Abstract
Glioblastoma is an aggressive brain cancer requiring improved treatments. Existing methods of drug discovery and development require years before new therapeutics become available to patients. Zebrafish xenograft models hold promise for prioritizing drug development. We have developed an embryo–larval zebrafish xenograft assay in which cancer cells are implanted in a brain microenvironment to discover and prioritize compounds that impact glioblastoma proliferation, migration, and invasion. We illustrate the utility of our assay by evaluating the well-studied, phosphatidylinositide 3-kinase inhibitor LY294002 and zinc oxide nanoparticles (ZnO NPs), which demonstrate selective cancer cytotoxicity in cell culture, but the in vivo effectiveness has not been established. Exposures of 3.125–6.25 μM LY294002 significantly decreased proliferation up to 34% with concentration-dependent trends. Exposure to 6.25 μM LY294002 significantly inhibited migration/invasion by ∼27% within the glioblastoma cell mass (0–80 μm) and by ∼32% in the next distance region (81–160 μm). Unexpectedly, ZnO enhanced glioblastoma proliferation by ∼19% and migration/invasion by ∼35% at the periphery of the cell mass (161+ μm); however, dissolution of these NPs make it difficult to discern whether this was a nano or ionic effect. These results demonstrate that we have a short, relevant, and sensitive zebrafish-based assay to aid glioblastoma therapeutic development.
Introduction
Despite 30 years of extensive research into developing drug therapies, the median survival rate of patients diagnosed with glioblastoma remains bleak at ∼15 months.1,2 This poor prognosis is due to a combination of the highly invasive nature and heterogeneous mutational spectrum of this deadly form of primary brain tumor making complete surgical resection virtually impossible and adjuvant therapies ineffective.2,3 There is a dire need to better understand the biological processes underlying glioblastoma invasion and progression to aid in the discovery of novel glioblastoma therapeutics that more effectively treat this aggressive disease. Further complicating new glioblastoma drug development is that studying invasion in an intact brain microenvironment can be especially difficult in traditional mammalian models. The opacity of the mammalian head makes directly observing how potential therapeutics influence individual invading glioblastoma cells problematic.4–9 Yet, research suggests that migrating cells will drastically alter morphology and mechanisms of movement in three-dimensional versus two-dimensional environments and the migrating cells receive signals from the microenvironment that dictate direction making studies on migration and invasion in an intact brain desirable.10 While investigating therapeutic efficacy in mammalian models is an important step in the drug development pipeline, testing during the early stages of discovery can be challenging. This is especially true in the expanding field of nanomedicine, where compound availability may be limited.11
Zebrafish xenograft models hold potential for studying human cancer progression, migration, invasion, metastases, and microenvironment interactions to aid in identifying new treatments.4,6,8,9,12 The embryo–larval zebrafish model has proved useful in quickly identifying and prioritizing the screening of promising new pharmaceuticals.13 The small size, high fecundity, and transparency of the embryo–larval zebrafish makes it amenable to conducting exposures in multiwell plates and noninvasively studying exposure-dependent effects on cancer progression using microscopy.4,6,8,9,12 Furthermore, embryo–larval zebrafish lack a fully functional adaptive immune system until ∼28 days making it possible to implant human cells without rejection.14 Existing embryo–larval zebrafish xenograft models have already demonstrated that human cancer cells, including glioma, can grow, divide, metastasize, and induce angiogenesis in zebrafish similarly to rodent xenograft models.5,6,8,9,15–17 A particular advantage of the zebrafish xenograft model is that only a small number of cancer cells are required for xenotransplantation, which better simulates earlier stages of cancer progression.8,9
Many embryo–larval zebrafish xenograft glioma models involve transplanting the cells into the yolk sac or perivitelline space.5,6,9,15 These transplant locations lack a complex brain microenvironment, which is filled with glial cells, neuronal processes, extracellular matrix, and a functional blood–brain barrier important for studying brain cancer.4 By transplanting glioblastoma cells into the 48–72 h postfertilization zebrafish brain, which possess a functioning blood–brain barrier similar to mammalian models, our laboratory has developed an efficient and relevant assay using high-content imaging to study glioblastoma progression and prioritize the development of new glioblastoma therapies.18
Our previous studies revealed that genetic knockdown of a protein important in glioblastoma invasion (calpain 2) reduces glioblastoma invasion by ∼90%.19 Implanting these modified cells in the zebrafish brain microenvironment further demonstrated that knockdown of calpain 2 reduced glioblastoma invasion by 2.9-fold, which was similar to the reduction observed in organotypic mouse brain tissues (2.3-fold).4 In this report, we expand upon previous research by developing a robust screening assay to determine if treatment of the zebrafish xenograft model with exogenous compounds could significantly impact glioblastoma proliferation, migration, and invasion. We validated the sensitivity of the assay through testing the selective phosphatidylinositide 3-kinase (PI 3-kinase) inhibitor LY294002, known to inhibit glioblastoma proliferation, migration, and invasion in both cell culture and rodent xenograft models.20–22 We also assessed novel zinc oxide nanoparticles (ZnO NPs), which demonstrate selective toxicity toward cancer cells in vitro,23 for conserved preferential anticancer efficacy in vivo in this refined zebrafish xenograft assay.
Materials and Methods
Zebrafish husbandry
Adult 5D tropical strain zebrafish (Danio rerio) were reared at Sinnhuber Aquatic Research Laboratory utilizing standardized procedures in accordance with Institutional Animal Care and Use Committee protocols at Oregon State University.24 Embryos were staged according to Kimmel et al. and at ∼4 h postfertilization, the chorion was enzymatically digested using pronase (63.6 mg/mL, >3.5 U/mg, P5147; Sigma-Aldrich, St. Louis, MO) assisted by a custom-automated instrument.25,26 Embryos were maintained in 1× strength E2 embryo medium sans methylene blue (ZIRC protocols, http://zebrafish.org/documents/protocols/pdf/Fish_Nursery/E2_solution.pdf) under a 14-h light–10-h dark photoperiod at 28°C ± 1°C. To inhibit pigment formation, 1-day postfertilization (DPF) zebrafish embryos were treated with 0.003% w/v N-phenylthiourea (Sigma-Aldrich), which was renewed daily until xenotransplantation occurred. Upon completion of experiments, zebrafish were euthanized by overdose to buffered Tricaine (MS-222; Sigma-Aldrich).
U87MG maintenance and fluorescent labeling
Human U87MG glioblastoma cells were purchased from the American Type Culture Collection (Manassas, VA) and grown in Dulbecco's modified Eagle's medium (DMEM; Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 1% l-glutamine (Cellgro). Cells were incubated at 37°C with 5% CO2 and 95% air in a humidified chamber. Chloromethylbenzamido (CellTracker™ CM-DiI; Invitrogen, Grand Island, NY), a fluorescent cell membrane intercalating dye, was used to label the U87MG cells according to the manufacturer's directions. Briefly, a 50 μg/μL stock was prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich), and diluted in phosphate-buffered saline to a working concentration of 2 μM. U87MG cells were incubated in 2 μM CM-DiI for 2 min at 37°C followed by 15 min at 4°C. Cells were resuspended in serum-free DMEM at a concentration of ∼10–20 million cells/mL.
