Abstract
Background
The need for reliable, valid tools to measure patient-reported outcomes (PROs) is critical for both research and for evaluating treatment effects in practice. The Patient Reported Outcome Measurement Information System (PROMIS) Fatigue-Short Form v1.0 –Fatigue 7a (PROMIS F-SF) has had limited psychometric evaluation in various populations.
Objectives
The aim of the study is to examine psychometric properties of PROMIS F-SF item responses across various populations.
Methods
Data from five studies with common data elements were used in this secondary analysis. Samples from patients with fibromyalgia, sickle cell disease, cardiometabolic risk, pregnancy, and healthy controls were used. Reliability was estimated using Cronbach’s alpha. Dimensionality was evaluated with confirmatory factor analysis. Concurrent validity was evaluated by examining Pearson’s correlations between scores from the PROMIS F-SF, the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF), and the Brief Fatigue Inventory (BFI). Discriminant validity was evaluated by examining Pearson’s correlations between scores on the PROMIS F-SF and measures of stress and depressive symptoms. Known groups validity was assessed by comparing PROMIS F-SH scores in the clinical samples to healthy controls.
Results
Reliability of PROMIS F-SF scores was adequate across samples, ranging from .72 in the pregnancy sample to .88 in healthy controls. Unidimensionality was supported in each sample. Concurrent validity was strong; across the groups, correlations with scores on the MFSI-SF and BFI ranged from .60–.85. Correlations of the PROMIS-SF with measures of stress and depressive mood were moderate to strong, ranging from .37–.64. PROMIS F-SF scores were significantly higher in clinical samples, compared to healthy controls.
Discussion
Reliability and validity of the PROMIS F-SF were acceptable. The PROMIS F-SF is a suitable measure of fatigue across the four diverse clinical populations included in the analysis.
Keywords: cardiometabolic risk, common data elements, fatigue, fibromyalgia, pregnancy, PROMIS, psychometrics, sickle cell disease
Reliable and valid tools to measure health outcomes from the patient’s perspective—patient-reported outcomes (PROs)—are essential for evaluating the effects of interventions and treatments. Our National Institute of Nursing Research (NINR)-funded P30 Center of Excellence for Biobehavioral Approaches to Symptom Management which targeted fatigue as a PRO. NINR focuses on symptom research and PROs, and to achieve this goal provides funding to support the development of centers of excellence (P30) that build symptom science (Redeker et al., 2015). Fatigue was measured in five studies supported by this P30 center. The studies included individuals with fibromyalgia syndrome (FMS), sickle cell disease (SCD), cardio-metabolic risk (CMR), pregnancy, and healthy controls from a breast cancer study. Three measures of fatigue were used across the five studies: the Patient Reported Outcomes Measurement Information System Fatigue-Short Form v1.0 –Fatigue 7a (PROMIS F-SF; National Institute of Health Patient Reported Outcomes Measurement Information System, [NIH], 2007); the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF; Stein, Jacobsen, Blanchard, & Thors, 2004; Stein, Martin, Hann, & Jacobsen, 1998); and the Brief Fatigue Inventory (BFI; Mendoza et al., 1999). Compared to the other two fatigue measures, the PROMIS F-SF has had limited psychometric evaluation in diverse populations. Therefore, in this cross-study analysis, we examined the psychometric properties of the PROMIS F-SF in these populations.
Fatigue is a symptom in great need of research because it is a common troublesome experience that is pervasive in today’s life (Christodoulou, Schneider, Junghaenel, Broderick, & Stone, 2014). In the general population, the prevalence of fatigue has been reported to range from 7% to 45% (Junghaenel, Christodoulou, Lai, & Stone, 2011). Among U.S. workers, 38% of those sampled reported being fatigued (Ricci, Chee, Lorandeau, & Berger, 2007). Among patients with chronic conditions, the prevalence is even higher. The occurrence of fatigue has been reported as high as 80%-90% among chronically ill patients (Franzén, Blomqvist, & Saveman, 2006; Prue, Rankin, Allen, Gracey, & Cramp, 2006), and fatigue is one of the most predominant features of chronic illness. For example, a recent study found that up to 35% of individuals referred to palliative care reported fatigue as a chronic refractory symptom from which little to no relief was found (Currow et al., 2015). Given the occurrence of fatigue, it would stand to reason that fatigue is one of the most frequent reasons for seeking medical care (Nikolaus, Bode, Taal, & van de Laar, 2013; Wessely, 2001).
Fatigue has been defined as “lack of energy and inability to maintain a usual routine” (NIH, 2015). Unlike acute fatigue—which is generally linked to a specific cause and often relieved by restorative techniques such as rest—persistent fatigue is viewed as being abnormal and pervasive, occurring in clinical populations who generally gain no relief from usual recuperative techniques (Christodoulou et al., 2014; Zautra, Fasman, Parish, & Davis, 2007). At such levels, fatigue can be overwhelming, debilitating, and lead to a sustained sense of exhaustion (DeWalt, Rothrock, Yount, & Stone, 2007; Junghaenel, et al, 2011). In fact, the experience of fatigue has been described by patients as “living with a loss of physical energy” that engulfs the whole body (Hägglund, Boman, & Lundman, 2008); out of balance with one’s physical and mental state that “drains life” from the body and fatigues the brain (Hodge, Itty, Cadogan, Martinez, & Pham, 2016); and having substantial negative consequences for daily life (Hägglund et al., 2008; Mengshoel, 2010).
The pathogenesis of fatigue, in general, is not well understood (Swain, 2006). Fatigue is thought to arise within the central nervous system with biochemical alterations leading to a cascade of events resulting in fatigue (Nozaki et al., 2009). Because the pathways are complex and multidimensional, it is suggested that possible etiologies of fatigue may be best viewed within the setting of the medical condition (Swain, 2006). In fibromyalgia, fatigue is a major symptom that is tremendously distressing and has been associated with chronic stress and with elevation of specific inflammatory cytokines and C-reactive protein (Menzies, Lyon, Elswick, McCain, & Gray, 2014). In sickle cell disease, fatigue is a hallmark symptom that is largely due to the hypoxemia from chronic anemia and to inflammation (Ameringer, Elswick, & Smith, 2014; Ameringer & Smith, 2011; Dampier et al., 2010; Levenson et al., 2008). In cardio-metabolic risk and cardiac disease, the pathophysiology may be attributable to alterations of insulin action, such as insulin resistance and the low-grade inflammatory state, due in part to cytokines produced by the excessive adipose tissue (Kaltsas, Vgontzas, & Chrousos, 2010). It is postulated that perceived stress and depressive symptoms contribute to fatigue and weight gain, which increases cardio-metabolic risk and, ultimately, cardiovascular disease (Robins, Elswick, Sturgill, & McCain, 2015). During pregnancy, the pathogenesis of fatigue is likely related to physiologic changes such as increased oxygen consumption, cardiovascular changes, metabolic effects, as well hormones such as progesterone (Poole, 1986). In addition, hypothalamic-pituitary-adrenal (HPA) activation dysfunction related to corticosteroid releasing hormone (CRH) may be important in the development of fatigue. Stress during pregnancy also activates the HPA axis, thus, contributing to fatigue (Chrousos, Torpy, & Gold, 1998).
