Abstract
The cognitive symptoms of schizophrenia are largely resistant to current treatment and are thus a life-long burden of the illness. Studies of cognitive symptoms have commonly focused on prefrontal cortex because of its demonstrated importance for executive function and working memory—key components of the deficit. The role of striatal-cortical circuitry and therefore the striatum itself has received much less attention. Here we review longstanding evidence that the striatum and its cortical connections are critical for complex cognition and discuss emerging evidence of the striatum’s potential involvement in cognitive symptoms. Finally, we suggest how mouse models might test ideas about the contribution of early striatal dysfunction to the cognitive symptoms of schizophrenia.
The cognitive symptoms of schizophrenia present early in the disease, and unlike positive symptoms, including hallucinations and delusions, cognitive symptoms are largely resistant to current treatment and therefore persist throughout life. Many studies of cognitive symptoms have focused on the prefrontal cortex because of its demonstrated importance for executive function and working memory—key components of the cognitive deficit. However, the role of striatal cortical circuitry and therefore the striatum itself has received much less attention than other brain structures affected in schizophrenia, such as the prefrontal cortex and temporal lobe. Here we review longstanding evidence that supports the idea that the striatum, and its cortical connections, are critical for complex cognitive functions, and we discuss the emerging evidence of the striatum’s potential involvement in generating cognitive symptoms of schizophrenia. Finally, we provide a general outline of how mouse models might test ideas about early striatal dysfunction’s contribution to the cognitive symptoms of schizophrenia.
The Importance of Understanding the Cognitive Symptoms of Schizophrenia
Schizophrenia is formally characterized by three distinct symptom clusters: positive, negative, and cognitive. The positive symptoms refer to hallucinations and delusions, psychotic abnormalities in perception and reality testing. The negative symptoms refer to social withdrawal, lack of motivation, and abnormalities in social interaction. The cognitive symptoms refer to inability to organize one’s life and to work sequentially and effectively. Although positive symptoms are the most dramatic and characteristic symptoms of schizophrenia, they are greatly improved with antipsychotic medication, whereas the negative and cognitive symptoms are not, and therefore, as a result, these two symptom clusters, and especially the cognitive symptoms that are so debilitating, have become a major focus in schizophrenia research (Keefe et al., 2007). The cognitive symptoms of schizophrenia include deficits in semantic and explicit memory, as well as deficits in attention, working memory, and executive function (Cirillo and Seidman, 2003; Goldman-Rakic, 1994). The cognitive deficits are not unique to schizophrenia. But because they affect mental abilities needed for day-to-day functioning as well as planning for the future, the severity of these symptoms are highly predictive of a patient’s long-term prognosis, more predictive than the positive symptoms (Green, 1996).
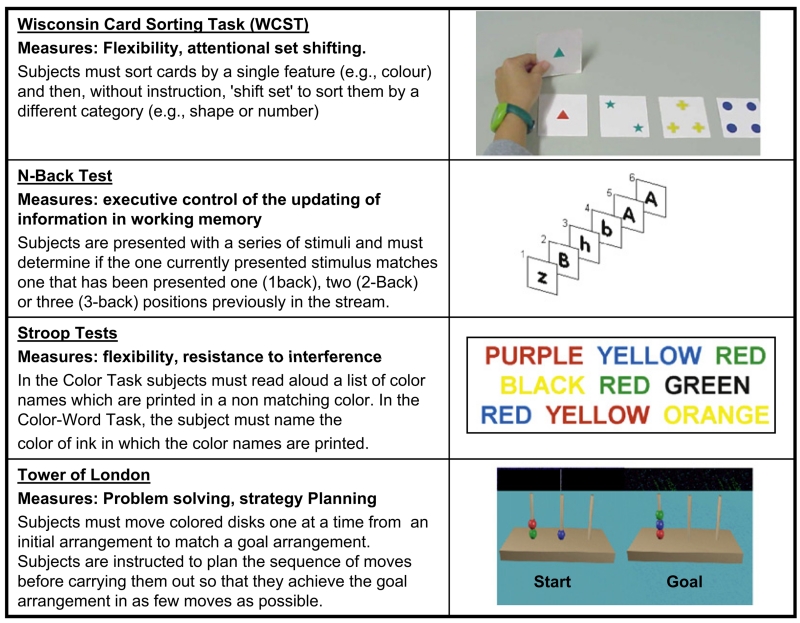
The relevance of the cognitive symptoms of schizophrenia was already understood more than a century ago by Kraepelin, who in 1893 divided the major psychotic illnesses into two groups by differentiating patients with “dementia praecox,” or premature dementia (later termed schizophrenia by Eugene Bleuler), from manic depressive illness based on their disordered thought. Kraeplin was also the first to recognize that patients with dementia praecox share many of the behavioral abnormalities observed in the dementia of patients with lesions of the frontal lobes (Kraepelin, 1919). Patients with frontal lobe damage and patients with schizophrenia suffer from defects in executive function that are measured on tasks such as the Wisconsin Card Sorting Test (WCST), the Stroop Task, the “Tower of London” task, and the N-back test (Kolb and Whishaw, 1983) (Figure 1). In nonhuman primates, success in executive tasks require optimal dopamine signaling (Brozoski et al., 1979), suggesting that cognitive dysfunction in schizophrenia involves disturbance of the dopamine system. This is one of many indirect observations consistent with the dopamine hypothesis of schizophrenia, the most enduring and continually provocative hypothesis in psychiatric research.
Figure 1. Tasks Used to Measure Prefrontal-Cortex-Dependent Cognitive Executive Function in Humans.
The Original Dopamine Hypothesis of Schizophrenia
Based on seminal experiments by Arvid Carlsson and others studying the effects of antipsychotic medications, Jacques Van Rossum proposed, in 1966, that “overstimulation of dopamine receptors could be part of the etiology” of schizophrenia (for a historical review, see Baumeister and Francis, 2002). Today, there is no question that intervention in the dopaminergic system is successful in treating the positive symptoms of schizophrenia. All established antipsychotic medications antagonize dopamine D2 receptors, and their clinical efficacy correlates directly with their occupancy of D2 receptors (Seeman et al., 1975; Creese et al., 1976). However, the clinical efficacy of any drug does not necessarily imply that its target is involved in an etiological mechanism, and as already stated, D2 blockade does not ameliorate all symptoms. But independent of antipsychotic actions, there is evidence (to be described below) that dopamine dysfunction could be involved in the pathogenesis of the disease.
The original dopamine hypothesis did not attempt to distinguish between the three major dopaminergic pathways that were identified using the Falck-Hillarp method: the mesostriatal, the mesolimbic, and the mesocortical (Moore and Bloom, 1978) (Figure 2). As a result, the original version of the hypothesis viewed schizophrenia as a general dopamine hyperfunction syndrome (Figure 1B).
Figure 2. The Dopamine Hypothesis of Schizophrenia.
(A) Organization of the nigrostriatal and mesocortical midbrain dopaminergic projections. The dopaminergic midbrain neurons topographically project to the striatum but with an inverse dorsal-to-ventral organization. The mesocortical projections arise from the dorsal and medial dopamine cells.
(B) The original dopamine hypothesis of schizophrenia. The original dopamine hypothesis proposed that a global hyperactivity of the dopaminergic projections in the brain may lead to the symptoms of schizophrenia.
(C) The revised dopamine hypothesis of schizophrenia. The revised dopamine hypothesis proposed that a hyperactive nigrostriatal dopaminergic projection leads to positive symptoms but a hypoactive mesocortical projection is responsible for cognitive and negative symptoms.
