Abstract
Expression of the pregnane X receptor (PXR) has been reported to be decreased in animal models of inflammatory bowel disease (IBD). To investigate the differential expression of PXR in children with Crohn’s disease, a type of IBD, RNA was extracted from archived intestinal biopsies from 18 children with Crohn’s disease (CD) and 12 age- and sex-matched controls (aged 7–17yrs). The aim of this investigation was to compare the relative mRNA expression of PXR, cytochrome p450 3A4 (CYP3A4), and villin 1 (VIL1) (a marker of epithelial cell integrity) in the inflamed terminal ileum (TI) versus noninflamed duodenum of children with CD. Relative expression was determined via reverse transcription real-time quantitative polymerase chain reaction, data normalized to glyceraldehyde 3-phosphate dehydrogenase, and differences in gene expression explored via paired t tests. PXR expression was decreased in the inflamed TI versus noninflamed duodenum (TI = 1.88 ± 0.89 versus duodenum = 2.5 ± 0.67; P < 0.001) in CD, but not controls (TI = 2.11 ± 0.41 versus duodenum = 2.26 ± 0.61; P = 0.52). CYP3A4 expression was decreased in CD (TI = –0.89 ± 3.11 versus duodenum = 1.90 ± 2.29; P < 0.05), but not controls (TI = 2.46 ± 0.51 versus duodenum = 2.60 ± 0.60; P = 0.61), as was VIL1 (CD TI = 3.80 ± 0.94 versus duodenum = 4.61 ± 0.52; P < 0.001; controls TI = 4.30 ± 0.35 versus duodenum = 4.47 ± 0.40; P = 0.29). PXR expression correlated with VIL1 (r = 0.78, P = 0.01) and CYP3A4 (r = 0.52, P = 0.01) expression. In conclusion, PXR, CYP3A4, and VIL1 expression was decreased only in the actively inflamed small intestinal tissue in children with CD. Our findings suggest that inflammation has the potential to influence expression of genes, and potentially intestinal proteins, important to drug disposition and response. The observed differential patterns of gene expression support further investigation of the role of PXR in the pathogenesis and/or treatment of pediatric Crohn’s disease.
Introduction
Human pregnane X receptor (PXR), a known transcriptional regulator of important drug-metabolizing enzymes [e.g., cytochrome p450 3A4 (CYP3A4)] and transporters (e.g., ABCB1), is a ligand-activated nuclear receptor expressed in the gastrointestinal (GI) tract, where it functions as a xenobiotic sensor (Zhang et al., 2008). It is well established that constant exposure to xenobiotics is not only capable of producing inflammation and injury (Zhou et al., 2006; Zhang et al., 2008) but can also impair immune function (Xie and Tian, 2006), raising the possibility that inappropriate adaptive responses to xenobiotics may contribute to the pathogenesis of immune-mediated inflammatory pathologies, including inflammatory bowel disease (IBD).
IBD is a chronic inflammatory condition of the GI epithelial mucosa that often manifests in childhood, with children comprising 20–25% of the total patient population (Leung et al., 2011). IBD is thought to have its origins in an incompletely understood, multifactorial, disease-causing combination of genetic predisposition, aberrant immune response, environmental triggers, and pathologic alterations in the intestinal epithelial barrier and microbiome (Sartor, 2006). A plausible mechanism linking xenobiotic exposure, inflammation, and immune (dys)function was recently explored in animal models of IBD, where evidence from the mouse model suggested a protective, anti-inflammatory effect of PXR activation in the GI tract (Shah et al., 2007; Ma et al., 2007; Cheng et al., 2010). The proposed mechanism involves PXR activation leading to suppression of nuclear factor κB, a key component of the inflammatory cascade and a known regulator of the immune response (Zhou et al., 2006). In children, the interpretation of such gene expression studies may be confounded by ontogeny. Our aim was to test the hypothesis that PXR and its target genes (e.g., CYP3A4) are underexpressed in the actively inflamed small intestinal tissue of children with Crohn’s disease, a type of IBD.
Materials and Methods
These research activities were approved and declared nonhuman-subjects research by the Institutional Review Board of the Children’s Mercy Hospital.
Tissue Specimens.
Archived biopsy tissue samples from the duodenum and terminal ileum (TI) were obtained from patients, 7–17 years old, with Crohn’s disease (CD, n = 18) and age- and sex-matched non-IBD patients (control, n = 12) who had normal-appearing duodenum and TI on esophagogastroduodenoscopy/colonoscopy performed as part of routine care. Diagnosis of Crohn’s disease was confirmed by clinical, radiographic, and histopathologic findings. All histopathology slides were independently reviewed by a single, experienced, board-certified pediatric pathologist to ensure presence of inflammation in CD TI, but not CD duodenum or any control tissues. Presence/absence of inflammation was additionally verified by IL8 and iNOS gene expression studies performed on all biopsy samples.
