Abstract
Cocaine use disorder is a persistent public health problem for which no widely effective medications exist. Self-administration procedures, which have shown good predictive validity in estimating the abuse potential of drugs, have been used in rodent, nonhuman primate, and human laboratory studies to screen putative medications. This review assessed the effectiveness of the medications development process regarding pharmacotherapies for cocaine use disorder. The primary objective was to determine whether data from animal and human laboratory self-administration studies predicted the results of clinical trials. In addition, the concordance between laboratory studies in animals and humans was assessed. More than 100 blinded, randomized, fully placebo-controlled studies of putative medications for cocaine use disorder were identified. Of the 64 drugs tested in these trials, only 10 had been examined in both human and well-controlled animal laboratory studies. Within all three stages, few studies had been conducted for each drug and when multiple studies had been conducted conclusions were sometimes contradictory. Overall, however, there was good concordance between animal and human laboratory results when the former assessed chronic drug treatment. Although only seven of the ten reviewed drugs showed fully concordant results across all three types of studies reviewed, the analysis revealed several subject-related, procedural, and environmental factors that differ between the laboratory and clinical trial settings that help explain the disagreement for other drugs. The review closes with several recommendations to enhance translation and communication across stages of the medications development process that will ultimately speed the progress toward effective pharmacotherapeutic strategies for cocaine use disorder.
I. Introduction
Development of pharmacotherapies for cocaine use disorder has been a priority for the National Institute on Drug Abuse for at least three decades (Schuster and Snyder, 1989). As part of this process, behavioral laboratory measures have been adopted for screening putative medications. These methods include self-administration and, to a lesser extent, drug discrimination techniques in humans and nonhuman animals, as well as subjective ratings in humans. These procedures were largely developed to study the behavioral pharmacology of abused drugs, particularly to predict the abuse potential of new compounds. Whether these behavioral measures demonstrate predictive validity regarding efficacy in treating cocaine use disorders has been a topic of debate, although self-administration measures appear to be the best screening tool (Mello and Negus, 1996; Comer et al., 2008; Haney and Spealman, 2008).
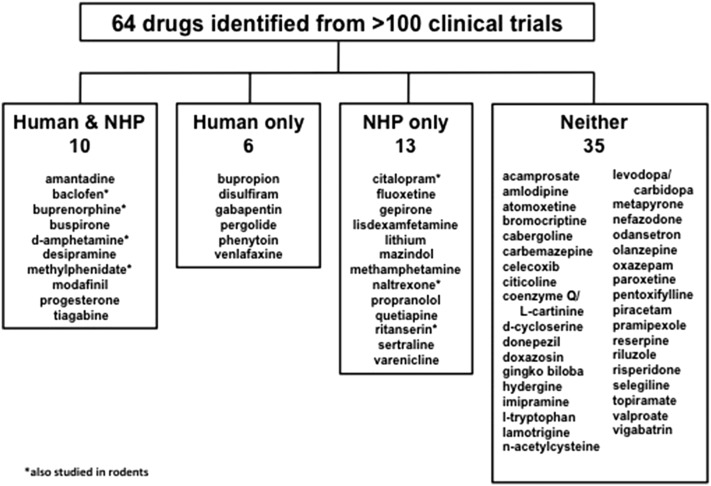
This review was undertaken partly to determine the predictive validity of animal and human laboratory efforts to develop medications for cocaine use disorder with respect to the results of clinical trials. In addition, the review was conceived as a way to evaluate the medications development process. At the outset, it was presumed that this process follows a rational “pipeline” moving from nonhuman animals (particularly nonhuman primates and rodents) to human laboratory studies to clinical trials. We found that this was rarely the case. Of the 64 medications identified from more than 100 blinded, randomized, fully placebo-controlled clinical trials, only 10 had also been tested in both nonhuman primate and human laboratory self-administration studies (Fig. 1; Table 1). By encouraging standardization of procedures and relevant dependent variables across laboratories and recommending increased communication and cooperation across preclinical and clinical settings, we believe this review will enhance the effectiveness of the medications development process for cocaine use disorder.
Fig. 1.
Categorization of the drugs identified by the searches for this review according to whether the drugs had been studied in both human and nonhuman primate (NHP) laboratory studies, only in one species, or in neither.
TABLE 1.
Published studies included in this review
Symbols indicate that the studies were interpreted as providing positive (+) or negative (-) results regarding the suitability of the drug as a pharmacotherapy. Under Laboratory Animal Studies, studies using rodent subjects are indicated by italics.
A. Epidemiology of Cocaine Use Disorder
Cocaine use is an unrelenting public health concern. Data from the National Survey on Drug Use and Health indicate that approximately 1.5 million Americans over 12 years of age report current (i.e., past month) cocaine use, making cocaine the most widely used illicit stimulant in the United States [Substance Abuse and Mental Health Services Administration (SAMHSA), 2014]. Importantly, the SAMHSA estimates, which do not include indigent and incarcerated populations, may vastly underestimate the actual number of cocaine users (Caulkins et al., 2015a,b). Nearly one million of those who reported use met cocaine abuse or dependence criteria in 2013. Despite prevention and intervention efforts, the prevalence of cocaine use and cocaine use disorders has remained relatively stable (SAMHSA, 2014), in part because there are currently no medications approved by the US Food and Drug Administration (FDA). The stable prevalence of problematic use (i.e., meeting use disorder criteria) indicates that novel approaches are necessary to help treatment seekers to achieve and maintain abstinence. Moreover, an important ramification of the passage of the Affordable Care Act will be a large influx of people with substance use disorders into the treatment setting—perhaps as many as 40 million (McLellan and Woodworth, 2014). Novel, effective medications to meet this need are currently lacking.
Chronic cocaine use produces persistent changes in vasculature that increase the likelihood of myocardial infarction, hypertension, atherosclerosis, and stroke (Daras et al., 1994; Mouhaffel et al., 1995; Brecklin and Bauman, 1999; Lange and Hillis, 2001; Patrizi et al., 2006; Lucena et al., 2010). Cocaine use disorder also increases risks for other health issues, including cigarette smoking, comorbid psychologic disorders, and acquiring and spreading sexually transmitted infections (Rounsaville et al., 1991; Budney et al., 1993; Van Tieu and Koblin, 2009). For example, after controlling for any history of injection drug use, the lifetime prevalence of HIV is more than 20-fold higher among current cocaine users relative to individuals not reporting current use (SAMHSA, 2014). Altogether, estimates have placed the economic cost of illicit drug use in the United States at more than $190 billion dollars annually (National Drug Intelligence Center, 2011). Research that identifies promising therapies for cocaine use disorder will have significant public health implications by reducing the prevalence of cocaine use and associated social, legal, and medical issues (Greberman and Wada, 1994). A substantial amount of research has been conducted to develop pharmacotherapies to manage stimulant use disorders and their attendant health and societal concerns, but a widely effective treatment remains elusive.
B. Pharmacology of Cocaine
A great deal of preclinical research has elucidated the mechanisms that mediate the abuse-related effects of cocaine. The primary pharmacological effects of cocaine are produced through its binding to, and inhibition of function of, neuronal transporters for the monoamine neurotransmitters dopamine, serotonin (5-HT), and norepinephrine (e.g., Koe, 1976). The normal function of these transporters is to terminate neuronal communication by transferring released neurotransmitter from the extracellular space (i.e., the synapse) back into neurons. Thus, the immediate effect of cocaine’s pharmacological action is to increase extracellular monoamines and prolong their interaction with pre- and postsynaptic receptors. It is this potentiation of neurotransmission that is understood to be responsible for cocaine’s psychomotor stimulant effects (e.g., Johanson and Fischman, 1989). Brain dopamine transporters have been implicated in these effects to a greater extent than cocaine’s actions on 5-HT or norepinephrine systems (Ritz et al., 1987; Koob and Volkow, 2010). For example, studies in laboratory animals have demonstrated that drugs that selectively block dopamine transporters are self-administered and produce cocaine-like interoceptive stimulus effects (Bergman et al., 1990; Balster et al., 1991; Roberts 1993; Katz et al., 2000). Dopamine receptor agonists maintain self-administration in monkeys (Woolverton et al., 1984; Weed and Woolverton, 1995; Grech et al., 1996; Sinnott et al., 1999), and antagonism of these receptors can attenuate cocaine self-administration (Woolverton and Virus, 1989; Bergman et al., 1990; Nader et al., 1999; Xi et al., 2005). Conversely, increased 5-HT function appears to attenuate the effects of cocaine (Czoty et al., 2002; Rothman et al., 2005; Howell and Cunningham, 2015). Considering the primary involvement of dopamine in the abuse-related behavioral effects of cocaine, it is unsurprising that brain dopamine transporters and receptors have been targeted frequently in the development of pharmacotherapies for cocaine use disorder (e.g., Davies et al., 1993; Carroll et al., 1999; Grabowski et al., 2004; Heidbreder and Newman, 2010). In addition, drugs have been developed to indirectly modulate the effects of cocaine through 5-HT, glutamate and γ-aminobutyric acid (GABA) and other systems (e.g., Cousins et al., 2002; Johnson, 2005; Negus et al., 2007; Bubar and Cunningham, 2008; Kalivas and Volkow, 2011; Shorter and Kosten, 2011; Li et al., 2013).
C. Development of Medications for Other Drug Use Disorders
An overarching theme of this review is that development of medications for cocaine use disorder will progress most efficiently by using a “pipeline” approach. With this approach, promising candidates are identified in the animal laboratory and subsequently studied in the human laboratory. These data are then used to prioritize candidate drugs for clinical trials. Before examining drugs that have been studied in the context of cocaine use disorders, it is worthwhile to consider development of currently approved medications for treating other drug use disorders. In this section, we briefly describe studies of naltrexone for alcohol use disorder, buprenorphine for opioid use disorder, and varenicline for cigarette smoking. Although the approval process may not have involved laboratory data for these specific drugs, evidence documenting concordance between clinical and laboratory studies strengthens the rationale for a “pipeline” approach.
Naltrexone has been approved by the FDA for treating alcohol use disorder since 1994, largely based on two clinical trials demonstrating that treatment with naltrexone significantly reduced alcohol drinking relative to placebo (O’Malley et al., 1992; Volpicelli et al., 1992). More recent work has supported these findings, demonstrating the efficacy of long-lasting “depot” naltrexone formulations for reducing drinking (Kranzler et al., 2004). Early research in monkeys demonstrated that naltrexone maintenance slightly increased intravenous alcohol self-administration over the first 5 days of treatment, but significantly reduced alcohol self-administration relative to saline maintenance during days 5–15 (Altshuler et al., 1980). More recent work has replicated this finding in rodents (Bienkowski et al., 1999; Middaugh et al., 2000) and monkeys (Rodefer et al., 1999; Williams et al., 2001). Human laboratory self-administration research published after FDA approval showed that naltrexone treatment reduced alcohol self-administration (Davidson et al., 1999) and increased latency to drink alcohol (Davidson et al., 1996).
Buprenorphine development and approval for treating opioid use disorder followed a similar progression. One of the earliest human self-administration studies demonstrated that maintenance on 4 or 8 mg of buprenorphine significantly reduced heroin self-administration relative to placebo (Mello and Mendelson, 1980). These data were followed by a study in monkeys showing that buprenorphine treatment selectively reduced opioid (heroin or hydromorphone) self-administration without reducing food intake (Mello et al., 1983). Following these systematic laboratory demonstrations that buprenorphine could reduce opioid self-administration, clinical trials showed that buprenorphine effectively reduced opioid use (Johnson et al., 1992, 1995), ultimately leading to the approval of buprenorphine for treating opioid use disorder by the FDA in 2002.
The FDA’s approval of varenicline for cigarette smoking in 2006 was supported by preclinical work showing that varenicline reduced nicotine self-administration in rats (Rollema et al., 2007) and clinical trials indicating that varenicline was more effective than placebo and bupropion for promoting smoking cessation (Gonzales et al., 2006; Jorenby et al., 2006). Human laboratory studies published after approval showed that chronic, but not acute, varenicline treatment significantly reduced cigarette self-administration in the laboratory (Stoops et al., 2008; Perkins et al., 2010).
Collectively, these studies demonstrate that laboratory self-administration models can be used to screen medications using a “pipeline” approach. Human laboratory studies were largely conducted after medication approval, likely reflecting regulatory hurdles for studying potential medications for drug use disorders (i.e., the need for drugs to be FDA-approved before administration to humans), as well as the more recent development of laboratory drug self-administration measures in humans (see Moeller and Stoops, 2015). Although these drugs may not have been tested specifically according to our suggested order (e.g., conducting laboratory research in both nonhuman animals and humans before progressing to a clinical trial), the laboratory results demonstrate that self-administration procedures generate outcomes consistent with those of clinical trials. The use of laboratory screening with naltrexone, buprenorphine, and varenicline, as well as the predictive validity of laboratory self-administration methods, shows that using a proposed “pipeline” development approach may more effectively identify treatments for cocaine use disorder. Thus, laboratory self-administration measures can and should be used to predict treatment efficacy before the conduct of more expensive and labor-intensive clinical trials.
II. Methods of Assessing Medication Effectiveness
A. Animal Laboratory Studies
Although a variety of procedures have been used in the preclinical laboratory to study the abuse-related effects of drugs (e.g., drug discrimination, conditioned place preference, locomotor activity, etc.), the standard for studying abuse potential has been drug self-administration. This technique relies on operant conditioning and delivery of drug, typically as an intravenous injection, under some schedule of reinforcement. All schedules of reinforcement are based on the relationship between prevailing environmental stimuli predictive of drug availability (i.e., discriminative stimuli), a required response and the consequent stimulus that is delivered (Skinner, 1938). Although the dependent variables are the same across multiple procedures (e.g., rates of responding, numbers of injections, drug intake), proper interpretation of results of drug self-administration studies depends on careful consideration of the particular schedule of reinforcement used. This section briefly describes procedures designed to assess two different endpoints used in the animal laboratory to predict a medication’s effectiveness.
1. Attenuation of the Reinforcing Effects of Cocaine.
Under most schedules of reinforcement, the rate of responding or reinforcer delivery is the primary dependent variable. Under such schedules, delivery of a stimulus (e.g., a drug injection) occurs after either a certain number of responses have been emitted (ratio-based schedules) or when a response is made after a certain amount of time has elapsed (interval-based schedules). For example, under a fixed-ratio 50 schedule (designated FR 50), the stimulus is delivered after every 50th response, whereas under a 300-second fixed-interval schedule (designated FI 300 second), the stimulus is delivered when a response is made after 300 seconds have elapsed. The drug injection is operationally defined as a reinforcer if the amount of responding leading to its presentation is significantly greater than the amount of responding that occurs when the consequent stimulus is an injection of the drug’s vehicle (typically saline or sterile water). FR and FI schedules are the most commonly used in the study of drug reinforcement.
One variation of this design is a second-order schedule. In this procedure, responding according to one schedule (e.g., FR) results in brief presentation of a stimulus (e.g., a light). Completion of this schedule constitutes a unitary response under another schedule of reinforcement (e.g., a FI), completion of which is reinforced by drug delivery accompanied by a longer presentation of the same stimulus. For example, under a FI 300(FR 50:S) schedule, the light (“S” for “stimulus”) is illuminated briefly after every 50th response (i.e., FR 50). The first FR 50 completed after 300 seconds have elapsed (i.e., the FI) results in delivery of the drug injection and a sustained illumination of the light. The advantages of second-order schedules in specific applications have been enumerated previously (e.g., Schindler et al., 2002; Di Ciano and Everitt, 2005). For example, because a great deal of responding occurs before presentation of drug, such schedules have been used as a model of drug “seeking.”
Under FR, FI, or second-order schedules, when rates of responding or reinforcement are plotted as a function of the self-administered dose, the relationship is represented by a biphasic (inverted U-shaped) function (e.g., Pickens and Thompson, 1968). Injection of vehicle or very low doses of drug maintains low rates of behavior; responding increases up to a maximum as the available drug dose increases. This dose range defines the ascending limb of dose-effect function. Further increases in the available dose produce dose-dependent decreases in rates of behavior and constitute the descending limb of dose-effect function. Although response rate and number of drug injections decrease on the descending limb as unit dose increases, total drug intake often increases over the entire range of doses.
The value of FR schedules lies in the ability to determine unambiguously whether a drug has reinforcing effects—that is, whether the drug injection increases the probability of occurrence of the behavior that led to its delivery. Whether a particular drug dose functions as a reinforcer can easily be determined by comparing response rates (or numbers of injections) during availability of that dose to response rates (or numbers of injections) when vehicle is available. This information has proven critical when the purpose of the experiment is to demonstrate whether a drug is likely to be abused by humans.
Some ambiguity is introduced when an FR schedule of reinforcement is used to determine whether a drug decreases cocaine self-administration. In the present context, if administration of a test drug before availability of a reinforcing dose of cocaine results in rates of responding that are not different from responding when saline is available, one possible interpretation is that the test drug blocked the reinforcing effects of cocaine. Under an FR schedule, a more detailed mechanistic interpretation is complicated by the fact that multiple drug effects can influence the location and slope of the dose effect curve. A drug-induced decrease in responding maintained by a certain dose of cocaine may arise because the pretreatment drug caused that cocaine dose to resemble a lower or higher dose on the curve. Subjects might respond less because they are satiated, because higher doses have aversive effects, or because high doses of some drugs can produce effects such as sedation or motor stereotypies that are incompatible with making responses. Consequently, the interpretation of self-administration data under FR or FI schedules is complicated by the integration of these effects into a single response-rate measure (see Zernig et al., 2004).
The ultimate behavioral mechanism by which a potential medication decreases cocaine use is of great importance. If cocaine use decreases because a pharmacotherapy causes sedation, emesis, or aversion, undesirable side effects would be expected in patients that would likely decrease compliance. If a medication potentiates the effects of cocaine (observed under an FR schedule as a decrease in response rates maintained by descending-limb doses), it is likely that the drug would also potentiate toxic effects, such as cardiovascular or seizure-inducing effects. Only medications that reduce the positive reinforcing effects of cocaine without producing undesired effects on behavior or health are likely to be successfully implemented in the clinic. Data from FR or FI schedules are ambiguous in this regard.