(D) Results of brain imaging studies in schizophrenia. Brain imaging studies show that an increase in amphetamine-induced dopamine release in the striatum is highest in the associative striatum. Moreover, the density and occupancy of striatal D2 receptors is increased in drug-free or drug-naive patients.
PFC, prefrontal cortex; dSTR, dorsal striatum; vSTR, ventral striatum; VTA, ventral tegmental area; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; D2R, dopamine D2 receptor.
A Reconceptualization of the Dopamine Hypothesis of Schizophrenia
Consistent with the potential importance of the frontal lobe first proposed by Kraepelin, the majority of brain imaging studies of patients with schizophrenia using both MRI and PET suggest that hypofunction of the prefrontal cortex contributes to the cognitive symptoms (Buchsbaum et al., 1982; Farkas et al., 1984; Ingvar and Franzén, 1974a, 1974b; for review, see Liddle et al., 1992; Ragland et al., 2007). Functional imaging studies (fMRI) also document a further hypofrontality in schizophrenia. During the Wisconsin Card Sorting Test (Weinberger et al., 1986), patients with schizophrenia show a decrease in regional cerebral blood flow in the frontal cortex compared to healthy controls. These alterations in prefrontal activation have now been demonstrated during a variety of tasks for executive function. It is thought that hypoactivation of the dorsolateral prefrontal cortex may be accompanied by compensatory hyperactivation of directly adjacent regions, reflecting a disturbance in the ability to engage the functional networks subserving executive functions (Barch, 2005; Glahn et al., 2005; Ragland et al., 2007).
There is no unequivocal evidence for a cortical dopamine hypofunction in schizophrenia; the relationship between metabolic function and dopaminergic activity is as yet indirect and conjectured. However, there are several lines of evidence that suggest that cortical dopamine hypofunction relates to the cortical functions affected in patients with schizophrenia. Here we describe six such independent lines of evidence, which provide indirect support for the hypothesis that the metabolic hypofunction of the prefrontal cortex observed by MRI might involve dopamine dysregulation. (1) In animal studies, pioneered by Pat Goldman-Rakic, dopamine signaling was identified as important for cognition mediated by the prefrontal cortex. Depletion of dopamine in the prefrontal cortex impairs working memory in nonhuman primates (Brozoski et al., 1979), as does blockade of dopamine D1 receptors in the PFC (Vijayraghavan et al., 2007; Williams and Goldman-Rakic, 1995). (2) Genetic studies support the idea that dopamine levels in the prefrontal cortex affect cognitive function also in humans. A common polymorphism in one of the enzymes that break down dopamine, the gene encoding catechol-O-methyl transferase (COMT), results in altered activity of the enzyme and presumably in the level of dopamine available at the synapse. In healthy subjects, the polymorphism modulates performance in the tests of working memory and executive function that are significantly affected in patients with schizophrenia (Bruder et al., 2005; Egan et al., 2001). In fact, a meta-analysis of such studies, including 12 published reports, concluded that there is a significant effect of this COMT genotype on executive function, specifically performance in the Wisconsin Card Sorting task, in healthy individuals (Barnett et al., 2007). (3) In patients with schizophrenia, a decrease in the immunoreactivity for both tyrosine hydroxylase, the dopamine synthetic enzyme, and for the dopamine transporter (Akil et al., 1999) has been found in the prefrontal cortex in the brains of patients with schizophrenia at post mortem. This finding still awaits replication, however. (4) In the cerebral spinal fluid of patients with schizophrenia, the basal concentration of the dopamine metabolite homovanilic acid correlates with the regional cerebral blood flow induced by mental activity in the PFC (Weinberger et al., 1988; Kahn et al., 1994). This suggests that in patients the level of prefrontal dopamine release could predict prefrontal function (Weinberger et al., 1988; see also Elsworth et al., 1987). Future imaging studies that directly measure dopamine release in the cortex are needed to further support this idea. (5) An increase in D1 receptor density has been found in the prefrontal cortex of patients with schizophrenia using [11C]NNC as a D1-specific PET ligand. This increase in receptor availability, which was not observed using the earlier tracer [11C]SCH 23390 (Okubo et al., 1997), correlates with deficits in the N-back task (Abi-Dargham et al., 2002) and could be a compensation for the hypoactive cortical dopaminergic projections. (6) A PET study also using the tracer [11C]NNC showed that the COMT genotype presumed to enhance dopamine metabolism predicts corticallimbic D1 receptor availability (Slifstein et al., 2008).
To reconcile the more recent evidence for dopamine hypoactivity in the frontal cortex with the original hypothesis of a general hyperdopaminergia syndrome, Weinberger and Davis proposed a variant of the original dopamine hypothesis of schizophrenia, according to which the three major dopaminergic pathways are not affected in similar ways in the disease. They proposed that only the mesostriatal and mesolimbic pathways became hyperactive and that these mediate the positive symptoms of schizophrenia. They further suggested that the hyperactivity in these two pathways is secondary to a hypofunction of the prefrontal dopamine system that is responsible for the cognitive deficits (Davis et al., 1991; Weinberger, 1987) (Figure 2C).
Although this model for the pathogenesis of schizophrenia has been supported by animal models, there is as yet no direct evidence derived from studies with patients. There are in fact alternate and equally valid reasons to suggest that if dopaminergic dysfunction is causative in the pathogenesis of schizophrenia the deficits may occur in the opposite order. We here discuss the possibility that the striatum may have a primary role in the pathogenesis of schizophrenia and that the striatum plays a critical role in initiating the cognitive deficits. We present evidence for a dopamine hyperfunction in the striatum of patients with schizophrenia and show how this hyperfunction might specifically contribute to the cognitive deficits of schizophrenia.
A Dopaminergic Hyperfunction in the Striatum as Causal in the Cognitive Deficits of Schizophrenia
The idea of hyperactive subcortical dopamine in schizophrenia was originally based on the efficacy of antipsychotic medication, but this idea has now been supported by a number of important findings, which converge on evidence for both an increase in striatal dopamine and an increase in striatal dopamine receptors. (Figure 2D). Evidence for increased dopamine release in the striatum came from several sources. For example, some, though not all, post mortem studies have detected an increase in the level of dopamine in the striatum or an increase, even in unmedicated patients, in the levels of the dopamine metabolite HVA (for a review, see Davis et al., 1991). This is consistent with the numerous findings of an increase in striatal uptake of dopamine in patients with schizophrenia, as determined by an increase in the uptake of Fluoro-Dopa, by the dopamine-synthesizing enzyme L-amino acid decarboxylase (Frankle, 2007; Hietala et al., 1999; Hietala et al., 1995; Lindström et al., 1999; Meyer-Lindenberg et al., 2002; Reith et al., 1994). Similarly, PET imaging has also found that in patients with schizophrenia, there is an increase in the striatum of amphetamine-induced displacement of D2 receptor binding, consistent with increased dopamine release (Abi-Dargham et al., 1998; Breier et al., 1997; Laruelle et al., 1996).
