Abstract
The aim of this propensity-matched cohort study was to evaluate the impact of prenatal SSRI exposure and a history of maternal depression on neonatal brain volumes and white matter microstructure. SSRI-exposed neonates (n = 27) were matched to children of mothers with no history of depression or SSRI use (n=54). Additionally, neonates of mothers with a history of depression, but no prenatal SSRI exposure (n=41), were matched to children of mothers with no history of depression or SSRI use (n=82). Structural magnetic resonance imaging and diffusion weighted imaging scans were acquired with a 3T Siemens Allegra scanner. Global tissue volumes were characterized using an automatic, atlas-moderated expectation maximization segmentation tool. Local differences in gray matter volumes were examined using deformation-based morphometry. Quantitative tractography was performed using an adaptation of the UNC-Utah NA-MIC DTI framework. SSRI-exposed neonates exhibited widespread changes in white matter microstructure compared to matched controls. Children exposed to a history of maternal depression but no SSRIs showed no significant differences in brain development compared to matched controls. No significant differences were found in global or regional tissue volumes. Additional research is needed to clarify whether SSRIs directly alter white matter development or whether this relationship is mediated by depressive symptoms during pregnancy.
Keywords: Magnetic Resonance Imaging, Depression, Fetal Development, Diffusion Tensor Imaging, Infant, Newborn, Antidepressant
1. Introduction
Approximately 18% of pregnant women in the U.S. suffer from depression (Waters et al., 2014). Untreated antenatal depression is associated with intense emotional distress, low fetal growth, preterm birth, neonatal complications, and conduct problems and antisocial behavior in offspring (Waters et al., 2014; Yonkers et al., 2009). While there is no definitive answer regarding optimal treatment for antenatal depression, the American Psychiatric Association and the American College of Obstetricians and Gynecologists provide algorithms for multiple scenarios that result in the initiation or maintenance of pharmacotherapy. Overall, approximately 13% of pregnant women report antidepressant use (Cooper et al., 2007) with selective serotonin reuptake inhibitors (SSRIs) being the most commonly prescribed class. Despite widespread use of SSRIs during pregnancy, effects on the fetus are not fully understood and are a source of concern for many pregnant women.
SSRIs are diffusible through the placenta and blood brain barrier and could potentially target the developing fetal brain (Velasquez et al., 2013). During this early period of neurodevelopment, serotonin from maternal, placental, and fetal sources is involved in neuronal proliferation, migration, and synaptogenesis (Whitaker-Azmitia, 2005). In rodents, exposure to SSRIs in the prenatal and/or early neonatal period disrupts dendritic organization and formation of thalamocortical afferents to the somatosensory cortex and results in aberrant axonal morphology, abnormal raphe circuitry, and altered cortical function (Lee, 2009; Liao and Lee, 2011; Simpson et al., 2011; Xu et al., 2004). These changes are accompanied by behavioral disturbances including decreased exploratory behavior and increased emotional reactivity, altered social behavior, and impaired motor performance (Borue et al., 2007; Glover et al., 2015; Xu et al., 2004).
Research on behavioral outcomes in humans exposed to SSRIs in utero has focused almost exclusively on measurements of cognitive development and IQ, where exposed children perform at typically developing levels (Nulman et al., 2012). However, motor development and control issues have been observed in a number of studies (Casper et al., 2003; Casper et al., 2011; Hanley et al., 2013; Pedersen et al., 2010; Rampono et al., 2009; Salisbury et al., 2011; Smith et al., 2013). A possible link between prenatal antidepressant exposure and autism spectrum disorders/symptoms has proved particularly controversial with several large studies reporting significant associations (Boukhris et al., 2016; Croen et al., 2011) and other large studies failing to replicate (Harrington et al., 2014; Hviid et al., 2013). Neuroimaging studies are extremely limited. One study reported benign caudothalamic cysts in 6 of 40 at term infants exposed to SSRIs but no unexposed comparison group was evaluated (Laine et al., 2003). We recently reported that children exposed to SSRIs prenatally exhibit a striking increase in Chiari I malformations, a condition resulting from the underdevelopment of the posterior cranial fossa and overcrowding of the normally developing hindbrain (Knickmeyer et al., 2014). The current study is the first to examine brain tissue volumes and white matter development of SSRI-exposed neonates. Based on the rodent literature and the motor deficits observed in human children exposed in utero to SSRIs, we hypothesized that prenatal SSRI exposure would impact diffusion parameters in corticothalamic and corticofugal white matter tracts originating/terminating in motor and somatosensory cortex, as well as gray matter volumes in these particular brain regions. To test the possibility that a history of maternal depression influences brain development in the absence of prenatal SSRI exposure we also examined a separate cohort of neonates whose mothers had a history of depression but were not treated with SSRIs during pregnancy.
2. Methods
2.1 Recruitment
Participants were drawn from three prospective longitudinal neuroimaging studies being carried out at UNC (Gilmore et al., 2010; Gilmore et al., 2012; Knickmeyer et al., 2014). Recruitment occurred through community physicians, relevant clinics at UNC, including the perinatal psychiatry clinic and general obstetrics clinics, and mass emails to the UNC community. Participants were recruited between November 2003 and December 2010.
Exclusion criteria in mothers were major medical illness or substance abuse during pregnancy. Exclusion criteria for neonates were gestational age at birth less than 32 weeks, major postnatal complications, major congenital anomalies, and metal in the body. After complete description of the study to subjects’ parent(s), written informed consent was obtained. Study protocols were in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by the Institutional Review Board of the UNC School of Medicine and the National Institutes of Health as well as Uniform Requirements for manuscripts submitted to Biomedical journals (http://www.icmje.org/).
2.2 Cohort 1 Participants (SSRI exposed)
This analysis included 27 SSRI-exposed (7 males; 21 singletons; 6 twins) and 54 matched control (20 males; 42 singletons; 12 twins) neonates. Diagnosis of a mood disorder and SSRI use in all three trimesters was confirmed through self-reports (oral interview) and review of medical records (primarily prenatal and labor & delivery). Nineteen mothers reported that they received a diagnosis of depression prior to study entry; medical records provided corroborating information in all cases. Twelve of these women reported active depression at entry and one additional woman reported active depression without a past history. Additionally, four women did not report active depression or a past diagnosis of depression at study entry, but review of medical records indicated such a diagnosis was made prior to or during pregnancy. Medical records were not sufficiently detailed to determine the exact date of diagnosis. The most common diagnoses were major depression and depressive disorder not elsewhere classified, one woman reported bipolar disorder, and seven were diagnosed with an anxiety disorder in addition to a mood disorder. Medical record review indicated fifteen mothers took SSRIs at the time of conception and six mothers began SSRI treatment during the first trimester. For three women, we could not determine whether treatment began before or after conception (data on SSRI type and dosage can be found in supplementary figure 1). Use of a psychiatric drug other than an SSRI was an exclusion criterion for the current analysis with the exception of trazodone (N=1 SSRI-exposed), low dose benzodiazepines (N=3 SSRI-exposed), and psychostimulants (N=1 SSRI-exposed). For data on non-SSRI medications during pregnancy see Supplementary Table 1.
Each exposed neonate was matched to two control neonates (offspring of pregnant women with no known history of mood disorders, anxiety, or antidepressant use) based on gender, gestational age at birth and MRI, maternal age, ethnicity, and education using propensity scores. Singletons were matched to singletons and twins to twins. See Table 1 for demographic information. Infants were considered positive for perinatal problems if there was a presence of a nuchal cord or if they experienced meconium aspiration, asphyxia or RH incompatibility. Infants were considered positive for postnatal problems if they experienced jaundice, seizures, sepsis, pneumonia, necrotizing enterocolitis, or respiratory distress syndrome.
Table 1.
