide useful information about whether CORT feedback alters the general central-mediated stress response or instead is selectively restricted to the HPA axis. Both CRH and proopiomelanocortin genes are rapidly induced after stress onset within hypothalamic paraventricular nucleus (PVN) CRH neurons and anterior pituitary corticotrophs, respectively (30, 31). This rapid gene induction can be observed by monitoring short-term changes in the primary transcript level of these two genes (hnRNA) (25, 26), and this may provide useful information about the relative activation state of these two populations of cells in response to our various CORT treatment conditions.
Materials and Methods
Subjects
Adult (290–320 g) male Sprague Dawley rats (Harlan Labs) were housed in polycarbonate tubs (47 × 23 × 20 cm) and maintained on a 12-hour light, 12-hour dark cycle with rat chow and tap water available ad libitum, unless otherwise indicated. All test day manipulations were performed during the first half of the rat's light phase. Rats were given 2 weeks to acclimate to the facility before exposure to surgical and experimental procedures. All animal use was approved by the University of Colorado's Institutional Animal Care and Use Committee.
Jugular vein catheters
Indwelling jugular catheters (SILASTIC brand tubing [Dow Corning Corp], 0.094 cm outer diameter × 0.043 cm inner diameter; Green Rubber) were pretreated with tridodecylmethyl ammonium chloride heparin (Polysciences Inc). One end of each catheter (exterior end) was attached to a 22-gauge threaded infusion cannula (Plastics One; number C313G-5UP/SPC 38172 22GA 5-UP), with its base secured to a Marlex mesh square (Bard Inc) using dental cement. A small square of Mersilene surgical mesh (General Medical) was attached near the other end of the catheter (atrial insertion tip) with glue. Jugular vein catheters were surgically positioned by advancing the SILASTIC brand tip into the right atrium and then secured in place by suture of the attached surgical mesh to surrounding tissue. The other end of the catheter-cannula was sc routed to the dorsal surface immediately behind the shoulder blades and positioned so that the threaded portion of the infusion cannula exited the skin. The infusion cannula was held in place by the attached Marlex mesh that remained sc. After surgery the rats were singly housed, and catheters were flushed daily with 0.5 mL saline-heparinized-gentamicin (0.9%, 20 U-5 U). All rats had a postsurgical recovery period of 4–5 days.
Jugular Infusion and serial blood samples
Rat home cages were outfitted with infusion and sampling apparatus that consisted of a single-axis counterweighted cage mount (number CM375BS; Instech Laboratories) attached to a fluid swivel (number 375/25; Instech Laboratories). Attached to each swivel inlet and exteriorized infusion cannula was a polyethylene tube (product number C313CT/SPC, inner diameter 0.58 × inner diameter 1.27 cm; Plastics One) surrounded by a 30-cm steel coil tether (number C313CS; Plastics One). Polyethylene extension tubing (∼60 cm long) was attached to each swivel outlet. Rats were acclimated to the test day conditions for at least 2 hours daily for 3 days prior to testing. The day before testing, the solution in each catheter line was replaced with 0.5 mL saline-gentamicin (0.9% 5 U; no heparin) to prevent heparin contamination of blood samples that can interfere with the subsequent ACTH assay values (32). On the test day, all catheters were flushed with 0.3 mL saline (0.9%), and each rat was attached to the extended catheter line for 1 hour or longer before testing onset. A bolus infusion of CORT (0.31 mg/kg) or vehicle (10% ethanol mixed with 30% propylene glycol and 60% sterile saline) preloaded into a syringe was slowly administered (∼5 sec) manually into the jugular catheter, followed by 0.3 mL saline infusion to ensure drug clearance through the catheter line. The CORT (Steraloids Inc) solution (0.31 mg/mL) was made fresh each test day. Repeated blood samples (∼0.3 mL) were drawn into disposable 3-mL sterile syringes. Rats received a 0.3-mL saline (0.9%) infusion after each blood sample to minimize hypovolemia. Plasma aliquots were snap frozen on dry ice and stored at −80 C.
Adrenalectomy
Adrenals were bilaterally removed from rats through dorsal-lateral incisions (experiments 1 and 4), immediately after completion of jugular catheter placement. After surgery, adrenalectomized (ADX) rats were given a drinking water solution of 0.9% saline that contained CORT (25 μg/mL) and 0.1% ethanol ad libitum (33). ADX rats were switched to a drinking solution containing only 0.9% saline 12 hours prior to testing to ensure complete clearance of exogenous CORT from the circulation.
Restraint stress
Acute restraint stress consisted of placing rats in clear Plexiglas tubes (length 23.5 cm, diameter 7 cm; with multiple air holes). The tubes restricted lateral, forward, and backward movement but did not interfere with breathing. Restraint is considered to be primarily a psychological rather than physical stressor (34).
Experimental procedures
Validation experiment 1
The time course of CORT plasma levels after infusion of CORT.
Rats were adrenalectomized to ensure that all plasma CORT levels reflected circulating levels produced by the exogenous CORT infusion (n = 10). On the test day, rats were placed in restraint tubes and then given a bolus infusion of CORT (0.31 mg/kg iv) through a chronic indwelling jugular catheter line. Blood samples (∼100 μL) were then taken from the tail vein (tail nick [35]) from each rat at 2, 5, 10, 15, 30, 45, 60, 90, and 120 minutes after the CORT treatment. Samples were collected from the tail vein instead of the jugular catheter to prevent the contamination of the samples by any residual CORT present in the jugular catheter after the CORT infusion. The small blood volumes and stress associated with this tail bleed method precludes ACTH measurement on these samples.
Validation experiment 2
Plasma hormone levels in adrenal-intact rats left in their home cage or placed in restraint immediately after vehicle infusion.
All rats (N = 12) were adrenal intact and surgically fitted with an indwelling jugular catheter. On test day an initial baseline blood sample was taken from all rats while in their home cage. Rats were then given a vehicle infusion, and 30 seconds after infusion half of the rats (n = 6) were placed in restraint tubes. Blood samples were collected 5, 15, and 30 minutes after vehicle infusion. Due to limited plasma volume, only CORT and ACTH were measured in these samples. Immediately after the last blood sample, the rats were decapitated in an area adjacent to the test room, and trunk blood was collected for direct comparison of plasma ACTH and prolactin levels. Plasma aliquots were snap frozen on dry ice and stored (−80°C).
Experiment 3
Test of CORT fast feedback, silent period, and delayed feedback in adrenal-intact rats.
Adrenal intact rats (n = 69, divided into three separate fully counterbalanced cohorts, n = 8–9 rats per treatment group) were surgically fitted with an indwelling jugular catheter. On the test day, rats were infused with either CORT (0.31 mg/kg) or vehicle 1 hour, 15 minutes, or 30 seconds prior to stress onset (30 min restraint) or 5 minutes after the stress onset. A single baseline sample was taken 5 minutes prior to the onset of the restraint. Additional jugular vein blood samples were taken 5, 15, and 30 minutes after the stress onset (Figure 1). The 5-minute poststress onset blood sample immediately preceded CORT/vehicle infusion for the groups that were given an infusion 5 minutes after the stress onset. Immediately after the last serial blood sample (30 minutes restraint), the rats were decapitated and trunk blood collected. Plasma ACTH levels were measured in the serial blood samples, and plasma ACTH and prolactin were measured in the trunk blood.
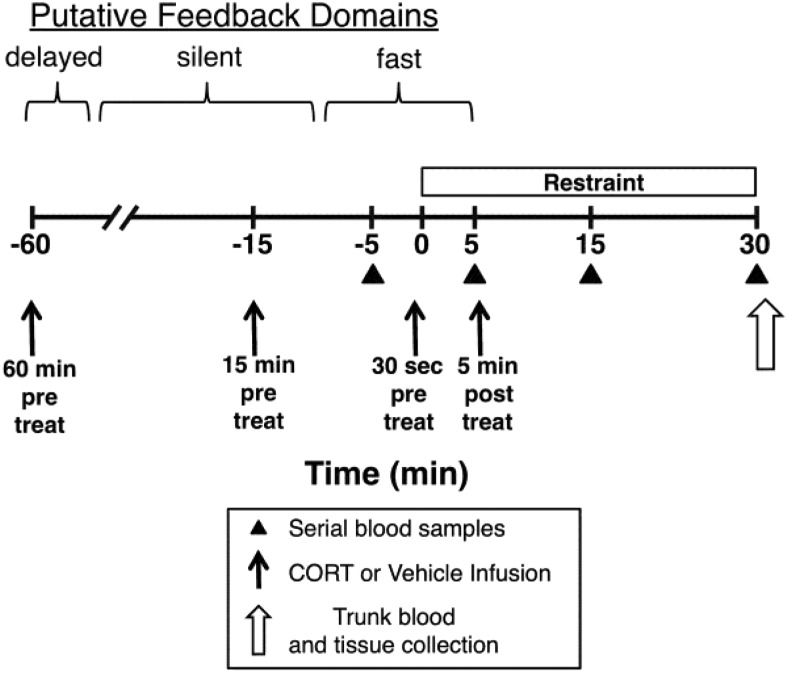
Figure 1. Test day time line for experiments 3 and 4.
