Abstract
Environmental factors may influence types and contents of active substances. This study investigated the influence of environmental factors on the active substance contents and antioxidant activity of Potentilla fruticosa L. from different regions of China. Also, HPLC fingerprint similarity analysis (SA) coupled with hierarchical cluster analysis (HCA) and discriminant analysis (DA) were further introduced for the accurate classification and quality assessment of P. fruticosa. The results showed that altitude was significantly and negatively correlated to the content of tannin (P < 0.05). Annual sunshine duration and altitude were significantly and positively correlated to the flavonoids content, rutin content and antioxidant activity (P < 0.05). Annual mean temperature was significantly and negatively correlated to the content of total phenolics, while altitude was significantly and positively correlated to the content of total phenolics (P < 0.05). Eight samples were unambiguously separated into three groups. Two types of discriminant functions with a 100% discrimination ratio were constructed. All data consistently supported the conclusion that P. fruticosa produced from Kangding, Sichuan Province had high quality among all samples, therefore, Kangding in Sichuan Province with favorable environmental conditions is recommended as a preferable production location.
The quality of traditional medicine, which plays a very important role in the health system of China, is determined by its active substances produced by the plants. Potentilla species have been used for a long time in traditional medicine for its curative properties. In Chinese traditional medicine Potentilla extracts have been used to treat diarrhoea, hepatitis, rheuma and scabies and as a remedy for detoxification1,2. Potentilla fruticosa L.is a species of hardy deciduous flowering shrub in the Potentilla genus of the family Rosaceae, native to the cool temperate and subarctic regions of the northern hemisphere, often growing at high altitudes in mountains3. Apart from common application as a garden plant, it also has numerous medicinal virtues4. Extracts of P. fruticosa have been shown to possess relatively high concentrations of phenolic acids and flavonoids and powerful radical scavenging capacity5,6,7. The activity of some extracts was higher than that of the synthetic antioxidant BHT (butylated hydroxytoluene) and of extracts isolated from Salvia officinalis L., which contains powerful antioxidants7. Moreover the leaves of P. fruticosa have applications as food additives and an ingredient in cosmetic products8. Similarly the same or other (local) Potentilla species have been used in traditional medicine of different cultures in Asia, Europe and Northern America. Modern scientific researches have confirmed that the material foundations of traditional Chinese herbal drugs are the different chemical constituents (most of them are secondary metabolites) that are contained in different raw plant materials9.
In China, P. fruticosa is commonly known as the “Jinlaomei medicine” and “Gesanghua” and is widely distributed in Qinghai, Gansu, Sichuan, Yunnan, Tibet and Heilongjiang, its altitude ranges from 400 to 5000 m10,11. For this widespread species, active substances that are contained in the same plant species may be different in types, contents, and proportions of the constituents because of the environmental differences in growing locations. Active substances are the result of the interaction between plants and the environment in the long evolution process, and its production and changes have a strong correlation and association with the environment12. Certain substances are only synthesized under specific environments, or the contents of certain substances may significantly increase under specific environments12. Studies have reported on the influences of growth environment on active substances of other medicinal plants. For example, altitude and annual mean temperature were significantly and positively correlated to the contents of chlorogenic acid and flavonoids (P < 0.05); annual sunshine duration was significantly and positively correlated to the content of geniposidic acid (P < 0.05), while annual mean temperature was significantly and negatively correlated to the content of geniposidic acid (P < 0.05) in Eucommia ulmoides Oliv9. In Betula pendula Roth., altitude was positively correlated to the contents of flavonoids13. Among the Sinopodophyllum hexandrum (Royle) T.S. Ying populations, the existing variations in podophyllotoxin content were proved to be coupled with geographical altitude and local ecological conditions (temperature, rainfall, humidity, soil pH, etc.) but not with genetic basis14,15. Previous studies have demonstrated that medicinal plants that grow in various environments produce different active substance contents because of their wide distribution in different geological zones. This will result in variations in their internal qualities in the same species from different growing regions9, making the quality assessment of widespread species P. fruticosa extremely crucial. Additionally, because applications for P. fruticosa are growing consistently, exploring a reliable quality assessment method is essential. Traditionally, the qualities of traditional Chinese herb medicines (TCHMs) were assessed by their external appearance, and by the experiences of medicinal practice of ancient physicians16. In this process, the concept of so called geo-authentic herbal drugs was established. It is assumed that most geo-authentic traditional herbs produced in their native geographical area contain adequate effective chemical constituents. For example, only Picrorhiza scrophulariiflora Pennell., one of the well-known herbal drugs in TCHM, produced in Tibet, China is officially recognized for use in medicinal practice17. By contrast, only Panax ginseng C. A. Mey., produced in northeastern China is officially recognized as medicinal drug17. Currently, high performance liquid chromatography (HPLC) techniques coupled with multivariate statistical methods (chemometric methods) have been employed extensively to classify and distinguish various herbs18,19,20, which is regarded as a reasonable approach for the quality evaluation of complicated TCHMs21. For example, twelve raw herbs of Artemisia selengensis Turcz ex Besser collected from five provinces of China were better clustered into three groups by HPLC combined with chemometrics methods viz. similarity analysis (SA), hierarchical clustering analysis (HCA) and principal component analysis (PCA); with fingerprint matching and discrimination, classification of samples becomes the preferred method of sample comparison for quality assurance requirements22. HPLC fingerprint analysis, PCA, and cluster analysis (CA) were introduced for quality assessment of Cortex cinnamomi and showed that 30 samples of Cortex cinnamomi from different species and geographic locations were rationally divided into three groups23. Chemical fingerprints of Lingzhi (Ganoderma) strains were evaluated statistically using HCA and discriminant analysis (DA) in order to classify the samples and to identify key categorizing parameters. The results indicated fifteen representative Lingzhi strains were separated into three groups, thereby confirming divisions based on morphological characteristics and providing reference data for its quality assessment20. Therefore, in order to produce qualified TCHMs, the influences of ecological factors should be investigated to determine the suitable production locations. On the other hand, quality evaluation of TCHMs should be performed by the HPLC fingerprint method coupled with multivariate statistical techniques.
In this study, the authors therefore investigated the influences of environmental factors on the main active substances (tannin, total flavonoids, rutin, total phenolics) and antioxidant activity of P. fruticosa from representative growing locations throughout China where P. fruticosa has been naturally growth according to historical records. Moreover, HPLC fingerprint similarity analysis (SA) coupled with hierarchical cluster analysis (HCA) and discrimination analysis (DA) were further introduced for accurate identification, classification and quality assessment of P. fruticosa. The present study aims at (1) clarifying the environmental factors affecting the production of active ingredients and antioxidant activity of P. fruticosa, (2) classifying the same P. fruticosa species from different regions based on HPLC fingerprint data for quality assessment, (3) suggestting the best production areas for this wild species, and (4) promoting its reasonable exploitation for the raw material production of medicinal drugs rather than arbitrary harvesting wild resources.
Results
Validation of the HPLC procedure
The precision and repeatability of the method were assessed using seven injections of sample solutions and six replicates of dry solid plant samples (re-extracting), respectively23. For the common peaks (Fig. 1, peaks 1–10) from duplicate injections, the relative standard deviations (RSD) of relative retention times (RRT) and relative peak areas (RPA) were found to be in the range of 0.02–0.06% and 0.22–2.91%, respectively (n = 7), which were calculated, respectively, to be 0.03–0.13% and 1.29–2.74% for six replicates of the solid samples (n = 6). To evaluate the accuracy of the method, we conducted a recovery experiment in which quantified analytes were mixed with specific amounts of standard components. The average percent recoveries for common peaks were in the range of 97.24 ± 0.02% to 104.31 ± 0.04%. The RSDs shifted from 1.19% to 2.31% (n = 6). The limit of detection (LOD) (signal/noise = 3) and the limit of quantification (LOQ) (signal/noise = 10) of the ten compounds (ten common peaks) varied within the range 1.95–3.12 ng/mL and 7.82–10.36 ng/mL, respectively. The stability of common peaks in the sample solutions that were maintained for 0–24 h was evaluated by determining their RPAs. The RSDs of RRTs and RPAs were found to be less than 3%. These results demonstrated that the conditions for the fingerprint analysis were optimal.
Figure 1. Chromatography of all the samples from eight different growing locations.
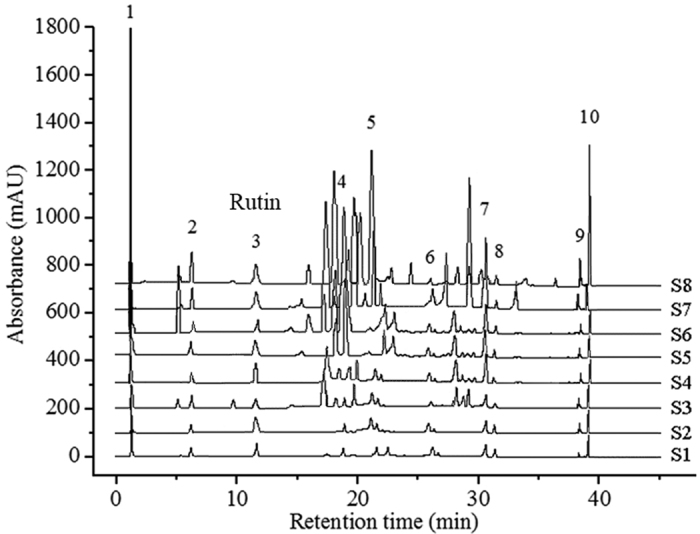
S1, Mei county, Shaanxi; S2, Diebu, Gansu; S3, Huzhu, Qinghai; S4, Jingyuan, Ningxia; S5, Yongdeng, Gansu; S6, Shangri-la, Yunnan; S7, Ningchi, Tibet; S8, Kangding, Sichuan.
Differences in active ingredient contents
The active ingredient contents of P. fruticosa leaves differed significantly because of their various growing locations (P < 0.05, Fig. 2). The highest tannin content (10.68%), flavonoids content (4.37%), and rutin content (0.79%) were found in leaves from the Kangding, Sichuan (S8), whereas the lowest tannin content (7.64%) and rutin content (0.19%) were observed in leaves from Shangri-la, Yunnan (S6). The lowest flavonoids content (3.29%) was found in leaves from Jingyuan, Ningxia (S4). Moreover, the leaves from Shangri-la, Yunnan (S6) had higher phenolic contents than any other growing location, with total phenolic contents at 9.40%. By contrast, leaves from the Jingyuan, Ningxia (S4) had the lowest total phenolic contents (5.29%). These rich differences may be due to ecological factors, genetic factors and the status of secondary metabolism in different growing locations.
Figure 2. Differences of active ingredient contents in the leaves of P. fruticosa in different growing locations.
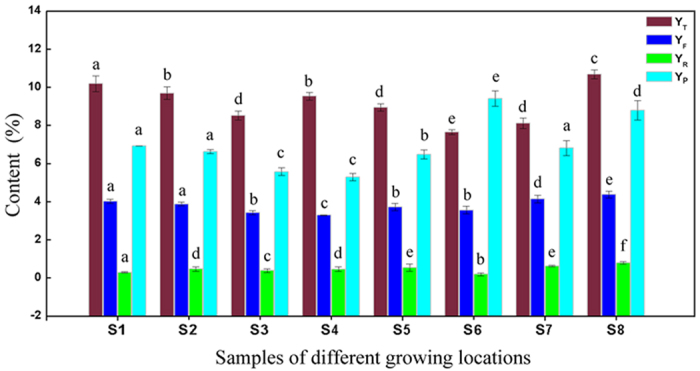
For the same variable, bars with no letters in common are significantly different (P < 0.05). YT (%), tannin content; YF (%), total flavonoids content; YR (%), rutin content; YP (%), total phenolics content. S1, Mei county, Shaanxi; S2, Diebu, Gansu; S3, Huzhu, Qinghai; S4, Jingyuan, Ningxia; S5, Yongdeng, Gansu; S6, Shangri-la, Yunnan; S7, Ningchi, Tibet; S8, Kangding, Sichuan.
