Abstract
Background: Endometrial cancer (ECa) is one of the serious healthy burden for female worldwide. The treatments of ECa focus on the application of endocrine therapy and aberrant signaling proteins expression recently years. Medroxyprogesterone acrtate (MPA) plays crucial role in the endocrine therapy for ECa patients. However, the outcomes are still not ideal in the advanced stage tumor, especially in the progesterone-resistant ECa. Thioridazine (THIO) is an anti-psychotic agent, which has been reported to suppress the development of several human cancers. In this study, we aimed at to explore the clinical significant of THIO in the treatment of ECa. Methods: Two ECa cell lines (ISK and KLE) were enrolled in this study, and were grouped into fore groups based on the treatment with different agents. Methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay was used to analyze the viability of ECa cell lines. The apoptosis of ECa cells was examined by using the flow cytometer. To investigate the expression of important proteins, we applied the quantitative real-time RT-PCR (qRT-PCR) method and western blot analysis. Results: The viability of ECa cells was downregulated, and the apoptosis of ECa cells was upregulated after treating with the THIO plus MPA. The expression of progesterone receptor B (PRB) and dopamine receptor D2 (DRD2) were increased, and epidermal growth factor receptor (EGFR) and p-AKT were decreased in the THIO+MPA group. All these results suggested that the THIO could promote MPA to inhibit the growth of cells in ECa, especially in the progesterone-resistant ECa. Conclusion: Taken together, all the data in the present study suggested that the THIO plus MPA might act as the suppressor of tumor growth in ECa by inhibiting the PI3K/AKT signal transduction pathway, which was mediated by PRB, DRD2 and EGFR.
Keywords: Thioridazine, MPA, endometrial cancer
Introduction
Endometrial cancer (ECa) is one of the most serious healthy problem among women around the world [1]. It is rank the most common gynecologic malignancy in female in USA [2,3]. Based on the statistics, there were 52630 new diagnosed ECa cases and 8590 deaths due to this disease in the United States in 2014 [4]. Evidences suggested that the risk factors for endometrial cancer include obesity, late menopause, hormonal imbalances, increases in postmenopausal bleeding, low fertility rate and anovulation [5,6]. Different characteristics are performed in different grades of ECa: type I ECa is usually well differentiated, express progesterone and estrogen receptors (PR and ER). While type II ECa is poorly differentiated and contributing the aggressive lesions [7,8]. The decreased expression of ER and PR leading to the resistance to the hormonal growth regulation in the type II ECa. To data, the advances in the treatments of ECa, but the survival rates of patients with this disease are still not ideal [9,10]. Thus, the mechanisms of the ECa development should be highlight to improve the therapeutic strategies for patients with ECa.
Recently years, endocrine therapy is one the most effective methods for ECa, and the agents correlated with PR are the important component of endocrine methods. Medroxyprogesterone acrtate (MPA) is the most common agent mediated by progesterone receptor B (PRB) used in the treatment of ECa [11]. However, the outcomes are not ideal in the ECa patients with progestin resistance. Studies suggest that the epidermal growth factor receptor (EGFR) is overexpressed in the ECa with progestin resistance, and plays an important role in reducing the sensitivity of progestin treatment in patients with ECa [12]. Thus, the expression of PRB and EGFR are essential for the improvements in treatment of ECa. Thioridazine (THIO) is a potent anti-anxiety and anti-psychotic agent belongs to the family of phenothiazine drug [13]. Recently years, several studies found that the THIO might correlate with the progression of human cancers. Sachlos et al. suggested that THIO suppresses the development in the stem cells of leucocythemia [14]. Kang and his colleagues demonstrated that THIO can inhibit the tumor growth in ECa patients [15]. Thus, we aim at exploring the clinical significant of THIO in ECa to improve the treatment of this disease.
Materials and methods
Cell cultures
In this study, we applied two human ECa cell line: type I ECa cell line ISK and type II ECa cell line KLE (ECa cell with progestin resistance), which are obtained from the American Type Culture Collection (ATCC). All these cells were cultured in the DMED-F12 medium (Hyclone, Logan, Calif) with 100 µg/mL streptomycin, 10% fetal bovine serum (FBS), 2 mM L-glu-tamine and 100 U/mL penicillin. These cells were cultured in the incubator at 37°C with the 5% CO2.