Demographic Characteristics of SSRI-exposed and Matched Control Neonates
| Characteristic | Matched Control | SSRI Exposed | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | Mean ± SD | N | Mean ± SD | P-Value | ||
| Gestational Age at Birth (days) | 54 | 271.3 ± 13.35 | 27 | 265.4 ± 15.93 | 0.118 | |
| Birth Length (cm) | 49 | 49.75 ± 3.32 | 25 | 49.07 ± 3.45 | 0.496 | |
| Postnatal Age at Neonatal MRI (days) | 54 | 24.83 ± 12.90 | 27 | 27.07 ± 13.56 | 0.581 | |
| Maternal Age (years) | 54 | 29.31 ± 5.31 | 27 | 30.89 ± 5.79 | 0.177 | |
| Maternal Education (years) | 54 | 15.57 ± 4.12 | 27 | 16.52 ± 3.27 | 0.313 | |
| Total Household Income ($) | 53 | 71,151 ± 47,166 | 25 | 78,124 ± 49,448 | 0.528 | |
| Birth weight (grams) | 54 | 3,176 ± 609.5 | 27 | 3,136 ± 617.1 | 0.912 | |
| N | % | N | % | P-Value | ||
| Perinatal Problems* | Yes | 24 | 44.44 | 13 | 50 | 0.811 |
| No | 30 | 55.56 | 13 | 50 | ||
| Postnatal Problems+ | Yes | 18 | 33.33 | 12 | 46.15 | 0.327 |
| No | 36 | 66.67 | 14 | 53.85 | ||
| NICU Stay | Yes | 5 | 9.26 | 5 | 19.23 | 0.280 |
| No | 49 | 90.74 | 21 | 80.77 | ||
| Sex | Female | 34 | 62.96 | 20 | 74.07 | 0.454 |
| Male | 20 | 37.04 | 7 | 25.93 | ||
| Maternal Ethnicity | White | 37 | 68.52 | 23 | 85.19 | 0.236 |
| Black | 14 | 25.93 | 4 | 14.81 | ||
| Asian | 3 | 5.56 | 0 | 0 | ||
| MDD history in a 1° Relative | Yes | 7 | 12.96 | 11 | 40.74 | 0.009 |
| No | 47 | 87.04 | 16 | 59.26 | ||
Perinatal problems include the presence of nuchal cord, meconium aspiration, asphyxia or RH incompatibility.
Postnatal problems include jaundice, seizures, sepsis, pneumonia, necrotizing enterocolitis, or respiratory distress syndrome.
2.2 Cohort 2 Participants (history of maternal depression)
We identified 41 neonates (21 males, 16 singletons, 25 twins) of mothers who received a diagnosis of depression prior to or during pregnancy but did not receive antidepressants in any trimester. This cohort may potentially share with the SSRI-exposed cohort a generally higher risk of un-identified behaviors (e.g. unreported smoking, substance misuse, exposure to other teratogens) as well as genetic factors associated with depression. Twenty-five mothers reported that they received a diagnosis of depression prior to study entry; medical records provided corroborating information in all cases. Three of these women reported active depression at entry. The other seven women did not report active depression or a past diagnosis of depression at study entry, but review of medical records indicated such a diagnosis was made prior to or during pregnancy. Medical records were not sufficiently detailed to determine the exact date of diagnosis. The most common diagnoses were major depression and depressive disorder not elsewhere classified. Additionally, one woman reported a lifetime history of bipolar disorder, and two women reported a lifetime history of anxiety disorder in addition to a mood disorder. One mother was also diagnosed with ADHD in addition to having a lifetime history of depression.
All neonates of untreated mothers were matched with 2 control neonates (exactly as described in section 2.1) using propensity scores (39 males, 24 singletons, 58 twins). When propensity scores failed to identify an appropriate match, comparison children were hand selected (6 of 82). See Table 2 for demographic information. For data on medication exposure during pregnancy see Supplementary Table 2.
Table 2.
Demographic Characteristics of Neonates with Mothers Diagnosed with Depression but Untreated during Pregnancy and Matched Control Neonates
| Characteristic | Matched Control | Maternal History of Depression, No SSRI Treatment during Pregnancy | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | Mean ± SD | N | Mean ± SD | P-Value | ||
| Gestational Age at Birth (days) | 82 | 262.6±14.81 | 41 | 261.1±16.78 | 0.964 | |
| Birth Length (cm) | 77 | 48.18±3.98 | 41 | 48.82±3.25 | 0.341 | |
| Postnatal Age at Neonatal MRI (days) | 82 | 27.1±12.94 | 41 | 27.98±14.49 | 0.959 | |
| Maternal Age (years) | 82 | 29.29±5.08 | 41 | 29.24±5.9 | 0.697 | |
| Maternal Education (years) | 82 | 14.79±3.77 | 41 | 14.4±3.13 | 0.477 | |
| Total Household Income ($) | 80 | 71000±53161 | 39 | 78669±63132 | 0.709 | |
| Birth weight (grams) | 82 | 2824±616 | 41 | 2795±599.8 | 0.891 | |
| N | % | N | % | P-Value | ||
| Perinatal Problems* | Yes | 31 | 37.8 | 18 | 43.9 | 0.561 |
| No | 51 | 62.2 | 23 | 56.1 | ||
| Postnatal Problems+ | Yes | 32 | 39.02 | 17 | 41.46 | 0.846 |
| No | 50 | 60.98 | 24 | 58.54 | ||
| NICU Stay | Yes | 13 | 15.85 | 7 | 17.07 | 1.000 |
| No | 69 | 84.15 | 34 | 82.93 | ||
| Sex | Female | 42 | 52.44 | 20 | 48.78 | 0.707 |
| Male | 39 | 47.56 | 21 | 51.22 | ||
| Maternal Ethnicity | White | 64 | 78.05 | 34 | 82.93 | 0.775 |
| Black | 16 | 19.51 | 6 | 14.63 | ||
| Indian | 1 | 1.22 | 0 | 0 | ||
| Asian | 1 | 1.22 | 1 | 2.44 | ||
| MDD history in a 1° Relative | Yes | 6 | 7.32 | 29 | 70.73 | <0.001 |
| No | 76 | 92.68 | 12 | 29.27 | ||
Perinatal problems include the presence of nuchal cord, meconium aspiration, asphyxia or RH incompatibility.
Postnatal problems include jaundice, seizures, sepsis, pneumonia, necrotizing enterocolitis, or respiratory distress syndrome.
2.3 MRI acquisition
MRI images were obtained using a Siemens Allegra head-only 3T scanner (Siemens Medical System, Inc., Earlangen, Germany) during natural sleep as in Gilmore et al., 2007. Neonatal scans were acquired at an average age of 27 days for SSRI exposed subjects and at 24 days for their matched controls. Scans were acquired at an average age of 27 days for neonates of mothers with a history of depression (untreated during pregnancy) and their matched controls. Children were scanned as close to birth as possible while still providing adequate recovery time for the baby and the mother. Structural T1-weighted images were acquired in 5 individuals in the first cohort (3 SSRI-exposed and 2 unexposed) and 4 individuals in the second cohort (1 neonate with an untreated mother and 3 controls) using a fast low angular shot sequence (FLASH, TR=15ms, TE=7ms, flip angle = 7°, spatial resolution = 1mm ×1 mm ×1 mm). Due to changes in protocol, T1-weighted images of remaining subjects in both cohorts were acquired using a 3D magnetization prepared rapid gradient echo sequence (MP-RAGE TR = 1820ms, TE = 4.38ms, flip angle = 7°, spatial resolution = 1mm × 1mm × 1mm). Proton density and T2 weighted images were acquired using a turbo-spin echo sequence (TSE, TR range = 5270ms-6200ms, TE1 range = 20ms-21ms, TE2 range = 119ms-124ms, flip angle = 150°, spatial resolution = 1.25mm × 1.25mm × 1.5 mm). We confirmed that differences in T1 and T2 acquisition protocols do not significantly impact tissue segmentation results in a sample of 561 unrelated neonates.
Diffusion tensor images were obtained using a single shot echo planar spin echo sequence following two protocols. Under the first protocol, 5 repetitions of 7 diffusion weighted images (in total 35) were generated: 1 without diffusion gradient (b=0) and 6 with b = 1,000 s/mm2 in unique directional diffusion gradients (TR = 5,200ms, TE = 73ms, slice thickness = 2 mm, in-plane resolution = 2mm x 2mm). Under the second protocol, a total of 49 images were acquired, 7 without diffusion gradients (b=0) and 42 with b=1,000 s/mm2 in unique directional diffusion gradients (TR= 7,680ms, TE= 82ms, slice thickness = 2mm, in-plane resolution = 2mm × 2mm). In the first cohort, 36 subjects (12 exposed, 24 unexposed) were scanned under the first protocol and 24 subjects (8 exposed, 16 unexposed) under the second. In the second cohort, 66 subjects (22 of untreated mothers, 44 control) were scanned under the first protocol and 24 subjects (8 of untreated mothers, 16 control) under the second. It is well known that different numbers of unique gradient directions can affect FA, MD, AD, and RD values and this is also true in our neonatal sample. For this reason, cases (SSRI exposed or untreated mothers with a history of depression) with 6 direction scans were always matched to controls with 6 direction scans and cases with 42 direction scans were always matched to controls with 42 direction scans. In addition, DTI direction (6 versus 42) was included as a covariate in the FADTTS model.
