Overview of Ion Channel Trafficking in the Heart
A remarkable aspect of cardiac ion channel biology is that individual ion channels have half-lives on the order of hours. For example, Connexin 43 (Cx43) gap junction proteins have a half-life of 1–3 hours 1, 2 while potassium channels, calcium channels, and the sodium-calcium exchanger have half-lives that are reported in the 2–8 hours range 3–6. The short life span of ion channels suggests there needs to be efficiency in their life-cycle and movements which follow the order of: formation, delivery to the correct subdomain on plasma membrane, behavior once in membrane, and internalization back into the cell. In order to maintain this efficiency in ion trafficking, thousands of individual proteins contribute to a functional equilibrium. Mutations in a single protein can disrupt this equilibrium and over time manifest as a generalized cardiomyopathy.
Cardiomyocytes use common intracellular organelles and machinery to produce and shuttle ion channel proteins to their specific organelles and functional subdomains at the cell membrane. After gene transcription in the nuclei, proteins are translated and subjected to post-translational modification in the ER and then further modified in the Golgi apparatus. For ion channels, sorting and delivery to their subcellular destination begins in the Golgi apparatus. The Golgi complex is usually found adjacent to the lateral side of each nucleus in mammalian ventricular cardiomyocytes. Co-localized with each Golgi is the centrosome at which microtubules are nucleated and extend throughout the cell 7. Sorting of proteins mainly takes place at the trans Golgi network (TGN) 8. Cargo proteins are sorted into post-Golgi carriers, which are docked onto molecular motors and delivered to the cell periphery along microtubules 9. These extending microtubules form an intricate and dynamic outgoing network capable of shuttling ion channel containing vesicles to their destinations. In the context of trafficking, one can consider the Golgi to be the “loading dock” and microtubules the “highways” along which packets of channels are delivered to the plasma membrane.
The mechanisms by which microtubules exert their specificity in interacting with the membrane subdomains are now being elucidated. Our report in 2007 10 and subsequent studies 11–13 have led to the Targeted Delivery model of ion channel delivery. The Targeted Delivery model has since been supported by multiple other reports 14–17.
Targeted Delivery
Targeted Delivery is the understanding that channels, once formed and exiting the Golgi, can be rapidly directed across the cytoplasm to their respective specific membrane subdomains. The highways for transport are microtubules whose negative ends originate at Golgi oriented microtubule-organizing centers and whose positive ends are growing outward and can be captured at the plasma membrane by membrane anchor proteins and complexes. Specificity of delivery is a combination of the individual channel, the plus-end-tracking proteins at the positive ends of microtubules which guide microtubule growth and capture, and the membrane bound anchor complex which captures the microtubule thus completing the highway for channel delivery. Actin cytoskeleton serves as en route rest-stop stations to redirect microtubules 18, providing additional sorting sites. This Targeted Delivery paradigm of ion channel forward trafficking has been generalizable to several cardiac ion channels and explored in terms of various cytoskeletal elements and anchor proteins 19.
In this review, Targeted Delivery is explored in detail for two different types of channels: Cx43 hemichannel trafficking to the intercalated discs and CaV1.2 channel trafficking to T-tubules.
Cx43 Trafficking in the Heart
Connexins are ubiquitous transmembrane proteins which are encoded by over 20 different genes in human with Cx43 being the most commonly expressed in all organ systems, particularly in the heart 20, 21. An extensively studied and well appreciated function of connexins is their ability to form gap junctions. To form a gap junction, six connexin monomers from one cell oligemarize to form a transmembrane channel referred to as a connexon or hemichannel. The connexons from one cell then dock and couple with apposing connexons on neighboring cells and coalesce into dense gap junction plaques 22–24. Gap junctions are specialized channels that aid in the intercellular exchange of small metabolites, secondary messengers and ions carrying electrical signals between neighboring cells, therefore allowing cells to cooperate both electrically and metabolically 22, 23, 25. Data also exist that Cx43 hemichannels can occur as free, non-junctional channels in the plasma membrane. These hemichannels are normally closed but may open in response to various triggers including cell depolarization, decreased extracellular Ca2+ ion concentration, increased intracellular Ca2+ concentration and alterations in the phosphorylation or redox status 26. Generally, the opening of plasma membrane Cx43 hemichannels is considered to be associated with pathological rather than physiological entities, contributing to cell swelling and cell death. In the heart and brain cells, excessive hemichannel opening allows the entry of Na+ and Ca2+ and the escape of K+, adenosine triphosphate (ATP) and other small metabolites, leading to osmotic shifts, energy depletion, Ca2+ overload and cell death promotion 26. Therefore, blockage of the Cx43 hemichannels using pharmacological inhibitors can possibly have protective effects against cardiac insult such as in the case of ischemia/reperfusion injury 26, 27.
In the heart, localization of Cx43 gap junctions at the intercalated discs is crucial to provide the intercellular coupling necessary for rapid action potential propagation through the myocardium and synchronized cardiac contraction 28–30. Altered Cx43 localization and losses in cell-cell gap junction coupling occur during cardiac disease 31 and contribute to abnormal impulse propagation and arrhythmogenic substrates leading to sudden cardiac death 32–38. A large number of studies in recent years have demonstrated a decrease in expression and/or lateralization and heterogeneous distribution of Cx43 in the myocardium of patients with hypertrophic cardiomyopathies (HCM) 39–41, dilated cardiomyopathies (DCM) 42, 43, ischemic cardiomyopathies 40–42 as well as clinical congestive heart failure 44. The presence of Cx43 at the lateral cell membrane has been shown in HCM patients and in different animal models to be associated with slowing of conduction velocity (CV) in early, compensated stage of hypertrophy that was followed by a reduction of Cx43 and development of arrhythmias 45. In a canine pacing-tachycardia model of DCM, dephosphorylation of Cx43 and its appearance at the lateral cell border were associated with decreased epicardial and endocardial CVs in both ventricles and with prolongation of the QRS interval 46. In another canine pacing-induced heart failure study, increased dephosphorylation of Cx43 was associated with later stages of heart failure while its presence at the lateral cell membrane occurred in an even later stage when the CV is significantly reduced 47. Localization of Cx43 at the lateral cell membrane regions has also been observed in the border zone of myocardial infarcts and has been correlated with development of reentrant circuits leading to ventricular tachycardia 48. It is also not clear whether the presence of lateral localized Cx43 correlated with low resistance cell-cell coupling. Electron microscopy and co-labeling studies have suggested that some of the lateral Cx43 may not be in plasma membrane but in nearby autophagosomes 49, suggesting a role of lateral Cx43 other than cell-cell communication.
In summary, altered Cx43 localization occurs in diseased myocardium, with consequences that include lethal arrhythmias. The mislocalization of Cx43 during disease reflects impaired forward trafficking to the intercalated discs. Exploring the mechanisms of forward trafficking and alterations in disease could pave the way for therapeutic rescue of cell-cell coupling in diseased myocardium.
Cx43 Forward Trafficking in Normal Heart Physiology
Cx43 oligomerizes into hemichannels at the TGN which is relatively late for such an event to occur, as other connexins oligomerize in the ER 22. Oligomerization of Cx43 at the TGN may represent a means of controlling heteromeric hemichannel formation with other connexin isoforms 30. Upon exiting the TGN, vesicles containing Cx43 hemichannels must navigate the complex cardiomyocyte intracellular environment, a feat they achieve by trafficking along dynamic microtubules.