Chemicals, antibodies and grouping design
THIO, MPA and other chemical agents were purchased from Sigma (St. Louis, MO). The antibodies, including anti-PRB, anti-dopamine receptor D2 (DRD2), anti-AKT and anti-p-AKT, were obtained from CST and used in our study. All the ECa cells were randomized into 4 group: black control group (no agent adding), MPA group, THIO group and MPA+THIO group. In these groups, cells were treated with 20 µM MPA in MPA group and 20 µM MPA plus 10 µM THIO in MPA+THIO group.
Cell viability assays
In the current study, we applied the methylthiazolyldiphenyl-tetrazolium bromide (MTT) analysis to calculate the viability of cell growth. Briefly, the cell lines were cultured in the DMEM medium with 10% FBS. Then the cells were distributed into the 96-well plates with the density of 3.4×103 cell/well. 24 hours later, the fresh medium, which contained 20 µl MTT (5 mg/ml) and 10% FBS, was added into all the wells. Then the 96-well plates were incubated at 37°C for 4 h. The score of viability assays was calculated with the absorbance at 540 nm by using the microculture plate reader. All the measurements were repeated three times.
Cell apoptosis assays
Flow cytometry was used in this study to measure the cell apoptosis. The cell lines were grown in the fresh medium for 24 h, then were incubated with the medium without FBS for 18 h. After treatments with the agents, the cells were processed with pancreatin, harvested by centrifugation, and rinsed with PBS buffer twice. All the cells were treated with the fluorescein isothiocyanate-labeled Annexin V (V-FITC) and propidium iodide (PI) for 15 min as per the supplier’s protocols. These products were analyzed by using the flow cytometer (FACScalibur, Becton-Dickinson, Franklin Lakes, NJ).
Quantitative real-time RT-PCR (qRT-PCR)
The expression of mRNAs of PRB, DRD2 and EGFR was examined by using the qRT-PCR. After treatments with the agents, the total RNA was extracted from the cell lines by using the TRIzol reagent (Incitrogen, Carlsbad, CA, USA). These RNAs were then reverstranscribed into cDNAs with the AMV reverse transcription system (Promega, USA). The qRT-PCR reaction was carried out by using the SYBR Green PCR master mix (Applied Biosystems, USA) with 7300 Real-Time PCR System (Applied Biosystems, USA). GAPDH gene was selected to act as the endogenous control gene. All the expression levels were calculated with the 2-ΔΔCt method.
Western blot analysis
72 hours after treatments with different agents, the cells were washed with PBS buffer twice. The total protein were extracted with SDS, and separated by SDS-PAGE, then electrotransferred to the polyvinylidene fluoride (PVDF) membrane. The membranes were bloched for 1 h at rome temperature. The membranes were incubated with the antibodies, including anti-PRB, anti-DRD2, anti-AKT and anti-p-AKT, at 4°C overnight, then incubated with the second antibodies for 1 h. The bands of protein were developed using chemiluminescence (ECL) reagents. The β-actin protein was used as the control protein.
Statistical analysis
Total of the data in the present study were analyzed by using the SPSS 21.0 statistical software. All the differences were performed by using the Student’s t test. The results with P value of <0.05 were considered significant.