RNA Extraction and Quantitative Reverse Transcription–Real-Time Quantitative Polymerase Chain Reaction.
Total RNA was prepared from four 10-μm sections of archived formalin-fixed paraffin-embedded intestinal biopsy samples using the RNeasy formalin-fixed paraffin-embedded RNA Isolation Kit (Qiagen, Valencia, CA), according to manufacturer’s protocol, including on-column DNase I treatment. RNA quantity was determined on a Nanodrop ND-1000 Spectrophotometer. Total RNA (200 ng) was reverse transcribed using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems/Thermo Fisher Scientific). RNA quality was estimated by performing glyceraldehyde 3-phosphate dehydrogenase (GAPDH) 3′:5′ assays (Nolan et al., 2006). RNA integrity is inferred from the ratio of quantities of the two PCR products targeting the 3′ and 5′ ends of GAPDH mRNA, which reflects successful reverse transcription across GAPDH. Ratios closest to one are indicative of good quality RNA.
Primers for the 3′ GAPDH assay were 5′ AGTCCCTGCCACACTCAG (forward) and 5′ TACTTTATTGATGGTACATGACAAGG (reverse); 5′ GTGAACCATGAGAAGTATGACAAC (forward) and 5′ CATGAGTCCTTCCACGATACC (reverse) for the 5′ GAPDH assay. GAPDH assays were performed with 2 μl cDNA, 250 nM each forward and reverse primers, and PerfeCta SYBR green SuperMix (Quanta Biosciences, Gaithersburg, MD). Gene expression assays for PXR (Hs00243666_m1), VIL1 (Hs00200229_m1), CYP3A4 (Hs00604506_m1), IL8 (Hs99999034_m1), and NOS2 (Hs01075529_m1) were performed using 2 μl cDNA, TaqMan Gene Expression Assay (Applied Biosystems), and PerfeCTa qPCR SuperMix (Quanta Biosciences). Standard curves were generated using serial dilutions of cDNA plasmids from 10 to 108 copies/μl. Molecule numbers were determined from linear regression of the standard curves. All gene expression assays were performed with triplicate determinations of molecule numbers for each sample. When sample replicates varied more than 0.5 Ct, the assay was repeated in triplicate.
Data Analysis.
Only tissue samples of adequate quality mRNA (i.e., GAPDH 3′:5′ ratios less than 10) were included in the study analysis. For relative mRNA expression quantification, replicate determinations of molecule numbers were averaged, normalized to the mean of GAPDH molecule numbers, and logarithmically transformed, as data were not normally distributed. Differences in normalized, log2-transformed, unit-less, relative gene expression were compared between the TI and the duodenum, using a two-tailed paired Student’s t test. Spearman’s correlation and linear regression analyses were carried out to explore relevant associations. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC) and SPSS version 20 (IBM) with a significance limit set at α = 0.05.
Results and Discussion
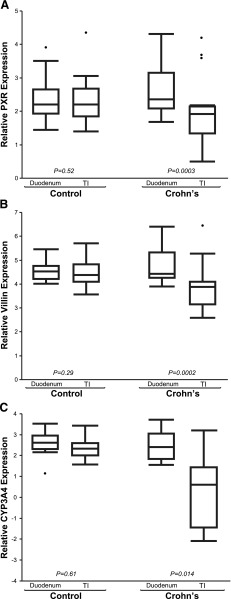
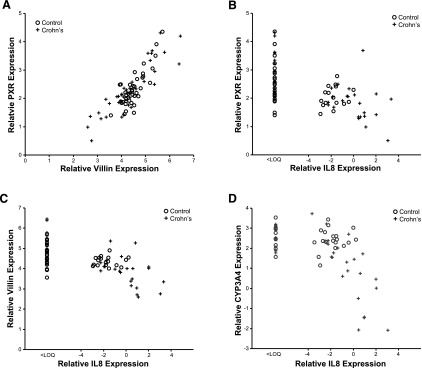
mRNA expression patterns of PXR, CYP3A4, and VIL1 observed in our pediatric patient cohorts are presented in Fig. 1. GAPDH expression did not differ with age, sex, or location along the small intestine in the CD or the control cohort (data not shown). In children with CD, PXR expression was decreased in inflamed TI versus noninflamed duodenum (TI = 1.88 ± 0.89 versus duodenum = 2.5 ± 0.67; P < 0.001), but not in controls (TI = 2.11 ± 0.41 versus duodenum = 2.26 ± 0.61; P = 0.52). Likewise, expression of CYP3A4 was decreased in inflamed TI of children with CD (TI = –0.89 ± 3.11 versus duodenum = 1.90 ± 2.29; P = 0.014), but not in controls (TI = 2.46 ± 0.51 versus duodenum = 2.60 ± 0.60; P = 0.61). VIL1 expression [indicator of epithelial cell integrity (Simms et al., 2008)] was also decreased in inflamed TI of children with CD (TI = 3.80 ± 0.94 versus duodenum = 4.61 ± 0.52; P < 0.001), but not in controls (TI = 4.30 ± 0.35 versus duodenum = 4.47 ± 0.40; P = 0.29). A positive correlation between PXR and CYP3A4 expression (Supplemental Fig. 1) was observed (r = 0.517, P = 0.01), as expected, given that CYP3A is a known target of PXR (Timsit and Negishi, 2007). Correlation