Given the nature and prevalence of fatigue across populations, interventions to reduce fatigue are likely to be applicable across various health states, such as pharmacological and nonpharmacological interventions. However, comparing the effectiveness of interventions is difficult as there are multiple measures used within the fatigue research, such as the Brief Fatigue Inventory, the Bidimensional Fatigue Scale (Chalder et al., 1993), and the Multidimensional Fatigue Inventory (Smets, Garssen, Bonke, & De Haes, 1995). Standardized measures of fatigue could be useful for determining effective interventions across conditions. A major step has been taken to develop standardized measures on the national level.
Fatigue is considered to be a patient-reported outcome (PRO). PRO is an umbrella term used to describe outcomes collected directly from patients without interpretation by clinicians or anyone else (Doward, Gnanasakthy, & Baker, 2010). In 2004, the National Institutes of Health (NIH) funded the Patient Reported Outcomes Measurement Information System (PROMIS) specifically to develop standardized tools for measuring PROs (NIH, 2007). PROMIS consists of two major frameworks—Adult Self-Reported Health and Pediatric Self- and Proxy-Reported Health—each containing physical, mental, and social health domains. Item banks and subsequent PROMIS measures were developed within each framework to assess illness-related concepts, or PROs, such as fatigue, emotional distress, and social participation across populations and disease conditions (Cella et al., 2010; Pilkonis et al., 2011). Within the PROMIS framework, fatigue is part of the physical health domain. In the early phases of PROMIS development, confirmatory factor analysis (CFA) was used to examine the unidimensionality of items within a domain, such as fatigue, but within limited clinical populations. The PROMIS team also used item response theory (IRT)—as opposed to classic test theory—to increase the quality, precision, and interpretation of the PROMIS measures. Specifically, IRT was used to improve the ability of scores to discriminate between various levels of a symptom and to allow tailoring the assessment of the symptom. PROMIS measures can be administered using computerized adaptive tests (CATs) or static short forms administered by paper or telephone. The NIH PROMIS website has complete information about the wide range of available forms (www.nihpromis.org/).
The PROMIS SF v1.0–Fatigue 7a (PROMIS F-SF) was a common data element in our P30 center studies. The short form was chosen because it consists of only seven items, and thus incurs little burden on the participant. The paper form was used because of the limited access to computers for the multiple studies, and because all other instruments in these studies were administered by paper. This paper reports on the reliability and validity of the PROMIS F-SF in four clinical populations, thus contributing important information for the overall validation effort for the new PROMIS instrumentation.
Methods
Design and Samples
This was a secondary analysis of datasets from five studies that focused on fatigue and were conducted within the P30 Center of Excellence for Biobehavioral Approaches to Symptom Management. Detailed information on the design and methods of four of the studies can be found in the original reports (Ameringer et al., 2014; Jallo, Ruiz, Elswick, & French, 2014; Menzies et al., 2014; Robins et al., 2015). One study examined fatigue in fibromyalgia (FMS) (Menzies et al., 2014), another in sickle cell disease (SCD) (Ameringer et al., 2014), a third in cardiometabolic risk (CMR) (Robins et al., 2015), and a fourth in pregnancy (Jallo et al., 2014). In the fifth study, fatigue was examined in healthy controls and women with breast cancer; here, data from the healthy control subsample were used to examine known groups validity only. Four of the five studies were longitudinal studies (baseline data were used for analysis); one was a cross-sectional study. Each of the study samples included adults; one also included adolescents (SCD). Four of the five studies included only women; one included both young women and men (SCD). Sample sizes ranged from 40 to 72. A comparison of levels of fatigue, depressive symptoms, and stress, as well as the full demographic profile across the four clinical samples can be found in a report published by Lyon et al. (2014); a snapshot is presented in Table 1. The Institutional Review Board approved protocols for each of the studies.
TABLE 1.
Participant Characteristics by Study Sample
| FMS (N = 72) | SCD (N = 60) | CMR (N = 63) | Pregnancy (N = 72) | HC (N = 40) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Characteristic | M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) |
| Age (years) | 46.9 | (13.4)a | 22.5 | (4.1)b | 43.9 | (4.4)c | 24.3 | (5.5)d | 33.4 | (13.5)e |
|
|
|
|
|
|
||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
|
|
|
|
|
|
||||||
| Gender (female) | 72 | (100.0) | 36 | (60.0) | 63 | (100.0) | 72 | (100.0) | 40 | (100.0) |
| Incomef | ||||||||||
| < 45,000 | 36 | (50.0) | 48 | (80.0) | 12 | (19.0) | 68 | (94.4) | 21 | (52.5) |
| ≥ 45,000 | 35 | (48.6) | 9 | (15.0) | 49 | (77.8) | 4 | (5.6) | 19 | (47.5) |
| Educationf | ||||||||||
| ≤High school | 15 | (20.8) | 33 | (55.0) | 3 | (4.8) | 35 | (49.0) | 3 | (7.5) |
| Post high school | 28 | (38.9) | 20 | (33.3) | 10 | (15.9) | 27 | (37.0) | 5 | (12.5) |
| Post college | 28 | (38.9) | 7 | (11.7) | 49 | (77.7) | 10 | (14.0) | 32 | (80.0) |
| Racef | ||||||||||
| Black/AA | 21 | (29.2) | 58 | (96.0) | 15 | (23.8) | 68 | (94.4) | 8 | (20.0) |
| White | 47 | (65.2) | 0 | (0.0) | 47 | (74.6) | 0 | (0.0) | 25 | (62.5) |
| Other | 4 | (5.6) | 2 | (3.4) | 0 | (0.0) | 4 | (5.6) | 7 | (17.5) |
Note. AA = African American; CMR = cardiometabolic risk; FMS = fibromyalgia syndrome; HC = healthy controls; SCD = sickle cell disease.
Range: 18–71.
Range: 15–30.
Range: 35–50.
Range: 18–39.
Range: 21–67.
Percents may not add to 100 due to missing data.
Procedures
A number of common measures were used across these studies—four of which were used to evaluate the psychometrics of the PROMIS F-SF. Two were measures of fatigue: the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) and the Brief Fatigue Inventory (BFI); one was a measure of stress (the Perceived Stress Scale; PSS); and one was a measure of depressive symptoms (Center for Epidemiological Studies-Depression; CES-D).
The approach to evaluating reliability and validity of the PROMIS F-SF across studies was as follows. Reliability was estimated using Cronbach’s alpha. Unidimensionality of scores on the PROMIS F-SF was evaluated with confirmatory factor analysis (CFA). Two aspects of validity were evaluated: concurrent and discriminant validity. Concurrent validity was evaluated by testing of the PROMIS F-SF with the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) and the Brief Fatigue Inventory (BFI). Discriminant validity was evaluated by testing the associations between fatigue and both stress and depressive symptoms—two concepts that are related to fatigue but distinct from it (Ream & Richardson, 1997). The known groups method was also used (means of study samples were compared with those of healthy controls).
Measures
PROMIS F-SF
The PROMIS F-SF consists of seven items that measure both the experience of fatigue and the interference of fatigue on daily activities over the past week (NIH, 2007). Examples of items are: “How often did you feel tired,” and “How often were you too tired to take a bath/shower”. Response options are on a 5-point Likert scale, ranging from 1 = never to 5 = always. One item, “How often did you have enough energy to exercise strenuously,” is reverse scored. The total score is used in the analysis and is obtained by summing keyed scores of all items. A summative score was obtained as recommended by the PROMIS developers when the PROMIS Assessment Center Scoring Services cannot be utilized (PROMIS, 2015) (NIH). Scores can range from 7 to 35, with higher scores indicating greater fatigue.