Glioblastoma transplantation procedure
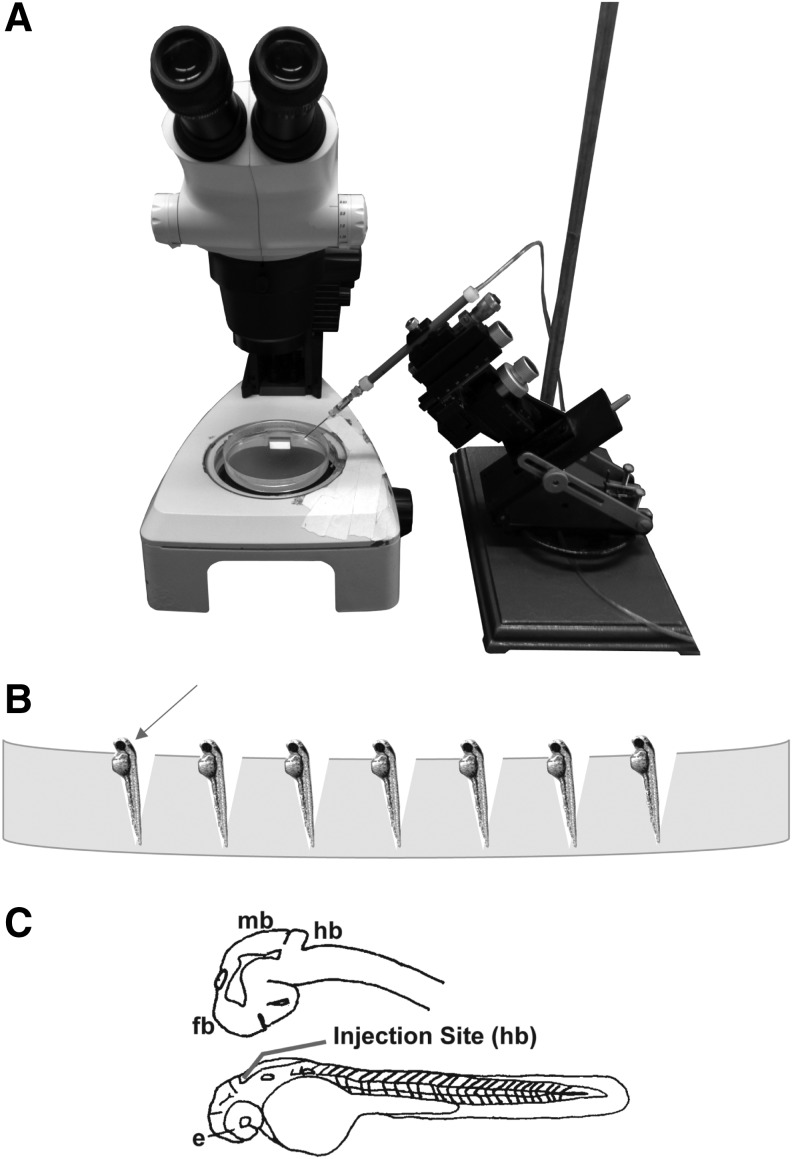
Borosilicate capillary tubes with an outer diameter of 1.14 mm and an inner diameter of 0.5 mm (World Precision Instruments, Sarasota, FL) were used to prepare microinjection needles on a Model P-97 micropipette puller (Sutter Instrument Co., Novato, CA). The micropipette puller settings were as follows: pressure = 200, heat = ramp +21°, pull = 20, velocity = 50, and time = 200. The needle was then broken back using forceps to create a slight bevel and allow passage of the U87 cells. Before transferring the fluorescently labeled U87 cells to the microinjection needle, Phenol Red (Sigma-Aldrich) was added to the cell solution at a 1:10 dilution (v/v). At 2 or 3 DPF, dechorionated zebrafish larvae were anesthetized in 200 mg/L buffered Tricaine, positioned on a 2% agarose gel (Fig. 1) and injected with ∼50–100, U87MG glioblastoma cells into the hindbrain ventricle near the midbrain hindbrain junction (Fig. 1C) using a ASI MPPI-2 air-driven pressure injector (Applied Scientific Instruments, Eugene, OR). Background control zebrafish were injected with the same volume of DMEM mixed with Phenol Red minus the U87MG cells. Following xenotransplantation, zebrafish were transferred to fresh embryo medium and kept in a dark, 33°C ± 1°C incubator. Active xenografts exhibiting no phenotypic malformations, such as edema or fin, axial, or craniofacial abnormalities 1 day postinjection were used for the subsequent assays, which ended at 7 DPF.
FIG. 1.
(A) Microinjector setup. (B) Placement of anesthetized 2 or 3 DPF zebrafish on 2% agarose gel for microinjection of human U87MG glioblastoma cells. (C) Closeup drawing depicting 2 DPF zebrafish brain (upper) and whole embryo (lower) showing the injection site (red line) in the hindbrain ventricle near midbrain–hindbrain junction. DPF, day postfertilization; e, eye; fb, forebrain; hb; hindbrain; mb: midbrain.
Imaging and analysis of U87MG behavior
Xenografts were anesthetized in 200 mg/L buffered Tricaine for imaging. This concentration of anesthetic was selected based on Kaufmann et al. for complete immobilization of zebrafish for several hours.27 Anesthetized xenografts were transferred to a 96-well imaging plate (Greiner BioOne, Monroe, NC) at one fish per well by hand and embedded in 0.1 mL of 0.8% (w/v) low-melt agarose (Promega, Madison, WI) containing 200 mg/L Tricaine. Before agarose solidified, xenograft heads were centered, dorsal side down, at the bottom of the well (Fig. 2A). To minimize the duration in low-melt agarose and reduce stress, xenografts were immobilized, embedded, and imaged in groups of 20–24 fish at a time.
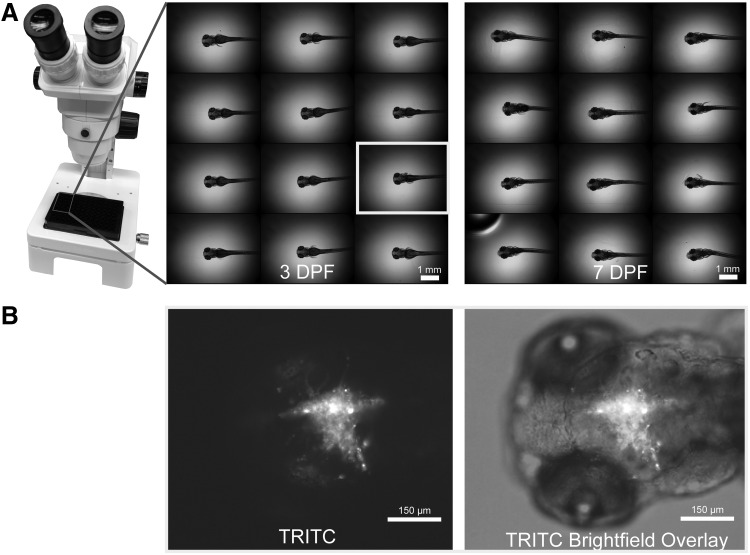
FIG. 2.
(A) Xenograft placement in a 96-well plate at 2× with heads centered and positioned dorsal side down in well using dissecting microscope. Images acquired using Molecular Devices High-Content Imager with 2× objective. (B) Representative image of 3 DPF xenograft (left: fluorescent 24 slice [8 μm per slice] Z-stack acquired 30 ms exposure with 2 × 2 binning and combined into one all-in-focus image using Best Focus stack arithmetic and right: one brightfield image acquired at 61 ms exposure overlayed with fluorescent image) depicting frame of view that is acquired and analyzed at 10× for all fish at 3 or 4 DPF and 7 DPF.
Images were acquired on an ImageXpress Micro (Molecular Devices, Sunnyvale, CA) using a 10× objective. The image frame of view focused on the most anterior portion of the head to the developing pharyngeal arches. All 10× images were acquired and analyzed using this frame of view for consistency (Fig. 2B). The TRITC filter (excitation 543/22 nm, emission 593/40 nm) was used to locate the U87MG cells and focus on the cancer cell mass. One brightfield image was acquired for locational reference before capturing a TRITC Z-stack. Typically, a 24-slice Z-stack at 8 μm per slice was of sufficient breadth within the z plane of the zebrafish brain to image all U87MG cells. Fluorescent Z-stacks were acquired with 30 ms exposures and 2 × 2 pixel binning (Fig. 2B). Zebrafish xenografts and background control zebrafish were imaged at 3 or 4 DPF and 7 DPF using identical acquisition settings.