DPPH radical scavenging activity
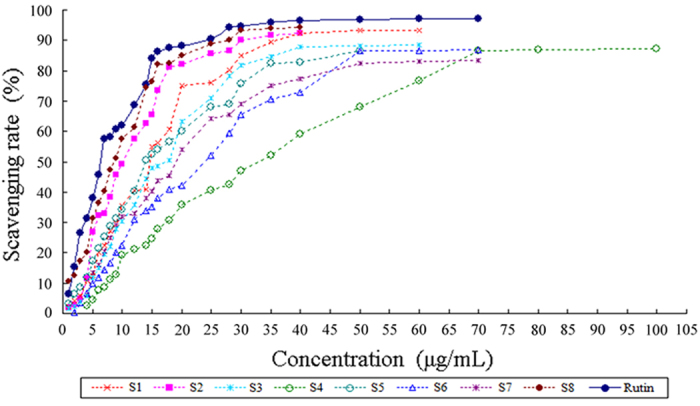
For evaluation of antioxidant activity of P. fruticosa, DPPH radical scavenging activity24 of different samples were compared and showed in Fig. 3. The scavenging effects of different locations increased with concentrations between 1 and 100 μg/mL. Among all samples, the highest radical scavenging activity was obtained for P. fruticosa collected from S8 (Kangding, Sichuan) with the lowest average IC50 value of 9.24 ± 0.04 μg/ml, followed by S2 (Diebu, Gansu, 10.01 ± 0.06 μg/ml). For P. fruticosa samples from eight regions, the IC50 values changed with the order: Rutin (5.85 ± 0.02 μg/ml) < S8 (9.24 ± 0.04 μg/ml) < S2 (10.01 ± 0.06 μg/ml) < S5 (14.25 ± 0.11 μg/ml) < S1 (14.76 ± 0.21 μg/ml) < S3 (17.63 ± 0.37 μg/ml) < S7 (18.94 ± 1.04 μg/ml) < S6 (23.68 ± 1.28 μg/ml) < S4 (32.35 ± 2.65 μg/ml). Compared with rutin standard, there was no significant difference (P < 0.05) on the antioxidant activities of S8, implying that S8 could have same scavenging effect with rutin standard at adequate concentration. These data indicated that P. fruticosa had high antioxidant activity, and the antioxidant activity of the same P. fruticosa species of different locations varied immensely from region to region.
Figure 3. DPPH radical scavenging activity of eight P. fruticosa samples from different growing locations.
Ferric reducing activity power (FRAP) assay
The antioxidant properties of the samples from different growing locations were evaluated based on their reducing ability using the FRAP assay. The FRAP values obtained from eight samples were significantly different (p < 0.05), which presented an inverted parabola (Fig. 4). In details, as to P. fruticosa derived from eight regions, the FRAP value ranged from 195.58 ± 6.86 to 416.58 ± 8.62 μmol equiv. Trolox/g. P. fruticosa sampled from S8 (Kangding, Sichuan) possessed the highest antioxidant capacity with a FRAP value value of 416.58 ± 8.62 μmol equiv. Trolox/g, followed by samples from S2 (Diebu, Gansu) and S1 (Mei county, Shaanxi) with values of 374.43 ± 6.47 and 337.91 ± 3.41 μmol equiv. Trolox/g, respectively.
Figure 4. Reducing power of eight P. fruticosa samples from different growing locations for ferric reducing activity power (FRAP) assay.
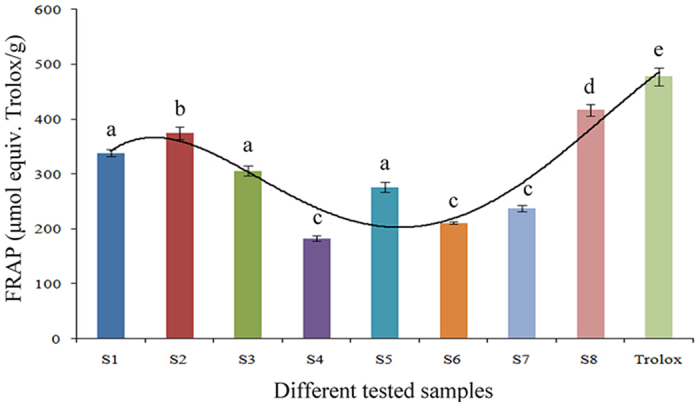
The FRAP values showed the same order of activity observed in the DPPH method: S8 > S2 > S1 > S3 > S5 > S7 > S6 > S4. Based on these results, we concluded that P. fruticosa samples presented not only free radical scavenge capacity but also reducing capacity. Also, P. fruticosa samples from Kangding, Sichuan (S8) was strongest in antioxidant capacity among all samples, which was close to the rutin and trolox standard. Furthermore, the antioxidant capacity of P. fruticosa samples exhibited significant differences in the same species from different regions (p < 0.05).
Analysis of environmental factors influencing the active ingredients and antioxidant activity
Principal component analysis (PCA) of environmental factors
The PCA was conducted to identify the principal variables from a lot of independent variables (environmental factors). Contribution rate reflects the quantity of original information contained within each factor. The accumulated contribution rate of the first three eigenvalues reached 95.867% (Table 1), which indicates that the first three main components nearly covered total original information of the eighteen environmental factors. Thus, these components can be extracted to obtain the loading level of each environmental factor based on SPSS 19.0 software (Fig. 5). Figure 5 indicated that important environmental factors that effected the first principal component (F1) were XN (0.872), XAAP (0.833), XALTI (0.753), XpH (0.651), XAAT (0.434), XAMT (−0.132), and XP (−0.472). For the second principal component (F2), important environmental factors were the X7 (0.853), X1 (0.815), XASD (0.757), and XTP (−0.123). The third principal component (F3) accounted for a larger proportion in the XOM (0.761), XTN (0.723), XALT (0.423), XAHT (0.411), XFFP (0.146), XTK (−0.387) and XK (−0.614) than that in other factors. However, its contribution rate was only 1.111%, and the F3 was not be considered. Two principal components of environmental factors, F1 (XN, XAAP, XALTI, XpH, XAAT, XAMT, and XP) and F2 (X7, X1, XASD, and XTP) were thus screened for further analysis.
Table 1. Eigenvalues and cumulative contribution rates of principal components.
| Ecological factors | Principal components | Eigenvalues | Contribution rates (%) | Cumulative contribution rates (%) |
|---|---|---|---|---|
| XN | F1 | 8.95 | 54.782 | 54.782 |
| XP | F2 | 7.784 | 39.974 | 94.756 |
| XK | F3 | 3.339 | 1.111 | 95.867 |
| XOM | F4 | 1.734 | 0.915 | 96.782 |
| XTN | F5 | 0.423 | 0.807 | 97.589 |
| XTP | F6 | 1.725 | 0.613 | 98.202 |
| XTK | F7 | 0.68 | 0.572 | 98.774 |
| XpH | F8 | 0.437 | 0.4105 | 99.184 |
| XAMT | F9 | 0.342 | 0.377 | 99.561 |
| X1 | F10 | 0. 175 | 0.3135 | 99.875 |
| X7 | F11 | 0.072 | 0.1065 | 99.981 |
| XAAT | F12 | 0.057 | 0.0065 | 99.988 |
| XAHT | F13 | 0.041 | 0.004 | 99.992 |
| XALT | F14 | 0.033 | 0.0024 | 99.994 |
| XAAP | F15 | 0.024 | 0.0021 | 99.996 |
| XASD | F16 | 0.022 | 0.0015 | 99.998 |
| XFFP | F17 | 0.018 | 0.001 | 99.999 |
| XALTI | F18 | 0.011 | 0.001 | 100 |
XN(mg/kg), rapidly available nitrogen; XP(mg/kg), rapidly available phosphorus; XK(mg/kg), rapidly available potassium; XOM(%), organic matter; XTN(%), total nitrogen; XTP(%), total phosphorus; XTK(%), total potassium; XpH, pH; XAMT(°C), annual mean temperature; X1(°C), january average temperature; X7(°C), july average temperature; XAAT(°C), annual accumulated temperature(≥10 °C); XAHT(°C), annual highest temperature; XALT(°C), annual lowest temperature; XAAP(mm), annual average precipitation; XASD(h), annual sunshine duration; XFFP(d), frost free period; XALTI(m), altitude.
Figure 5. Loading plot generated from principal component analysis (PCA) for each environmental factor.
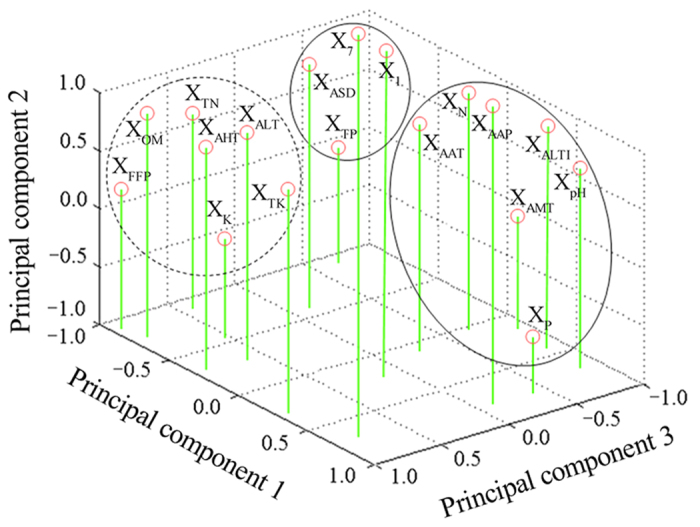
XN(mg/kg), rapidly available nitrogen; XP(mg/kg), rapidly available phosphorus; XK(mg/kg), rapidly available potassium; XOM(%), organic matter; XTN(%), total nitrogen; XTP(%), total phosphorus; XTK(%), total potassium; XpH, pH; XAMT(°C), annual mean temperature; X1(°C), january average temperature; X7(°C), july average temperature; XAAT(°C), annual accumulated temperature(≥10 °C); XAHT(°C), annual highest temperature; XALT(°C), annual lowest temperature; XAAP(mm), annual average precipitation; XASD(h), annual sunshine duration; XFFP(d), frost free period; XALTI(m), altitude.
Gray correlation analysis (GCA)
A GCA was conducted between the contents of active ingredients, antioxidant activity, and the principal components of environmental factors (F1, F2) to deduce primary environmental factors25,26. The results of GCA (Table 2) demonstrated that environmental factors imposed different degrees of influences on different active ingredients and antioxidant activity: the primary environmental factor for the accumulation of tannin was altitude (XALTI); for the accumulation of total flavonoids, rutin and antioxidant activity, were annual sunshine duration (XASD) and altitude (XALTI); for the accumulation of total phenolics, were annual mean temperature (XAMT), annual average precipitation (XAAP), and altitude (XALTI).