Results
The proliferation and apoptosis of cell lines
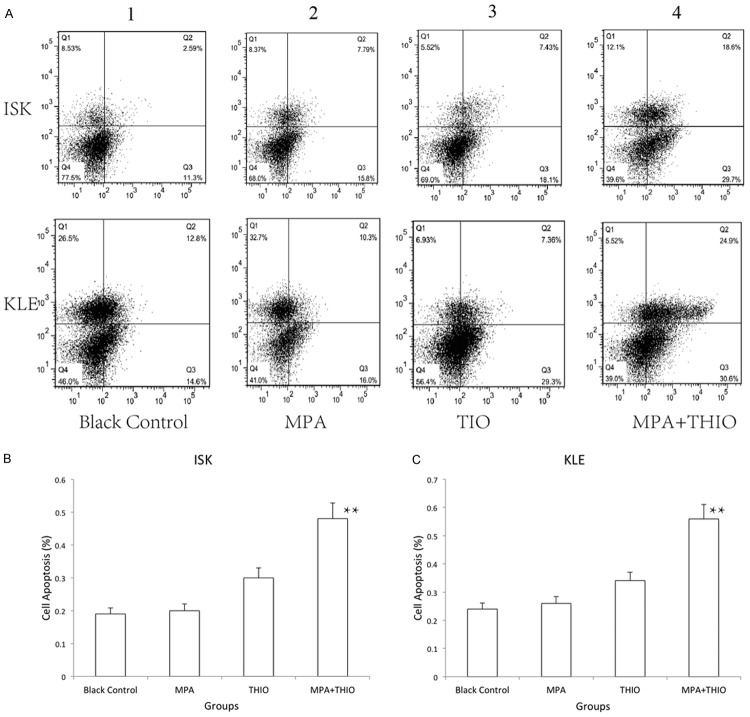
In order to investigate the effect of THIO on the proliferation and apoptosis of cell lines of ECa, all the cells were treated with the THIO (10 µM) in the THIO group. The cells were treated with 20 µM MPA in MPA group and 20 µM MPA plus 10 µM THIO in MPA+THIO group. The viability of cell lines were significant reduced after treatment with MPA+THIO compared with the black group (P<0.05, Figure 1). The numbers of viable ISK cells after 96 h treated with more than one drug were cut down half. In LKE cells, the single using of MPA was not effective as that in ISK cell lines. The apoptosis of cells was measured by using the flow cytometer, which measure by right bottom quadrant. The results of flow cytometric shown that the apoptosis of cell lines significantly increased in the MPA+THIO group for both two cell lines, which were more than two fold than that in the black group (P<0.01, Figure 2). All these foundings suggested that MPA and THIO repress cell proliferation by improving apoptosis in ECa cell lines.
Figure 1.
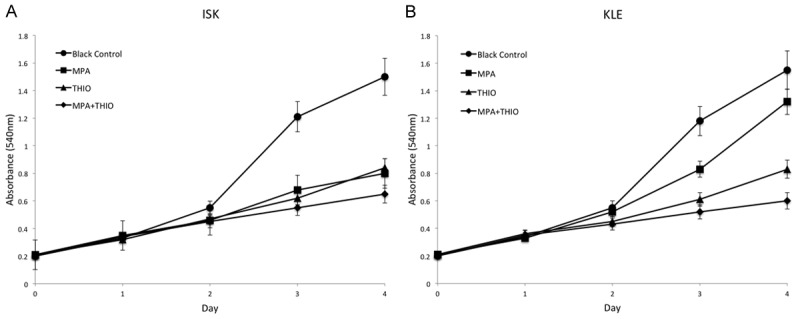
The cell viability in two ECa cell lines in different groups. The cell viability was downregulated in the MPA+THIO group compared with the black control group. A. ISK cell line; B. KLE cell line.
Figure 2.
The cell apoptosis in two ECa cell lines measured by flow cytometer. 1, black control group; 2, MPA group; 3, THIO group; 4, MPA+THIO group. *P<0.05 and **P<0.01. A. Flow cytometry; B. Apoptosis of ISK cells; C. Apoptosis of KLE cells.
The expression of mRNA of PRB, DRD2 and EGFR in ECa cell lines
In the current study, we used qRT-PCR to examine the expression level of mRNAs of PBR, DRD2 and EGFR in the ECa cell lines. From the results, QRT-PCR were used to examine the expression level of mRNAs of PBR, DRD2 and EGFR in the ECa cell lines. From the results, we found that the PBR and DRD2 were significantly increased 10-fold after the treatment with THIO plus MPA in both two cells lines. PBR was sensitive to MPA in ISK cell lines, while DRD2 was sensitive to THIO in ISK cell line. Then, the expression level of EGFR was cut down 40% in the MPA+THIO group in both cell lines (P<0.01, Figure 3). EGR was sensitive to THIO in ISK cell lines, and MPA in KLE cell line.
Figure 3.