Multidimensional Fatigue Symptom Inventory-Short Form
The Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) measures fatigue over the past week (Stein et al., 2004; Stein et al., 1998). The MFSI-SF consists of 30-items with five subscales: general, physical, emotional, and mental fatigue, and vigor. Response options are on a 5-point Likert-type scale and range from 0 = not at all to 4 = extremely. Subscale scores are obtained by summing item responses on each subscale. To obtain a total fatigue score, scores on the four fatigue subscales (general, physical, emotional, mental) are summed and then the vigor subscale score is subtracted from that score. Total scores can range from 24 to 86, with higher scores indicating greater fatigue. Scores on the MFSI-SF have evidence of being reliable and valid (Donovan et al., 2015; Stein et al., 2004). Reported reliabilities for scores on the total scale have ranged from .86 to .96 (Clayton, Dudley, & Musters, 2008; Roepke et al., 2009; Stein et al., 1998). Construct validity has been supported with confirmatory factor analysis of the five subscales (Stein et al., 2004); concurrent validity has been supported with significant correlations between the MFSI-SF and Profile of Mood States (POMS) fatigue scale scores in both African Americans and Caucasians (Bardwell et al., 2006) and with scores on the Fatigue Symptom Inventory (Stein et al., 2004).
Brief Fatigue Inventory
The Brief Fatigue Inventory (BFI) measures fatigue severity and interference with daily function over the past 24 hours (Mendoza et al., 1999). The BFI consists of nine items: three items assess severity and six items assess interference on an 11-point numeric rating scale. A mean of the nine items is obtained for the total fatigue score—with higher scores indicating greater fatigue. Scores on the BFI have demonstrated excellent reliability and validity. Reliabilities have ranged from .88 to .96 (Mendoza et al., 2010; Radbruch et al., 2003). Concurrent validity has been supported with significant correlations between the BFI and “feeling tired” on the Medical Outcomes Study Short Form (SF-36; Radbruch et al., 2003) and with fatigue subscales on the Functional Assessment of Cancer Therapy (FACT) and the POMS (Mendoza et al., 1999). Construct validity has been supported with confirmatory factor analysis (Radbruch et al., 2003).
Perceived Stress Scale
The Perceived Stress Scale (PSS) consists of 10 items and measures the frequency with which perceived stressful life situations are experienced (Cohen, 1988). Response options are on a 4-point scale and range from 0 = never to 4 = very often. A total score is obtained by summing across items; the potential range of scores is 0 to 40. Higher scores indicate greater perceived stress. The PSS has been used extensively, and scores have strong evidence of being reliable and valid. Reliabilities have ranged from .78 to .91 (Cohen, Kamarck, & Mermelstein, 1983; Cohen, 1988; Lee, 2012). Construct validity has been supported by confirmatory factor analysis (Leung, Lam, & Chan, 2010; Ramírez & Hernández, 2007; Reis, Hino, & Añez, 2010).
Center for Epidemiological Studies-Depression
The Center for Epidemiological Studies-Depression (CES-D) is a 20-item measure of the frequency at which individuals experience symptoms of depressive mood over the past week (Radloff, 1977). Response options are on a 4-point scale, ranging from less than 0 = 1 day per week/none to 3 = most of the time. A total score is calculated by summing the scores of all items; scores can range from 0 to 60. Higher scores indicate more depressive symptomatology. The CES-D has been used extensively in research, and scores are reliable and valid (Radloff & Rae, 1979; Radloff, 1977, 1991). Reliability estimates have ranged from .89 to .93 (Choi, Schalet, Cook, & Cella, 2014; Makambi, Williams, Taylor, Rosenberg, & Adams-Campbell, 2009; Radloff, 1977). Construct validity has been supported in several studies (Björgvinsson, Kertz, Bigda-Peyton, McCoy, & Aderka, 2013; Makambi et al., 2009).
Statistical Analysis
Data from the five studies were used to evaluate the psychometrics of the PROMIS F-SF. The healthy controls data were used to examine known groups validity only. The unidimensionality of the PROMIS F-SF was examined with confirmatory factor analysis (CFA) using OpenMx v2.3.1 software run in R. Initially, we examined whether data could be combined across studies for the CFA by testing the null hypothesis that the population covariance matrices were equal. Using Box’s M test (Box, 1949), the null hypothesis was rejected (p < .001), indicating the covariance matrices were not equal and the data should not be combined. Therefore, CFAs were conducted on each sample separately. The χ2 test statistic, root mean squared error of approximation (RMSEA), comparative fit index (CFI), and Tucker-Lewis index (TLI) were used to assess CFA solutions. To be consistent, the remainder of the reliability and validity testing was also conducted on each sample separately. Reliability was estimated using Cronbach’s alpha. Cronbach’s alpha was used because it is an adequate approach to estimating reliability for a single test with a single administration (Sijtsma & van der Ark, 2015). Reliability was also calculated for the PROMIS F-SF in the healthy controls to verify its reliability for its use in the known groups validity assessment. Concurrent validity was examined using Pearson’s correlation among the fatigue measures. Discriminant validity was examined using Pearson’s correlation between PROMIS F-SF and the PSS and CES-D. For known groups validity, an ANOVA was used to examine mean differences between the four study samples and healthy controls on the PROMIS F-SF; Tukey’s HSD multiple comparison adjustment for multiplicity. These analyses were done in SAS 9.4* or JMP 11.1*.
Results
Fatigue Severity
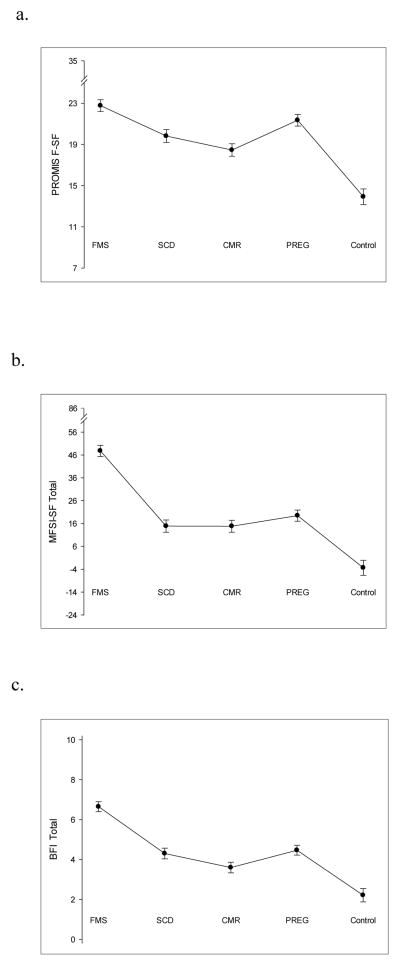
Fatigue was moderate to severe in all of the study populations (FMS, SCD, CMR, and pregnancy), and very low in the healthy controls as would be expected (see Figure 1). Mean fatigue scores in the four study samples ranged from 18.5 to 22.8 on the PROMIS F-SF (potential range 7–35); from 14.8 to 47.8 on the MFSI-SF (potential range 24–86); and from 3.6 to 6.6 on the BFI (potential range 0–10). For the healthy controls, the mean score on the PROMIS F-SF was 13.9, on the MFSI-SF was 3.4; and on the BFI was 2.2. The observed ranges on the PROMIS F-SF were 11–34 (FMS), 8–30 (SCD), 9–30 (CMR), 13–31 (pregnancy), and 8–29 (healthy controls), demonstrating a broad distribution of possible scores in each group.