Table 2. Gray correlation coefficients between the active ingredients, antioxidant activity and related environmental factors.
| Environmental factors | Correlation degree |
||||
|---|---|---|---|---|---|
| Tannin | Total flavonoids | Rutin | Total phenolics | IC50 | |
| XN | 0.313 | 0.456 | 0.501 | 0.444 | 0.478 |
| XAAP | 0.351 | 0.511 | 0.421 | 0.602 | 0.507 |
| XALTI | 0.717 | 0.758 | 0.661 | 0.724 | 0.776 |
| XpH | 0.471 | 0.376 | 0.357 | 0.376 | 0.444 |
| XAAT | 0.402 | 0.413 | 0.364 | 0.461 | 0.305 |
| XAMT | 0.357 | 0.301 | 0.462 | 0.598 | 0.287 |
| XP | 0.316 | 0.406 | 0.456 | 0.336 | 0.321 |
| X7 | 0.579 | 0.432 | 0.477 | 0.458 | 0.429 |
| X1 | 0.481 | 0.338 | 0.328 | 0.294 | 0.328 |
| XASD | 0.357 | 0.674 | 0.733 | 0.328 | 0.613 |
| XTP | 0.322 | 0.329 | 0.353 | 0.422 | 0.515 |
XN, XP, ···, XALTI was performed in the Table 1. IC50 was used to indicate antioxidant activity.
Path analysis (PA)
Although primary environmental factors could be clarified by GCA, the correlations between active ingredient contents, antioxidant activity, and the primary environmental factors remain unclear. So, the correlation between active ingredient contents, antioxidant activity, and the primary environmental factors were further explored by PA, which makes the multiple statistical analyses more rational because the path coefficient possesses directional features27. The results of PA (Table 3) in this study demonstrated the effect of primary factors was significant on active ingredient contents and antioxidant activity. XALTI (altitude) was key environmental factor for the content of tannin, XASD (annual sunshine duration) and XALTI (altitude) were key ones for flavonoids, rutin and antioxidant activity, while XAMT (annual mean temperature) and XALTI (altitude) were key ones for total phenolics. The content of tannin was significantly and negatively correlated to altitude (P < 0.05). The flavonoids content, rutin content and antioxidant activity were significantly and positively correlated to annual sunshine duration and altitude (P < 0.05). The content of total phenolics was significantly and negatively correlated to annual mean temperature, while the content of total phenolics was significantly and positively correlated to altitude (P < 0.05). No significant correlation was observed between the soil factors and the contents of each active substance or antioxidant activity.
Table 3. Path analysis between the active ingredient contents, antioxidant activity and primary factors.
| Items | Primary factors | Direct Path coefficient | Indirect Path coefficient |
Significance level(P-value) | ||||
|---|---|---|---|---|---|---|---|---|
| Total | →XAMT | →XAAP | →XASD | →XALTI | ||||
| Tannin | XAMT | 0.447 | −0.362 | −0.398 | 0.673 | −0.637 | 0.229 | |
| XAAP | −0.821 | 0.533 | 0.081 | −0.413 | 0.324 | 0.327 | ||
| XASD | 0.541 | 0.202 | 0.230 | −0.169 | 0.141 | 0.073 | ||
| XALTI | −0.739 | 0.210 | −0.045 | 0.001 | 0.254 | 0.006 | ||
| Total flavonoids | XAMT | 0.422 | 0.473 | 0.062 | 0.142 | 0.269 | 0.272 | |
| XAAP | 0.501 | 0.322 | 0.360 | −0.368 | 0.322 | 0.345 | ||
| XASD | 0.916 | −0.837 | −0.442 | 0.126 | −0.521 | 0.009 | ||
| XALTI | 0.569 | 0.334 | 0.976 | 0.188 | −0.830 | 0.000 | ||
| Rutin | XAMT | 0.851 | −0.143 | 0.508 | −0.775 | 0.124 | 0.073 | |
| XAAP | −0.368 | −0.315 | −0.683 | 0.135 | 0.233 | 0.062 | ||
| XASD | 0.711 | −0.436 | 0.227 | −0.543 | −0.12 | 0.012 | ||
| XALTI | 0.413 | 0.529 | 0.124 | 0.264 | 0.141 | 0.000 | ||
| Total phenolics | XAMT | −0.429 | −0.334 | 0.380 | −0.53 | 0.576 | 0.006 | |
| XAAP | −0.501 | 0.322 | 0.268 | −0.168 | 0.222 | 0.109 | ||
| XASD | 0.633 | 0.281 | 0.324 | 0.388 | −0.431 | 0.302 | ||
| XALTI | 0.736 | −0.425 | −0.315 | −0.376 | 0.266 | 0.015 | ||
| IC50 | XAMT | −0.513 | 0.796 | 0.612 | 0.430 | −0.246 | 0.461 | |
| XAAP | 0.808 | −0.316 | 0.108 | −0.185 | −0.239 | 0.248 | ||
| XASD | −0.602 | −0.319 | 0.065 | 0.272 | −0.656 | 0.003 | ||
| XALTI | −0.635 | 0.241 | 0.768 | −0.651 | 0.124 | 0.000 | ||
Primary factors including XAMT, XAAP, XASD, XALTI were performed in the Table 1. IC50 was used to indicate antioxidant activity, a lower IC50 representing stronger antioxidant capacity.
The statistical significance demonstrated that P. fruticosa samples from Kangding, Sichuan Province (S8) was selected as the most valuable materials due to the higher phytochemical contents and significant antioxidant activity. Furthermore, Kangding, Sichuan Province was proved to be the most suitable region for producing P. fruticosa through the comprehensive investigation and analysis of the effect of environmental factors.
Quality assessment of P. fruticosa based on RP-HPLC (reverse phase-high performance liquid chromatography) fingerprint data
Similarity analysis (SA)
A standard HPLC fingerprints of 8 batches of samples from different regions of China were obtained under the optimized HPLC conditions (Fig. 1). These herbs showed different chromatographic profiles. After carefully analyzing the fingerprint profiles of these samples, ten common peaks with acceptable heights and good resolution were selected as characteristic peaks for the identification and classification of the P. fruticosa samples from different production regions16,19,21,22. The relative retention time and relative areas of these constituents (characteristic peaks) were calculated with respect to the reference peak eluting at a retention time of 18.0 min (peak 4). The similarities among the 8 chromatograms were evaluated based on a matrix comprising the relative areas of the 10 constituents of samples 1–8 by the CASE software. The results showed that the similarity of chromatograms of eight samples had a wide range and varied within the range of 0.21–0.89 (Table 4), which indicated that the chromatographic patterns of the samples varied greatly in different production regions.
Table 4. Similarity of the chromatograms of P. fruticosa samples from eight locations.
| No. | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|
| S1 | 1.00 | |||||||
| S2 | 0.91 | 1.00 | ||||||
| S3 | 0.67 | 0.61 | 1.00 | |||||
| S4 | 0.73 | 0.57 | 0.79 | 1.00 | ||||
| S5 | 0.81 | 0.70 | 0.68 | 0.85 | 1.00 | |||
| S6 | 0.76 | 0.32 | 0.39 | 0.66 | 0.53 | 1.00 | ||
| S7 | 0.34 | 0.50 | 0.53 | 0.41 | 0.68 | 0.62 | 1.00 | |
| S8 | 0.27 | 0.46 | 0.38 | 0.52 | 0.21 | 0.31 | 0.56 | 1.00 |
S1, Mei county, Shaanxi; S2, Diebu, Gansu; S3, Huzhu, Qinghai; S4, Jingyuan, Ningxia; S5, Yongdeng, Gansu; S6, Shangri-la, Yunnan; S7, Ningchi, Tibet; S8, Kangding, Sichuan.
Hierarchical clustering analysis (HCA)
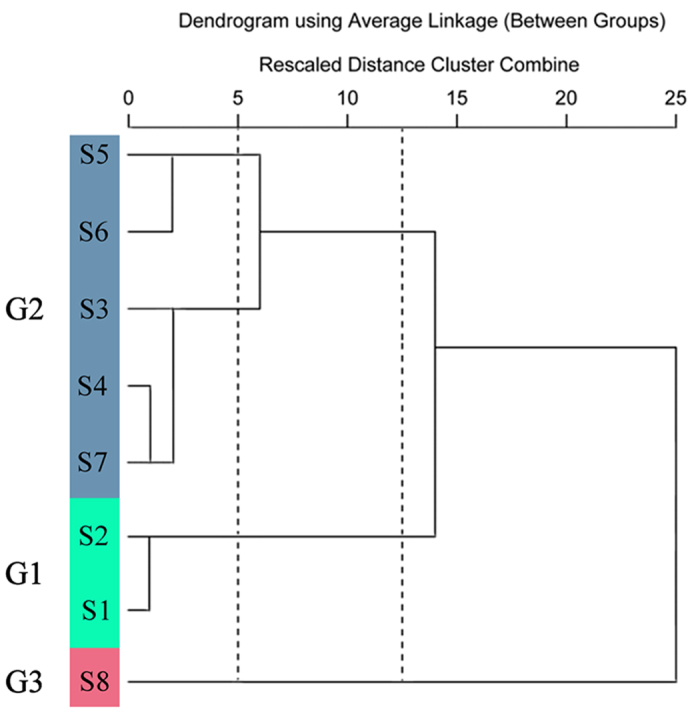
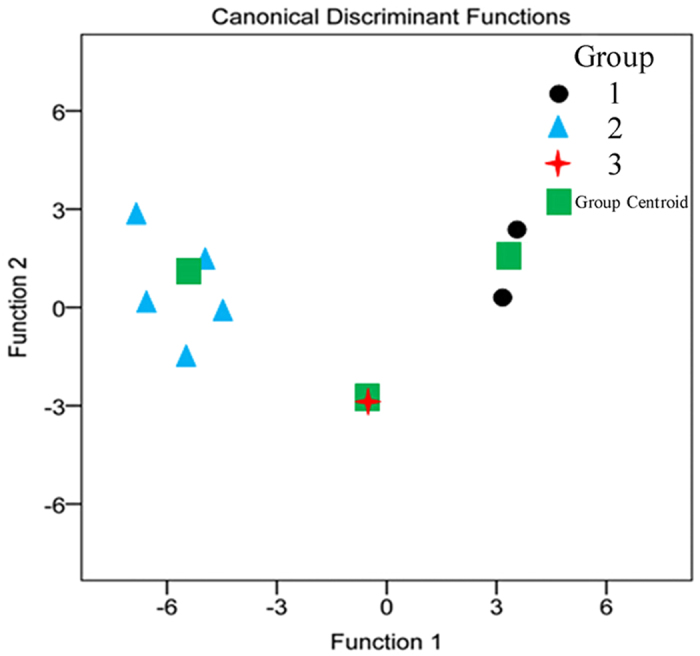
Prior to HCA, we compared the fingerprints visually and simply divided the samples into three distinct groups, namely A, B, and C (Fig. 6). Although it was possible to differentiate chromatograms on the basis of visual comparison, this process was subjective and non-quantitative. HCA can provide a quantitative and objective analysis of fingerprints20. HCA was performed according to the relative peak areas of ten constituents and a dendrogram was acquired (Fig. 7). At the rescaled distance of 12.5, HCA can divide the eight samples from different locations into three groups consistent with the results of visual comparisons. S1 and S2 were merged into a group G1; S3, S4, S5, S6, and S7 were grouped in G2; and S8 was found in G3.
Figure 6. Visual classification for HPLC chromatograms of eight P. fruticosa samples from different growing locations.