Trafficking of Cx43 hemichannels to the intercalated discs involves a major plus-end binding protein, EB1, which is known to be necessary for targeted delivery of Cx43 hemichannels to adherens junction complexes 10 (Figure 1). Through interaction with another plus-end protein, p150GLUED, the EB1-tipped microtubule complexes specifically with β-catenin molecules at the adherens junctions of intercalated discs. Vesicular cargo is unloaded and subsequently inserted into the plasma membrane at nearby gap junctions. Other reports propose a less specific paradigm of connexin delivery, whereby connexons are inserted indiscriminately into the lateral membrane of the cell and freely diffuse to gap junction structures 22. The free diffusion model may serve as an alternative pathway for Cx43 delivery. However, the inefficiency of lateral diffusion to a few specific subdomains, the short half-life of connexins (1–3 hours in the myocardium) 1, 2, and the complex interactions between a single cell with multiple neighboring cardiomyocytes, all suggest directed targeting can be a more effective form of connexon localization to the intercalated discs. Free diffusion of hemichannels likely does occur, but over a restricted region of membrane such as post delivery of channels to adherens junction and into nearby plaque.
Figure 1.
Cx43 trafficking in healthy and failing hearts. Cx43 proteins are synthesized by ribosomes and they oligomerize into hemichannels at the trans Golgi network (TGN). Multiple isoforms of Cx43 are produced as a result of alternative translation. The Cx43 hemichannels are then sorted into vesicular carriers and docked onto microtubules at the TGN, and subsequently delivered to the intercalated discs with actin “way stations” along the route. (Top panel) In healthy hearts, the interaction between EB1, a microtubule plus-end binding protein, and the adherens junction (N-cadherin) complex ensures targeted delivery of Cx43 hemichannels to the intercalated discs. Cx43 gap junctions on the plasma membrane undergo internalization for degradation. (Bottom panel) In failing cardiomyocytes, expression of Cx43 gap junctions on the cell surface is altered. As highlighted in the light green boxed areas, possible mechanisms underlying this change include (1) dissociation of microtubule plus-end binding proteins from microtubules; and (2) increased internalization of Cx43 gap junctions. Under oxidative stress EB1 dissociates from the tip of microtubules, impairing the attachment of microtubules to the adherens junction and the delivery of Cx43 to the intercalated discs. During acute cardiac ischemia 14-3-3 mediated internalization of Cx43 is increased, diminishing the amount of Cx43 channels on the plasma membrane.
In Targeted Delivery, specificity of delivery occurs near the membrane, in which a key aspect is the differential membrane bound anchor proteins at distinct membrane subregions. Membrane anchors are critical in capturing with specificity a subgroup of microtubules allowing channel delivery directly to regions of membrane that happen to contain the particular anchor. For Cx43 delivery to the intercalated disc, EB1-tipped microtubules bind to N-Cadherin associated β-catenin and also p150GLUED 10. Desmoplakin may also be involved in capturing the EB1-tiped microtubule for Cx43 delivery 50, although the transmembrane domain still appears to be N-Cadherin rather than desmosomal desmoglein 51. Non-sarcomeric actin has also been shown to be necessary for Cx43 forward delivery 18, 52. However, it remains to be determined how actin interacts with channels and the microtubule apparatus. At any given point in time, the majority of intracellular Cx43 channels are not moving rapidly on microtubules, but rather are stationary and associated with non-sarcomeric actin 18, 52.
Other than microtubules, there is increasing appreciation for the involvement of non-sarcomeric actin cytoskeleton in Targeted Delivery of Cx43. The fundamental question remains with regard to why actin is involved in Cx43 trafficking. If vesicles containing Cx43 can depart the Golgi and ride a microtubule highway straight to its proper subdomain, is there a need for actin filaments which appear to slow down vesicle transport? Actin can have at least two important roles in forward delivery of Cx43. The first is to help contribute specificity to delivery. Vesicles transported along microtubules on kinesin motors move rapidly, at a rate of about 1 micron per second 10. Thus delivery to most locations at a cell membrane can occur within a minute. Association with important accessory proteins and post-translational modification of channels, both of which can affect delivery destination, probably also happen en route between the Golgi and membrane. Hopping off the microtubule highway on an actin “way station”, which is analogous to a highway rest stop with convenience stores, could allow Cx43 and the vesicle containing it to pick up accessory proteins and allow for needed post-translational modification. Such rest stops would occur at Z-disc, subcortical locations, or other important cytoskeleton intersections in the cytoplasm. These actin rest stops could also allow the Cx43 containing vesicles to use multiple microtubule highways in their delivery path. The Golgi exiting microtubule could be destined for an actin rest stop, allowing for a different membrane domain specific microtubule to finish the delivery. The second potential role for actin in microtubule based forward delivery pertains to the microtubules themselves. In non-myocyte systems, actin can help stabilize and guide microtubules 53, 54. Actin could be the blueprint along and across which microtubule highways are patterned. In this respect, actin involvement could be an upstream to microtubules in determining location of Cx43 delivery.
Cx43 Forward Trafficking in Heart Pathophysiology
We have found that when isolated cardiomyocytes are subjected to oxidative stress, Cx43 gap junction delivery to intercalated discs is impaired due to disruption of the forward trafficking machinery 12. Specifically, oxidative stress causes the microtubule plus-end protein EB1 to disassociate from the tips of microtubules, impairing microtubule attachment to adherens junction structures and subsequent delivery of Cx43 hemichannels to plasma membrane 12 (Figure 1). Manipulation of EB1 as well as the upstream regulators of EB1 localization at microtubules could potentially preserve or enhance gap junction coupling during stress. As many ion channels rely on microtubules for their transport, it is likely that such disruption of microtubule trafficking machinery inhibits delivery of many essential channels to the sarcolemma.
Such studies provide evidence that forward trafficking of Cx43 is impaired in acquired heart failure. At present we do not know how oxidative stress causes EB1 displacement and disassembly of the forward trafficking apparatus. We have preliminary investigations on the role of actin in maintaining EB1 based microtubule integrity, and the response of these cytoskeletal fibers to stress conditions. This remains an active area of investigation.
Regulation of Cx43 Forward Trafficking by its Alternatively Translated Isoforms
Ion channel function and trafficking are usually dependent and regulated by auxiliary protein subunits 55. With regard to accessory proteins, Cx43 hemichannels are notable for, despite extensive examination, not being associated with their own unique β-subunits that assist in their trafficking. It turns out that Cx43 mRNA, through alternative translation, encodes its own trafficking subunits 56 which are N-terminal truncations of the full protein. These isoforms have potential roles in non-canonical functions of Cx43 as well.
