Significance
Arc lavas from Martinique have nonmidocean ridge basalt Mg isotopic composition, which is consistent with the incorporation of subducted Mg. This is, to our knowledge, the first report of mantle-derived lavas with Mg isotopic composition heavier than oceanic basalts, heretofore shown to be isotopically homogenous. More importantly, our results provide insight into the strongly debated origins of Martinique arc lavas and suggest that contributions of Mg from fluids supplied by the subducted slab may play a significant control in the Mg isotopic systematics of arc lavas.
Keywords: magnesium isotopes, arc magmatism, mantle wedge, Lesser Antilles arc, Martinique Island
Abstract
Incorporation of subducted slab in arc volcanism plays an important role in producing the geochemical and isotopic variations in arc lavas. The mechanism and process by which the slab materials are incorporated, however, are still uncertain. Here, we report, to our knowledge, the first set of Mg isotopic data for a suite of arc lava samples from Martinique Island in the Lesser Antilles arc, which displays one of the most extreme geochemical and isotopic ranges, although the origin of this variability is still highly debated. We find the δ26Mg of the Martinique Island lavas varies from −0.25 to −0.10, in contrast to the narrow range that characterizes the mantle (−0.25 ± 0.04, 2 SD). These high δ26Mg values suggest the incorporation of isotopically heavy Mg from the subducted slab. The large contrast in MgO content between peridotite, basalt, and sediment makes direct mixing between sediment and peridotite, or assimilation by arc crust sediment, unlikely to be the main mechanism to modify Mg isotopes. Instead, the heavy Mg isotopic signature of the Martinique arc lavas requires that the overall composition of the mantle wedge is buffered and modified by the preferential addition of heavy Mg isotopes from fluids released from the altered subducted slab during fluid−mantle interaction. This, in turn, suggests transfer of a large amount of fluid-mobile elements from the subducting slab to the mantle wedge and makes Mg isotopes an excellent tracer of deep fluid migration.
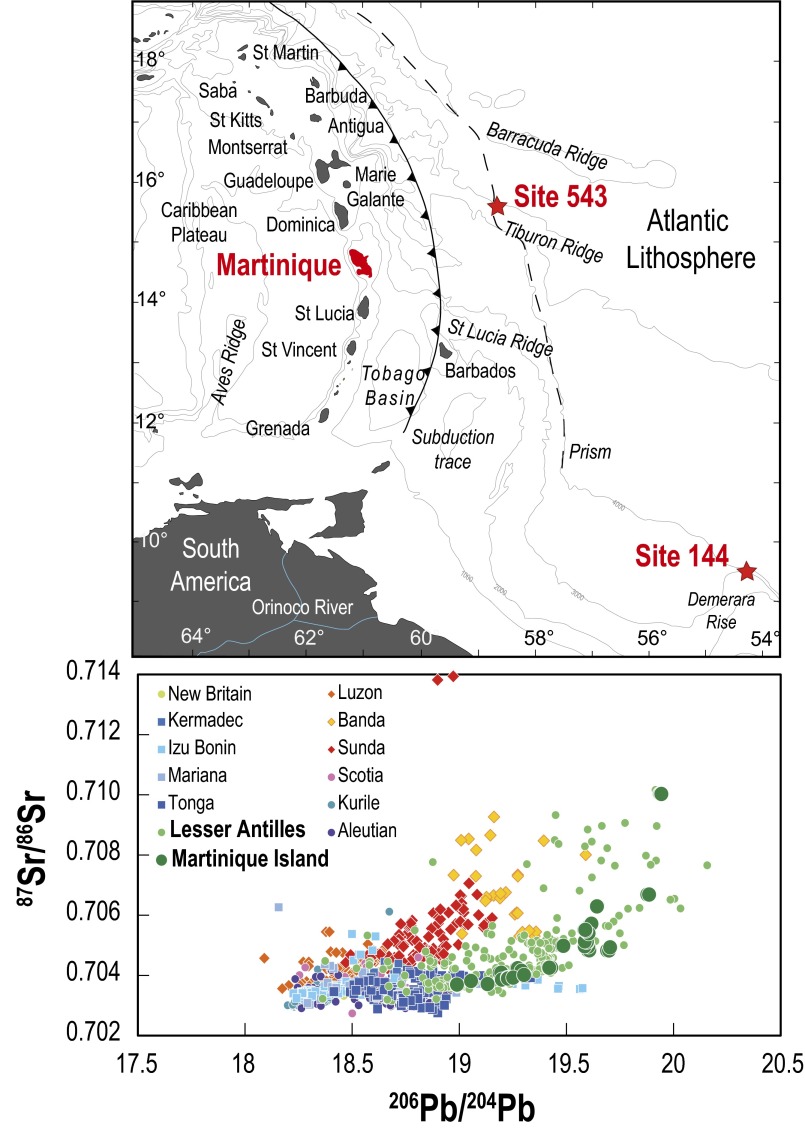
Arc volcanism records the elemental cycling between the subducting slab and subarc mantle. Of particular interest is the mechanism by which the subducted material is incorporated into the arc lava. Except for the rare case where arc lava is the direct melting product of a subducted slab (1), most scenarios suggest that mantle wedge is the major magma source that melts after being modified by fluids or melts derived from the subducted