Ascribing drug-induced reductions in cocaine self-administration to a decrease in cocaine reinforcement as opposed to other mechanisms described above is critical. For this reason, a criterion for inclusion in this review is that a study in laboratory animals assessed behavioral selectivity. The majority of studies have accomplished this by studying the effects of putative medications on schedule-controlled responding maintained by cocaine as well as a non-drug reinforcer (typically food) under identical conditions. In this review, we considered that a drug produced selective reductions in cocaine self-administration (Table 2) only when at least one dose of the drug significantly decreased rates of cocaine-maintained responding and lacked significant effects on food-maintained responding. If a study showed that a test drug reduced both cocaine- and food-maintained responding over the same dose range, it was scored as a negative result. One caveat that remains even with this control assessment is that an effect could appear selective if responding maintained by the non-drug reinforcer is more difficult to attenuate than responding maintained by drug (that is, if the non-drug stimulus is a stronger reinforcer). When comparing cocaine injections to food pellets, this would seem to be a minor concern. Nonetheless, some studies have taken the innovative step of attempting to equate the reinforcing strength of the drug and non-drug reinforcers or of examining drug effects on a range of magnitudes of the non-drug reinforcer (e.g., different concentrations of liquid food in water; Barrett et al., 2004; Thomsen et al., 2013).
TABLE 2.
Effect of reviewed drugs on cocaine taking
Parentheses indicate the number of studies showing significant (and, in animal studies, selective) reductions in cocaine taking (numerator) out of total reviewed studies (denominator).
| Drug | Animal Laboratory | Human Laboratory | Clinical Trials |
|---|---|---|---|
| Drugs that Target Monoamine Transporters | |||
| d-Amphetamine | ⇓(9/12) | ⇓(1/1) | ⇓(4/4) |
| Methylphenidate | −(0/2) | ⇓(1/1) | −(1/3) |
| Modafinil | ⇓(1/1) | ⇔(1/2) | −(2/7) |
| Desipramine | −(0/1) | −(0/1) | ⇔(1/2) |
| Amantadine | −(0/1) | −(0/1) | −(1/3) |
| Drugs that Facilitate GABA Function | |||
| Baclofen | ⇔(5/8) | ⇔(1/2) | ⇔(1/2) |
| Tiagabine | −(0/1) | −(0/1) | −(0/2) |
| Drugs that Target Other Mechanisms | |||
| Buspirone | −(1/5) | -(0/1) | −(0/2) |
| Buprenorphine | ⇓(6/8) | ⇓(2/2) | ⇔(2/4) |
| Progesterone | ⇓(1/1) | −(0/1) | −(0/1) |
⇓, Drug selectively reduced cocaine taking in majority of reviewed studies. ⇔, Drug had mixed effects on cocaine taking across reviewed studies. −, Drug had no selective effect on cocaine taking in majority of reviewed studies.
2. Attenuation of the Reinforcing Strength of Cocaine.
Whereas FR and FI schedules provide qualitative information regarding whether a drug serves as a reinforcer, it is often of greater relevance to determine how a potential pharmacotherapy alters the reinforcing strength of cocaine, a quantitative measure. Changes in the reinforcing strength of cocaine (sometimes called “reinforcing efficacy”) can be more unambiguously determined using more complex behavioral procedures. Two general approaches used to provide such quantitative information are progressive-ratio (PR) schedules and choice procedures.
A progressive-ratio schedule is similar to an FR schedule, except that the response requirement for delivery of successive reinforcers increases according to a predefined equation (Hodos, 1961). For example, the first drug injection might be delivered after 50 responses, the second after an additional 100 responses, the third after an additional 200 responses, and so on. In addition to assessing whether a drug functions as a positive reinforcer, PR schedules provide a measure of how many responses a subject will make to receive a drug injection before they cease responding. PR schedules have proven very useful in preclinical drug self-administration research (Richardson and Roberts, 1996; Stafford et al., 1998; Rowlett, 2000). The primary dependent variable under PR schedules is the break point, defined as the final response ratio requirement completed either after a predetermined period of time without a drug injection or the at end of an experimental session. Because break point is not a continuous variable, the actual number of drug injections delivered is typically used to analyze and plot the data (i.e., number of injections is plotted as a function of the available drug dose). Dose-effect functions under PR schedules produce a monotonic increase in responding over a wider range of doses compared to FR and FI schedules. However, because the dependent measure is still a rate of behavior that integrates both reinforcement-dependent and -independent effects, there is a unit drug dose that produces maximal responding. Further increases in unit drug doses generate less responding, resulting in an inverted U-shaped dose-response curve.
Another complex schedule of reinforcement adds a second, simultaneously active schedule that results in delivery of a different reinforcer. For example, responding on one lever may result in delivery of a drug injection, whereas responding on a second lever results in delivery of food. Because both schedules of reinforcement are concurrently active, the term for this type of schedule is a concurrent schedule of reinforcement. The two schedules can be of any type and need not be the same (e.g., an FR:FI schedule). Such a schedule is also called a choice procedure because the subject can choose to respond for either reinforcer.
Although underutilized, choice procedures have made important contributions to the understanding of drug self-administration, owing in large part to the translational value of this procedure (Johanson, 1975; Bergman and Paronis, 2006; Banks and Negus, 2012). The primary dependent variable under a choice procedure reflects the distribution of responding across the two alternatives. Thus, choice procedures uniquely encompass the fundamental clinical reality that drug use disorder represents a choice to allocate time, effort, and resources toward obtaining drug to the exclusion of other potential reinforcers in the environment such as food, employment, family, or other enjoyable activities (e.g., Kalivas and Volkow, 2005). When percent drug choice is plotted as a function of unit drug dose, the dose-effect function generates a monotonic increase in choice for drug over the alternative reinforcer, up to a maximum of 100% drug choice. Larger unit drug doses typically maintain exclusive choice for drug and only at the largest doses that suppress responding is it not possible to determine percent drug choice.
Beyond simply determining whether a putative medication completely blocks the reinforcing effects of cocaine, PR schedules and choice procedures can be used to determine the extent to which a drug reduces cocaine self-administration. This information goes beyond what is provided by self-administration procedures that characterize drugs according to response rates under FR or FI schedules of reinforcement. In the context of medications development, data from these procedures may be more useful in that they can show whether a potential pharmacotherapy would be expected to reduce cocaine use, even if it may not completely eliminate self-administration. Moreover, choice procedures quantify the extent to which a candidate medication promotes reallocation of from drug-maintained responding to responding maintained by an alternative reinforcer.
B. Human Laboratory Studies
Two measures have been used to predict pharmacotherapeutic efficacy in the human laboratory: subjective ratings and drug self-administration. Efficacy of a potential medication is evaluated by administering cocaine in combination with acute doses of the putative pharmacotherapy, or, more preferred, after a maintenance regimen with the pharmacotherapy. Medications shown to attenuate the effects of cocaine on these outcomes (e.g., those that reduce “liking” of cocaine or number of cocaine doses earned) are considered to be worthy of further investigation.
1. Attenuation of the Subjective Effects of Cocaine.
Subjective ratings are typically collected through responses on visual analog, true/false, or Likert-type ratings scales. Information is usually collected for groups of similar items, then scored as single measures (e.g., good effects or rush) or as multiple ratings grouped into a single scale (e.g., the Stimulant subscale of the Adjective Rating Scale; Oliveto et al., 1992). In general, the likelihood that a stimulant will be abused has been attributed to its ability to produce positive subjective effects (e.g., like drug; Johanson et al., 1983; Fischman and Foltin, 1991). Although subjective ratings can be rapidly and easily assess abuse potential and the efficacy of potential interventions (Griffiths et al., 2003), these methods have been criticized as an indirect measure of drug taking (Katz, 1990) and for significant variability across subjects due to variations in interpretation across time and individuals (Kelly et al., 2003). Perhaps the most important criticism is that subjective ratings can produce false positives when used to screen putative pharmacotherapies (Comer et al., 2008; Haney and Spealman, 2008). The use of multiple subjective ratings items, with most studies including 10 to 20 individual subjective ratings, also complicates interpretation because different effects may be observed across questions or responses to these items may covary (Bolin et al., 2013; Strickland et al., 2014). Using many subjective ratings can also increase the likelihood of false-positive conclusions.
2. Attenuation of Cocaine Self-Administration.
Self-administration methods evaluate the reinforcing effects of drugs and represent a more direct assessment of naturalistic drug taking in humans than subjective ratings. This approach frequently provides a single outcome with purportedly better predictive validity for intervention efficacy (Comer et al., 2008; Haney and Spealman, 2008). Although a number of behavioral arrangements and schedules of reinforcement have been used in a manner comparable to that described above for laboratory animals, subjects typically first sample a dose of drug and are then given the opportunity to work to earn that dose, or portions of that dose, again (for a review, see Jones and Comer, 2013).
Unlike in the animal laboratory, in humans the reinforcing effects of cocaine have predominantly been evaluated using choice procedures wherein subjects choose between cocaine and some alternative reinforcer such as food or money (Hart et al., 2000; Stoops et al., 2010, 2012a; Vosburg et al., 2010; Walsh et al., 2010). As described above, the use of choice procedures is thought to best model the natural ecology wherein drug users make choices between taking drugs and engaging in behaviors maintained by alternative behaviors. Choice procedures also model abstinence reinforcement treatment (Higgins et al., 2004) and allow for a determination of not only behavioral selectivity but can provide evidence of reallocation of behavior (e.g., subjects allocate choices to the alternative instead of cocaine). In this regard, choice procedures may be particularly advantageous because responding maintained by drug and an alternative reinforcer occurs at the same time in the same subject. The noted predictive validity and single outcome (e.g., number of drug choices, break point within a choice context) usually provided by self-administration measures, along with the direct comparison afforded between findings with laboratory animals and humans with these measures, led to inclusion of human laboratory studies of the reinforcing effects of cocaine in this review rather than subjective effects.
C. Clinical Trials
Clinical trials of medications for managing cocaine use disorder have used a number of outcomes to indicate efficacy, but abstinence from cocaine use and retention in treatment have been used most frequently (for reviews, see Donovan et al., 2012; Carroll et al., 2014). Complete abstinence from cocaine use for a specified period of time during a clinical trial is frequently the primary outcome and is best verified with biochemical analysis (e.g., quantitative or qualitative urine testing for the cocaine metabolite benzoylecgonine). This measure can be expressed as either percentage of urine samples that are indicative of use or dichotomously as abstinent/nonabstinent. Results can also be expressed quantitatively as the level of benzoylecgonine in urine. Retention in treatment is usually defined as patient attendance at scheduled clinic visits and/or time to dropout from a protocol. Retention does not directly measure the traditional primary variable of interest (drug use), but it is linked to improved treatment outcome (Simpson et al., 1999; Ciraulo et al., 2003; Carroll et al., 2014).
Ratings scales that assess global functioning and quality of life can indirectly indicate drug use and have been used to evaluate treatment success (Ghitza et al., 2007; Donovan et al., 2012; Carroll et al., 2014). Self-reported cocaine use with standardized tools like the Timeline Follow Back questionnaire can also be valuable (Preston et al., 1997; Preston et al., 2002). New target outcomes, such as reductions in drug use, were recently proposed as potential indicators of success. However, questions about the extent to which reductions in cocaine use result in clinically meaningful changes have prevented widespread adoption of these indicators in clinical trials for cocaine use disorder (Winchell et al., 2012; Carroll et al., 2014; McCann et al., 2015; Kiluk et al., 2016). Cocaine abstinence, verified as observation of urine samples testing negative for cocaine metabolites, thus remains the standard for demonstrating treatment efficacy in clinical trials. For these reasons, results of urine screening were selected as the primary clinical trial outcome to be considered in this review.
D. Variables Affecting Translation
The preceding sections describe differences in the variables typically used to assess the effectiveness of a putative medication across the animal laboratory, human laboratory, and clinical trial environment. Most animal studies use FR schedules of reinforcement and report medication effects on rates of drug self-administration, whereas most human laboratory studies use choice schedules and report medication effects on proportion of drug choices. Many other parameters and variables also influence the ability to translate from one setting to the others. Differences between humans and nonhuman animals, as well as between laboratory and clinical settings, create obstacles to standardizing procedures and measures across all experimental environments. However, attention to these variables when designing experiments can clearly enhance translation; specific examples will be highlighted below during discussion of individual drugs.
1. Medication Type.
Investigators working in different environments often have different reasons for selecting drugs to study. Preclinical research in animals has tended to focus on the development of novel compounds rather than investigation of older, well-known compounds. This may occur because an older drug has already been deemed ineffective in clinical trials. However, as illustrated below, clinical data are often limited to one or two trials in distinct subject groups. Premature dismissal of a drug based on limited clinical data may discourage preclinical researchers from investigating that drug or similar compounds under different conditions. In addition, preclinical researchers frequently use pharmacologically selective drugs to test hypotheses related to specific mechanisms of action. Drugs used for this purpose often have not been approved for use in humans, limiting the ability to assess whether animal data translate to the human laboratory or clinical population. Conversely, clinical trials often favor more established drugs, already approved for other indications, that are of less interest to preclinical researchers investigating biologic targets.
2. Medication Dose.
Even when a drug can be studied in humans, regulatory concerns may limit how much of a drug can be given to human subjects (see Negus and Henningfield, 2015); drugs can typically be safely tested at higher doses and with more varied routes of administration in animals than in humans. One example is lisdexamfetamine, a pro-drug for d-amphetamine that showed positive results in nonhuman primates (Banks et al., 2015) but negative results in a subsequent clinical trial (Mooney et al., 2015). Although this discordance appears to indicate a lack of predictive validity of the nonhuman primate study, Mooney and colleagues noted that the doses that could be administered in their trial were limited to those used to treat attention-deficit hyperactivity disorder (ADHD). They suggested that higher doses, which have been safely used in humans, were likely to be necessary to produce positive results. Thus, even when scientific interests align, regulatory issues such as those related to preparing an Investigational New Drug application can reduce the likelihood of preclinical and clinical researchers collaborating to study the same drug. Comparison of results between animal and human laboratory studies and clinical trials should include an assessment of whether poor concordance may reflect a failure to test equivalent dose ranges in animals and humans.
3. Treatment Regimen.
A critical difference between the experimental designs of typical laboratory studies and clinical trials is the duration of drug treatment. Acute drug treatments are common in laboratory animal studies, whereas chronic or at least repeated drug treatment regimens predominate in the human laboratory and clinical trials. Studies have shown that drugs can have different or even opposite effects after acute versus chronic administration; preclinical studies could provide an early indication that tolerance to the therapeutic effects or sensitization to toxic effects of a drug is likely to occur. In this review, we consider preclinical studies that examine both acute and chronic administration of putative medications and highlight instances of disagreement. The results indicate that characterizing the effects of chronic drug treatment in animal studies is a critical step in evaluation of a potential pharmacotherapy. Poor concordance may reflect comparison of acute drug effects in preclinical studies to chronic drug effects in clinical trials.
4. Contingencies Associated with Medication Administration.
Consideration of experimental parameters such as the drug under study, duration of treatment, and dose ranges tested are likely to enhance translation of findings from animal models. Other characteristics of laboratory and clinical studies in humans may be more difficult to incorporate into animals. One consideration is the extent to which administration of the medication is contingent on the subject’s behavior. In the laboratory, medications are taken voluntarily by human subjects but are administered noncontingently to animal subjects by the experimenter (excepting some oral drug administration procedures). In both cases, however, investigators can be certain that the medication has been administered. Outpatient clinical trials represent a third scenario, in which medications are administered by the subjects but compliance is not assured, underreported, and often overestimated (see King and Pryce, 2014). Although this issue has not received much attention as an important variable that may influence translation, whether drug taking is voluntary or noncontingent may alter the response to the drug. Low compliance may lead to the false negative conclusion that a drug is ineffective.
5. Inclusion of Behavioral Treatments.
Finally, it should be noted that behavioral interventions, including contingency-management approaches and cognitive behavioral therapy, are a valuable part of treatment and may interact positively with medications; nearly all clinical trials include some form of psychotherapy. However, there is no correlate of psychotherapy included in laboratory studies.
In summary, there are a number of factors that can hinder translation between animal and human laboratories and clinical trials. Some of these can be addressed when designing laboratory studies to enhance the strength of translation to the clinical setting. Overall, concordance of translational studies is likely to be greatest when comparing effects of the same doses of the same drug administered using a chronic regimen. Other factors, such as the incorporation of behavioral interventions, will require continued development of animal and, in some cases, human laboratory models.
E. Study Selection
For this review, articles reporting results of clinical trials were initially identified through PubMed searches and review of references within identified articles. Only blinded, randomized, fully placebo-controlled studies were included for review. Next, for each of the 64 drugs identified through this search, we identified published journal articles that tested the ability of the drugs to reduce cocaine-self administration in the nonhuman primate or human laboratory. Human laboratory studies met criteria only if they included both a placebo cocaine and pharmacotherapy condition. When possible, human laboratory studies and clinical trials were limited to populations that did not have diagnosed comorbidities (e.g., co-occurring alcohol and cocaine use disorder). It is worth noting that a number of studies were excluded because they only included opioid-dependent cocaine users. Two exceptions were made such that studies of amphetamine or methylphenidate in cocaine users with ADHD and studies of buprenorphine in opioid-dependent cocaine users were included. Studies in monkeys only qualified if they included an assessment of behavioral selectivity (typically, examination of drug effects on food-maintained responding, see section II.A.1). For the 29 drugs that had been studied in either or both settings, we next identified published studies in rodents that included an assessment of behavioral selectivity and included these in our assessment. In the final analysis, there were 10 clinically tested drugs for which we identified articles that met criteria in both animal and human laboratories. These 10 drugs served as the basis for our assessment of concordance across experimental settings.