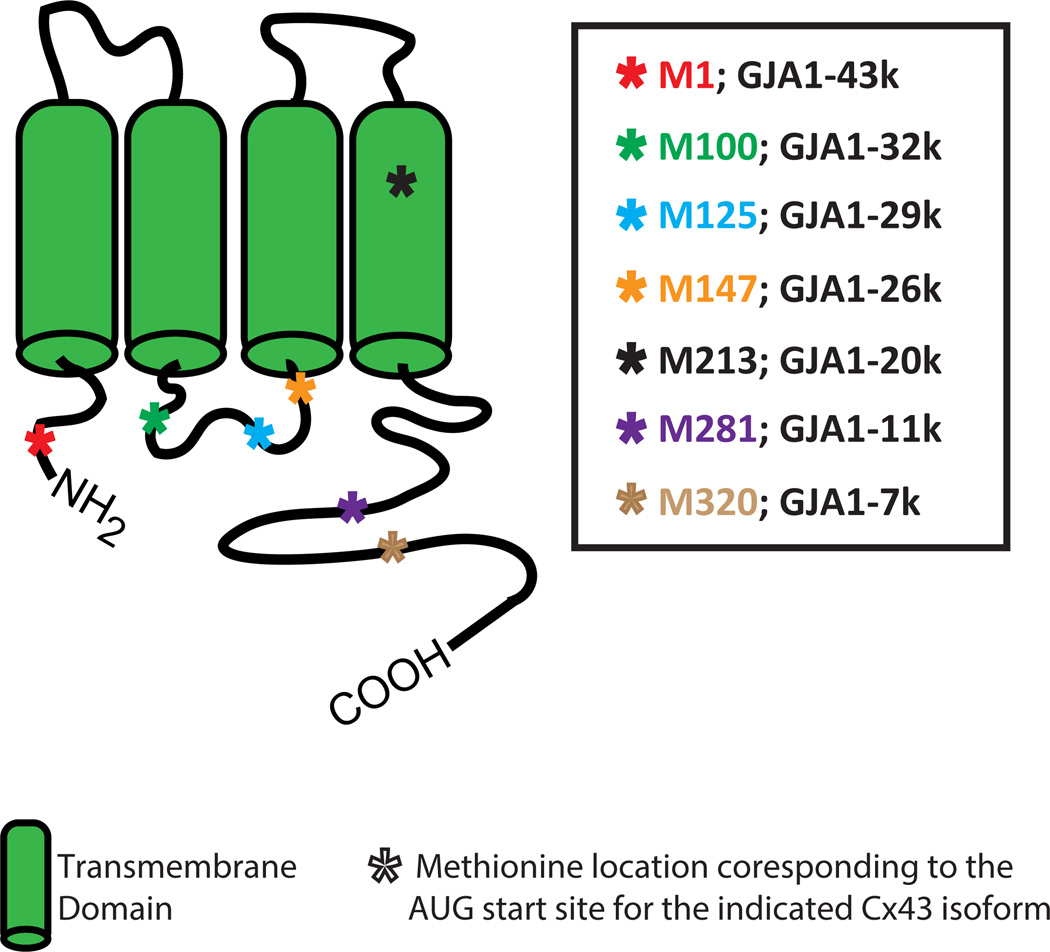
In order to understand alternative translation, it should be recognized that traditional translation of mRNA begins with the first coding triplet, which is always an AUG (Methionine). Most transcribed genes (mRNA strands) have other AUG sites downstream of the first one. The Cx43 protein has six Methionines, corresponding to the different AUG triplet translation start sites, beyond the first one (Figure 2). Alternative translation occurs when ribosomal translation initiates not at the first triplet, but at a downstream triplet. By initiating translation at downstream sites, alternative translation creates truncated proteins that lack the respective non-translated upstream (N-terminal) portions of the proteins.
Figure 2.
Alternatively translated isoforms of Cx43. Schematic showing the protein structure of full length Cx43 (GJA1–43k) with the Methionine locations corresponding to the respective AUG start sites of the various Cx43 isoforms marked by asterisks and color coded. Cx43 alternative translation creates N-terminal truncated proteins lacking the respective non-translated upstream (N-terminal) portions of the Cx43 protein. The six different Cx43 isoforms resulting from alternative translation are GJA1–32k, GJA1–29k, GJA1–26k, GJA1–20k, GJA1–11k and GJA1–7k.
Cx43 is a product of the GJA1 gene and we have recently reported that the coding region of GJA1 mRNA occurs as a polycistronic molecule with different N-terminal truncated isoforms of Cx43 protein arising from internal translation of the same mRNA molecule 56. We have found that the GJA1 mRNA produces the expected full-length 43 kDa protein as well as proteins that are approximately 32 kDa, 29 kDa, 26 kDa, 20 kDa, 11 kDa and 7 kDa in size (Figure 2) with the 20 kDa isoform (GJA1–20k) being the predominate isoform in human heart tissue and several other cell lines 56. This is the first evidence that alternative translation is possible for human ion channels and in human heart. These results have since been supported by a separate report showing that the GJA1–20k isoform is expressed in many cell lines that express high levels of full length Cx43 57. In addition, it has also been reported that this 20 kDa isoform is induced by hypoxic stimuli in the mouse brain and is the result of internal translation from an IRES element 58.
We have found that at least one of the alternatively translated isoforms, GJA1–20k, is important for increasing trafficking of Cx43 to the plasma membrane 56. Loss of all four of the following Cx43 isoforms; GJA1–32k, GJA1–29k, GJA1–26k and GJA1–20k, severely abrogated the formation of Cx43 gap junctions at the membrane 56. Interestingly, reintroduction of the GJA1–20k isoform was sufficient to rescue the transport of Cx43 to the cell surface. The majority of ectopically expressed GJA1–20K remained localized primarily at cytoplasmic reticular structures which were confirmed to be the ER/Golgi network. The interaction between full length GJA1–43k and GJA1–20k in the ER was confirmed using co-immunoprecipitation assays where Brefeldin A (an inhibitor of protein transport from the ER to the Golgi) resulted in increased interactions between these two peptides. This data suggests a role of GJA1–20k isoform early in the Cx43 vesicular transport pathway and that this isoform may function as a cytoplasmic chaperone auxiliary protein for trafficking of de novo GJA1–43k molecules through the ER/Golgi complex to the membrane 56. We also found that the mTOR signaling pathway increases expression of the GJA1–20K isoform and Cx43 trafficking 56. It remains to be determined how GJA1–20k contributes specificity to trafficking delivery. We expect that GJA1–20k is involved in cytoskeleton organization.
Cx43 Internalization in Healthy Cardiomyocytes
Endocytosis of Cx43 can occur either through internalization of uncoupled hemichannels or entire gap junctions, which requires engulfment of gap junctions from the opposing neighboring cell plasma membrane as well. The internalized double-membrane intracellular structures are known as nonfunctional annular gap junctions. Both the lysosome and the proteasome have been implicated in degradation of Cx43 59 and interestingly, autophagy is now known to be involved in degradation of annular gap junctions in failing hearts 49. Studies have shown that recycling of gap junctions occur during cell cycle progression in cell lines 60, but whether gap junctions are recycled in cardiomyocytes remains a controversial issue. It is exciting to consider the possibility that there exists a delicate balance and competition between the various posttranslational modifications of Cx43 including phosphorylation 2, 61, 62 and ubiquitination 63–66 that may act as checkpoints within the same connexin molecule, or connexon hemichannel. This would then allow specific series of events to permit internalization and degradation of the hemichannel, or annular gap junction.
In the case of Cx43, phosphorylation is most well studied, and the importance of phosphorylation has been highlighted by recent findings that casein kinase-dependent phosphorylation alters gap junction remodeling and decreases arrhythmic susceptibility 61. Many residues on the C-terminus of Cx43, specifically 22 serines, 5 tyrosines, and 4 threonines, are potentially subjected to phosphorylation. To make matters even more complex, Cx43 exists as a hexamer on the plasma membrane, and it is currently not known how phosphorylation differs between individual connexins of the same connexon. It is likely that a cascade of phosphorylation events occurs preceding ubiquitination of Cx43, which then leads to channel internalization and degradation 62, 67. For example, it has been shown that Cx43 phosphorylation by EGF (epidermal growth factor) and TPA (12-O-tetradecanoylphorbol-13-acetate) promotes interaction between Cx43 and the E3 ubiquitin ligase Nedd4 (neuronal precursor cell-expressed developmentally down-regulated 4) leading to the subsequent ubiquitination of Cx43 66, 68.
There is increasing evidence that Cx43 ubiquitination plays an important role in regulating gap junction internalization and degradation and several E3 ubiquitin ligases have been shown to regulate Cx43 internalization from the plasma membrane including TRIM21 69, Smurf2 70, and Nedd4 66, 68. Since these studies were done in cell culture systems, it still remains largely unclear how Cx43 ubiquitination is regulated in the cardiomyocytes. Nedd4 was the first described E3 ubiquitin ligase to be implicated in regulating Cx43 ubiquitination, internalization and autophagic degradation through a mechanism involving recruitment of the endocytotic adaptor Eps15 (epidermal growth factor receptor substrate 15) and the autophagic receptor p62 in cell lines 66, 71. In neonatal rat cardiomyocytes, Nedd4 has also been reported to interact with Cx43 possibly regulating its ubiquitination and internalization in response to norepinephrine 72. Further evidence implicating the role of Nedd4 in regulating Cx43 in myocytes was recently reported showing that only under basal conditions, silencing of Nedd4 in the HL-1 mouse atrial cell line led to increased Cx43 protein with a decrease in its ubiquitination levels 64. Wwp1, which is a close family member to Nedd4, has been recently shown to interact with, ubiquitinate and degrade Cx43 in cell lines 65. In addition, cardiomyocyte specific overexpression of Wwp1 in an inducible transgenic mouse model led to a significant reduction in Cx43 protein levels in the heart thus highlighting the importance of Wwp1 in regulating Cx43 turn over in the myocardium 65.
