To the Editor
Asthma is characterized by airway inflammation and bronchial obstruction due to airway smooth muscle (ASM) contraction. However, the underlying basis of the ASM hyper-contractile state in asthma is not known. It remains equally unclear whether asthmatic ASM has an intrinsic (genetic or epigenetic) property of increased basal tone and enhanced contractile responses.1–5 Furthermore, current dogma suggests that any altered mechanical property of the smooth muscle, if it exists in the disease, is from airway inflammation. Here we sought to establish, using highly quantitative methods, whether the contractile state of ASM from asthmatics has an inflammation-independent component. We applied recently developed single-cell technologies to probe the mechanical properties of isolated, passaged, primary human ASM cells.6,7 This approach potentially avoids the interactions between airway epithelium and smooth muscle that is encountered in bronchial sections, the limited availability of fresh tissues from asthmatic and non-asthmatic donors, and the nonspecific effects of acute dissociation of ASM cells from other tissues in biopsies. This approach may also minimize the acute effects of drugs such as β-agonists that would be expected to be administered during attempts to treat a severe asthma exacerbation. The methods utilized, Fourier transform traction microscopy and magnetic twisting cytometry, can be performed on the living cells adhered to matrices of varying rigidities across a pathophysiologic spectrum.
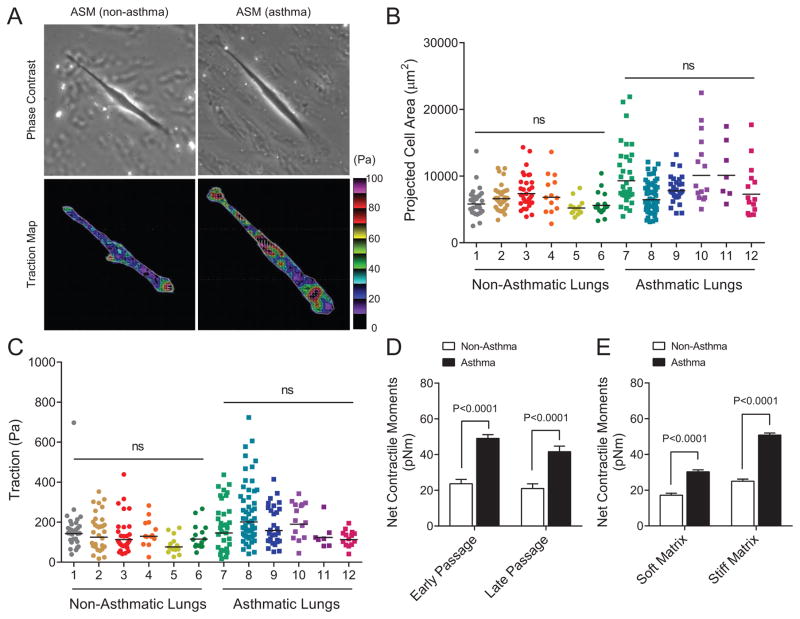
As shown in Fig. 1a, we employed Fourier transform traction microscopy to measure ASM mechanics, with direct measurement of traction stress, and the derived net contractile moments, so as to characterize the physical properties of human ASM cells derived from donor lungs from asthmatic and non-asthmatic patients (Table E1 in the Online Repository shows subject demographics). For these studies, we used cells that had been passaged in standard culture media, in the absence of inflammatory mediators, and studied them under identical experimental conditions using tuned elastic matrices (i.e. mimicking the physiological extremes of airway wall rigidity). Human ASM cells isolated from both sets of donor lungs showed the expected between-cell and -donor variation in cell spreading (Fig. 1b) and traction (root mean square) averaged over the entire cell projected area (Fig. 1c). Nested design analysis8 revealed significant differences in both the projected area (7779 ± 303 vs 6345 ± 199 μm2; P=0.0136, nested ANOVA) and the average traction stress (166 ± 10 vs 119 ± 8 Pa; P=0.0316, nested ANOVA) between asthmatics and non-asthmatics; there were no within group differences. In particular, compared with non-asthmatic ASM, asthmatic ASM showed an approximately twofold higher (47.4 ± 4.8 vs 26.4 ± 2.4 pNm; P=0.0015, nested ANOVA) net contractile moment, which is a scalar measure of the cell’s resting contractile amplitude. These differences in physical force generation between asthmatics and non-asthmatics were maintained in culture with increasing passage number (Fig. 1d), and across the range of matrix rigidity of ~1–8 kPa (Fig. 1e). These results establish an unequivocal difference in resting force of cultured ASM cells between asthmatic and non-asthmatic, which is persistent and is apparent across a wide range of matrix rigidities mimicking that of healthy and diseased airways–in the absence of the inflammatory airway milieu.
Figure 1.