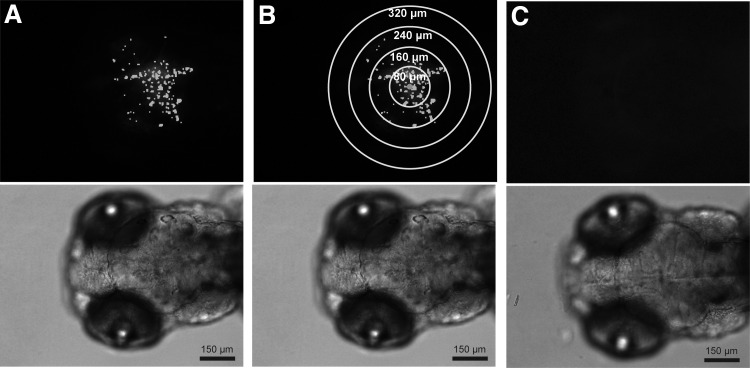
All images were analyzed using MetaXpress High-Content Image Acquisition and Analysis Software Version 5 (Molecular Devices). Each Z-stack was processed with proprietary best focus stack arithmetic similar to maximum projection to create one fluorescent image (Fig. 3). The images were analyzed using the Multi Wavelength Cell Scoring Module to obtain cell counts. Individual cells were identified based on an image intensity of 100 gray levels above background with a minimum cell diameter of 4 μm and maximum cell diameter of 10 μm (Fig. 3A). For cell migration and invasion, individual cells were identified using Multi Wavelength Cell Scoring using the same parameters as stated above. A custom MetaXpress journal located the centroid of the image based on the locations of all identified cells and calculated the distance from the image centroid to all cells. Then the number of cells located at increasing radial distance regions (0–80, 81–160, 161–240, 241–320, and 321+ μm) from the image centroid were quantified (Fig. 3B). To quantify changes in cell migration/invasion, the cell count obtained from each distance region in each final xenograft image (7 DPF) was first normalized through division by the total cell count in the corresponding initial xenograft image (3 or 4 DPF). Then the fold change in cell migration/invasion for each treatment group within a distance region was compared with the control for that distance region. No autofluorescence signal was identified as cells during the analysis of the background control zebrafish (Fig. 3C).
FIG. 3.
Representative 10× fluorescent images (top) with corresponding brightfield images showing position of cells in zebrafish head directly below. (A) Glioblastoma-injected zebrafish imaged at 3 DPF with Multi Wavelength Cell Scoring Mask (white) used to delineate and count individual cells. (B) Same xenograft as image (A) with added concentric circles (yellow) used to delineate regions for quantifying cell migration/invasion in 80 μm distances from the centroid of the cell mass (blue circle). (C) Mock-injected zebrafish imaged at 3 DPF. No fluorescent cell count data obtained from zebrafish mock injection, which consisted of cell medium only.
Xenograft exposure assay
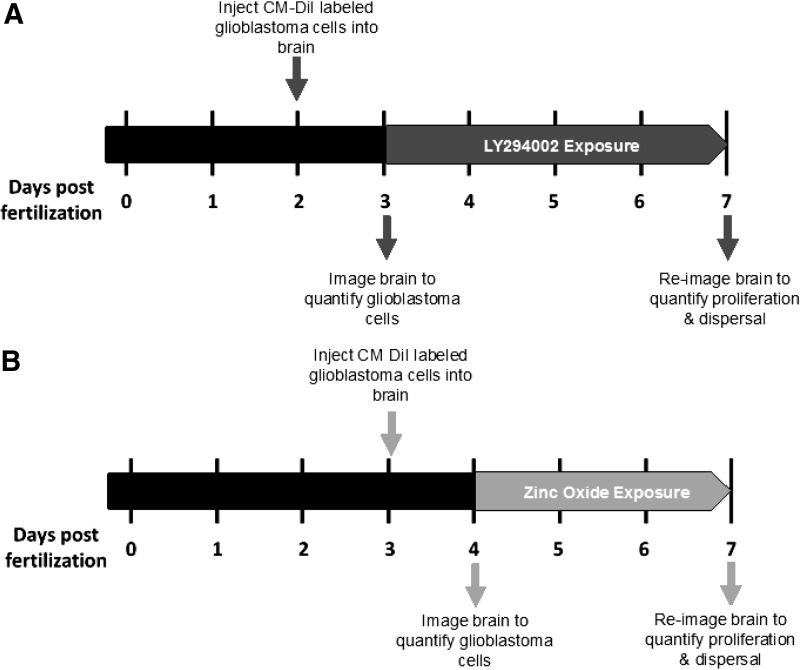
After imaging, zebrafish were carefully removed from the low-melt agarose, rinsed in 1× embryo medium or sterile, deionized water (ultrapure water; Invitrogen) and transferred to a sterile polystyrene 96-well tissue culture, flat bottom plate (Falcon, Manassas, VA) at one fish per well. Each well was preloaded with 0.1 mL of 1× embryo medium at 0.003% PTU for the LY294002 exposure or sterile, deionized water at 0.003% PTU for the ZnO NP exposure. Any xenograft exhibiting damage from imaging or transfer was euthanized and excluded from the experiment. The timeline of each assay is presented in Figure 4.
FIG. 4.
Diagram depicting the timeline of the zebrafish xenograft assay. (A) Overview of the 4-day LY294002 assay. (B) Overview of the 3-day ZnO NP assay. ZnO NP, zinc oxide nanoparticle.
Before conducting the xenograft assay, exposure concentrations were selected based on the results of an initial concentration–response test. Briefly, uninjected age-matched, larval zebrafish were exposed to a range of compound under conditions mimicking the xenograft assay to identify a maximum tolerable exposure concentration, which resulted in insignificant mortality or phenotypical malformations compared with the vehicle control. The maximum tolerable concentration was used to set the upper limit of the concentration–response series for the xenograft assay.
Phosphatidylinositol 3-kinase inhibitor LY294002
The selective pan PI 3-kinase inhibitor LY294002 (Enzo Life Sciences, Farmingdale, NY) was chosen as a positive control for the inhibition of U87MG proliferation in the xenograft assay. LY294002 was dissolved in DMSO to prepare a 16.2 mM stock. A twofold dilution series was prepared at 2× (6.25–25 μM LY294002) with PTU and DMSO maintained at 0.003% and 1%, respectively, and 0.1 mL was transferred to the 96-well plate containing the xenografts. This resulted in a 4-day static nonrenewal, exposure to 1× (3.125–12.5 μM) LY294002 with 0.003% PTU and vehicle control of 0.5% DMSO.
Zinc oxide nanoparticle
ZnO NPs with a primary particle size of 8.37 nm were synthesized using forced hydrolysis as described previously in Wehmas et al.28 One gram of zinc acetate dehydrate and 99.5:0.5 mL diethylene glycol:nanopure water mix were heated to 160°C for 90 min followed by multiple ethanol rinses and separation using centrifugation for 20 min at 20,000 rpm after each rinse. Immediately before exposure, an aliquot of ZnO NP and ZnO bulk material control were suspended in 100% DMSO at a concentration of 10 mg/mL and water bath sonicated (Ultrasonik NDI; NEY, Inc., Bloomfield, CT) for 20 min at room temperature. The ZnO bulk material acted as a size control because the primary particle size is in the micrometer range as opposed to the size operationally defined as nano (possessing at least one dimension between 1 and 100 nm) for the ZnO NPs. A twofold dilution series was prepared in sterile, deionized water (ultrapure water; Invitrogen) at 2× for NP and bulk (0.3125–2.5 mg/L) with PTU and DMSO maintained at 0.003% and 1%, respectively. A 0.1 mL of the 2× NP and bulk was transferred to the 96-well plate containing the xenografts preloaded in 0.1 mL deionized water at 0.003% PTU. This resulted in a 3-day static nonrenewal, exposure to 1× (0.156–1.25 mg/L) ZnO NP or ZnO bulk control with 0.003% PTU and vehicle at 0.5% DMSO.28 To minimize agglomeration with salts present in zebrafish embryo medium, the ZnO NP exposures were conducted in ultrapure water.28,29 As previously reported in Wehmas et al., this ZnO NP has a mean hydrodynamic diameter on the order of microns when prepared in zebrafish embryo medium, whereas its mean hydrodynamic diameter is ∼400 nm and zeta potential (an indication of charge) is ∼20.2 mV when prepared in ultrapure water creating a more stable exposure suspension.28
Following addition of compound, the 96-well plates were sealed with Parafilm, wrapped in aluminum foil to block incidental light and maintained in a 33°C ± 1°C incubator until 7 DPF. At 7 DPF, xenografts were assessed for mortality and phenotypic malformations. Living 7 DPF xenografts were reimaged to obtain U87MG proliferation and dispersal data as described in Imaging and Analysis of U87MG Behavior.