These alterations in dopamine availability are paralleled by changes in striatal dopamine receptors. First observed by Cross et al. (1981) and then by others (for a review, see Davis et al., 1991), an upregulation of striatal D2 receptors was discovered in post mortem studies of patients who were drug-free. Similarly, an increase in the density and occupancy of D2 receptors in the striatum has been found in PET imaging (Abi-Dargham et al., 2000; Seeman and Kapur, 2000; Wong et al., 1986; Laruelle, 1998). Although there has been inconsistency in these findings, the inconsistency seems to be related to the functional properties of the different ligands that have been used. For example, M-spiperone is more lipid soluble and more readily penetrates cell membranes to reach internalized receptors than do the benzamides that generally are water-soluble. These different classes of ligands may also differ in their binding to monomeric versus dimeric receptors (Seeman and Kapur, 2000). Moreover, elevated occupancy of D2 receptors was found to be predictive for a good treatment response of positive symptoms with antipsychotic drugs (Abi-Dargham et al., 2000). Although antipsychotic medications increase the density of D2 receptors in the striatum, thereby complicating the interpretations of the PET imaging studies, a mild (12%) but highly significant elevation in D2 receptor binding potential has also been observed in drug-free and drug-naive patients (see meta-analysis: Laruelle, 1998).
An increase in D2 receptor binding potential does not, however, necessarily imply an increase in the number of receptors—it could also be due to an increase in the affinity of the receptors (Seeman et al., 2006). To clarify this issue, studies using D2 receptor ligands that preferentially bind to D2 receptors in the high-affinity state are needed. In addition, there is a need to determine whether the binding potential of D2 receptors is already high in patients in the prodromal phase of the disease, and during remission, or whether the phenotype fluctuates with the phase of the illness, like the observed increase in amphetamine-induced dopamine. This would have important implications for the role of striatal D2 receptor binding in the etiology of the disease.
With the improved resolution of PET imaging, Kegeles et al. (2006) have been able to determine that the increase in amphetamine-induced dopamine secretion in patients with schizophrenia is most pronounced in the dorsal or associative striatum, the region also known as the head of the caudate nucleus. These findings were surprising because animal models had suggested that antipsychotic medication improves positive symptoms by acting on the ventral striatum, the nucleus accumbens, rather than the dorsal striatum, the caudate nucleus.
To date there are no ways for determining whether patients with schizophrenia have excess D2 receptors during development. But, if striatal dopaminergic hyperactivity is a causative event in the disease process, then variants in genes coding for components of dopamine signaling may be associated with schizophrenia. Indeed, genetic studies have identified associations between variations in genes involved in dopamine signaling and schizophrenia (Allen et al., 2008; Talkowski et al., 2008). The continually updated meta-analyses of published schizophrenia association studies, which currently include over 1400 studies, continues to put the D2 receptor gene (DRD2) in the top ten “most strongly associated genes” (Allen et al., 2008). Of the six individual SNPs in the D2 receptor gene subjected to the meta-analysis, rs2677 (C957T) has the highest odds ratio of 1.37. This SNP does not affect the amino acid sequence of the protein, but the C allele increases mRNA stability (Duan et al., 2003) and has now been associated with schizophrenia in five independent studies (Betcheva et al., 2009; Hänninen et al., 2006; Hoenicka et al., 2006; Lawford et al., 2005; Monakhov et al., 2008). There are also two studies that did not find a significant association (Gupta et al., 2009; Sanders et al., 2008). In healthy subjects, the C allele is negatively associated with cognitive performance in the Wisconsin Card Sorting Test, linking D2 receptor function with cognition (Rodriguez-Jimenez et al., 2006). Moreover, this allele interacts with the Val158Met polymorphism in the COMT gene in the six-word serial position task, a test of working memory (Xu et al., 2007). While genetic data do not support a role for any individual SNP as a significant causative agent in schizophrenia, these data may imply that genetic variation in the D2 receptor could contribute to the disease and therefore warrant continued investigation. In further support of a genetic component to D2 receptor upregulation, unaffected monozygotic co-twins of patients with schizophrenia show increased D2-receptor density in the caudate (Hirvonen et al., 2005).
In summary, considerable evidence has accumulated for a specific hyperdopaminergic state in the striatum of patients with schizophrenia. Because the striatum is involved in aspects of cognition and, as we discuss in the next section, the prefrontal cortex and the striatum are anatomically interconnected, it raises the interesting possibility that the striatum may be involved directly in cognitive functions traditionally thought of as strictly mediated by the prefrontal cortex. To examine this possibility further, we review the evidence for the direct role of the striatum in cognitive domains related to the deficits in schizophrenia.
The Striatum Has an Important Role in Cognition
The striatum, which is made up of the caudate and the putamen, is the input component of the basal ganglia, a group of subcortical nuclei that traditionally has been associated with the control of motor movement. The striatum receives its inputs from the cortex, the thalamus, the hippocampus, and the amygdala. The main output nuclei of the basal ganglia are the globus pallidus, the subthalamic nucleus, the substantia nigra, and the ventral tegmental area. These output structures mainly project to the thalamus, the principal target of the basal ganglia. The thalamus projects back to the cortex, thereby completing a loop that involves the cortex, the striatum, and the thalamus (Alexander et al., 1986). The main reason that the focus of study for the striatum has been on its role in motor control is due to the established importance of the striatum in the pathophysiology of Parkinson and Huntington disorders, two neurodegenerative diseases that affect motor control. The striatum is recruited for the refinement of motor actions with the procedural learning of motor habits. As a result, after extensive numbers of trials, performance becomes insensitive to changes in the expected reward values (Graybiel, 2005). Patients with schizophrenia show subtle deficits in these types of behaviors, suggesting an impairment in striatal function (Horan et al., 2008; Siegert et al., 2008). This impairment is mild, however, compared to the deficits observed in Huntington and Parkinson disorders, whose phenotypes are comparable to those produced in animal models by extensive lesions of the striatum.
Work in monkeys and rats beginning in the 1960s by Steven Chorover, Haldor Rosvold, and others showed that lesions in the dorsal striatum not only affect procedural learning but also result in cognitive deficiencies similar to those produced by prefrontal lesions (Battig et al., 1960; Chorover and Gross, 1963). Detailed anatomical tracing studies revealed that within the corticostriatal pathways there are two types of projections from the prefrontal cortex to the dorsal (associative) striatum: focal, circumscribed projections and diffuse, widespread projections (Eblen and Graybiel, 1995). The focal projections to the associative striatum (the head of the caudate) include some areas of convergence between terminal fields from different functional regions of the prefrontal cortex, such striatal nodes may therefore be critical for integrating information encoded in the cortex (Calzavara et al., 2007; Graybiel, 1998; Reep et al., 2003). Consistent with this idea, Ann Graybiel has found that information derived from the cortex is transformed in the striatum, where representations of motor and cognitive action sequences can be integrated and developed into more complex units for implementing more efficient behavioral responses (Graybiel, 1998). In vivo recordings reveal dramatic learning-related changes in striatal neuronal activity as animals learn procedural tasks requiring conditional associations (Barnes et al., 2005; Jog et al., 1999). Taken together, these results, and those obtained with in vivo imaging in nonhuman primates (Levy et al., 1997) and human subjects provide strong evidence that, in addition to modulating motor control and motor learning, the striatum plays an important role in the formation of more complex behaviors, including decision making and executive function (Graybiel, 2008).