FIGURE 1.
Mean fatigue scores in study samples using the PROMIS F-SF (Panel a), the MFSI-SF (Panel b), and the BFI (Panel c). PROMIS F-SF = Patient Reported Outcomes Measurement Information System Fatigue-Short Form v1.0 –Fatigue 7a; MFSI-SF= Multidimensional Fatigue Symptom Inventory-Short Form; BFI = Brief Fatigue Inventory; FMS = fibromyalgia syndrome; SCD = sickle cell disease; CMR = cardiometabolic risk; PREG = pregnancy; Control = healthy control. Error bars show the standard error of the mean.
Reliability
Cronbach’s alpha reliability coefficients for the total scale of the PROMIS F-SF ranged from .72 to .86 across the study samples; it was .88 in the healthy controls (see Table 2). The reliability analyses revealed that in each of the four study samples (FMS, SCD, CMR, pregnancy), the reliability increases if Item 7, “How often did you have enough energy to exercise strenuously?” is excluded. However, the increase in the CMR sample was insignificant, increasing from .85 to .86.
TABLE 2.
Reliability Estimates for PROMIS F-SF Total Scores
| Estimate | FMS | SCD | CMR | Pregnancy | HC |
|---|---|---|---|---|---|
| α | .76 | .83 | .85 | .72 | .88 |
| 95% CI | [.67, .84] | [.76, .89] | [.80, .91] | [.63, .82] | [.83, .94] |
| α (if item deleted) | |||||
| Q1: feel tired | .75 | .79 | .83 | .70 | .86 |
| Q2: extreme exhaustion | .69 | .79 | .84 | .66 | .86 |
| Q3: out of energy | .66 | .78 | .81 | .62 | .85 |
| Q4: limits work | .70 | .79 | .83 | .67 | .85 |
| Q5: think clearly | .71 | .79 | .81 | .66 | .85 |
| Q6: bathe/shower | .75 | .81 | .85 | .69 | .87 |
| Q7: strenuous exercise | .81 | .88 | .86 | .79 | .91 |
Note. Reliability estimates using Cronbach’s alpha; 95% CIs were calculated using the method of Lacobucci and Duchachek (2003). CI = confidence interval; CMR = cardiometabolic risk; FMS = fibromyalgia; HC = healthy controls; SCD = sickle cell disease.
Unidimensionality
We evaluated unidimensionality of PROMIS F-SF responses with confirmatory factor analysis (CFA). Fit was assessed using root mean square error of approximation (RMSEA) (< 0.05 ideal; 0.05–0.10 adequate), comparative fit index (CFI) (> 0.95 ideal; > 0.90 adequate), and Tucker-Lewis index (TLI) (> 0.95 ideal; > 0.90 adequate).
Correlation matrices used for the analysis are shown in Table 3. A two-factor model was attempted first, with items 1 through 6 mapped to one factor and item 7 mapped to a second factor, and either did not fit or did not fit well. Thus, it was concluded that a two-factor model was not appropriate. Based on the reliability results and the performance of Item 7 in the CFA, one-factor models were fit to two item sets. The first included all seven items from the PROMIS F-SF and the second excluded Item 7. Factor loadings and fit indexes are shown in Table 4 (see Table, Supplemental Digital Content 1 for complete factor solutions). For the fibromyalgia sample, the unidimensionality model was plausible when Item 7 was not part of the item set. Virtually no differences existed between the fit indices with or without Item 7 for both the SCD and CMR samples. However, even though the fit indices were not significantly different in the CMR sample, the unidimensionality model was plausible when Item 7 was included. In the pregnancy sample, unidimensionality did not seem plausible using either six or seven items; this group is healthy compared to the others, which could contribute to the difference.
TABLE 3.
PROMIS F-SF Items: Correlations, Means, and Standard Deviations in Five Samples
| Fibromyalgia syndrome (FMS) (N = 72 )a | Sickle cell disease (N = 60) | ||||||||||||||
|
|
|
||||||||||||||
| Item | 1. | 2. | 3. | 4. | 5. | 6. | 7. | Item | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
| 1 | 1.00 | .52 | .46 | .28 | .21 | .14 | .01 | 1 | 1.00 | .56 | .59 | .57 | .58 | .53 | .19 |
| 2 | .52 | 1.00 | .68 | .58 | .50 | .35 | .07 | 2 | .56 | 1.00 | .65 | .54 | .56 | .59 | .05 |
| 3 | .46 | .68 | 1.00 | .59 | .54 | .32 | .31 | 3 | .59 | .65 | 1.00 | .72 | .60 | .52 | .06 |
| 4 | .28 | .58 | .59 | 1.00 | .50 | .35 | .15 | 4 | .57 | .54 | .72 | 1.00 | .60 | .39 | .16 |
| 5 | .21 | .50 | .54 | .50 | 1.00 | .45 | −.06 | 5 | .58 | .56 | .60 | .60 | 1.00 | .44 | .07 |
| 6 | .14 | .35 | .32 | .35 | .45 | 1.00 | .02 | 6 | .53 | .59 | .52 | .39 | .44 | 1.00 | −.03 |
| 7 | .01 | .07 | .31 | .15 | −.06 | .02 | 1.00 | 7 | .19 | .05 | .06 | .16 | .07 | −.03 | 1.00 |
| M | 4.31 | 3.47 | 3.72 | 3.68 | 3.04 | 2.53 | 3.99 | M | 3.55 | 2.50 | 2.97 | 2.78 | 2.50 | 2.00 | 3.52 |
| SD | 0.66 | 0.84 | 0.97 | 0.90 | 1.03 | 1.11 | 1.12 | SD | 0.85 | 1.08 | 1.06 | 1.12 | 1.14 | 1.04 | 1.14 |
|
|
|
||||||||||||||
| Cardio-metabolic risk (N = 63)a | Pregnancy (N = 72)a | ||||||||||||||
|
|
|
||||||||||||||
| Item | 1. | 2. | 3. | 4. | 5. | 6. | 7. | Item | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
| 1 | 1.00 | .54 | .54 | .52 | .52 | .30 | .44 | 1 | 1.00 | .36 | .50 | .25 | .07 | .21 | .11 |
| 2 | .54 | 1.00 | .57 | .42 | .52 | .37 | .22 | 2 | .36 | 1.00 | .62 | .44 | .43 | .31 | −.12 |
| 3 | .54 | .57 | 1.00 | .62 | .74 | .52 | .42 | 3 | .50 | .62 | 1.00 | .45 | .51 | .33 | .10 |
| 4 | .52 | .42 | .62 | 1.00 | .69 | .33 | .33 | 4 | .25 | .44 | .45 | 1.00 | .49 | .35 | −.05 |
| 5 | .52 | 0.52 | .74 | .69 | 1.00 | .42 | .39 | 5 | .07 | .43 | .51 | .49 | 1.00 | .47 | .04 |
| 6 | .30 | .37 | .52 | .33 | .42 | 1.00 | .23 | 6 | .21 | .31 | .33 | .35 | .47 | 1.00 | −.05 |
| 7 | .44 | .22 | .42 | .33 | .39 | .23 | 1.00 | 7 | .11 | −.12 | .10 | −.05 | .04 | −.05 | 1.00 |
| M | 3.67 | 2.16 | 2.78 | 2.43 | 2.32 | 1.63 | 3.46 | M | 3.93 | 2.97 | 3.13 | 2.89 | 2.49 | 2.07 | 3.88 |
| SD | 0.86 | 1.03 | 0.97 | 1.15 | 0.93 | 0.89 | 1.09 | SD | 0.91 | 1.06 | 1.13 | 1.19 | 1.05 | 1.04 | 1.17 |
|
|
|||||||||||||||
| Healthy controls (N = 40)a | |||||||||||||||
|
|
|||||||||||||||
| Item | 1. | 2. | 3. | 4. | 5. | 6. | 7. | ||||||||
| 1 | 1.00 | .68 | .70 | .63 | .64 | .47 | .34 | ||||||||
| 2 | .68 | 1.00 | .76 | .65 | .65 | .46 | .32 | ||||||||
| 3 | .70 | .76 | 1.00 | .72 | .72 | .51 | .36 | ||||||||
| 4 | .63 | .65 | .72 | 1.00 | .86 | .59 | .38 | ||||||||
| 5 | .64 | .65 | .72 | .86 | 1.00 | .72 | .30 | ||||||||
| 6 | .47 | .46 | .51 | .59 | .72 | 1.00 | .13 | ||||||||
| 7 | .34 | .32 | .36 | .38 | .30 | .13 | 1.00 | ||||||||
| M | 2.75 | 1.65 | 2.13 | 1.55 | 1.65 | 1.33 | 2.88 | ||||||||
| SD | 1.03 | 1.00 | 0.97 | 0.93 | 0.95 | 0.69 | 1.28 | ||||||||
Note. SD = standard deviation.