2.4 Segmentation and lobar parcellation
Segmentation of brain tissue into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) was performed using an atlas-based expectation-maximization segmentation algorithm with T1 and T2 weighted images as in (Gilmore et al., 2007). Intracranial volume (ICV) represents the sum of GM, WM, and CSF. Left and right hemispheres were subdivided along the anterior-posterior axis to generate prefrontal, frontal, parietal, and occipital regions by nonlinear warping of a neonate parcellation atlas template (Gilmore et al., 2007).
2.5 Deformation based morphometry (DBM)
To identify local differences in gray matter volumes, DBM was performed using T2-weighted images. Images were corrected for intensity inhomogeneity, skull stripped, and aligned using both rigid and affine registration methods. Intensity histogram matching was applied on the affinely aligned images and an unbiased large deformation nonrigid group-wise registration method (Joshi et al., 2004) was used to produce the atlas and corresponding deformation fields.
2.6 Diffusion tensor imaging (DTI) analysis
DTI analysis was performed using a neonate specific adaptation of the UNC-Utah NA-MIC DTI pipeline (Verde et al., 2014). First, automated quality control (QC) was performed using DTIPrep. This included controlling for correct image dimensions and gradient directions, detecting slice-wise intensity change and excessive motion artifacts, and correcting for motion and eddy current effects. Diffusion images with large motion artifacts and missing or corrupted sections were excluded from later analysis. Diffusion tensors were estimated using weighted least squares fitting. Additional expert-guided QC was performed using 3D Slicer. The most common reason for a subject to fail DTI quality control was poor image quality (rather than underlying neuroanatomical differences or abnormalities. In particular, because DTI image acquisition is highly sensitive to motion, many of the images that failed quality control were those with large amounts of head motion artifacts.
Next, non-brain tissue was excluded as described in Verde et al. (2014). Resulting images were mapped into the space of a neonate DTI atlas consisting of the unbiased symmetric diffeomorphic transformed average of 144 neonatal DTI images. In total, 47 fiber tracts of interest are defined on this atlas (Figure 1 and Supplementary Appendix 1). Warped DTI images were visually compared with the atlas to confirm successful registration. Finally, atlas fibers were mapped into individual subject space and profiles of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) were extracted using DTIAtlasFiberAnalyzer. For all individuals, FA, MD, AD, and RD metrics along each tract were plotted against the atlas for visual comparison. FA profiles with correlation values below 0.7 were considered poorly mapped into atlas space and excluded from analysis on a tract-by-tract basis (drop-out rates did not differ between SSRI exposed neonates, neonates with a history of maternal depression, and unexposed controls). Two tracts (temporoparietal segment of the left arcuate and hippocampal segment of the left cingulum) were not examined further as more than 25% of FA fiber profiles did not pass QC. For certain tracts, fiber profiles were not examined in terminal arc lengths due to high levels of noise.
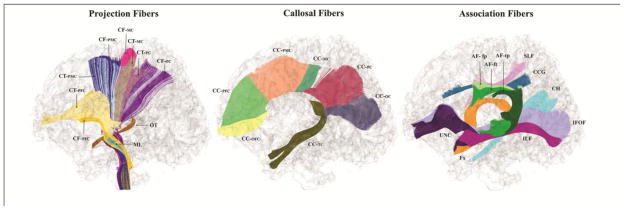
Figure 1.
Sagittal view. 11 projection pathways (bilateral, left hemisphere segments not shown). CF-PFC, corticofugal prefrontal cortex; CF-PMC, corticofugal premotor cortex; CF-MC, corticofugal motor cortex; CF-PC, corticofugal parietal cortex; CT-PFC, corticothalamic prefrontal cortex; CT-PMC, corticothalamic premotor cortex; CT-MC, corticothalamic motor cortex; CT-PC, corticothalamic parietal cortex; OT, optic tract; ML, medial lemniscus. 7 callosal pathways. CC-OFC, corpus callosum: orbitofrontal cortex (rostrum); CC-PFC, corpus callosum: prefrontal cortex (genu); CC-PMC, corpus callosum: premotor cortex; CC-MC, corpus callosum: motor cortex; CC-PC, corpus callosum: parietal cortex; CC-OC, corpus callosum: occipital cortex (splenium); CC-TC, corpus callosum: temporal cortex (tapetum). 9 association pathways (bilateral, right hemisphere segments not shown). UNC, uncinate; AF-fp, Arcuate frontoparietal segment; AF-ft, Arcuate frontotemporal segment; AF-tp, Arcuate temporoparietal segment; SLF, superior longitudinal fasciculus; CCG, cingulum cingulate gyrus segment; CH, cingulum: hippocampal segment; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; Fx, fornix
2.7 Statistical analysis
2.7.1 Demographics and global/lobar brain volumes
Statistical analyses were performed using SAS statistical software, version 9.2. For demographic data, two-sided Fisher’s exact tests were used to evaluate group differences in categorical variables. Two-sided nonparametric Wilcoxon tests were used for continuous variables. Subjects with missing data were excluded on a variable-by-variable basis.
Mixed effects models were used to test for group differences in global and lobar brain tissue volumes in order to adjust for non-independence within twin pairs. Postnatal age at MRI, gestational age at birth, gender, and ICV were included as covariates. All statistical hypothesis tests were two-tailed and conducted at a significance level of 0.003 (Bonferroni corrected for 16 brain volume phenotypes).
2.7.2 DBM
Associations between local gray matter volumes and SSRI-exposure were examined by fitting a multiscale adaptive generalized estimation equation (MAGEE) model to the Jacobian determinant of the deformation matrix at individual voxels (Li et al., 2013). Postnatal age at MRI, gestational age at birth, gender, and ICV were included as covariates. Analysis was restricted to GM and a linear model for the jth subject of the i-th twin pair at the dth voxel was generated.
Cluster-based inference was used to identify significant group differences. Cluster-based inference is based on random field theory, a widely used multiple testing method for determining corrected significances while accounting for the high level of spatial dependencies between adjacent voxels (Worsley et al., 1996). Following the recommendation of (Silver et al., 2011), we used a high cluster-forming threshold (p< 0.001) and cluster extent criterion of p < 0.05. Full Width at Half Maximum (FWHM) was set to 4. Anatomical locations of significant clusters were established using a 90-region neonate atlas (Gilmore et al., 2012).
2.7.3 DTI
Functional analysis of diffusion tensor tract statistics (FADTTS) (Zhu et al., 2011) was used to test for group differences in FA, MD, AD, and RD along 45 major white matter tracts. Postnatal age at MRI, gestational age at birth, gender, ICV, and DTI protocol (6 versus 42 directions) were included as covariates. FADTTS provides a global test statistic and local test statistics along the fiber tract. Local p-values within each tract are corrected for multiple comparisons with false discovery rate. Test statistics and local p-values were merged onto the corresponding fiber locations for visualization. To ensure results were not confounded by prematurity or genetic relatedness within subjects, we performed sensitivity analyses removing infants born under 34 gestational weeks and also removing related subjects for all outcomes. To check whether group differences were affected by DTI protocol, we examined the overlap between tract regions showing a significant effect of SSRI exposure and those showing a significant effect of DTI direction and performed additional sensitivity analyses without DTI protocol as a covariate.
3. Results
3.1 Participants
Demographic and obstetric characteristics did not differ between SSRI-exposed and matched control neonates (Table 1) or between neonates whose mothers had a history of depression and matched controls (Table 2). Mothers using SSRIs had more first-degree relatives with mood disorders suggesting high genetic vulnerability to depression. Similar results were found for untreated mothers with a history of depression also suggesting higher genetic vulnerability to depression compared to control mothers.