Early in the morning of the test day (4–5 d after surgical implantation of indwelling jugular vein catheters), adrenal-intact rats were transferred to a test room (within their home cage) and connected to an extension catheter line that permitted subsequent jugular vein CORT/vehicle infusion and serial blood samples without handling of the rat. Experimental manipulations were initiated approximately 2 hours later. Rats were given an iv infusion of CORT (0.31 mg/kg) or vehicle 60 minutes (experiment 3), 15 minutes (experiment 3), or 30 seconds (experiments 3 and 4) prior to or 5 minutes after (experiments 3 and 4) placement of rats in Plexiglas restraint tubes. Blood samples were taken 5 minutes before restraint onset (baseline) and 5, 15, and 30 minutes after restraint onset. Immediately after the 30-minute blood sample, rats were decapitated and trunk blood (experiment 3) or tissue (experiment 4) was collected. The putative glucocorticoid-negative feedback time domains for fast feedback, delayed feedback, and an intervening silent period are depicted (12).
Experiment 4
CORT-negative feedback in ADX rats: test of stress-state resistance to fast feedback.
Rats (N = 24, n = 6) were ADX and surgically fitted with an indwelling jugular catheter. After surgery the rats were placed on a CORT drinking water regimen (see above; Adrenalectomy section) to minimize the substantial up-regulation of the HPA axis activity that accompanies the long-term absence of tonic CORT activity (36). On the test day, the rats were infused with either CORT (0.31 mg/kg) or vehicle 30 seconds prior to the stress onset or 5 minutes after the stress onset. The infusion and blood sample procedures were the same as for experiment 3 (Figure 1). Rats were decapitated immediately after 30 minutes of restraint and brains and pituitaries were removed for subsequent measure of Crh hnRNA and Pomc hnRNA, respectively.
Hormone assays
ACTH (picograms per milliliter) was determined in duplicate (100 μL plasma) by a competitive RIA protocol (37, 38). Primary ACTH antiserum Rb 7 was obtained from William Engeland (University of Minnesota, Minneapolis, Minnesota) and diluted to a final concentration of 1:15 000 to 1:30 000. The intraassay and interassay coefficients of variability for ACTH measurements were all less than 8%. Plasma CORT levels were determined using an enzyme immunoassay kit (Assay Design). Plasma was diluted 1:50 in an assay buffer and placed in a heated water bath (70°C) for 60 minutes to inactivate corticosteroid binding globulin. Plasma prolactin levels were determined using an enzyme immunoassay kit (number 589701; Cayman Chemical) in accordance with the manufacturer's protocol.
In situ hybridization and densitometry
Brains and pituitaries were rapidly extracted (experiment 4) after decapitation, frozen in chilled isopentane (between −30°C and −40°C), and stored at −80°C. With the use of a cryostat, coronal brain sections (12 μm thick) were collected from the PVN (∼1.80 mm posterior to bregma) (39), and horizontal pituitary sections (12 μm thick) were collected from the middle portion of the tissue. Tissue sections were thaw mounted onto Superfrost Plus slides (Fisher Scientific) and stored at −80°C. For in situ hybridization, tissue was postfixed in buffered 4% paraformaldehyde (1 h, 22°C) and then processed as previously described (40). 35S-labeled antisense riboprobes were generated from plasmids containing an intronic portion of the cDNA sequences encoding Crh hnRNA (courtesy of Robert Thompson, University of Michigan, Ann Arbor, Michigan) or Pomc hnRNA (courtesy of Stanley Watson, University of Michigan, Ann Arbor, Michigan). X-ray film (Kodak Biomax MR film) was exposed to slides for 28 days (Crh hnRNA) or 48 hours (Pomc hnRNA). Densitometry by an individual blinded to treatment condition was performed on digitized images using IMAGEJ software (National Institutes of Health, Bethesda, Maryland), as described previously (41).
Statistical analysis
Statistical analyses used IBM SPSS Software (SPSS Inc;, version 21.0, for Macintosh OS). Experiment 1 used a repeated-measures ANOVA followed by a Bonferroni post hoc analysis. Experiments 2–4 used mixed-design ANOVAs with a repeated-measures factor for within-subject serial ACTH values and between-subject factors for restraint, drug infusion time, and drug condition, followed by a Fisher's least significant difference test for post hoc tests (significance level was P < .05). For some subjects we were not able to obtain enough blood from one of their serial blood samples to measure plasma ACTH. So as to not exclude the entire case from the mixed-design ANOVA, we estimated the missing value by either substituting the mean group value (for baseline sample) or interpolating from that subject's other values (for poststress samples). The overall significant main factor effects and interactions observed were the same whether we included or did not include the missing data estimates in the analysis. F values reported in Results include the estimated values for the missing data. All graphs depict the group means ± SEM and do not include the estimated values for the missing data.
Results
Validation experiment 1: time course of CORT plasma levels after infusion of CORT
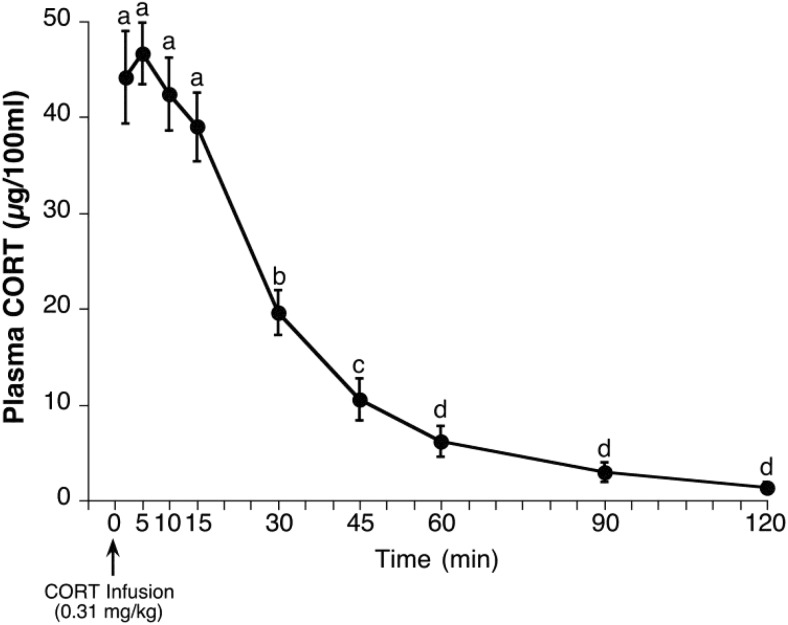
Infusion of CORT into ADX rats produced peak plasma levels of CORT (∼45 μg/100 mL) within 2 minutes after the infusion (earliest postinfusion blood sample time), which remained near peak levels for 15 minutes but then declined substantially during the next 15 minutes and approached undetectable levels by 2 hours after infusion (Figure 2). Post hoc analyses of differing CORT levels after infusion supports dividing the CORT time course into four distinct phases: 1) rapidly rising phase (<2 min), 2) high-plateau phase (2–15 min), 3) declining phase (>15 min and <60 min), and 4) restored basal phase (≥60 min). The peak plasma levels attained were well within the physiological range produced by moderate intensity stressors (27, 42). The CORT clearance closely followed first-order kinetics, and the estimated half-life of CORT clearance in this experiment (16.0 min) is similar to published reports of CORT half-life in the rat (43–45).
Figure 2. Validation experiment 1: time course of CORT plasma levels after bolus iv infusion of CORT in ADX rats.