Cx43 Internalization in Diseased Cardiomyocytes
Our experience with Cx43 protein is that post-translational modification preferentially affects ion channel internalization. Pathological gap junction remodeling is strongly associated with altered phosphorylation of Cx43 30, 73, 74. Rather than individual independent phosphorylation events of singular residues at the C-terminus, it is likely that internalization results from a sophisticated cascade of posttranslational modifications. The Cx43 C-terminus contains a phosphorylation-dependent 14-3-3 binding motif at Serine 373 (within 10 amino acids of the end of the protein). 14-3-3 proteins are known to regulate protein transport and have been implicated in facilitating de novo Cx43 transport from ER to Golgi apparatus 75, 76. Phosphorylation of Ser373 and subsequent 14-3-3 binding provide a gateway to a signaling cascade of downstream phosphorylation of Ser368, leading to gap junction ubiquitination, internalization and degradation during acute cardiac ischemia 2.
The C-terminus of Cx43 is the main protein-protein interaction domain responsible for Cx43 binding to its partners within the cell 77. In close proximity to the Cx43 14-3-3 binding motif is a PDZ domain at the distal end of the C-terminus. It is through this PDZ domain that Cx43 interacts with ZO-1 78, and this interaction has been demonstrated to regulate Cx43 gap junction plaque size and assembly 79, 80. Disruption of Cx43/ZO-1 complexing has been reported to increase gap junction plaque size in cultured cells 81, 82. Phosphorylation of Cx43 Serine373 can disrupt interaction with ZO-1 83, and indeed it would be sterically unlikely for both 14-3-3 and ZO-1 to bind the same Cx43 protomer simultaneously. However, increased Cx43/ZO-1 interaction has also been associated with gap junction remodeling, highlighting the complex nature of these dynamic posttranslational and protein complexing events 84, 85.
Acute cardiac ischemic injury in isolated rat hearts has been shown to cause increased ubiquitination of Cx43 at the intercalated discs accompanied by increased interaction between Cx43 and Nedd4 63. However, silencing of Nedd4 in HL-1 mouse atrial cells subjected to ischemic conditions did not have any significant effect on Cx43 ubiquitination nor degradation and only under basal conditions did the knockdown of Nedd4 prevent ubiquitination and degradation of Cx43 64. This suggests that other E3 ubiquitin ligases besides Nedd4 may regulate Cx43 ubiquitination and degradation in cardiac injury. Indeed it has been recently reported that cardiomyocyte specific overexpression of Wwp1 in an inducible transgenic mouse model caused a significant reduction in Cx43 protein levels in the heart leading to the development of lethal left ventricular arrhythmias 65.
CaV1.2 Channel Trafficking in the Heart
The calcium handling proteins that are important in cardiac excitation-contraction coupling, in particular the voltage-gated LTCCs, are mostly enriched in T-tubules. Enrichment of the LTCCs (with pore forming subunit CaV1.2) at the T-tubules helps bring these channels in close proximity (~15 nm) to intracellular sarcoplasmic reticulum (SR)-based calcium sensing and releasing channel ryanodine receptors (RyR) (Figure 3). This is important for efficient calcium-induced-calcium-release (CICR) process during each heartbeat. Upon membrane depolarization, initial calcium influx occurs through CaV1.2 channels and the close association between CaV1.2 and RyR permits efficient CICR and subsequent sarcomeric contraction 86. In addition, a recent report showed that the membrane scaffolding protein bridging integrator 1 (BIN1) which organizes T-tubule microfolds 87 is important to bridge the dyadic cleft spanning between CaV1.2 channels at the t-tubules and phosphorylated RyR at the SR membrane, thus maintaining the LTCC-RYR couplons at the dyads and regulating calcium transient development 88.
Figure 3.
CaV1.2 trafficking in healthy and failing hearts. CaV1.2, a voltage-gated L-type calcium channel protein, is synthesized by ribosomes, translocated to the rough endoplasmic reticulum, transported through the Golgi apparatus and then to the TGN. CaV1.2 proteins are then sorted into vesicular carriers, docked onto microtubules and subsequently delivered to their subcellular destinations (T-Tubules). The association of microtubules with bridging integrator 1 (BIN1), a membrane scaffolding protein, warrants the delivery of CaV1.2 to the T-tubules. (Top panel) In the healthy heart, BIN1 is responsible for creating T-tubule folds thus affecting extracellular ion diffusion and controlling the driving force of CaV1.2 channel activity. BIN1-folded subdomains within T-tubules also limit LTCC lateral diffusion once the channels are inserted into T-tubule membrane, in order to maintain functional LTCC-Ryanodine receptor (RyR) dyads and healthy excitation-contraction coupling. (Bottom panel) In the failing heart, BIN1 expression is reduced and the dense membrane folds in T-tubules are lost due to low expression of BIN1. This leads to defective CaV1.2 delivery to the T-tubules, decreased LTCC-RyR dyads and altered excitation-contraction coupling.
T-tubules, which are continuously extended from surface sarcolemma, are lipid bilayers embedded with transmembrane or lipid-associated proteins 89. Cardiac T-tubules occur at regular intervals along the lateral sides of the cell, closely coincident with the sarcomeric Z-discs. The physiological function of cardiac T-tubules depends on the proteins that are localized at and within the vicinity of the T-tubules, including transmembrane ion channels and ion handling proteins. Specific membrane scaffolding proteins and cytoskeletal structural proteins are required to localize to T-tubules for the organization and regulation of T-tubule network and structure. By differentially compartmentalizing proteins involved in ion handling and signaling, T-tubules serve as a signaling hub-like organelle to regulate myocyte function. The expression of transmembrane ion channels, ion transporters, and pumps have been well characterized in cardiac T-tubules 90.
It has also been reported that a subset of CaV1.2 channels is localized within caveolae to aid in calcium signaling 91. Caveolae are distinct membrane microdomains “little caves” that exist in both T-tubules and the lateral sarcolemma of ventricular cardiomyocytes. A caveolae is a flask-shaped structure enriched with cholesterol and sphingolipids formed by the cholesterol-binding scaffolding protein Caveolin-3 (Cav-3). Biochemical fractionation and electron microscopy studies have identified a subpopulation of many ion channels at caveolae, and loss of caveolae is associated with arrhythmogenesis 91. The precise role of caveolae on ion channel regulation and its significance still awaits further investigation. In addition, the mechanisms affecting CaV1.2 enrichment at caveolae are unknown, but close interactions between caveolae and the cytoskeleton present an appealing possibility of targeted ion channel delivery to these sarcolemmal microdomains 92.
CaV1.2 Forward Trafficking in Normal Heart Physiology
Enrichment of CaV1.2 channels in the T-tubules is essential for the efficient contractile function of the myocardium. We found that trafficking of CaV1.2 vesicles from the TGN to T-tubules also occurs in a microtubule-dependent manner 11 (Figure 3). Moreover, consistent with the Targeted Delivery model just as with Cx43 connexons, dynamic microtubules preferentially interact with a specific membrane anchor protein, BIN1, in order to insure targeted delivery of CaV1.2 to the T-Tubules 11 (Figure 3). BIN1 contains a membrane curvature BAR-domain (which confers the ability to form membrane curvature), a coiled-coil domain, and an SH3 protein-protein interaction domain. Perhaps most compelling for BIN1 utilizing the cytoskeleton is the finding that deletion of the coiled-coil and SH3 domains does not affect membrane invagination, but abrogates CaV1.2 colocalization with these structures. Therefore, it is through interaction specifically with the BIN1 membrane scaffolding protein, and not T-tubule structures, that targeting of CaV1.2 delivery is achieved 11. The specificity of Targeted Delivery is also contributed by the +TIP proteins at the plus ends of growing microtubules. For example, EB1 works in concert with p150GLUED to target Cx43 channels to adherens junctions at intercalated discs 10 while the other +TIP protein ClIP170 has been reported to interact with BIN1 93, possibly facilitating BIN1 directed delivery of LTCCs to T-tubules.