basalt and sediment (2, 3). In addition, processes such as polybaric crystallization and crustal assimilation can also modify the composition of arc magmas on their way to the surface. These different processes have different implications on subduction dynamics and elemental cycling, but, in many cases, they are difficult to distinguish. One of the best examples comes from studies of island arc lavas from the Lesser Antilles arc (Fig. 1). Geochemical and Sr, Nd, Pb, Hf, and Li isotopic studies suggest that the Lesser Antilles arc lavas incorporated a variable but to some extent significant amount of subducted sediments (4–8). However, the exact mechanism by which the sediment was incorporated into the lavas is still highly debated and involves various processes such as crustal contamination, subarc mantle metasomatism by fluids released from the slab, or melts derived by partial melting of the subducted sediments (4–17).
Fig. 1.
(Upper) Geological map of the Lesser Antilles island arc and the two DSDP sites (sites 144 and 543). (Lower) Comparison of Martinique Island basalts with other Lesser Antilles and worldwide island arcs in 87Sr/86Sr versus 206Pb/204Pb isotopic space (data compiled by Geochemistry of Rocks of the Oceans and Continents database). Modified from ref. 4 with permission from Elsevier; www.sciencedirect.com/science/journal/0012821X.
Magnesium isotopes have the potential to provide new and independent constraints on both source composition and processes operating during the formation of arc magmas, not only because Mg is a major element in all magmas but also because surficial and low-temperature processes fractionate Mg isotopes whereas high-temperature magmatic processes do not (18, 19) (Fig. 2). Subducted marine sediments and altered basalts have isotopic compositions different from those of the normal mantle as sampled by global peridotite xenoliths (Fig. 2); however, they generally have low Mg concentrations (18–25, *). In comparison, altered abyssal peridotites have Mg concentrations similar to the normal mantle whereas their Mg isotopic compositions are heavier because of the impact of hydrothermal circulation during accretion and residence in the deep ocean (Fig. 2).†,‡ Finally, although the mechanism is still not well understood, studies of a few arc peridotites show that they also have slightly heavier Mg isotopic composition than the normal mantle (Fig. 2). Given these observed ranges, Mg isotopes may help in understanding the relative contributions of crustal and mantle components to arc magmatism, but no systematic study of either continental or island arc lavas has been carried out yet.