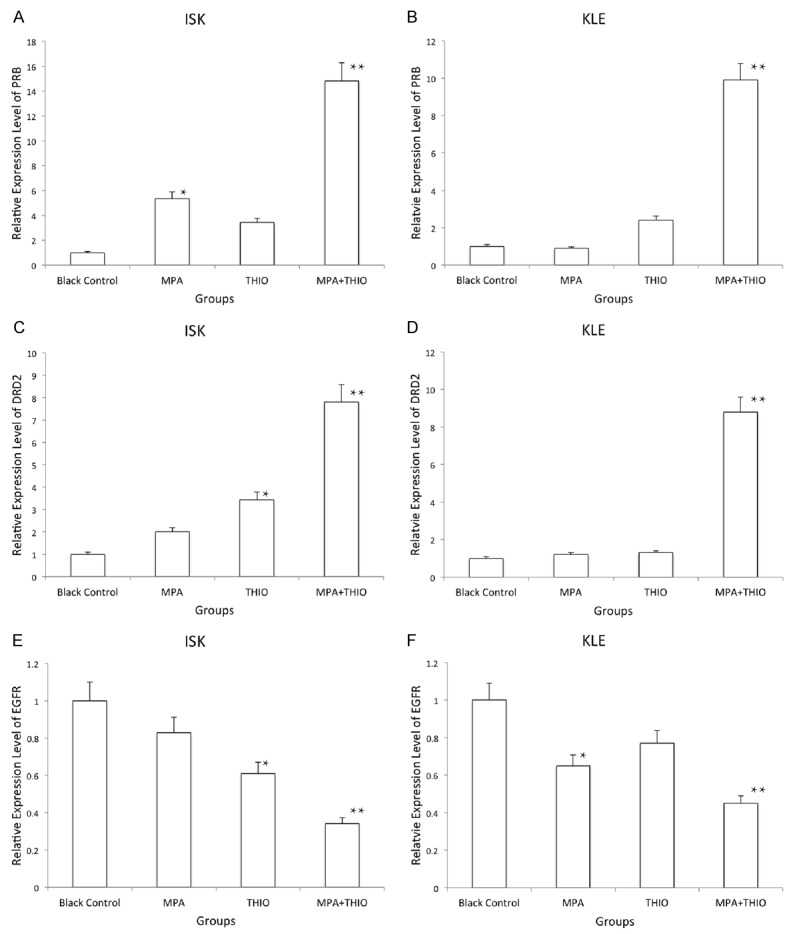
The expression of PRB, DRD2 and EGFR detected by qRT-PCR in two ECa cell lines in different groups. 1, black control group; 2, MPA group; 3, THIO group; 4, MPA+THIO group. *P<0.05 and **P<0.01. A. PRB in ISK cells; B. PRB in KLE cells; C. DRD2 in ISK cells; D. DRD2 in KLE cells; E. EGFR in ISK cells; F. EGFR in KLE cells.
The expression of PBR, DRD2, AKT and p-AKT protein in the ECa cell lines
Western blot was used to investigate the expression of PBR, DRD2, AKT and p-AKT protein. The results of this analysis suggested that the PBR, DRD2 were increased in the MPA+THIO group, while the p-AKT was decreased after treatment with THIO plus MPA (all P<0.05, Figure 4). There was no significant difference of AKT between the black group and the agents treatment group. The EGFR was significantly decreased after treatment with THIO plus MPA, which is consistent with mRNA expression level (Figure 5).
Figure 4.
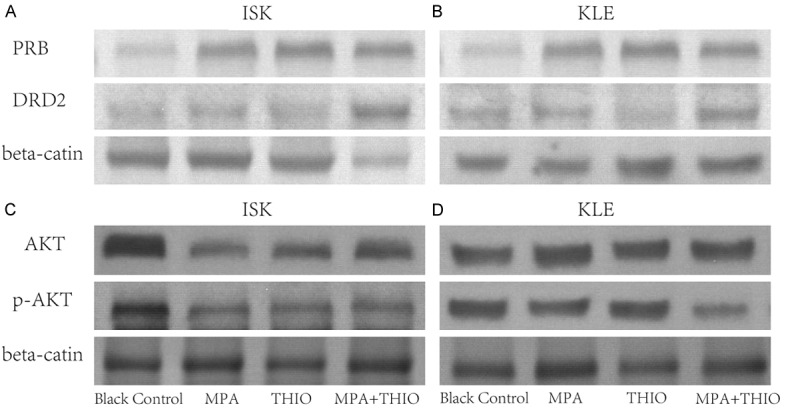
The expression of PRB, DRD2, AKT and p-AKT measured by western blot in two ECa cell lines in different groups. 1, black control group; 2, MPA group; 3, THIO group; 4, MPA+THIO group. A. PRB and DRD2 in ISK cells; B. PRB and DRD2 in KLE cells; C. AKT and p-AKT in ISK cells; D. AKT and p-AKT in KLE cells.