In retrospect, this view is not surprising. As we have seen, the striatum and cortex are anatomically and functionally linked by means of several parallel frontostriatal associative loops (Alexander et al., 1986). In primates, the “associative loop” projects from the dorsolateral prefrontal cortex directly to the associative component of the dorsal striatum (the head of the caudate nucleus). Consequently, lesions of either the prefrontal cortex or the dorsal striatum impair working memory (Battig et al., 1960; Divac et al., 1967). Additional evidence for a function of the striatum in working memory comes from physiological studies. Neurons that show increased activity during the delay period of a working memory task may code for the actual memory or associated attentional or motivational information. These “memory cells,” originally identified in the prefrontal cortex by Joaquin Fuster (Fuster, 1973), have also been found in the caudate nucleus and other areas of the cortex (Hikosaka et al., 1989). In rodents, the homologous “associative loop” includes the medial prefrontal cortex and the dorsomedial striatum (the medial caudate putamen). Lesioning this region of the striatum affects spatial working memory (Mair et al., 2002; Voorn et al., 2004) in the same way that lesions of the prefrontal cortex do. Lesions of the striatum or its associative loops also affect various attentional processes. In the Five-Choice Serial Reaction Time Task, which measures attention in rodents, lesions of either the dorsomedial prefrontal cortex or the medial caudate putamen reduce response accuracy and increase premature and perseverative responding, implicating the striatum in attention and cognitive control (Chudasama and Robbins, 2006). A function of the striatum in attentional processes and cognitive control is further suggested in humans because attentional set shifting measured within the Wisconsin Card Sorting Task activates the caudate nucleus in healthy human subjects (Rogers et al., 2000).
These findings suggest that the prefrontal cortex and the striatum function in concert to mediate working memory and other executive functions. The finding that the cognitive deficits in schizophrenia may arise not simply from alterations in the prefrontal cortex but also from a deficit in the functioning of the striatum places the nature of the cognitive symptom in a new light. In this regard it is interesting that the increase in amphetamine-induced dopamine secretion in patients with schizophrenia is most pronounced in the associative striatum (Kegeles et al., 2006), the area of the striatum that receives direct input from the dorsolateral prefrontal cortex. Therefore, increased dopamine release in the caudate may have a direct modulatory effect on the efficacy of the corticostriatal synapse in the associative striatum, potentially affecting cognitive processes such as working memory or attentional-set shifting.
If indeed the corticostriatal pathway is critical in the generation of cognitive symptoms of schizophrenia, it may be that components of the loop are disabled in a particular etiological sequence. A primary defect in the cortex has been proposed, but direct evidence is lacking. It may therefore be interesting to investigate the possibility that the striatum is the primary site of dysfunction. This idea is an elaboration of the previous suggestion that the striatum and associated basal ganglia are cognitive pattern generators working in concert with the prefrontal cortex to allow new cognitive patterns to form and that a deficit in this striatum-based function may be a core defect in schizophrenia (Graybiel, 1997). The hypothesis that a primary defect originates in the striatum rather than the cortex, does not imply that the striatal deficit is necessarily more profound than the defect in the cortex. As we have mentioned, the cognitive behaviors that are sensitive to striatal lesions, such as habit learning and implicit memory, are less affected in schizophrenia than executive functions, and this may be because they are less sensitive to the modulation of dopamine.
Testing the Specific Hypothesis that Striatal Dysfunction Can Result in Cognitive Deficits Related to Those Observed in Schizophrenia: The Use of Mouse Models
The finding that hypodopaminergic function in the cortex and hyperdopaminergic function in the striatum coexist in patients with schizophrenia poses a number of questions. Does a single common primary etiologic factor drive both dopaminergic dysfunctions? Or does one perturbation drive the other? For example, the alteration in the prefrontal cortex may precede that of the striatum, or the alteration in the striatum may precede that of the cortex. Of these three possibilities, only the second has been formally proposed (Davis et al., 1991; Weinberger, 1987); we believe it is also important to investigate the other two possibilities.
PET studies that combined measurement of regional cerebral blood flow in the prefrontal cortex and F-Dopa uptake in the striatum have found that prefrontal hypofunction correlates with increased F-Dopa uptake in the striatum, suggesting that both are functionally linked (Meyer-Lindenberg et al., 2002). While PET studies are powerful in detecting differences in patient populations, they do not establish causality and therefore cannot be used to identify the origins of these dysfunctions. It may be highly informative of the etiology of schizophrenia if we could identify any sequential events that could be predicted to unfold into schizophrenia. Some insights into the question could come from studying prodromal patients. Cognitive deficits begin to emerge in the prodromal period years before the full onset of the disease in the patient. The early emergence of cognitive symptoms has given rise to the idea that the prefrontal abnormality may occur earlier than the other symptom clusters. However, as we have seen, because the preservation of the corticostriatal synapses is essential for prefrontal cognitive functions, it may be impossible to use symptomatology to isolate the origin of the pathology. Evidence supporting an early perturbation has been found in both the prefrontal cortex and the striatum. MRI has identified a reduction in frontal gray matter volume in patients at risk for schizophrenia (Borgwardt et al., 2007). As expected, this reduction is milder than that observed in schizophrenic patients. Evidence for early hyperactivity of the striatal dopamine system comes from a recent imaging study that found that the uptake of F-Dopa is already increased in the striatum of prodromal patients (Howes et al., 2009). On a behavioral level, subtle abnormalities in motor as well as cognitive and emotional development have been observed in children who later develop schizophrenia (Isohanni et al., 2005; Mittal et al., 2008; Walker et al., 1994), pointing to an early involvement of both structures in the disease.
One useful and direct way for exploring causal mechanisms in the pathophysiology of disease comes from animal models. These can prove specifically useful here because they allow perturbations that assist the investigation of the mechanisms by which schizophrenia might possibly develop (Kellendonk et al., 2009). In rats, dopamine depletion induced in the prefrontal cortex by 6-OHDA increases subcortical dopamine turnover and dopamine D2 receptor numbers (Pycock et al., 1980). Similar observations have been made in primates (Roberts et al., 1994), suggesting that prefrontal dopaminergic hypofunction can indeed induce a hyperactive subcortical dopamine system. However, studies with genetically modified mice have recently presented evidence that modulation in the opposite direction is also possible.
To address the causal relationship between striatal dopamine dysfunction and cognition and to test the specific hypothesis that striatal D2R hyperactivity can result in the cognitive deficits observed in schizophrenia, Kellendonk et al. generated a genetically modified mouse that modeled the observed increase in striatal D2 receptor availability, one of the specific in vivo findings of striatal dysfunction in schizophrenia (Kellendonk et al., 2006).
In this transgenic mouse, the expression of dopamine D2 receptors was selectively increased in the striatum, including the caudate putamen, nucleus accumbens, and olfactory tubercle. To obtain temporal control of transgene expression, the mice were created using the bi-transgenic tetracycline-sensitive expression system (Mayford et al., 1996). Striatal D2 overexpressing mice show an impairment in prefrontal-dependent cognitive tasks that are associated with schizophrenia as well as a decrease in dopamine turnover and an increase in D1 receptor activation in the prefrontal cortex (Bach et al., 2008; Kellendonk et al., 2006). These results suggest that a selective and primary increase in dopamine signaling restricted to the striatum can result in deficits in both the cognitive behaviors affected in schizophrenia and in the perturbation of the prefrontal cortical dopamine system. Most interestingly, the cognitive deficits persisted long after expression of the transgene had been switched off. In fact, expression during development was sufficient to cause cognitive deficits in adulthood.
These findings suggest two important new ideas. First, a hyperfunctioning of the mesostriatal pathway could be an early event in the etiology of the disease, but this hyperfunction gives rise to compensatory changes in the prefrontal cortex that are induced during development and maintained even after treating the mesostriatal hyperactivity. Second, the reason D2 receptor blockade has minimal, if any, beneficial effects on the cognitive symptoms of schizophrenia is because antipsychotic medications are given too late, long after irreversible compensatory changes have taken place.