In cohort 1, SSRI-exposed neonates differed from matched control neonates in prenatal exposure to corticosteroids, thyroid medications, and benzodiazepines (Supplementary Table 1). In cohort 2, neonates of mothers with a history of depression differed from matched controls in prenatal exposure to antibiotics, antifungals, and sleep aids (Supplementary Table 2).
3.2 Brain volumes
For cohort 1, no significant group differences were observed in global GM, WM, CSF, or for GM and WM in prefrontal, frontal, parietal, and occipital divisions (Table 3). DBM analysis did not reveal any regional GM differences between SSRI-exposed and matched control neonates. The pattern of results was highly similar in sensitivity analyses excluding neonates born before 34 gestational weeks and related subjects (Supplementary Tables 3 and 4), although in the DBM analysis, a new cluster of reduced GM volume was observed in the right thalamus of SSRI-exposed neonates when we excluded children born before 34 weeks (Supplementary Figure 2.
Table 3.
Brain Volumes of SSRI-Exposed and Matched Control Neonates
| Matched Controls (N=52) | SSRI Exposed (N=26) | Test Statistic | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Global Tissue Volume (mm3) | LS Means | SE | LS Means | SE | T | df | P-Value |
| Total GM | 241999 | 1081 | 244421 | 1298 | −1.48 | 72 | 0.144 |
| Total Myelinated WM | 10629 | 504 | 9672 | 829 | 1.01 | 72 | 0.316 |
| Total Unmyelinated WM | 151758 | 1500 | 153704 | 1514 | −0.94 | 72 | 0.349 |
| Total CSF | 56404 | 1035 | 53729 | 1479 | 1.52 | 72 | 0.13 |
| Cortical Hemispheric GM | 201609 | 1150 | 203259 | 1578 | −0.87 | 72 | 0.388 |
| Cortical Hemispheric WM | 153869 | 1178 | 154519 | 1515 | −0.35 | 72 | 0.728 |
| Prefrontal GM | 28226 | 578 | 29130 | 610 | −1.11 | 72 | 0.27 |
| Prefrontal WM | 25148 | 596 | 25253 | 500 | −0.14 | 72 | 0.889 |
| Frontal GM | 44021 | 468 | 43095 | 594 | 1.26 | 72 | 0.211 |
| Frontal WM | 38279 | 391 | 38329 | 532 | −0.08 | 72 | 0.938 |
| Parietal GM | 60293 | 632 | 59962 | 775 | 0.34 | 72 | 0.735 |
| Parietal WM | 48339 | 474 | 47943 | 512 | 0.59 | 72 | 0.559 |
| Occipital GM | 69092 | 693 | 70912 | 949 | −1.59 | 72 | 0.115 |
| Occipital WM | 42153 | 558 | 42901 | 547 | −0.99 | 72 | 0.325 |
| Total Cerebellar | 27739 | 419 | 27179 | 629 | 0.76 | 72 | 0.449 |
WM = white matter, GM = gray matter
For cohort 2, no significant differences (after Bonferroni correction) were observed in global or regional brain volumes between neonates whose mothers had a history of depression and matched controls (Table 4). Global volume results remained similar when controlling for neonates born before 34 gestational weeks and for related subjects (Supplementary Tables 5 and 6). In the DBM analysis, while no clusters were significant when adjusting the gestational age at birth cutoff to 34 weeks, one cluster (in the right calcarine and lingual region) was found to be significantly smaller when removing related subjects (Supplementary Figure 3).
Table 4.
Brain Volumes of Neonates with Mothers Diagnosed with Depression but Untreated during Pregnancy and Matched Control Neonates
| Matched Controls (N = 82) | History of maternal depression, no treatment during pregnancy (N = 41) | Test Statistic | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Global Tissue Volume (mm3) | LS Means | SE | LS Means | SE | T | df | P-Value |
| Total GM | 236703 | 633 | 237911 | 870 | −1.12 | 117 | 0.266 |
| Total Myelinated WM | 10429 | 438 | 10412 | 530 | 0.02 | 117 | 0.98 |
| Total Unmyelinated WM | 158442 | 630 | 159743 | 661 | −1.41 | 117 | 0.162 |
| Total CSF | 60748 | 752 | 59057 | 1102 | 1.26 | 117 | 0.209 |
| Cortical Hemispheric GM | 195597 | 652 | 196952 | 873 | −1.24 | 117 | 0.218 |
| Cortical Hemispheric WM | 149447 | 594 | 151478 | 712 | −2.17 | 117 | 0.032 |
| Prefrontal GM | 26932 | 339 | 28016 | 550 | −1.68 | 117 | 0.096 |
| Prefrontal WM | 24083 | 318 | 25233 | 359 | −2.4 | 117 | 0.018 |
| Frontal GM | 42833 | 305 | 42510 | 475 | 0.57 | 117 | 0.568 |
| Frontal WM | 37781 | 216 | 37640 | 449 | 0.28 | 117 | 0.778 |
| Parietal GM | 59065 | 362 | 58609 | 560 | 0.68 | 117 | 0.495 |
| Parietal WM | 47201 | 335 | 47265 | 566 | −0.1 | 117 | 0.923 |
| Occipital GM | 66808 | 480 | 67734 | 800 | −0.99 | 117 | 0.324 |
| Occipital WM | 40359 | 289 | 40887 | 408 | −1.05 | 117 | 0.295 |
| Total Cerebellar | 23523 | 300 | 23444 | 360 | 0.17 | 117 | 0.866 |
WM = white matter, GM = gray matter
3.3 White matter microstructure
3.3.1 SSRI-exposure
Global test statistics revealed widespread differences between SSRI-exposed versus matched control neonates (uncorrected p-values presented in Table 5, findings surviving Bonferroni correction for 45 tracts (p < 0.001) shaded in gray). Strongest associations were observed for MD and RD in corticofugal and corticothalamic projection tracts. To better understand these associations, local statistics were computed along each tract. In general, FA values were decreased while MD, RD, and AD values were increased in SSRI-exposed compared to matched controls. The overall pattern of results remained similar when removing neonates born prior to 34 gestational weeks, when removing neonates with genetic relatedness (see Supplementary Table 7), or when removing DTI direction as a covariate (see Supplementary Table 8). Regions of localized significance along the length of white matter fibers are plotted and visualized in 3D (Figure 2, 3, and Supplementary Figure 4).
Table 5.
Global Significance of SSRI Exposure on Major White Matter Pathways in Neonates.