relationships for all genes of interest, across all samples, are summarized in the supplemental documents (Supplemental Table 1). Unexpectedly, a significant correlation between PXR and VIL1 expression was observed across all samples (r = 0.780, P = 0.01; Fig. 2A), as well as between CYP3A4 and VIL1 (Supplemental Fig. 2). Expression of PXR (Fig. 2B), VIL1 (Fig. 2C), and CYP3A4 (Fig. 2D) correlated inversely with IL8 (marker of inflammation) in the TI of CD, but not controls. The observed trend in inverse correlation between IL8 and the genes of interest reached statistical significance for CYP3A4 (P < 0.01) and approached significance for PXR (P = 0.06) and VIL1 (P = 0.07) in the inflamed TI of CD (Supplemental Table 2). This inverse correlation was not observed in noninflamed tissues (e.g., control TI).
Fig. 1.
Relative gene expression of PXR (A), VIL1 (B), and CYP3A4 (C) in the TI and the duodenum of children with Crohn’s disease (CD; n = 18) versus age- and sex-matched controls without IBD (n = 12). Mean differences in relative gene expression (triplicate determinations of molecule number per sample, normalized to GAPDH, and log2-transformed) were compared between the TI and the duodenum in the same children, using a two-tailed paired Student’s t test (α = 0.05).
Fig. 2.
Correlation relationships (Spearman’s correlation; α = 0.05) in relative gene expression (triplicate determinations of molecule number per sample, normalized to GAPDH, and log2-transformed) between (A) PXR and VIL1 across all tissue samples (n = 30; r = 0.78, P = 0.01); (B) PXR and IL8 in the TI of CD (r = –0.45, P = 0.06) and controls (r = 0.18, P = 0.6), (C) VIL1 and IL8 in the TI of CD (r = –0.44, P = 0.07) and controls (r = 0.27, P = 0.4), and (D) CYP3A4 and IL8 in the TI of CD (r = –0.68, P < 0.01) and controls (r = 0.21, P = 0.5). Crohn’s disease (CD) tissue denoted by cross (n = 18), control tissue by circle (n = 12).
Our study results demonstrate a significant decrease in PXR expression only in the inflamed small intestinal tissue (i.e., TI) in children with CD, relative to uninflamed small intestinal tissue from the same children (i.e., duodenum); Fig. 1A. The strength of our study resides in utilization of noninflamed duodenal tissue from each subject as a negative control for that subject, effectively eliminating interindividual genetic variability as a confounding factor in our mRNA analysis. As no difference in PXR expression was observed between the TI and the duodenum in age- and sex-matched controls, the observed decrease in PXR in the CD TI cannot be attributed to biopsy site location along the small intestinal tract. More probably, the observed decrease represents the effect of inflammation or disease on the intestinal epithelium, and the diverse population of enzymes, proteins, and receptors that reside within it. Taken together, our observations of the differential patterns of expression in PXR, CYP3A4, and VIL1 in children with CD raise several important implications.
First, expression of all genes of interest was lower in CD versus controls, implying that disease/inflammation per se have the potential to produce a significant effect on the expression of genes important to drug disposition and response, including drug-metabolizing enzymes (e.g., CYP3A4) and presumably transporters (e.g., ABCB1). In the case of CYP3A4, the observed disease-associated finding could potentially impact intestinal drug biotransformation, and the rate/extent of bioavailability, of perorally administered drugs. This disease effect could be quite dynamic in a given pediatric patient, as the extent of inflammation changes between the time of disease onset/flare and disease remission and mucosal repair. Given apparent age-related differences in biotransformation of certain CYP3A4 substrates (e.g., cyclosporine, tacrolimus, midazolam) (Kearns et al., 2003), ontogeny could also be an important covariate; although, we did not detect any age-related changes over the investigated age range (7–17yrs).