Figure 5.
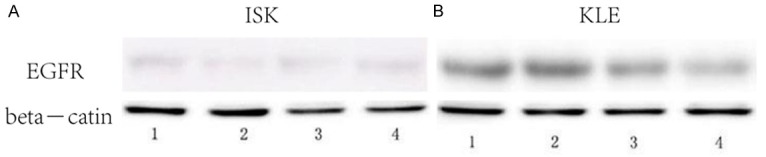
The expression of EGFR measured by western blot in two ECa cell lines in different groups, 1, black control group; 2, MPA group; 3, THIO group; 4, MPA+THIO group.
Discussion
ECa represents the seventh most common malignancy in female around the world [16]. Based on the statistics, the incidence and mortality of ECa have been on the rise recently decades [17-19]. The treatment of ECa has been improved continually, but the outcomes for patients with ECa are not ideal, especially for the ECa patients at the advanced stage [20,21]. The characteristic of tumor at the advanced stage is the serious drug resistance [22]. Therefore, the survival rates of patients with ECa is still needed to improve. In the recently studies, the endocrine therapy was act as an effective new therapeutic method for patients with ECa [23]. MPA is one of the most common endocrine agents, which can suppress the growth of ECa tumor cells mediated by PRB [24]. However, the outcomes are not improved in the patients with progesterone-resistant ECa [25,26]. Evidences demonstrated that the expression of PRB is downregulated in the progesterone-resistant ECa, but the upregulated expression of EGFR is found in ECa with progestin resistance [27]. EGFR is the important member of ErbB/HER receptor tyrosine kinase family, which has been reported to involved in the development of human cancer [28]. The upregulated EGFR has been found in some human cancers, including ECa. This increased EGFR can promote the proliferation and metastasis of tumors by activating the downstream phosphatidylinositol 3-kinase (PI3K)/AKT signal transduction [29]. In the previous studies, the PI3K/AKT signal transdution is correlated with the growth of human cancers by suppressing the apoptosis of tumor cells [30-33]. Moreover, the upregulated EGFR contributes the downregulated of PRB, leading the resistance to progestin [12,34]. Thus, these upregulated or downreuglated signaling proteins might play crucial role in the progression of ECa, and could be used to study the treatment of ECa.
THIO is a member of anti-psychotic drugs, which has been used to treat the cancer related sweating and depression [35,36]. Recently evidences demonstrated that the THIO can inhibit the proliferation and induce the apoptosis of several human cancers mediated by DRD2 [37]. In the clinical field of ECa, THIO has also been found correlate with the apoptosis of ECa cells by targeting the PI3K/AKT/mTOR pathway [15]. However, the understanding of THIO in the treatment of ECa is not enough. Therefore, we aimed at to explore the significant of THIO in the treatment of ECa.
In the current study, two ECa cell line ISK and KLE were treated with the THIO to perform the function of this agent. Meanwhile, we applied MPA to act as the control agent to examine the role of THIO in the progesterone-resistant ECa. Thus, four groups were carried out, including black control, MPA, THIO and MPA+THIO group. From the results of MTT analysis, we found that the viability of two cell lines were decreased in the THIO, MPA and MPA+THIO groups compared with the black group, and the lowest one was in the MPA+THIO groups. The results of flow cytometric detection suggested that the apoptosis of two cell lines were significantly higher in the MPA+THIO groups than that in the black control group.
The important proteins correlated with THIO and MPA in ECa cells were analyzed by qRT-PCR and western blot. The results shown that the PRB and DRD2 were significantly increased in the MPA+THIO group compared with the black control group. While the EGFR and p-AKT were downregulated after treating with the THIO plus MPA. Thus, all the data suggested that the THIO plus MPA might involved in the progression of ECa by upregulating the PRB and DRD2 and downregulating the EGFR. Moreover, the PI3K/AKT signal transduction pathway was suppressed after treating with the THIO, especially with the MPA+THIO in the ECa cell lines.