| Tract | N | N | FA | MD | RD | AD |
|---|---|---|---|---|---|---|
| Case | Control | |||||
| Corticofugal | ||||||
| Left Prefrontal | 20 | 40 | 0.294 | < 0.001 | 0.002 | 0.017 |
| Right Prefrontal | 20 | 40 | 0.156 | < 0.001 | 0.004 | 0.065 |
| Left Premotor | 20 | 40 | 0.422 | 0.007 | 0.013 | 0.072 |
| Right Premotor | 20 | 40 | 0.164 | < 0.001 | 0.002 | 0.181 |
| Left Motor | 20 | 40 | 0.48 | 0.009 | 0.025 | 0.048 |
| Right Motor | 20 | 40 | 0.534 | 0.002 | 0.018 | 0.021 |
| Left Parietal | 20 | 40 | 0.389 | 0.004 | 0.014 | 0.071 |
| Right Parietal | 20 | 40 | 0.501 | 0.022 | 0.038 | 0.227 |
| Corticothalamic | ||||||
| Left Prefrontal | 20 | 40 | 0.123 | 0.002 | 0.014 | 0.005 |
| Right Prefrontal | 20 | 40 | 0.435 | 0.014 | 0.027 | 0.1 |
| Left Premotor | 20 | 40 | 0.369 | 0.004 | 0.003 | 0.096 |
| Right Premotor | 20 | 40 | 0.093 | < 0.001 | < 0.001 | 0.024 |
| Left Motor | 20 | 40 | 0.122 | 0.004 | 0.004 | 0.023 |
| Right Motor | 20 | 40 | 0.412 | 0.027 | 0.052 | 0.035 |
| Left Parietal | 20 | 40 | 0.072 | 0.006 | 0.005 | 0.078 |
| Right Parietal | 20 | 40 | 0.417 | 0.023 | 0.051 | 0.066 |
| Medial Lemniscus | ||||||
| Left | 20 | 40 | 0.0697 | 0.257 | 0.468 | 0.347 |
| Right | 19 | 38 | 0.447 | 0.227 | 0.362 | 0.159 |
| Optic Tract | ||||||
| Left | 20 | 38 | 0.211 | 0.008 | 0.034 | 0.01 |
| Right | 20 | 38 | 0.873 | < 0.001 | < 0.001 | 0.011 |
| Corpus Callosum | ||||||
| Orbitofrontal | 20 | 40 | 0.046 | 0.058 | 0.035 | 0.137 |
| Prefrontal | 20 | 40 | 0.285 | 0.157 | 0.171 | 0.119 |
| Premotor | 20 | 40 | 0.495 | 0.465 | 0.531 | 0.222 |
| Motor | 20 | 40 | 0.511 | 0.418 | 0.409 | 0.25 |
| Parietal | 20 | 40 | 0.855 | 0.328 | 0.382 | 0.296 |
| Occipital | 20 | 40 | 0.902 | 0.516 | 0.63 | 0.429 |
| Temporal | 20 | 40 | 0.069 | 0.11 | 0.137 | 0.068 |
| Arcuate fasciculus | ||||||
| Left Frontoparietal | 18 | 39 | 0.952 | 0.33 | 0.369 | 0.419 |
| Left Frontotemporal | 19 | 39 | 0.491 | 0.252 | 0.177 | 0.399 |
| Right Frontoparietal | 15 | 36 | 0.73 | 0.402 | 0.567 | 0.219 |
| Right Frontotemporal | 19 | 40 | 0.489 | 0.356 | 0.288 | 0.661 |
| Right Temporoparietal | 17 | 40 | 0.776 | 0.119 | 0.159 | 0.139 |
| Cingulum | ||||||
| Left Cingulate Gyrus | 19 | 40 | 0.029 | 0.134 | 0.056 | 0.475 |
| Right Cingulate Gyrus | 18 | 37 | 0.359 | 0.009 | 0.028 | 0.046 |
| Right Hippocampal | 12 | 39 | 0.512 | 0.077 | 0.059 | 0.36 |
| Fornix | ||||||
| Left | 20 | 40 | 0.529 | 0.151 | 0.242 | 0.067 |
| Right | 19 | 37 | 0.349 | 0.424 | 0.389 | 0.37 |
| Inferior Fronto-occipital fasciculus (IFOF) | ||||||
| Left | 20 | 40 | 0.397 | 0.055 | 0.085 | 0.056 |
| Right | 20 | 40 | 0.933 | 0.032 | 0.048 | 0.06 |
| Inferior Longitudinal Fasciculus (ILF) | ||||||
| Left | 20 | 40 | 0.969 | 0.611 | 0.595 | 0.65 |
| Right | 20 | 40 | 0.163 | 0.366 | 0.272 | 0.39 |
| Superior longitudinal fasciculus (SLF) | ||||||
| Left | 14 | 38 | 0.733 | 0.868 | 0.885 | 0.69 |
| Right | 12 | 36 | 0.033 | 0.136 | 0.05 | 0.452 |
| Uncinate Fasciculus | ||||||
| Left | 20 | 40 | 0.076 | 0.024 | 0.017 | 0.045 |
| Right | 20 | 40 | 0.1 | 0.012 | 0.021 | 0.056 |
Bold/italicized text indicates p-values below 0.05 (unadjusted for multiple comparisons), gray shading indicates p-values surviving Bonferroni correction (0.05/45 = 0.001)
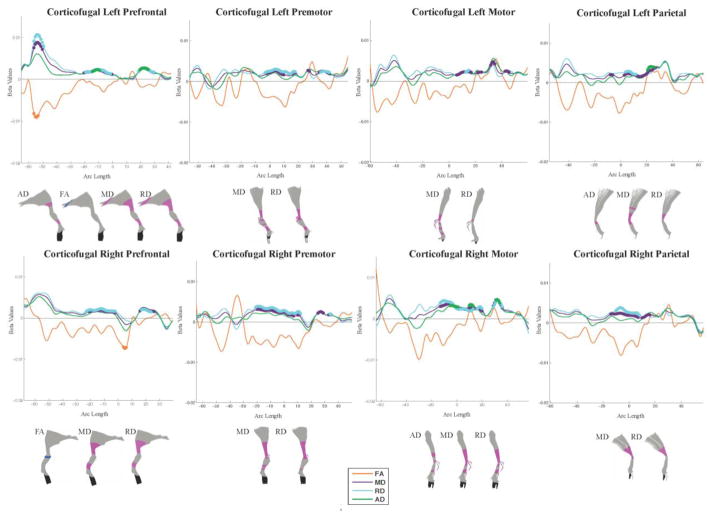
Figure 2.
Local differences in anisotropy and diffusivity along the corticofugal tracts. Beta plots show diffusion parameter values along the arc length of each fiber. Positive beta values correspond to higher diffusion or anisotropy in SSRI-exposed neonates and negative beta values correspond to lower diffusion or anisotropy in SSRI-exposed neonates. Colored circles indicate regions of significance post FDR correction. Significant points are also visualized on the fiber itself for each parameter. Magenta indicates significant positive beta values. Yellow indicates significant negative beta values. Black regions were not analyzed due to increased subject variability.
Figure 3.
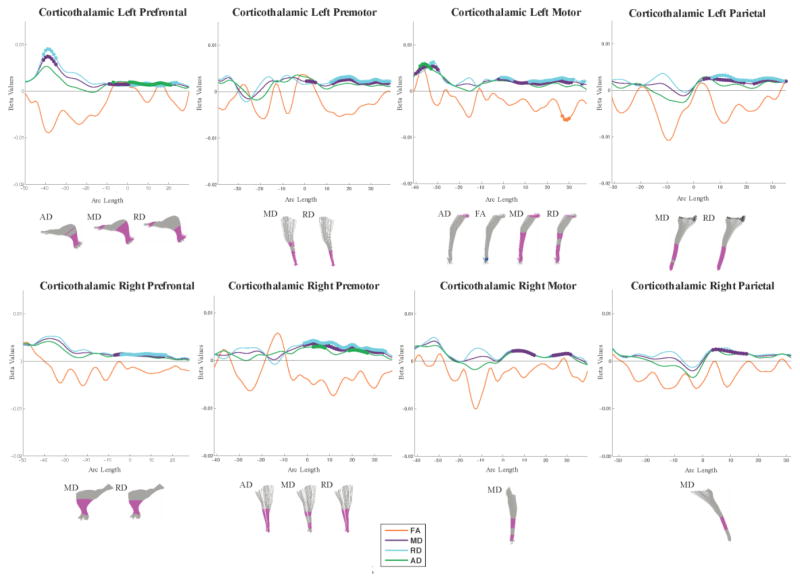
Local differences in anisotropy and diffusivity along the corticothalamic tracts. Beta plots show diffusion parameter values along the arc length of each fiber. Positive beta values correspond to higher diffusion or anisotropy in SSRI-exposed neonates and negative beta values correspond to lower diffusion or anisotropy in SSRI-exposed neonates. Colored circles indicate regions of significance post FDR correction. Significant points are also visualized on the fiber itself for each parameter. Magenta indicates significant positive beta values. Yellow indicates significant negative beta values. Black regions were not analyzed due to increased subject variability.
Regarding projection fibers, all corticofugal and corticothalamic tracts showed regions of increased MD and RD in SSRI-exposed as compared to matched controls, with the exception of the right motor and parietal corticothalamic tracts which only showed increased MD. Increases in AD were observed in fewer tracts, namely the left prefrontal, left parietal and right motor corticofugal tracts and the left motor, left prefrontal, and right premotor corticothalamic tracts. Significant differences were observed in regions passing through the cortex, internal capsule, thalamus, and brain stem. The optic tracts showed a pattern of increased MD and RD in the right and decreased FA and AD in the left segment. Regions of lower FA were also observed in the projection fibers of SSRI-exposed neonates, albeit less consistently. No significant group differences were observed along the left or right medial lemniscus.
Regarding commissural fibers, higher MD was observed in a small region of the temporal segment (tapetum) of the CC. Otherwise, no differences were observed along the orbitofrontal, motor, prefrontal (genu), premotor, parietal or occipital (splenium) segments.