All particpants were women.
TABLE 4.
Factor Loadings and Fit for Unidimensional Confirmatory Factor Analysis Models for Six and Seven Items from the PROMIS F-SF
| Seven item model
|
Six item model
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Item | FMS (N = 72) | SCD (N = 60) | CMR (N = 62) | Pregnancy (N = 72) | FMS (N = 72) | SCD (N = 60) | CMR (N = 62) | Pregnancy (N = 72) |
| 1 | .34 | .63 | .57 | .44 | .35 | .63 | .56 | .44 |
| 2 | .69 | .82 | .66 | .77 | .69 | .83 | .67 | .77 |
| 3 | .82 | .90 | .85 | .93 | .80 | .90 | .85 | .93 |
| 4 | .64 | .88 | .86 | .73 | .64 | .88 | .86 | .73 |
| 5 | .65 | .85 | .80 | .66 | .66 | .85 | .80 | .66 |
| 6 | .49 | .66 | .47 | .50 | .49 | .66 | .47 | .50 |
| 7 | .20 | .13 | .52 | .02 | -- | -- | -- | -- |
| Fit | ||||||||
| χ2 | 27.35* | 14.84 | 13.59 | 29.42** | 12.74 | 10.97 | 9.47 | 23.32** |
| df | 14 | 14 | 14 | 14 | 9 | 9 | 9 | 9 |
| CFI | 0.91 | 0.99 | 1.0 | .87 | 0.97 | 0.99 | 1.0 | .88 |
| TLI | 0.87 | 0.99 | 1.0 | .81 | 0.96 | 0.98 | 1.0 | .80 |
| RMSEAa | 0.12 | 0.03 | 0.0 | .12 | 0.08 | 0.06 | .03 | .15 |
| 95%CI | [.03, .19] | [0, .14] | [0, .13] | [.04, .20] | [0, .18] | [0, .18] | [0, .16] | [.06, .24] |
Note. Complete factor solutions (unstandardized factor loadings, uniquenesses, and standard errors) are available in Supplemental Digital Content 2. CFI = comparative fit index; CI = confidence interval; CMR = cardiometabolic risk; df = degrees of freedom; FMS = fibromyalgia syndrome; RMSEA = root mean squared error of approximation; SCD = sickle cell disease; SE = standard error; TLI = Tucker-Lewis Index.
When df > χ2 , RMSEA is set to 0 (Browne & Cudeck, 1993). In simulations with small sample sizes, small degrees of freedom, and a correctly specified model, RMSEA indicates a poor fitting model more often than theoretically expected (Kenny, Kaniskan, & McCoach, 2015).
p < .05,
p < .01.
Validity
Concurrent
Correlations between the PROMIS F-SF and the MFSI-SF ranged from r = .70 to .85, and between the PROMIS F-SF and the BFI ranged from r = .60 to .85. (See Table 5.) Correlations between measures of like constructs are expected to be strong (Jensen, 2003). Thus, as these were all measures of fatigue, strong correlations were expected.
TABLE 5.
Correlations: PROMIS F-SF and Fatigue Measures, and Stress and Depressive Symptoms Measures within Study Samples
| Correlate | FMS | SCD | CMR | Pregnancy | HC |
|---|---|---|---|---|---|
| Fatigue | |||||
| MFSI-SF | .71 | .70 | .85 | .73 | .73 |
| BFI | .60 | .65 | .70 | .65 | .71 |
| Stress/depressive sxs | |||||
| PSS | .51 | .37 | .62 | .44 | .52 |
| CES-D | .55 | .45 | .64 | .47 | .57 |
Note. All correlations were significant at p < .01. Complete correlation matrices for each group are available in Supplemental Digital Content 2. BFI = Brief Fatigue Inventory; CES-D = Center for Epidemiological Studies-Depression; CMR = cardiometabolic risk; FMS = fibromyalgia; MFSI-SF = Multidimensional Fatigue Symptom Inventory-Short Form; PSS = Perceived Stress Scale; SCD = sickle cell disease; sxs = symptoms.
Discriminant
Pearson correlations between the PROMIS F-SF and stress (PSS) and depressive symptoms (CES-D) were calculated to evaluate the discriminant validity of PROMIS F-SF scores. Correlations between measures of constructs that are related, but not alike, are expected to be weak to moderate (Jensen, 2003). Correlations between the PROMIS F-SF and the PSS ranged from r = .37 to .62, and between the PROMIS F-SF and the CES-D ranged from r = .45 to .64 (Table 5). Complete correlation matrices for all variables in each group are available (see Table, Supplemental Digital Content 2).
Known groups
For known groups validity, each of the four study samples had significantly higher levels of fatigue on the PROMIS F-SF than the healthy controls. (See Table 6.)
TABLE 6.
Known Groups Validity of the PROMIS F-SF: Clinical Samples Compared to Healthy Controls
| Sample | N | M | (SE) | pa |
|---|---|---|---|---|
| FMS | 72 | 22.8 | (0.57) | <.0001 |
| SCD | 60 | 19.8 | (5.30) | <.0001 |
| CMR | 63 | 18.5 | (0.61) | <.0001 |
| Pregnancy | 72 | 21.3 | (0.76) | <.0001 |
| Healthy controls | 40 | 13.9 | (0.76) |
Note. FMS = fibromyalgia; SCD = sickle cell disease; CMR = cardiometabolic risk
Single Factor ANOVA with Tukey HSD adjusted p-values; each clinical sample was compared with healthy controls.