In addition to self-administration, drug discrimination techniques and subjective effects measures have played a role in assessing the abuse potential of drugs. Initially we planned to include data from studies using these techniques in this review, but this approach was abandoned for several reasons. First, of the 10 drugs we identified as having been tested in both animal and human laboratories, only one had been tested in a cocaine discrimination experiment in humans—tiagabine, which did not affect the discriminative stimulus effects of cocaine (Lile et al., 2004b)—and none had been tested in monkeys. Moreover, only four of the other drugs tested in nonhuman primates only or humans only had been studied in a cocaine discrimination experiment. Thus, there were insufficient data available to include drug discrimination or subjective effects studies in this review. Second, previous reviews have concluded that subjective effects are not a reliable indicator of medication efficacy (Comer et al., 2008; Haney and Spealman, 2008). Third, as described above (section II.C), clinical trials do not use attenuation of subjective or discriminative stimulus effects of cocaine as an outcome.
III. Putative Medications Assessed in the Animal and Human Laboratory
This section describes the results of our search for drugs that have been tested in both humans and animals in the laboratory setting as well as in clinical trials for treatment of cocaine use disorder. As described above, animal studies were only included if an assessment of behavioral selectivity was conducted. Clinical trials were only included if they were blinded, randomized, and fully placebo controlled. The 10 drugs that were found to fit these criteria were grouped according to their pharmacological mechanisms of action. We first describe each study, indicating whether the results represent positive or negative data with respect to the potential of the drug as a medication. These findings are collated in Table 1. Next, for each drug in each setting, we determined an overall conclusion as to whether the available data indicated that the drug produced significant and selective decreases in cocaine self-administration. If >50% of the studies with the drug were positive (Table 1), it was scored as decreasing cocaine self-administration. Otherwise (<50%), it was scored as having no selective effect on cocaine self-administration in that setting. In some instances an equal number of studies supported either conclusion (i.e., = 50%). In these cases, we identified the result of that drug in that setting as “mixed.” These determinations are summarized in Table 2. Finally, we used these determinations to assess the extent of concordance across the three experimental settings: the animal laboratory, the human laboratory, and clinical trials. We operationally defined results as “concordant” when the determination was the same across the three settings. In this framework, a “mixed” result neither supported nor prevented a claim of concordance. Rather, concordance was based on results scored as positive or negative.
A. Drugs Targeting Monoamine Transporters
1. d-Amphetamine.
Among putative medications that target monoamine transporters, d-amphetamine has been the most widely studied in laboratory animals. The findings of these studies are remarkably consistent in demonstrating that chronic d-amphetamine treatment can attenuate the reinforcing effects of cocaine under a variety of conditions. Regarding acute treatment, an early study used rhesus monkeys whose responding was reinforced by either food or cocaine (0.01 or 0.033 mg/kg per infusion) in separate components of the same behavioral session (i.e., a “multiple schedule”; Mansbach and Balster, 1993). Both food- and cocaine-maintained behavior were decreased by acute injections of d-amphetamine (0.1–1 mg/kg, i.v.). Subsequent experiments in rodents reported similar results of acutely administered d-amphetamine. In groups of rats self-administering cocaine (0.03–1.0 mg/kg per injection) or liquid food (3–100% Ensure in water) under an FR 5 schedule (Barrett et al., 2004), acute d-amphetamine (1.8 mg/kg, i.p.) shifted the cocaine dose-response curve to the left and increased responding when low concentrations of food were available. In a later study using a food-cocaine choice procedure (Thomsen et al., 2013), d-amphetamine (0.32–0.56 mg/kg, i.p.) did not significantly alter overall responding but produced increases in choice of cocaine (0.03–1.0 mg/kg per injection). Taken together, these data indicate that acute d-amphetamine either increases or does not affect cocaine self-administration at doses that do not alter food-maintained responding.
Studies of the effects of chronic d-amphetamine treatment on cocaine self-administration produced opposite results. Negus and Mello demonstrated that chronic treatment with d-amphetamine (0.01–0.1 mg/kg per hour, i.v., for 7–28 days) decreased cocaine, but not food, self-administration under a second-order schedule, a PR schedule and a food-drug choice procedure (Negus, 2003; Negus and Mello, 2003a,b). The latter results have been replicated in studies designed to extend these results to other monoamine-releasing drugs (Banks et al., 2013, 2015) and have been extended to rats choosing between food and cocaine (Thomsen et al., 2013), in which rats received 0.1 or 0.32 mg/kg per hour, s.c. via implanted osmotic pumps. Rodent studies also replicated the ability of chronic d-amphetamine (5 mg/kg per day, s.c. via osmotic pump for 14 days) to selectively decrease cocaine versus food pellet self-administration under a PR schedule (Chiodo et al., 2008). In subsequent nonhuman primate studies using a PR procedure designed specifically to mimic clinical conditions of cocaine use and treatment, continuous infusion of intravenous d-amphetamine (0.01–0.1 mg/kg per hour over several weeks) similarly produced long-lasting decreases in cocaine self-administration, whereas other measures designed to assess potential side effects of d-amphetamine (including disruption of food-maintained responding and observation of agitation or increased locomotion) were unaltered or only transiently affected (Czoty et al., 2010, 2011).
One human laboratory experiment that met review criteria assessed cocaine reinforcement in subjects receiving d-amphetamine (Rush et al., 2010). In that study, nine cocaine-dependent subjects received d-amphetamine (0 and 40 mg/day) for 3–5 days. Conditions were tested in a counterbalanced fashion. During five experimental sessions under each maintenance condition, subjects first sampled placebo (i.e., 4 mg intranasal cocaine) identified as Drug A. Subjects sampled a second intranasal drug dose (4, 10, 20, or 30 mg cocaine) identified as Drug B. Subjects then made six discrete choices between Drug A and Drug B. All doses of cocaine were chosen significantly more times than placebo during both maintenance conditions (i.e., placebo and d-amphetamine). Choice of the 20 mg dose of cocaine was significantly lower during d-amphetamine maintenance relative to when this cocaine dose was tested during placebo maintenance.
Clinical trial results suggest that amphetamine isomers are effective for treating cocaine dependence (Grabowski et al., 2001; Shearer et al., 2003; Schmitz et al., 2012; Levin et al., 2015). In the seminal trial, cocaine-dependent subjects were randomly assigned to receive d-amphetamine (15 or 30 mg/day; n = 26 and 28, respectively) or placebo (n = 40) for 25 weeks (Grabowski et al., 2001). During the fifth week, the d-amphetamine dose was doubled. Subjects maintained on the higher d-amphetamine dosing regimen (30/60 mg/day) used significantly less cocaine during the trial than subjects maintained on either the lower dosing regimen (15/30 mg/day) or placebo as determined by benzoylecgonine-free urines. In the next study, dependent cocaine injectors were assigned to placebo (n = 14) or 60 mg/day d-amphetamine (n = 16) for 14 weeks (Shearer et al., 2003). In the d-amphetamine maintenance group, the percent of cocaine-positive urines decreased from 94% at baseline to 56% by the end of the trial. In contrast, the percent of cocaine-positive urines in the placebo maintenance group remained stable at approximately 79% from the beginning to the end of the study. In a recent trial, cocaine-dependent subjects were assigned to placebo (n = 16) or 60 mg/day d-amphetamine (n = 22) for 16 weeks (Schmitz et al., 2012). Two other conditions were tested in this study: modafinil (see below) and modafinil combined with d-amphetamine (not reviewed). d-Amphetamine maintenance decreased the proportion of cocaine-positive urine samples provided by subjects across the trial. Finally, Levin et al. (2015) found that extended-release mixed amphetamine salts (60 and 80 mg), combined with cognitive behavioral therapy, were effective in reducing cocaine use in a population of individuals with comorbid cocaine use disorder and ADHD.
2. Methylphenidate.
The dopamine/norepinephrine uptake inhibitor methylphenidate has been evaluated as a potential cocaine pharmacotherapy in one rodent study and one nonhuman primate study. In rats, acute administration of methylphenidate (3.2–32 mg/kg by mouth) progressively shifted the dose-response curve for cocaine self-administration to the left, indicating an increase in the potency of cocaine as a reinforcer (Hiranita et al., 2009). Those doses of methylphenidate did not affect food-reinforced responding. In monkeys (Czoty et al., 2013), effects of chronic treatment with methylphenidate were examined under the same conditions as was d-amphetamine described above (Czoty et al., 2011). Initially, methylphenidate was delivered via constant intravenous infusion (0.003–0.056 mg/kg per hour); doses were increased at approximately 2-week intervals if no effect on cocaine self-administration was observed. Cocaine self-administration was decreased by approximately 50% in one monkey after 2–4 weeks of treatment with the highest dose, but the experiment was discontinued in two other subjects because of adverse health and behavioral effects such as agitation, heightened aggression, and a marked disruption in food-maintained responding. These effects dissipated almost immediately upon cessation of intravenous administration of methylphenidate and were not present in other monkeys who received methylphenidate orally (1.0–9.0 mg/kg twice a day), with the exception of some mild disruption of the pattern of food-maintained responding. Despite lacking overt effects on health and behavior, oral methylphenidate had equivocal effects on cocaine self-administration. During treatment, cocaine self-administration decreased 30–50% in two monkeys but increased in two other subjects.
Only one study assessed the effects of methylphenidate on cocaine reinforcement in humans (Collins et al., 2006). In that study, cocaine-dependent subjects with comorbid ADHD (N = 7) were maintained on methylphenidate (0, 40, and 60 mg/day for 4 or 5 days). The reinforcing effects of intravenous cocaine (0, 16, and 48 mg) were assessed using a choice procedure wherein subjects sampled a dose of cocaine and were then given five opportunities to choose between it and a $2.00 token. Subjects chose the 48 mg cocaine dose four of five times, on average, during placebo maintenance. Maintenance on 60 mg/day methylphenidate significantly reduced choice of the 48 mg cocaine dose to approximately two of five choices.
The clinical trial results with methylphenidate are largely negative. Methylphenidate was tested in what was most likely the first trial to evaluate “agonist replacement” for cocaine use disorders with a double-blind, placebo-controlled, randomized design (Grabowski et al., 1997). In that study, 24 cocaine-dependent subjects were randomly assigned to receive placebo or methylphenidate (5 mg immediate-release plus 20 mg sustained-release formulations) daily. The two groups had similar levels of benzoylecgonine-positive urines in the trial, approximately 40%. Two trials tested methylphenidate as a putative agonist replacement therapy in cocaine-dependent subjects with comorbid ADHD (Schubiner et al., 2002; Levin et al., 2007), which is a comparable population to that used in the one human laboratory study in which methylphenidate reduced cocaine self-administration (Collins et al., 2006). In the earlier trial, 48 subjects were randomly assigned to placebo or methylphenidate in a 12-week protocol (Schubiner et al., 2002). The methylphenidate dose was titrated upward to a target dose of 90 mg/day. The placebo- and methylphenidate-treated groups did not differ in terms of cocaine use as verified by drug urine testing. In the more recent trial, 48 subjects were randomly assigned to placebo or methylphenidate over 14 weeks (Levin et al., 2007). The methylphenidate dose was titrated upward to a target dose of 60 mg/day. Methylphenidate-treated individuals demonstrated a significant decrease in the probability of providing a cocaine-positive urine sample during the trial relative to their placebo-treated counterparts.
3. Modafinil.
Modafinil was evaluated in rhesus monkeys self-administering cocaine under a second-order schedule of reinforcement (Newman et al., 2010). In that study, chronic treatment with 10 mg/kg modafinil did not affect cocaine- or food-maintained responding. Chronic treatment with a higher dose of modafinil (32 mg/kg per day, i.v., for 5–10 days) selectively decreased self-administration of low and intermediate doses of cocaine, but self-administration of higher cocaine doses and food was unaffected. Testing of 56 mg/kg/day dose was terminated because of behavioral toxicity (stereotypies and decreases in food-maintained responding).
Two human laboratory studies have evaluated modafinil as a putative pharmacotherapy for cocaine use disorder using self-administration methodology (Hart et al., 2008; Verrico et al., 2014). In the earlier study, the reinforcing effects of smoked cocaine (0, 12, 25, and 50 mg) were assessed in eight subjects maintained on modafinil (0, 200, and 400 mg/day for 7 days; Hart et al., 2008). Subjects first sampled the available cocaine dose and then made five choices between receiving another drug dose and $5.00. As expected, cocaine choices increased as a function of dose. Cocaine choices were decreased during maintenance on both doses of modafinil. In the more recent experiment, the reinforcing effects of intravenous cocaine (0 or 20 mg) were assessed in 16 subjects maintained on placebo or modafinil (200 mg/day) for 5 days (Verrico et al., 2014). Subjects first sampled the available cocaine dose and then made five choices between another drug dose and $1.00. Cocaine was chosen to a greater degree than placebo, and although modafinil reduced the number of cocaine choices relative to placebo maintenance, this effect did not reach statistical significance.
Seven double-blind, placebo-controlled, randomized clinical trials have investigated modafinil for managing cocaine dependence (Dackis et al., 2005, 2012; Anderson et al., 2009; Schmitz et al., 2012, 2014; Kampman et al., 2015; Karila et al., 2016). In the earliest trial (Dackis et al., 2005), cocaine-dependent subjects were randomly assigned to receive 400 mg modafinil per day (n = 30) or placebo (n = 32) for 8 weeks. The modafinil-treated subjects provided significantly more benzoylecgonine-free urines than the placebo-treated participants. A 12-week multisite trial then compared placebo (n = 72) and modafinil [200 (n = 69) and 400 mg (n = 68); Anderson et al., 2009]. The initial analysis showed little difference between placebo and either dose of modafinil in terms of biologically verified cocaine abstinence across the trial. Post hoc analyses, however, showed that modafinil increased cocaine abstinence in subjects who did not have a history of alcohol dependence. In the third trial, 210 subjects were randomized to placebo (n = 75), 200 mg/day modafinil (n = 65), or 400 mg/day modafinil (n = 70) combined with cognitive behavioral therapy for 8 weeks (Dackis et al., 2012). Although modafinil did not reduce cocaine use in the overall sample relative to placebo, post hoc analyses revealed that men receiving 400 mg/day modafinil tended to have greater levels of cocaine abstinence than those maintained on placebo. In more recent trials, cocaine-dependent participants received either placebo or 400 mg/day of modafinil for 16 weeks (Schmitz et al., 2012) or 12 weeks (Schmitz et al., 2014). Relative to placebo, modafinil maintenance increased the proportion of cocaine-positive urines across one trial (Schmitz et al., 2012) but did not change the proportion of cocaine-positive urines in the other trial (Schmitz et al., 2014). Most recently, the observation in the Anderson et al. (2009) trial that modafinil showed positive effects in those without a history of alcohol dependence was investigated directly (Kampman et al., 2015). That 8-week trial specifically excluded individuals who were dependent on alcohol. The main finding was that 300 mg modafinil was superior to placebo in increasing abstinence. In the most recent trial (Karila et al., 2016), 29 cocaine-dependent men were treated with a descending-dose regimen of modafinil (400 mg/day × 26 days, then 300 mg/day × 30 days, then × 200 mg/day for 31 days), and abstinence was assessed over the subsequent 10 weeks. Significantly more dropouts and positive urine samples were observed in the modafinil- compared with the placebo-treated group.
4. Desipramine.
Mello and colleagues (1990a) studied the effects of 5 days of treatment with the norepinephrine uptake inhibitor desipramine (0.56–10.0 mg/kg per day) on responding maintained by cocaine injections or food pellet deliveries. Lower desipramine doses (up to 1.78 mg/kg per day) increased self-administration in most monkeys. Higher doses produced less consistent effects; only one subject showed a selective decrease in cocaine versus food self-administration. One study evaluated the influence of desipramine on the reinforcing effects of cocaine in humans (Fischman et al., 1990). In that study, the reinforcing effects of intravenous cocaine (0, 8, 16, and 32 mg) were first evaluated using a drug versus placebo choice in six cocaine-using subjects. Those individuals were then maintained on desipramine for 3–4 weeks. The maximum desipramine dose was 350 mg/day, but doses varied across subjects such that stable blood levels of desipramine (between 80 and 150 ng/ml) were maintained for 2 weeks. After achieving stable desipramine blood levels, the cocaine self-administration dose response curve was redetermined. During baseline testing, active cocaine doses were chosen to a greater degree than placebo, with subjects allocating approximately 5 or 6 choices (out of 7) to the 8, 16, or 32 mg doses. Desipramine maintenance did not change allocation of drug choices.
Two studies meeting inclusion criteria for this review evaluated desipramine for managing cocaine use disorder (Gawin et al., 1989; Campbell et al., 2003). In the first study, which lasted 6 weeks, cocaine-dependent subjects were assigned to receive placebo (n = 24) or 2.5 mg/kg desipramine daily (Gawin et al., 1989). Subjects who received desipramine were more likely to achieve abstinence for longer periods, as verified by a combined use indicator of a cocaine-negative urine sample and self-report of no cocaine use, than subjects assigned to receive placebo. In the more recent study, cocaine-dependent subjects were maintained on placebo (n = 50) or desipramine (n = 49) in an 8-week trial (Campbell et al., 2003). The desipramine dose started at 50 mg/day and was titrated up to 200 mg/day. Groups did not differ in their ability to sustain cocaine abstinence or in proportion of cocaine-positive urine samples.
5. Amantadine.
Chronic intravenous administration of amantadine (10 or 32 mg/kg per day) did not alter self-administration of cocaine (0.32 mg/kg per injection) under an FR schedule in baboons (Sannerud and Griffiths, 1988). There is also one study that evaluated the influence of amantadine on the reinforcing effects of cocaine in humans (Collins et al., 2003). In that study, the reinforcing effects of smoked cocaine (0, 12, 25, and 50 mg) were evaluated in a drug-versus-money ($5.00) choice procedure in 10 cocaine-using subjects after 5 days of maintenance on placebo or 200 mg/day amantadine. Active cocaine doses were chosen over money to a greater degree than placebo, with subjects allocating approximately four or five choices (out of 5) to the 12, 25, or 50 mg doses. Amantadine maintenance did not change allocation of drug choices.