How the increase in developmental D2 receptor expression produces these phenotypes may therefore be highly informative for the possible pathophysiological steps in the development of the disorder. We will now discuss, in turn, how altered subcortical dopamine may impact the functioning of both the striatum and the prefrontal cortex.
The Possible Consequences of Altered Subcortical Dopamine on Striatal Function
Several lines of evidence suggest that alterations in the subcortical dopamine system affect the global functioning of the striatum, producing both metabolic and structural changes. Decreased metabolic activity in the basal ganglia and the striatum (caudate nucleus) has been consistently found by PET imaging studies in unmedicated patients (for review, see Buchsbaum and Hazlett, 1998). In contrast, in patients treated with D2 receptor antagonists, there is an increase in striatal glucose metabolism (Buchsbaum and Hazlett, 1998; Buchsbaum et al., 1992; Shihabuddin et al., 1998). These two findings suggest that D2 receptor activity negatively controls striatal metabolic rate (see Figure 3, which discusses how overstimulation of striatal D2 receptor alters physiology on the cellular level [Bamford et al., 2004; Surmeier et al., 2007]). Data from mice that selectively overexpress D2 receptors in the striatum support this idea. D2 transgenic mice show decreased glucose metabolism in the striatum, phenocopying the finding in unmedicated patients (Kellendonk et al., 2006). Switching off the additional transgenic receptor expression in the adult animal returned glucose metabolism to normal levels, suggesting that the changes in metabolism were due to concurrent D2 receptor activity and not due to a developmental compensatory change.
Figure 3. Three Potential Cellular Mechanisms by which Increased Striatal Dopamine Transmission via D2 Receptors Could Affect Striatal Function in Patients with Schizophrenia.
(A) Heterosynaptic inhibition of corticostriatal terminals. It has been shown that activation of D2 receptors at the presynaptic terminal of the incoming cortical projections inhibits corticostriatal glutamate release. Heterosynaptic inhibition is selective for the least active terminals, thereby filtering out less active inputs (Bamford et al., 2004). Because PET imaging does not have the resolution to determine which exact cells contribute to the elevated binding potential of D2 receptors in the striatum, D2 receptors could be upregulated in the corticostriatal terminals. This in combination with increased dopamine release may lead to a hypereffective heterosynaptic inhibition that may prevent the transmission of important cognitive information from the cortex to the striatum.
(B) Inhibition of medium spiny neurons of the direct pathway. Both D1 and D2 receptors are present on medium spiny neurons, which represent 95% of striatal neurons and are the main output neurons of the striatum. D1 receptors are preferentially present on medium spiny neurons that project directly to the substantia nigra pars reticularis and the internal segment of the globus pallidus. D1 receptor activation enhances excitability of these cells. In contrast, D2 receptors are preferentially expressed on medium spiny neurons that project indirectly to the substantia nigra pars reticularis and the internal segment of the globus pallidus. D2 receptor activation reduces excitability of these cells (Surmeier et al., 2007). Upregulation of D2 receptors may therefore affect the balance between direct and indirect pathways and alter the striatal output of the incoming cortical information.
(C) Global inhibition of medium spiny neurons. Activation of D2 receptors on the cholinergic interneurons diminishes acetylcholine release from these cells. The released acetylcholine affects glutamatergic and dopaminergic terminals. Acetylcholine generally enhances the glutamatergic excitation of medium spiny neurons (Surmeier et al., 2007). Increased activation of D2 receptors on cholinergic interneurons may therefore globally reduce the excitability of the striatum. Although the mechanism for the reduced metabolic activity observed in patients with schizophrenia is unknown, reduced cholinergic activity could be a contributing factor. MSN, medium spiny neuron; ChAT, choline-acetyl-transferase as a marker for cholinergic interneurons; GLU, glutamate; DA, dopamine.
Chronic alterations in dopamine signaling and neuronal metabolism may lead to structural changes. In most structural MRI studies of patients with schizophrenia, there is an increase in striatal volume (Shenton et al., 2001). These increases appear to be a consequence of medication, because never-medicated patients have smaller caudate nucleus (Keshavan et al., 1998; Shihabuddin et al., 1998), and longitundinal studies have found that first-episode patients that are treated with neuroleptics show increases in striatal volumes within 12–18 month (Chakos et al., 1994; Keshavan et al., 1994). We have made similar observations in D2 receptor overexpressing mice, which, like unmedicated patients, show decreased striatal volume (unpublished data). This is consistent with the observation that, when rats are treated chronically with the D2 blocker haloperidol, striatal volume and the area of neuronal terminals increase whereas synaptic density decreases (Andreassen et al., 2001; Roberts et al., 1995).
If a reduction in striatal volume is indeed an early event in the development of the disease, or an underlying risk trait, then it may be highly valuable to measure striatal volume in individuals identified to be in the prodromal phase of the disease. It has already been shown that young offspring of patients with schizophrenia show reduced striatal volumes compared to young people with no family history of mental illness (Rajarethinam et al., 2007). This finding suggests that reduced striatal volume is a genetic risk factor or perhaps even an early marker, since a significant proportion of the at-risk group are expected to develop schizophrenia. A replication of this study would also add weight to the hypothesis of early involvement of the striatum. More importantly, longitudinal studies of individuals with high genetic risk from an even earlier age could determine when during development the differences in striatal volume become apparent (Rajarethinam et al., 2007).
How Can Striatal D2 Receptors Affect the Functioning of the Prefrontal Cortex?
There are at least three possible ways by which altered dopamine signaling in the striatum could lead to prefrontal-dependent cognitive deficits. First, dopamine signaling may disrupt or alter the cognitive information that is coming in from the cortex. As discussed, the striatum and cortex are anatomically and functionally linked via several parallel frontostriatal loops, and in addition, nodes of convergence exist to which different cortical areas project (Calzavara et al., 2007; Eblen and Graybiel, 1995; Reep et al., 2003). One such node is located in the associative striatum, which is where the increase in amphetamine-induced dopamine release has been observed in patients with schizophrenia. The associative striatum receives input from the dorsolateral prefrontal cortex and the orbitofrontal cortex (Eblen and Graybiel, 1995; Haber et al., 2006). Increased D2 receptor signaling at the level of the medium spiny neuron in the associative striatum may therefore disturb the integration of neuronal information coming from different areas of the prefrontal cortex (Figure 4A).
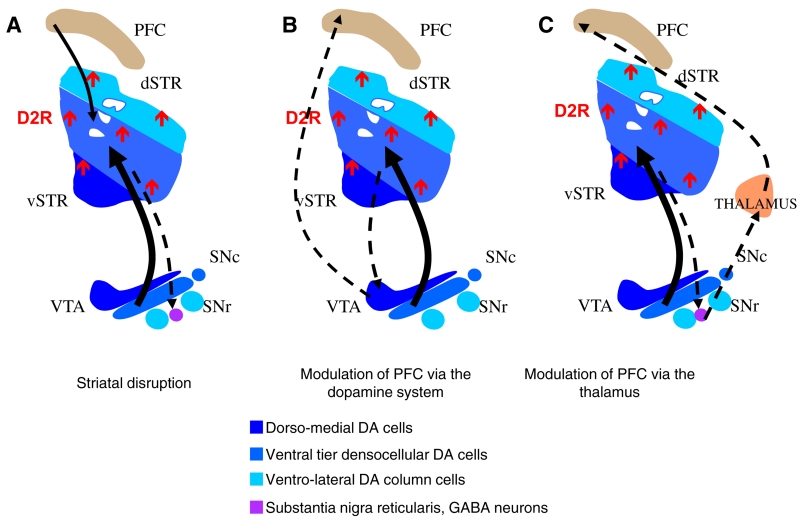
Figure 4. Mechanisms on the Circuit Level by which Increased Striatal Dopamine Transmission via D2 Receptors Could Lead to Cognitive Deficits.