Prior to the test day, rats were adrenalectomized and implanted with jugular vein catheters. On the test day, rats (n = 10) were placed in restraint tubes and then infused with CORT (0.31 mg/kg). Serial blood samples were taken from the tip of the tail at 2, 5, 10, 15, 30, 45, 60, 90, and 120 minutes after infusion. CORT values at time points marked with different letters are significantly different from each other (Student's dependent sample t test with Bonferroni correction).
Validation experiment 2: Plasma hormone levels in adrenal-intact rats left in their home cage or placed in restraint immediately after vehicle infusion
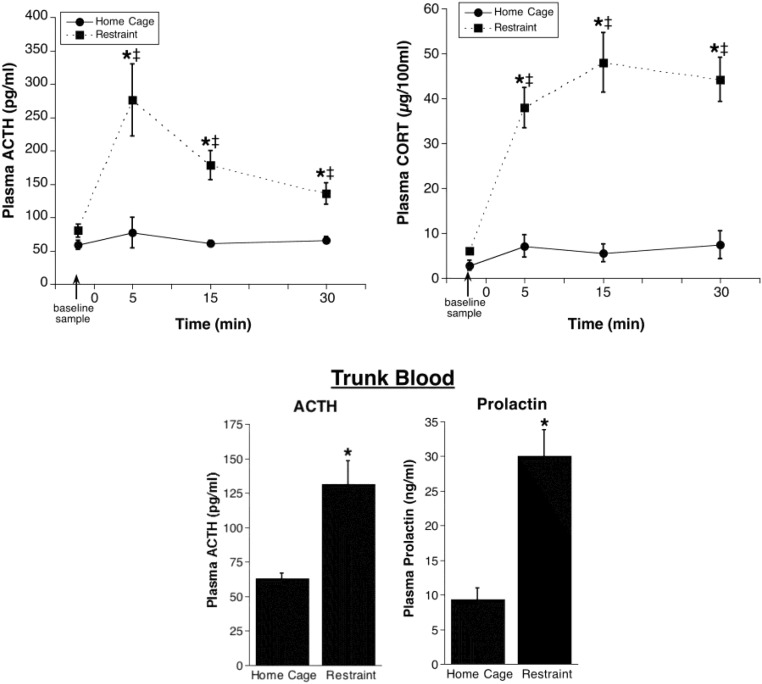
Baseline plasma ACTH (<100 pg/mL) and CORT (<5 μg per 100 mL) were low, and these levels are consistent with normal low basal HPA axis hormone activity (3). As expected, restraint produced a large increase in HPA axis activity, with peak plasma ACTH and CORT evident 5 minutes and 15 minutes, respectively, after restraint onset (Figure 3). Rats that remained in their home cage continued to have low plasma ACTH and CORT levels across the 30-minute repeated sample time. There was an overall significant effect of restraint and restraint by blood sample time interaction for both ACTH (restraint: F1,10 = 18.6, P < .01; restraint by time: F3,30 = 6.2, P < .01) and CORT (restraint: F1,10 = 25.3, P < .01; restraint by time: F3,30 = 8.0, P < .001). Only ACTH and CORT levels of the restrained rats were significantly elevated relative to the baseline sample (P < .05).
Figure 3. Validation experiment 2: plasma hormone levels in adrenal-intact rats left in their home cage or placed in restraint immediately after vehicle infusion.
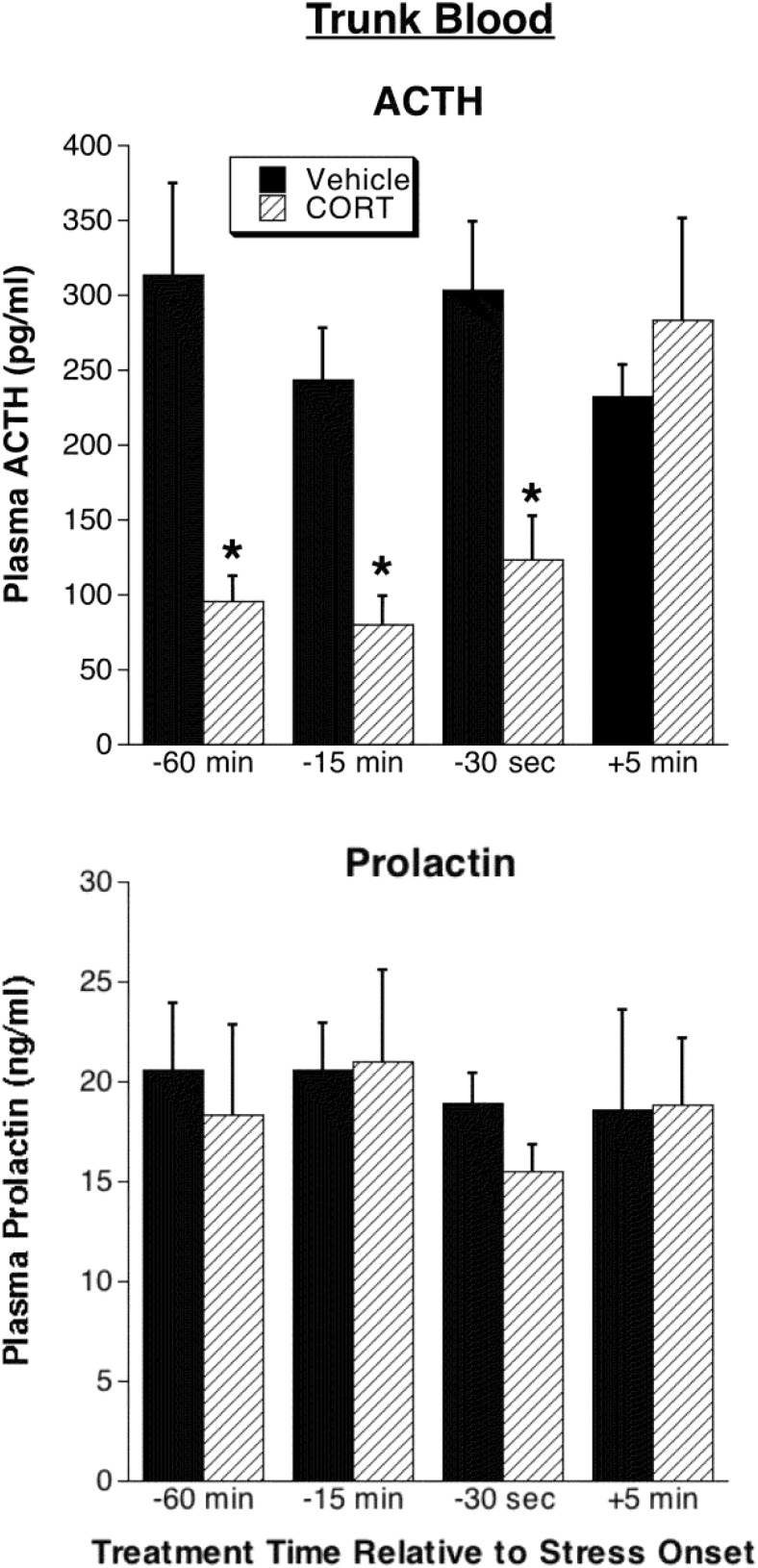
Plasma ACTH (upper left line graph) and plasma CORT (upper right line graph) from repeated jugular vein blood samples are shown. Rats were given a vehicle injection immediately after a baseline blood sample and were then either placed into a restraint tube (n = 6) or remained in their home cage (n = 6). *, Significantly different from home cage rats at the same time point (P < .05); ‡, significantly different from baseline for the same treatment condition (P < .05). The lower bar graphs show plasma ACTH and prolactin in trunk blood collected from rats immediately after the last repeated blood sample (∼30 minutes after restraint onset). *, Significantly different from home cage (P < .05).
We also examined plasma ACTH and prolactin levels in trunk blood collected immediately after the last jugular catheter blood sample (30 min after stress onset). Both anterior pituitary-derived hormones displayed a significant increase 30 minutes after the restraint (Figure 3). The trunk blood ACTH levels were similar to those measured in the final sample withdrawn from the jugular catheter line, indicating that there was minimal distortion of plasma ACTH levels present in samples collected via the catheter line.