Discussion
The PROMIS F-SF demonstrated good reliability and validity in four diverse samples: fibromyalgia, sickle cell disease, cardio-metabolic risk, and pregnancy. The samples were not only diverse in health issues, but also in race, income, and education. For example, two of the study samples consisted primarily of African Americans (SCD and pregnancy), while the majorities in the remaining samples were White. Nonetheless, the evidence of reliability and validity was adequate within each sample.
PROMIS F-SF scores had adequate reliabilities in the FMS and pregnancy samples (Cronbach’s alphas > .70), and good reliabilities in the SCD and CMR samples (Cronbach’s alphas alpha > .80); Nunnally & Bernstein, 1994). However, in all the samples, for one particular item, the reliability coefficient of the total scale would increase if the item were to be deleted. Although the increase in reliability would not be substantial if Item 7 was deleted in the CMR sample (from .85 to .86), the increase would be substantial in the FMS, SCD, and pregnancy samples. This item (“In the past seven days, how often did you have enough energy to exercise strenuously?”) is most probably not suitable in the FMS, SCD, or pregnancy populations for various reasons. For individuals with FMS, exercising strenuously is a challenge because they live with chronic widespread pain, fatigue, and sleep disorders, and frequently report comorbidities that may include, but are not limited to diagnoses of obesity, hypothyroidism, restless legs, or other rheumatic conditions (Shillam, Jones, & Miller, 2011). In SCD, strenuous exercise is generally contraindicated because it puts the individual in an anaerobic metabolic state, which can be unsafe because it can lead to metabolic acidosis, hyperventilation, and eventually, sickling and vaso-occlusion (Myers & Ashley, 1997). During pregnancy, strenuous exercise would not be encouraged. In fact, there is some evidence to suggest strenuous activity during the second trimester may compromise the well-being of the fetus (Salvesen, Hem, & Sundgot-Borgen, 2012). Consideration could be given to either modifying or omitting this item in these populations. Of note, neither the BFI nor MFSI-SF asks about strenuous exercise. The BFI asks questions about how much fatigue has interfered with “general activity” or “walking” in the past 24 hours, but it does not use the word “exercise.” As for the MFSI-SF, while having two subscales that measure “physical” and “vigor,” there are no items within this instrument that specifically refer to exercise activity.
The CFAs demonstrated a unidimensional measure but with mixed results between samples as to the performance of Item 7. These results are not inconsistent with some of the reliability assessments. For example, in the FMR sample, unidimensionality was plausible for the six-item set but not for the complete set of seven items included in the PROMIS F-SF, and likewise, the reliability improves if Item 7 is deleted. Similarly, in the CMR sample, the fit was plausible for both the six- and seven-item sets, and the reliabilities did not change substantially. Less clear was why the fit was plausible for both the six- and seven-item sets for the SCD sample when the factor loading on the CFA for Item 7 was low (.13), and the reliability increased from .83 to .88 if Item 7 was deleted. Either way, as previously mentioned, individuals with SCD are instructed to avoid strenuous exercise.
Concurrent and discriminant validity was supported in each of the samples. Concurrent validity was supported in each of the samples with significant, strong correlations between the PROMIS F-SF and two other validated fatigue measures: the MFSI-SF and BFI. Strong correlations were expected because they would indicate that the instruments are measuring the same or very similar constructs. Discriminant validity was supported in each of the samples with significant, moderate to moderately strong correlations between the PROMIS F-SF and other factors associated with fatigue—namely perceived stress (PSS) and depressive symptoms (CES-D). Weak to moderate correlations were expected because fatigue is associated with both stress and depressive symptoms in these populations, but is not the same construct. Thus, the moderate correlations in both the SCD and pregnancy samples are consistent with expectations. The correlations in the FMS and CMR samples were stronger, though interpretation of correlations is limited due to the nature of correlational data. Both stress and depressive mood is higher in individuals with FMS compared to healthy individuals (McInnis, Matheson, & Anisman, 2014) as is fatigue; thus a stronger correlation may indicate one symptom is causing the other or there is a bidirectional influence. A third, unidentified variable, such as pain, may also be influencing the relationships. Perhaps stress, depressive mood, and fatigue overlap to such an extent in the perceived experience of the individual that obtaining precise or distinct measures of each construct may be challenging.
For known groups validity, groups are expected to differ based on certain characteristics. Specifically, for the PROMIS F-SF, mean fatigue scores were expected to be significantly higher in the study samples compared to the healthy controls. As expected, the fatigue scores on the PROMIS F-SF were significantly higher, thus, indicating worse fatigue, compared to healthy controls, supporting its ability to distinguish between known groups expected to have fatigue vs. those expected to have no/low fatigue. For example, pregnant women had a significantly higher mean fatigue score on the PROMIS F-SF than the healthy control group.
Some characteristics of the PROMIS F-SF make it appealing for use in research and, potentially, in the clinical setting. The tool consists of only seven items; therefore, patient burden to complete the tool is low as opposed to the MFSI-SF, which has 30 items. In addition, the PROMIS F-SF assesses fatigue over the past week as compared to the BFI, which assesses fatigue over the past 24 hours. The time period of the past week as compared to the past 24 hours may be more applicable for examining effects of certain interventions to improve fatigue, such as new medication regimens, physical activity, or nutritional interventions, which would not likely show immediate effects.
Limitations
There are several limitations of this psychometric evaluation of the PROMIS F-SF to note. The study samples included only women, except for the SCD study, which comprised both young women and men. The majority of the samples did not have data on the elderly. Across all studies, the ages ranged from 15 to 67 years. Regarding validity testing, concurrent validity was limited to evaluation with other self-report (subjective) measures of fatigue. While validation with objective measures could strengthen the evidence for validity, there are no objective, validated measures of fatigue as defined here: “the overwhelming, debilitating, and sustained sense of exhaustion” (Lee, Bardwell, Ancoli-Israel, & Dimsdale, 2010). For example, hemoglobin levels may be a surrogate biomarker of fatigue to consider, but if low hemoglobin is long standing, as in SCD, individual adaptation can occur, in which case, hemoglobin level may not be a valid biomarker of the subjective fatigue experience. Further psychometric testing in larger samples from these populations will be needed to support this initial evidence of reliability and validity of the PROMIS F-SF.
Conclusion
These findings contribute to the evidence of reliability and validity of PROMIS F-SF scores in diverse populations; it performed satisfactorily in each of these samples. The PROMIS F-SF is short which makes it easy for researchers, providers, and participants/patients to use. The PROMIS F-SF may have value for evaluating the effectiveness of interventions to reduce fatigue in these populations. However, researchers and clinicians should determine whether Item 7—the item that assesses the frequency with which one is able to exercise strenuously—is applicable to their population. A valuable lesson learned from this cross-studies analysis of the PROMIS F-SF was the advantage of using a common data element of fatigue to be able to compare this PRO across diverse samples.
Supplementary Material
Table that presents unidimensional factor analysis models. .doc
Table that provides complete factor solutions (unstandardized factor loadings, uniquenesses, and standard errors). .doc
Acknowledgments
The authors acknowledge that research was supported by a grant from the National Institute of Health, M. J. Grap, (PI). P30 NR011403 (2009-2014), Center of Excellence for Biobehavioral Approaches to Symptom Management; National Institute of Nursing Research; and CTSA award No. UL1TR000058 from the National Center for Advancing Translational Sciences.