In conclusion, we considered that THIO could promote MPA to suppress the growth of ECa cells by inhibiting the PI3K/AKT signal transduction pathway, which was mediated by PRB, DRD2 and EGFR.
Acknowledgements
Fund: (National Nature Science Foundation of China, 81572547, 81172477; Natural Science Foundation of Science and Technology Commission of Shanghai Municipality, 11ZR1440800, 13JC1401303; New Hundred Talents Program of Shanghai, XBR2013097).
Disclosure of conflict of interest
None.
References
- 1.Platz CE, Benda JA. Female genital tract cancer. Cancer. 1995;75:270–294. doi: 10.1002/1097-0142(19950101)75:1+<270::aid-cncr2820751312>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG. Endometrial carcinoma. N Engl J Med. 1996;335:640–649. doi: 10.1056/NEJM199608293350907. [DOI] [PubMed] [Google Scholar]
- 3.Arnett-Mansfield RL, DeFazio A, Mote PA, Clarke CL. Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J Clin Endocrinol Metab. 2004;89:1429–1442. doi: 10.1210/jc.2003-031111. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MA. Effect of angiotensin II type 1 receptor blocker on renal function, arterial blood pressure and parathyroid hormone related protein over expression in cadmium induced nephrotoxicity in adult male rats. Int J Physiol Pathophysiol Pharmacol. 2013;5:109–119. [PMC free article] [PubMed] [Google Scholar]
- 5.Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem-Maghami S. Endometrial cancer. BMJ. 2011;343:d3954. doi: 10.1136/bmj.d3954. [DOI] [PubMed] [Google Scholar]
- 6.Galaal K, Al Moundhri M, Bryant A, Lopes AD, Lawrie TA. Adjuvant chemotherapy for advanced endometrial cancer. Cochrane Database Syst Rev. 2014;5:CD010681. doi: 10.1002/14651858.CD010681.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26:1268–1274. doi: 10.1016/0046-8177(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 8.Sherman ME, Sturgeon S, Brinton L, Kurman RJ. Endometrial cancer chemoprevention: implications of diverse pathways of carcinogenesis. J Cell Biochem Suppl. 1995;23:160–164. doi: 10.1002/jcb.240590921. [DOI] [PubMed] [Google Scholar]
- 9.Bevis KS, Kilgore LC, Alvarez RD, Straughn JM Jr, Leath CA 3rd. Combination therapy with paclitaxel, carboplatin and megestrol acetate for the management of advanced stage or recurrent carcinoma of the endometrium: a phase II study. J Reprod Med. 2014;59:113–120. [PubMed] [Google Scholar]
- 10.Ray M, Fleming G. Management of advanced-stage and recurrent endometrial cancer. Semin Oncol. 2009;36:145–154. doi: 10.1053/j.seminoncol.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Yuan X, Wang LU, Xue J, Li LI, Zhang J. Endocrine MPA enhances the effects of TAC chemotherapy on improvement of prognosis and increase in long-term survival rates for patients with endometrial cancer. Oncol Lett. 2015;10:1902–1906. doi: 10.3892/ol.2015.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai Z, Wang J, Wang Y, Lu L, Tong J, Teng Y. Overexpressed epidermal growth factor receptor (EGFR)-induced progestin insensitivity in human endometrial carcinoma cells by the EGFR/mitogen-activated protein kinase signaling pathway. Cancer. 2010;116:3603–3613. doi: 10.1002/cncr.25220. [DOI] [PubMed] [Google Scholar]
- 13.Vibe CB, Fenaroli F, Pires D, Wilson SR, Bogoeva V, Kalluru R, Speth M, Anes E, Griffiths G, Hildahl J. Thioridazine in PLGA nanoparticles reduces toxicity and improves rifampicin therapy against mycobacterial infection in zebrafish. Nanotoxicology. 2015:1–9. doi: 10.3109/17435390.2015.1107146. [DOI] [PubMed] [Google Scholar]