Experiment 3: test of CORT fast feedback, silent period, and delayed feedback in adrenal-intact rats
The time course of the plasma CORT levels produced by our infusion procedure (validation experiment 1) indicates that a rapid rise in CORT occurs only within the first 2 minutes after infusion, and those elevated levels almost completely clear the system within 60 minutes (Figure 2). Taking advantage of the different phases of changing CORT levels after CORT infusion, this experiment (Figure 4) compared the negative feedback effectiveness present when stress onset coincides with CORT's rapidly rising phase (30 sec before treatment), high-plateau phase (15 min before treatment), or restored basal phase (60 min before treatment). This experiment also examined the effectiveness of CORT infusion that occurs after the onset of stress (5 min after stress onset treatment).
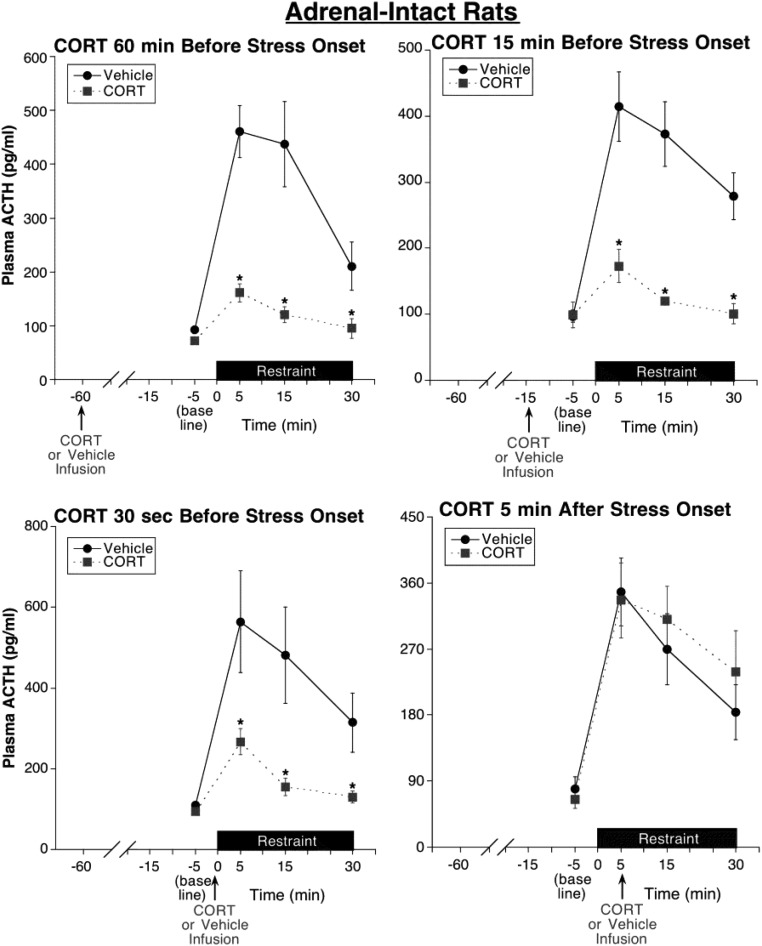
Figure 4. Experiment 3: test of CORT fast feedback, silent period, and delayed feedback in adrenal-intact rats.
CORT infusion (0.31 mg/kg) 60 minutes (delayed feedback test), 15 minutes (silent period test), or 30 seconds (fast feedback test) prior to restraint onset substantially suppressed restraint-induced ACTH levels compared with time-matched vehicle infusion. In contrast, CORT infusion 5 minutes after restraint onset (fast feedback test) had no significant effect on subsequent stress-induced ACTH levels for the time period examined, suggesting a stress-state fast feedback resistance. Note the substantial inhibition produced by the very short (30 sec) prestress treatment interval and the similarly effective inhibition produced by the 15-minute CORT prestress treatment, a pretreatment interval resulting in declining CORT levels at the time of stress onset (see Figure 2), suggesting an absence of a putative feedback silent period. *, Significantly different from vehicle-treated rats at same time point (P < .05; n = 8–9).
We found that infusions of CORT 60 minutes, 15 minutes, or 30 seconds prior to restraint onset were similarly effective in substantially suppressing the overall ACTH response to restraint (Figure 4). Consequently, when comparing ACTH data for the three prestress treatment conditions, there was an overall drug effect (F1,61 = 40.6, P < .001) but no significant effect of treatment time and no significant interaction between treatment time and drug condition. In each CORT prestress treatment case, the suppression was fully evident by 5 minutes after restraint onset, the time of peak ACTH secretion in vehicle-treated rats (Figure 4). In contrast, an infusion of CORT 5 minutes after restraint onset had no effect on the subsequent ongoing ACTH response to restraint (Figure 4). Consequently, when comparing ACTH data for all four treatment time conditions, there was a significant interaction between treatment time and drug condition (F3,61 = 6.5, P = .001). When examining each treatment time separately, there was an overall significant drug effect for each CORT prestress treatment time (60 min CORT pretreatment: F1,16 = 59.0, P < .001; 15 min CORT pretreatment: F1,15 = 23.7, P < .001; 30 sec CORT pretreatment: F1,15 = 11.6, P < .01) but not for the 5-minute poststress treatment time. When comparing ACTH data of only vehicle-treated rats for all four treatment time conditions, there was no significant effect of treatment time (P > .05) and no significant treatment time by blood sample time interaction (P > .05), indicating that the ACTH responses to restraint were similar, regardless of the time of vehicle infusion.
Consistent with the jugular catheter-derived data, trunk blood plasma ACTH was substantially suppressed by each of the CORT prestress onset treatments (Figure 5) but not by the 5 minute-poststress-onset treatment (drug by treatment time interaction: F3,39= 4.2, P < .05, followed by post hoc tests). None of the CORT treatments had a significant effect on stress-induced trunk blood prolactin levels (Figure 5).
Figure 5. Experiment 3: comparison of trunk blood plasma ACTH and prolactin levels.
Trunk blood was collected immediately after the last serial blood sample (30 min after restraint onset; see Figure 4) in rats given CORT or vehicle infusions 60 minutes, 15 minutes, or 30 seconds prior to restraint onset or 5 minutes after restraint onset. *, Significantly different from rats receiving vehicle treatment at the same time relative to stress onset (P < .05; n = 8–9).
Experiment 4: CORT-negative feedback in ADX rats: test of stress state resistance to fast feedback
We considered two possible explanations for the inability of a CORT infusion in experiment 3 to alter the ongoing ACTH response to restraint when CORT was administered 5 minutes after stress onset (see Figure 4). First, CORT may not be able to produce fast feedback effects after stress onset (stress state complete resistance). Alternatively, exogenous CORT may not be able to produce fast feedback effects in addition to the feedback effects produced by stress-induced endogenous CORT secretion (stress state partial resistance, ceiling effect). To discriminate between these two possibilities, this experiment compared the ability of the 30-second CORT prestress treatment with the 5-minute CORT poststress onset treatment in ADX rats (Figure 6).
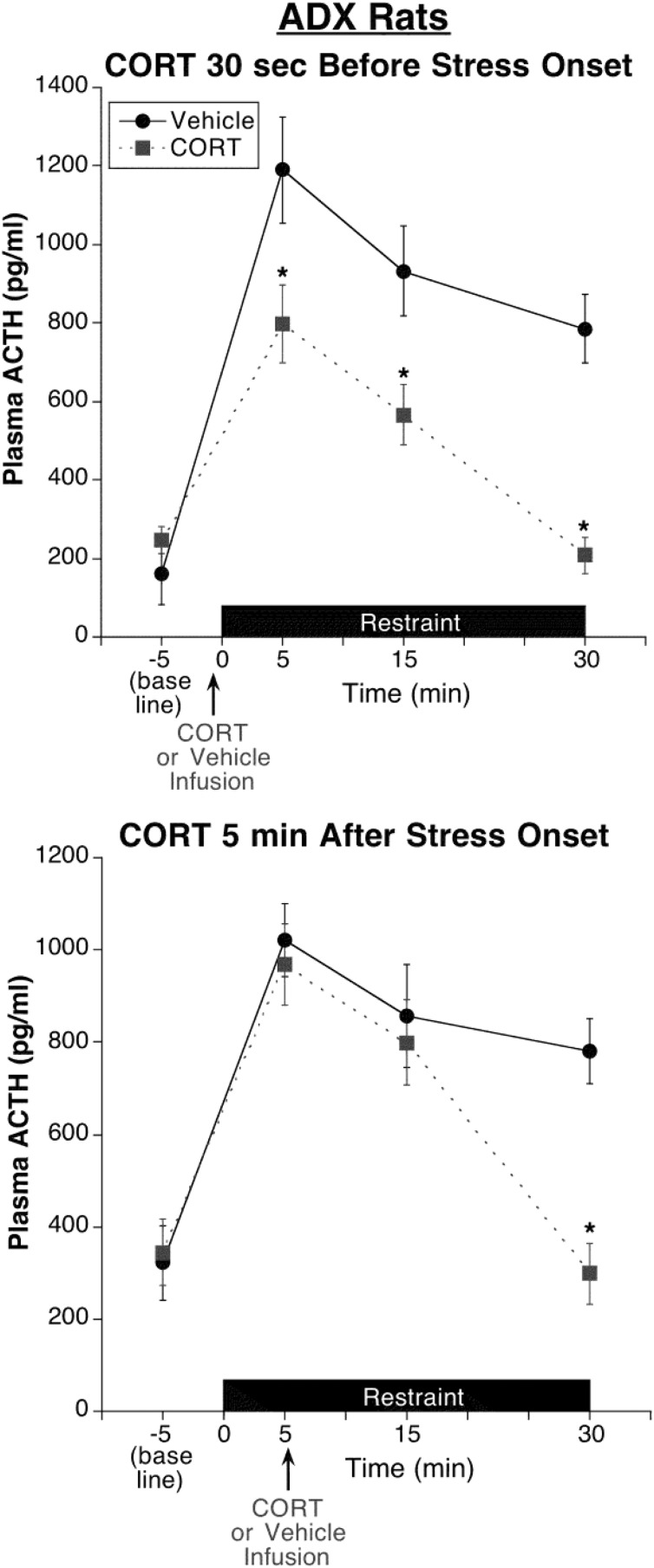
Figure 6. Experiment 4: test of stress state resistance to CORT fast feedback in ADX rats.