Statistical methods
Mortality data were analyzed using Fisher's Exact Test comparing each treatment to the vehicle control. Zebrafish xenograft proliferation and migration/invasion data were analyzed by one-way analysis of variance using Tukey's multiple comparison procedure. If assumptions of normality or equal variance were violated according to SigmaPlot, data were analyzed by Kruskal–Wallis one-way analysis of variance on Ranks using a Dunn's multiple comparison procedure. Analyses were completed using SigmaPlot version 11 software and significance was defined as p-value <0.05 (SigmaPlot 11.0, San Jose, CA).
Results
Transplantation conditions in brain support glioblastoma growth
Our previous studies demonstrated the utility of using a zebrafish xenograft model in which glioblastoma cells are injected into the brain ventricle to study invasion in real time.4 We also established the importance of injection location for studying human glioblastoma cells by demonstrating that glioblastoma cells implanted in the zebrafish yolk sac do not invade as they do when transplanted into the brain compartment.4 To adapt the model for compound screening against glioblastoma, we decided to inject the glioblastoma cells into the zebrafish hindbrain ventricle near the midbrain–hindbrain junction (Fig. 1C) between 2 and 3 DPF. At this time, minimal mortality was observed due to injection (∼1% and ∼20% for 2 and 3 DPF, respectively) and most major organ systems are fully developed during this time period, including a rudimentary blood–brain barrier allowing us to more closely simulate tests conducted in mammalian models.18,25 Based on work by others, we maintained our xenografts at 33°C, which is lower than the standard 37°C for culturing glioblastoma cells, but higher than the typical 28°C for zebrafish maintenance.6,15 This temperature caused no observable zebrafish toxicity, yet resulted in adequate human glioblastoma growth and migration/invasion over the 3–4 day assay period to detect exposure-dependent differences between treatment groups.
Control xenografts maintained at 33°C from 4 to 7 DPF typically demonstrated ∼1.6-fold increases in glioblastoma proliferation, whereas controls maintained at the same temperature for 3 to 7 DPF typically demonstrated ∼1.3- to 2-fold increases in growth (Figs. 5A and 7A controls). Cell migration/invasion experiments conducted from 3 or 4 to 7 DPF display greater movement (between 0.66 and 0.84-fold) near the main cell mass (0–80 μm), which tapers off as distance from the center increases with 0.21- to 0.34-fold changes for distances between 161 and 240 μm (Figs. 6 and 8 controls). The relative change in cell spread for distances 241+ μm was insignificant (<0.06-fold) and not depicted in Figures or discussed in subsequent results.
FIG. 5.
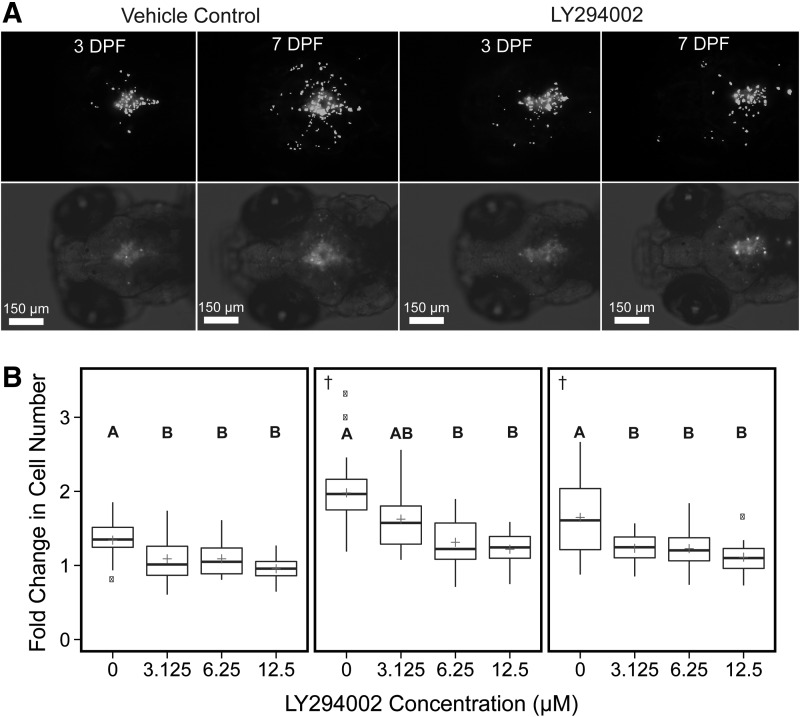
Comparison of the effects of increasing exposure to LY294002 on the fold change in human glioblastoma (U87MG) cell count data of each zebrafish xenograft within each treatment group. (A) consists of representative 10× images of two zebrafish imaged at 3 and 7 DPF. Each image panel consists of a 10× monochromatic fluorescent image with Multi Wavelength Cell Scoring mask (white) identifying individual cells (top) and a 10× monochromatic fluorescent image with brightfield overlay of the same xenograft showing injection placement in zebrafish brain (bottom). Each image was selected with an initial cell count of 45 and 54 cells. (B) The fold change as determined by the fluorescent signal above background from the CM-DiI dyed U87MG cells in each xenograft from each treatment group from initiation of the experiment at 3 DPF to the end at 7 DPF. The 0 treatment contained 0.5% DMSO, which was maintained across all groups. The plus indicates the overall mean of each treatment group. These values were determined from three independent experiments (left, middle, and right panels, n = 13–32 per experiment). Significant differences for all pairwise comparisons between treatments are indicated by letters (A, B), p < 0.05. †Datasets for which Kruskal–Wallis one-way ANOVA was performed. DMSO, dimethyl sulfoxide.
FIG. 7.
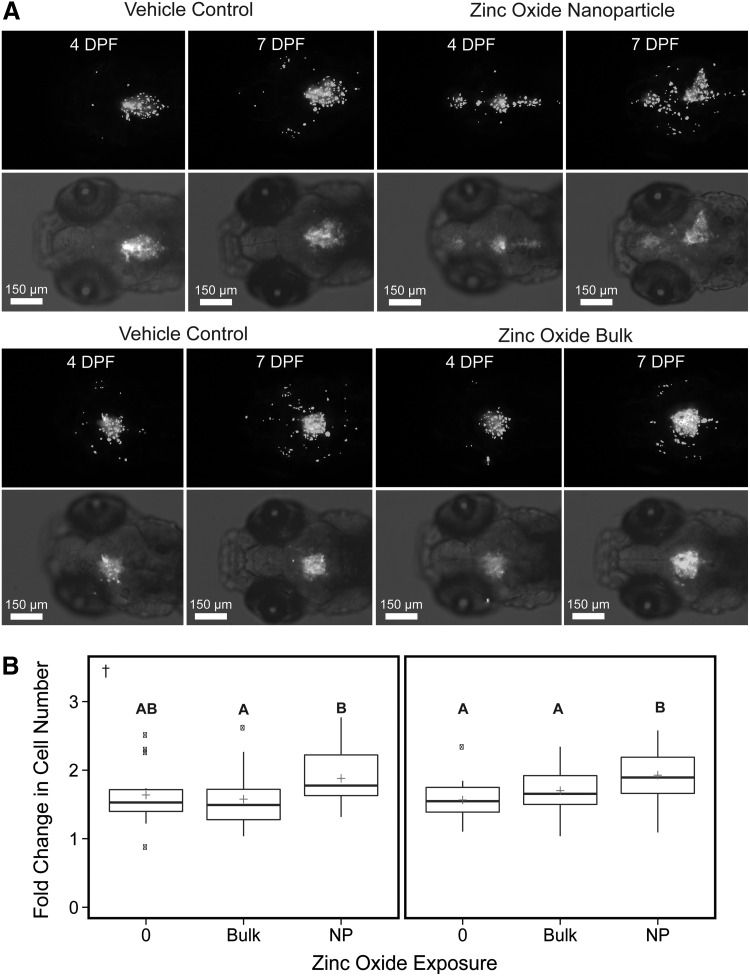
Comparison of the effects of exposure to ZnO NPs, bulk material control and vehicle control on the fold change in human glioblastoma (U87MG) cell count data of each zebrafish xenograft within each treatment group. (A) Consists of representative 10× images of four zebrafish imaged at 4 and 7 DPF. Each image panel consists of a 10× monochromatic fluorescent image with Multi Wavelength Cell Scoring mask (white) identifying individual cells (top) and a 10× monochromatic fluorescent image with brightfield overlay of the same xenograft showing injection placement in zebrafish brain (bottom). All images were selected with an initial cell count ranging from 51 to 59 cells. (B) The fold change as determined by the fluorescent signal above background from the CM-DiI dyed U87MG cell mass in each xenograft from each treatment group from initiation of the experiment at 4 and 7 DPF. The 0 treatment contained 0.5% DMSO, which was maintained across all groups. The plus indicates the overall mean of each treatment group. These values were determined from two independent experiments (left and right panels, n = 14–30 per experiment). Significant differences for all pairwise comparisons between treatments are indicated by letters (A, B), p < 0.05. †Datasets for which Kruskal–Wallis one-way ANOVA was performed.