Regarding association fibers, regions of increased MD and RD were observed in the uncinate fasciculus of SSRI-exposed neonates. Several other tracts connecting frontal and temporal cortex showed focal areas of increased diffisivity including increased AD and MD in the left fornix and increased MD and RD in the right temporoparietal segment of the arcuate fasciculus (AF). Higher MD and RD values were also observed along the right inferior fronto-occipital fasciculus while higher AD was observed along the left. Within the cingulum, decreased FA was observed in the left cingulate gyrus segment and higher MD and RD values were observed along the right cingulate gyrus segment. Higher RD values were also observed along the hippocampal segment of the cingulum. Finally, higher RD values were observed in a small region of the right frontoparietal segment of the AF and decreased FA values were observed in the right inferior longitudinal fasciculus (ILF). No significant differences were observed along the left and right frontotemporal segment and the left frontoparietal segment of the AF, the left and right superior longitudinal fasciculi, the right fornix, or the left ILF.
3.3.2 Neonates of mothers with a history of depression
Global test statistics revealed no differences between neonates of mothers diagnosed with depression but untreated during pregnancy and their matched controls (uncorrected p-values presented in Table 6, no findings surviving Bonferroni correction). The overall pattern of results remained nonsignificant when removing neonates born prior to 34 gestational weeks and when removing neonates with genetic relatedness (see Supplementary Table 9).
Table 6.
Global Significance of Major White Matter Pathways in Neonates with Mothers Diagnosed with Depression but Untreated during Pregnancy and Matched Control Neonates
| Tract | N | N | FA | MD | RD | AD |
|---|---|---|---|---|---|---|
|
| ||||||
| Case | Control | |||||
| Corticofugal | ||||||
| Left Prefrontal | 41 | 82 | 0.473 | 0.609 | 0.595 | 0.507 |
| Right Prefrontal | 41 | 82 | 0.252 | 0.257 | 0.262 | 0.131 |
| Left Premotor | 41 | 82 | 0.105 | 0.556 | 0.38 | 0.366 |
| Right Premotor | 41 | 82 | 0.122 | 0.509 | 0.295 | 0.421 |
| Left Motor | 41 | 82 | 0.076 | 0.753 | 0.589 | 0.218 |
| Right Motor | 41 | 82 | 0.068 | 0.224 | 0.325 | 0.008 |
| Left Parietal | 41 | 82 | 0.063 | 0.854 | 0.975 | 0.353 |
| Right Parietal | 41 | 82 | 0.171 | 0.456 | 0.856 | 0.093 |
| Corticothalamic | ||||||
| Left Prefrontal | 41 | 82 | 0.752 | 0.918 | 0.919 | 0.835 |
| Right Prefrontal | 41 | 82 | 0.384 | 0.823 | 0.764 | 0.43 |
| Left Premotor | 41 | 82 | 0.125 | 0.456 | 0.439 | 0.152 |
| Right Premotor | 41 | 82 | 0.229 | 0.366 | 0.271 | 0.381 |
| Left Motor | 41 | 82 | 0.187 | 0.76 | 0.657 | 0.289 |
| Right Motor | 41 | 82 | 0.238 | 0.679 | 0.483 | 0.748 |
| Left Parietal | 41 | 82 | 0.257 | 0.789 | 0.484 | 0.776 |
| Right Parietal | 41 | 82 | 0.173 | 0.754 | 0.685 | 0.471 |
| Medial Lemniscus | ||||||
| Left | 41 | 82 | 0.114 | 0.764 | 0.522 | 0.782 |
| Right | 41 | 79 | 0.397 | 0.281 | 0.301 | 0.246 |
| Optic Tract | ||||||
| Left | 41 | 80 | 0.486 | 0.407 | 0.504 | 0.432 |
| Right | 41 | 81 | 0.908 | 0.878 | 0.935 | 0.703 |
| Corpus Callosum | ||||||
| Orbitofrontal | 41 | 82 | 0.452 | 0.753 | 0.775 | 0.55 |
| Prefrontal | 41 | 82 | 0.968 | 0.922 | 0.939 | 0.895 |
| Premotor | 41 | 82 | 0.546 | 0.254 | 0.284 | 0.345 |
| Motor | 41 | 82 | 0.431 | 0.605 | 0.478 | 0.849 |
| Parietal | 39 | 82 | 0.111 | 0.536 | 0.33 | 0.897 |
| Occipital | 41 | 82 | 0.374 | 0.454 | 0.527 | 0.195 |
| Temporal | 41 | 82 | 0.29 | 0.41 | 0.72 | 0.09 |
| Arcuate fasciculus | ||||||
| Left Frontoparietal | 38 | 76 | 0.805 | 0.679 | 0.695 | 0.617 |
| Left Frontotemporal | 40 | 79 | 0.76 | 0.624 | 0.665 | 0.519 |
| Right Frontoparietal | 30 | 69 | 0.668 | 0.758 | 0.88 | 0.538 |
| Right Frontotemporal | 40 | 80 | 0.798 | 0.542 | 0.701 | 0.306 |
| Right Temporoparietal | 41 | 80 | 0.161 | 0.569 | 0.857 | 0.199 |
| Cingulum | ||||||
| Left Cingulate Gyrus | 41 | 82 | 0.449 | 0.758 | 0.536 | 0.896 |
| Right Cingulate Gyrus | 40 | 78 | 0.403 | 0.466 | 0.496 | 0.292 |
| Right Hippocampal | 36 | 68 | 0.801 | 0.935 | 0.927 | 0.908 |
| Fornix | ||||||
| Left | 41 | 82 | 0.024 | 0.534 | 0.699 | 0.219 |
| Right | 37 | 75 | 0.401 | 0.387 | 0.446 | 0.234 |
| Inferior Fronto-occipital fasciculus (IFOF) | ||||||
| Left | 41 | 82 | 0.865 | 0.837 | 0.791 | 0.935 |
| Right | 41 | 82 | 0.842 | 0.892 | 0.94 | 0.663 |
| Inferior Longitudinal Fasciculus (ILF) | ||||||
| Left | 41 | 82 | 0.669 | 0.461 | 0.433 | 0.544 |
| Right | 41 | 81 | 0.49 | 0.849 | 0.921 | 0.478 |
| Superior longitudinal fasciculus (SLF) | ||||||
| Left | 41 | 75 | 0.718 | 0.741 | 0.671 | 0.779 |
| Right | 41 | 72 | 0.879 | 0.978 | 0.995 | 0.801 |
| Uncinate Fasciculus | ||||||
| Left | 41 | 82 | 0.718 | 0.556 | 0.76 | 0.301 |
| Right | 41 | 82 | 0.382 | 0.544 | 0.58 | 0.549 |
Bold/italicized text indicates p-values below 0.05 (unadjusted for multiple comparisons), no values survived Bonferroni correction (0.05/45 = 0.001)
3.3.3 Effects of DTI direction
Examination of local test statistics revealed some overlap between tract regions showing a significant effect of SSRI exposure and those showing a significant effect of DTI direction (results for the corticofugal and corticothalamic tracts are shown in Supplementary Figures 5 and 6), confirming the importance of matching SSRI exposed subjects with control subjects based on DTI direction. There are also numerous regions where there is a significant effect of SSRI exposure and no effect of DTI direction.
4. Discussion
We report results from the first quantitative neuroimaging study of brain development following prenatal SSRI exposure. While no group differences in global or regional brain volumes were found, SSRI-exposed neonates exhibited lower FA and increased MD, RD, and AD across multiple fiber bundles. Differences were most pronounced for MD and RD in the corticothalamic and corticofugal tracts. In contrast, children of mothers who received a diagnosis of depression prior to or during pregnancy but did not use SSRIs exhibited no differences in white microstructure compared to their matched controls. The data support one of two interpretations: SSRIs alter white matter development, or other factors which differentiate women treated with SSRIs from untreated women with a history of depression impact white matter development. Factors that may be of particular importance are genetic risk for depression and severity of depressive symptoms during pregnancy.
Regarding genetic risk, the percentage of children whose mothers reported a family history of MDD/BPD was higher in the history of depression cohort compared to the SSRI exposed cohort, but widespread changes in DTI parameters were only observed in the SSRI exposed cohort. In addition, a DTI study of healthy adolescents with familial high-risk for depression revealed reduced FA in the left cingulum, the splenium of the CC, and superior longitudinal, uncinate, and inferior fronto-occipital fasciculi (Huang et al., 2011), a pattern of results which does not match the pattern observed in our SSRI-exposed sample. This suggests that altered DTI parameters in the SSRI exposed cohort are not simply the result of heightened genetic risk for depression. Genes predisposing to depression may impact postnatal white development (e.g. myelination and axonal pruning) leading to the pattern of changes observed in the Huang et al. study, but these differences are not evident in neonates.