All rats were adrenalectomized and implanted with jugular vein catheters 5 days prior to test day. CORT infusion (0.31 mg/kg) 30 seconds prior to restraint onset significantly suppressed restraint-induced ACTH levels at each of the poststress time points examined, including the first time point 5 minutes after restraint onset. CORT infusion 5 minutes after restraint onset produced a delayed inhibitory effect that was evident by 25 minutes but not 10 minutes after infusion. Note the absence of this delayed inhibitory effect in adrenal-intact rats given CORT infusion 5 minutes after restraint onset (Figure 4). *, Significantly different from vehicle-treated rats at the same time point (P < .05; n = 6).
Although these ADX rats were given CORT in their drinking water to help restrain the dramatic up-regulation of the HPA axis activity that develops with long-term ADX, these rats still had elevated basal and stress-induced ACTH levels that were approximately 2–3 times greater than were observed in adrenal-intact rats, (experiment 3, see Figure 4). As was the case in adrenal-intact rats (experiment 3), the 30-second CORT prestress treatment significantly reduced ACTH levels at all poststress time points (Figure 6), including the first 5-minute poststress time point (30 sec CORT pretreatment: F1,10 = 9.5, P < .05; followed by post hoc test). The overall magnitude of peak ACTH suppression by CORT, however, was less (∼33%) than we observed in adrenal-intact rats (∼47%), perhaps due to the up-regulated state of the HPA axis. In contrast to the rapid inhibitory effect of the 30-second CORT pretreatment, the 5-minute CORT poststress-onset treatment produced a delayed suppressive effect that was evident 25 minutes, but not 10 minutes, after infusion (5 min CORT posttreatment by blood sample time interaction: F3,33 = 11.7, P < .001; followed by post hoc tests). Despite the delayed inhibitory influence of CORT poststress-onset treatment, this CORT treatment produced an inhibitory effect in ADX rats (Figure 6) that was not evident in adrenal-intact rats (experiment 3, see Figure 4).
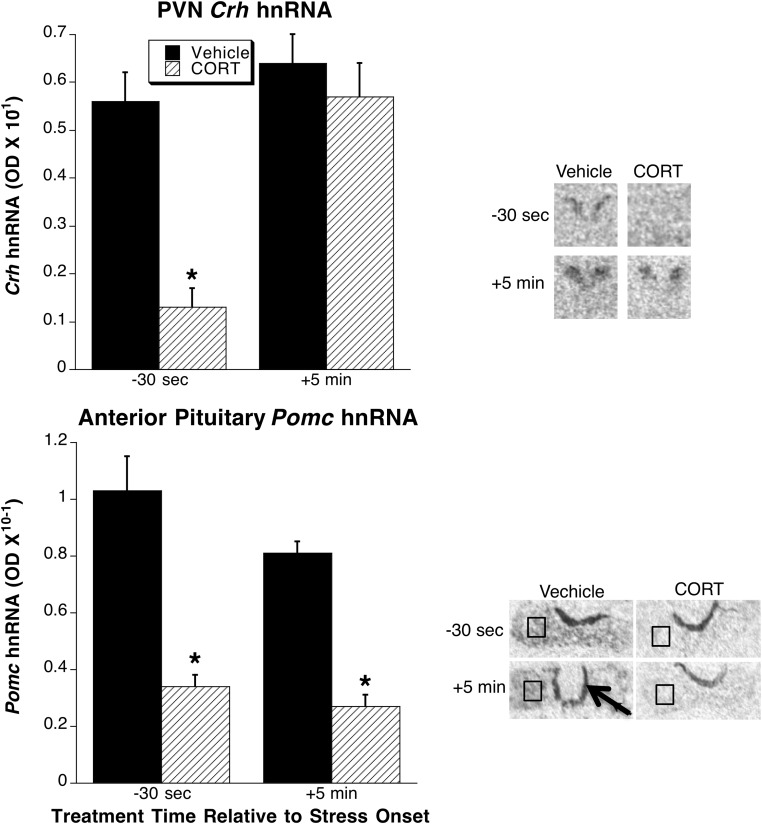
In this experiment we also examined PVN Crh hnRNA and anterior pituitary Pomc hnRNA in postmortem tissue collected immediately after the last jugular catheter blood sample (30 min after stress onset, see Figure 7). In prior studies we found that in adrenal-intact rats, restraint produces a transient increase in Crh hnRNA that returns to near basal levels within 30 minutes after restraint onset, whereas in ADX rats, this Crh hnRNA increase is prolonged (38), thereby allowing for assessment of stress-induced Crh hnRNA levels in PVN tissue collected in this final experiment. We found that the 30-second CORT prestress treatment substantially blunted both stress-induced Crh and Pomc hnRNA (Figure 7). In contrast, the 5-minute CORT poststress-onset treatment substantially blunted stress-induced Pomc hnRNA but had no effect on stress-induced Crh hnRNA (Figure 7). Consequently, there was a significant CORT by treatment time interaction for Crh hnRNA (F1,20 = 8.43, P < .01) but not for Pomc hnRNA.
Figure 7. Experiment 4: comparison of PVN Crh hnRNA and anterior pituitary Pomc hnRNA.
Tissue was collected immediately after the last serial blood sample (30 min after restraint onset; see Figure 6) in rats given CORT or vehicle infusions 30 seconds prior to restraint onset or 5 minutes after restraint onset. A representative in situ hybridization sample autoradiogram from each treatment group is shown to the right of the bar graphs for each tissue region. The PVN-containing region of coronal brain sections are shown. The entire transverse portion of a pituitary section is shown, with a black box illustrating the region of interest centered over a portion of the anterior pituitary (note the very strong Pomc hnRNA expression in the intermediate lobe of the pituitary (black arrow), which serves as a landmark for separation of anterior pituitary from intermediate and posterior pituitary). *, Significantly different from rats receiving vehicle treatment at the same time relative to stress onset (P < .05; n = 6).
Discussion
In this study we observed four notable findings that provide important revisions to our understanding of in vivo aspects of glucocorticoid fast feedback: 1) CORT infusion 30 seconds prior to stress onset in male rats was sufficient to dramatically suppress the entire stress-induced ACTH response, including the peak response present within 5 minutes after stress onset; 2) CORT infusion 15 minutes prior to stress onset, a time interval previously believed to be a silent period for CORT-negative feedback, was equally effective at suppressing the stress-induced ACTH response; 3) CORT infusion 5 minutes after the stress onset produced no suppression of stress-induced ACTH in adrenal-intact rats and a delayed suppression in adrenalectomized rats, suggesting a stress-state resistance to CORT fast feedback; and 4) there was a parallel stress-state resistance in the ability of CORT to rapidly suppress stress-induced Crh gene expression in the PVN but not stress-induced Pomc gene expression in the anterior pituitary.