Single-cell analyses on the identity and state of ASM mechanics as measured by Fourier transform traction microscopy. (A) Representative traction maps of single human ASM cells obtained from non-asthma and asthma lung donors. The white lines show the cell boundary, and the colors show the magnitude of the traction in Pascal (Pa) indexed to the color bar at the right. Arrows represent the direction and relative magnitude of the tractions. Scale bars represent 50 microns. (B and C) cell projected area and traction of isolated human ASM cells (non-asthma, n = 134; asthma, n = 154 individual cell measurements). For these studies, cells were derived from donor lungs of 6 non-asthmatics and 6 asthmatics (Table E1), and plated onto an inert elastic gel (8 kPa) coated with type I collagen. Bars are the geometric means of the respective lung donors. (D) Net contractile moments of human ASM cells in terms of early (passages 1–4: non-asthmatics, n = 69; asthmatics, n = 81) versus late (passages 6–11: non-asthmatics, n = 65; asthmatics, n = 73). (E) Net contractile moments of human ASM cells measured on the relatively soft (1 kPa: non-asthmatics, n = 43; asthmatics, n = 46) versus the relatively hard (8 kPa: non-asthmatics, n = 34; asthmatics, n = 28) elastic gels. Data are presented as geometric mean ± SE.
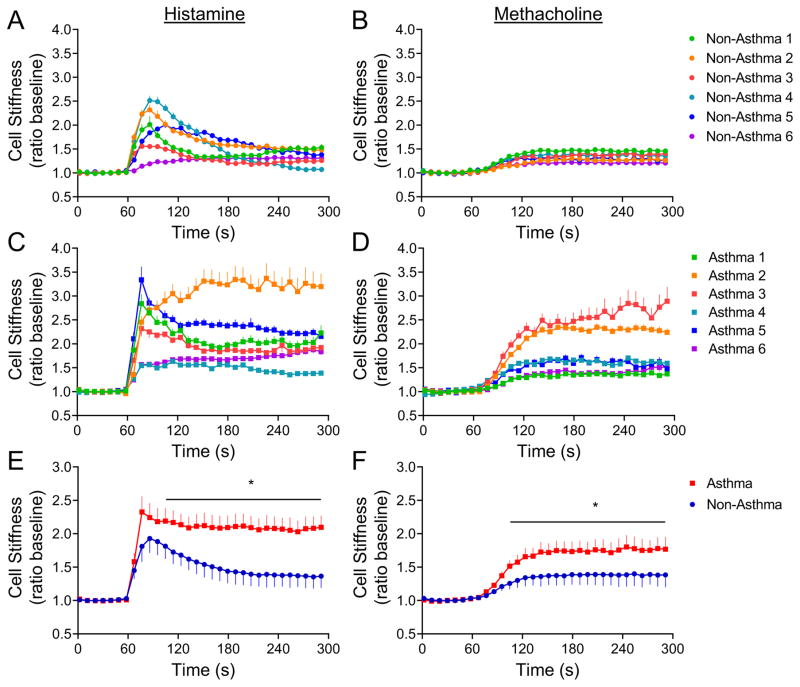
To ascertain if asthmatic ASM cells display increased responsiveness to locally generated spasmogens, we then measured dynamic changes in cytoskeleton stiffness in response to histamine and methacholine with magnetic twisting cytometry, as an index of single-cell contractility.6,7 Here, RGD-coated ferrimagnetic beads are attached to ASM cell surface integrin receptors and subjected to an external magnetic field, with measurement of lateral displacement of the beads during drug exposure (see Methods). For these experiments, we used primary cells derived from 12 additional lungs of asthmatics and non-asthmatics (Table E2). Based on the dose-response relationships (Fig. E1), and previous experience using isolated human ASM cells, herein we chose a single dose (10μM) of histamine or methacholine to contract the cells (Fig. 2). There was heterogeneity in the responses from both non-asthmatic and asthmatic cells, although the latter showed greater within-group variability in both the responses to histamine (Fig. 2a,c) and methacholine (Fig. 2b,d). Using the nested effect model8 to control for random effects from multiple cells from the same donor, and the repeated measurements, we found significant differences in the cell stiffening response to histamine (Fig. 2e) and methacholine (Fig. 2f) between asthmatics and non-asthmatics. These series of studies, taken together, establish for the first time that, in the resting state, asthmatic ASM has increased basal tone and enhanced contractility to known asthmatic spasmogens.
Figure 2.
Asthmatic ASM exhibits increased reactivity than non-asthmatic ASM as measured by magnetic twisting cytometry. Dynamic changes in cell stiffness in response to 10μM histamine (left) and 10μM methacholine (right) of ASM derived from individual non-asthma (A and B) and asthma (C and D) lung donors. Cells were derived from 12 additional donor lungs (Table E2). For each individual human ASM cell, baseline stiffness was measured for the first 60 s, and after drug addition stiffness was measured continuously for the next 240 s. For each cell, stiffness was normalized to its baseline stiffness prior to the agonist stimulation. Data are presented as mean ± SE (n = 68–562 individual cell measurements per donor lung). (E and F) Nested model shows increased cell stiffening response to spasmogens in asthmatic ASM than nonasthmatic ASM. Data are presented as mean ± SE from 6 asthma and 6 non-asthma donor lungs.