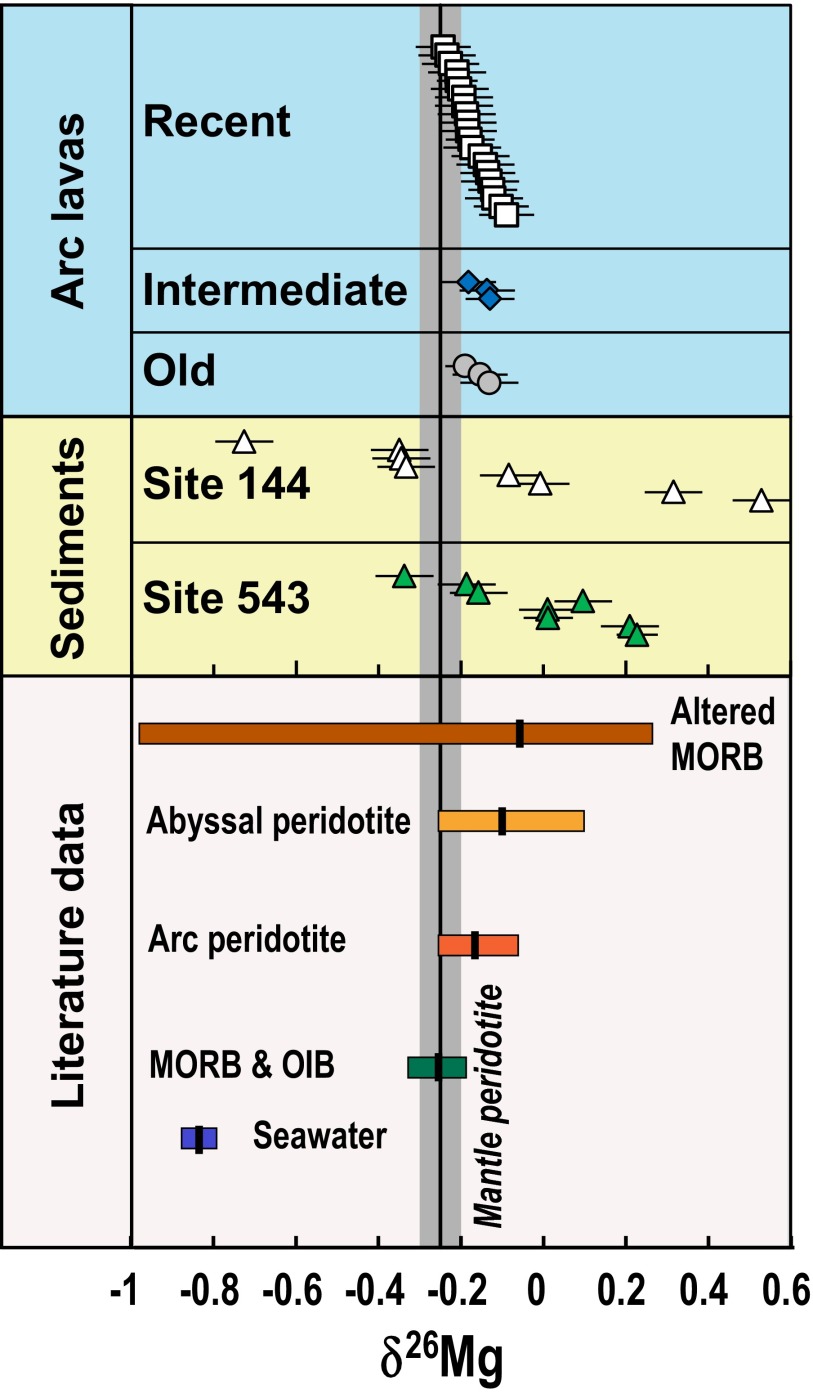
Fig. 2.
Magnesium isotopic composition of Martinique arc lavas and subducting forearc sediments (sites 144 and 543). (Data are reported in Tables S1 and S2.) Data sources for the other reservoirs are from several references (18, 22–24, 36, 42, 43,*,†,‡). The vertical solid line and gray bar represent the average δ26Mg and 2 SD of normal mantle, as sampled by global peridotite xenoliths (−0.25 ± 0.04) (18). The short bold black vertical lines represent the mean δ26Mg value of each individual reservoir.
Here, we report Mg isotopic data for 27 arc lavas and 17 subducting forearc sediment samples. The lava samples are from the Martinique Island and cover most of the chemical and isotopic variations in the Lesser Antilles arc (4, 5) (Fig. 1). The sediment samples are from Deep Sea Drilling Project (DSDP) sites 543 and 144 (NE and SE of Martinique Island, respectively); they cover the whole compositional spectrum of subducting sediments and range in lithology from chalky ooze to terrigenous and pelagic deposits (6, 7).