Three prospective trials have tested the efficacy of amantadine for treating patients with cocaine use disorder (Kampman et al., 1996, 2006; Shoptaw et al., 2002). In the earliest study, cocaine-dependent subjects were assigned to placebo (n = 30) or 300 mg/day amantadine (n = 31) for 4 weeks (Kampman et al., 1996). The proportion of urine samples indicating cocaine use was not significantly different across groups, with 57.5% of samples being positive in the placebo group and 49.6% of samples being positive in the amantadine group when counting missing samples as positive. In the next study, cocaine-dependent subjects were assigned to receive placebo (n = 35) or 200 mg/day amantadine (n = 34) for 18 weeks (Shoptaw et al., 2002). Amantadine maintenance increased the probability that subjects would provide a cocaine-negative urine sample, with statistically significant differences observed at a priori comparison time points (i.e., weeks 8 and 16). In the most recent study, 199 cocaine-dependent subjects with severe withdrawal symptoms were assigned to receive placebo, 300 mg/day amantadine, 100 mg/day propranolol or combined amantadine and propranol for 10 weeks (Kampman et al., 2006). There was no difference between the amantadine-treated and the placebo-treated groups on cocaine use outcomes. Taken together, the results are equivocal. Differences in the subjects’ severity of cocaine use may have played a role in the discrepancy.
6. Summary.
When viewed in light of relevant experimental and subject factors, largely consistent results have been found in nonhuman and human laboratory studies and clinical trials regarding the effectiveness of drugs that target monoamine transporters to reduce cocaine self-administration (Table 2). The only human laboratory study of d-amphetamine reported results similar to those of rodent and nonhuman primate laboratory studies and several clinical trials that all support the effectiveness of chronic d-amphetamine to decrease cocaine use. Although three animal studies did not show that reductions in self-administration were selective for cocaine (versus food) self-administration, all three of those studies examined acute d-amphetamine treatment, whereas all studies that showed positive results involved chronic d-amphetamine administration. Thus it is clear that the predictive validity of these animal models, at least with respect to d-amphetamine, is critically dependent on chronic treatment with the putative medication.
Data with methylphenidate that may appear equivocal at first glance are reconciled when the ADHD status of subjects is considered. Results were negative in rats (Hiranita et al., 2009), rhesus monkeys (Czoty et al., 2013), and a clinical trial in subjects without comorbid ADHD (Grabowski et al., 1997). When tested in an ADHD population, however, more encouraging results were found in the only human laboratory study (Collins et al., 2006) and one of two clinical trials (Levin et al., 2007). The effects of modafinil were positive in the one preclinical study in monkeys and one study in humans. Four of the six reviewed clinical trials reported negative results, although in some cases positive results were found in subsets of the subjects based on sex or history of alcohol dependence. Likewise, negative results were found with the norepinephrine uptake blocker desipramine in laboratory studies in nonhuman primates and humans and in one of two clinical trials (Campbell et al., 2003). However, positive results were seen with lower doses of desipramine in the other clinical trial (Gawin et al., 1989). Finally, negative results with amantadine were reported in monkey and human laboratory studies as well as two of three clinical trials.
Taken together, results with d-amphetamine (when administered chronically) and amantadine are clearly consistent across settings; negative results with desipramine have been found in all but one study and apparent discrepancies with methylphenidate can largely be explained when ADHD status is taken into consideration. Only modafinil resulted in clearly discordant conclusions across settings. However, as noted above, recent data suggest that the effectiveness in clinical trials may require the absence of lifetime alcohol dependence: if true, this would bring clinical trial results more in line with the only nonhuman primate study conducted to date (Newman et al., 2010). Moreover, investigators have enumerated other reasons for the discordance across clinical trials that may affect translation. For example, differences in medication compliance rates and motivation to quit may influence results. In addition, it is important to note that the effective dose in monkeys (32 mg/kg per day, equal to 2240 mg/day in a 70-kg human) was much higher than the highest dose studied in human laboratory studies or clinical trials, 400 mg, which is less than 6 mg/kg per day) and that the effects in the nonhuman primate study were relatively small and were not dose-dependent.
B. Drugs that Facilitate γ-Aminobutyric Acid Function
1. Baclofen.
Drugs described in the preceding section directly interact with the neurobiological substrates of cocaine, monoamine transporters. Another strategy for development of medications for cocaine use disorder has been to target neurotransmitter systems that indirectly modulate brain monoamine function. To this end, drugs that enhance the function of GABA, the ubiquitous and primary inhibitory neurotransmitter in the brain, have been examined in rodents and monkeys self-administering cocaine. Roberts et al. (1996) extensively studied the effects of baclofen and other GABAB agonists on cocaine self-administration in rats; several publications fit the inclusion criteria for this review. In the earliest study, acute baclofen (1.25–5.0 mg/kg) produced a downward/rightward shift in the cocaine self-administration dose-effect curve determined under a PR schedule. Food-maintained responding was not affected significantly. Subsequent studies replicated this effect and its behavioral selectivity and extended the conditions under which it is observed to an FR 1 schedule (Brebner et al., 2000a) and a discrete-trials procedure in which rats were presented with an opportunity to self-administer one cocaine injection under an FR 5 schedule at 30-minute intervals, 24 hours per day, for several weeks (Roberts and Andrews, 1997). The same effect was found when baclofen (56 ng) was delivered directly into the ventral tegmental area (Brebner et al., 2000b). Other investigators reported a similar antagonism of cocaine reinforcement by baclofen without disruption of food-maintained responding under varied conditions including a multiple FR 5 schedule of food (45-mg pellet) and cocaine (0.66 mg/kg per infusion) delivery (Shoaib et al., 1998). In that study, 2.5–10.0 mg/kg baclofen was effective acutely and 2.55.0 mg/kg baclofen was effective after 35 days of treatment. In two other studies, baclofen was found to decrease both cocaine- and food-maintained responding at similar doses. Barrett and colleagues (2005) reported that baclofen (1.85.6 mg/kg) decreased self-administration of a range of cocaine doses (0.033.2 mg/kg per injection) under an FR 5 schedule, but also decreased self-administration of a range of concentrations of liquid food (3–100% Ensure in water). Subsequently, Filip and colleagues (2007) demonstrated that self-administration of cocaine (0.5 mg/kg per injection) and food (sweetened milk) were reduced by baclofen when made available under an FR 5 schedule. It is likely that the ability to vary the magnitude of food consumption in the latter studies explains the differences in behavioral selectivity of the effect of baclofen (see Barrett et al., 2004, 2005 and Thomsen et al., 2013 for discussion). It also interesting to speculate that the use of a liquid versus solid food reinforcer may have affected results. Although the difference in the effects of solid versus liquid food reinforcement per se has not been examined, it is a fundamental tenet of behavioral pharmacology that the effects of drugs on behavior can vary according to the stimulus that maintains that behavior (e.g., McKearney, 1976; Barrett, 1976).
In contrast to the extensive studies in rats, only one published study examined the ability of GABAB receptor agonists to decrease cocaine self-administration in nonhuman primates (Weerts et al., 2005). Baboons self-administered a relatively low dose of cocaine (0.032 mg/kg) or food pellets. The GABAB agonists baclofen (0.1–1.7 mg/kg, i.m.) and CGP44532 (0.1–1.0 mg/kg, i.m.) produced dose-dependent decreases in both cocaine- and food-maintained responding. The lack of behavioral selectivity of effects across reinforcers suggests that the observed decreases in cocaine self-administration may be related to factors other than an attenuation of the reinforcing effects of cocaine.
Two studies have tested the influence of baclofen administration on the reinforcing effects of cocaine in humans (Lile et al., 2004a; Haney et al., 2006). In the earlier study, seven cocaine-using subjects first received acute doses of oral baclofen (0, 10, 20, or 30 mg; Lile et al., 2004a). Approximately 90 minutes later, 4 mg (active placebo) or 45 mg intranasal cocaine was administered. The reinforcing effects of the cocaine doses were evaluated using the Multiple-Choice Procedure. This procedure is a contingency-based questionnaire in which subjects make a number of choices between a drug dose, in this case 4 or 45 mg intranasal cocaine after pretreatment with baclofen, and a range of money values (i.e., $0.25–$64.00). The highest value at which a subject chooses drug over money for any given dose condition is termed the “crossover point.” One of the choices made by the subject is selected at random and delivered later. The active cocaine dose increased crossover point relative to placebo cocaine. The average crossover point was $6.48 for cocaine and $0.35 for placebo across baclofen pretreatment conditions. Baclofen did not change this outcome. In the more recent study, 10 cocaine-dependent subjects received 0, 30, and 60 mg oral baclofen for 7 days (Haney et al., 2006). Midway through and at the end of the maintenance period for each baclofen dose, the reinforcing effects of smoked cocaine (0, 12, 25, and 50 mg) were determined using a drug-versus-money choice procedure. All active doses of cocaine were chosen over money to a greater degree than placebo, with subjects generally making near maximal choices for the 12, 25, and 50 mg doses across baclofen maintenance conditions. However, 60 mg/day baclofen significantly reduced choice of the 12 mg cocaine dose relative to placebo maintenance (to approximately three out of five choices). As observed with animal experiments with d-amphetamine, human laboratory results with baclofen clearly indicate different effects of acute and chronic administration.
Two clinical trials evaluated baclofen for managing cocaine use disorder, with mixed results (Shoptaw et al., 2003; Kahn et al., 2009). In the earlier study, cocaine-dependent subjects were randomly assigned to receive baclofen (60 mg/day; n = 35) or placebo (n = 35) for 16 weeks (Shoptaw et al., 2003). Although initial analyses indicated no significant difference between those assigned to placebo and baclofen, a subsequent longitudinal analysis showed that those individuals maintained on baclofen were significantly more likely to provide benzoylecgonine-free urine samples relative to those maintained on placebo across the length of the trial. In the more recent trial, “severely” dependent cocaine users were assigned to placebo (n = 80) or 60 mg/day baclofen (n = 80) for 8 weeks (Kahn et al., 2009). Baclofen did not change the number of cocaine non-use days, verified by benzoylecgonine urine testing, relative to placebo.
2. Tiagabine.
Acute administration of the GABA uptake inhibitor tiagabine (0.1–1.0 mg/kg, i.m.) produced similar effects to baclofen in the nonhuman primate study described above (Weerts et al., 2005). There is one report in humans describing two experiments that tested the effects of tiagabine administration on the reinforcing effects of cocaine (Lile et al., 2004b). In the first experiment, four cocaine-using subjects received acute doses of oral tiagabine (0 and 4 mg) in combination with doses of oral cocaine (0, 25, 50, 100, and 150 mg). In the second experiment, six cocaine-using subjects received acute doses of oral tiagabine (0 and 8 mg) in combination with doses of oral cocaine (0, 25, 50, 100, and 150 mg). The reinforcing effects of each cocaine dose combined with tiagabine were evaluated using the multiple-choice procedure. A statistically significant effect of cocaine dose was observed for crossover point in the data from the 8 mg, but not the 4 mg, tiagabine group. Cocaine increased crossover point on the multiple-choice procedure. For example, the crossover point for the high cocaine dose was $22.00. Tiagabine (8 mg) reduced crossover point for this dose to $11.00, but this effect did not reach statistical significance.
Two clinical trials evaluated the efficacy of tiagabine for treating cocaine use disorder, and the results of these two studies are concordant (Winhusen et al., 2005, 2007). In the first trial, conducted using the Cocaine Rapid Efficacy Screening Trial model, subjects were randomized to receive placebo (n = 17) or 20 mg/day tiagabine (n = 17) for 10 weeks (Winhusen et al., 2005). Tiagabine showed a trend to decrease quantitative levels of benzoylecgonine in urine samples, but this effect did not reach statistical significance when compared with placebo (P = 0.17). In the second trial, subjects were also randomized to receive placebo (n = 70) or 20 mg/day tiagabine (n = 71) but for 12 weeks (Winhusen et al., 2007). The tiagabine group did not differ from their placebo-treated counterparts, either in proportion of cocaine non-use days, verified by urinalysis, or quantitative benzoylecgonine levels in urine.
3. Summary.
Conclusions regarding the promise of baclofen as a putative pharmacotherapy for treating cocaine use disorder are similar across animal, human laboratory, and clinical studies in that all three settings have produced mixed results. Acute baclofen treatment in rodents produced positive results (i.e., a selective decrease in cocaine versus food self-administration) only in the laboratory that used food pellets as a reinforcer but not in two others where liquid food was used. Baclofen did not selectively decrease cocaine self-administration in monkeys. Thus we concluded mixed findings as 5 of 8 studies showed that baclofen reduced cocaine self-administration (Table 2) similar to studies in the other two settings. The two human laboratory studies produced opposite results and the results of clinical trials were also mixed, perhaps based on the extent of cocaine use of the subjects. In addition, because of the relatively short duration of action of baclofen and documented side effects (e.g., Brebner et al., 2002; Bowery, 2006), it is tempting to speculate that experimental parameters, such as dose, duration of treatment, and drug pretreatment times (in acute experiments), may contribute to the discordant results obtained with baclofen. The tiagabine results are more clearly concordant, showing no differential effects on cocaine taking observed as a function of tiagabine treatment across nonhuman primate and human laboratory and clinical trial research (Lile et al., 2004b; Weerts et al., 2005; Winhusen et al., 2005, 2007).
C. Drugs Targeting other Mechanisms
1. Buspirone.
Buspirone is clinically available as an anxiolytic with effects attributed to its ability to function as a 5-HT1A receptor partial agonist. In an early study in rhesus monkeys, acute administration of buspirone (0.1 and 0.3 mg/kg, i.v.) increased cocaine self-administration under an FR 10 schedule without affecting food-maintained consumption (Gold and Balster, 1992). This was interpreted as encouraging because the effects were similar to those of a D2 receptor antagonist. However, tolerance developed to the effect when it was administered over 10 days. In addition to its serotonergic effects, buspirone can block D2-like dopamine receptors, with some selectivity for the D3 and D4 subtype versus the D2 subtype (Bergman et al., 2013). In the early 2010s, this selectivity generated interest in buspirone as a tool to test hypotheses related to the utility of D3 receptor antagonists as medications for substance abuse (e.g., Heidbreder and Newman, 2010). Bergman et al. (2013) studied buspirone’s effects on cocaine self-administration using a three-component procedure. In the first and third components, food pellets were self-administered under an FR 30 schedule for 5 minutes. These were separated by 5-minute timeout periods from a 100-minute middle component during which monkeys self-administered cocaine under an FR 60 schedule. Acute buspirone treatment (0.1–0.32 mg/kg) decreased cocaine self-administration in all monkeys. However, doses necessary to produce this effect decreased food-maintained responding in the first component by 100% in two monkeys and by 20–40% in two other subjects. In contrast, selective effects of buspirone were observed in monkeys self-administering cocaine under a second-order schedule of reinforcement (Mello et al., 2013). In that study, intravenous infusion of buspirone 23 hours per day for 7–10 days shifted the cocaine self-administration dose-effect curve downward. In more recent studies using a food-drug choice procedure, buspirone (0.03–0.056 mg/kg, i.v. acutely or 0.03–0.3 mg/kg, i.m. for 5 days) was ineffective overall in altering food-cocaine choice. One study was conducted in group-housed cynomolgus monkeys and one involved rhesus monkeys (Czoty and Nader, 2015; John et al., 2015, respectively). Interestingly, in the former study, buspirone decreased cocaine choice in socially dominant monkeys, suggesting that the efficacy of buspirone might be enhanced in enriched environments. However, decreases in overall responding were observed, particularly early in the session (as in Bergman et al., 2013), indicating a lack of behavioral selectivity of the effect. In the latter study in rhesus monkeys (John et al., 2015), buspirone was unequivocally ineffective in decreasing cocaine self-administration. In addition to the difference in schedules of reinforcement (FR and second-order versus a concurrent FR-based choice procedure), daily cocaine intake may have played a role in the discrepant results across these studies; monkeys self-administered much less cocaine each day under the choice procedure (see John et al., 2015).
One study tested the effect of buspirone maintenance on cocaine self-administration in humans (Bolin et al., 2015). In that study, nine subjects who met criteria for cocaine abuse or dependence were maintained on 0 or 30 mg/day oral buspirone in counterbalanced order for 3 days. The reinforcing effects of intranasal cocaine (0, 15, and 45 mg) were then tested under each of these conditions using a forced drug versus money ($0.25) choice procedure wherein each reinforcer was available under concurrent, independent PR schedules (see Stoops et al., 2010). The active cocaine doses were chosen over money to a greater degree than placebo. There was no effect of buspirone maintenance condition on cocaine choice.
Two clinical trials evaluated the efficacy of buspirone for treating cocaine use disorder (Moeller et al., 2001; Winhusen et al., 2014). In the earlier study, cocaine-dependent subjects were randomized to receive placebo (n = 18) or 45 mg/day buspirone (n = 17) for 12 weeks (Moeller et al., 2001). The two groups did not differ in percent of cocaine negative urines nor did they differ in semiquantitative levels of cocaine metabolites in urine samples. In the second study, which lasted 16 weeks and was designed to evaluate the ability of buspirone to prevent cocaine relapse, subjects were first admitted to an inpatient treatment unit to achieve cocaine abstinence. While on the unit, subjects were randomized to receive placebo (n = 27) or 60 mg/day buspirone (n = 35; Winhusen et al., 2014). There were no differences between the groups assigned to receive placebo or buspirone in their ability to maintain cocaine abstinence after discharge from the inpatient unit or in the number days using cocaine after discharge from the inpatient unit.