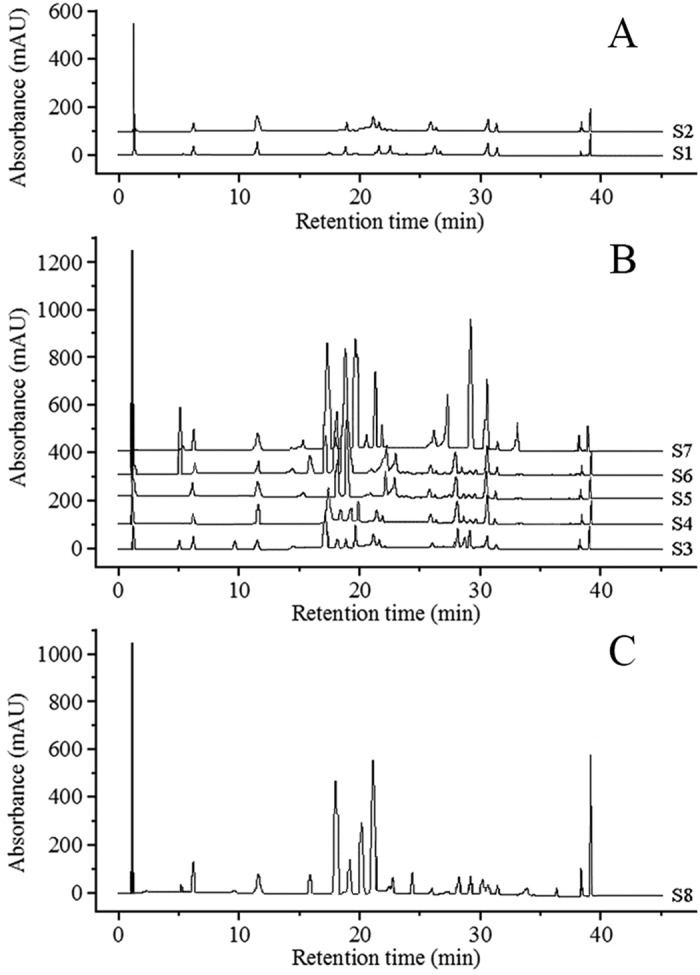
Figure 7. Dendrograms of hierarchical cluster analysis (HCA) for eight P. fruticosa samples from different growing locations.
The correlation coefficients of each chromatogram within groups G1, G2 and G3 corresponding to software-generated group simulated mean chromatograms, and the correlation coefficients between these simulated mean chromatograms were shown in Table 5. The chromatograms within a particular group were generally consistent. Correlation coefficients for each chromatogram which were classified into a particular group to the corresponding simulative mean were higher than 0.90. However, the chromatograms within a particular group were markedly different from the chromatograms in different groups, and the differences in the similarity values for the three groups (Table 5) reflected the HCA-generated data. Meanwhile, the correlation coefficient between the software-generated group simulated mean chromatograms was lower than 0.65, and ANOVA investigations indicated the difference between groups was significant (P = 0.041). These results demonstrate that HCA can distinguish P. fruticosa species with the same origin from different districts.
Table 5. Correlation coefficients between individual chromatograms within a group and the group simulative mean chromatogram, and between the group simulative mean chromatograms.
aCorrelation coefficient of individual chromatograms to the simulative mean chromatogram of the corresponding group.
bCorrelation coefficient between simulative mean chromatograms.
Discrimination analysis (DA)
Ten common peaks were selected from the fingerprints, thereby creating 10 variables. However, not all these variables are of value to establish discriminant function. The DA will generate discriminant functions only by use of valuable variables. Two types of discriminant functions [equations (1) and (2)] were obtained using the SPSS software (SPSS for Windows 19.0, SPSS Inc., USA).
Canonical discriminant function:
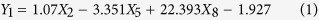 |
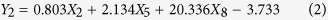 |
Discrimination standard:
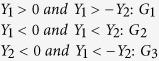 |
Fisher’s discrimination function:
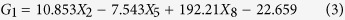 |
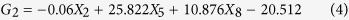 |
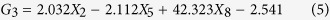 |
Discriminant standard: each sample has three functional values and is assigned to the group corresponding to the highest of these function values. G1 [equation (3)], G2 [equation (4)] and G3 [equation (5)] denote the samples from groups G1, G2 and G3 respectively, and X denotes the variable.
Only three variables, corresponding to the areas of peaks 2, 5 and 8, were used to generate the discriminant functions. In order to place an unknown sample, the values of the three variables are inserted into the equations, and the unknown sample was grouped according to the discriminant standard value obtained. Use of the three most discriminating variables enable test samples belonging to groups G1, G2 and G3 to be classified with 100% accuracy. The well-resolved DA plots for the three groups are shown in Fig. 8. DA approach supported a division of the 8 samples into 3 major groups for which HPLC SA confirmed the considerable variation in the active substances and quality of P. fruticosa from different growing locations. Furthermore, the samples from Kangding, Sichuan Province (S8) was separately assigned into a special group Group 3 (Fig. 8), and also far away from Group 1 and 2. There was a huge overlap between the center of the samples contained by Group3 (S8) and group centroid, indicating that S8 was in high quality.
Figure 8. Discrimination analysis (DA) for eight P. fruticosa samples from different growing locations.
Discussion
Variation in active substance contents and antioxidant activity
Altitude, temperature, illumination, and moisture are important factors to influence the metabolism and accumulation of secondary metabolites28. Environmental differences (such as altitude, temperature, illumination, precipitation, humidity, soils) in different production locations contribute to the differences in active ingredient contents and antioxidant activity of medicinal plants9,16,19. In this study, significant differences were observed in the contents of the chemical compositions and antioxidant activity of P. fruticosa leaves obtained from different growing locations (Figs 1~4). The contents of tannin in the leaves of P. fruticosa planted in S8 (Kangding, Sichuan) were higher than those planted in other locations, followed by S1 (Mei county, Shaanxi). The contents of total flavonoids were high in those planted in S8 (Kangding, Sichuan), followed by S7 (Ningchi, Tibet). Li et al. also discovered that the contents of total flavonoids in P. fruticosa leaves from different environments displayed great differences29, which agrees with our findings. The contents of rutin were also high in S8 (Kangding, Sichuan), followed by S7 (Ningchi, Tibet). The contents of total phenolics were high in S6 (Shangri-la, Yunnan), followed by S8 (Kangding, Sichuan). The antioxidant activity was high in S8 (Kangding, Sichuan), followed by S2 (Diebu, Gansu). The results indicate that the contents and properties of active substances are closely related to the growing locations, and are determined by environmental factors of the growing locations. Integrating the active substance contents, antioxidant activity ability and the effect of environmental factors, P. fruticosa from Kangding, Sichuan Province (S8) was selected as the most valuable material, and Kangding, Sichuan Province was considered as the most suitable region for producing P. fruticosa. The rich variation in chemical compositions and antioxidant activity may lead to significant differences in effectiveness as medicines, functional foods and nutritional supplements. HPLC fingerprint SA coupled with HCA and DA were further introduced to establish an effective method for the accurate classification and quality assessment of P. fruticosa. This method performed well for the quality evaluation of P. fruticosa samples and also confirmed that S8 had high quality.
Altitude and active substance contents
Altitude is an overall reflection of multiple ecological factors, such as temperature, humidity, and solar radiation. Many studies have confirmed that the contents of flavonoids are positively correlated to the altitude of the growing location. For example, in Leontodon species, analyses of samples originating from different altitudes (13 samples of Leontodon helveticus Mérat from 1600–2950 m, 19 samples of Leontodon autumnalis L. from 30–2075 m, and 61 samples of Leontodon hispidus L. from 40–2550 m) showed a significant positive correlation between altitudes of collection sites and total contents of flavonoids28. Dong et al. selected Eucommia ulmoides that is a medicinal plant from six altitudes (550 m, 690 m, 780 m, 845 m, 950 m and 1180 m) as study materials and found that altitude was significantly and positively correlated to the contents of flavonoids (P < 0.05)9. Ghasem et al. studied the influence of environmental factors on flavonoids contents of walnut (Juglans regia L.) green husks and found the good correlation coefficient was existed between the flavonoid contents and collection altitudes30. The altitude and other information about locales of sample collection were shown in Table 6. Similarly, in this study, the altitude in Kangding is higher than other locations, the contents of total flavonoids were the highest, and significantly different from other locations (Fig. 2). GCA and PA showed that maximum degree of correlation was found between altitude and the contents of total flavonoids, and positive relationship existed (Tables 2 and 3). Flavonoids contain ortho-dihydroxylated structure, which exhibits the characteristics of absorbing ultraviolet, and scavenging free radicals. Significant positive correlation between the contents of total flavonoids and altitude are responses of the P. fruticosa species distributed at higher altitude to the strong ultraviolet radiation. Also, UV-absorbing compounds such as flavonoids and phenolic acids have been proven to be important for plants to protect their vegetative organs from harmful UV radiation13. By contrast, the total amount of flavonoids of some medicinal plants, such as Buxus sempervirens L., decreased with increased altitude31. The reduction of flavonoids in B. sempervirens leaves with altitude might be a consequence of an increased synthesis of wax in response to the decrease in temperature32 since both, cuticular waxes and flavonoids, are derived from acetyl-CoA33.
Table 6. Main environmental factors of eight locations throughout China.
| Items | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|
| XN | 6.31 ± 0.03 abcd | 28.12 ± 0.02 cde | 32.65 ± 0.04 abc | 8.09 ± 0.07 ab | 7.43 ± 0.04 abc | 8.13 ± 0.03 abcd | 32.21 ± 0.02 cde | 35.82 ± 0.03 abc |
| XP | 6.42 ± 0.06 ab | 7.58 ± 0.05 a | 8.56 ± 0.02 b | 11.37 ± 0.15 de | 8.25 ± 0.24 ab | 7.24 ± 0.06 ab | 8.85 ± 0.05 a | 10.13 ± 0.02 b |
| XK | 107.69 ± 0.02 cde | 357.53 ± 0.04 e | 96.71 ± 2.14 de | 132.04 ± 4.30 e | 160.41 ± 0.01 de | 135.96 ± 0.02 cde | 384.35 ± 0.03 e | 112.71 ± 0.04 de |
| XOM | 5.00 ± 0.01 bcde | 6.52 ± 0.01 bcd | 5.40 ± 0.01 b | 10.02 ± 0.06 de | 6.38 ± 0.03 cde | 4.09 ± 0.01 bcde | 7.22 ± 0.03 bcd | 6.50 ± 0.01 b |
| XTN | 0.23 ± 0.02 e | 0.25 ± 0.001 bc | 0.20 ± 0.004 e | 0.29 ± 0.005 de | 0.32 ± 0.004 e | 0.17 ± 0.001 e | 0.33 ± 0.001 bc | 0.16 ± 0.005 e |
| XTP | 0.03 ± 0.001 ab | 0.11 ± 0.002 cde | 0.14 ± 0.003 e | 0.21 ± 0.005 bc | 0.05 ± 0.001 de | 0.02 ± 0.001 ab | 0.15 ± 0.002 cde | 0.32 ± 0.003 e |
| XTK | 2.45 ± 0.01 e | 1.49 ± 0.02 de | 1.62 ± 0.04 de | 1.77 ± 0.03 abc | 2.46 ± 0.05 e | 2.60 ± 0.01 e | 1.56 ± 0.02 de | 2.35 ± 0.04 de |
| XpH | 6.20 ± 0.05 abc | 7.31 ± 003 bcd | 6.59 ± 0.05 cde | 6.39 ± 0.07 cde | 6.12 ± 0.06 abcd | 6.40 ± 0.05 abc | 7.09 ± 003 bcd | 6.20 ± 0.05 cde |
| XAMT | 7.00 ± 0.05 ab | 6.90 ± 0.08 d | 6.40 ± 0.04 e | 4.32 ± 0.02 e | 6.80 ± 0.05 cde | 9.00 ± 0.05 ab | 6.90 ± 0.08 d | 7.60 ± 0.04 e |
| X1 | −7.0 ± 0.01 bcd | −4.0 ± 0.04 abcd | −8.2 ± 0.02 de | −7.6 ± 0.05 bcd | −2.2 ± 0.04 bcd | −5.6 ± 0.01 bcd | 1.5 ± 0.03 abcd | −5.0 ± 0.02 de |
| X7 | 19.0 ± 0.01 abc | 22.5 ± 0.07 ab | 23.4 ± 0.03 bcd | 21.7 ± 0.04 d | 15.5 ± 0.01 ab | 13.4 ± 0.02 abc | 16.3 ± 0.03 ab | 16.8 ± 0.05 bc |
| XAAT | 1847.43 ± 0.03 bc | 2390.80 ± 0.05 ab | 3643.87 ± 0.06 c | 4108.22 ± 0.04 bcd | 1265.20 ± 0.04 bcde | 1526.70 ± 0.03 bc | 2263.20 ± 0.05 ab | 1931.52 ± 0.03 bcd |
| XAHT | 40.44 ± 0.08 cd | 32.80 ± 0.04 bc | 39.50 ± 0.01 abc | 35.00 ± 0.03 ab | 31.70 ± 0.01 cd | 24.60 ± 0.04 cd | 30.60 ± 0.05 bc | 33.90 ± 0.02 abc |
| XALT | −24.00 ± 0.04 de | −25.50 ± 0.06 d | −26.62 ± 0.03 bcd | −24.24 ± 0.01 bc | −28.91 ± 0.04 abc | −23.40 ± 0.04 de | −14.4 ± 0.02 d | −20.30 ± 0.03 bcd |
| XAAP | 616.32 ± 0.01 abc | 635.44 ± 0.04 bc | 620.30 ± 0.04 abc | 530.27 ± 0.06 cde | 880.30 ± 0.04 ab | 627.20 ± 0.01 abc | 650.45 ± 0.04 bc | 642.41 ± 0.06 abc |
| XASD | 2370.12 ± 0.04 ab | 2021.35 ± 0.07 bcde | 1226.55 ± 0.03 bc | 2659.62 ± 0.06 abc | 1738.34 ± 0.03 a | 2206.40 ± 0.04 ab | 2020.53 ± 0.07 bcde | 2246.20 ± 0.01 bc |
| XFFP | 132.61 ± 15.38 abc | 158.00 ± 9.66 a | 230.54 ± 11.55 ab | 121.52 ± 8.12 abc | 180.00 ± 1.23 a | 167.00 ± 15.38 abc | 180.00 ± 9.66 a | 147.35 ± 11.55 ab |
| XALTI | 2061.25 ± 36.55 a | 2747 ± 87.32 c | 2800 ± 56.72 c | 2311.5 ± 23.65 b | 2525.25 ± 121.66 b | 3200 ± 67.54 d | 3345.25 ± 210.4 d | 3373.75 ± 121.41 d |
Eighteen environmental factors were performed in the Table 1. For the same variable, values with no letters in common are significantly different (P < 0.05).