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Suzanne Ameringer, Virginia Commonwealth University, Richmond, VA.
R. K. Elswick, Jr., Virginia Commonwealth, University Richmond, VA.
Victoria Menzies, Virginia Commonwealth University, Richmond, VA.
Jo Lynne Robins, Virginia Commonwealth University, Richmond, VA.
Angela Starkweather, Virginia Commonwealth University, Richmond, VA.
Jeanne Walter, Virginia Commonwealth University, Richmond, VA.
Amanda Elswick Gentry, Virginia Commonwealth University, Richmond, VA.
Nancy Jallo, Virginia Commonwealth University, Richmond, VA.
References
- Ameringer S, Elswick RK, Jr, Smith WR. Fatigue in adolescents and young adults with sickle cell disease: Biological and behavioral correlates and health-related quality of life. Journal of Pediatric Oncology Nursing. 2014;31:6–17. doi: 10.1177/1043454213514632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameringer S, Smith WR. Emerging biobehavioral factors of fatigue in sickle cell disease. Journal of Nursing Scholarship. 2011;43:22–29. doi: 10.1111/j.1547-5069.2010.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell WA, Burke SC, Thomas KS, Carter C, Weingart K, Dimsdale JE. Fatigue varies by social class in African Americans but not Caucasian Americans. International Journal of Behavioral Medicine. 2006;13:252–258. doi: 10.1207/s15327558ijbm1303_9. [DOI] [PubMed] [Google Scholar]
- Björgvinsson T, Kertz SJ, Bigda-Peyton JS, McCoy KL, Aderka IM. Psychometric properties of the CES-D-10 in a psychiatric sample. Assessment. 2013;20:429–436. doi: 10.1177/1073191113481998. [DOI] [PubMed] [Google Scholar]
- Box GEP. A general distribution theory for a class of likelihood criteria. Biometrika. 1949;36:317–346. doi: 10.2307/2332671. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, … Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, Wallace EP. Development of a fatigue scale. Journal of Psychiosomatic Research. 1993;37:147–153. doi: 10.1016/0022-3999(93)90081-P. [DOI] [PubMed] [Google Scholar]
- Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: Linking the BDI-II, CES-D, and PHQ-9 to PROMIS Depression. Psychological Assessment. 2014;26:513–527. doi: 10.1037/a0035768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou C, Schneider S, Junghaenel DU, Broderick JE, Stone AA. Measuring daily fatigue using a brief scale adapted from the Patient-Reported Outcomes Measurement Information System (PROMIS) Quality of Life Research. 2014;23:1245–1253. doi: 10.1007/s11136-013-0553-z. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic- pituitary-adrenal axis and the female reproductive system: Clinical implications. Annals of Internal Medicine. 1998;129:229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Clayton MF, Dudley WN, Musters A. Communication with breast cancer survivors. Health Communication. 2008;23:207–221. doi: 10.1080/10410230701808376. [DOI] [PubMed] [Google Scholar]
- Cohen S. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: The Claremont symposium on applied social psychology. Thousand Oaks, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- Cohen S, Kamarch T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Currow DC, Clark K, Kamal A, Collier A, Agar MR, Lovell MR, … Ritchie C. The population burden of chronic symptoms that substantially predate the diagnosis of a life-limiting illness. Journal of Palliative Medicine. 2015;18:480–485. doi: 10.1089/jpm.2014.0444. [DOI] [PubMed] [Google Scholar]
- Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, … Wang W. Health-related quality of life in children with sickle cell disease: A report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatric Blood & Cancer. 2010;55:485–494. doi: 10.1002/pbc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWalt DA, Rothrock N, Yount S, Stone AA. Evaluation of item candidates: The PROMIS qualitative item reviews. Medical Care. 2007;45(5 Suppl 1):S12–S21. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KA, Stein KD, Lee M, Leach CR, Ilozumba O, Jacobsen PB. Systematic review of the Multidimensional Fatigue Symptom Inventory-Short Form. Supportive Care in Cancer. 2015;23:191–212. doi: 10.1007/s00520-014-2389-7. [DOI] [PubMed] [Google Scholar]
- Doward LC, Gnanasakthy A, Baker MG. Patient reported outcomes: Looking beyond the label claim. Health and Quality of Life Outcomes. 2010;8:89. doi: 10.1186/1477-7525-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén K, Blomqvist K, Saveman BI. Impact of chronic heart failure on elderly persons’ daily life: A validation study. European Journal of Cardiovascular Nursing. 2006;5:137–145. doi: 10.1016/j.ejcnurse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hägglund L, Boman K, Lundman B. The experience of fatigue among elderly women with chronic heart failure. European Journal of Cardiovascular Nursing. 2008;7:290–295. doi: 10.1016/j.ejcnurse.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Hodge FS, Itty TL, Cadogan MP, Martinez F, Pham A. The cultural constructs of cancer-related ftigue among American Indian cancer survivors. Supportive Care in Cancer. 2016;24:1235–1240. doi: 10.1007/s00520-015-2902-7. [DOI] [PubMed] [Google Scholar]
- Jallo N, Ruiz RJ, Elswick RK, French E. Guided imagery for stress and symptom management in pregnant African American women. Evidence-Based Complementary and Alternative Medicine, 2014. 2014 doi: 10.1155/2014/840923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP. Questionnaire validation: A brief guide for readers of the research literature. Clinical Journal of Pain. 2003;19:345–352. doi: 10.1097/00002508-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Junghaenel DU, Christodoulou C, Lai JS, Stone AA. Demographic correlates of fatigue in the US general population: Results from the patient-reported outcomes measurement information system (PROMIS) initiative. Journal of Psychosomatic Research. 2011;71:117–123. doi: 10.1016/j.jpsychores.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltasas G, Vgontzas A, Chrousos G. Fatigue, endocrinopathies, and metabolic disorders. PM&R. 2010;2:393–398. doi: 10.1016/j.pmrj.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kaniskan B, McCoach DB. The Performance of RMSEA in models with small degrees of freedom. Sociological Methods & Research. 2015;44:486–507. doi: 10.1177/0049124114543236+. [DOI] [Google Scholar]
- Lee EH. Review of the psychometric evidence of the Perceived Stress Scale. Asian Nursing Research. 2012;6:121–127. doi: 10.1016/j.anr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Lee IS, Bardwell WA, Ancoli-Israel S, Dimsdale JE. Number of lapses during the psychomotor vigilance task as an objective measure of fatigue. Journal of Clinical Sleep Medicine. 2010;6:163–168. https://www.researchgate.net/profile/Wayne_Bardwell/publication/43297388_Number_of_lapses_during_the_psychomotor_vigilance_task_as_an_objective_measure_of_fatigue/links/00463529a0d49b7257000000.pdf. [PMC free article] [PubMed] [Google Scholar]
- Leung DYP, Lam TH, Chan SSC. Three versions of Perceived Stress Scale: Validation in a sample of Chinese cardiac patients who smoke. BMC Public Health. 2010;10:513. doi: 10.1186/1471-2458-10-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JL, McClish DK, Dahman BA, Bovbjerg VE, de Citero AV, Penberthy LT, … Smith WR. Depression and anxiety in adults with sickle cell disease: The PiSCES Project. Psychosomatic Medicine. 2008;70:192–196. doi: 10.1097/PSY.0b013e31815ff5c5. [DOI] [PubMed] [Google Scholar]
- Lyon D, McCain N, Elswick RK, Sturgill J, Ameringer S, Jallo N, … Grap MJ. Biobehavioral examination of fatigue across populations: Report from a P30 Center of Excellence. Nursing Outlook. 2014;62:322–331. doi: 10.1016/j.outlook.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makambi KH, Williams CD, Taylor TR, Rosenberg L, Adams-Campbell LL. An assessment of the CES-D scale factor structure in black women: The Black Women’s Health Study. Psychiatry Research. 2009;168:163–170. doi: 10.1016/j.psychres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis OA, Matheson K, Anisman H. Living with the unexplained: Coping, distress, and depression among women with chronic fatigue syndrome and/or fibromyalgia compared to an autoimmune disorder. Anxiety, Stress, & Coping. 2014;27:601–618. doi: 10.1080/10615806.2014.888060. [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1186::AID-CNCR24>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Laudico AV, Wang XS, Guo H, Matsuda ML, Yosuico VD, … Cleeland CS. Assessment of fatigue in cancer patients and community dwellers: Validation study of the Filipino version of the Brief Fatigue Inventory. Oncology. 2010;79:112–117. doi: 10.1159/000320607. [DOI] [PubMed] [Google Scholar]
- Mengshoel AM. Life strain–related tiredenss and illness-related fatigue in indvidiuals with ankylosing spondylitis. Artthritis Care & Research. 2010;62:1272–1277. doi: 10.1002/acr.20216. doi:10/1002acr/20216. [DOI] [PubMed] [Google Scholar]
- Menzies V, Lyon DE, Elswick RK, Jr, McCain NL, Gray DP. Effects of guided imagery on biobehavioral factors in women with fibromyalgia. Journal of Behavioral Medicine. 2014;37:70–80. doi: 10.1007/s10865-012-9464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J, Ashley E. Dangerous curves: A perspective on exercise, lactate, and the anaerobic threshold. Chest Journal. 1997;111:787–795. doi: 10.1378/chest.111.3.787. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Medical encyclopedia: Fatigue. 2015 Retrieved from https://www.nlm.nih.gov/medlineplus/ency/article/003088.htm.