A subpopulation of CaV1.2 channels, on the other hand, can be delivered to caveolae through interaction between subunits of LTCC channel complex and the caveolae structural protein caveolin 3 94. In addition, the fibroblast growth factor homologous factors have been shown to be potent regulators of CaV1.2 localization to the sarcolemmal membrane 95 by interacting with C-terminal domains of ion channels.
CaV1.2 Forward Trafficking in Heart Pathophysiology
In failing heart, forward trafficking of CaV1.2 channels to T-tubules is also impaired 13 (Figure 3). Biochemical assessment of CaV1.2 channel content in failing heart indicates no difference in total channel content compared to healthy muscle, yet channel localization to T-tubules is impaired 13. A difference between impaired forward delivery of Cx43 channels and CaV1.2 channels in failing hearts exists with their respective anchor proteins. Even in diseased heart muscle, the adherens junction structures for Cx43 delivery to intercalated discs remain intact 12, whereas transcription of BIN1 protein, needed to anchor microtubules for CaV1.2 delivery to T-tubules, is reduced by half 13. In animal models, successful treatment of heart failure and recovery of function correlates with recovery of muscle BIN1 levels 96, 97.
Accessory Proteins Involved in CaV1.2 Targeted Delivery
As mentioned before, Ion channel function and trafficking are usually dependent and regulated by auxiliary protein subunits 55 including their own unique β-subunits that assist in their trafficking. In the case of LTCC, accessory β-subunits exist with the expression of four different isoforms (β1-β4) varying across species in the myocardium. In the mouse hearts, only β2 subunit (with five splice variants β2a-2e) 98 has been detected, whereas all of the four isoforms have been detected in canine myocardium 99. By masking the ER retention signal at the intracellular I–II loop of CaV1.2 protein, β-subunits are critical in facilitating the ER exiting of CaV1.2 channel 100. Due to the essential role of the LTCC β subunits in regulating trafficking and surface expression of the calcium ion channels, different β subunit mutations have been implicated in human disease 101, 102. More specifically, two point mutations in the β2b subunit, which is the most abundant LTCC β subunit isoform in the heart 103, have been implicated in disease. A S481L mutation, which occurs in the C-terminus of β2b, contributes to a sudden death syndrome characterized by a short QT interval and an elevated ST-segment 104. A T11I mutation occurs in the β2b N-terminus and causes accelerated inactivation of cardiac L-type channels and is linked to Brugada syndrome 105.
The role of β-subunit in targeted delivery of LTCCs remains unclear. We speculate that the β-subunit may be the one directly binding to membrane anchor proteins to facilitate delivery of LTCCs to membrane subdomains. We also speculate that the specificity of LTCCs delivery can be determined by binding of BIN1 or caveolin-3 like membrane anchor proteins with different β -subunit isoforms and splice variants.
T-tubules and CaV1.2 Regulation in Normal Heart Physiology
A recent development in cardiac membrane biology is the finding that T-tubule invaginations are not simply straight and planar, but instead contain complex folds which are tight and narrow enough to limit the free flow of extracellular ions 87. We found that BIN1 is responsible for these minifolds within the T-tubules, thus affecting extracellular ion diffusion and controlling the driving force of CaV1.2 channel activity 87. BIN1-folded subdomains within T-tubules may also limit LTCC lateral diffusion once the channels are inserted into T-tubule membrane, in order to maintain functional LTCC-RyR dyads. Therefore, BIN1-like membrane scaffold proteins may help localize particular pools of ion channel proteins to membrane subdomains for compartmentalized regulation of ion channel activity and function.
T-tubules and CaV1.2 Regulation in Heart Pathophysiology
In failing hearts, L-type calcium channels also have diminished forward trafficking resulting in intracellular accumulation of the channels 13. There already exists significant evidence that gross T-tubule network remodeling occurs in failing heart 106–108. It is an area of active research with regard to the mechanisms of T-tubule remodeling in failing hearts. Junctophilin-2 trafficked by microtubules has been implicated in impaired T-tubule maintenance during heart failure 109. However the role of junctophilin-2 in T-tubule remodeling during heart failure has been questioned due to a lack of decrease with heart failure as T-tubule structures are diminished 96, 97 or return with recovery of T-tubule structures in treated heart failure 96. In these same studies, BIN1 decreased with decrease in T-tubule density in heart failure 96, 97, and then BIN1 recovered along with T-tubule density during functional recovery of the myocardium 96.
During extended in vitro culture, isolated mature ventricular myocytes loose T-tubules in 3 days. Interestingly, actin stabilization by cytochalasin D can preserve T-tubules in cultured myocytes 87, 110, 111. To that end, the cardiac isoform of BIN1, which we described recently, was found to be able to promote N-WASP dependent actin polymerization 87. Exogenous BIN1 introduced by adenovirus not only rescued T-tubule membrane intensity 87 but also surface CaV1.2 channels 13 in isolated cardiomyocytes cultured in vitro. Taken together, we have found that T-tubule inner folds are rescued only by the BIN1 cardiac specific isoform, which promotes N-WASP dependent actin polymerization to stabilize T-tubule membrane at cardiac Z-discs to help recruit CaV1.2 channels 87.
In mice with cardiac Bin1 deletion, T-tubule folding is decreased which does not change overall cardiomyocyte morphology, but frees diffusion of local extracellular calcium and potassium ions, prolonging action potential duration, and increasing susceptibility to ventricular arrhythmias 87. In addition, these cardiac specific BIN1-deificient mice exhibit T-tubule remodeling similar to what is observed in failing hearts. Thus BIN1 cardiac specific isoform recruits actin to fold T-tubule membrane, creating a fuzzy space that protectively restricts ionic flux. When cardiac BIN1 is decreased, as occurs in acquired cardiomyopathy, T-tubule morphology is altered and arrhythmias can result 87.
Cav1.2 Internalization
General internalization of LTCCs is poorly understood with particular lack of studies in cardiomyocytes. In oocytes, the LTCC β-subunit can enhance dynamin-dependent internalization 112, and in neurons CaV1.2 channels may undergo depolarization and calcium dependent internalization 113. We found in cardiomyocytes that a dynamin GTPase inhibitor dynasore can increase surface LTCC expression, indicating dynamin dependent endocytosis of cardiac CaV1.2 channels 11. Furthermore, a small GTPase Rab11 is implicated in endosomal transport of LTCCs, thereby limiting surface expression of LTCCs 114.
Channelopathies as a Result of Altered Trafficking in Heart Pathophysiology
Numerous channelopathies in heart disease are caused by mutations negatively affecting trafficking. For instance, Anderson et al. have found that of 28 clinically relevant mutations in Kv11.1, most reduce hERG current not by altering Kv11.1 expression or kinetics, but by diminishing Kv11.1 trafficking to the membrane 115. In accordance with this finding, different trafficking-deficient mutations in several regions of the hERG channel protein have been identified to cause LQT2 syndrome. Such mutations include; (T65P) in the N-terminus region, (N470D and A561V) in the transmembrane region, (G601S, Y611H, V612L, T613M, and L615V) in the pore region and (R752W, F805C, V822M, R823W, and N861I) in the C terminus 116. In addition, the missense mutation (A558P) in hERG has been shown to exert a dominant negative effect causing trafficking deficiency of the channel and fever-induced QT interval prolongation in patients 117.