FIG. 6.
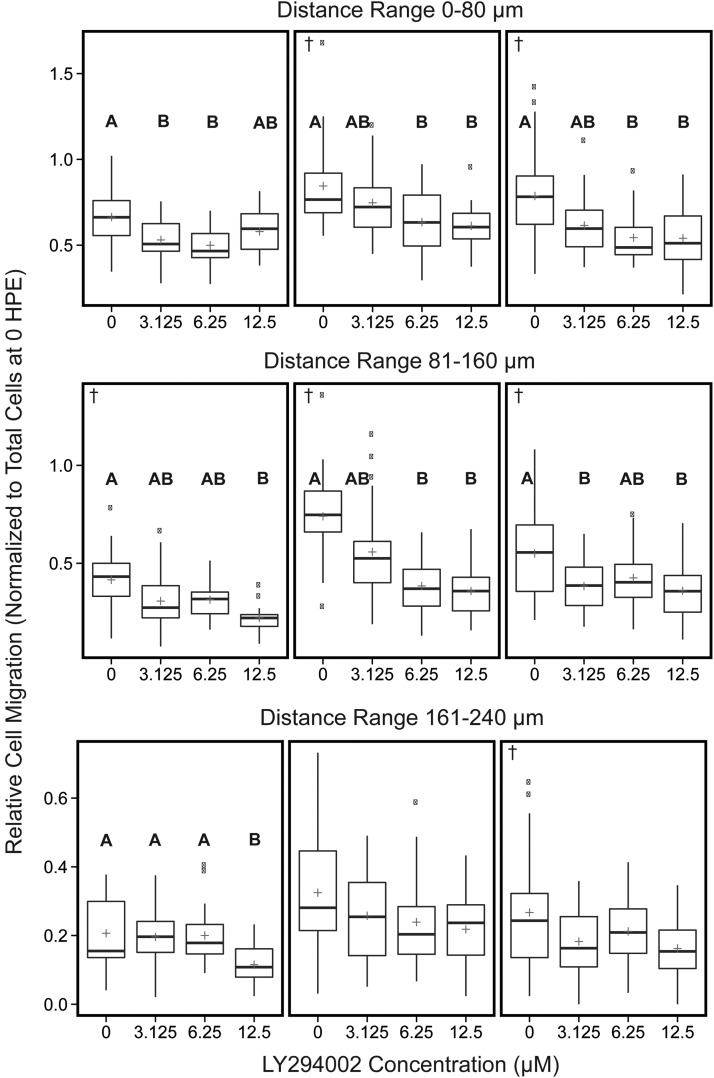
Quantitative analysis of human glioblastoma (U87MG) cell spread following LY294002 or vehicle control exposure. The number of migrated cells within concentric 80 μm distance regions from the centroid of U87MG mass was measured. Data reported as relative number of cells migrated within each distance region normalized, by division, to the total number of cells at 3 DPF. The 0 treatment contained 0.5% DMSO, which was maintained across all groups. The plus indicates the overall mean of each treatment group. These values were determined from three independent experiments (left, middle, and right panels, n = 13–32 per experiment). Note changes in y axis scale between the different distance regions. Significant differences for all pairwise comparisons between treatments are indicated by letters (A, B), p < 0.05. †Datasets for which Kruskal–Wallis one-way ANOVA was performed.
FIG. 8.
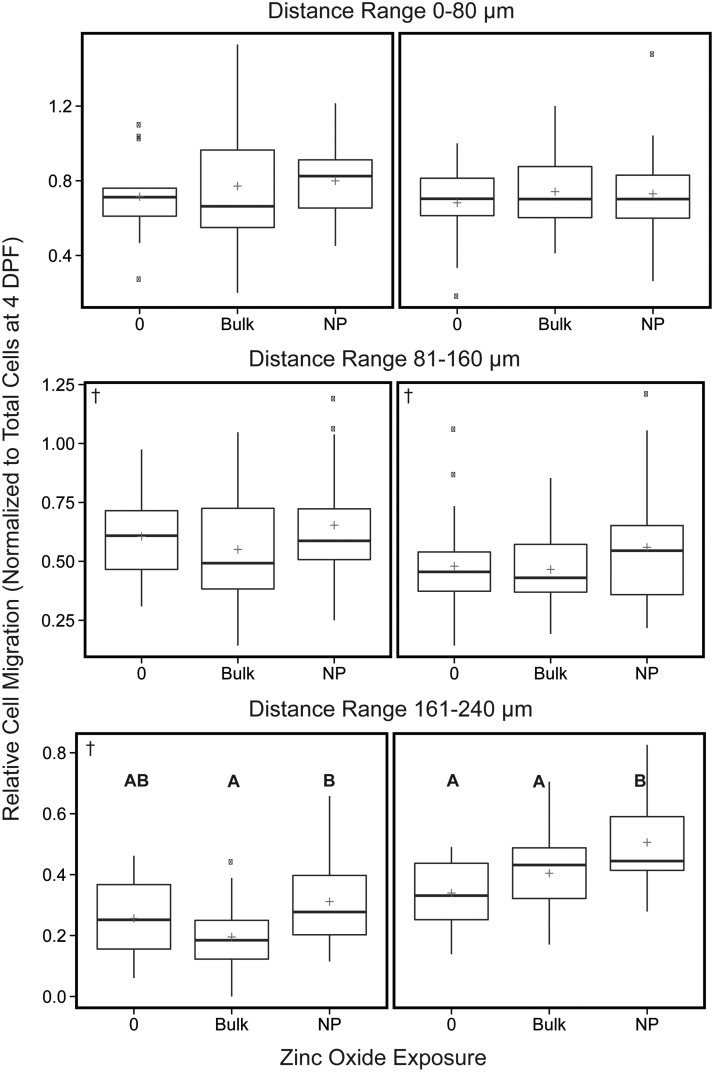
Quantitative analysis of human glioblastoma (U87MG) cell spread following ZnO NP, zinc oxide bulk (BULK), or vehicle control exposure. The number of migrated cells within concentric 80 μm distance regions from the centroid of U87MG mass was measured. Data reported as relative number of cells migrated within each distance region normalized, by division, to the total number of cells at 4 DPF. The 0 treatment contained 0.5% DMSO, which was maintained across all groups. The plus indicates the overall mean of each treatment group. These values were determined from two independent experiments (left and right panels, n = 14–30 per experiment). Note changes in y axis scale between the different distance ranges. Significant differences for all pairwise comparisons between treatments indicated by letters (A, B), p < 0.05. †Datasets for which Kruskal–Wallis one-way ANOVA was performed.