(A) Striatal disruption. The striatum and cortex are anatomically and functionally linked via several parallel frontostriatal loops. Neuronal information encoding working memory and executive function may be altered by increased D2 receptor signaling at the level of the medium spiny neuron in the associative striatum, which is one synapse down-stream of the dorsolateral prefrontal cortex.
(B) Striatal modulation of prefrontal function via the dopaminergic system. The striatum harbors the main input to the dopaminergic neurons of the ventral tegmental area (VTA) and substantia nigra (SN). By modulating the activity of neurons in the VTA, it may modulate the dopaminergic input of the prefrontal cortex. In mice with upregulated D2 receptors in the striatum, prefrontal dopamine turnover is decreased and D1 receptor activation increased. Since optimal D1 receptor activation in the prefrontal cortex is crucial for working memory, this could be a mechanism by which striatal D2 receptor overactivation may affect prefrontal function.
(C) Striatal modulation of cortical function via the thalamus. The striatum projects back to the prefrontal cortex via the thalamus and may thereby modulate activity in the prefrontal cortex (for simplicity only the direct pathway via the substantia nigra reticularis is drawn). These mechanisms on the circuit level could already arise during development. In mice, selective upregulation of D2 receptors in the striatum during development leads to prefrontal-dependent cognitive deficits that cannot be reversed by switching off the additional receptors in the adult animal. This suggests that early upregulation of D2 receptors during development leads to compensatory changes potentially in the discussed circuits that cannot be easily reversed in the adult animal.
Second, the striatum is the main structure projecting to the dopaminergic midbrain neurons in the ventral tegmental area (Frankle et al., 2006). Increased signaling through striatal D2 receptors may regulate the activity of the dopaminergic neurons, including those that project to the cortex, thereby affecting dopamine release in the prefrontal cortex (Figure 4B). Third, altered activity in the striatum could indirectly change cortical functioning via the thalamus, which is part of the parallel corticostriatal loops (Figure 4C).
The data from these genetically modified mice suggest that mechanisms on the circuit level that in principle could contribute to the cognitive symptoms of schizophrenia already emerge during prenatal development. In these mice, the selective upregulation of D2 receptors in the striatum during development leads to prefrontal-dependent cognitive deficits that cannot be reversed by switching off the additional receptors in the adult animal (Bach et al., 2008; Kellendonk et al., 2006).
Perhaps the most useful direction in which to take the animal model approach is to study developmental mechanisms that are extremely difficult to study in humans. Increasing our understanding of the changes in early development that result in cognitive dysfunctions in adulthood would be a major step forward. In mouse genetic models, we can perturb individual molecules at any time point and look at the effect at anytime point after. For example, in the striatal D2R overexpression model, it is possible to quantify the neuronal projections in the corticostriatal pathway using quantitative tracing techniques. Such experiments, combined with immunohistochemistry, may identify specific cell types and molecular markers involved in any circuit abnormalities, and the relevance to schizophrenia of such markers may be investigated by studying them in post mortem tissue. Other novel mouse models that could be informative might include those that express cell-type-specific, easily imaged markers, which would allow for the monitoring of corticostriatal projections as they develop. Such marker mice could be subjected to the numerous pre-, peri-, or postnatal alterations, including environmental insults that have been determined to influence risk for schizophrenia, and one could then see when and how such perturbations affect development of the tagged circuit(s). Separate cohorts of mice that have not been sacrificed for imaging could be allowed to develop and their adult cognitive functions measured. By using different developmental insults and inducing them at different time points in development, one presumes that subtly different anatomical phenotypes will ensue. A systematic comparison of the anatomical and cognitive defects might allow one to make more detailed inferences about the relative importance of specific pathways on specific cognitive functions. By combining such studies with quantitative assays of gene or protein expression, specifically in the tagged cells, new candidate molecules may be identified, which one could study in post mortem tissue to determine if similar changes in expression occur in the brains of patients with schizophrenia. One limitation of the approach is that some of the expression changes identified may occur only during development and therefore be undetectable in post mortem studies.
Conclusion
We begin our conclusion with an open disclaimer. To focus attention on the striatum, a relatively neglected area in schizophrenia research, the discussion presented here focuses on a specific hypothesis. Thus, this perspective is not aimed to be a comprehensive review of the entire literature of schizophrenia research but rather aimed to bring more attention to a possible role of the striatum in schizophrenia. While there is solid evidence for the involvement of the striatum, the vast majority of studies so far have focused on the prefrontal cortex and hippocampus. Indeed, we have not described important studies on the involvement of the temporal lobe (Harrison, 2004; Seidman et al., 2003). For example, hippocampal volume, which may be genetically determined, has been found to be reduced already at onset of schizophrenia and continues to decrease in volume as the disease progresses. Altered function of the hippocampus could be responsible for some of the cognitive deficits in patients with schizophrenia, such as deficits in explicit memory. Moreover, animal models suggest that hippocampal hyperactivity induces subcortical dopaminergic hyperfunction. We have deliberately not included these studies in this perspective.
The ultimate goal of understanding the biological basis for the cognitive symptoms of schizophrenia is threefold. First and foremost is the development of treatments that will ameliorate these symptoms and thereby help patients with schizophrenia to live a more functional life in society. Based on the data reviewed here, we suggest that treatment strategies should not overlook the striatum and any compensatory changes it may induce in the prefrontal cortex as an important structure involved in the generation of cognitive symptoms. Second, insights into the biology of schizophrenia should give us insights into the biology of human mental processes in general: into the sense of self, into volition, and short-term memory.
The findings in mice raised a third consideration. They illustrate that mice can teach one a great deal about possible mechanism underlying the pathogenesis of schizophrenia. They show that these mechanisms may already have been induced during development and therefore may not be reversible at late adolescence or early adulthood, when schizophrenia usually precipitates. Medications given at the time of diagnosis must therefore be directed against the compensatory changes that have been induced by developmental alterations rather than the specific, as yet unknown, underlying etiologic factors. One limitation is, of course, that a specific pharmacological compound may ameliorate a dysfunction in one structure, e.g., the striatum, but at the same time may have detrimental effects in other areas of the brain, such as cortex or thalamus. Treatment strategies should therefore take advantage of differences in dopamine signaling that may occur in different anatomical structures or pathways in the brain. Dopamine receptor heterodimer complex formation, the phosphorylation or subcellular localization of receptors, or the recruitment of downstream signaling cascades may be distinct in different pathways or cell types in the brain. One example of this strategy is the selective inhibitor of the phosphodiesterase PDE10A, which targets the striatum because PDE10A is predominantly expressed in the putamen and caudate nucleus, exclusively in medium spiny neurons (Xie et al., 2006). Pharmacological PDE10A inhibition has been shown to be efficacious in preclinical models of the positive, negative, and cognitive symptoms of schizophrenia (Grauer et al., 2009), and one PDE10A inhibitor is currently undergoing clinical trial. A recent comprehensive review of the potential of PDE10A inhibitors has recently been published (Chappie et al., 2009). Forty years after the dopamine hypothesis of schizophrenia was first proposed, we are now in a position to benefit from a substantial investment in understanding the different dopamine pathways in molecular terms, in the healthy and diseased brain.