Defective cardiac ion channel trafficking in inherited arrhythmia has also been reported for KCNQ1 (LQT1 syndrome), KCNE1 (LQT5 syndrome), and SCN5A (Brugada syndrome). Such SCN5A trafficking-deficient mutations in Brugada syndrome include T351I, R367H, R1232W, R1232W/T1620M, R1432G, and G1743R 116. Also mutations in Nav1.5 which limit binding of Nav1.5 to a membrane anchor protein ankyrin-G have been shown to cause aberrant Nav1.5 trafficking to the intercalated discs and result in human Brugada syndrome 118. An additional ankyrin isoform found in ventricular cardiomyocytes, ankyrin-B, has been described to be associated with targeting and maintenance of the Na+/Ca2+ exchanger (NCX), Na+/K+ ATPase (NKA), at T-tubules where they proximate with the IP3 receptor (InsP3) of the sarcoplasmic reticulum (SR) and regulate Ca2+ export. Mutations in ankyrin-B ablating its interaction with NCX/NKA/InsP3 result in arrhythmogenic cardiac disorders in humans, including type-4 long-QT syndrome 119.
Conclusions
The individual cardiomyocyte is a highly complex and dynamic system with internal organization designed to maintain efficient cell-cell communication and excitation-contraction coupling. To maintain intracellular homeostasis as well as overall synchrony across the myocardium, cardiomyocytes regulate ion channel intracellular movement and localization through highly sophisticated and highly efficient protein trafficking machineries. In diseased hearts, cardiomyocyte structures and organization are negatively affected by environmental conditions of stress, impacting channel trafficking and function. As the physiologic movements of cardiac channels are elucidated, and then disease related changes of these movements are understood, interventions can be designed to promote positive intracellular remodeling. Therefore, new therapies for failing heart should focus on the specific organelles and pathways that regulate cardiomyocyte channel trafficking.
Acknowledgments
Sources of Funding: This work was supported by National Institute of Health grants HL094414 (R.M.S.), and by the America Heart Association (R.M.S.).
Footnotes
Disclosures: None.
References
- 1.Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 2.Smyth JW, Zhang SS, Sanchez JM, Lamouille S, Vogan JM, Hesketh GG, Hong T, Tomaselli GF, Shaw RM. A 14-3-3 mode-1 binding motif initiates gap junction internalization during acute cardiac ischemia. Traffic. 2014;15:684–699. doi: 10.1111/tra.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colley BS, Biju KC, Visegrady A, Campbell S, Fadool DA. Neurotrophin b receptor kinase increases kv subfamily member 1.3 (kv1.3) ion channel half-life and surface expression. Neuroscience. 2007;144:531–546. doi: 10.1016/j.neuroscience.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized beta subunit in the formation and targeting of functional l-type ca2+ channels. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 5.Di Biase V, Tuluc P, Campiglio M, Obermair GJ, Heine M, Flucher BE. Surface traffic of dendritic cav1.2 calcium channels in hippocampal neurons. J Neurosci. 2011;31:13682–13694. doi: 10.1523/JNEUROSCI.2300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egger M, Porzig H, Niggli E, Schwaller B. Rapid turnover of the “functional” na(+)-ca2+ exchanger in cardiac myocytes revealed by an antisense oligodeoxynucleotide approach. Cell Calcium. 2005;37:233–243. doi: 10.1016/j.ceca.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.De Forges H, Bouissou A, Perez F. Interplay between microtubule dynamics and intracellular organization. Int J Biochem Cell Biol. 2012;44:266–274. doi: 10.1016/j.biocel.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Gu F, Crump CM, Thomas G. Trans-golgi network sorting. Cell Mol Life Sci. 2001;58:1067–1084. doi: 10.1007/PL00000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luini A, Mironov AA, Polishchuk EV, Polishchuk RS. Morphogenesis of post-golgi transport carriers. Histochem Cell Biol. 2008;129:153–161. doi: 10.1007/s00418-007-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. Bin1 localizes the l-type calcium channel to cardiac t-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong TT, Smyth JW, Chu KY, Vogan JM, Fong TS, Jensen BC, Fang K, Halushka MK, Russell SD, Colecraft H, Hoopes CW, Ocorr K, Chi NC, Shaw RM. Bin1 is reduced and cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm. 2012;9:812–820. doi: 10.1016/j.hrthm.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8:808–819. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 15.Levy JR, Holzbaur EL. Special delivery: Dynamic targeting via cortical capture of microtubules. Dev Cell. 2007;12:320–322. doi: 10.1016/j.devcel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Hendricks AG, Lazarus JE, Perlson E, Gardner MK, Odde DJ, Goldman YE, Holzbaur EL. Dynein tethers and stabilizes dynamic microtubule plus ends. Curr Biol. 2012;22:632–637. doi: 10.1016/j.cub.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chkourko HS, Guerrero-Serna G, Lin X, Darwish N, Pohlmann JR, Cook KE, Martens JR, Rothenberg E, Musa H, Delmar M. Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin43 lateralization. Heart Rhythm. 2012;9:1133–1140. doi: 10.1016/j.hrthm.2012.03.003. e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, Shaw RM. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res. 2012;110:978–989. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 associates with na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol. 2012;245:411–422. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 21.Beyer EC. Gap junctions. Int Rev Cytol. 1993;137C:1–37. [PubMed] [Google Scholar]
- 22.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noorman M, van der Heyden MA, van Veen TA, Cox MG, Hauer RN, de Bakker JM, van Rijen HV. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47:23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Unwin PN, Zampighi G. Structure of the junction between communicating cells. Nature. 1980;283:545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- 25.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 26.Schulz R, Gorge PM, Gorbe A, Ferdinandy P, Lampe PD, Leybaert L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther. 2015;153:90–106. doi: 10.1016/j.pharmthera.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas FF, Sipido KR, Heusch G, Schulz R, Bultynck G, Leybaert L. Selective inhibition of cx43 hemichannels by gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108:309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–322. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue Roles of the sodium and l-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 30.Hesketh GG, Van Eyk JE, Tomaselli GF. Mechanisms of gap junction traffic in health and disease. J Cardiovasc Pharmacol. 2009;54:263–272. doi: 10.1097/FJC.0b013e3181ba0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saffitz JE, Hames KY, Kanno S. Remodeling of gap junctions in ischemic and nonischemic forms of heart disease. J Membr Biol. 2007;218:65–71. doi: 10.1007/s00232-007-9031-2. [DOI] [PubMed] [Google Scholar]
- 32.Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, Fishman GI. Heterogeneous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 33.Van Rijen HV, van Veen TA, Gros D, Wilders R, de Bakker JM. Connexins and cardiac arrhythmias. Adv Cardiol. 2006;42:150–160. doi: 10.1159/000092567. [DOI] [PubMed] [Google Scholar]
- 34.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in cx43-deficient mice. Circulation. 2000;101:547–552. doi: 10.1161/01.cir.101.5.547. [DOI] [PubMed] [Google Scholar]
- 36.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–H1770. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 37.Lo CW. Role of gap junctions in cardiac conduction and development: Insights from the connexin knockout mice. Circ Res. 2000;87:346–348. doi: 10.1161/01.res.87.5.346. [DOI] [PubMed] [Google Scholar]