- National Institutes of Health. PROMIS domain framework/definitions. 2007 Retrieved from http://www.nihpromis.org/measures/domainframework.
- Nikolaus S, Bode C, Taal E, van de Laar MA. Fatigue and factors related to fatigue in rheumatoid arthritis: A systematic review. Arthritis Care & Research. 2013;65:1128–1146. doi: 10.1002/acr.21949. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Tanaka M, Mizuno K, Ataka S, Mizuma H, Tahara T, … Watanabe Y. Mental and physical fatigue-related biochemical alterations. Nutrition. 2009;25:51–57. doi: 10.1016/j.nut.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric theory. 3. New York, NY: McGraw-Hill; 1994. [Google Scholar]
- Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, anxiety, and anger. Assessment. 2011;18:263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CI. Fatigue during the first trimeter of pregnancy. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 1986;15:375–379. doi: 10.1111/j.1552-6909.1986.tb01409.x. [DOI] [PubMed] [Google Scholar]
- PROMIS Scoring Manuals. PROMIS Fatigue Scoring Manual. 2015 Retrieved from https://www.assessmentcenter.net/documents/PROMIS%20Fatigue%20Scoring%20Manual.pdf.
- Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. European Journal of Cancer. 2006;42:846–863. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Radbruch L, Sabatowski R, Elsner F, Everts J, Mendoza T, Cleeland C. Validation of the German version of the brief fatigue inventory. Journal of Pain and Symptom Management. 2003;25:449–458. doi: 10.1016/s0885-3924(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: Sex differences and similarities. Journal of Abnormal Psychology. 1979;88:174–181. doi: 10.1037/0021-843X.88.2.174. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of Youth and Adolescence. 1991;20:149–166. doi: 10.1007/bf01537606. [DOI] [PubMed] [Google Scholar]
- Ramírez MTG, Hernández RL. Factor structure of the Perceived Stress Scale (PSS) in a sample from Mexico. Spanish Journal of Psychology. 2007;10:199–206. doi: 10.1017/S1138741600006466. [DOI] [PubMed] [Google Scholar]
- Ream E, Richardson A. Fatigue in patients with cancer and chronic obstructive airways disease: A phenomenological enquiry. International Journal of Nursing Studies. 1997;34:44–53. doi: 10.1016/S0020-7489(96)00032-6. [DOI] [PubMed] [Google Scholar]
- Redeker NS, Anderson R, Bakken S, Corwin E, Docherty S, Dorsey SG, … Grady P. Advancing symptom science through use of common data elements. Journal of Nursing Scholarship. 2015;47:379–388. doi: 10.1111/jnu.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis RS, Hino AAF, Añez CRR. Perceived stress scale: Reliability and validity study in Brazil. Journal of Health Psychology. 2010;15:107–114. doi: 10.1177/1359105309346343. [DOI] [PubMed] [Google Scholar]
- Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the U.S. workforce: Prevalence and implications for lost productive work time. Journal of Occupational and Environmental Medicine. 2007;49:1–10. doi: 10.1097/01.jom.0000249782.60321.2a. [DOI] [PubMed] [Google Scholar]
- Robins JL, Elswick RK, Jr, Sturgill J, McCain NL. The effects of Tai Chi on cardiovascular risk in women. American Journal of Health Promotion. 2015 doi: 10.4278/ajhp.140618-QUAN-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke SK, Mausbach BT, von Känel R, Ancoli-Israel S, Harmell AL, Dimsdale JE, … Grant I. Relations between personal mastery, chronic caregiving stress, and multiple dimensions of fatigue. International Journal of Geriatric Psychiatry. 2009;24:1453–1462. doi: 10.1002/gps.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen KA, Hem E, Sundgot-Borgen J. Fetal wellbeing may be compromised during strenuous exercise among pregnant elite athletes. British Journal of Sports Medicine. 2012;46:279–283. doi: 10.1136/bjsm.2010.080259. [DOI] [PubMed] [Google Scholar]
- Shillam CR, Jones KD, Miller L. Fibromyalgia symptoms, physical function, and comorbidity in middle-aged and older adults. Nursing Research. 2011;60:309–317. doi: 10.1097/NNR.0b013e31822bbdfa. [DOI] [PubMed] [Google Scholar]
- Sijtsma K, van der Ark LA. Conceptions of reliability revisited and practical recommendations. Nursing Research. 2015;64:128–136. doi: 10.1097/NNR.0000000000000077. [DOI] [PubMed] [Google Scholar]
- Smets EMA, Garssen B, Bonke BD, De Haes JCJM. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the Multidimensional Fatigue Symptom Inventory-Short Form. Journal of Pain and Symptom Management. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Practice. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- Swain MG. Fatigue in liver disease: Pathophysiology and clinical management. Canadian Journal of Gastroenterology. 2006;20:181–188. doi: 10.1155/2006/624832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely S. Chronic fatigue: Symptom and syndrome. Annals of Internal Medicine. 2001;134:838–843. doi: 10.7326/0003-4819-134-9_Part_2-200105011-00007. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128:128–135. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table that presents unidimensional factor analysis models. .doc
Table that provides complete factor solutions (unstandardized factor loadings, uniquenesses, and standard errors). .doc