The sediments display a large range of δ26Mg (−0.76 to +0.52) with an average of −0.10 ± 0.61 (2 SD) (Table S1). This large variation is mainly controlled by sediment mineralogy, with carbonate-rich samples at site 144 generally having light Mg isotopic compositions, whereas clays, the dominant type of sediments at site 543, have heavy isotopic compositions (Fig. 2). This mineralogical control is also evident in studies of loess, shale, mudrock, and carbonates as well as leaching experiments that show preferential enrichment of light Mg isotopes in carbonates over silicates (26, 27).
Table S1.
Magnesium isotopic compositions of standards and subducting sediments from DSDP sites 144 and 543 (SE and NE of Martinique Island, respectively)
| Sample | Depth, m | MgO, wt% | LOI | 87Sr/86Sr | 143Nd/144Nd | 206Pb/204Pb | δ26Mg | 2 SD | δ25Mg | 2 SD |
| Standards | ||||||||||
| San Carlos olivine | −0.27 | 0.07 | −0.14 | 0.06 | ||||||
| Duplicate | −0.25 | 0.07 | −0.11 | 0.06 | ||||||
| Replicate | −0.29 | 0.09 | −0.14 | 0.06 | ||||||
| Replicate | −0.24 | 0.05 | −0.12 | 0.05 | ||||||
| Average | −0.26 | 0.05 | −0.13 | 0.03 | ||||||
| Hawaiian seawater | −0.86 | 0.07 | −0.43 | 0.06 | ||||||
| Duplicate | −0.82 | 0.07 | −0.44 | 0.05 | ||||||
| Replicate | −0.86 | 0.11 | −0.45 | 0.10 | ||||||
| Duplicate | −0.86 | 0.10 | −0.45 | 0.07 | ||||||
| Replicate | −0.87 | 0.07 | −0.45 | 0.05 | ||||||
| Duplicate | −0.84 | 0.07 | −0.43 | 0.05 | ||||||
| Duplicate | −0.88 | 0.10 | −0.46 | 0.07 | ||||||
| Duplicate | −0.88 | 0.09 | −0.45 | 0.06 | ||||||
| Duplicate | −0.84 | 0.06 | −0.44 | 0.04 | ||||||
| Average | −0.86 | 0.04 | −0.45 | 0.02 | ||||||
| Rec. value† | −0.83 | 0.09 | −0.43 | 0.06 | ||||||
| JB-1* | −0.27 | 0.07 | −0.14 | 0.05 | ||||||
| Duplicate | −0.27 | 0.09 | −0.12 | 0.06 | ||||||
| Duplicate | −0.25 | 0.06 | −0.13 | 0.04 | ||||||
| Average | −0.26 | 0.04 | −0.14 | 0.03 | ||||||
| Rec. value† | −0.27 | 0.10 | −0.15 | 0.04 | ||||||
| SCo-1* | −0.86 | 0.10 | −0.46 | 0.07 | ||||||
| Duplicate | −0.85 | 0.09 | −0.43 | 0.06 | ||||||
| Duplicate | −0.87 | 0.06 | −0.46 | 0.04 | ||||||
| Average | −0.86 | 0.05 | −0.45 | 0.03 | ||||||
| Rec. value† | −0.89 | 0.08 | −0.47 | 0.05 | ||||||
| SGR-1* | −1.03 | 0.11 | −0.54 | 0.10 | ||||||
| Rec. value† | −0.98 | 0.12 | −0.50 | 0.06 | ||||||
| DSDP site 144: South Antilles (9.454°N, 54.342°W) | ||||||||||
| 144A-2–2W-79–80.5* | −0.32 | 0.09 | −0.16 | 0.06 | ||||||