ACKNOWLEDGMENTS
We would like to thank David Lewis and Suzanne Haber for their insightful comments on an earlier version of this manuscript and Ann Graybiel for helpful discussions. Our research is supported by NARSAD (www.narsad.org), the National Institute of Mental Health Silvio O. Conte Center for Schizophrenia Research (www.nimh.nih.gov), and the Lieber Center for Schizophrenia Research. We also are grateful to Harold and Shari Levy, whose generous contributions have supported our schizophrenia research.
REFERENCES
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am. J. Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am. J. Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat. Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Meshul CK, Moore C, Jørgensen HA. Oral dyskinesias and morphological changes in rat striatum during long-term haloperidol administration. Psychopharmacology (Berl.) 2001;157:11–19. doi: 10.1007/s002130100767. [DOI] [PubMed] [Google Scholar]
- Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc. Natl. Acad. Sci. USA. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu. Rev. Clin. Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Müller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executivefunction: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol. Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Battig K, Rosvold HE, Mishkin M. Comparison of the effects of frontal and caudate lesions on delayed response and alternation in monkeys. J. Comp. Physiol. Psychol. 1960;53:400–404. doi: 10.1037/h0047392. [DOI] [PubMed] [Google Scholar]
- Baumeister AA, Francis JL. Historical development of the dopamine hypothesis of schizophrenia. J. Hist. Neurosci. 2002;11:265–277. doi: 10.1076/jhin.11.3.265.10391. [DOI] [PubMed] [Google Scholar]
- Betcheva ET, Mushiroda T, Takahashi A, Kubo M, Karachanak SK, Zaharieva IT, Vazharova RV, Dimova II, Milanova VK, Tolev T, et al. Case-control association study of 59 candidate genes reveals the DRD2 SNP rs6277 (C957T) as the only susceptibility factor for schizophrenia in the Bulgarian population. J. Hum. Genet. 2009;54:98–107. doi: 10.1038/jhg.2008.14. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher-Rössler A, Dazzan P, Chitnis X, Aston J, Drewe M, Gschwandtner U, Haller S, Pflüger M, Rechsteiner E, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol. Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc. Natl. Acad. Sci. USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, Gilliam TC. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol. Psychiatry. 2005;58:901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Hazlett EA. Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr. Bull. 1998;24:343–364. doi: 10.1093/oxfordjournals.schbul.a033331. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Ingvar DH, Kessler R, Waters RN, Cappelletti J, van Kammen DP, King AC, Johnson JL, Manning RG, Flynn RW, et al. Cerebral glucography with positron tomography. Use in normal subjects and in patients with schizophrenia. Arch. Gen. Psychiatry. 1982;39:251–259. doi: 10.1001/archpsyc.1982.04290030001001. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Potkin SG, Siegel BV, Jr., Lohr J, Katz M, Gottschalk LA, Gulasekaram B, Marshall JF, Lottenberg S, Teng CY, et al. Striatal metabolic rate and clinical response to neuroleptics in schizophrenia. Arch. Gen. Psychiatry. 1992;49:966–974. doi: 10.1001/archpsyc.1992.01820120054008. [DOI] [PubMed] [Google Scholar]
- Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur. J. Neurosci. 2007;26:2005–2024. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am. J. Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chappie T, Humphrey J, Menniti F, Schmidt C. PDE10A inhibitors: an assessment of the current CNS drug discovery landscape. Curr. Opin. Drug Discov. Devel. 2009;12:458–467. [PubMed] [Google Scholar]
- Chorover SL, Gross CG. Caudate nucleus lesions: Behavioral effects in the rat. Science. 1963;141:826–827. doi: 10.1126/science.141.3583.826. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol. Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol. Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Crow TJ, Owen F. 3H-Flupenthixol binding in postmortem brains of schizophrenics: evidence for a selective increase in dopamine D2 receptors. Psychopharmacology (Berl.) 1981;74:122–124. doi: 10.1007/BF00432676. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J. Comp. Physiol. Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, Gejman PV. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J. Neurosci. 1995;15:5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Leahy DJ, Roth RH, Redmond DE., Jr. Homovanillic acid concentrations in brain, CSF and plasma as indicators of central dopamine function in primates. J. Neural Transm. 1987;68:51–62. doi: 10.1007/BF01244639. [DOI] [PubMed] [Google Scholar]
- Farkas T, Wolf AP, Jaeger J, Brodie JD, Christman DR, Fowler JS. Regional brain glucose metabolism in chronic schizophrenia. A positron emission transaxial tomographic study. Arch. Gen. Psychiatry. 1984;41:293–300. doi: 10.1001/archpsyc.1984.01790140083010. [DOI] [PubMed] [Google Scholar]
- Frankle WG. Neuroreceptor imaging studies in schizophrenia. Harv. Rev. Psychiatry. 2007;15:212–232. doi: 10.1080/10673220701679812. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Laruelle M, Haber SN. Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology. 2006;31:1627–1636. doi: 10.1038/sj.npp.1300990. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J. Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum. Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Grauer SM, Pulito VL, Navarra RL, Kelly M, Kelley C, Graf R, Langen B, Logue S, Brennan J, Jiang L, et al. PDE10A inhibitor activity in preclinical models of the positive, cognitive and negative symptoms of schizophrenia. Pharmacol. Exper. Ther. 2009;331:574–590. doi: 10.1124/jpet.109.155994. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophr. Bull. 1997;23:459–469. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr. Opin. Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Gupta M, Chauhan C, Bhatnagar P, Gupta S, Grover S, Singh PK, Purushottam M, Mukherjee O, Jain S, Brahmachari SK, Kukreti R. Genetic susceptibility to schizophrenia: role of dopaminergic pathway gene polymorphisms. Pharmacogenomics. 2009;10:277–291. doi: 10.2217/14622416.10.2.277. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen K, Katila H, Kampman O, Anttila S, Illi A, Rontu R, Mattila KM, Hietala J, Hurme M, Leinonen E, Lehtimäki T. Association between the C957T polymorphism of the dopamine D2 receptor gene and schizophrenia. Neurosci. Lett. 2006;407:195–198. doi: 10.1016/j.neulet.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl.) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvälahti E, Vuorio K, Räkköläinen V, Bergman J, Haaparanta M, Solin O, Kuoppamäki M, Kirvelä O, Ruotsalainen U, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvälahti E, Vilkman H, Vuorio K, Räkkölaïnen V, Bergman J, Haaparanta M, Solin O, Kuoppamäki M, Eronen E, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr. Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J. Neurophysiol. 1989;61:814–832. doi: 10.1152/jn.1989.61.4.814. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, van Erp TG, Huttunen J, Aalto S, Någren K, Huttunen M, Lönnqvist J, Kaprio J, Hietala J, Cannon TD. Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch. Gen. Psychiatry. 2005;62:371–378. doi: 10.1001/archpsyc.62.4.371. [DOI] [PubMed] [Google Scholar]
- Hoenicka J, Aragüés M, Rodríguez-Jiménez R, Ponce G, Martínez I, Rubio G, Jiménez-Arriero MA, Palomo T, Psychosis and Addictions Research Group (PARG) C957T DRD2 polymorphism is associated with schizophrenia in Spanish patients. Acta Psychiatr. Scand. 2006;114:435–438. doi: 10.1111/j.1600-0447.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- Horan WP, Green MF, Knowlton BJ, Wynn JK, Mintz J, Nuechterlein KH. Impaired implicit learning in schizophrenia. Neuropsychology. 2008;22:606–617. doi: 10.1037/a0012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Ingvar DH, Franzén G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr. Scand. 1974a;50:425–462. doi: 10.1111/j.1600-0447.1974.tb09707.x. [DOI] [PubMed] [Google Scholar]