2. Buprenorphine.
Based on the safety and effectiveness of the mixed-action opioid receptor modulator buprenorphine in treating opiate use disorder and evidence of interactions between opioid and dopaminergic systems, buprenorphine was evaluated as a potential pharmacotherapy for cocaine use disorder. An initial study in rhesus monkeys showed that 0.40 and 0.70 mg/kg per day, i.v. suppressed self-administration of cocaine for at least 30 days under a second-order schedule of reinforcement (Mello et al., 1989). Although food-maintained responding was also affected, the decrease in reinforcement frequency was much smaller, normalized over time, and was determined to be “unlikely [to be] biologically significant.” Next, these investigators compared the effects of buprenorphine and the mu opiate receptor antagonist naltrexone on self-administration of cocaine (0.05 mg/kg per injection) or food pellets under a second-order schedule (Mello et al., 1990b). Drugs were administered intravenously over 1 hour each day for at least 15 consecutive days. Both food and cocaine self-administration were reduced on the first session of treatment with buprenorphine (0.237–0.7 mg/kg per day). Over time, tolerance developed to the suppression of food-reinforced responding in most subjects under most conditions, whereas the effects on cocaine self-administration were sustained or increased. Moreover, cocaine self-administration remained suppressed for several weeks after termination of buprenorphine treatment. The orderly dose- and time-dependent effects of buprenorphine contrasted with those of naltrexone, which were lower in magnitude and less consistent. Subsequent studies from this laboratory and others extended these encouraging data to other cocaine doses and routes of administration (i.e., smoked cocaine base; Carroll et al., 1992), although the effect of buprenorphine (0.03–0.8 mg/kg per day, i.m. for 5 days) on smoked cocaine base was not found to be behaviorally selective. In addition, it was shown that tolerance did not develop to effects of buprenorphine on cocaine self-administration for up to 120 days of treatment, that intermittent buprenorphine treatment (i.e., every 48 or 72 hours) was less effective than daily treatment, and that buprenorphine’s partial agonist effects at mu opioid receptors were likely responsible for its ability to decrease cocaine self-administration (Mello et al., 1992, 1993a,b; Lukas et al., 1995). In studies that followed, the efficacy of buprenorphine to reduce cocaine self-administration was extended to self-administration of cocaine-heroin combinations (i.e., “speedball”; Mello and Negus, 1998, 2001, 2007). One study in rodents met inclusion criteria for this review (Carroll and Lac, 1992). In that study, responding under an FR 4 schedule was reinforced with injections of self-administered cocaine (0.1–0.4 mg/kg, i.v.) or presentations of 0.01 ml of a glucose + saccharin solution. Buprenorphine (0.1–0.4 mg/kg) was given once daily for 5 days. In combination with lower cocaine doses, buprenorphine substantially reduced the number of infusions delivered on the first day of treatment. Tolerance developed gradually to this effect over days 2–5. The same doses of buprenorphine also reduced intake of the glucose + saccharin solution, although, unlike the effect on cocaine self-administration, the reduction of glucose + saccharin intake developed progressively over the 5 days of buprenorphine treatment.
Two experiments have evaluated whether buprenorphine maintenance could alter the reinforcing effects of cocaine in humans (Foltin and Fischman, 1994, 1996). In the earlier study, seven subjects with histories of cocaine and opioid use who were not physically dependent on opioids were treated with 0, 2, or 4 mg of sublingual buprenorphine (Foltin and Fischman, 1994). Subjects then sampled two intravenous cocaine doses (i.e., 4 and 8 mg/70 kg, 8 and 16 mg/70 kg, or 16 and 32 mg/70 kg) and made four choices between those two doses and token alternative reinforcers that were exchangeable for inpatient unit privileges. After pretreatment with placebo, subjects made a similar number of choices between the high cocaine dose and the tokens. Subjects rarely chose the low dose. Both buprenorphine pretreatment doses significantly decreased high cocaine dose choices in the 16 and 32 mg/70 kg condition. Subjects reallocated their behavior to choose tokens over high cocaine doses relative to placebo pretreatment. In the second experiment, 12 opioid-dependent cocaine users were maintained on 8 mg/day sublingual buprenorphine (Foltin and Fischman, 1996). Subjects then made six choices between individual intravenous cocaine doses (16, 32, and 48 mg/70 kg) and $5.00. Because subjects were opioid dependent, a placebo buprenorphine condition could not be tested. Instead, cocaine self-administration was compared between buprenorphine maintenance and methadone maintenance periods. Buprenorphine significantly decreased choice of the 16 and 32 mg doses, but not the 48 mg dose, relative to when subjects were maintained on methadone.
We identified four trials that evaluated sublingual buprenorphine in individuals diagnosed with cocaine use disorder (Schottenfeld et al., 1993, 1997, 2005; Montoya et al., 2004). A number of other studies evaluated the effects of buprenorphine on cocaine use in opioid-dependent subjects, but these studies were excluded because they did not explicitly enroll individuals meeting diagnostic criteria for cocaine use disorder (e.g., Strain et al., 1994a,b; Petitjean et al., 2001). As with one of the human laboratory studies described above, because those enrolled in these trials were opioid-dependent, no placebo comparison could be included for ethical reasons. Instead, different doses of buprenorphine were compared with one another or to methadone. As such, no conclusions can be made about whether buprenorphine is different from placebo. Nonetheless, a comparison between methadone and buprenorphine can yield important information about treating cocaine use disorder in individuals physically dependent on opiates, given the high comorbidity between these disorders.
In the first study, 30 cocaine- and opioid-dependent subjects received ascending daily doses of buprenorphine (2, 4, 8, 12, and 16 mg, doses varied across individual subjects) for 21 days at each dose (Schottenfeld et al., 1993). The buprenorphine dose was then tapered. During the taper, the proportion of cocaine-positive urines was lower than during the dose escalation period, with similar effects across doses. In the next study, 116 opioid-dependent cocaine abusers were randomly assigned to receive 4 or 12 mg/day buprenorphine or 20 or 65 mg/day methadone for 24 weeks (Schottenfeld et al., 1997). None of the treatment groups differed in rates of cocaine use. The third study evaluated 2, 8, or 16 mg/day buprenorphine or 16 mg buprenorphine every other day in 200 cocaine and opioid-dependent subjects (Montoya et al., 2004). Urine toxicology testing revealed significantly reduced benzoylecgonine concentrations in the subjects randomized to 8 or 16 mg buprenorphine daily. The 16 mg/day buprenorphine group also displayed significant reductions in the number of cocaine-positive urines during withdrawal from opioid maintenance. The most recent trial compared maintenance on 12–16 mg buprenorphine to maintenance on 65–85 mg methadone in 162 individuals assigned to contingency management or performance feedback using a 2 × 2 factorial design over 24 weeks (Schottenfeld et al., 2005). Subjects assigned to methadone, regardless of behavioral therapy platform, were significantly more likely to provide cocaine-negative urines and achieved longer consecutive periods of abstinence from cocaine than their buprenorphine-treated counterparts.
3. Progesterone.
Over the past several decades, a great deal of data has indicated that men and women differ in sensitivity to the abuse-related effects of cocaine (e.g., Lynch, 2006; Greenfield et al., 2010). In particular, observations that women are less sensitive to cocaine during the luteal phase of the menstrual cycle, when concentrations of the steroid hormone progesterone are high, have led to the hypothesis that progesterone may have efficacy in reducing cocaine abuse. Although experiments in monkeys have not provided strong evidence for differences in the reinforcing effects of cocaine across the menstrual cycle (Mello et al., 2007; Cooper et al., 2013), one study examined exogenous administration of progesterone (Mello et al., 2011). In that study, acute doses of progesterone (0.1, 0.2, and 0.3 mg/kg, i.m.) produced downward shifts in the dose-response curve for intravenous cocaine self-administration under an FR 30 schedule in female rhesus monkeys without affecting food self-administration.
One human laboratory study evaluated the influence of progesterone on cocaine self-administration in humans (Reed et al., 2011). In that study, 10 female cocaine users completed a total of three inpatient admissions during 1) a normal follicular phase of their menstrual cycle, 2) a normal luteal phase of their menstrual cycle, and 3) a follicular phase of their menstrual cycle in which 150 mg oral micronized progesterone was administered twice daily for at least 1 day before cocaine testing. During cocaine challenge sessions, subjects smoked 0, 12, 25, or 50 mg cocaine, then had five opportunities to self-administer the sampled dose at a cost of $5.00 per dose. Cocaine was self-administered to a greater degree than placebo regardless of menstrual cycle phase and progesterone pretreatment.
One clinical trial met review criteria examining the efficacy of progesterone for treating cocaine dependence (Yonkers et al., 2014). In that study, postpartum women with cocaine use disorder were randomized to receive placebo or 100 mg oral micronized progesterone twice daily for 12 weeks. The two groups did not differ in the proportion of cocaine-positive urine samples across the trial, although women assigned to receive progesterone self-reported less weekly cocaine use than those assigned to receive placebo.
4. Summary.
With the exception of one study in nonhuman primates (Mello et al., 2013), agreement was found across studies with buspirone. At least four studies in monkeys under multiple conditions including a FR schedule, a second-order schedule and a choice procedure reported that effects of buspirone on cocaine self-administration were absent, nonselective, or transient; these data are consistent with the only human laboratory study and two clinical trials. Similarly, regarding buprenorphine good concordance was found between the positive results of nonhuman primate and human laboratory self-administration studies. Important caveats to this conclusion include the fact that the positive results, all obtained in one laboratory, did not extend to another laboratory which examined smoked (versus intravenous) cocaine and also studied buprenorphine’s effects in rodents. Moreover, the positive laboratory results were not consistent with the mixed clinical trial outcomes. It should be reiterated, however, that assessment of concordance of buprenorphine results is complicated by the fact that subjects in the human laboratory study and clinical trials were opioid dependent and that, for ethical reasons, a placebo condition was not included in those trials. Finally, progesterone decreased cocaine self-administration in monkeys but not in a human laboratory study. Progesterone also failed to decrease urine samples indicative of cocaine use in a clinical trial, although women reported less cocaine use.
IV. Conclusions and Future Directions
A. Overarching Findings Regarding the Medications Development Process
Laboratory research in animals and humans is an important part of the process of developing medications to treat human disease. Presumably, a more specific function of laboratory research is to serve as a “pipeline” to guide decisions regarding which drugs and doses to test in clinical trials, which methods of assessment to use, and which endpoints to track as indicators of success or failure. These are not trivial considerations given the tremendous time and expense involved in conducting such trials. Optimizing clinical trials in this manner seems particularly important in developing pharmacotherapies for conditions that currently lack FDA-approved medications such as cocaine use disorder. This review was undertaken to assess the predictive validity of nonhuman primate and human laboratory studies that tested putative medications for cocaine use disorder relative to clinical trials and to each other. Our premise was that a drug that decreased cocaine self-administration in the laboratory should demonstrate positive results in clinical trials (Mello and Negus, 1996; Comer et al., 2008; Haney and Spealman, 2008). By using this approach, we were also able to assess the extent to which the “pipeline” approach has been implemented and whether attempts have been made to translate laboratory findings to the clinic as well as the extent to which compounds showing clinical effectiveness have been examined in the laboratory.
We identified over 100 blinded, fully placebo-controlled studies using PubMed searches. For the most part, we excluded studies that involved subjects that were dependent on more than one substance or had comorbid psychiatric conditions. Two exceptions were studies examining studies of amphetamine or methylphenidate in cocaine users with ADHD and studies of buprenorphine in opioid-dependent cocaine users. These searches identified 64 drugs given alone or in combination across the clinical trials that fit review criteria (see Fig. 1). Next, we searched for published articles that assessed these drugs’ ability to selectively decrease cocaine self-administration in the nonhuman primate or human laboratory and, subsequently, the rodent laboratory.
Strikingly, of the 64 drugs tested in clinical trials, 35 had not been assessed in either monkey or human laboratory studies in the context of selectively reducing cocaine self-administration. Nineteen drugs were studied in only one of the species (6 only in humans and 13 only in monkeys). Thus this review was based on the remaining 10 drugs. Four of these drugs (and three of the “monkey only” drugs) have been examined in rodent studies that met review criteria, and those data are included in the evaluation of the translational capability of these models. That so few drugs have been examined across all levels of analysis indicates that translation of preclinical findings to clinical trials using a “pipeline” approach is the exception rather than the rule. The results also indicate that there are few instances of “reverse translation” (i.e., laboratory studies of drugs for which clinical data have been generated).
There are several likely reasons for the dearth of compounds that have been examined at all three stages of the medications development process. As mentioned in section II.D, the rationale for selecting drugs to test can differ in preclinical laboratories and clinical trials. Advances in molecular biology and medicinal chemistry over the past three decades have enabled the development of extremely pharmacologically selective drugs, which are useful for testing specific hypotheses relating to the mechanisms of action of abused drugs and potential pharmacotherapies. These tools have proven useful in preclinical research that aims to identify specific receptor systems that can be targeted to decrease cocaine self-administration. Older drugs that act at a broad range of targets are less suitable for this purpose. Conversely, human laboratory studies and clinical trials with new, more selective drugs cannot easily be conducted because of the need for evidence of safety in humans and for sufficient dosing information and quantities of drug to conduct such trials. In addition, preclinical researchers are often hesitant to study drugs that have already been tested in the clinic. Because clinical work has occurred, the apparent significance of studying the drug in the laboratory is lessened. Finally, the urgency to find an effective medication can lead to drugs being moved to clinical trials too quickly, without the support of preclinical data. In light of these considerations, it is perhaps not surprising that few drugs tested in the laboratory are examined in clinical trials and vice versa.
B. Concordance
In addition to determining the extent to which preclinical and clinical studies of medications for cocaine use disorder inform each other, an important goal of this review was to assess concordance between laboratory results in animals and humans, as well as to assess the predictive validity of these data with respect to the results of clinical trials. An initial finding was that, even when a drug had been studied in monkeys, humans, and clinical trials, there were few published studies in each setting (Table 1). The generally low number of published articles we identified complicates a clear understanding of the concordance of these studies, necessitating a determination of “mixed” results for some drugs across some settings (Table 2). Specifically, there are only two drugs (baclofen and buprenorphine) for which there was more than one study available for review at every level of analysis. Thus we (along with the scientific community) are forced to draw conclusions based on extremely limited data collected under varying conditions. The concern extends to clinical trials as well. For example, we found only one clinical trial with progesterone (Yonkers et al., 2014). It is important to note that for many medications, clinical trials yielded mixed results, forcing us to base conclusions on the conclusions of the majority of studies (Table 2). Thus the risk of false negatives in our analyses (i.e., a premature determination that conclusions about drugs do not agree across animals/humans/clinical trials) is high. For example, if a drug decreased cocaine choice in the human laboratory but did not decrease cocaine self-administration under an FR schedule in monkeys, we concluded that it had different effects in the two species. However, if the drug were to be tested in a choice procedure in monkeys it may have similar effects to those in humans. An important extension of this concern is that the therapeutic potential of a compound could be overestimated, leading to further testing of a drug that is doomed to fail. Conversely, the risk exists that a drug that might eventually be found to be an effective pharmacotherapy may be dismissed prematurely based on limited evidence. It is concerning that critical decisions to pursue or abandon development, testing, and approval of specific drugs are currently based on a limited amount of data.
1. Concordance across Animal Laboratory, Human Laboratory, and Clinical Trials.
Overall, seven of the ten medications (d-amphetamine, amantadine, baclofen, tiagabine, buspirone, desipramine, and buprenorphine) showed concordant results, although it should be noted that the latter two drugs showed mixed results in clinical trials (Table 2). That this proportion is not higher is disheartening, considering that previous reviews suggested that drug self-administration measures are the best way to screen potential medications for drug use disorders (Mello and Negus, 1996; Comer et al., 2008; Haney and Spealman, 2008). It is important to note that those reviews included findings with opioids, for which FDA-approved medications have been developed (i.e., methadone, buprenorphine, and naltrexone). Without an FDA-approved medication for cocaine use disorder, we must rely on clinical trial findings. This task was complicated by the observation that, for six of the ten drugs, discordant findings were reported across clinical trials (Table 2).
A closer look at the data for drugs that did not show concordance (methylphenidate, baclofen, and progesterone) often revealed differences in specific experimental parameters that may have played a role in the disagreement. For example, concordant results were observed between the human laboratory study and the clinical trial that demonstrated efficacy of methylphenidate for cocaine use disorder in ADHD-diagnosed cocaine users that used the same dose (i.e., 60 mg/day; Collins et al., 2006; Levin et al., 2007). Studies that failed to demonstrate efficacy in this population used different doses (e.g., Schubiner et al., 2002). Thus, when the results of experiments with methylphenidate are viewed according to treatment dose and ADHD diagnosis, the concordance of results across species increases. As another example, there appears to be a critical influence of alcohol on the ability of modafinil to reduce cocaine use (see section III.A.3). Thus it is possible that, in some cases, apparent discordance may result from the implementation of different experimental parameters rather than poor predictive validity of the models themselves. Importantly, there were no cases under which animal and human laboratory studies were concordant with each other but not with clinical trials. Rather, clinical trials were concordant with animal results for methylphenidate and with human laboratory results for modafinil and progesterone. This provides evidence to suggest that clinical trials might be warranted only if both animal and human laboratory studies suggest effectiveness.
One encouraging result of this review is that the medication that appears to show the most efficacy for treating cocaine use disorder, d-amphetamine, produced the strongest concordant results across rodent, nonhuman primate, and human laboratory studies as well as clinical trials. Every experiment that tested chronic d-amphetamine treatment found decreases in cocaine self-administration/use. Lessons should be taken from the methodologies used to assess d-amphetamine for managing cocaine use disorders across these three types of studies to develop more predictive assays. For example, the d-amphetamine data clearly demonstrate the importance of studying chronic treatment; the three reviewed studies that used acute treatment reached opposite conclusions to the majority that studied chronic treatment. Particularly valuable in this regard is the Thomsen et al. (2013) study, which examined both acute and chronic treatment. With so few studies to review, and with those studies using different procedures, adopting a more unified screening process will be necessary to make stronger conclusions about the concordance of animal laboratory, human laboratory, and clinical trial outcomes.
2. Concordance between Animal and Human Laboratory Studies.
In assessing the concordance of animal and human laboratory data, we found similar consistency of results (Table 2). Comparable conclusions were drawn for seven of the ten drugs reviewed (d-amphetamine, desipramine, amantadine, baclofen, tiagabine, buspirone, and buprenorphine) in animal and in human laboratory studies. Regarding two of the drugs that did not show similar results (methylphenidate and modafinil), some caveats exist. Comparison of effects of methylphenidate across species appeared to be complicated by the ADHD status of the human subjects. Results in monkeys and rodents were largely negative (Hiranita et al., 2009; Czoty et al., 2013), whereas methylphenidate reduced cocaine choice in the laboratory in cocaine abusers with comorbid ADHD (Collins et al., 2006). No human laboratory study has been conducted in cocaine abusers without ADHD; thus it is unknown if an experiment in this population would be more concordant with the results in laboratory animals. Regarding modafinil, the two human studies arrived at opposite conclusions (Hart et al., 2008; Verrico et al., 2014), and only one study has been conducted in nonhuman primates (Newman et al., 2010). Progesterone was the only drug to show clearly different effects in monkeys and humans, although the comparison is limited to only one study in each. In summary, concordant results between animal and human laboratory studies were found for seven of the ten drugs included in this review. For two drugs for which conflicting data were obtained (methylphenidate and modafinil), experimental factors were identified that may have played a role in the discrepant results.