Second, our observation of decreased PXR expression in actively inflamed versus noninflamed tissue in patients with CD suggests that PXR may play a role in the disease phenotype expression of CD, accounting for the pathognomonic skip lesions characteristic of the disease. Potential mechanisms to support the role of PXR in CD pathogenesis can be inferred from animal studies that demonstrate a mutual and reciprocal repression between PXR and NF-κB (Ma et al., 2007; Shah et al., 2007; Zhou et al., 2006). Alternatively, given the recent implication of PXR activation in epithelial cell repair mechanisms via the p38 mitogen-activated protein kinase–dependent pathway in mice (Terc et al., 2014), our observed decrease in PXR (Fig. 1A) may be related to impaired epithelial barrier integrity thought to contribute to the development of IBD. This is supported by our observation of decreased VIL1 expression (marker of epithelial integrity) in the inflamed CD TI (Fig. 1B).
Although our mRNA data would be strengthened by protein studies, such studies could not be performed, as archived biopsy tissues of limited quantity were used and depleted during the gene experiments described above. A prospective study utilizing fresh biopsy tissue samples from children is underway to address this study limitation.
Herein, we provide preliminary evidence concerning a complex interplay between intestinal inflammation in pediatric patients with Crohn’s disease and the expression of PXR, VIL1, and CYP3A4 in the GI tract. Prospective studies will differentiate whether our observations have a structural (e.g., tissue damage/loss) or a mechanistic (e.g., disease modification) basis. In the meantime, our data raise the question of what happens to PXR, its target genes, epithelial cell repair mechanisms, and mucosal healing when PXR is exogenously activated by ligands. Adult studies exploring the utility of PXR agonists (e.g., rifaximin) in the treatment of IBD are ongoing (Prantera et al., 2012). Further exploration of PXR’s potential as a novel/adjunct therapeutic target in pediatric Crohn’s disease is warranted.
Abbreviations
- CD
Crohn’s disease
- CYP3A4
cytochrome p450 3A4
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- PXR
pregnane X receptor
- TI
terminal ileum
- VIL1
villin 1
Authorship Contributions
Participated in research design: Shakhnovich, Vyhlidal, Friesen, Daniel, Leeder.
Conducted experiments: Shakhnovich, Vyhlidal, Hildreth, Singh.
Contributed new reagents or analytic tools: Shakhnovich, Vyhlidal, Singh.
Performed data analysis: Shakhnovich, Vyhlidal, Leeder, Kearns, Friesen.
Wrote or contributed to the writing of the manuscript: Shakhnovich, Vyhlidal, Friesen, Hildreth, Singh, Daniel, Kearns, Leeder.
Footnotes
This work was supported in part by a Pediatric/Developmental Clinical Pharmacology training grant from the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda MD [1T32HD069038-05].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, Krausz KW, Gonzalez FJ. (2010) Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther 335:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. (2003) Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167. [DOI] [PubMed] [Google Scholar]
- Leung Y, Heyman MB, Mahadevan U. (2011) Transitioning the adolescent inflammatory bowel disease patient: guidelines for the adult and pediatric gastroenterologist. Inflamm Bowel Dis 17:2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Shah YM, Guo GL, Wang T, Krausz KW, Idle JR, Gonzalez FJ. (2007) Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther 322:391–398. [DOI] [PubMed] [Google Scholar]
- Nolan T, Hands RE, Bustin SA. (2006) Quantification of mRNA using real-time RT-PCR. Nat Protoc 1:1559–1582. [DOI] [PubMed] [Google Scholar]
- Prantera C, Lochs H, Grimaldi M, Danese S, Scribano ML, Gionchetti P, Retic Study Group (Rifaximin-Eir Treatment in Crohn’s Disease) (2012) Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn’s disease. Gastroenterology 142:473–481.e4. [DOI] [PubMed] [Google Scholar]
- Sartor RB. (2006) Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3:390–407. [DOI] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. (2007) Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol 292:G1114–G1122. [DOI] [PubMed] [Google Scholar]
- Simms LA, Doecke JD, Walsh MD, Huang N, Fowler EV, Radford-Smith GL. (2008) Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn’s disease. Gut 57:903–910. [DOI] [PubMed] [Google Scholar]
- Terc J, Hansen A, Alston L, Hirota SA. (2014) Pregnane X receptor agonists enhance intestinal epithelial wound healing and repair of the intestinal barrier following the induction of experimental colitis. Eur J Pharm Sci 55:12–19. [DOI] [PubMed] [Google Scholar]
- Timsit YE, Negishi M. (2007) CAR and PXR: the xenobiotic-sensing receptors. Steroids 72:231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xie W, Krasowski MD. (2008) PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics 9:1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, et al. (2006) Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116:2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W and Tian Y (2006) Xenobiotic receptor meets NF-kappaB, a collision in the small bowel. Cell Metab 4:177–178. [DOI] [PubMed] [Google Scholar]