Temperature and active substance contents
The production of secondary metabolites by temperature stress is the expression of self defense mechanism of medicinal plants. Recently a significant increase of the biosynthesis of phenolics was demonstrated for plants growing in a low temperature regime34. Similarly, in this study, annual mean temperature was significantly and negavely correlated to the contents of total phenolics. This result agreed with the previous researches for total phenolics contents involved in other medicinal plants. For example, in walnut (Juglans regia L.), a negative correlation was found between temperature and total phenolic contents30. Albert et al. reported that enhanced UV-B radiation is probably not the key factor triggering shifts in the phenolic composition in Arnica montana L. cv. ARBO grown at higher altitudes but rather temperature, which displays negative correlation with the phenolics35. Reyes et al. studied total phenolics during development of Solanum tuberosum L. in cultivars grown in Texas and Colorado and found cooler temperatures in Colorado favored about a 1.4-times higher total phenolics content than in Texas-grown tubers36. The present study also showed phenolic compounds increased with altitude, similar the trends as the results for Buxus sempervirens obtained in a previous study31. Usually a decrease of the mean temperature of 0.55 °C per 100 m of altitude is observed37. Hence, the contents of phenolic compounds increased with the decrease of temperature. However, temperature is positively correlated to the contents of active ingredients in some plants. The contents of hypericin in Hypericum perforatum L. increased with temperature38. The content of anthocyanin in sugarcane that was under high temperature obviously increased39. Plants in alpine habitats are exposed to many environmental stresses, in particular temperature and radiation extremes. Another laboratory studies confirm that the biosynthesis of certain phenolic compounds is increased under increased UV radiation, whereas shading leads to a decrease in the biosynthesis of these compounds40. Therefore, plant could have a chemical adaption to the alpine environment, indicating that the impacts of ecological factors on the secondary metabolites are related to their chemical types, structures and characteristics.
Sunshine duration and active substance contents
Illumination also affects the synthesis and accumulation of secondary metabolites in medicinal plants. The increase of illumination time can increase the contents of secondary metabolites. For example, the amount of flavonoids in Arabidopsis increased after long time illumination41. The contents of ginsenogsides in Panax quinquefolius L. were positively correlated to the annual sunshine duration42. Dong et al. investigated the influence of environmental factors on the contents of secondary metabolites in the leaves of Eucommia ulmoides and found that annual sunshine duration was significantly and positively correlated to the contents of geniposidic acid (P < 0.05)9. In this study, PA showed that the contents of active substances (tannin, total flavonoids, rutin, and total phenolics) were highly associated to annual sunshine duration, positive correlation between them was observed (Table 3). The results were also verified in the study on production of active substances of other medicinal plant such as Sinopodophyllum hexandrum16. These data indicated that annual sunshine duration is a key environmental factor to the synthesis and accumulation of active substances. P. fruticosa is a heliophilous plant, locations with long time sunshine would be favorable for its secondary metabolism, resulting in adequate substrate to catalyze the synthesis of the active substances and thereby increasing their contents.
Precipitation and active substance contents
In this study, the contents of tannin, rutin and total phenolics were negatively correlated to the annual average precipitation. This finding was consistent with the previous research results about other active substances. For example, the quinine content of Cinchona ledgeriana (Howard) Bern. Moens ex Trimen was higher under drought conditions and significantly lower under excessive soil humidity, even to the point of not being synthesized at all16. The atropine content of Anisodus carniolicoides (C.Y. Wu & C. Chen) D’Arcy & Zhi Y. Zhang could be as high as 1% under dry conditions, which is 2.5-times greater than that obtained under humid conditions (0.4%)16. In the meantime, the study showed that the contents of total flavonoids were positively correlated to annual average precipitation. Although the contents of tannin, rutin and total phenolics were negatively correlated to the annual average precipitation, and the contents of total flavonoids were positively correlated to annual average precipitation, the impacts of annual average precipitation on each secondary metabolite were not significant (Table 3). Therefore, precipitation is not a limited factor to the synthesis and accumulation of secondary metabolites for P. fruticosa. Additionally, 500 mm annual precipitation can meet the demand of the growth of P. fruticosa according to the biological features and the demands for environmental conditions. In the field investigation, the annual average precipitations in 8 locations are all over 500 mm, they all can meet the moisture demand from the growth of P. fruticosa. This could explain why the influence of annual average precipitation was not significant on active substances of P. fruticosa.
Soil and active substance contents
Some researchers have stated that the variations in the active ingredient contents of the plants are related to soil fertility. For example, the leaf polyphenol content of young greenhouse tomato plants was increased considerably in response to low N availability43. The saponin contents of Panax quinquefolius were increased by 27.86% by the repeated applications of high-quality organic fertilizer and the functionality of potassic fertilizer was the same as that of a total nutrient admixture in terms of saponin contents44. Liu et al. reported that organic matter was significantly and positively correlated with the active substances of Sinopodophyllum hexandrum16. In this study, no correlation was observed between the soil factors and the contents of each secondary metabolite of P. fruticosa. GCA that screened primary factors for active substances demonstrated that no soil factors were included in primary factors, indicating soil factors were not main drive force contributing the contents of active substances. Similarly, Liu et al. also found the soil factors contributed less to the active ingredient contents in Sinopodophyllum hexandrum16 than did the climate factors. The field investigation found all the growing locations and soil were well protected and managed under national conservation policy. Soil with abundant nutrients could meet the growth and development of P. fruticosa. Compared with soil factors, variation of climatic factors played an important role in the accumulation of active substances, which may explain the reason why no correlation existed between the soil factors and the contents of active substances of P. fruticosa. A deep reason should be explored by the investigation of metabolism of soil microorganism, soil trace elements and key enzymes combined with molecular techniques.
Environmental factors and antioxidant activity
Many reports strongly suggested that environmental conditions influenced the antioxidant properties of the plants. For instance, Yu et al.45 reported that significant effects of growing conditions, including number of hours exceeding 32 °C, on the antioxidant properties of Akron, a hard red winter wheat variety. The average temperature at growing locations was shown to have effects on the antioxidant properties of strawberries46. Mpofu et al. reported that location effects were highly significant (P < 0.0001), and wheat grown in Winnipeg with the highest altitude had the highest antioxidant activity47. Similarly, altitude showed significant and positive correlation to the antioxidant activity in this study. These data suggest potential influences of growing conditions on the antioxidant properties of P. fruticosa and the possibility of producing a selected wild species with a desired antioxidant property by optimizing growing conditions. The significant effects of environment must, therefore, be considered. However, few specific data are available in the literature on the relationship between environmental factors and antioxidant activity of P. fruticosa. More research is required to further investigate the cause of environmental factor effects using a greater sample of both field locations and varieties. Going forward, we would conduct further studies in cells and living bodies to fully reflect the antioxidant properties of P. fruticosa, which could not be achieved by the DPPH scavenging capacity assay and FRAP assay carried out above.
Quality assessment of P. fruticosa
Traditional Chinese medicine (TCM), which has a 5000-years history of application, is still in wide demand48. TCMs are being closely examined for the development of novel pharmaceuticals49. However, quality control of TCM has always been a bottleneck for their adoption worldwide because of their complexity, the presence of unknown components and lack of quality control. Chromatographic fingerprint analysis has been introduced as a rational strategy for the assessment of complex TCMs. Unlike the traditional practice that one or more compounds were selected as active markers for identification and quality assessment, fingerprint relies on the inherent relationship of multiple compounds and displays the chemical pattern of TCM. Among available quality control methods, chromatographic fingerprint has gained more and more attention recently. In this study, HPLC fingerprint coped with chemometric methods were applied to deal with the classification for the quality assessment of P. fruticosa from different regions of China. The results showed that the eight samples were divided into 3 clusters. Two types of discriminant functions were generated using three selected predictor variables and the ratio of discrimination was 100%. The results of HPLC fingerprint SA, HCA, and DA in this study were in agreement, and the techniques can identify and classify the same species from different regions and performed well for the quality assessment of P. fruticosa. Also, it can be used to compare and control other natural products prepared from them. Many similar studies on other medical plants have been published for the quality evaluation20,21,22,23. The quality of herbal drugs is affected by many composite factors. Climatoecological type, symbiosis ecotype, and soil ecotype in growing locations are biologically essential to produce high-quality geoauthentic herbal drugs9. For the widespread plant species, because the large span in geological zones, differences in chemical constituents within the same plant species would occur. P. fruticosa belongs to one of widespread plant species, distributed in the Asia, Europe and North America. Currently, the influence of environmental factors in other locations on active substances of P. fruticosa, and its quality assessment are less studied. Further research include more samples from other regions of China and other countries should be carried out. The present study provides meaningful information for the collection and application of wild P. fruticosa herbs in both healthcare and the food industry. Moreover, although this present study relates only to P. fruticosa, the methodology has wider relevance for quality assessment of other edible and medicinal plants.