3. Concordance between Nonhuman Primate- or Human-Only Studies and Clinical Trials.
As depicted in Fig. 1, of the 64 drugs identified during our searches, there were 19 drugs that had been studied in the laboratory either in nonhuman primates (and, in some cases, rodents) or humans but not both. Thus they did not meet the main criterion to be included in the review. Nonetheless, the concordance of these laboratory studies and clinical data are worth examining (Table 3). Although clinical trial results with lisdexamfetamine were negative (Mooney et al., 2015), the authors of the study conceded that they were limited in the doses that could be tested, and anticipated that future studies with higher doses would generate positive results to match the only nonhuman primate study (Banks et al., 2015). Thus it is premature to draw any conclusions regarding the concordance of preclinical and clinical lisdexamfetamine data.
TABLE 3.
Published studies of drugs that were not tested in both animal and human laboratories
Symbols indicate that the studies were interpreted as providing positive (+) or negative (−) results regarding the suitability of the drug as a pharmacotherapy. Under Laboratory Animal Studies, studies using rodent subjects are indicated by italics.
Of the remaining 18 drugs, predictions of laboratory data regarding the potential clinical utility of the drug matched the results of the clinical trial(s) for 11 drugs (Table 3). Concordant negative data were found for fluoxetine, gabapentin, gepirone, mazindol, pergolide, propranolol, quetiapine, sertraline, and venlafaxine. Data for naltrexone were mixed, and data for methamphetamine were, on the whole, positive across clinical trials and laboratory studies. Discordant conclusions were reported for the other seven drugs (citalopram, bupropion, disulfiram, lithium, phenytoin, ritanserin, and varenicline). As with the comparisons discussed above, a greater number of studies under a wider variety of conditions might lead to enhanced concordance.
Methodological details may explain some of the apparent discordance between laboratory and clinical trial data for some of these drugs. Human laboratory studies administered bupropion and phenytoin acutely (Sofuoglu et al., 1999; Stoops et al., 2012b), whereas clinical trials gave medications chronically (Crosby et al., 1996; Shoptaw et al., 2008). The only clinical trial to meet inclusion criteria for disulfiram (i.e., the only one not conducted in opioid-maintained patients) showed positive results (Carroll et al., 2004), unlike the only human laboratory study (Haile et al., 2012). In the latter study, however, although 250 mg per day disulfiram did not decrease choice of cocaine versus saline, follow up analysis indicated that, when calculated on a milligram per kilogram basis, disulfiram dose was negatively correlated with cocaine choices. Regarding ritanserin, clinical trial data are negative but laboratory data are mixed. However, it should be noted that the study that showed positive effects of ritanserin (Meert et al., 1991) was conducted in rats and used a procedure very different from that of the vast majority of studies in this review. Meert et al. (1991) reported that rats’ preference for an oral cocaine solution (versus water) was reversed by ritanserin without changing total fluid intake, whereas ritanserin increased intravenous cocaine self-administration in squirrel monkeys (Howell and Byrd, 1995). Finally, citalopram was found to be ineffective in selectively reducing self-administration in one rodent study (Hiranita et al., 2009) and one monkey study (Howell et al., 2007), but reduced cocaine-positive urines in the only clinical trial (Moeller et al., 2007). It is important to note that the clinical trial found citalopram to be effective when combined with contingency management—an aspect not incorporated into animal laboratory studies.
Taken together, the conclusions from studies with these drugs largely mirror the conclusions from the group of 10 drugs that were tested in all three settings. Specifically, generally good agreement was found with clinical trials, some clear explanations exist for discordance, and the scarcity of published data point to the need for a greater number of laboratory studies and clinical trials to enhance our ability to assess translation.
C. Laboratory versus Clinical Endpoints
A critical area of departure between preclinical studies and clinical trials is the selection of endpoints used to quantify effectiveness of putative medications. Animal and human laboratory studies use an array of dependent measures to assess whether a potential pharmacotherapy changes the quantity of drug used. On the other hand, clinical trials typically compare cocaine-positive urine tests across medication- and placebo-treated groups. This difference in experimental design leads to divergent definitions of success in the laboratory versus the clinic that undoubtedly complicates the translation of findings. In preclinical studies, reduction of cocaine intake by a putative medication represents a positive result. It is not necessary that cocaine self-administration be completely eliminated. In contrast, the reliance on qualitative urine screens renders clinical trials able to measure changes only in the general frequency and not the quantity of cocaine use. In fact, it is also possible for frequency of drug use to be reduced without affecting the number of positive urine screens. The recent trial of progesterone (Yonkers et al., 2014) provides an intriguing example. Although progesterone- and placebo-treated subjects submitted cocaine-positive urines at the same rate, self-reported use was lower and time to relapse was longer in those that received progesterone. These data suggest that the progesterone treatment produced meaningful improvements despite similar results of urinalysis.
The reliance on total abstinence has historically been attributed to the requirements of the FDA for development of new medications. It is intuitive, however, that a significant reduction in drug use is likely to have beneficial consequences even in the absence of complete cessation. This understanding is reflected in the endorsement by the FDA of “percent subjects with no heavy drinking days” as a meaningful endpoint in trials for medications for alcohol use disorder (FDA, 2006; see Falk et al., 2010). More recently, some have called for a similar approach in trials of medications for cocaine use disorders (Winchell et al., 2012; McCann et al., 2015; Kiluk et al., 2016). As elucidated by McCann et al. (2015), the critical hurdle to adopting such measures is a clear demonstration that reductions in drug use lead to clinically measureable benefits to the patient and a determination of the extent of reduction necessary to produce measureable improvements.
D. Behavioral Phenotypes as Predictors of Clinical Efficacy
Researchers at all levels of the medications development process would likely agree that there will be no single medication that is universally effective in treating cocaine use disorder. The ability to identify subpopulations of patients in whom a particular medication is likely to be effective would be a critical advance in treatment. One encouraging conclusion that can be drawn from the findings of this review is that such phenotypes are likely to exist with respect to a number of subject characteristics and drug use variables. This section highlights some clinical variables that emerged from the present analysis as being potentially influential.
1. Psychiatric Comorbidity.
As discussed above (section IV.B.2), there appear to be differences in the effectiveness of methylphenidate to reduce cocaine use depending on whether the individual has been diagnosed with ADHD. Such a diagnosis could lead a clinician to consider methylphenidate as part of a treatment strategy. This approach has not been generally successful in patients dually diagnosed with cocaine dependence and major depressive disorder. A 2005 review and meta-analysis did not find evidence for prescribing antidepressants to reduce cocaine use in dually diagnosed patients, although few published studies were available for review (Torrens et al., 2005). More recently, positive results have been found in depressed cocaine abusers using sertraline (Oliveto et al., 2012; Mancino et al., 2014), but not venlafaxine (Raby et al., 2014). Taken together, these results raise the possibility that comorbid psychiatric disorders may influence the efficacy of specific medications for cocaine use disorder.
2. Severity of Cocaine Use or Withdrawal.
Severity of cocaine use also likely contributes to medication efficacy. Baseline number of cocaine-positive urine drug tests as well as scores on the Cocaine Selective Severity Assessment predict treatment outcome (Kampman et al., 2002; Ahmadi et al., 2006). The influence of drug use severity on medication efficacy in the present review is exemplified when evaluating the discordant results for amantadine (e.g., Shoptaw et al., 2002; Kampman et al., 2006). Shoptaw and colleagues demonstrated the efficacy of amantadine (200 mg/day) for reducing cocaine use; however, this was not observed with prospectively enrolled subjects with high scores on the Cocaine Selective Severity Assessment, in whom a higher dose was used (300 mg/day; Kampman et al., 2006). This indicates that drug use severity must be considered when selecting enrollment criteria. Similarly, Kampman et al. (2001) demonstrated a greater effectiveness of propranolol in cocaine users with more severe withdrawal symptoms. Thus is it possible that attention to heterogeneity in cocaine use and in symptomatology may help identify medications with effectiveness in subpopulations of cocaine users.
3. Polysubstance Abuse.
In individuals diagnosed with substance use disorders, polysubstance abuse is common. For example, up to 90% of cocaine abusers also abuse alcohol (Helzer and Pryzbeck 1988; Grant and Harford 1990; Kampman et al., 2013). Despite this clinical reality, most animal laboratory studies involve subjects who have never been exposed to more than one drug. Similarly, humans who are dependent on multiple substances are typically excluded from clinical studies. It is reasonable to conclude that the behavioral pharmacology of cocaine and/or potential pharmacotherapies is altered by a history of exposure to other psychoactive substances. Clinical trial data with modafinil suggest that the drug may be effective in reducing cocaine use in individuals without a history of heavy alcohol use/dependence (Anderson et al., 2009; Kampman et al., 2015). This raises the intriguing possibility that the effectiveness of a treatment to reduce cocaine use may be modulated by the kinds and quantity of use of other substances.
4. Other Factors.
In addition to those mentioned above, many other variables can influence the ability of drugs to decrease cocaine self-administration in the laboratory and in the clinic. The clinical trial with buspirone (Winhusen et al., 2014) points to the importance of a subject’s sex. In that trial, there was no statistically significant effect of buspirone treatment on the ability of subjects to maintain abstinence. When men and women were compared, however, it was revealed that the probability of maintaining abstinence until the end of the trial was slightly but not significantly better for men who received buspirone versus placebo (21% versus 13%, respectively) but significantly worse for women who received buspirone versus placebo (18% versus 33%, respectively). Greater attention to sex as a critical biologic variable is likely to enhance the conclusions of studies of putative medications for cocaine use disorder in the laboratory and the clinic. In recognition of this fact, in June 2015 the NIH announced its expectation that sex be considered as an important variable in study designs (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html).
E. Overall Conclusions and Recommendations
A strikingly low proportion of drugs that have been tested in clinical trials for treatment of cocaine use disorder have also been tested in well-controlled animal and human laboratory studies. As such, it can be concluded that a demonstration of good therapeutic potential in animal or human laboratory studies is rarely part of the rationale for testing a drug in a clinical trial. Conversely, it is apparent that few preclinical researchers study drugs that have already been tested in clinical trials. Despite this lack of a translational “pipeline” approach, the studies reviewed here indicate generally good correspondence between animal and human laboratory studies in predicting drugs’ ability to decrease cocaine self-administration. Similar concordance was also observed between laboratory and clinical trial results.
Although the predictive power of laboratory studies is not perfect, the concordance is certainly better than what has been observed when using subjective effects to evaluate the efficacy of putative medications (Mello and Negus, 1996; Comer et al., 2008; Haney and Spealman, 2008). Moreover, where discordant results were observed across settings, a closer examination of subject characteristics, regimens of drug treatment (i.e., dose/duration), and dependent variables used to assess treatment success revealed clear differences between preclinical and clinical studies that may have contributed to inconsistent results. We close the review with some recommendations that may lead to enhanced translation and, ultimately, more efficient and successful identification of efficacious treatments for cocaine use disorder.
First, preclinical researchers should take into account subject characteristics noted above, including sex and polysubstance use. The important modulatory effect of sex on cocaine reinforcement is well-documented (e.g., Lynch, 2006) but seldom investigated directly. Investigators should ensure that their experiments are appropriately powered and that statistical approaches are appropriately selected to detect sex differences. The effect of other licit and illicit substances on cocaine use has received even less attention. However, those studies that have modeled polysubstance abuse in animals have identified drug interactions that can enhance cocaine reinforcement (e.g., Mattox et al., 1997; Mello et al., 2014; Czoty, 2015). Severity of cocaine use (in terms of duration or total lifetime doses) could also be incorporated as an independent variable, particularly in animal laboratory studies where pharmacological variables can be easily controlled. Other subject factors, such as comorbid psychiatric conditions, will require creativity in model development in animals, but would be more readily incorporated into human laboratory studies and clinical studies. Although this additional stratification would require recruitment of a larger number of subjects, human laboratory studies would directly inform clinical trials as to which comorbid psychiatric disorders are worthy of consideration.
Second, we strongly agree with previous reviews on the topic (e.g., Haney and Spealman, 2008) in concluding that concordance is greatly enhanced when studies across laboratories use similar experimental parameters. Specifically, the data reviewed here and elsewhere advocate that animal researchers adopt the parameters and research designs used in the human laboratory to whatever extent is possible. For example, predictive validity is enhanced in studies that assess medication effects on cocaine self-administration rather than other endpoints such as cocaine discrimination, subjective effects, reinstatement of extinguished responding, conditioned place preference or other cocaine-induced behaviors. Assessment of chronic administration of putative pharmacotherapies (rather than acute) also emerged as a critically important procedural variable. In addition, we advocate for assessment of selectivity of medication effects—an inclusion criterion of this review—as a critical experimental feature to reduce false-positive conclusions. Although there are many schedules of reinforcement available to measure cocaine self-administration, procedures that require the subject to choose between drug and an alternative reinforcer appear to be the most translational. Choice procedures, which have been adapted for use in animal and human laboratories (Comer et al., 2008; Banks and Negus, 2012; Thomsen et al., 2013; Moeller and Stoops, 2015), reflect a critical aspect of the disorder in that drug abusers choose to allocate their time and resources toward procuring and using drugs and away from other commodities and activities that could serve as reinforcers. A greater effort to test potential medications using similar methods across laboratories will help to clarify whether results in preclinical and clinical settings support the same conclusions. Researchers are currently working to build such models to enhance translation across species (e.g., Foltin et al., 2015). We encourage continued prospective development of these models, particularly with respect to dependent measures used to assess the success or failure of a medication, subsets of patients who might respond to different medications and replication of results of the relatively small number of studies we were able to include in this review.
Third, the reviewed data suggest that drugs should be tested under multiple conditions in laboratory studies in animals and humans before a determination is made regarding the likelihood of clinical effectiveness. "Conditions" in this case can include any procedural, subject, drug, or environment-related factors. The majority of drugs included in the present review have been examined in only one or two studies at most levels of analysis (Table 1). Studying a drug under a limited set of conditions (for example, only in male subjects or only under an FR schedule) enhances the likelihood of misleading conclusions and greatly hampers the ability to use the existing preclinical framework as a “pipeline” to filter drugs with potential clinical efficacy.
Fourth, laboratory studies and clinical trials diverge in the primary dependent variables used to assess medication success or failure. We advocate that clinical researchers should transition away from strict reliance on positive-versus-negative urine screens as the sole measure of medication efficacy. Indeed, the process of developing alternative outcome measures has already begun (Kiluk et al., 2016). Furthermore, we encourage the perspective that reducing cocaine use can be a meaningful clinical endpoint. Determining what extent of reduction of drug use is necessary to yield real benefits, and the point in treatment when that occurs, is a formidable task (see Kiluk et al., 2016). Preclinical researchers are well-positioned to assist in this area. By identifying biomarkers of the behavioral and physiologic processes that are adversely affected by cocaine, it will be possible to assess the extent and speed of recovery of these variables when cocaine intake is decreased, but not eliminated. Full recovery of a biomarker in the absence of total abstinence may endorse that measure as a potentially useful endpoint of clinical trials.
Fifth, the overarching conclusions of this review also suggest ways that the efforts of researchers to enhance translation can be supported by funding agencies. Preclinical study of drugs that have already been clinically tested should be supported as necessary steps to understanding the basis for reported clinical success or failure (“reverse translation”) rather than unnecessary confirmation of clinical data. Similarly, efforts to replicate and extend preclinical data should be viewed as vital rather than duplicitous by both funding agencies and editors of scientific journals. The NIH recently expressed a commitment to enhancing the rigor and reproducibility of research findings (http://www.nih.gov/science/reproducibility/principles-guidelines.htm) that has been embraced by the major scientific publishing companies (Nosek et al., 2015). In addition, investigators in both the laboratory and clinic should be supported in their efforts to determine the extent of reduction of drug intake necessary to achieve clinically meaningful effects and the physiologic and behavioral measures that can best serve as biomarkers in this endeavor. To facilitate this process, we recommend that National Institute on Drug Abuse convenes a roundtable of animal laboratory, human laboratory, and clinical researchers to discuss and refine the process of evaluating medications at each level as well as the process of prioritizing drugs for testing.
Sixth, we believe that the “pipeline” approach advocated in this review will clarify strategies that should be used to identify effective pharmacotherapies. However, steps are required by the scientific and healthcare communities to ensure this information affects drug abuse treatment and policy. For example, Table 2 identifies seven drugs that decreased cocaine use in at least one clinical trial. None of these are approved for clinical use, and the extent of off-label use of such drugs is unknown. Thus there is clearly another critical step: the translation of data from clinical trials to use in treatment. What evidence, at the level of clinical trials, is necessary to hasten the FDA approval process and, subsequently, willingness of physicians to prescribe a drug? To the extent that such drugs are prescribed, treatment data should be collected and used to assess and refine laboratory and clinical research. In some cases, even drugs found to be effective at all levels of analysis will face significant political and economic obstacles (regarding d-amphetamine, see Negus and Henningfield, 2015). Developing strategies to ensure that drugs that emerge from the “pipeline” can overcome such obstacles and make a meaningful impact on treatment will require the creativity and cooperation of scientists, physicians, and makers of policy.
Acknowledgments
The authors are grateful to Justin C. Strickland for thoughtful comments and assistance during preparation of this review.
Abbreviations
- ADHD
attention-deficit hyperactivity disorder
- 5-HT
5-hydroxytryptamine (serotonin)
- FDA
Food and Drug Administration
- FI
fixed interval
- FR
fixed ratio
- GABA
γ-aminobutyric acid
- PR
progressive ratio
- SAMHSA
Substance Abuse and Mental Health Services Administration
Authorship Contributions
Participated in research design: Czoty, Stoops, and Rush.