- 38.Saffitz JE. Arrhythmogenic cardiomyopathy and abnormalities of cell-to-cell coupling. Heart Rhythm. 2009;6:S62–S65. doi: 10.1016/j.hrthm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Sepp R, Severs NJ, Gourdie RG. Altered patterns of cardiac intercellular junction distribution in hypertrophic cardiomyopathy. Heart. 1996;76:412–417. doi: 10.1136/hrt.76.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters NS. New insights into myocardial arrhythmogenesis: Distribution of gap-junctional coupling in normal, ischaemic and hypertrophied human hearts. Clin Sci (Lond) 1996;90:447–452. doi: 10.1042/cs0900447. [DOI] [PubMed] [Google Scholar]
- 41.Jongsma HJ, Wilders R. Gap junctions in cardiovascular disease. Circ Res. 2000;86:1193–1197. doi: 10.1161/01.res.86.12.1193. [DOI] [PubMed] [Google Scholar]
- 42.Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, Bauer EP, Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- 43.Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, Galeano EJ, Kubo S, Hayashi Y, Itoh H, Yokoyama M. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:865–870. doi: 10.1046/j.1540-8167.2002.00865.x. [DOI] [PubMed] [Google Scholar]
- 44.Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, Kaprielian R, Yacoub MH, Severs NJ. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 45.Fontes MS, van Veen TA, de Bakker JM, van Rijen HV. Functional consequences of abnormal cx43 expression in the heart. Biochim Biophys Acta. 2012;1818:2020–2029. doi: 10.1016/j.bbamem.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 46.Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 47.Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1223–H1230. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 48.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 49.Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, Yen TE, Kass DA, Machamer CE, Van Eyk JE, Tomaselli GF. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–1163. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel DM, Dubash AD, Kreitzer G, Green KJ. Disease mutations in desmoplakin inhibit cx43 membrane targeting mediated by desmoplakin-eb1 interactions. J Cell Biol. 2014;206:779–797. doi: 10.1083/jcb.201312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw RM. Desmosomal hotspots, microtubule delivery, and cardiac arrhythmogenesis. Dev Cell. 2014;31:139–140. doi: 10.1016/j.devcel.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang SS, Hong S, Kleber AG, Lee LP, Shaw RM. A micropatterning approach for imaging dynamic cx43 trafficking to cell-cell borders. FEBS Lett. 2014;588:1439–1445. doi: 10.1016/j.febslet.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartolini F, Ramalingam N, Gundersen GG. Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mdia1. Mol Biol Cell. 2012;23:4032–4040. doi: 10.1091/mbc.E12-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of leading edge microtubule and actin dynamics downstream of rac1. J Cell Biol. 2003;161:845–851. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smyth JW, Shaw RM. Forward trafficking of ion channels: What the clinician needs to know. Heart Rhythm. 2010;7:1135–1140. doi: 10.1016/j.hrthm.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smyth JW, Shaw RM. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013;5:611–618. doi: 10.1016/j.celrep.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salat-Canela C, Sese M, Peula C, Ramon y Cajal S, Aasen T. Internal translation of the connexin 43 transcript. Cell Commun Signal. 2014;12:31. doi: 10.1186/1478-811X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ul-Hussain M, Olk S, Schoenebeck B, Wasielewski B, Meier C, Prochnow N, May C, Galozzi S, Marcus K, Zoidl G, Dermietzel R. Internal ribosomal entry site (ires) activity generates endogenous carboxyl-terminal domains of cx43 and is responsive to hypoxic conditions. J Biol Chem. 2014;289:20979–20990. doi: 10.1074/jbc.M113.540187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laing JG, Tadros PN, Westphale EM, Beyer EC. Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp Cell Res. 1997;236:482–492. doi: 10.1006/excr.1997.3747. [DOI] [PubMed] [Google Scholar]
- 60.Boassa D, Solan JL, Papas A, Thornton P, Lampe PD, Sosinsky GE. Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic. 2010;11:1471–1486. doi: 10.1111/j.1600-0854.2010.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, Liu FY, Zhang J, Lent DS, Morley GE, Fishman GI. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108:1459–1466. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leithe E, Rivedal E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-o-tetradecanoylphorbol 13-acetate treatment. J Biol Chem. 2004;279:50089–50096. doi: 10.1074/jbc.M402006200. [DOI] [PubMed] [Google Scholar]
- 63.Martins-Marques T, Catarino S, Marques C, Matafome P, Ribeiro-Rodrigues T, Baptista R, Pereira P, Girao H. Heart ischemia results in connexin43 ubiquitination localized at the intercalated discs. Biochimie. 2015;112:196–201. doi: 10.1016/j.biochi.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 64.Martins-Marques T, Catarino S, Zuzarte M, Marques C, Matafome P, Pereira P, Girao H. Ischaemia-induced autophagy leads to degradation of gap junction protein connexin43 in cardiomyocytes. Biochem J. 2015;467:231–245. doi: 10.1042/BJ20141370. [DOI] [PubMed] [Google Scholar]
- 65.Basheer WA, Harris BS, Mentrup HL, Abreha M, Thames EL, Lea JB, Swing DA, Copeland NG, Jenkins NA, Price RL, Matesic LE. Cardiomyocyte-specific overexpression of the ubiquitin ligase wwp1 contributes to reduction in connexin 43 and arrhythmogenesis. J Mol Cell Cardiol. 2015;88:1–13. doi: 10.1016/j.yjmcc.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Girao H, Catarino S, Pereira P. Eps15 interacts with ubiquitinated cx43 and mediates its internalization. Exp Cell Res. 2009;315:3587–3597. doi: 10.1016/j.yexcr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Leithe E, Rivedal E. Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci. 2004;117:1613–1613. doi: 10.1242/jcs.00951. [DOI] [PubMed] [Google Scholar]
- 68.Leykauf K, Salek M, Bomke J, Frech M, Lehmann WD, Durst M, Alonso A. Ubiquitin protein ligase nedd4 binds to connexin43 by a phosphorylation-modulated process. J Cell Sci. 2006;119:3634–3642. doi: 10.1242/jcs.03149. [DOI] [PubMed] [Google Scholar]
- 69.Chen VC, Kristensen AR, Foster LJ, Naus CC. Association of connexin43 with e3 ubiquitin ligase trim21 reveals a mechanism for gap junction phosphodegron control. J Proteome Res. 2012;11:6134–6146. doi: 10.1021/pr300790h. [DOI] [PubMed] [Google Scholar]
- 70.Fykerud TA, Kjenseth A, Schink KO, Sirnes S, Bruun J, Omori Y, Brech A, Rivedal E, Leithe E. Smad ubiquitination regulatory factor-2 controls gap junction intercellular communication by modulating endocytosis and degradation of connexin43. J Cell Sci. 2012;125:3966–3976. doi: 10.1242/jcs.093500. [DOI] [PubMed] [Google Scholar]
- 71.Bejarano E, Girao H, Yuste A, Patel B, Marques C, Spray DC, Pereira P, Cuervo AM. Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin-dependent manner. Mol Biol Cell. 2012;23:2156–2169. doi: 10.1091/mbc.E11-10-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mollerup S, Hofgaard JP, Braunstein TH, Kjenseth A, Leithe E, Rivedal E, Holstein-Rathlou NH, Nielsen MS. Norepinephrine inhibits intercellular coupling in rat cardiomyocytes by ubiquitination of connexin43 gap junctions. Cell Commun Adhes. 2011;18:57–65. doi: 10.3109/15419061.2011.611920. [DOI] [PubMed] [Google Scholar]
- 73.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 74.Marquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012;1818:1985–1992. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Majoul IV, Onichtchouk D, Butkevich E, Wenzel D, Chailakhyan LM, Duden R. Limiting transport steps and novel interactions of connexin-43 along the secretory pathway. Histochem Cell Biol. 2009;132:263–280. doi: 10.1007/s00418-009-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Batra N, Riquelme MA, Burra S, Jiang JX. 14-3-3theta facilitates plasma membrane delivery and function of mechanosensitive connexin 43 hemichannels. J Cell Sci. 2014;127:137–146. doi: 10.1242/jcs.133553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second pdz domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 79.Laing JG, Chou BC, Steinberg TH. Zo-1 alters the plasma membrane localization and function of cx43 in osteoblastic cells. J Cell Sci. 2005;118:2167–2176. doi: 10.1242/jcs.02329. [DOI] [PubMed] [Google Scholar]