Almost 150 years ago Henry Hyde Salter9 posited that ‘the vice in asthma consists, not in the production of any special irritant, but in the irritability of the part irritated.’ Defining the asthmatic ASM mechanical phenotype, however, has been somewhat elusive.1–5 We hypothesized that an asthmatic mechanical phenotype of ASM, if it existed, would be intrinsic to asthmatic cells, and thus allow us to study cultured ASM in the absence of inflammatory mediators, drugs used in the treatment of asthma such as corticosteroids, β-agonists, and antagonists to histamine and acetylcholine receptors, or influences from the epithelium. Our results from the current study indicate that asthmatic ASM cells have increased cell traction forces at baseline, and enhanced stiffening (contraction) in response to activation of physiologically relevant G protein-coupled receptors, the M3-muscarinic receptor and the H1-histamine receptors, than non-asthmatic ASM cells. We thus conclude that ASM from asthmatics has intrinsic mechanical properties that are hard-wired to the development of AHR. These phenotypes are presumably from genetic or epigenetic mechanisms. To date, we have not been successful in ascertaining the distinct polymorphisms or methylation-specific variants that are common across our group of ASM cells derived from asthmatic subjects that might account for this mechanical phenotype (data not shown). As might be expected, the number of variants found dictates that a much larger number of ASM samples are needed to infer statistical significance after correcting for multiple comparisons. Lastly, we have not addressed any additional contribution to ASM phenotypes that arise from airway inflammation, and indeed we contend that asthma susceptibility and exacerbations are dependent on both inflammation-dependent and independent mechanisms.
Supplementary Material
A short summary: ‘for a clinically oriented audience’.
Patients with asthma typically experience periodic or persistent decreases in airflow from bronchospasm, and virtually all patients exhibit an exaggerated bronchoconstrictive response to exogenously administered agents such as histamine and methacholine, termed airway hyperresponsiveness (AHR). Clinically, this hyperresponsiveness may represent the physiological outcome of an enhanced airway smooth muscle (ASM) contractile sensitivity, which can lead to asthma exacerbations from a variety of stimuli such as exercise, cold air, allergens, air pollution, and viral infections. However, whether ASM from asthmatics is intrinsically more responsive to such stimulation (more “twitchy”) has not been established at the level of isolated smooth muscle. Here we show for the first time asthmatic ASM cells have increased basal tone and enhanced contractile responses that are independent of immune inflammatory responses. These results strongly support the concept of a distinct set of inflammation-independent ASM mechanical properties, which are hard-wired to the development of AHR, and point to genetic and/or epigenetic forces driving contraction mechanophenotype in asthma.
Acknowledgments
Funding: This work was supported by US National Heart, Lung, and Blood Institute grants: HL107361 (to S.S.A.); HL114471 (to S.S.A., S.B.L., R.A.P.); HL097805 (to J.S); and HL45967 (to S.B.L.). S.S.A was also supported by American Asthma Foundation (Sandler: 108183) grant.
Tissues for some of our studies were derived from the Gift of Hope Organ & Tissue Donor Network donor families; we thank them for their selfless gift.
Footnotes
Competing financial interests: The authors declare no competing financial interests.
Supplementary Information accompanies this paper in the Online Repository.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven S. An, Email: san3@jhu.edu.
Stephen B. Liggett, Email: sliggett@health.usf.edu.
References
- 1.de Jongste JC, Mons H, Bonta IL, Kerrebijin KF. In vitro responses of airways from an asthmatic patient. Eur J Respir Dis. 1987;71:23–29. [PubMed] [Google Scholar]
- 2.Bai TR. Abnormalities in airway smooth muscle in fatal asthma. Am Rev Respir Dis. 1990;141:552–557. doi: 10.1164/ajrccm/141.3.552. [DOI] [PubMed] [Google Scholar]
- 3.Bjorck T, Gustafsson LE, Dahlen SE. Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberating of leukotrienes and histamine. Am Rev Respir Dis. 1992;145:1087–1091. doi: 10.1164/ajrccm/145.5.1087. [DOI] [PubMed] [Google Scholar]
- 4.Chin LY, Bossé Y, Pascoe C, Hackett TL, Seow CY, Paré PD. Mechanical properties of asthmatic airway smooth muscle. Eur Respir J. 2012;40:45–54. doi: 10.1183/09031936.00065411. [DOI] [PubMed] [Google Scholar]
- 5.Ijpma G, Kachmar L, Matusovsky OS, Bates JH, Benedetti A, Martin JG, Lauzon AM. Human trachealis and main bronchi smooth muscle are normoresponsive in asthma. Am J Respir Crit Care Med. 2015;191:884–893. doi: 10.1164/rccm.201407-1296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol. 2006;35:55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande DA, Wang WC, Mcllmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krzywinski M, Altman N, Blainey P. Points of significance: nested designs. Nat Methods. 2014;11:977–978. doi: 10.1038/nmeth.3137. [DOI] [PubMed] [Google Scholar]
- 9.Salter HH. Asthma: Its Pathology and Treatment. 2. London: Churchill; 1868. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.