Regarding severity of depressive symptoms, the parent studies did not collect direct measures of symptom severity during pregnancy. In addition, medical records did not provide sufficient information to determine how many mothers reporting non-active depression at study entry developed symptoms after the initial interview. Since SSRI treatment during pregnancy does not guarantee remission of depression (Hanley and Oberlander, 2014), we cannot distinguish between the possibility that SSRIs directly impact white matter development and the possibility that this relationship is mediated by severity of depressive symptoms during pregnancy. We note that maternal depressive symptoms during pregnancy are associated with cortical thinning in preschool and school-age children (Sandman et al., 2015) and higher postnatal maternal depressive symptoms are associated with lower white matter diffusivity in frontal regions in children age 2–5 (Boukhris et al., 2016).
Regarding mechanisms, active depressive symptoms during pregnancy could impact white matter development via poor nutrition and neuroendocrine alterations, including disruption of the maternal and infant HPA axis (Waters et al., 2014). Macronutrients and vitamins such as iron and choline are key contributors to myelin composition and production. Neuroendocrine factors, including cortisol, modulate oligodendrocyte differentiation and myelogenesis (Howell et al., 2013; Monk et al., 2013). Cortisol levels in infancy are also associated with altered white matter microstructure in adolescent monkeys exposed to early life stress (Howell et al., 2013).
Regarding the possibility of a direct, causal relationship between SSRI exposure and altered white matter development, the increases in MD and RD we observed in the parietal corticothalamic tracts are in keeping with the rodent literature which indicates that prenatal SSRI exposure disrupts formation of thalamocortical afferents to the somatosensory cortex (Borue et al., 2007). However, white matter abnormalities were not limited to corticothalamic tracts in the present study. This is not entirely surprising as the serotonin transporter (5HTT) is expressed broadly during development (Narboux-Neme et al., 2008). Furthermore, in vitro studies suggest that elevated extracellular 5-HT levels adversely affect the survival and development of oligodendrocytes (Fan et al., 2015) while early life exposure to SSRIs produces ultrastructural abnormalities of white matter oligodendrocytes and myelin sheaths in corpus callosum (Simpson et al., 2011) and downregulation of myelination-related genes in the hippocampus of adult rats,(Kroeze et al., 2015), suggesting SSRIs alter key myelination processes in diverse brain regions.
We do note that women who elect to stay on medication during pregnancy often constitute a more severely affected group of women than those that discontinue use during pregnancy. As our first cohort took SSRIs in all 3 trimesters, it is possible their offspring experienced a ‘triple hit’ consisting of exposure to pharmacotherapy, exposure to maternal depression, and high genetic vulnerability to depression. Results may not generalize to offspring of women with shorter duration of SSRI exposure. In addition, the current sample size is not sufficient to assess how the changes we observed vary based on medication dose or differences in the type of SSRI prescribed.
Interpreting changes in DTI parameters is complex. White matter development can be divided into 3 stages thought to be reflected by distinct changes in FA, MD, RD, and AD. During stage 1, fibers are organized into fascicles, often increasing FA without impacting MD (Yoshida et al., 2013). During stage 2, there is proliferation and maturation of glial cells that is generally captured by changes in MD. During stage 3, which largely occurs postnatally, there is premyelination and myelination, largely indexed by RD (Feldman et al., 2010). Increased MD observed in SSRI-exposed neonates suggests disrupted glial proliferation and maturation. Increases in RD likely reflect disrupted premyelination and perhaps myelination in early maturing areas such as the brainstem. Complementary studies using mcDESPOT, CHARMED or NODDI (Deoni et al., 2012; Kunz et al., 2014) would allow a better understanding of this cellular microstructure. In addition, follow-up studies are necessary to determine whether observed differences reflect a maturational delay or persistent alterations with functional consequences.
In conclusion, neonatal offspring of depressed mothers treated with SSRIs during pregnancy exhibit widespread changes in white matter microstructure, but no differences in brain volumes compared to unexposed offspring of women with no history of mood disorder. Offspring of untreated depressed mothers show no differences in gray matter volumes or white matter microstructure compared to matched controls. Strengths of the current study include detailed exposure data reflecting both maternal report and medical record review, comprehensive analysis of brain structure and connectivity, and use of propensity matching to minimize differences between exposed and unexposed infants. Limitations include small sample size, heterogeneity of SSRIs and dosage, and incomplete measures of symptom severity. The widespread white matter abnormalities observed in SSRI-exposed children likely reflect a complex interplay of factors including SSRIs, maternal depression, and genetic risk. Future research should focus on disentangling these possibilities.
Supplementary Material
Highlights.
13% of pregnant women report antidepressant use.
Effects of antidepressants on the fetal brain are not fully understood.
We used MRI to study brain structure in neonates exposed to SSRIs during pregnancy.
Neonates exposed to SSRIs in pregnancy exhibit minimal differences in brain volumes.
They exhibit widespread changes in white matter microstructure.
Acknowledgments
This work was supported by the National Institutes of Health (MH064065 and MH070890 to Dr. Gilmore, MH083045 to Dr. Knickmeyer, MH085165 to Dr. Meltzer-Brody, SES-1357666, DMS-1407655, 1UL1TR001111, and MH086633 to Dr. Zhu, HD-003110 and EB005149 to Dr. Styner, and NS007431 to Ms. Jha) and the Foundation of Hope for the Research and Treatment of Mental Illness. The sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. We thank our participating families and staff of the UNC MRI Research Center, the UNC Neuro Image Research and Analysis Laboratories, and the UNC Early Brain Development Program. A special thanks also to Joseph Blocher, Dianne Evans, Jenny Prater, Wendy Neuheimer, Jessica Bullins, and Jean Noel.
Footnotes
Author Contributions
Shaili C. Jha: Made substantial contributions to the analysis and interpretation of data for the work and drafted the manuscript.
Samantha Meltzer-Brody: Made substantial contributions to the conception and design of the work, as well as the interpretation of data, and revised the manuscript for important intellectual content.
Rachel J. Steiner: Made substantial contributions to the analysis of data for the work and revised the manuscript for important intellectual content.
Sandra Woolson: Made substantial contributions to the analysis of data for the work and revised the manuscript for important intellectual content.
Emil Cornea: Made substantial contributions to the analysis of data for the work and revised the manuscript for important intellectual content.
Mihye Ahn: Made substantial contributions to the analysis of data for the work and revised the manuscript for important intellectual content.
Audrey R. Verde: Made substantial contributions to the analysis of data for the work and revised the manuscript for important intellectual content.
Robert M. Hamer: Made substantial contributions to the conception and design of the work, as well as analysis of the data, and revised the manuscript for important intellectual content.
Hongtu Zhu: Made substantial contributions to the analysis of data for the work and revised the manuscript for important intellectual content.
Martin Styner: Made substantial contributions to the analysis of data for the work and revised the manuscript for important intellectual content.
John H. Gilmore: Made substantial contributions to the conception and design of the work, as well as the interpretation of data, and revised the manuscript for important intellectual content.
Rebecca C. Knickmeyer: Had primary responsibility for the conception and design of the work. Made substantial contributions to analysis and interpretation of data and revised the manuscript for important intellectual content.