Rapid CORT-negative feedback effect on stress-induced ACTH
There is considerable scientific interest in the rapid negative feedback effect of CORT, not only because of its potential importance in HPA axis physiology, but also because it is one of the best examples of a glucocorticoid action in mammals that is too rapid to depend on alteration of gene expression. In our study, pretreatment with CORT just 30 seconds prior to stress onset remarkably suppressed the entire ACTH response. This substantial suppression (>60%) was evident by 5 minutes after the stress onset. The suppression of the initial response may largely account for the reduced ACTH levels seen at all subsequent time points. Other studies of unanesthetized rats have seen rapid (within 10–30 min) and inhibitory (30%–80%) effects of systemic or intra-PVN glucocorticoid treatment on stress-induced ACTH and CORT secretion (45–48). Our results illustrate the most rapid and substantial inhibition reported to date. The effectiveness of our treatment may be due to the fact that the glucocorticoid pretreatment was systemic and administered in a stress-free manner. These features were also present in a study that observed substantial inhibition of the ACTH response to air-puff stress in rats infused with CORT 5 minutes before stress onset (48). The effectiveness of our treatment may also be due to the use of the rat's natural endogenous glucocorticoid, CORT, rather than synthetic glucocorticoids. The fact, however, that other studies have also observed fast feedback effects when using different synthetic glucocorticoids (45, 46) raises the intriguing prospect that these actions are mediated by GR rather than a novel CORT binding site that happens to share a ligand specificity profile similar to GR. Two recent studies provide support for the GR dependence of glucocorticoid fast feedback effects on PVN CRH neurons and anterior pituitary corticotrophs (9, 49).
Consideration of a silent period of CORT-negative feedback
Initial studies of glucocorticoid-negative feedback identified a silent period of feedback function during which glucocorticoid treatment approximately 10–45 minutes prior to stimulus-induced HPA axis activity failed to produce an inhibitory effect on HPA axis hormone secretion (12). In contrast to those initial findings of a silent period, we found that an infusion of CORT 15 minutes before the stress onset effectively suppressed stress-induced ACTH secretion. This fast feedback effect was present despite the fact that circulating CORT levels were no longer rising at the time of the stress onset (Figure 2). Although we cannot rule out the possibility that CORT pretreatment intervals longer than 15 minutes would exhibit a silent period, our results demonstrate that fast feedback can be expressed, even if stress onset does not occur during the rising phase of prior CORT infusion. Moreover, our results indicate that fast feedback can be initiated within 30 seconds, and once initiated its actions last at least 15 minutes. Our in vivo results are consistent with ex vivo hypothalamic slice studies that find that the expression of rapid inhibition of CRH neuron electrophysiological activity by glucocorticoids is not rate dependent and is relatively long lasting, even after glucocorticoid washout (up to 60 min) (6, 20, 49). In contrast, this extended duration of fast feedback expression may not be characteristic of fast feedback actions initiated at the level of corticotrophs because Deng et al (9) saw rapid, but short-lasting CORT inhibition (∼5 min after washout) of ACTH response to CRH in cultured corticotrophs.
The discrepancy between our results and early studies of glucocorticoid fast feedback are likely due to the extensive methodological differences between these studies. Most notably, prior studies that observed a silent period and/or rate-dependent glucocorticoid fast feedback were typically performed in anesthetized animals (eg, references 11, 21, 22, and 50). The tonic alteration in GABAergic tone present with anesthesia may substantially alter basal and stimulus-induced activity of CRH neurons. For example, activation of CRH neurons is largely dependent on altered GABAergic activity within the PVN and proximal hypothalamic regions (8, 51–54).
It is unlikely that the mechanisms responsible for the expression of fast feedback that we observed with 30 seconds and 15 minutes of CORT pretreatment are the same mechanisms that contribute to the inhibitory actions that we observed after 60 minutes of CORT pretreatment (delayed feedback). In contrast to the shorter pretreatment intervals, with the 60-minute CORT pretreatment, we found that plasma CORT had returned to low basal levels by the time of stress onset. Thus, those delayed feedback effects must depend on glucocorticoid receptor-mediated processes that do not require substantial receptor occupancy around the time of stress onset. Other in vivo and in vitro studies indicate that glucocorticoid-negative feedback effects observed an hour or more after glucocorticoid pretreatment depends on the activation of the intracellular GR and subsequent alteration of gene transcription and de novo protein synthesis (12, 31, 55–57).
Stress-state resistance to CORT fast feedback
A somewhat unexpected finding of this study was the very limited fast feedback effects evident when CORT infusion occurred 5 minutes after stress onset. This corresponds to the time when stress-induced endogenous CORT levels begin to surge as a result of the time lag necessary for de novo steroidogenesis after adrenal cortical stimulation by ACTH. Our result raises the provocative question of whether fast feedback is manifest under physiological conditions, ie, during stress activation of the HPA axis. We saw no fast feedback effect of poststress CORT infusion in adrenal-intact rats. One possibility for the absence of this fast feedback may simply pertain to the kinetics of stress-induced ACTH and CORT secretion. If the endogenous CORT rise occurs after an initial large stress-induced bolus release of ACTH, then the fast feedback inhibitory influence may not be evident until after clearance of the initial ACTH surge. However, the half-life of ACTH is very short (∼4.5 min) (58), so it appears that the high levels of ACTH present 15 minutes after stress onset is due to some ongoing HPA activation and ACTH secretion that extends beyond the initial moments of stress onset. This ongoing response should then be subject to modulation by CORT fast feedback inhibition. Consequently, our observation of the absence of any subsequent inhibitory effect of CORT infusion over the 30-minute stress response in adrenal-intact rats suggests a genuine stress-state difference in the efficacy of fast feedback inhibition.
Our results may indicate that HPA axis activity is completely resistant to fast feedback induction after stress-induced onset of axis activation. However, an alternative possibility that we considered was that CORT elevation after stress onset is capable of producing some fast feedback inhibition of the ongoing HPA axis response, but the magnitude of that inhibitory response is substantially diminished, ie, partial stress-state resistance. In this case, the stress-induced endogenous CORT secretion may have been sufficient to produce a ceiling level of fast feedback expression such that no greater suppression is seen with the infusion of exogenous CORT. Our last experiment, in which rats were adrenalectomized, supports this prospect. In the absence of endogenous CORT, exogenous CORT infusion 5 minutes after stress onset did produce some fast feedback. However, there was a delay of more than 10 minutes before any suppression was observed. This again suggests some stress-state resistance to fast feedback expression. It should be noted that another study observed a delayed (30 min) ACTH suppression of poststress (2 min) cortisol treatment in adrenal-intact rats (59). A delay in the expression of fast feedback may be physiologically adaptive because it would not limit the initial magnitude of the CORT response to stress, but it would constrain the duration of that response.
How may stress state modulate glucocorticoid fast feedback processes? Recent ex vivo studies indicate that acute stress leads to rapid and in some cases relatively long-lasting changes in synaptic function at CRH neuron dendritic and somatic sites within the PVN. These changes include enhanced excitability of CRH neurons due to a shift of γ-aminobutyric acidA receptor-dependent currents from inhibition to excitation (7, 54), and an emergent capability for activity-dependent rapid potentiation of GABAergic (60) and glutamatergic synaptic activity (61). Whether stress-induced CORT acts rapidly to constrain these net excitatory actions on CRH neurons has yet to be tested.
Site of CORT fast feedback action
Glucocorticoids produce feedback inhibition of HPA axis activity by directly modulating activity of the intrinsic anatomical elements of the axis (CRH neurons in the PVN and corticotrophs in the anterior pituitary) as well as indirectly by modulating activity of the neural circuits that ultimately drive basal and stress-induced HPA axis activity (3–5). The systemic CORT treatment that we used in this study does not permit direct determination of the anatomical site(s) of negative feedback action at play. However, our prolactin hormone and gene expression measures allow us to make some inferences about the site of fast feedback and stress-state alteration of fast feedback efficacy. Prior studies suggest that acute glucocorticoid-negative feedback does not produce a generalized suppression of the central neural response to stress. For example, acute glucocorticoid manipulations have limited effects on stress-induced immediate early gene expression throughout the brain (62–64). In this study we also observed no inhibitory effect of any of the CORT treatment times on stress-induced prolactin secretion. The neural pathways responsible for stress-induced activation of lactotrophs and subsequent prolactin secretion remain to be established, but CRH does not appear to be a neurosecretagogue for prolactin (28, 29). It appears then that if CORT alters stress-induced transynaptic input to CRH neurons, the effect is restricted to neural input that selectively converges on PVN CRH neurons.