LY294002 inhibits U87MG proliferation and dispersal
To validate our newly developed assay, we evaluated the PI 3-kinase inhibitor LY294002, which is a well-studied compound known to inhibit glioblastoma proliferation, migration, and invasion in cell culture and rodent models.20,21,30,31 Exposure of zebrafish xenografts to 3.125–12.5 μM LY294002 resulted in significant decreases in glioblastoma proliferation compared with the vehicle control (Fig. 7) with trends toward concentration-dependent effects. However, exposure to 12.5 μM LY294002 caused significant mortality (Table 1), so any effects on proliferation and migration/invasion associated with this exposure concentration were excluded from further analyses. Exposure to 3.125–6.25 μM LY294002 resulted in minimal xenograft toxicity and up to 34% reduction in glioblastoma proliferation compared with the vehicle control (Fig. 5). LY294002 significantly reduced the number of cells that migrated/invaded in a concentration-dependent manner nearer the main cell mass (0–160 μm) with less influence at further distances (161+ μm) (Fig. 8). Within the distance range of 0–80 μm, 6.25 μM LY294002 significantly diminished the number of cells by 25% to 30% compared with vehicle control across all three experiments (Fig. 6). Exposure to 6.25 μM LY294002 reduced the number of cells that migrated/invaded outward to 81–160 μm by 23% to 48% compared with vehicle control (Fig. 6). While exposure to 3.125 μM LY294002 did not always significantly reduce migration/invasion compared with vehicle control, a trend is apparent across all experiments for all distance increments depicted (Fig. 6).
Table 1.
Mortality Associated with the Three LY294002 Exposures
| Treatment (μM) | N | Mortality (%) | Date |
|---|---|---|---|
| 0 | 33 | 6.06 | 1/8/2015 |
| 3.125 | 33 | 6.06 | |
| 6.25 | 33 | 3.03 | |
| 12.5 | 33 | 45.45a | |
| 0 | 28 | 0 | 12/12/2014 |
| 3.125 | 27 | 14.81 | |
| 6.25 | 27 | 0 | |
| 12.5 | 27 | 51.85b | |
| 0 | 23 | 4.35 | 12/3/2014 |
| 3.125 | 24 | 0 | |
| 6.25 | 24 | 8.33 | |
| 12.5 | 24 | 29.17 |
p-Value <0.01.
p-Value <0.001.
Zinc oxide enhances U87MG proliferation and dispersal
After validating that our embryo–larval zebrafish xenograft assay of glioblastoma was sensitive to detect exposure-dependent decreases in brain cancer proliferation, migration, and invasion using LY294002, we tested a putative anticancer nanotherapeutic (ZnO NPs). Previous research demonstrated these ZnO NPs significantly and preferentially inhibit cancer cell proliferation in the presence of normal human cells in culture, but whether that selective cancer toxicity is conserved in vivo has not been investigated.23 The minimal quantity of compound needed for assessment in our assay (∼1 μg per experiment) made it ideal for evaluating systemic toxicity as well as the cancer-specific efficacy of these ZnO NPs in vivo. Exposure to ZnO NP and bulk ZnO did not result in significant lethality, but there was a slight material-dependent increase in mortality compared with the vehicle control (Table 2). Exposure of xenografts to ZnO NP slightly enhanced glioblastoma proliferation by 17% to 23% compared with the vehicle control (Fig. 7). Xenografts exposed to ZnO NP also demonstrated between a 13% and 19% increase in glioblastoma proliferation compared with the ZnO bulk material control. ZnO NP exposure tended to augment glioblastoma migration/invasion slightly in both experiments across all distance increments compared with vehicle and ZnO bulk controls; however, significant increases were only observed near the perimeter of the cell mass (161–240 μm) (Fig. 8). At this distance, exposure to ZnO NP enhanced the number of glioblastoma cells migrating/invading outward by 22% to 49% compared with vehicle and by 25% to 59% compared with the ZnO bulk across the two experiments (Fig. 8).
Table 2.
Mortality Associated with the Two Zinc Oxide Nanoparticle Exposures
| Treatment (mg/L) | N | Mortality (%) | Date |
|---|---|---|---|
| 0 | 26 | 0 | 7/1/2013 |
| Bulk (0.312–1.25) | 32 | 6.25 | |
| NP (0.312–1.25) | 32 | 18.75 | |
| 0 | 15 | 0 | 11/30/2012 |
| Bulk (0.312–1.25) | 32 | 6.25 | |
| NP (0.312–1.25) | 32 | 18.75 |
NP, nanoparticle.
Discussion
Zebrafish xenograft assay supports study of glioblastoma biology
The main objective of this study was to create a robust zebrafish xenograft assay for the prioritization of putative anticancer compounds to advance glioblastoma drug development. Using this assay, we demonstrate the U87MG glioblastoma cells proliferate and disperse well in the zebrafish brain microenvironment over the assay period. We observed a 1.3- to 2-fold increase in growth over a 3–4 day period under vehicle control (0.5% DMSO) conditions (Figs. 5 and 7). Proliferation was quantified by counting individual cells based on size and fluorescent signal above background using image-based software analyses. While this automation increases the throughput of measuring proliferation, migration, and invasion in our zebrafish xenograft assay, compression of Z-stacks into one all-in-focus image needed for the analysis likely underestimates the growth and migration of glioblastoma cells due to a loss of information in the z-plane. Despite this limitation, other researchers have observed similar growth (∼1.5-fold) over 4 days.6,32 For instance, Geiger et al. observed similar fold increases in glioblastoma proliferation using red fluorescent protein-transfected U251 glioblastoma cells in an embryo–larval zebrafish xenograft assay that quantifies proliferation by measuring relative fluorescence.6 However, the 1.5-fold increase assumes that the U251 growth directly corresponds to a 1.5-fold increase in relative fluorescence.6 Also, Welker et al. quantified similar fold increases in an extrapolated measure of tumor volume using image-based analysis methods with GBM9 neurospheres implanted within the zebrafish brain at a similar location to the model described in this article and with similar cell implantation numbers (∼50).32
Under control experimental conditions, the U87MG cells also move from the main cancer cell mass (Figs. 6 and 8), which we quantify as a surrogate for invasion and migration. Measurements of migration and invasion in embryonic zebrafish xenograft models has typically involved qualitative scoring or simply counting the number of cells to move away from the initial cell mass with no information on distance.5,15,16 Our method provides a quantitative method to measure changes in the cells spread outward over concentric distances from the center of the cancer mass. This approach is similar to Zhang et al. who measured chemical inhibition of leukemia stem cell migration in a zebrafish xenograft model.33 However, Zhang et al. reported the migration results in a way in which the distance information was lost making it impossible to tell how far the cells metastasized from the main mass.33 Differences in approaches make it difficult to compare our results to others reported in the literature, except to say that migration and invasion are observed in some zebrafish xenograft models. However, our migration/invasion results for glioblastoma differ from those reported by Geiger et al., where no migration or invasion of U251 cells was observed.6 This may be partially explained by differences in injection site locations between the two experiments.6 Geiger et al. implanted U251 cells in the embryonic zebrafish yolk sac and Lal et al. reported that glioblastoma cells do not migrate or invade when implanted into the zebrafish yolk compared with implantation in the brain.4 Differences in cell lines used between the two experiments do not justify the variation in results by Geiger et al. as ongoing experiments in our laboratory demonstrate that U251 cells grow, migrate, and invade similarly to U87MG cells (data not shown). Another group studying glioblastoma invasion in an embryonic zebrafish xenograft model only observed invasion of U87 cells implanted in the zebrafish yolk sac after injecting a subpopulation enriched for stem cells.15 In a more similar xenograft model to the one described in this article, Welker et al. quantified GBM9 neurosphere migration in the zebrafish brain up to 1.2 mm from the injection site after 7 days posttransplantation or ∼8.5 DPF.32 However, this value is difficult to compare with our results since the measurements were made in a more mature zebrafish after 3–4 day longer growth period. Furthermore, without reviewing all the initial and final images in the dataset, it is not apparent what proportion of these cells migrated 1.2 mm within each fish and how much the initial spread of the cells from the injection influenced that distance. No normalized change in migration is reported, only the final measured endpoint. Yet, this work and work by Lal et al. exemplify the importance of injection site location and microenvironment in influencing realistic glioblastoma behavior.4,15,32
Application of zebrafish xenograft assay for drug prioritization against cancer
Our embryo–larval zebrafish xenograft model supports reproducible human glioblastoma proliferation, migration, and invasion beneficial to studying the biological processes controlling this deadly disease. We then utilized the well-studied PI 3-kinase inhibitor LY294002 to validate that our model, over a short 4-day assay period, is sensitive to exposure-induced decreases in glioblastoma proliferation, migration, and invasion. We selected LY294002 as a model antiproliferative and anti-invasive agent because glioblastomas frequently possess a genomic mutation that results in the loss of tumor suppressor PTEN expression, thereby escalating PI 3-kinase activation. The PTEN mutation is present in 30% to 40% of malignant gliomas and U87MG cells.34–36 PTEN dephosphorylates phosphatidylinositol (3,4,5)-trisphosphate (PtdIns (3,4,5)-P3)-mediated activation of serine/threonine kinase (AKT), thereby controlling cell proliferation, survival, migration, and invasion.22,30,37 Inhibiting PI 3-kinase activity with LY294002 decreases glioblastoma proliferation, migration, and invasion by mitigating enhanced AKT phosphorylation.