Methods
Plant materials and soil samples
P. fruticosa were collected from eight representative locations located in seven provinces of China during 10–25th July in 2014 (Fig. 9). Specifically, a total of eighty healthy specimens from four populations (each population was separated geographically by at least 30 km, and 5 m for adjacent individuals) with similar growth stature were collected in every test region, as shown in Table 7. Matured leaves were picked up respectively from four directions (north, south, east, and west) of three positions (up, middle and low part) of the plant and then mixed as one sample for each test location, thereby obtaining eight test samples. All samples were dried under the vacuum at 40 °C. After grinding into powders, the samples were stored in the dark at −20 °C before further use. Simultaneously, soil rhizosphere were collected and treated for each study site, thereby obtained eight soil samples from eight study sites for the measurement of soil factors. Voucher specimens from all populations were identified by professor Jianjun Liu of Northwest A&F University and were deposited at the Herbarium of Northwest A&F University (WUK0781792-0781823).
Figure 9. Growing locations of P. fruticosa samples involved in seven provinces of China sampled for this study.
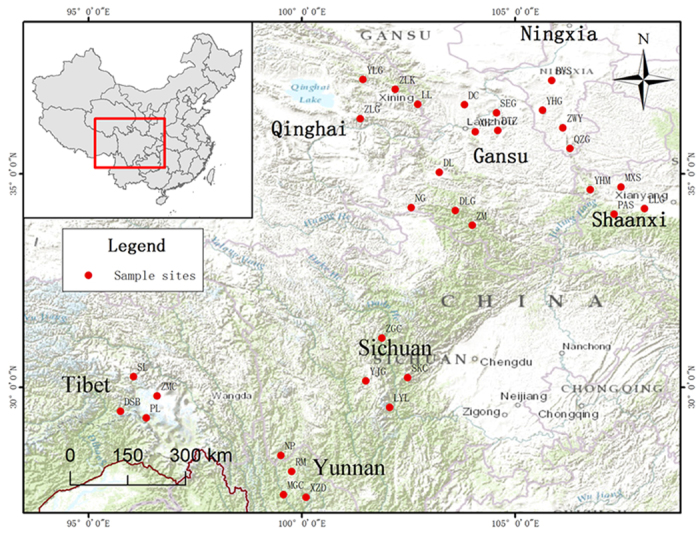
Maps generated using ArcGIS 10.0 (ESRI Inc. 2014; http://www.esri.com/).
Table 7. P. fruticosa samples collected from the eight different regions.
| No. | Locations | Population | Code | Coordinates | N | Altitude (m) | Soil type | Climate zones |
|---|---|---|---|---|---|---|---|---|
| S1 | Mei county, Shaanxi | Pingansi | PAS | E107°43′N34°1′ | 20 | 2815 | Dark brown soil | Semi-humid warm temperate continental monsoon climate |
| Mingxingsi | MXS | E107°44′N34°0′ | 20 | 2637 | Dark brown soil | Semi-humid warm temperate continental monsoon climate | ||
| Yuhuangmiao | YHM | E107°22′N34°5′ | 20 | 1780 | Dark brown soil | Semi-humid warm temperate continental monsoon climate | ||
| Liulingou | LLG | E108°10′N33°52′ | 20 | 1013 | Dark brown soil | Semi-humid warm temperate continental monsoon climate | ||
| S2 | Diebu, Gansu | Zemo | ZM | E103°21′N33°45′ | 20 | 2728 | Alpine meadow soil | Mountain continental climate zone |
| Dalong | DL | E103°14′N35°2′ | 20 | 2620 | Alpine meadow soil | Mountain continental climate zone | ||
| Dalagou | DLG | E103°22′N33°52′ | 20 | 2677 | Alpine meadow soil | Mountain continental climate zone | ||
| Nagai | NG | E103°14′N33°51′ | 20 | 2963 | Alpine meadow soil | Mountain continental climate zone | ||
| S3 | Huzhu, Qinghai | Zhalongkou | ZLK | E102°34′N36°53′ | 20 | 2264 | Alpine meadow soil | Semi-arid continental plateau monsoon climate zone |
| Zhalonggou | ZLG | E102°37′N36°47′ | 20 | 2698 | Alpine meadow soil | Semi-arid continental plateau monsoon climate zone | ||
| Yuanlongogu | YLG | E102°27′N36°54′ | 20 | 3069 | Alpine meadow soil | Semi-arid continental plateau monsoon climate zone | ||
| Lalagou | LL | E102°42′N36°44′ | 20 | 3169 | Alpine meadow soil | Semi-arid continental plateau monsoon climate zone | ||
| S4 | Jingyuan, Ningxia | Baiyunshan | BYS | E106°15′N35°37′ | 20 | 2232 | Gray cinnamonic soil | Humid and semi-humid temperate climate zone |
| Yehegu | YHG | E106°13′N35°31′ | 20 | 2370 | Gray cinnamonic soil | Humid and semi-humid temperate climate zone | ||
| Zhiwuyuan | ZWY | E106°18′N35°22′ | 20 | 2080 | Gray cinnamonic soil | Humid and semi-humid temperate climate zone | ||
| Qiaozigou | QZG | E106°22′N35°15′ | 20 | 2564 | Gray cinnamonic soil | Humid and semi-humid temperate climate zone | ||
| S5 | Yongdeng, Gansu | Suoergou | SEG | E102°43′N36°40′ | 20 | 2389 | Alpine meadow soil | Semi-arid continental cold temperate climate zone |
| Xiahe | XH | E102°43′N36°35′ | 20 | 2733 | Alpine meadow soil | Semi-arid continental cold temperate climate zone | ||
| Dachang | DC | E102°44′N36°44′ | 20 | 2449 | Alpine meadow soil | Semi-arid continental cold temperate climate zone | ||
| Datanzigou | DTZ | E102°46′N36°33′ | 20 | 2530 | Alpine meadow soil | Semi-arid continental cold temperate climate zone | ||
| S6 | Shangri-la, Yunnan | Rime | RM | E99°37′N27°51′ | 20 | 3528 | Subalpine shrub soil | Mountains cool temperate monsoon climate zone |
| Naipi | NP | E99°36′N28°2′ | 20 | 3432 | Subalpine shrub soil | Mountains cool temperate monsoon climate zone | ||
| Xiaozhongdian | XZD | E99°56′N27°28′ | 20 | 3590 | Subalpine shrub soil | Mountains cool temperate monsoon climate zone | ||
| Mugaocun | MGC | E99°34′N27°30′ | 20 | 2250 | Subalpine shrub soil | Mountains cool temperate monsoon climate zone | ||
| S7 | Nyingchi, Tibet | Zhangmaicun | ZMC | E94°20′N29°40′ | 20 | 3097 | Subalpine shrub soil | Temperate continental plateau climate zone |
| Selong | SL | E94°11′N29°44′ | 20 | 3173 | Subalpine shrub soil | Temperate continental plateau climate zone | ||
| Pula | PL | E94°22′N29°27′ | 20 | 3256 | Subalpine shrub soil | Temperate continental plateau climate zone | ||
| Duosongba | DSB | E94°13′N29°37′ | 20 | 3855 | Subalpine shrub soil | Temperate continental plateau climate zone | ||
| S8 | Kangding, Sichuan | Yajaigeng | YJG | E101°57′N30°0′ | 20 | 2946 | Rich in humus loam | Humid subtemperate plateau climate zone |
| Laoyulin | LYL | E101°59′N29°55′ | 20 | 3788 | Rich in humus loam | Humid subtemperate plateau climate zone | ||
| Shengkangcun | SKC | E102°1′N30°4′ | 20 | 3207 | Rich in humus loam | Humid subtemperate plateau climate zone | ||
| Zhonggucun | ZGC | E101°54′N30°16′ | 20 | 3554 | Rich in humus loam | Humid subtemperate plateau climate zone |
Related data of environmental factors
Soil samples were used to determine the key soil parameters including rapidly available nitrogen (XN), rapidly available phosphorus (XP), rapidly available potassium (XK), organic matter (XOM), total nitrogen (XTN), total phosphorus (XTP), total potassium (XTK), and pH (XpH) using the modified soil chemistry analysis methods described by Sparks et al.50. Related data of climate factors including annual mean temperature (XAMT), january average temperature (X1), july average temperature (X7), annual accumulated temperature (≥10 °C) (XAAT), annual highest temperature (XAHT), annual lowest temperature (XALT), annual average precipitation (XAAP), annual sunshine duration (XASD), and frost free period (XFFP) in the 50 years (1964~2013) was collected from local meteorological bureaus (stations) for the eight study sites. The environmental factors of the eight study sites were summarized in Table 6.
Preparation of the extracts
Considering the impact of various factors, the extraction process was optimized using a response surface method51. The optimized extraction process was as follows: ethanol concentration, 70%; extraction temperature, 45 °C; extraction time, 2 h; extraction times, three; and liquid-solid ratio, 15:1. Each powdered sample (5.0 g) was treated as described in the optimized extraction process. The obtained filtrates were evaporated at 40 °C under vacuum using a rotary evaporator, and were then stored in the dark at 4 °C for further use. Extracts were diluted if necessary. Each sample was performed in triplicate.
Measurement of tannin
Tannin contents were determined using the Folin-Denis method52. 1.0 mL of the diluted samples (2 mg/mL) was transferred to a 25 mL calibration flask to which were added 1.0 mL of Folin-Denis reagent and 5 mL of sodium carbonate (1 mol/L). The solution was diluted to 25 mL by the addition of methanol. After incubating for 30 min at room temperature, the absorbance at 720 nm was measured against a blank. Tannin acid (1 mg/L to 10 mg/L) was used for the standard curve calibration. All measurements were performed in triplicate.
Measurement of total flavonoids
Total flavonoids contents were determined by sodium nitrite-aluminum nitrate colorimetric method53. Sample solution (1.0 mL, 2 mg/mL) was transferred into 25 mL volumetric flasks, then 0.3 mL NaNO2 (5%) was added and held for 6 min. Next, 0.3 mL Al(NO3)3 (10%) was added and held for another 6 min. Finally, 4 mL NaOH (1 mol/L) was added and the solution was diluted to 25 mL with 70% ethanol solution. After 30 min of incubation at room temperature, the absorbance at 510 nm was measured against a blank. Rutin (4 mg/L to 40 mg/L) was used for the standard curve calibration. All measurements were performed in triplicate.
Measurement of total phenolics
The total phenolic contents were determined using a modified Folin-Ciocalteau colourimetric method54. Sample solution (1 mL, 2 mg/mL) was transferred to a 25 mL calibration flask, 0.5 mL of Folin-Ciocateu reagent and 2.5 mL of sodium carbonate (1 mol/L) was added and final volume was marked with methanol. After 30 min of incubation at 30 °C, the solution was centrifuged at 4,000 rpm (10 min) and the absorbance at 760 nm was measured against a reagent blank. Gallic acid (0 mg/L to 6 mg/L) was used for the standard curve calibration. Three replicates were made for each sample.
RP-HPLC analysis
Sample solution (1 mg/mL) was filtered through a 0.22 μm microporous filtering film and were then separated by RP-HPLC at ambient temperature to obtain chromatograms55. The amounts of rutin were also quantified by RP-HPLC. The contents of rutin were identified and calculated by a comparison of the relative retention time (RRT) and relative peak areas (RPAs) with those of the standards. HPLC analyses were conducted on an Agilent Series 1260 liquid chromatograph, equipped with a quaternary gradient pump, a variable wavelength detector system and a reversed-phase SB-C18 column (5 μm, 4.6 × 250 mm, Agilent, USA). The mobile phase consisted of water with 0.5% acetic acid (mobile phase A) and methanol with 0.5% acetic acid (mobile phase B). The flow rate was 1 mL/min. The gradient program was as follows: 0–15 min, 25% to 35% B; 15–30 min, 35% to 60% B; 30–40 min, 60% to 100% B; 40–45 min, hold B at 100%. The injection volume and detect wavelength were 20 μL and 360 nm, respectively56,57. Analyses were performed in triplicate.