It is likely that at least some of the fast feedback effects that we observed were exerted at the level of the PVN and/or corticotrophs. Microinfusion of glucocorticoids in the PVN is sufficient to inhibit stress-induced ACTH secretion when administered either 1 hour or immediately before stress onset (46, 65). This inhibition could be due to a direct effect on CRH neurons that uncouples excitation from secretion or a more indirect effect, such as triggering of a retrograde endocannabinoid signal from CRH neurons that modulates glutamate synaptic transmission onto CRH neurons (66). As noted above, the relatively long-lasting effect of fast feedback that we observed once initiated (at least 15 min), is consistent with ex vivo glucocorticoid fast feedback actions observed in PVN slices but not corticotrophs (6, 9, 20, 49). The suppression of CRH gene induction with 30 seconds of CORT pretreatment also points to some rapid glucocorticoid action that is expressed at the level of CRH neurons. Whether the CORT regulation of Crh gene expression reflects altered intracellular signal transduction or a direct GR-dependent alteration of Crh gene expression cannot be determined from our data, but there is support for both prospects (30, 31, 67, 68).
Interestingly, in our study the 5-minute poststress CORT treatment had no effect on stress-induced Crh hnRNA while completely suppressing stress-induced Pomc hnRNA. This result supports the prospect that the stress-state fast feedback resistance that we observed was also manifest at the level of the PVN rather than the anterior pituitary. The lack of a stress-state effect on CORT suppression of Pomc gene expression may be due to a direct repressive effect of activated GR on Pomc gene expression that does not vary with the corticotroph activation state (26).
Physiological and clinical implications
Altered glucocorticoid negative feedback function is associated with a wide range of physiological and mental disorders (eg, references 69–74). Pioneering research illustrated the multiple temporal and cellular processes that contribute to this glucocorticoid-negative feedback process (3). Recent studies of glucocorticoid effects on ex vivo PVN and anterior pituitary tissue have provided new insights into potential cellular and molecular mechanisms of glucocorticoid-negative feedback (6, 7, 9). The in vivo physiological features of glucocorticoid-negative feedback provide an essential context for the interpretation of these ex vivo studies. For example, this study illustrates the critical importance of considering the recent stress state of the PVN and the target tissue sites of action when unraveling mechanisms of glucocorticoid fast feedback.
Acknowledgments
We thank Angela Tomczik for her assistance in computing the corticosterone half-life, Vanessa Thompson for the tissue processing, image capture, and quantification help, and Ryan Bachtell and Serge Campeau for sharing equipment and expertise with use of chronic indwelling jugular catheters.
This work was supported by National Institutes of Health Grant MH075968 and the University of Colorado Boulder Undergraduate Research Opportunity Program.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADX
- adrenalectomized
- CORT
- corticosterone
- GABAergic
- secretion of γ-aminobutyric acid
- GR
- glucocorticoid receptor
- hnRNA
- heteronuclear RNA
- HPA
- hypothalamus-pituitary-adrenal
- PVN
- hypothalamic paraventricular nucleus.
References
- 1. McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharmacol. 2008;585(1):64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. [DOI] [PubMed] [Google Scholar]
- 4. Fink G, Rosie R, Sheward WJ, Thomson E, Wilson H. Steroid control of central neuronal interactions and function. J Steroid Biochem Mol Biol. 1991;40(1–3):123–132. [DOI] [PubMed] [Google Scholar]
- 5. Myers B, McKlveen JM, Herman JP. Neural regulation of the stress response: the many faces of feedback. Cell Mol Neurobiol. 2012;32(5):683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23(12):4850–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wamsteeker Cusulin JI, Füzesi T, Inoue W, Bains JS. Glucocorticoid feedback uncovers retrograde opioid signaling at hypothalamic synapses. Nat Neurosci. 2013;16(5):596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wamsteeker JI, Bains JS. A synaptocentric view of the neuroendocrine response to stress. Eur J Neurosci. 2010;32(12):2011–2021. [DOI] [PubMed] [Google Scholar]
- 9. Deng Q, Riquelme D, Trinh L, et al. Rapid glucocorticoid feedback inhibition of ACTH secretion involves ligand-dependent membrane association of glucocorticoid receptors. Endocrinology. 2015; 156(9):3215–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verkuyl JM, Karst H, Joëls M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21(1):113–121. [DOI] [PubMed] [Google Scholar]
- 11. Dallman MF, Yates FE. Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann NY Acad Sci. 1969;156(2):696–721. [DOI] [PubMed] [Google Scholar]
- 12. Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1–24. [DOI] [PubMed] [Google Scholar]
- 13. Hodges JR, Jones MT. The effect of injected corticosterone on the release of adrenocorticotrophic hormone in rats exposed to acute stress. J Physiol (Lond). 1963;167:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dallman MF, JONES MT. Corticosteroid feedback control of ACTH secretion: effect of stress-induced corticosterone secretion on subsequent stress responses in the rat. Endocrinology. 1973;92(5):1367–1375. [DOI] [PubMed] [Google Scholar]
- 15. Keller-Wood ME, Shinsako J, Dallman MF. Feedback inhibition of adrenocorticotropic hormone by physiological increases in plasma corticosteroids in conscious dogs. J Clin Invest. 1983;71(4):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dallman MF, Akana SF, Scribner KA, et al. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1992;4(5):517–526. [DOI] [PubMed] [Google Scholar]
- 17. Jones MT, Hillhouse EW, Burden JL. Dynamics and mechanics of corticosteroid feedback at the hypothalamus and anterior pituitary gland. J Endocrinol. 1977;73(3):405–417. [DOI] [PubMed] [Google Scholar]
- 18. Jones MT, Tiptaft EM, Brush FR, Fergusson DA, Neame RL. Evidence for dual corticosteroid-receptor mechanisms in the feedback control of adrenocorticotrophin secretion. J Endocrinol. 1974;60(2):223–233. [DOI] [PubMed] [Google Scholar]
- 19. Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26(3–4):103–108. [DOI] [PubMed] [Google Scholar]
- 20. Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci. 2010;30(33):11188–11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones MT, Brush FR, Neame RL. Characteristics of fast feedback control of corticotrophin release by corticosteroids. J Endocrinol. 1972;55(3):489–497. [DOI] [PubMed] [Google Scholar]
- 22. Kaneko M, Hiroshige T. Fast, rate-sensitive corticosteroid negative feedback during stress. Am J Physiol. 1978;234(1):R39–R45. [DOI] [PubMed] [Google Scholar]
- 23. Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res. 2000;17(10):1273–1277. [DOI] [PubMed] [Google Scholar]
- 24. Joëls M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64(4):901–938. [DOI] [PubMed] [Google Scholar]
- 25. Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol. 1992;6(7):1061–1069. [DOI] [PubMed] [Google Scholar]
- 26. Autelitano DJ, Lundblad JR, Blum M, Roberts JL. Hormonal regulation of POMC gene expression. Annu Rev Physiol. 1989;51:715–726. [DOI] [PubMed] [Google Scholar]
- 27. Armario A, Lopez-Calderon A, Jolin T, Castellanos JM. Sensitivity of anterior pituitary hormones to graded levels of psychological stress. Life Sci. 1986;39(5):471–475. [DOI] [PubMed] [Google Scholar]
- 28. Krulich L. The role of central monoamines in the stress-related changes of the secretion of prolactin and TSH. Psychopharmacol Bull. 1976;12(4):22–24. [PubMed] [Google Scholar]
- 29. Gala RR. The physiology and mechanisms of the stress-induced changes in prolactin secretion in the rat. Life Sci. 1990;46(20):1407–1420. [DOI] [PubMed] [Google Scholar]
- 30. Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26(3–4):109–130. [DOI] [PubMed] [Google Scholar]