| Duplicate | −0.32 | 0.06 | −0.17 | 0.04 | ||||||
| Average | 41 | 0.88 | 36.7 | 0.708098 | 0.511942 | 19.3454 | −0.32 | 0.05 | −0.17 | 0.03 |
| 144–1-4W-98–99* | −0.78 | 0.11 | −0.40 | 0.10 | ||||||
| Duplicate | −0.76 | 0.06 | −0.39 | 0.04 | ||||||
| Average | 62 | 0.56 | 36.2 | 0.707944 | 0.511998 | 19.4381 | −0.76 | 0.05 | −0.39 | 0.03 |
| 144A-3–1W-79–80* | 0.53 | 0.11 | 0.27 | 0.10 | ||||||
| Duplicate | 0.52 | 0.06 | 0.26 | 0.04 | ||||||
| Average | 141 | 1.33 | 22.8 | 0.708335 | 0.511988 | 19.7053 | 0.52 | 0.05 | 0.26 | 0.03 |
| 144A-3–3W-125–126* | −0.35 | 0.09 | −0.17 | 0.06 | ||||||
| Duplicate | −0.42 | 0.06 | −0.22 | 0.04 | ||||||
| Duplicate | −0.37 | 0.09 | −0.20 | 0.06 | ||||||
| Duplicate | −0.41 | 0.06 | −0.21 | 0.04 | ||||||
| Duplicate | −0.42 | 0.06 | −0.22 | 0.04 | ||||||
| Average | 144 | 0.52 | 36.5 | 0.708301 | 0.511780 | 19.6460 | −0.40 | 0.03 | −0.21 | 0.02 |
| 144–3-1W-120–121* | 0.32 | 0.11 | 0.18 | 0.10 | ||||||
| Duplicate | 0.30 | 0.06 | 0.14 | 0.04 | ||||||
| Average | 163 | 0.75 | 30.3 | 0.708694 | 0.511730 | 20.0424 | 0.30 | 0.05 | 0.15 | 0.03 |
| 144A-6–1W-125–130 | −0.23 | 0.06 | −0.12 | 0.04 | ||||||
| Duplicate | −0.25 | 0.07 | −0.13 | 0.05 | ||||||
| Average | 190 | 0.56 | 37.7 | 0.707904 | 0.511840 | 22.9249 | −0.24 | 0.05 | −0.13 | 0.03 |
| 144–6-1W-46–48* | −0.41 | 0.09 | −0.20 | 0.06 | ||||||
| Duplicate | −0.38 | 0.07 | −0.19 | 0.05 | ||||||
| Duplicate | −0.40 | 0.09 | −0.21 | 0.06 | ||||||
| Duplicate | −0.40 | 0.06 | −0.21 | 0.04 | ||||||
| Average | 295 | 1.02 | 13.5 | 0.709158 | 0.512101 | 19.1781 | −0.39 | 0.04 | −0.20 | 0.03 |
| 144–7-1W-80–82* | −0.04 | 0.07 | −0.03 | 0.06 | ||||||
| Duplicate | −0.04 | 0.07 | −0.02 | 0.05 | ||||||
| Duplicate | −0.03 | 0.07 | −0.01 | 0.05 | ||||||
| Average | 299 | 2.03 | 11.5 | 0.710830 | 0.512105 | 18.8081 | −0.04 | 0.04 | −0.02 | 0.03 |
| 144–7-1W-125–130 | 299 | 1.63 | 12.2 | 0.709141 | 0.512105 | 18.9347 | −0.16 | 0.07 | −0.08 | 0.06 |
| DSDP site 543: North Antilles (15.712°N, 58.654°W) | ||||||||||
| 543–23-2W-73–75 | 0.04 | 0.07 | 0.03 | 0.05 | ||||||
| Duplicate | 0.03 | 0.07 | 0.02 | 0.05 | ||||||
| Duplicate | 0.03 | 0.07 | 0.01 | 0.05 | ||||||
| Duplicate | 0.03 | 0.05 | 0.01 | 0.05 | ||||||
| Average | 220 | 1.96 | 15.1 | 0.718270 | 0.511953 | 19.4023 | 0.03 | 0.03 | 0.02 | 0.03 |
| 543–26-1W-120–122* | −0.19 | 0.09 | −0.08 | 0.06 | ||||||