DPPH radical scavenging activity
DPPH assay has been widely used for the determination of antioxidant activity of pure antioxidant compounds as well as of different plant extracts, a lower IC50 representing stronger antioxidant capacity24. IC50 obtained by interpolation from linear regression analysis is the effective sample concentration at which DPPH radicals is scavenged by 50%. A slightly modified DPPH method was used to determine the radical scavenging property of P. fruticosa leaves58. Amounts of 2 mL of the tested samples (1–100 μg/mL) and the positive controls (rutin, 1–70 μg/mL) were mixed with 2.0 mL of 0.1 mol/L DPPH in methanol. The mixture was vortexed thoroughly and allowed to stand in the dark for 30 min. The absorbance was measured at 517 nm spectrophotometrically against a blank. All measurements were performed in triplicate. DPPH free radical scavenging activity (SA) can be expressed with the following equation (6):
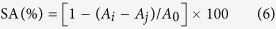 |
where Ai: absorbance of tested samples (2 mL) mixed with DPPH (2 mL); Aj: absorbance of tested samples (2 mL) mixed with methanol (2 mL); A0: absorbance of methanol (2 mL) mixed with DPPH (2 mL).
Ferric reducing antioxidant power (FRAP) assay
In this assay, the colour changed from yellow to green depending on the reducing power of the sample. Fe3+/ferricyanide complex was reduced to the ferrous form by reductants in the solution. Thus, Fe2+ can be monitored as the reducing power index by measuring the absorbance. The reducing capacity of the extract was determined by the method of Oyaizu59. Varying concentrations of the extracts in the methanol (2.5 mL, 50–500 μg/mL) were mixed with phosphate buffer (2.5 mL, 0.2 mol/L, pH 6.6) and potassium ferricyanide (2.5 mL, 1%), and incubated at 50 °C for 20 min. Aliquots (2.5 mL) of 10% trichloroacetic acid were added to the reaction mixture, which was then centrifuged at 4,000 rmp (10 min). The upper layer of the solution (2.5 mL) was mixed with distilled water (2.5 mL) and ferric chloride (0.5 mL, 0.1%). The absorbance was measured at 593 nm. The FRAP results were expressed in terms of micromoles trolox equivalent per gram dry weight of samples (μmol equiv. Trolox/g). All measurements were performed in triplicate.
Data analyses
Three methodologies were performed step-by-step to analysis systematically the influence of environmental factors on the active substance contents and antioxidant activity of P. fruticosa leaves, which also represented a quantitative comparative analysis of the dynamic process of plant and the environment. Firstly, principal component analysis (PCA) was carried out using SPSS software (SPSS for Windows 19.0, SPSS Inc., USA). PCA has the strong advantage of significantly reducing the dimension of the complex data while preserving most of the variance within by using dependencies among large numbers of variables without requiring knowledge of the data set in order to visualize high dimensional data and identify the most important variables60. PCA in the present study was used to find out principal components of environmental factors. Secondly, gray correlation analysis (GCA) between the contents of active substances, antioxidant activity and the principal components of environmental factors obtained by PCA were conducted by DPS7.5 software (Date Processing System, Science Press, China) to screen primary factors from principal components of environmental factors. The result of GCA reflects the close degree of the relationship between principal behavior factors and other factors, to deduce primary factors and secondary factors based on the gray correlation degrees of different factors9. High correlation degree indicates high influence of the factor to the accumulation of active substances and antioxidant activity25,26. GCA is usually applied in the indefinite system, including small sampling and poor data information system, in which limited information is available, others are not known25,26. Therefore, it is suitable to be used for the analysis of the data obtained in this study. Thirdly, path analysis (PA) deals with the quantitative relationship between dependent and independent variables to explain the relative significance of each factor to the dependent variables9,27. Figure 10 showed the path network of four independent variables included in PA. PA can determine whether the effect of Xi on Y is significant or not, and can identify not only the direct effect of Xi on Y (bi, Xi—Y) but also indirect effect of Xi on Y through Xj (rijbj, Xi—Xj—Y, i≠j). Thereby, the correlation coefficient (riy) contains the direct path coefficient (bi, Xi—Y) and the indirect path coefficient (rijbj, Xi—Xj—Y, i≠j) 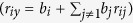 . PA was conducted by statistical software SAS 9.1 (SAS Institute, Cary, NC, USA) to evaluate correlation between the active ingredients, antioxidant activity and primary environmental factors obtained by GCA. Environmental factors were used as independent variables, and active ingredients and antioxidant activity were used as dependent variables in each test. Furthermore, in order to further evaluate the quality of P. fruticosa from different growing locations, another three methods viz. HPLC fingerprint analysis coupled with chemometric methods were applied. Firstly, HPLC fingerprint similarity analysis (SA) was performed using Computer Aided Similarity Evaluation software (CASE 2004, Zhejiang University, Hangzhou, China) as recommended by the Chinese Pharmacopoeia Committee. The software is used for evaluating similarities between different chromatograms based on the cosine values of vectorial angel61. The cosine values of the two chromatograms approaching 1 means they are highly similar. This software was also used to compute the mean chromatogram as a representative standard chromatogram for a group of chromatograms. The standard HPLC fingerprint is set up with the median of all chromatograms62,63,64. Subsequently, hierarchical clustering analysis (HCA) and discrimination analysis (DA) were performed using SPSS 19.019,20. The ‘average linkage between groups’ method was applied and the Pearson correlation was selected as a measurement65. DA can be used to build a predictive model of the group membership based on observed characteristics in each case. This procedure can generate a discrimination function or a set of discriminant functions from the samples with known membership based on linear combinations of the predictor variables that provide the best discrimination among the groups. The functions can be applied to discriminate and classify new cases with measurements for the predictor variables but with unknown group membership20. A detailed description/theory of statistical methods/models was provided in supplementary Text S1. Normalization of data was performed by the Z score transformation normalization method if necessary66.
. PA was conducted by statistical software SAS 9.1 (SAS Institute, Cary, NC, USA) to evaluate correlation between the active ingredients, antioxidant activity and primary environmental factors obtained by GCA. Environmental factors were used as independent variables, and active ingredients and antioxidant activity were used as dependent variables in each test. Furthermore, in order to further evaluate the quality of P. fruticosa from different growing locations, another three methods viz. HPLC fingerprint analysis coupled with chemometric methods were applied. Firstly, HPLC fingerprint similarity analysis (SA) was performed using Computer Aided Similarity Evaluation software (CASE 2004, Zhejiang University, Hangzhou, China) as recommended by the Chinese Pharmacopoeia Committee. The software is used for evaluating similarities between different chromatograms based on the cosine values of vectorial angel61. The cosine values of the two chromatograms approaching 1 means they are highly similar. This software was also used to compute the mean chromatogram as a representative standard chromatogram for a group of chromatograms. The standard HPLC fingerprint is set up with the median of all chromatograms62,63,64. Subsequently, hierarchical clustering analysis (HCA) and discrimination analysis (DA) were performed using SPSS 19.019,20. The ‘average linkage between groups’ method was applied and the Pearson correlation was selected as a measurement65. DA can be used to build a predictive model of the group membership based on observed characteristics in each case. This procedure can generate a discrimination function or a set of discriminant functions from the samples with known membership based on linear combinations of the predictor variables that provide the best discrimination among the groups. The functions can be applied to discriminate and classify new cases with measurements for the predictor variables but with unknown group membership20. A detailed description/theory of statistical methods/models was provided in supplementary Text S1. Normalization of data was performed by the Z score transformation normalization method if necessary66.
Figure 10. Network of path analysis.
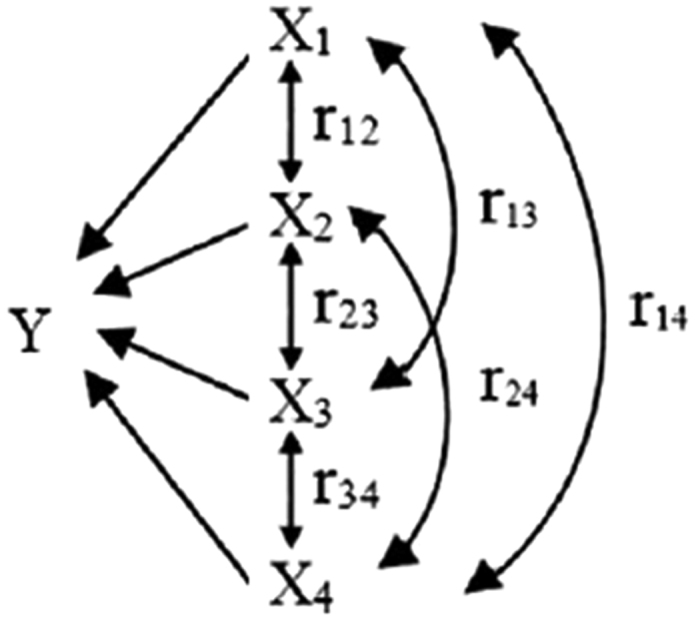
X1, X2, X3 and X4 are independent variables; Y is dependent variable. rab represents the correlation coefficient between independent variables a and b.
The results were presented as the mean value ± SD (standard deviation). The data was analyzed by one-way ANOVA (Analysis of Variance) followed by Duncan multiple comparison (p < 0.05) based on SPSS software (SPSS for Windows 19.0, SPSS Inc., USA).
Additional Information
How to cite this article: Liu, W. et al. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla fruticosa L. and Its Quality Assessment. Sci. Rep. 6, 28591; doi: 10.1038/srep28591 (2016).
Supplementary Material
Acknowledgments
The program was supported by the Special Fund for Forestry Scientific Research in the Public Interest of China (200904004) and Program for Innovative Research Team (in Science and Technology) in University of Henan Province (14TRTSTHN014). We are grateful to all the members for their assistance and helpful comments in the field and lab. We also thank Yanzheng Yang for the technical support.
Footnotes
Author Contributions D.L. and J.L. conceived of and proposed the idea. W.L. and D.W. designed the study. W.L., D.Y. and N.L. carried out the experiments. W.L., D.Y. and N.L. performed the data analysis. W.L. wrote the paper. Professor X.H., D.W., D.L. and J.L. contributed to editing and proof-reading the manuscript. All the authors discussed the results and commented on the manuscript.
References
- Xue P. F., Luo G., Zeng W. Z., Zhao Y. Y. & Liang H. Secondary metabolites from Potentilla multifida L. (Rosaceae). Biochem. Syst. Ecol. 33, 725–728 (2005). [Google Scholar]
- Xue P. F., Zhao Y. Y., Wang B. & Liang H. Secondary metabolites from Potentilla discolor Bunge (Rosaceae). Biochem. Syst. Ecol. 34, 825–828 (2006). [Google Scholar]
- Barkley T. & Cronquist A. A Synonymized Checklist of the Vascular Flora of the United States, Canada and Greenland. Volume II, The Biota of North America. Brittonia 32, 573–573 (1980). [Google Scholar]
- Mitich L. W. Cinquefoils (Potentilla spp.), the five finger weeds. Weed Technol. 9, 857–861 (1995). [Google Scholar]
- Tomczyk M., Pleszczyńska M. & Wiater A. Variation in total polyphenolics contents of aerial parts of Potentilla species and their anticariogenic activity. Molecules 15, 4639–4651 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliauskas G., Venskutonis P. & Van Beek T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 85, 231–237 (2004). [Google Scholar]
- Miliauskas G. et al. Antioxidant activity of Potentilla fruticosa. J. Sci. Food Agr. 84, 1997–2009 (2004). [Google Scholar]
- Nkiliza J.; Berkem, Gardonne, France. Process for extraction of catechin polyphenols from Potentillas, extract obtained and its utilization. United States patent US 5,928,646. 1999 Jul 27.