- 80.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–1528. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hunter AW, Jourdan J, Gourdie RG. Fusion of gfp to the carboxyl terminus of connexin43 increases gap junction size in hela cells. Cell Commun Adhes. 2003;10:211–214. doi: 10.1080/cac.10.4-6.211.214. [DOI] [PubMed] [Google Scholar]
- 83.Chen J, Pan L, Wei Z, Zhao Y, Zhang M. Domain-swapped dimerization of zo-1 pdz2 generates specific and regulatory connexin43-binding sites. EMBO J. 2008;27:2113–2123. doi: 10.1038/emboj.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with zo-1. Cardiovasc Res. 2008;77:757–765. doi: 10.1093/cvr/cvm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–1112. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orchard C, Brette F. T-tubules and sarcoplasmic reticulum function in cardiac ventricular myocytes. Cardiovasc Res. 2008;77:237–244. doi: 10.1093/cvr/cvm002. [DOI] [PubMed] [Google Scholar]
- 87.Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marban E, Jan LY, Shaw RM. Cardiac bin1 folds t-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. 2014;20:624–632. doi: 10.1038/nm.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu Y, Shaw SA, Naami R, Vuong CL, Basheer WA, Guo X, Hong T. Isoproterenol promotes rapid ryanodine receptor movement to bridging integrator 1 (bin1)-organized dyads. Circulation. 2016;133:388–397. doi: 10.1161/CIRCULATIONAHA.115.018535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lindner E. [Submicroscopic morphology of the cardiac muscle] Z Zellforsch Mikrosk Anat. 1957;45:702–746. [PubMed] [Google Scholar]
- 90.Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology (Bethesda) 2007;22:167–173. doi: 10.1152/physiol.00005.2007. [DOI] [PubMed] [Google Scholar]
- 91.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98:149–160. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 93.Meunier B, Quaranta M, Daviet L, Hatzoglou A, Leprince C. The membrane-tubulating potential of amphiphysin 2/bin1 is dependent on the microtubule-binding cytoplasmic linker protein 170 (clip-170) Eur J Cell Biol. 2009;88:91–102. doi: 10.1016/j.ejcb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 94.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac l-type ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hennessey JA, Wei EQ, Pitt GS. Fibroblast growth factor homologous factors modulate cardiac calcium channels. Circ Res. 2013;113:381–388. doi: 10.1161/CIRCRESAHA.113.301215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lyon AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, Hulot JS, Kohlbrenner E, Hajjar RJ, Peters NS, Korchev YE, Macleod KT, Harding SE, Gorelik J. Plasticity of surface structures and β(2)-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ Heart Fail. 2012;5:357–365. doi: 10.1161/CIRCHEARTFAILURE.111.964692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caldwell JL, Smith CE, Taylor RF, Kitmitto A, Eisner DA, Dibb KM, Trafford AW. Dependence of cardiac transverse tubules on the bar domain protein amphiphysin ii (bin-1) Circ Res. 2014;115:986–996. doi: 10.1161/CIRCRESAHA.116.303448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meissner M, Weissgerber P, Londono JE, Prenen J, Link S, Ruppenthal S, Molkentin JD, Lipp P, Nilius B, Freichel M, Flockerzi V. Moderate calcium channel dysfunction in adult mice with inducible cardiomyocyte-specific excision of the cacnb2 gene. J Biol Chem. 2011;286:15875–15882. doi: 10.1074/jbc.M111.227819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Foell JD, Balijepalli RC, Delisle BP, Yunker AM, Robia SL, Walker JW, McEnery MW, January CT, Kamp TJ. Molecular heterogeneity of calcium channel beta-subunits in canine and human heart: Evidence for differential subcellular localization. Physiol Genomics. 2004;17:183–200. doi: 10.1152/physiolgenomics.00207.2003. [DOI] [PubMed] [Google Scholar]
- 100.Shaw RM, Colecraft HM. L-type calcium channel targeting and local signalling in cardiac myocytes. Cardiovasc Res. 2013;98:177–186. doi: 10.1093/cvr/cvt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buraei Z, Yang J. The b subunit of voltage-gated ca2+ channels. Physiol Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buraei Z, Yang J. Structure and function of the beta subunit of voltage-gated ca(2)(+) channels. Biochim Biophys Acta. 2013;1828:1530–1540. doi: 10.1016/j.bbamem.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haissaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by st-segment elevation, short qt intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cordeiro JM, Marieb M, Pfeiffer R, Calloe K, Burashnikov E, Antzelevitch C. Accelerated inactivation of the l-type calcium current due to a mutation in cacnb2b underlies brugada syndrome. J Mol Cell Cardiol. 2009;46:695–703. doi: 10.1016/j.yjmcc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, Claus P, Dymarkowski S, Maes F, Bogaert J, Rademakers F, D’Hooge J, Sipido K. Remodeling of t-tubules and reduced synchrony of ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102:338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 107.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, Luther S, Hasenfuss G, Parlitz U, Jafri MS, Hell SW, Lederer WJ, Lehnart SE. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of t-tubule membrane structures after myocardial infarction. Circ Res. 2012;111:402–414. doi: 10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang C, Chen B, Guo A, Zhu Y, Miller JD, Gao S, Yuan C, Kutschke W, Zimmerman K, Weiss RM, Wehrens XH, Hong J, Johnson FL, Santana LF, Anderson ME, Song LS. Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and ca2+ handling dysfunction in heart failure. Circulation. 2014;129:1742–1750. doi: 10.1161/CIRCULATIONAHA.113.008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on l-type ca channel distribution in rat ventricular myocytes. Cell Calcium. 2005;38:515–526. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 111.Tian Q, Pahlavan S, Oleinikow K, Jung J, Ruppenthal S, Scholz A, Schumann C, Kraegeloh A, Oberhofer M, Lipp P, Kaestner L. Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin d supplement. J Mol Cell Cardiol. 2012;52:113–124. doi: 10.1016/j.yjmcc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Gonzalez-Gutierrez G, Miranda-Laferte E, Neely A, Hidalgo P. The src homology 3 domain of the beta-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J Biol Chem. 2007;282:2156–2162. doi: 10.1074/jbc.M609071200. [DOI] [PubMed] [Google Scholar]
- 113.Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE. The tumor suppressor eif3e mediates calcium-dependent internalization of the l-type calcium channel cav1.2. Neuron. 2007;55:615–632. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Best JM, Foell JD, Buss CR, Delisle BP, Balijepalli RC, January CT, Kamp TJ. Small gtpase rab11b regulates degradation of surface membrane l-type cav1.2 channels. Am J Physiol Cell Physiol. 2011;300:C1023–C1033. doi: 10.1152/ajpcell.00288.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. Most lqt2 mutations reduce kv11.1 (herg) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 116.Balijepalli SY, Anderson CL, Lin EC, January CT. Rescue of mutated cardiac ion channels in inherited arrhythmia syndromes. J Cardiovasc Pharmacol. 2010;56:113–122. doi: 10.1097/FJC.0b013e3181dab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amin AS, Herfst LJ, Delisle BP, Klemens CA, Rook MB, Bezzina CR, Underkofler HA, Holzem KM, Ruijter JM, Tan HL, January CT, Wilde AA. Fever-induced qtc prolongation and ventricular arrhythmias in individuals with type 2 congenital long qt syndrome. J Clin Invest. 2008;118:2552–2561. doi: 10.1172/JCI35337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V. Nav1.5 e1053k mutation causing brugada syndrome blocks binding to ankyrin-g and expression of nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohler PJ, Davis JQ, Bennett V. Ankyrin-b coordinates the na/k atpase, na/ca exchanger, and insp3 receptor in a cardiac t-tubule/sr microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]