| Duplicate | −0.13 | 0.07 | −0.07 | 0.05 | ||||||
| Duplicate | −0.18 | 0.07 | −0.09 | 0.05 | ||||||
| Duplicate | −0.15 | 0.07 | −0.08 | 0.05 | ||||||
| Duplicate | −0.17 | 0.07 | −0.09 | 0.03 | ||||||
| Average | 248 | 2.17 | 12.1 | 0.717401 | 19.1260 | −0.16 | 0.03 | −0.08 | 0.02 | |
| 543–29-4W-45–47* | 0.11 | 0.09 | 0.06 | 0.06 | ||||||
| Replicate* | 0.09 | 0.10 | 0.05 | 0.07 | ||||||
| Duplicate | 0.10 | 0.07 | 0.05 | 0.05 | ||||||
| Average | 280 | 1.45 | 15 | 0.719727 | 0.511947 | 19.5323 | 0.10 | 0.05 | 0.05 | 0.03 |
| 543–31-1W-76–78* | 0.01 | 0.09 | 0.01 | 0.06 | ||||||
| Duplicate | 0.00 | 0.07 | 0.01 | 0.05 | ||||||
| Duplicate | 0.00 | 0.07 | −0.01 | 0.05 | ||||||
| Average | 295 | 1.67 | 15.2 | 0.718082 | 0.511880 | 19.5283 | 0.00 | 0.04 | 0.00 | 0.03 |
| 543–33-2W-71–73* | −0.13 | 0.07 | −0.05 | 0.06 | ||||||
| Replicate* | −0.13 | 0.11 | −0.06 | 0.10 | ||||||
| Duplicate | −0.15 | 0.07 | −0.08 | 0.05 | ||||||
| Duplicate | −0.15 | 0.07 | −0.08 | 0.05 | ||||||
| Duplicate | −0.15 | 0.07 | −0.07 | 0.03 | ||||||
| Average | 315 | 2.63 | 13.7 | 0.716811 | 0.511918 | 19.1515 | −0.14 | 0.03 | −0.07 | 0.02 |
| 543A-5–3W-47–49* | 0.19 | 0.07 | 0.11 | 0.05 | ||||||
| Duplicate | 0.25 | 0.07 | 0.13 | 0.05 | ||||||
| Replicate* | 0.18 | 0.09 | 0.09 | 0.06 | ||||||
| Duplicate | 0.20 | 0.07 | 0.10 | 0.05 | ||||||
| Duplicate | 0.24 | 0.07 | 0.13 | 0.05 | ||||||
| Duplicate | 0.23 | 0.07 | 0.13 | 0.05 | ||||||
| Average | 364 | 3.07 | 14.6 | 0.722128 | 0.511936 | 19.2605 | 0.22 | 0.03 | 0.12 | 0.02 |
| 543A-8–1W-116–118* | −0.33 | 0.09 | −0.17 | 0.06 | ||||||
| Duplicate | −0.37 | 0.07 | −0.18 | 0.05 | ||||||
| Average | 390 | 4.94 | 19.7 | 0.731781 | 0.511965 | 19.3564 | −0.36 | 0.05 | −0.18 | 0.04 |
| 543A-10–1W-25–27* | 0.06 | 0.09 | 0.04 | 0.06 | ||||||
| Duplicate | 0.05 | 0.06 | 0.02 | 0.04 | ||||||
| Average | 408 | 2.38 | 28.8 | 0.709340 | 0.512077 | 19.1270 | 0.05 | 0.05 | 0.03 | 0.03 |
The depth, MgO, LOI, and Sr, Nd, Pb isotopic data are from Carpentier et al. (6, 7); 2 SD = 2 times the SD of the population of n (n > 20) repeated measurements of the standards during an analytical session. Duplicate refers to repeated measurement of Mg isotopic ratios on the same purified Mg cuts at different days. Replicate refers to repeated column chemistry and measurement of different aliquots of a stock solution. The average value and associated 2 SD are error-weighted values calculated by Isoplot.