Performed data analysis: Czoty and Stoops.
Wrote or contributed to the writing of the manuscript: Czoty, Stoops, and Rush.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R21DA034095, R21DA035376, R01DA036553, R01DA032254, R01DA036827,P50DA06634, R37DA10584, R01DA017763].
References
- Ahmadi J, Kampman K, Dackis C. (2006) Outcome predictors in cocaine dependence treatment trials. Am J Addict 15:434–439. [DOI] [PubMed] [Google Scholar]
- Altshuler HL, Phillips PE, Feinhandler DA. (1980) Alteration of ethanol self-administration by naltrexone. Life Sci 26:679–688. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, et al. (2009) Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend 104:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Carroll FI, Graham JH, Mansbach RS, Rahman MA, Philip A, Lewin AH, Showalter VM. (1991) Potent substituted-3 beta-phenyltropane analogs of cocaine have cocaine-like discriminative stimulus effects. Drug Alcohol Depend 29:145–151. [DOI] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. (2011) Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol 22:824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. (2013) Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend 131:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Blough BE, Poklis JL, Negus SS. (2015) Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int J Neuropsychopharmacol 18:pyv009 10.1093/ijnp/pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. (2012) Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci 2012:281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JE. (1976) Effects of alcohol, chlordiazepoxide, cocaine and pentobarbital on responding maintained under fixed-interval schedules of food or shock presentation. J Pharmacol Exp Ther 196:605–615. [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. (2004) Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 47 (Suppl 1):256–273. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Negus SS, Mello NK, Caine SB. (2005) Effect of GABA agonists and GABA-A receptor modulators on cocaine- and food-maintained responding and cocaine discrimination in rats. J Pharmacol Exp Ther 315:858–871. [DOI] [PubMed] [Google Scholar]
- Batki SL, Washburn AM, Delucchi K, Jones RT. (1996) A controlled trial of fluoxetine in crack cocaine dependence. Drug Alcohol Depend 41:137–142. [DOI] [PubMed] [Google Scholar]
- Berger SP, Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Leiderman DB, Montgomery MA, Goldsmith RJ, Bloch DA, Singal BM, et al. (2005) A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction 100 (Suppl 1):58–67. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. (1990) Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol 1:355–363. [DOI] [PubMed] [Google Scholar]
- Bergman J, Paronis CA. (2006) Measuring the reinforcing strength of abused drugs. Mol Interv 6:273–283. [DOI] [PubMed] [Google Scholar]
- Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, Skolnick P. (2013) Modification of cocaine self-administration by buspirone (buspar®): potential involvement of D3 and D4 dopamine receptors. Int J Neuropsychopharmacol 16:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W, Koros E. (1999) Ethanol-reinforced behaviour in the rat: effects of naltrexone. Eur J Pharmacol 374:321–327. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. (2006) A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend 81:267–274. [DOI] [PubMed] [Google Scholar]
- Bolin BL, Lile JA, Marks KR, Rush CR, Stoops WW. (2015) Influence of buspirone maintenance on the pharmacodynamic effects of cocaine and sexual risk taking in cocaine users. Drug Alcohol Depend 156:e23. [Google Scholar]
- Bolin BL, Reynolds AR, Stoops WW, Rush CR. (2013) Relationship between oral D-amphetamine self-administration and ratings of subjective effects: do subjective-effects ratings correspond with a progressive-ratio measure of drug-taking behavior? Behav Pharmacol 24:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG. (2006) GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol 6:37–43. [DOI] [PubMed] [Google Scholar]
- Brebner K, Childress AR, Roberts DC. (2002) A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol 37:478–484. [DOI] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. (2000a) Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology (Berl) 148:314–321. [DOI] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. (2000b) Intra-VTA baclofen attenuates cocaine self-administration on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav 66:857–862. [DOI] [PubMed] [Google Scholar]
- Brecklin CS, Bauman JL. (1999) Cardiovascular effects of cocaine: focus on hypertension. J Clin Hypertens (Greenwich) 1:212–217. [PubMed] [Google Scholar]
- Brutcher RE, Nader MA. (2015) Effects of quetiapine treatment on cocaine self-administration and behavioral indices of sleep in adult rhesus monkeys. Psychopharmacology (Berl) 232:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2008) Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res 172:319–346. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. (1993) Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse 5:117–130. [DOI] [PubMed] [Google Scholar]
- Campbell J, Nickel EJ, Penick EC, Wallace D, Gabrielli WF, Rowe C, Liskow B, Powell BJ, Thomas HM. (2003) Comparison of desipramine or carbamazepine to placebo for crack cocaine-dependent patients. Am J Addict 12:122–136. [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. (1999) Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem 42:2721–2736. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. (2004) Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry 61:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, DeVito EE, Decker S, LaPaglia D, Duffey D, Babuscio TA, Ball SA. (2014) Toward empirical identification of a clinically meaningful indicator of treatment outcome: features of candidate indicators and evaluation of sensitivity to treatment effects and relationship to one year follow up cocaine use outcomes. Drug Alcohol Depend 137:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Carmona GN, May SA, Buzalsky S, Larson C. (1992) Buprenorphine’s effects on self-administration of smoked cocaine base and orally delivered phencyclidine, ethanol and saccharin in rhesus monkeys. J Pharmacol Exp Ther 261:26–37. [PubMed] [Google Scholar]
- Carroll ME, Lac ST. (1992) Effects of buprenorphine on self-administration of cocaine and a nondrug reinforcer in rats. Psychopharmacology (Berl) 106:439–446. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. (1990) Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav 35:237–244. [DOI] [PubMed] [Google Scholar]
- Caulkins JP, Kilmer B, Reuter PH, Midgette G. (2015a) Cocaine’s fall and marijuana’s rise: questions and insights based on new estimates of consumption and expenditures in US drug markets. Addiction 110:728–736. [DOI] [PubMed] [Google Scholar]
- Caulkins JP, Sussell J, Kilmer B, Kasunic A. (2015b) How much of the cocaine market are we missing? Insights from respondent-driven sampling in a mid-sized American city. Drug Alcohol Depend 147:190–195. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Läck CM, Roberts DC. (2008) Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 200:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciraulo DA, Piechniczek-Buczek J, Iscan EN. (2003) Outcome predictors in substance use disorders. Psychiatr Clin North Am 26:381–409. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Sarid-Segal O, Knapp CM, Ciraulo AM, LoCastro J, Bloch DA, Montgomery MA, Leiderman DB, Elkashef A. (2005) Efficacy screening trials of paroxetine, pentoxifylline, riluzole, pramipexole and venlafaxine in cocaine dependence. Addiction 100 (Suppl 1):12–22. [DOI] [PubMed] [Google Scholar]
- Collins ED, Vosburg SK, Hart CL, Haney M, Foltin RW. (2003) Amantadine does not modulate reinforcing, subjective, or cardiovascular effects of cocaine in humans. Pharmacol Biochem Behav 76:401–407. [DOI] [PubMed] [Google Scholar]
- Collins SL, Levin FR, Foltin RW, Kleber HD, Evans SM. (2006) Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug Alcohol Depend 82:158–167. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. (2008) The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend 96:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Foltin RW, Evans SM. (2013) Effects of menstrual cycle phase on cocaine self-administration in rhesus macaques. Horm Behav 63:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JW, Maany I, Fudala PJ, Ehrman RN, Robbins SJ, O’Brien CP. (2001) A randomized, double-blind, placebo-controlled study of ritanserin pharmacotherapy for cocaine dependence. Drug Alcohol Depend 61:183–189. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Roberts DC, de Wit H. (2002) GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend 65:209–220. [DOI] [PubMed] [Google Scholar]
- Crosby RD, Pearson VL, Eller C, Winegarden T, Graves NL. (1996) Phenytoin in the treatment of cocaine abuse: a double-blind study. Clin Pharmacol Ther 59:458–468. [DOI] [PubMed] [Google Scholar]
- Czoty PW. (2015) Effects of chronic binge-like ethanol consumption on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend 153:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. (2002) Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 300:831–837. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. (2011) Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology 36:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. (2010) Effects of chronic d-amphetamine administration on the reinforcing strength of cocaine in rhesus monkeys. Psychopharmacology (Berl) 209:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Martelle SE, Gould RW, Nader MA. (2013) Effects of chronic methylphenidate on cocaine self-administration under a progressive-ratio schedule of reinforcement in rhesus monkeys. J Pharmacol Exp Ther 345:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. (2015) Effects of oral and intravenous administration of buspirone on food-cocaine choice in socially housed male cynomolgus monkeys. Neuropsychopharmacology 40:1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. (2005) A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 30:205–211. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, O’Brien CP. (2012) A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat 43:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daras M, Tuchman AJ, Koppel BS, Samkoff LM, Weitzner I, Marc J. (1994) Neurovascular complications of cocaine. Acta Neurol Scand 90:124–129. [DOI] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. (1999) Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res 23:195–203. [PubMed] [Google Scholar]
- Davidson D, Swift R, Fitz E. (1996) Naltrexone increases the latency to drink alcohol in social drinkers. Alcohol Clin Exp Res 20:732–739. [DOI] [PubMed] [Google Scholar]
- Davies HM, Saikali E, Sexton T, Childers SR. (1993) Novel 2-substituted cocaine analogs: binding properties at dopamine transport sites in rat striatum. Eur J Pharmacol 244:93–97. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. (2005) Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. Eur J Pharmacol 526:186–198. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, Hamilton JA, Huestis MA, Hughes JR, Lindblad R, et al. (2012) Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction 107:694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. (2010) Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res 34:2022–2034. [DOI] [PubMed] [Google Scholar]
- FDA (2006) Medical Review of Vivitrol, U.S. Government, Rockville, MD. [Google Scholar]
- Filip M, Frankowska M, Przegaliński E. (2007) Effects of GABA(B) receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination. Eur J Pharmacol 574:148–157. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. (1991) Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict 86:1563–1570. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. (1990) Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther 253:760–770. [PubMed] [Google Scholar]
- Focchi GR, Leite MC, Andrade AG, Scivoletto S. (2005) Use of dopamine agonist pergolide in outpatient treatment of cocaine dependence. Subst Use Misuse 40:1169–1177. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. (1994) Effects of buprenorphine on the self-administration of cocaine by humans. Behav Pharmacol 5:79–89. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. (1996) Effects of methadone or buprenorphine maintenance on the subjective and reinforcing effects of intravenous cocaine in humans. J Pharmacol Exp Ther 278:1153–1164. [PubMed] [Google Scholar]
- Foltin RW, Haney M, Rubin E, Reed SC, Vadhan N, Balter R, Evans SM. (2015) Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and non-human primates. Pharmacol Biochem Behav 134:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Ward AS, Collins ED, Haney M, Hart CL, Fischman MW. (2003) The effects of venlafaxine on the subjective, reinforcing, and cardiovascular effects of cocaine in opioid-dependent and non-opioid-dependent humans. Exp Clin Psychopharmacol 11:123–130. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD, Byck R, Rounsaville BJ, Kosten TR, Jatlow PI, Morgan C. (1989) Desipramine facilitation of initial cocaine abstinence. Arch Gen Psychiatry 46:117–121. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Epstein DH, Preston KL. (2007) Psychosocial functioning and cocaine use during treatment: strength of relationship depends on type of urine-testing method. Drug Alcohol Depend 91:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Balster RL. (1992) Effects of buspirone and gepirone on i.v. cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 108:289–294. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Gonzalez FA. (1976) Effects of propranolol on behavior maintained under fixed-ratio schedules of cocaine injection or food presentation in squirrel monkeys. J Pharmacol Exp Ther 198:626–634. [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, Varenicline Phase 3 Study Group (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296:47–55. [DOI] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Nader SH, Nader MA. (2011) Effects of varenicline on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 339:678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, Davis C, Creson D, Kirby K. (1995) Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled double-blind trials. J Clin Psychopharmacol 15:163–174. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. (2001) Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol 21:522–526. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. (1997) Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 17:485–488. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29:1439–1464. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC. (1990) Concurrent and simultaneous use of alcohol with cocaine: results of national survey. Drug Alcohol Depend 25:97–104. [DOI] [PubMed] [Google Scholar]
- Greberman SB, Wada K. (1994) Social and legal factors related to drug abuse in the United States and Japan. Public Health Rep 109:731–737. [PMC free article] [PubMed] [Google Scholar]
- Grech DM, Spealman RD, Bergman J. (1996) Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology (Berl) 125:97–104. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. (2010) Substance abuse in women. Psychiatr Clin North Am 33:339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. (2003) Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend 70(3, Suppl)S41–S54. [DOI] [PubMed] [Google Scholar]
- Haile CN, De La Garza R, 2nd, Mahoney JJ, 3rd, Nielsen DA, Kosten TR, Newton TF. (2012) The impact of disulfiram treatment on the reinforcing effects of cocaine: a randomized clinical trial. PLoS One 7:e47702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. (1998) Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology (Berl) 137:15–24. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW. (2006) Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology 31:1814–1821. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. (2008) Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 199:403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Collins ED, Rubin E, Foltin RW. (2007a) Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend 86:274–277. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. (2000) Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol 11:87–91. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. (2007b) Gabapentin does not reduce smoked cocaine self-administration: employment of a novel self-administration procedure. Behav Pharmacol 18:71–75. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. (2008) Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology 33:761–768. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. (2004) Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend 73:279–287. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. (2010) Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci 1187:4–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Pryzbeck TR. (1988) The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol 49:219–224. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. (1996) The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther 277:1247–1258. [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. (2004) Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol 55:431–461. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. (2009) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. (1961) Progressive ratio as a measure of reward strength. Science 134:943–944. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. (1995) Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther 275:1551–1559. [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. (2007) Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320:757–765. [DOI] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA. (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SW, Warfield NA, Blaine JD, Cornish J, Ling W, Rosen MI, Urschel H, 3rd, Wesson D, Ziedonis D. (1992) A pilot trial of gepirone vs. placebo in the treatment of cocaine dependency. Psychopharmacol Bull 28:21–26. [PubMed] [Google Scholar]
- Johanson CE. (1975) Pharmacological and environmental variables affecting drug preference in rhesus monkeys. Pharmacol Rev 27:343–355. [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. (1989) The pharmacology of cocaine related to its abuse. Pharmacol Rev 41:3–52. [PubMed] [Google Scholar]
- Johanson CE, Kilgore K, Uhlenhuth EH. (1983) Assessment of dependence potential of drugs in humans using multiple indices. Psychopharmacology (Berl) 81:144–149. [DOI] [PubMed] [Google Scholar]
- John WS, Banala AK, Newman AH, Nader MA. (2015) Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology (Berl) 232:1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. (2005) Recent advances in the development of treatments for alcohol and cocaine dependence: focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs 19:873–896. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Chen YR, Swann AC, Schmitz J, Lesser J, Ruiz P, Johnson P, Clyde C. (1997) Ritanserin in the treatment of cocaine dependence. Biol Psychiatry 42:932–940. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. (1995) A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend 40:17–25. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. (1992) A controlled trial of buprenorphine treatment for opioid dependence. JAMA 267:2750–2755. [PubMed] [Google Scholar]
- Jones JD, Comer SD. (2013) A review of human drug self-administration procedures. Behav Pharmacol 24:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study Group (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63. [DOI] [PubMed] [Google Scholar]
- Kahn R, Biswas K, Childress AR, Shoptaw S, Fudala PJ, Gorgon L, Montoya I, Collins J, McSherry F, Li SH, et al. (2009) Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug Alcohol Depend 103:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Dackis C, Lynch KG, Pettinati H, Tirado C, Gariti P, Sparkman T, Atzram M, O’Brien CP. (2006) A double-blind, placebo-controlled trial of amantadine, propranolol, and their combination for the treatment of cocaine dependence in patients with severe cocaine withdrawal symptoms. Drug Alcohol Depend 85:129–137. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C, O’Brien CP. (2015) A double blind, placebo controlled trial of modafinil for the treatment of cocaine dependence without co-morbid alcohol dependence. Drug Alcohol Depend 155:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. (2013) A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend 133:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman K, Volpicelli JR, Alterman A, Cornish J, Weinrieb R, Epperson L, Sparkman T, O’Brien CP. (1996) Amantadine in the early treatment of cocaine dependence: a double-blind, placebo-controlled trial. Drug Alcohol Depend 41:25–33. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, et al. (2001) Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend 63:69–78. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, Weinrieb RM, O’Brien CP. (2002) Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav 27:251–260. [DOI] [PubMed] [Google Scholar]
- Karila L, Leroy C, Dubol M, Trichard C, Mabondo A, Marill C, Dubois A, Bordas N, Martinot JL, Reynaud M, et al. (2016) Dopamine transporter correlates and occupancy by modafinil in cocaine-dependent patients: a controlled study with high-resolution PET and [(11)C]-PE2I. Neuropsychopharmacology (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL. (1990) Models of relative reinforcing efficacy of drugs and their predictive utility. Behav Pharmacol 1:283–301. [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Terry P. (2000) Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacology (Berl) 148:90–98. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Stoops WW, Perry AS, Prendergast MA, Rush CR. (2003) Clinical neuropharmacology of drugs of abuse: a comparison of drug-discrimination and subject-report measures. Behav Cogn Neurosci Rev 2:227–260. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Carroll KM, Duhig A, Falk DE, Kampman K, Lai S, Litten RZ, McCann DJ, Montoya ID, Preston KL, et al. (2016) Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug Alcohol Depend 158:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MA, Pryce RL. (2014) Evidence for compliance with long-term medication: a systematic review of randomised controlled trials. Int J Clin Pharm 36:128–135. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. (1993) Effects of three monoamine uptake inhibitors on behavior maintained by cocaine or food presentation in rhesus monkeys. Drug Alcohol Depend 31:149–158. [DOI] [PubMed] [Google Scholar]
