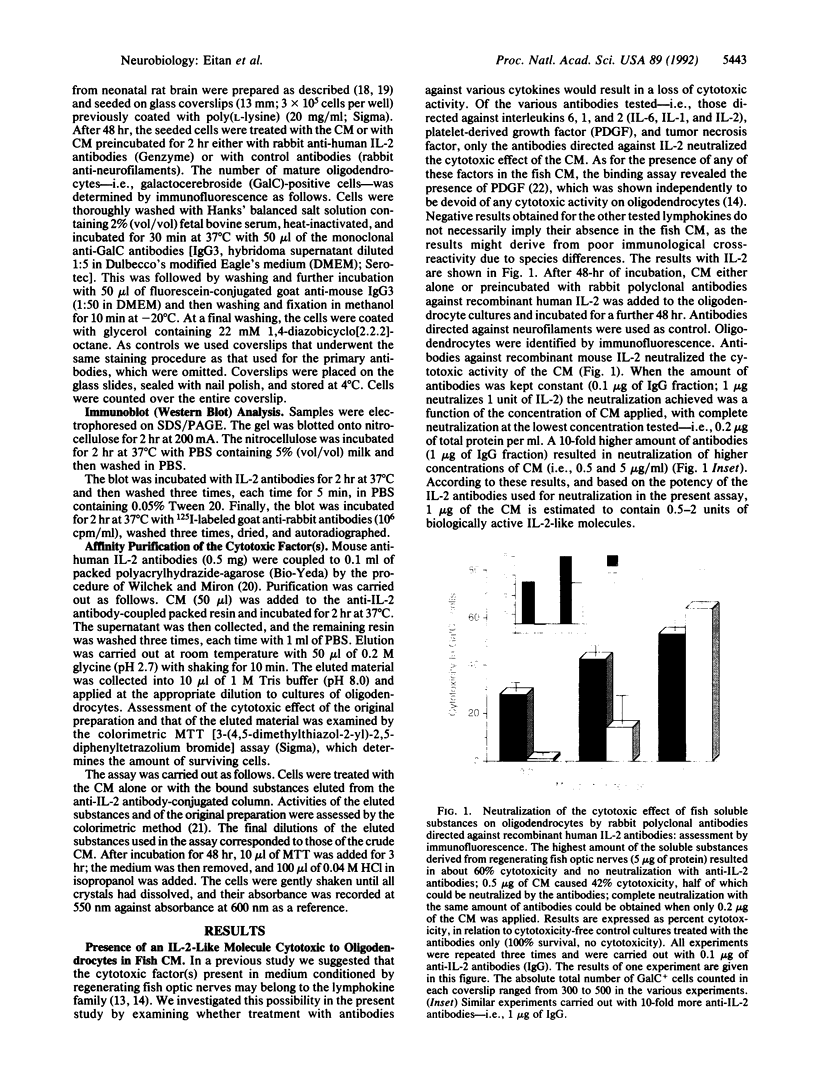
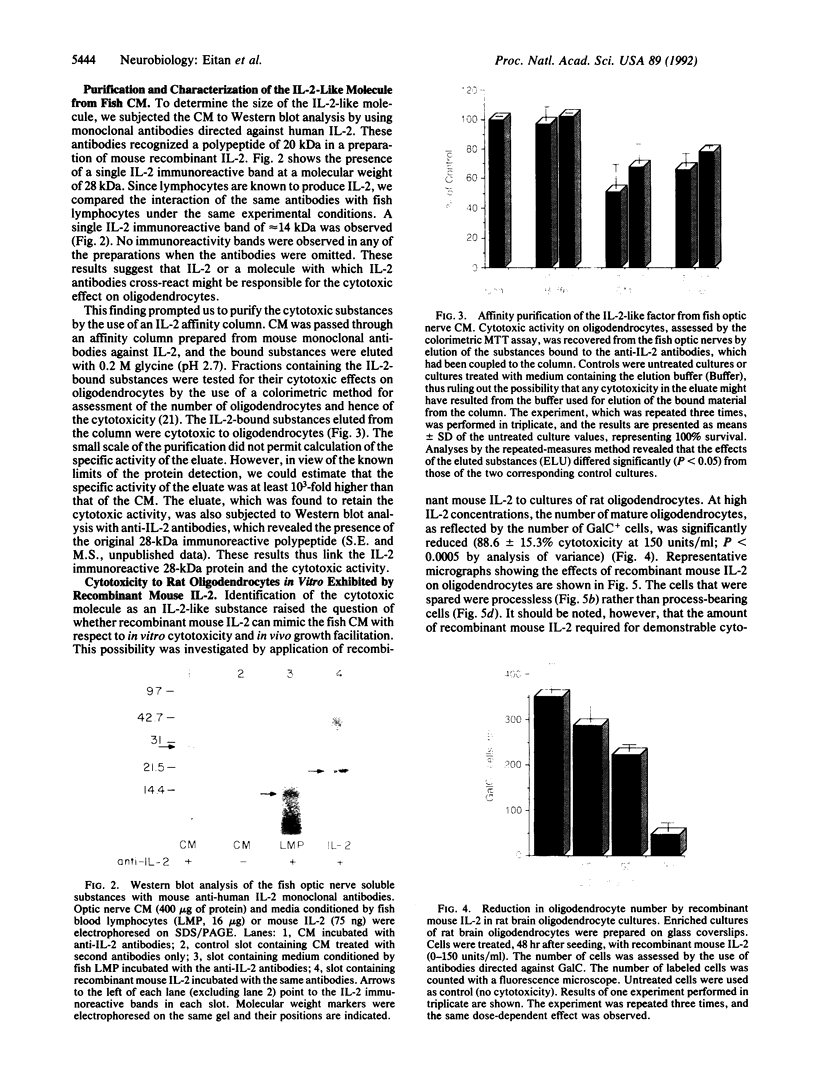
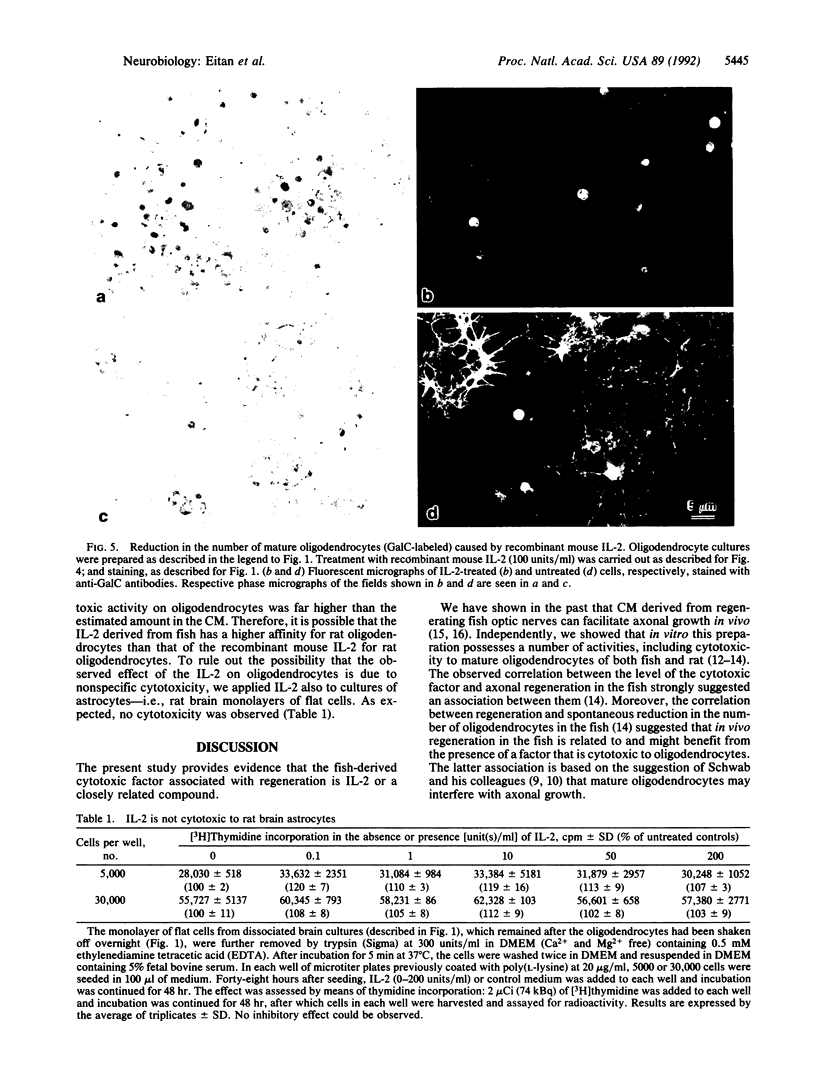
Abstract
Axons of the central nervous system in adult mammals do not regenerate spontaneously after injury, partly because of the presence of oligodendrocytes that inhibit axonal growth. This is not the case in lower vertebrates (e.g., in fish), where regeneration of the optic nerve does occur spontaneously and has been correlated with the presence of factors cytotoxic to oligodendrocytes. The present study provides evidence that the substance originating from the fish optic nerves, which is cytotoxic to oligodendrocytes, is an interleukin 2-like substance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTARDI D. G., SPERRY R. W. Preferential selection of central pathways by regenerating optic fibers. Exp Neurol. 1963 Jan;7:46–64. doi: 10.1016/0014-4886(63)90093-1. [DOI] [PubMed] [Google Scholar]
- Allcutt D., Berry M., Sievers J. A qualitative comparison of the reactions of retinal ganglion cell axons to optic nerve crush in neonatal and adult mice. Brain Res. 1984 Nov;318(2):231–240. doi: 10.1016/0165-3806(84)90028-2. [DOI] [PubMed] [Google Scholar]
- Araujo D. M., Lapchak P. A., Collier B., Quirion R. Localization of interleukin-2 immunoreactivity and interleukin-2 receptors in the rat brain: interaction with the cholinergic system. Brain Res. 1989 Oct 2;498(2):257–266. doi: 10.1016/0006-8993(89)91104-9. [DOI] [PubMed] [Google Scholar]
- Benveniste E. N., Merrill J. E. Stimulation of oligodendroglial proliferation and maturation by interleukin-2. Nature. 1986 Jun 5;321(6070):610–613. doi: 10.1038/321610a0. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carbonetto S., Evans D., Cochard P. Nerve fiber growth in culture on tissue substrata from central and peripheral nervous systems. J Neurosci. 1987 Feb;7(2):610–620. doi: 10.1523/JNEUROSCI.07-02-00610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Schwab M. E. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988 Mar;1(1):85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Avtalion R. R. Evidence for the existence of an IL-2-like lymphocyte growth promoting factor in a bony fish, Cyprinus carpio. Dev Comp Immunol. 1984 Winter;8(1):51–60. doi: 10.1016/0145-305x(84)90009-0. [DOI] [PubMed] [Google Scholar]
- Cohen A., Sivron T., Duvdevani R., Schwartz M. Oligodendrocyte cytotoxic factor associated with fish optic nerve regeneration: implications for mammalian CNS regeneration. Brain Res. 1990 Dec 24;537(1-2):24–32. doi: 10.1016/0006-8993(90)90335-9. [DOI] [PubMed] [Google Scholar]
- GAZE R. M. Regeneration of the optic nerve in Amphibia. Int Rev Neurobiol. 1960;2:1–40. doi: 10.1016/s0074-7742(08)60118-x. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Ingoglia N. A. Intracranial transection of the optic nerve in adult mice: preliminary observations. Exp Neurol. 1982 May;76(2):318–330. doi: 10.1016/0014-4886(82)90212-6. [DOI] [PubMed] [Google Scholar]
- Lavie V., Murray M., Solomon A., Ben-Bassat S., Belkin M., Rumelt S., Schwartz M. Growth of injured rabbit optic axons within their degenerating optic nerve. J Comp Neurol. 1990 Aug 15;298(3):293–314. doi: 10.1002/cne.902980304. [DOI] [PubMed] [Google Scholar]
- Liang S. M., Liang C. M., Chiueh C. C. Visualization of interleukin-2-like molecules in MPP(+)-lesioned rat brain. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1312–1318. doi: 10.1016/0006-291x(89)92746-0. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray M. Regeneration of retinal axons into the goldfish optic tectum. J Comp Neurol. 1976 Jul 15;168(2):175–195. doi: 10.1002/cne.901680202. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M., Chandy K. G. Interleukin-2-like activity in injured rat brain. Neurochem Res. 1987 Aug;12(8):723–727. doi: 10.1007/BF00970528. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Issa V. M., Shemie S. Regeneration and retrograde degeneration of axons in the rat optic nerve. J Neurocytol. 1982 Dec;11(6):949–966. doi: 10.1007/BF01148310. [DOI] [PubMed] [Google Scholar]