Data that were measured on a Nu Plasma HR-MC-ICP-MS; all other data were measured on a Nu Plasma II MC-ICP-MS.
Rec. value is recommended value, from Teng et al. (44).
The δ26Mg values of Martinique lavas define a smaller range from −0.25 to −0.10, and are, on average (−0.18 ± 0.07, 2 SD) (Fig. 2), higher than midocean ridge basalt (MORB) (δ26Mg = −0.25 ± 0.06, 2 SD) and mantle peridotite (δ26Mg = −0.25 ± 0.04 2 SD) (18, 23, 28, 29). This difference indicates that the source of Martinique lavas is different from that of MORB, which could be related to a diversity of processes that include seawater alteration for submarine lavas, melting of a mantle source with different δ26Mg, or crustal contamination during magma ascent.
Chemical weathering and seawater alteration can potentially modify the Mg isotopic composition of arc basalts, and can shift their δ26Mg to higher values if clays are the dominant alteration products (24). However, the analyzed lava samples are all fresh [loss on ignition (LOI) < 2% with one exception; Table S2], and most erupted above sea level (4, 5). A previous Li isotopic study on the same suite of samples has shown that only the three samples that erupted as submarine lava have high δ7Li due to interaction with isotopically heavy seawater (8). These three samples, however, have Mg isotopic compositions similar to the other samples. In addition, δ26Mg of Martinique arc lavas does not correlate with their LOI. Therefore, different from Li isotopes, interaction with seawater has little effect on the δ26Mg. The different behavior between Li and Mg isotopes likely reflects the higher concentration of Mg over Li in basalts, which results in an easier isotopic fractionation of Li than Mg during weathering and alteration.
Table S2.
Magnesium isotopic compositions of arc lavas from Martinique Island, Lesser Antilles arc
| Sample | Age, ka | MgO, wt% | LOI | 87Sr/86Sr | 143Nd/144Nd | 206Pb/204Pb | δ26Mg | 2 SD | δ25Mg | 2 SD |
| Recent arc | ||||||||||
| 06MT50* | −0.21 | 0.09 | −0.12 | 0.06 | ||||||
| Duplicate | −0.22 | 0.08 | −0.12 | 0.05 | ||||||
| Duplicate | −0.23 | 0.07 | −0.12 | 0.03 | ||||||
| Average | 1.929 | 2.27 | 0.23 | 0.704269 | 0.512744 | 19.4160 | −0.22 | 0.04 | −0.12 | 0.03 |
| 06MT51* | −0.19 | 0.09 | −0.10 | 0.06 | ||||||
| Duplicate* | −0.19 | 0.10 | −0.09 | 0.07 | ||||||
| Duplicate | −0.21 | 0.09 | −0.13 | 0.06 | ||||||
| Duplicate | −0.25 | 0.06 | −0.13 | 0.04 | ||||||
| Duplicate | −0.21 | 0.06 | −0.10 | 0.04 | ||||||
| Average | 1.929 | 2.09 | −0 | 0.704261 | 0.512766 | 19.4235 | −0.22 | 0.03 | −0.11 | 0.02 |