- 14.Sachlos E, Risueno RM, Laronde S, Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn A, Graham M, Levadoux-Martin M, Lee JB, Giacomelli AO, Hassell JA, Fischer-Russell D, Trus MR, Foley R, Leber B, Xenocostas A, Brown ED, Collins TJ, Bhatia M. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Kang S, Dong SM, Kim BR, Park MS, Trink B, Byun HJ, Rho SB. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis. 2012;17:989–997. doi: 10.1007/s10495-012-0717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banno K, Kisu I, Yanokura M, Masuda K, Kobayashi Y, Ueki A, Tsuji K, Yamagami W, Nomura H, Susumu N, Aoki D. Endometrial Cancer and Hypermethylation: Regulation of DNA and MicroRNA by Epigenetics. Biochem Res Int. 2012;2012:738274. doi: 10.1155/2012/738274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YS, Lee KE. The Significance of miR-34a Expression in Endometrial Carcinogenesis: Correlation With Expression of p16 and Ki-67 Proteins in Endometrial Cancers. J Cancer Prev. 2015;20:268–274. doi: 10.15430/JCP.2015.20.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 19.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 20.Jin C, Liang R. miR-205 promotes epithelial-mesenchymal transition by targeting AKT signaling in endometrial cancer cells. J Obstet Gynaecol Res. 2015;41:1653–1660. doi: 10.1111/jog.12756. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Zhang X, Zhang R, Ge Q. MicroRNA heterogeneity in endometrial cancer cell lines revealed by deep sequencing. Oncol Lett. 2015;10:3457–3465. doi: 10.3892/ol.2015.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalogiannidis I, Bobos M, Papanikolaou A, Makedos A, Amplianitis I, Vergote I, Nenopoulou E, Makedos G. Immunohistochemical bcl-2 expression, p53 overexpression, PR and ER status in endometrial carcinoma and survival outcomes. Eur J Gynaecol Oncol. 2008;29:19–25. [PubMed] [Google Scholar]
- 23.Prior JC. Progesterone or progestin as menopausal ovarian hormone therapy: recent physiology-based clinical evidence. Curr Opin Endocrinol Diabetes Obes. 2015;22:495–501. doi: 10.1097/MED.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Sun X, Zhang H, Wang Y, Li Y. MPA influences tumor cell proliferation, migration, and invasion induced by RANKL through PRB involving the MAPK pathway in endometrial cancer. Oncol Rep. 2015;33:799–809. doi: 10.3892/or.2014.3651. [DOI] [PubMed] [Google Scholar]
- 25.Hanekamp EE, Gielen SC, van Oosterhoud SA, Burger CW, Grootegoed JA, Huikeshoven FJ, Blok LJ. Progesterone receptors in endometrial cancer invasion and metastasis: development of a mouse model. Steroids. 2003;68:795–800. doi: 10.1016/j.steroids.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Koval OA, Sakaeva GR, Fomin AS, Nushtaeva AA, Semenov DV, Kuligina EV, Gulyaeva LF, Gerasimov AV, Richter VA. Sensitivity of endometrial cancer cells from primary human tumor samples to new potential anticancer peptide lactaptin. J Cancer Res Ther. 2015;11:345–351. doi: 10.4103/0973-1482.157301. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Chen X, Lu X, Yu Y, Feng Y. Epidermal growth factor receptor signaling enhanced by long-term medroxyprogesterone acetate treatment in endometrial carcinoma. Gynecol Oncol. 2007;105:45–54. doi: 10.1016/j.ygyno.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 29.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 31.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 33.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, Testa JR. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 34.Konecny GE, Santos L, Winterhoff B, Hatmal M, Keeney GL, Mariani A, Jones M, Neuper C, Thomas B, Muderspach L, Riehle D, Wang HJ, Dowdy S, Podratz KC, Press MF. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer. 2009;100:89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhukovsky DS. Fever and sweats in the patient with advanced cancer. Hematol Oncol Clin North Am. 2002;16:579–588. viii. doi: 10.1016/s0889-8588(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 36.Ly KL, Chidgey J, Addington-Hall J, Hotopf M. Depression in palliative care: a systematic review. Part 2. Treatment. Palliat Med. 2002;16:279–284. doi: 10.1191/0269216302pm570oa. [DOI] [PubMed] [Google Scholar]
- 37.Park MS, Dong SM, Kim BR, Seo SH, Kang S, Lee EJ, Lee SH, Rho SB. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget. 2014;5:4929–4934. doi: 10.18632/oncotarget.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]