- Saneto R. P., Altman A., Knobler R. L., Johnson H. M., de Vellis J. Interleukin 2 mediates the inhibition of oligodendrocyte progenitor cell proliferation in vitro. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9221–9225. doi: 10.1073/pnas.83.23.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneto R. P., Chiappelli F., de Vellis J. Interleukin-2 inhibition of oligodendrocyte progenitor cell proliferation depends on expression of the TAC receptor. J Neurosci Res. 1987;18(1):147–154. doi: 10.1002/jnr.490180122. [DOI] [PubMed] [Google Scholar]
- Schnell L., Schwab M. E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990 Jan 18;343(6255):269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988 Jul;8(7):2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Belkin M., Harel A., Solomon A., Lavie V., Hadani M., Rachailovich I., Stein-Izsak C. Regenerating fish optic nerves and a regeneration-like response in injured optic nerves of adult rabbits. Science. 1985 May 3;228(4699):600–603. doi: 10.1126/science.3983646. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Solomon A., Lavie V., Ben-Bassat S., Belkin M., Cohen A. Tumor necrosis factor facilitates regeneration of injured central nervous system axons. Brain Res. 1991 Apr 5;545(1-2):334–338. doi: 10.1016/0006-8993(91)91309-o. [DOI] [PubMed] [Google Scholar]
- Sivron T., Cohen A., Duvdevani R., Jeserich G., Schwartz M. Glial response to axonal injury: in vitro manifestation and implication for regeneration. Glia. 1990;3(4):267–276. doi: 10.1002/glia.440030406. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Miron T. Polymers coupled to agarose as stable and high capacity spacers. Methods Enzymol. 1974;34:72–76. doi: 10.1016/s0076-6879(74)34008-6. [DOI] [PubMed] [Google Scholar]