- Dong J. E., Ma X. H., Wei Q., Peng S. B. & Zhang S. C. Effects of growing location on the contents of secondary metabolites in the leaves of four selected superior clones of Eucommia ulmoides. Ind. Crop Prod. 34, 1607–1614 (2011). [Google Scholar]
- Shimono A. et al. Range shifts of Potentilla fruticosa on the Qinghai-Tibetan Plateau during glacial and interglacial periods revealed by chloroplast DNA sequence variation. Heredity 104, 534–542 (2009). [DOI] [PubMed] [Google Scholar]
- Editorial committee of Flora of China. Flora of China (Vol. 9). (Science Press & Missouri Botanical Garden Press, Beijing & St. Louis, 2003). [Google Scholar]
- Peñuelas J. & Llusià J. Effects of carbon dioxide, water supply, and seasonally on terpene content and emission by Rosmarinus officinalis. J. Chem. Ecol. 23, 979–993 (1997). [Google Scholar]
- Wulff A. et al. Birch (Betula pendula Roth) responses to high UV-B radiation. Boreal. Environ. Res. 4, 77–88 (1999). [Google Scholar]
- Alam A., Naik P. K., Gulati P., Gulati A. K. & Mishra G. P. Characterization of genetic structure of Podophyllum hexandrum populations, an endangered medicinal herb of Northwestern Himalaya, using ISSR-PCR markers and its relatedness with podophyllotoxin content. Afr. J. Biotechnol. 7, 1028–1040 (2008). [Google Scholar]
- Alam M. A., Gulati P., Aswini K. G., Gyan P. M. & Pradeep K. N. Assessment of genetic diversity among Podophyllum hexandrum genotypes of Northwestern Himalayan region for podophyllotoxin production. Indian J. Biotechnol. 8, 391–399 (2009). [Google Scholar]
- Liu W., Liu J., Yin D. & Zhao X. Influence of ecological factors on the production of active substances in the anti-cancer plant Sinopodophyllum hexandrum (Royle) T.S. Ying. PLoS ONE 10, e0122981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Pharmacopoeia Committee. Chinese Pharmacopoeia of People’s Republic of China. (Chemical Industry Press, Beijing, 2010). [Google Scholar]
- Feng Y. et al. Analysis of Cnidium monnieri fruits in different regions of China. Talanta 53, 1155–1162 (2001). [DOI] [PubMed] [Google Scholar]
- Wang D. M., He F. Y., Lv Z. J. & Li D. W. Phytochemical composition, antioxidant activity and HPLC fingerprinting profiles of three Pyrola species from different regions. PLoS ONE 9, e96329 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. M. et al. Fingerprint analysis of Lingzhi (Ganoderma) strains by high-performance liquid chromatography coupled with chemometric methods. World J. Microbiol. Biotechnol. 24, 2443–2450 (2008). [Google Scholar]
- Yang D. F., Liang Z. S., Duan Q. M. & Zhang Y. J. Quality assessment of cardiotonic pills by HPLC fingerprinting. Chromatographia 66, 509–514 (2007). [Google Scholar]
- Peng L., Wang Y. Z., Zhu H. B. & Chen Q. M. Fingerprint profile of active components for Artemisia selengensis Turcz by HPLC–PAD combined with chemometrics. Food chem. 125, 1064–1071 (2011). [Google Scholar]
- Yang J., Chen L. H., Zhang Q., Lai M. X. & Wang Q. Quality assessment of Cortex cinnamomi by HPLC chemical fingerprint, principle component analysis and cluster analysis. J. Sep. Sci. 30, 1276–1283 (2007). [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M. E. & Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28, 25–30 (1995). [Google Scholar]
- Liu G. & Yu J. Gray correlation analysis and prediction models of living refuse generation in Shanghai city. Waste Manage. 27, 345–351 (2007). [DOI] [PubMed] [Google Scholar]
- Yeh Y. L. & Chen T. C. Application of grey correlation analysis for evaluating the artificial lake site in Pingtung Plain, Taiwan. Can. J. Civ. Eng. 31, 56–64 (2004). [Google Scholar]
- Garson G. D. Path Analysis. (Statistical Associates Publishers, Asheboro, 2014). [Google Scholar]
- Zidorn C. & Stuppner H. Evaluation of chemosystematic characters in the genus Leontodon (Asteraceae). Taxon 50, 115–133 (2001). [Google Scholar]
- Li H. C., Sun H. Z. & Hu X. Analysis on total flavonoid in leaves of Potentilla fruticoca in different environment and related mechanism. Journal of West China Forestry Science 36, 71–73 (2007). [Google Scholar]
- Ghasemi K. et al. Influence of environmental factors on antioxidant activity, phenol and flavonoids contents of walnut (Juglans regia L.) green husks. J. Med. Plants Res. 5, 1128–1133 (2011). [Google Scholar]
- Bernal M., Llorens L., Julkunen-Tiitto R., Badosac J. & Verdaguer D. Altitudinal and seasonal changes of phenolic compounds in Buxus sempervirens leaves and cuticles. Plant Physiol. Biochem. 70, 471–482 (2013). [DOI] [PubMed] [Google Scholar]
- Shepherd T. & Griffiths W. The effects of stress on plant cuticular waxes. New Phytol. 171, 469–499 (2006). [DOI] [PubMed] [Google Scholar]
- Kunst L. & Samuels A. L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 42, 51–80 (2003). [DOI] [PubMed] [Google Scholar]
- Bilger W., Rolland M. & Nybakken L. UV screening in higher plants induced by low temperature in the absence of UV-B radiation. Photochem. Photobiol. Sci. 6, 190–5 (2007). [DOI] [PubMed] [Google Scholar]
- Albert A., Sareedenchai V., Heller W., Seidlitz H. K. & Zidorn C. Temperature is the key to altitudinal variation of phenolics in Arnica montana L. cv. ARBO. Oecologia 160, 1–8 (2009). [DOI] [PubMed] [Google Scholar]
- Reyes L. F., Miller J. C. & Cisneros-Zevallos L. Environmental conditions influence the content and yield of anthocyanins and total phenolics in purple-and red-flesh potatoes during tuber development. Am. J. Potato Res. 81, 187–193 (2004). [Google Scholar]
- Leist N. & Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. J. Plant Physiol. 157, 140–141 (2000). [Google Scholar]
- Zobayed S. M. A., Afreen F. & Kozai T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’s wort. Plant Physiol. Bioch. 43, 977–984 (2005). [DOI] [PubMed] [Google Scholar]
- Wahid A. & Ghazanfar A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 163, 723–730 (2006). [DOI] [PubMed] [Google Scholar]
- Ganzera M., Guggenberger M., Stuppner H. & Zidorn C. Altitudinal variation of secondary metabolite profiles in flowering heads of Matricaria chamomilla cv. BONA. Planta medica 74, 453–457 (2008). [DOI] [PubMed] [Google Scholar]
- Fuglevand G., Jackson J. A. & Jenkins G. I. UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell 8, 2347–2357 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R. B., Wu Q. S. & Wan Z. L. Influence of altitudes on effective compositions of American Ginseng in mountains of west Anhui. Chin. J. Agri. Meteorol. 22, 19–22 (2001). [Google Scholar]
- Dumas Y., Suniaga Quijada J. & Bonafous M. Influence of nitrogen availability on growth and development of tomato plants until fruit-setting. Optimization of Plant Nutrition. Fragoso M. A. C. & van Beusichem M. L. (ed.). Kluwer Academic Publishers (1993). [Google Scholar]
- Liu T. C. Panax quinquefolium of China. (People’s Medical Publishing House, Beijing, 1995). [Google Scholar]
- Yu L., Perret J., Harris M., Wilson J. & Haley S. Antioxidant properties of bran extracts from “Akron” wheat grown at different locations. J. Agric. Food Chem. 51, 1566–1570 (2003). [DOI] [PubMed] [Google Scholar]
- Wang S. Y. & Zheng W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 49, 4977–4982 (2001). [DOI] [PubMed] [Google Scholar]
- Mpofu A., Sapirstein H. D. & Beta T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 54, 1265–1270 (2006). [DOI] [PubMed] [Google Scholar]
- Feng Y., Wu Z. H., Zhou X. Z., Zhou Z. M. & Fan W. Y. Knowledge discovery in traditional Chinese medicine: state of the art and perspectives. Artif. Intell. Med. 38, 219–236 (2006). [DOI] [PubMed] [Google Scholar]
- Yuan R. & Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol. Ther. 86, 191–198 (2000). [DOI] [PubMed] [Google Scholar]
- Sparks D. L. et al. Methods of soil analysis. Part 3-Chemical methods. (Soil Science Society of America Inc., Madison, 1996). [Google Scholar]
- Kalil S. J., Maugeri F. & Rodrigues M. I. Response surface analysis and simulation as a tool for bioprocess design and optimization. Process Biochem. 35, 539–550 (2000). [Google Scholar]
- Helrich K. C. Official Methods of Analysis of the Association of Official Analytical Chemists (Volume 2). (Association of Official Analytical Chemists Inc., Rockville, 1965). [Google Scholar]
- Jia Z. S., Tang M. C. & Wang J. M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559 (1999). [Google Scholar]
- Singleton V. & Rossi J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965). [Google Scholar]
- Sagdic O. et al. RP-HPLC-DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chem. 126, 1749–1758 (2011). [DOI] [PubMed] [Google Scholar]
- Liu W., Wang D., Liu J., Li D. & Yin D. Quality evaluation of Potentilla fruticosa L. by High Performance Liquid Chromatography fingerprinting associated with chemometric methods. PLoS ONE 11, e0149197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Wang D. M., Pu W. J. & Li D. W. Phytochemical profiles, antioxidant and antimicrobial activities of three Potentilla species. BMC Complem. Altern. M. 13, 775–779 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois M. S. Antioxidant determinations by the use of a stable free radical. Nature 26, 1199–1200 (2002). [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 44, 307–315 (1986). [Google Scholar]
- Wold S., Esbensen K. & Geladi P. Principal component analysis. Chemometr. Intell. Lab. 2, 37–52 (1987). [Google Scholar]
- Fang K. T., Liang Y. Z., Yin X. L., Chan K. & Lu G. H. Critical value determination on similarity of fingerprints. Chemometr. Intell. Lab. 82, 236–240 (2006). [Google Scholar]
- Yang L. W. et al. Fingerprint quality control of Tianjihuang by high-performance liquid chromatography–photodiode array detection. J Chromatogr. A 1070, 35–42 (2005). [DOI] [PubMed] [Google Scholar]
- Lu G. H. et al. Development of high-performance liquid chromatographic fingerprints for distinguishing Chinese Angelica from related umbelliferae herbs. J Chromatogr. A 1073, 383–341 (2005). [DOI] [PubMed] [Google Scholar]
- Cai M., Zhou Y., Gesang S. L., Bianba C. & Ding L. S. Chemical fingerprint analysis of rhizomes of Gymnadenia conopsea by HPLC–DAD–MSn. J Chromatogr. B 844, 301–307 (2006). [DOI] [PubMed] [Google Scholar]
- Zhao K. J., Dong T. T. X. & Tsim K. W. K. Molecular genetic and chemical assessment of Radix angelica (Danggui) in China. J. Agric. Food. Chem. 51, 2576–2583 (2003). [DOI] [PubMed] [Google Scholar]
- Noy-Meir I., Walker D. & Williams W. T. Data transformations in ecological ordination: II. On the meaning of data standardization. J. Ecol. 63, 779–800 (1975). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