- 31. Osterlund C, Spencer RL. Corticosterone pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis activity via multiple actions that vary with time, site of action, and de novo protein synthesis. J Endocrinol. 2011;208(3):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orth DN. Methods of hormone radioimmunoassay. In: Jaffe B, ed. Adrenocorticotropic Hormone (ACTH). 2nd ed San Diego: Academic Press; 1978:245–284. [Google Scholar]
- 33. Osterlund CD, Jarvis E, Chadayammuri A, Unnithan R, Weiser MJ, Spencer RL. Tonic, but not phasic corticosterone, constrains stress activated extracellular-regulated-kinase 1/2 immunoreactivity within the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2011;23(12):1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14(7):1143–1152. [DOI] [PubMed] [Google Scholar]
- 35. Vahl TP, Ulrich-Lai YM, Ostrander MM, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 2005;289(5):E823–E828. [DOI] [PubMed] [Google Scholar]
- 36. Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122(4):1343–1348. [DOI] [PubMed] [Google Scholar]
- 37. Nicholson WE, Davis DR, Sherrell BJ, Orth DN. Rapid radioimmunoassay for corticotropin in unextracted human plasma. Clin Chem. 1984;30(2):259–265. [PubMed] [Google Scholar]
- 38. Pace TWW, Gaylord RI, Jarvis E, Girotti M, Spencer RL. Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat. Stress (Amsterdam, Netherlands). 2009;12(5):400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2007. [Google Scholar]
- 40. Girotti M, Pace TWW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138(4):1067–1081. [DOI] [PubMed] [Google Scholar]
- 41. Osterlund CD, Thompson V, Hinds L, Spencer RL. Absence of glucocorticoids augments stress-induced Mkp1 mRNA expression within the hypothalamic-pituitary-adrenal axis. J Endocrinol. 2014;220(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pace TWW, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer RL. Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur J Neurosci. 2005;22(7):1679–1690. [DOI] [PubMed] [Google Scholar]
- 43. Glenister DW, Yates FE. Sex difference in the rate of disappearance of corticosterone-4-C14 from plasma of intact rats: further evidence for the influence of hepatic δ4-steroid hydrogenase activity on adrenal cortical function. Endocrinology. 1961;68(5):747–758. [DOI] [PubMed] [Google Scholar]
- 44. Schapiro S, Percin CJ, Kotichas FJ. Half-life of plasma corticosterone during development. Endocrinology. 1971;89(1):284–286. [DOI] [PubMed] [Google Scholar]
- 45. Andrews MH, Wood SA, Windle RJ, Lightman SL, Ingram CD. Acute glucocorticoid administration rapidly suppresses Basal and stress-induced hypothalamo-pituitary-adrenal axis activity. Endocrinology. 2012;153(1):200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151(10):4811–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jacobson L, Sapolsky R. Augmented ACTH responses to stress in adrenalectomized rats replaced with constant, physiological levels of corticosterone are partially normalized by acute increases in corticosterone. Neuroendocrinology. 1993;58(4):420–429. [DOI] [PubMed] [Google Scholar]
- 48. Thrivikraman KV, Nemeroff CB, Plotsky PM. Sensitivity to glucocorticoid-mediated fast-feedback regulation of the hypothalamic-pituitary-adrenal axis is dependent upon stressor specific neurocircuitry. Brain Res. 2000;870(1–2):87–101. [DOI] [PubMed] [Google Scholar]
- 49. Nahar J, Haam J, Chen C, et al. Rapid Nongenomic glucocorticoid actions in male mouse hypothalamic neuroendocrine cells are dependent on the nuclear glucocorticoid receptor. Endocrinology. 2015;156(8):2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaneko M, Hiroshige T. Site of fast, rate-sensitive feedback inhibition of adrenocorticotropin secretion during stress. Am J Physiol. 1978;234(1):R46–R51. [DOI] [PubMed] [Google Scholar]
- 51. Bali B, Kovács KJ. GABAergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Eur J Neurosci. 2003;18(6):1518–1526. [DOI] [PubMed] [Google Scholar]
- 52. Verkuyl JM, Hemby SE, Joëls M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci. 2004;20(6):1665–1673. [DOI] [PubMed] [Google Scholar]
- 53. Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213(1–2):63–72. [DOI] [PubMed] [Google Scholar]
- 54. Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31(50):18198–18210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dayanithi G, Antoni FA. Rapid as well as delayed inhibitory effects of glucocorticoid hormones on pituitary adrenocorticotropic hormone release are mediated by type II glucocorticoid receptors and require newly synthesized messenger ribonucleic acid as well as protein. Endocrinology. 1989;125(1):308–313. [DOI] [PubMed] [Google Scholar]
- 56. Shipston MJ. Mechanism(s) of early glucocorticoid inhibition of adrenocorticotropin secretion from anterior pituitary corticotropes. Trends Endocrinol Metab. 1995;6(8):261–266. [DOI] [PubMed] [Google Scholar]
- 57. Jeanneteau FD, Lambert WM, Ismaili N, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci USA. 2012;109(4):1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vázquez DM, Morano MI, Taylor L, Akil H. Kinetics of radiolabeled adrenocorticotropin hormone in infant and weanling rats. J Neuroendocrinol. 1997;9(7):529–536. [DOI] [PubMed] [Google Scholar]
- 59. Young EA. Normal glucocorticoid fast feedback following chronic 50% corticosterone pellet treatment. Psychoneuroendocrinology. 1995;20(7):771–784. [DOI] [PubMed] [Google Scholar]
- 60. Inoue W, Baimoukhametova DV, Füzesi T, et al. Noradrenaline is a stress-associated metaplastic signal at GABA synapses. Nat Neurosci. 2013;16(5):605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kuzmiski JB, Marty V, Baimoukhametova DV, Bains JS. Stress-induced priming of glutamate synapses unmasks associative short-term plasticity. Nat Neurosci. 2010;13(10):1257–1264. [DOI] [PubMed] [Google Scholar]
- 62. Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2003;15(11):1075–1083. [DOI] [PubMed] [Google Scholar]
- 63. Fevurly RD, Spencer RL. Fos expression is selectively and differentially regulated by endogenous glucocorticoids in the paraventricular nucleus of the hypothalamus and the dentate gyrus. J Neuroendocrinol. 2004;16(12):970–979. [DOI] [PubMed] [Google Scholar]
- 64. Kononen J, Honkaniemi J, Alho H, Koistinaho J, Iadarola M, Pelto-Huikko M. Fos-like immunoreactivity in the rat hypothalamic-pituitary axis after immobilization stress. Endocrinology. 1992;130(5):3041–3047. [DOI] [PubMed] [Google Scholar]
- 65. Weiser MJ, Osterlund C, Spencer RL. Inhibitory effects of corticosterone in the hypothalamic paraventricular nucleus (PVN) on stress-induced adrenocorticotrophic hormone secretion and gene expression in the PVN and anterior pituitary. J Neuroendocrinol. 2011;23(12):1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress (Amsterdam, Netherlands). 2011;14(4):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bali B, Ferenczi S, Kovács KJ. Direct inhibitory effect of glucocorticoids on corticotrophin-releasing hormone gene expression in neurones of the paraventricular nucleus in rat hypothalamic organotypic cultures. J Neuroendocrinol. 2008;20(9):1045–1051. [DOI] [PubMed] [Google Scholar]
- 68. Evans AN, Liu Y, Macgregor R, Huang V, Aguilera G. Regulation of hypothalamic corticotropin-releasing hormone transcription by elevated glucocorticoids. Mol Endocrinol. 2013;27(11):1796–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pariante CM, Papadopoulos AS, Poon L, et al. A novel prednisolone suppression test for the hypothalamic-pituitary-adrenal axis. Biol Psychiatry. 2002;51(11):922–930. [DOI] [PubMed] [Google Scholar]
- 70. Yehuda R, Golier JA, Halligan SL, Meaney M, Bierer LM. The ACTH response to dexamethasone in PTSD. Am J Psychiatry. 2004;161(8):1397–1403. [DOI] [PubMed] [Google Scholar]
- 71. Bruehl H, Rueger M, Dziobek I, et al. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J Clin Endocrinol Metab. 2007;92(7):2439–2445. [DOI] [PubMed] [Google Scholar]
- 72. Jerjes WK, Taylor NF, Wood PJ, Cleare AJ. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology. 2007;32(2):192–198. [DOI] [PubMed] [Google Scholar]
- 73. Galli U, Gaab J, Ettlin DA, Ruggia F, Ehlert U, Palla S. Enhanced negative feedback sensitivity of the hypothalamus-pituitary-adrenal axis in chronic myogenous facial pain. Eur J Pain. 2009;13(6):600–605. [DOI] [PubMed] [Google Scholar]
- 74. Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007;61(10):1179–1186. [DOI] [PubMed] [Google Scholar]