Financial Disclosures
Regarding financial disclosures, Dr. Knickmeyer is a collaborator on an investigator-initiated grant from Pfizer. Dr. Meltzer-Brody has received research grant funding from Astra Zeneca (investigator initiated study). Dr. Hamer has served on an advisory board, consulted on a clinical trial, or served on a DSMB, for Abbott, Allergan, Alkermes, Cenerx, Columbia University, Endo, Lilly, Novartis, Pfizer, Roche, Wyeth, and has served as an expert witness in cases involving Forest, Lundbeck, Sun, Caraco, Teva, Barr, Mylan, Eurand, Cephalon, Anesta, and Marial. Ms. Woolson has received compensation for professional services from CeNeRx and Janssen Pharmaceuticals. Ms. Jha, Ms. Steiner, Dr. Ahn, Dr. Verde, Dr. Zhu, Dr. Styner, and Dr. Gilmore report no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borue X, Chen J, Condron BG. Developmental effects of SSRIs: lessons learned from animal studies. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2007;25:341–347. doi: 10.1016/j.ijdevneu.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhris T, Sheehy O, Mottron L, Berard A. Antidepressant Use During Pregnancy and the Risk of Autism Spectrum Disorder in Children. JAMA pediatrics. 2016;170:117–124. doi: 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
- Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. The Journal of pediatrics. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Casper RC, Gilles AA, Fleisher BE, Baran J, Enns G, Lazzeroni LC. Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacology. 2011;217:211–219. doi: 10.1007/s00213-011-2270-z. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. American journal of obstetrics and gynecology. 2007;196:544, e541–545. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Archives of general psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- Deoni SC, Dean DC, 3rd, O'Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63:1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Bhatt A, Tien LT, Zheng B, Simpson KL, Lin RC, Cai Z, Kumar P, Pang Y. Exposure to serotonin adversely affects oligodendrocyte development and myelination in vitro. Journal of neurochemistry. 2015;133:532–543. doi: 10.1111/jnc.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Yeatman JD, Lee ES, Barde LH, Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. Journal of developmental and behavioral pediatrics : JDBP. 2010;31:346–356. doi: 10.1097/DBP.0b013e3181dcaa8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Schmitt JE, Knickmeyer RC, Smith JK, Lin W, Styner M, Gerig G, Neale MC. Genetic and environmental contributions to neonatal brain structure: A twin study. Human brain mapping. 2010;31:1174–1182. doi: 10.1002/hbm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Zhu H, Hamer RM, Styner M, Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral cortex. 2012;22:2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover ME, Pugh PC, Jackson NL, Cohen JL, Fant AD, Akil H, Clinton SM. Early-life exposure to the SSRI paroxetine exacerbates depression-like behavior in anxiety/depression-prone rats. Neuroscience. 2015;284:775–797. doi: 10.1016/j.neuroscience.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley GE, Brain U, Oberlander TF. Infant developmental outcomes following prenatal exposure to antidepressants, and maternal depressed mood and positive affect. Early human development. 2013;89:519–524. doi: 10.1016/j.earlhumdev.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Hanley GE, Oberlander TF. The effect of perinatal exposures on the infant: antidepressants and depression. Best practice & research. Clinical obstetrics & gynaecology. 2014;28:37–48. doi: 10.1016/j.bpobgyn.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics. 2014;133:e1241–1248. doi: 10.1542/peds.2013-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, McCormack KM, Grand AP, Sawyer NT, Zhang X, Maestripieri D, Hu X, Sanchez MM. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biol Mood Anxiety Disord. 2013;3:21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fan X, Williamson DE, Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. The New England journal of medicine. 2013;369:2406–2415. doi: 10.1056/NEJMoa1301449. [DOI] [PubMed] [Google Scholar]
- Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. NeuroImage. 2004;23(Suppl 1):S151–160. doi: 10.1016/j.neuroimage.2004.07.068. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Meltzer-Brody S, Woolson S, Hamer RM, Smith JK, Lury K, Gilmore JH. Rate of Chiari I Malformation in Children of Mothers with Depression with and without Prenatal SSRI Exposure. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze Y, Peeters D, Boulle F, van den Hove DL, van Bokhoven H, Zhou H, Homberg JR. Long-term consequences of chronic fluoxetine exposure on the expression of myelination-related genes in the rat hippocampus. Translational psychiatry. 2015;5:e642. doi: 10.1038/tp.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz N, Zhang H, Vasung L, O'Brien KR, Assaf Y, Lazeyras F, Alexander DC, Huppi PS. Assessing white matter microstructure of the newborn with multi-shell diffusion MRI and biophysical compartment models. NeuroImage. 2014;96:288–299. doi: 10.1016/j.neuroimage.2014.03.057. [DOI] [PubMed] [Google Scholar]
- Laine K, Heikkinen T, Ekblad U, Kero P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Archives of general psychiatry. 2003;60:720–726. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- Lee LJ. Neonatal fluoxetine exposure affects the neuronal structure in the somatosensory cortex and somatosensory-related behaviors in adolescent rats. Neurotoxicity research. 2009;15:212–223. doi: 10.1007/s12640-009-9022-4. [DOI] [PubMed] [Google Scholar]
- Li Y, Gilmore JH, Shen D, Styner M, Lin W, Zhu H. Multiscale adaptive generalized estimating equations for longitudinal neuroimaging data. NeuroImage. 2013;72:91–105. doi: 10.1016/j.neuroimage.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CC, Lee LJ. Neonatal fluoxetine exposure affects the action potential properties and dendritic development in cortical subplate neurons of rats. Toxicology letters. 2011;207:314–321. doi: 10.1016/j.toxlet.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Monk C, Georgieff MK, Osterholm EA. Research review: maternal prenatal distress and poor nutrition - mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry. 2013;54:115–130. doi: 10.1111/jcpp.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narboux-Neme N, Pavone LM, Avallone L, Zhuang X, Gaspar P. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs) Neuropharmacology. 2008;55:994–1005. doi: 10.1016/j.neuropharm.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Nulman I, Koren G, Rovet J, Barrera M, Pulver A, Streiner D, Feldman B. Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. The American journal of psychiatry. 2012;169:1165–1174. doi: 10.1176/appi.ajp.2012.11111721. [DOI] [PubMed] [Google Scholar]
- Pedersen LH, Henriksen TB, Olsen J. Fetal exposure to antidepressants and normal milestone development at 6 and 19 months of age. Pediatrics. 2010;125:e600–608. doi: 10.1542/peds.2008-3655. [DOI] [PubMed] [Google Scholar]
- Rampono J, Simmer K, Ilett KF, Hackett LP, Doherty DA, Elliot R, Kok CH, Coenen A, Forman T. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry. 2009;42:95–100. doi: 10.1055/s-0028-1103296. [DOI] [PubMed] [Google Scholar]
- Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM. Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depression and anxiety. 2011;28:1008–1019. doi: 10.1002/da.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, Davis EP. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry. 2015;77:324–334. doi: 10.1016/j.biopsych.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M, Montana G, Nichols TE Alzheimer's Disease Neuroimaging, I. False positives in neuroimaging genetics using voxel-based morphometry data. NeuroImage. 2011;54:992–1000. doi: 10.1016/j.neuroimage.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Perinatal antidepressant exposure alters cortical network function in rodents. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MV, Sung A, Shah B, Mayes L, Klein DS, Yonkers KA. Neurobehavioral assessment of infants born at term and in utero exposure to serotonin reuptake inhibitors. Early human development. 2013;89:81–86. doi: 10.1016/j.earlhumdev.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez JC, Goeden N, Bonnin A. Placental serotonin: implications for the developmental effects of SSRIs and maternal depression. Frontiers in cellular neuroscience. 2013;7:47. doi: 10.3389/fncel.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde AR, Budin F, Berger JB, Gupta A, Farzinfar M, Kaiser A, Ahn M, Johnson H, Matsui J, Hazlett HC, Sharma A, Goodlett C, Shi Y, Gouttard S, Vachet C, Piven J, Zhu H, Gerig G, Styner M. UNC-Utah NA-MIC framework for DTI fiber tract analysis. Front Neuroinform. 2014;7:51. doi: 10.3389/fninf.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CS, Hay DF, Simmonds JR, van Goozen SH. Antenatal depression and children's developmental outcomes: potential mechanisms and treatment options. Eur Child Adolesc Psychiatry. 2014;23:957–971. doi: 10.1007/s00787-014-0582-3. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Worsley K, Marrett S, Neelin P, Vandal AC, Friston K, Evans AC. A unified statistical approach for determining significant voxels in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sari Y, Zhou FC. Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain research. Developmental brain research. 2004;150:151–161. doi: 10.1016/j.devbrainres.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31:403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Oishi K, Faria AV, Mori S. Diffusion tensor imaging of normal brain development. Pediatric radiology. 2013;43:15–27. doi: 10.1007/s00247-012-2496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Kong L, Li R, Styner M, Gerig G, Lin W, Gilmore JH. FADTTS: functional analysis of diffusion tensor tract statistics. Neuroimage. 2011;56:1412–1425. doi: 10.1016/j.neuroimage.2011.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.