- Koe BK. (1976) Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther 199:649–661. [PubMed] [Google Scholar]
- Kohut SJ, Bergman J, Blough BE. (2016) Effects of L-methamphetamine treatment on cocaine- and food-maintained behavior in rhesus monkeys. Psychopharmacology (Berl) 233:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L, DrugAbuse Sciences Naltrexone Depot Study Group (2004) Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res 28:1051–1059. [DOI] [PubMed] [Google Scholar]
- Lange RA, Hillis LD. (2001) Cardiovascular complications of cocaine use. N Engl J Med 345:351–358. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F. (2007) Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend 87:20–29. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Specker S, Mooney M, Mahony A, Brooks DJ, Babb D, Bai Y, Eberly LE, Nunes EV, et al. (2015) Extended-release mixed amphetamine salts vs placebo for comorbid adult attention-deficit/hyperactivity disorder and cocaine use disorder: a randomized clinical trial. JAMA Psychiatry 72:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xi ZX, Markou A. (2013) Metabotropic glutamate 7 (mGlu7) receptor: a target for medication development for the treatment of cocaine dependence. Neuropharmacology 66:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Allen TS, Glaser PE, Hays LR, Rush CR. (2004a) Baclofen does not alter the reinforcing, subject-rated or cardiovascular effects of intranasal cocaine in humans. Psychopharmacology (Berl) 171:441–449. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. (2004b) Acute administration of the GABA reuptake inhibitor tiagabine does not alter the effects of oral cocaine in humans. Drug Alcohol Depend 76:81–91. [DOI] [PubMed] [Google Scholar]
- Lucena J, Blanco M, Jurado C, Rico A, Salguero M, Vazquez R, Thiene G, Basso C. (2010) Cocaine-related sudden death: a prospective investigation in south-west Spain. Eur Heart J 31:318–329. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Mello NK, Drieze JM, Mendelson JH. (1995) Buprenorphine-induced alterations of cocaine’s reinforcing effects in rhesus monkey: a dose-response analysis. Drug Alcohol Depend 40:87–98. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. (2006) Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol 14:34–41. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Kajdasz DK, Herron J, Anton RF, Brady KT. (2000) A double-blind, placebo-controlled outpatient trial of pergolide for cocaine dependence. Drug Alcohol Depend 60:161–168. [DOI] [PubMed] [Google Scholar]
- Mancino MJ, McGaugh J, Chopra MP, Guise JB, Cargile C, Williams DK, Thostenson J, Kosten TR, Sanders N, Oliveto A. (2014) Clinical efficacy of sertraline alone and augmented with gabapentin in recently abstinent cocaine-dependent patients with depressive symptoms. J Clin Psychopharmacol 34:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Balster RL. (1993) Effects of mazindol on behavior maintained or occasioned by cocaine. Drug Alcohol Depend 31:183–191. [DOI] [PubMed] [Google Scholar]
- Mattox AJ, Thompson SS, Carroll ME. (1997) Smoked heroin and cocaine base (speedball) combinations in rhesus monkeys. Exp Clin Psychopharmacol 5:113–118. [DOI] [PubMed] [Google Scholar]
- McCann DJ, Ramey T, Skolnick P. (2015) Outcome measures in medication trials for substance use disorders. Curr Treat Options Psychiatry, in press. [Google Scholar]
- McKearney JW. (1976) Punishment of responding under schedules of stimulus-shock termination: effects of d-amphetamine and pentobarbital. J Exp Anal Behav 26:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Woodworth AM. (2014) The affordable care act and treatment for “substance use disorders:” implications of ending segregated behavioral healthcare. J Subst Abuse Treat 46:541–545. [DOI] [PubMed] [Google Scholar]
- Meert TF, Awouters F, Niemegeers CJ, Schellekens KH, Janssen PA. (1991) Ritanserin reduces abuse of alcohol, cocaine, and fentanyl in rats. Pharmacopsychiatry 24:159–163. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. (1983) Comparison of buprenorphine and methadone effects on opiate self-administration in primates. J Pharmacol Exp Ther 225:378–386. [PubMed] [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ, Bergman J. (2013) Effects of chronic buspirone treatment on cocaine self-administration. Neuropsychopharmacology 38:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ, Carroll FI. (2014) Effects of chronic varenicline treatment on nicotine, cocaine, and concurrent nicotine+cocaine self-administration. Neuropsychopharmacology 39:1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Kamien JB, Lukas SE, Mendelson JH, Drieze JM, Sholar JW. (1993a) Effects of intermittent buprenorphine administration on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 264:530–541. [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Kelly M, Fivel PA, Mendelson JH. (2011) Effects of progesterone and testosterone on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology 36:2187–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. (2007) Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology 32:1956–1966. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Kamien JB, Mendelson JH, Drieze J, Cone EJ. (1992) The effects of chronic buprenorphine treatment on cocaine and food self-administration by rhesus monkeys. J Pharmacol Exp Ther 260:1185–1193. [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Bree MP, Mendelson JH. (1990a) Desipramine effects on cocaine self-administration by rhesus monkeys. Drug Alcohol Depend 26:103–116. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH, Drieze J. (1993b) Naltrexone-buprenorphine interactions: effects on cocaine self-administration. Neuropsychopharmacology 9:211–224. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. (1980) Buprenorphine suppresses heroin use by heroin addicts. Science 207:657–659. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. (1990b) Buprenorphine and naltrexone effects on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 254:926–939. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. (1989) Buprenorphine suppresses cocaine self-administration by rhesus monkeys. Science 245:859–862. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. (1998) The effects of buprenorphine on self-administration of cocaine and heroin “speedball” combinations and heroin alone by rhesus monkeys. J Pharmacol Exp Ther 285:444–456. [PubMed] [Google Scholar]
- Mello NK, Negus SS. (2001) Effects of indatraline and buprenorphine on self-administration of speedball combinations of cocaine and heroin by rhesus monkeys. Neuropsychopharmacology 25:104–117. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. (2007) Effects of d-amphetamine and buprenorphine combinations on speedball (cocaine+heroin) self-administration by rhesus monkeys. Neuropsychopharmacology 32:1985–1994. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Lee AM, Bandy AL. (2000) Ethanol reinforcement in nondeprived mice: effects of abstinence and naltrexone. Alcohol Clin Exp Res 24:1172–1179. [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. (2001) The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat 21:193–198. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. (2007) Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse 33:367–378. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Stoops WW. (2015) Cocaine choice procedures in animals, humans, and treatment-seekers: Can we bridge the divide? Pharmacol Biochem Behav 138:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, Lange WR, Contoreggi C, Johnson RE, Fudala PJ. (2004) Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther 75:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. (2009) Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 101:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Specker S, Babb D, Levin FR, Grabowski J. (2015) Pilot study of the effects of lisdexamfetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 153:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouhaffel AH, Madu EC, Satmary WA, Fraker TD., Jr (1995) Cardiovascular complications of cocaine. Chest 107:1426–1434. [DOI] [PubMed] [Google Scholar]
- Nader MA, Green KL, Luedtke RR, Mach RH. (1999) The effects of benzamide analogues on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 147:143–152. [DOI] [PubMed] [Google Scholar]
- National Drug Intelligence Center (2011) National Drug Threat Assessment, United States Department of Justice, Washington, DC. [Google Scholar]
- Negus SS. (2003) Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology 28:919–931. [DOI] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. (2015) Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology 40:1815–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. (2003a) Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 167:324–332. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. (2003b) Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend 70:39–52. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. (2007) Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320:627–636. [DOI] [PubMed] [Google Scholar]
- Newman JL, Negus SS, Lozama A, Prisinzano TE, Mello NK. (2010) Behavioral evaluation of modafinil and the abuse-related effects of cocaine in rhesus monkeys. Exp Clin Psychopharmacol 18:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek BA, Alter G, Banks GC, Borsboom D, Bowman SD, Breckler SJ, Buck S, Chambers CD, Chin G, Christensen G, et al. (2015) SCIENTIFIC STANDARDS. Promoting an open research culture. Science 348:1422–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. (1992) Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther 261:885–894. [PubMed] [Google Scholar]
- Oliveto A, Poling J, Mancino MJ, Williams DK, Thostenson J, Pruzinsky R, Gonsai K, Sofuoglu M, Gonzalez G, Tripathi S, et al. (2012) Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction 107:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. (1992) Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry 49:881–887. [DOI] [PubMed] [Google Scholar]
- Patrizi R, Pasceri V, Sciahbasi A, Summaria F, Rosano GM, Lioy E. (2006) Evidence of cocaine-related coronary atherosclerosis in young patients with myocardial infarction. J Am Coll Cardiol 47:2120–2122. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Fonte CA, Mercincavage M, Stitzer ML, Chengappa KN, Jain A. (2010) Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clin Pharmacol Ther 88:109–114. [DOI] [PubMed] [Google Scholar]
- Petitjean S, Stohler R, Déglon JJ, Livoti S, Waldvogel D, Uehlinger C, Ladewig D. (2001) Double-blind randomized trial of buprenorphine and methadone in opiate dependence. Drug Alcohol Depend 62:97–104. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. (1968) Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther 161:122–129. [PubMed] [Google Scholar]
- Plebani JG, Lynch KG, Yu Q, Pettinati HM, O’Brien CP, Kampman KM. (2012) Results of an initial clinical trial of varenicline for the treatment of cocaine dependence. Drug Alcohol Depend 121:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Epstein DH, Cone EJ, Wtsadik AT, Huestis MA, Moolchan ET. (2002) Urinary elimination of cocaine metabolites in chronic cocaine users during cessation. J Anal Toxicol 26:393–400. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. (1997) Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr 167:130–145. [PubMed] [Google Scholar]
- Raby WN, Rubin EA, Garawi F, Cheng W, Mason E, Sanfilippo L, Lord S, Bisaga A, Aharonovich E, Levin F, et al. (2014) A randomized, double-blind, placebo-controlled trial of venlafaxine for the treatment of depressed cocaine-dependent patients. Am J Addict 23:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Evans SM, Bedi G, Rubin E, Foltin RW. (2011) The effects of oral micronized progesterone on smoked cocaine self-administration in women. Horm Behav 59:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223. [DOI] [PubMed] [Google Scholar]
- Roberts DC. (1993) Self-administration of GBR 12909 on a fixed ratio and progressive ratio schedule in rats. Psychopharmacology (Berl) 111:202–206. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM. (1997) Baclofen suppression of cocaine self-administration: demonstration using a discrete trials procedure. Psychopharmacology (Berl) 131:271–277. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM, Vickers GJ. (1996) Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology 15:417–423. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Campbell UC, Cosgrove KP, Carroll ME. (1999) Naltrexone pretreatment decreases the reinforcing effectiveness of ethanol and saccharin but not PCP or food under concurrent progressive-ratio schedules in rhesus monkeys. Psychopharmacology (Berl) 141:436–446. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, et al. (2007) Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. (2005) Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther 313:1361–1369. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. (1991) Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry 48:43–51. [DOI] [PubMed] [Google Scholar]
- Rowlett JK. (2000) A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology (Berl) 153:1–16. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. (2010) Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol 30:152–159. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Griffiths RR. (1988) Amantadine: evaluation of reinforcing properties and effect on cocaine self-injection in baboons. Drug Alcohol Depend 21:195–202. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. (2002) Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 163:327–344. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Green CE, Stotts AL, Lindsay JA, Rathnayaka NS, Grabowski J, Moeller FG. (2014) A two-phased screening paradigm for evaluating candidate medications for cocaine cessation or relapse prevention: modafinil, levodopa-carbidopa, naltrexone. Drug Alcohol Depend 136:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Rathnayaka N, Green CE, Moeller FG, Dougherty AE, Grabowski J. (2012) Combination of modafinil and d-amphetamine for the treatment of cocaine dependence: A preliminary investigation. Front Psychiatry 3:77 10.3389/fpsyt.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. (2001) Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav 26:167–180. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. (2005) Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. Am J Psychiatry 162:340–349. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. (1997) Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry 54:713–720. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR. (1993) Buprenorphine: dose-related effects on cocaine and opioid use in cocaine-abusing opioid-dependent humans. Biol Psychiatry 34:66–74. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, Edwards A, Donlin J, Pihlgren E. (2002) Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol 10:286–294. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Snyder M. (1989) NIDA’s Medication Development Program--1989. NIDA Res Monogr 95:64–73. [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. (2003) Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction 98:1137–1141. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW. (1998) The GABAB agonist baclofen modifies cocaine self-administration in rats. Behav Pharmacol 9:195–206. [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Kao UH, Wang PC, Bholat MA, Ling W. (2008) Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis 27:13–23. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Kintaudi PC, Charuvastra C, Ling W. (2002) A screening trial of amantadine as a medication for cocaine dependence. Drug Alcohol Depend 66:217–224. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. (2003) Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry 64:1440–1448. [DOI] [PubMed] [Google Scholar]
- Shorter D, Kosten TR. (2011) Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med 9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD. (1999) A national evaluation of treatment outcomes for cocaine dependence. Arch Gen Psychiatry 56:507–514. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Mach RH, Nader MA. (1999) Dopamine D2/D3 receptors modulate cocaine’s reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend 54:97–110. [DOI] [PubMed] [Google Scholar]
- Skinner BF. (1938) The Behavior of Organisms, Appleton-Century-Crofts, New York. [Google Scholar]
- Sofuoglu M, Pentel PR, Bliss RL, Goldman AI, Hatsukami DK. (1999) Effects of phenytoin on cocaine self-administration in humans. Drug Alcohol Depend 53:273–275. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. (1998) Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 139:169–184. [DOI] [PubMed] [Google Scholar]
- Stine SM, Krystal JH, Kosten TR, Charney DS. (1995) Mazindol treatment for cocaine dependence. Drug Alcohol Depend 39:245–252. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. (2010) Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. Eur J Pharmacol 644:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. (2012a) Alternative reinforcer response cost impacts cocaine choice in humans. Prog Neuropsychopharmacol Biol Psychiatry 36:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. (2012b) Influence of acute bupropion pre-treatment on the effects of intranasal cocaine. Addiction 107:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Vansickel AR, Glaser PE, Rush CR. (2008) The influence of acute varenicline administration on smoking and eating behavior in humans. Pharmacol Biochem Behav 91:165–169. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. (1994a) Buprenorphine versus methadone in the treatment of opioid-dependent cocaine users. Psychopharmacology (Berl) 116:401–406. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. (1994b) Comparison of buprenorphine and methadone in the treatment of opioid dependence. Am J Psychiatry 151:1025–1030. [DOI] [PubMed] [Google Scholar]
- Strickland JC, Lile JA, Rush CR, Stoops WW. (2014) Relationship between intranasal cocaine self-administration and subject-rated effects: predictors of cocaine taking on progressive-ratio schedules. Hum Psychopharmacol 29:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF, Sengpiel T, Mackler SA, Volpicelli JR, O’Brien CP, Vogel WH. (2002) Effect of naltrexone on oral consumption of concurrently available ethanol and cocaine in the rat. Alcohol 28:169–179. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2014) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Tapp A, Wood AE, Kennedy A, Sylvers P, Kilzieh N, Saxon AJ. (2015) Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend 149:18–24. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. (2013) Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav 99:211–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens M, Fonseca F, Mateu G, Farré M. (2005) Efficacy of antidepressants in substance use disorders with and without comorbid depression. A systematic review and meta-analysis. Drug Alcohol Depend 78:1–22. [DOI] [PubMed] [Google Scholar]
- Van Tieu H, Koblin BA. (2009) HIV, alcohol, and noninjection drug use. Curr Opin HIV AIDS 4:314–318. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Haile CN, Mahoney JJ, 3rd, Thompson-Lake DG, Newton TF, De La Garza R., 2nd (2014) Treatment with modafinil and escitalopram, alone and in combination, on cocaine-induced effects: a randomized, double blind, placebo-controlled human laboratory study. Drug Alcohol Depend 141:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. (1992) Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49:876–880. [DOI] [PubMed] [Google Scholar]
- Vosburg SK, Haney M, Rubin E, Foltin RW. (2010) Using a novel alternative to drug choice in a human laboratory model of a cocaine binge: a game of chance. Drug Alcohol Depend 110:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Donny EC, Nuzzo PA, Umbricht A, Bigelow GE. (2010) Cocaine abuse versus cocaine dependence: cocaine self-administration and pharmacodynamic response in the human laboratory. Drug Alcohol Depend 106:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Woolverton WL. (1995) The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 275:1367–1374. [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Griffiths RR. (2005) Effects of GABAergic modulators on food and cocaine self-administration in baboons. Drug Alcohol Depend 80:369–376. [DOI] [PubMed] [Google Scholar]
- Williams KL, Kane EC, Woods JH. (2001) Interaction of morphine and naltrexone on oral ethanol self-administration in rhesus monkeys. Behav Pharmacol 12:325–333. [DOI] [PubMed] [Google Scholar]
- Winchell C, Rappaport BA, Roca R, Rosebraugh CJ. (2012) Reanalysis of methamphetamine dependence treatment trial. CNS Neurosci Ther 18:367–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Kropp F, Lindblad R, Douaihy A, Haynes L, Hodgkins C, Chartier K, Kampman KM, Sharma G, Lewis DF, et al. (2014) Multisite, randomized, double-blind, placebo-controlled pilot clinical trial to evaluate the efficacy of buspirone as a relapse-prevention treatment for cocaine dependence. J Clin Psychiatry 75:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Ciraulo DA, Harrer JM, Goldsmith RJ, Grabowski J, Coleman FS, Mindrum G, Kahn R, Osman S, et al. (2007) A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug Alcohol Depend 91:141–148. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, Coleman FS, Bloch DA, Leiderman DB, Singal BM, et al. (2005) A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction 100 (Suppl 1):68–77. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. (1979) The effects of lithium on choice between cocaine and food in the rhesus monkey. Commun Psychopharmacol 3:309–318. [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. (1984) Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther 230:678–683. [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. (1989) The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav 32:691–697. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. (2005) Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci 21:3427–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Forray A, Nich C, Carroll KM, Hine C, Merry BC, Shaw H, Shaw J, Sofuoglu M. (2014) Progesterone reduces cocaine use in postpartum women with a cocaine use disorder: a randomized, double-blind study. Lancet Psychiatry 1:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A. (2004) Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate “sensitization” to “drug wanting”? Psychopharmacology (Berl) 171:349–351, author reply 352–363. [DOI] [PubMed] [Google Scholar]