Exposure to LY294002 significantly inhibited glioblastoma proliferation by ∼26% (Fig. 5). Several in vitro and in vivo experiments with glioblastoma corroborate these results.20,21,30,31 For instance, our proliferation data are similar to results by Su et al., where dosing orthotopic mouse xenografts with LY294002 resulted in ∼40% inhibition of glioblastoma growth compared with controls after 5 days. The similarity in results is somewhat surprising as Su et al. utilized a repeated dosing regimen compared with the static nonrenewal exposure in our experiment.21 Another mouse study using a subcutaneous glioblastoma xenograft model identified that intratumoral administration of LY294002 significantly reduced tumor volume by ∼50% after 12 days.20 Besides directly impacting the growth of the glioblastoma cell, LY294002 can influence the surrounding environment through antiangiogenic effects, which can also reduce glioblastoma proliferation.21 The reduction in glioblastoma proliferation by LY294002 in our xenograft assay could be partly due to a reduction in angiogenesis. Despite assay and model differences, the zebrafish xenografts absorb sufficient LY294002 to significantly inhibit glioblastoma proliferation similar to rodent models. In vitro research reveals that LY294002 exposure reduces the number of glioblastoma cells that migrate by 20% to 50% at 5–10 μM, which is not much different from the 30% to 48% reductions in migration/invasion observed upon 6.25 μM exposure from our assay (Fig. 6).20,38 Interestingly, Joy et al. demonstrated that migrating glioblastoma cells become more resistant to apoptosis than migration-restricted cells. This suggests that identifying therapeutics that inhibit glioblastoma migration and invasion may be important in sensitizing glioblastoma to apoptosis.22 This embryo–larval zebrafish xenograft model allows for identification of anti-invasive as well as antiproliferative therapeutics in one assay, which is very challenging in orthotopic rodent models.
After validating our assay with LY294002, we assessed the putative anticancer efficacy of ZnO NPs against glioblastoma. These ZnO NPs were selected for our assay because of ∼28- to 35-fold preferential toxicity to cancerous T cells relative to normal peripheral blood mononuclear cells in vitro23 and existing literature demonstrates that other ZnO NPs selectively inhibit glioblastoma proliferation in culture.39,40 However, these in vitro anticancer effects are often seen at high exposure concentrations (mg/L or mM levels), which may call into question the relevancy of the results. Yet, it was not known if this selective toxicity would be conserved in vivo. Scaling up the production of nanomaterials to the quantities necessary to conduct typical in vivo rodent assays for toxicity or efficacy assessment can be challenging especially during the early stages of nanomaterial development. With our zebrafish xenograft assay of glioblastoma, we could quickly determine whether the ZnO NPs were good in vivo candidates for further anticancer development against glioblastoma using only ∼1–2 μg quantities of material per assay.
The high ZnO NP exposure concentrations used in the in vitro studies23,39,40 caused toxicity in the embryo–larval zebrafish assay. At lower, nontoxic exposure concentrations (0.156–1.25 mg/L), the ZnO NPs slightly enhanced glioblastoma proliferation and migration/invasion compared with the vehicle control (Figs. 7 and 8), which is the opposite of what we hypothesized would occur based on the in vitro data. However, the exposure concentrations used in the zebrafish xenograft assay were lower than the effective inhibitory concentrations in in vitro studies,23,39,40 and we also identified that the ZnO NP agglomerated and underwent considerable dissolution (∼47%–50%) when prepared in water (data previously published28). Therefore, we cannot distinguish whether this increase in glioblastoma proliferation is due to a low-exposure NP effect, an ionic effect, or a combination of both. Research in breast and prostate cancer cells with overexpression of zinc ion importers demonstrates that exposure to small amounts of ionic zinc can enhance cancer cell proliferation and invasion.41,42 Lin et al. identified several zinc ion importers with increased expression in malignant glioma samples and waterborne exposure of embryonic zebrafish to zinc can result in distribution to the brain.43,44 Furthermore, invasion of glioblastoma cells is partly controlled by matrix metalloproteinases (MMPs), which require zinc as cofactor. Malignant gliomas often possess upregulation of MMP2 and MMP9.45,46 Based on this data, we hypothesize that the ZnO NP exposure could be delivering enough zinc to the glioblastoma in the zebrafish brain to enhance growth, migration, and invasion. Our short, 3-day assay allowed us to identify that this ZnO NP is unstable and slightly enhances glioblastoma growth in vivo. These results, in addition to evidence that ZnO NPs seem to only be effective at extremely high concentrations (∼62 and 814 mg/L for instance) in in vitro assays,39,40 suggest that these ZnO NPs in their current state are not a good candidate for future preclinical assessment against glioblastoma in mammalian models. These results illustrate how this short assay successfully combines the rapid economy of in vitro assays with the relevance of slower in vivo rodent assays to aid advances in drug and nanotherapeutic development.
Conclusions
Glioblastoma is an aggressive brain cancer requiring improved treatment options to increase patient survival. Under existing drug development paradigms, any new glioblastoma drugs will typically require several years to transition from discovery phase to approved human use, which demonstrates a dire need to accelerate the drug development process. Incorporation of embryonic zebrafish assays into the drug development pipeline already promises to enhance discovery and prioritization of novel pharmaceuticals, including nanomaterials. The results of this assay demonstrate that we have a short duration, relevant and sensitive embryonic zebrafish-based assay to detect compound induced alterations in glioblastoma proliferation and migration/invasion, which will assist in identifying and prioritizing novel glioblastoma therapeutic agents.
Ongoing research in the laboratory will determine whether typical aggressive/invasive glioblastoma behavior (i.e., growth patterns, migration and invasion) is conserved using other glioblastoma cell types. Current efforts are examining whether other well-studied compounds targeting different cancer-associated signaling pathways in glioblastoma remain effective in our zebrafish xenograft model. Research by others in a similar but longer duration assay already suggests this to be the case, and these results, in conjunction with our contributions, strengthen the relevance of this model and assay.32 Future research will investigate whether ionic zinc, at exposure concentrations comparable to the dissolved zinc present in the ZnO NP experiments, significantly enhances glioblastoma proliferation and dispersal like ZnO NP. Additional research will involve identifying a compound that strictly targets glioblastoma migration/invasion without the confounding effects of proliferation inhibition. This will be useful as a control in future assays and in classifying potential therapeutics targeting migration and invasion. Some MMP inhibitors have proven effective in selectively inhibiting glioblastoma dispersal in other zebrafish xenograft assays.15 Use of transgenic fli1:eGFP zebrafish with fluorescent vasculature will aid in distinguishing between migrating and invading glioblastoma cells and help identify compounds that affect angiogenesis as well as glioblastoma proliferation, migration, and invasion. Three-dimensional reconstruction of confocal time series images will augment the sensitivity of the zebrafish xenograft model by providing information about movement and proliferation of cells in the z plane of the head in real time. Quantifying molecular changes associated with exposure to identify and confirm therapeutic targets will also improve model and assay significance. Increasing the incubation temperature of the xenografts to 35°C or incorporating heat-tolerant transgenic zebrafish so that xenografts can be maintained at 37°C may increase the sensitivity of this assay by enhancing glioblastoma growth without adding to the length of the assay.