- Ingvar DH, Franzén G. Distribution of cerebral activity in chronic schizophrenia. Lancet. 1974b;2:1484–1486. doi: 10.1016/s0140-6736(74)90221-9. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Lauronen E, Moilanen K, Isohanni I, Kemppainen L, Koponen H, Miettunen J, Mäki P, Räsänen S, Veijola J, et al. Predictors of schizophrenia: evidence from the Northern Finland 1966 Birth Cohort and other sources. Br. J. Psychiatry Suppl. 2005;48:s4–s7. doi: 10.1192/bjp.187.48.s4. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Harvey PD, Davidson M, Keefe RS, Apter S, Neale JM, Mohs RC, Davis KL. Neuropsychological correlates of central monoamine function in chronic schizophrenia: relationship between CSF metabolites and cognitive function. Schizophr. Res. 1994;11:217–224. doi: 10.1016/0920-9964(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, et al. CATIE Investigators Neurocognitive Working Group Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Frankle WG, Gil R, Narendran R, Slifstein M, Hwang DR, Cangiano C, Haber SN, Abi-Dargham A, Laruelle M. Schizophrenia is associated with increased synaptic dopamine in associative rather than limbic regions of the striatum: implications for mechanisms of action of antipsychotic drugs. J. Nucl. Med. 2006;47:139P. [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW. Decreased caudate volume in neuroleptic-naive psychotic patients. Am. J. Psychiatry. 1998;155:774–778. doi: 10.1176/ajp.155.6.774. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Performance of schizophrenic patients on tests sensitive to left or right frontal, temporal, or parietal function in neurological patients. J. Nerv. Ment. Dis. 1983;171:435–443. doi: 10.1097/00005053-198307000-00008. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenia. Livingstone; Edinburgh: 1919. [Google Scholar]
- Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q. J. Nucl. Med. 1998;42:211–221. [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Swagell CD, Barnes M, Burton SC, Ward WK, Heslop KR, Shadforth S, van Daal A, Morris CP. The C/C genotype of the C957T polymorphism of the dopamine D2 receptor is associated with schizophrenia. Schizophr. Res. 2005;73:31–37. doi: 10.1016/j.schres.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J. Neurosci. 1997;17:3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Frackowiak RS. Cerebral blood flow and mental processes in schizophrenia. J. R. Soc. Med. 1992;85:224–227. doi: 10.1177/014107689208500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström LH, Gefvert O, Hagberg G, Lundberg T, Bergström M, Hartvig P, Långström B. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol. Psychiatry. 1999;46:681–688. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Mair RG, Koch JK, Newman JB, Howard JR, Burk JA. A double dissociation within striatum between serial reaction time and radial maze delayed nonmatching performance in rats. J. Neurosci. 2002;22:6756–6765. doi: 10.1523/JNEUROSCI.22-15-06756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch. Gen. Psychiatry. 2008;65:165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- Monakhov M, Golimbet V, Abramova L, Kaleda V, Karpov V. Association study of three polymorphisms in the dopamine D2 receptor gene and schizophrenia in the Russian population. Schizophr. Res. 2008;100:302–307. doi: 10.1016/j.schres.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu. Rev. Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Carter CJ, Kerwin RW. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J. Neurochem. 1980;34:91–99. doi: 10.1111/j.1471-4159.1980.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int. Rev. Psychiatry. 2007;19:417–427. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarethinam R, Upadhyaya A, Tsou P, Upadhyaya M, Keshavan MS. Caudate volume in offspring of patients with schizophrenia. Br. J. Psychiatry. 2007;191:258–259. doi: 10.1192/bjp.bp.106.029017. [DOI] [PubMed] [Google Scholar]
- Reep RL, Cheatwood JL, Corwin JV. The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J. Comp. Neurol. 2003;467:271–292. doi: 10.1002/cne.10868. [DOI] [PubMed] [Google Scholar]
- Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, Bachneff S, Cumming P, Diksic M, Dyve SE, et al. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc. Natl. Acad. Sci. USA. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J. Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Gaither LA, Gao XM, Kashyap SM, Tamminga CA. Ultrastructural correlates of haloperidol-induced oral dyskinesias in rat striatum. Synapse. 1995;20:234–243. doi: 10.1002/syn.890200307. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Jimenez R, Hoenicka J, Jimenez-Arriero MA, Ponce G, Bagney A, Aragues M, Palomo T. Performance in the Wisconsin Card Sorting Test and the C957T polymorphism of the DRD2 gene in healthy volunteers. Neuropsychobiology. 2006;54:166–170. doi: 10.1159/000098652. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J. Cogn. Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, Burrell GJ, Rice JP, Nertney DA, Olincy A, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am. J. Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc. Natl. Acad. Sci. USA. 2000;97:7673–7675. doi: 10.1073/pnas.97.14.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Chau-Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc. Natl. Acad. Sci. USA. 1975;72:4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, et al. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Pantelis C, Keshavan MS, Faraone SV, Goldstein JM, Horton NJ, Makris N, Falkai P, Caviness VS, Tsuang MT. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophr. Bull. 2003;29:803–830. doi: 10.1093/oxfordjournals.schbul.a007048. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr. Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin L, Buchsbaum MS, Hazlett EA, Haznedar MM, Harvey PD, Newman A, Schnur DB, Spiegel-Cohen J, Wei T, Machac J, et al. Dorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch. Gen. Psychiatry. 1998;55:235–243. doi: 10.1001/archpsyc.55.3.235. [DOI] [PubMed] [Google Scholar]
- Siegert RJ, Weatherall M, Bell EM. Is implicit sequence learning impaired in schizophrenia? A meta-analysis. Brain Cogn. 2008;67:351–359. doi: 10.1016/j.bandc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, Gordon Frankle W, Weinberger DR, Laruelle M, Abi-Dargham A. COMT genotype predicts cortical-limbic D1 receptor availability measured with [(11)C]NNC112 and PET. Mol. Psychiatry. 2008;13:821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Talkowski ME, Kirov G, Bamne M, Georgieva L, Torres G, Mansour H, Chowdari KV, Milanova V, Wood J, McClain L, et al. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum. Mol. Genet. 2008;17:747–758. doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr. Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch. Gen. Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Arch. Gen. Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wong DF, Wagner HN, Jr., Tune LE, Dannals RF, Pearlson GD, Links JM, Tamminga CA, Broussolle EP, Ravert HT, Wilson AA, et al. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234:1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- Xie Z, Adamowicz WO, Eldred WD, Jakowski AB, Kleiman RJ, Morton DG, Stephenson DT, Strick CA, Williams RD, Menniti FS. Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience. 2006;139:597–607. doi: 10.1016/j.neuroscience.2005.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kellendonk CB, Simpson EH, Keilp JG, Bruder GE, Polan HJ, Kandel ER, Gilliam TC. DRD2 C957T polymorphism interacts with the COMT Val158Met polymorphism in human working memory ability. Schizophr. Res. 2007;90:104–107. doi: 10.1016/j.schres.2006.10.001. [DOI] [PubMed] [Google Scholar]