Acknowledgments
The author thanks Dr. Cliff Pereira for guidance on statistical analyses. The author also acknowledges Jane LaDu and Dr. Sangeet Lal for helping establish the zebrafish xenograft model, members of the Punnoose laboratory for supplying ZnO NPs, and the Sinnhuber Aquatic Research Laboratory for providing zebrafish. Thanks also go to Dr. Josephine Bonventre for assistance with the article editing.
The authors alone are responsible for the content and writing of the article. This research was funded by NSF 1134468 and 1310657 and NIH T32 ES07060 and P30 ES000210.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. New Engl J Med 2005;352:997–1003 [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis LM. Brain tumors. New Engl J Med 2001;344:114–123 [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008;455:1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lal S, La Du J, Tanguay RL, Greenwood JA. Calpain 2 is required for the invasion of glioblastoma cells in the zebrafish brain microenvironment. J Neurosci Res 2012;90:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marques IJ, Weiss FU, Vlecken DH, Nitsche C, Bakkers J, Lagendijk AK, et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009;9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiger GA, Fu W, Kao GD. Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res 2008;68:3396–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci U S A 2007;104:17406–17411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoli S, Ribatti D, Cotelli F, Presta M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res 2007;67:2927–2931 [DOI] [PubMed] [Google Scholar]
- 9.Haldi M, Ton C, Seng WL, McGrath P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 2006;9:139–151 [DOI] [PubMed] [Google Scholar]
- 10.Webb DJ, Horwitz AF. New dimensions in cell migration. Nat Cell Biol 2003;5:690–692 [DOI] [PubMed] [Google Scholar]
- 11.Fako VE, Furgeson DY. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv Drug Deliv Rev 2009;61:478–486 [DOI] [PubMed] [Google Scholar]
- 12.Stoletov K, Kato H, Zardouzian E, Kelber J, Yang J, Shattil S, et al. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci 2010;123:2332–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 2005;4:35–44 [DOI] [PubMed] [Google Scholar]
- 14.Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol 2004;28:9–28 [DOI] [PubMed] [Google Scholar]
- 15.Yang XJ, Cui W, Gu A, Xu C, Yu SC, Li TT, et al. A novel zebrafish xenotransplantation model for study of glioma stem cell invasion. PLoS One 2013;8:e61801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SL, Rouhi P, Dahl Jensen L, Zhang D, Ji H, Hauptmann G, et al. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci U S A 2009;106:19485–19490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn 2005;233:1560–1570 [DOI] [PubMed] [Google Scholar]
- 18.Jeong J-Y, Kwon H-B, Ahn J-C, Kang D, Kwon S-H, Park JA, et al. Functional and developmental analysis of the blood–brain barrier in zebrafish. Brain Res Bull 2008;75:619–628 [DOI] [PubMed] [Google Scholar]
- 19.Jang H, Lal S, Greenwood J. Calpain 2 is required for glioblastoma cell invasion: regulation of matrix metalloproteinase 2. Neurochem Res 2010;35:1796–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han L, Yang Y, Yue X, Huang K, Liu X, Pu P, et al. Inactivation of PI3K/AKT signaling inhibits glioma cell growth through modulation of beta-catenin-mediated transcription. Brain Res 2010;1366:9–17 [DOI] [PubMed] [Google Scholar]
- 21.Su JD, Mayo LD, Donner DB, Durden DL. PTEN and phosphatidylinositol 3′-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res 2003;63:3585–3592 [PubMed] [Google Scholar]
- 22.Joy AM, Beaudry CE, Tran NL, Ponce FA, Holz DR, Demuth T, et al. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci 2003;116:4409–4417 [DOI] [PubMed] [Google Scholar]
- 23.Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, et al. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008;19:295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reimers MJ, La Du JK, Periera CB, Giovanini J, Tanguay RL. Ethanol-dependent toxicity in zebrafish is partially attenuated by antioxidants. Neurotoxicol Teratol 2006;28:497–508 [DOI] [PubMed] [Google Scholar]
- 25.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253–310 [DOI] [PubMed] [Google Scholar]
- 26.Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, Lang C, et al. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom 2012;17:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann A, Mickoleit M, Weber M, Huisken J. Multilayer mounting enables long-term imaging of zebrafish development in a light sheet microscope. Development 2012;139:3242–3247 [DOI] [PubMed] [Google Scholar]
- 28.Wehmas LC, Anders C, Chess J, Punnoose A, Pereira CB, Greenwood JA, et al. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicology Rep 2015;2:702–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KT, Truong L, Wehmas L, Tanguay RL. Silver nanoparticle toxicity in the embryonic zebrafish is governed by particle dispersion and ionic environment. Nanotechnology 2013;24:115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubiatowski T, Jang T, Lachyankar MB, Salmonsen R, Nabi RR, Quesenberry PJ, et al. Association of increased phosphatidylinositol 3-kinase signaling with increased invasiveness and gelatinase activity in malignant gliomas. J Neurosurg 2001;95:480–488 [DOI] [PubMed] [Google Scholar]
- 31.Shingu T, Yamada K, Hara N, Moritake K, Osago H, Terashima M, et al. Growth inhibition of human malignant glioma cells induced by the PI3-K-specific inhibitor. J Neurosurg 2003;98:154–161 [DOI] [PubMed] [Google Scholar]
- 32.Welker AM, Jaros BD, Puduvalli VK, Imitola J, Kaur B, Beattie CE. Standardized orthotopic xenografts in zebrafish reveal glioma cell-line specific characteristics and tumor cell heterogeneity. Dis Model Mech 2016;9:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, Shimada Y, Kuroyanagi J, Umemoto N, Nishimura Y, Tanaka T. Quantitative phenotyping-based in vivo chemical screening in a zebrafish model of leukemia stem cell xenotransplantation. PLoS One 2014;9:e85439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst 2001;93:1246–1256 [DOI] [PubMed] [Google Scholar]
- 35.Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, et al. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res 1997;57:4183–4186 [PubMed] [Google Scholar]
- 36.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997;275:1943–1947 [DOI] [PubMed] [Google Scholar]
- 37.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A 1999;96:4240–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HC, Park IC, Park MJ, An S, Woo SH, Jin HO, et al. Sulindac and its metabolites inhibit invasion of glioblastoma cells via down-regulation of Akt/PKB and MMP-2. J Cell Biochem 2005;94:597–610 [DOI] [PubMed] [Google Scholar]
- 39.Ostrovsky S, Kazimirsky G, Gedanken A, Brodie C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res 2009;2:882–890 [Google Scholar]
- 40.Wahab R, Kaushik N, Verma A, Mishra A, Hwang I, Yang Y, et al. Fabrication and growth mechanism of ZnO nanostructures and their cytotoxic effect on human brain tumor U87, cervical cancer HeLa, and normal HEK cells. J Biol Inorg Chem 2011;16:431–442 [DOI] [PubMed] [Google Scholar]
- 41.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci 2007;98:692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A 2007;104:18636–18641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brun NR, Lenz M, Wehrli B, Fent K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: importance of zinc ions. Sci Total Environ 2014;476–477:657–666 [DOI] [PubMed] [Google Scholar]
- 44.Lin Y, Chen Y, Wang Y, Yang J, Zhu VF, Liu Y, et al. ZIP4 is a novel molecular marker for glioma. Neuro Oncol 2013;15:1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, et al. Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg 1994;81:69–77 [DOI] [PubMed] [Google Scholar]
- 46.Nakano A, Tani E, Miyazaki K, Yamamoto Y, Furuyama J. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gliomas. J Neurosurg 1995;83:298–307 [DOI] [PubMed] [Google Scholar]