| 06MT40* | 189 | 2.49 | 0.82 | 0.704014 | 0.512832 | 19.3014 | −0.23 | 0.07 | −0.10 | 0.07 |
| 06MT37 | −0.19 | 0.09 | −0.09 | 0.06 | ||||||
| Duplicate | −0.19 | 0.06 | −0.10 | 0.04 | ||||||
| Average | 322 | 4.36 | 1.12 | 0.704986 | 0.512426 | 19.4802 | −0.19 | 0.05 | −0.10 | 0.03 |
| 04MT07* | 341 | −0.15 | 0.07 | −0.06 | 0.06 | |||||
| 06MT18* | 346 | 2.78 | 1.03 | 0.703931 | 0.512853 | 19.2506 | −0.19 | 0.07 | −0.12 | 0.06 |
| 06MT28* | 543 | 2.58 | 1.2 | 0.703901 | 0.512882 | 19.2263 | −0.20 | 0.07 | −0.07 | 0.06 |
| IAR* | 640 | 12.4 | −0 | 0.703821 | 0.512951 | 19.0545 | −0.19 | 0.07 | −0.09 | 0.06 |
| 06MT21* | −0.20 | 0.07 | −0.08 | 0.07 | ||||||
| Duplicate* | −0.22 | 0.07 | −0.11 | 0.05 | ||||||
| Average | 893 | 1.9 | 0.92 | 0.705718 | 0.512410 | 19.6166 | −0.21 | 0.05 | −0.10 | 0.04 |
| 06MT36* | −0.13 | 0.07 | −0.07 | 0.07 | ||||||
| Duplicate | −0.13 | 0.07 | −0.08 | 0.05 | ||||||
| Duplicate | −0.13 | 0.09 | −0.06 | 0.06 | ||||||
| Duplicate | −0.12 | 0.08 | −0.04 | 0.05 | ||||||
| replicate | −0.16 | 0.07 | −0.08 | 0.05 | ||||||
| Duplicate | −0.15 | 0.06 | −0.07 | 0.04 | ||||||
| Duplicate | −0.13 | 0.08 | −0.06 | 0.05 | ||||||
| Average | 998 | 2.01 | 1.55 | 0.706307 | 0.512293 | 19.6448 | −0.14 | 0.03 | −0.07 | 0.02 |
| 06MT61* | 1,175 | 7.9 | 0.1 | 0.703716 | 0.512818 | 19.1318 | −0.20 | 0.07 | −0.12 | 0.06 |
| 06MT55* | 1,332 | 3.02 | 1.19 | 0.704157 | 0.512782 | 19.2893 | −0.18 | 0.07 | −0.07 | 0.06 |
| 06MT14* | 1,530 | 3.69 | 0.99 | 0.705131 | 0.512616 | 19.5857 | −0.17 | 0.07 | −0.10 | 0.06 |
| 06MT19* | −0.24 | 0.07 | −0.10 | 0.07 | ||||||
| Duplicate* | −0.21 | 0.07 | −0.09 | 0.05 | ||||||
| Average | 1,750 | 2.84 | 1.4 | 0.705120 | 0.512612 | 19.5904 | −0.22 | 0.05 | −0.10 | 0.04 |
| 06MT04* | −0.13 | 0.07 | −0.07 | 0.06 | ||||||
| Duplicate | −0.13 | 0.09 | −0.06 | 0.06 | ||||||
| Average | 1,870 | 2.02 | 1.33 | 0.704972 | 0.512629 | 19.5847 | −0.13 | 0.06 | −0.07 | 0.04 |
| 06MT10* | −0.13 | 0.07 | −0.04 | 0.06 | ||||||
| Duplicate | −0.16 | 0.09 | −0.08 | 0.06 | ||||||
| Average | 2,111 | 2.94 | 1.85 | 0.705098 | 0.512558 | 19.5970 | −0.14 | 0.05 | −0.05 | 0.04 |
| 06MT13* | 2,550 | 3.08 | 1.17 | 0.704960 | 0.512650 | 19.6018 | −0.10 | 0.07 | −0.06 | 0.06 |
| 06MT30* | 3,010 | 3.66 | 3.1 | 0.704836 | 0.512627 | 19.5997 | −0.24 | 0.07 | −0.13 | 0.06 |
| 06MT34* | −0.15 | 0.07 | −0.06 | 0.06 | ||||||
| Duplicate | −0.15 | 0.09 | −0.08 | 0.06 | ||||||
| Average | 4,100 | 2.33 | 0.64 | 0.703920 | 0.512910 | 19.2247 | −0.15 | 0.06 | −0.07 | 0.04 |
| 06MT23* | −0.20 | 0.07 | −0.11 | 0.05 | ||||||
| Duplicate | −0.19 | 0.08 | −0.10 | 0.05 | ||||||
| Replicate* | −0.19 | 0.11 | −0.12 | 0.10 | ||||||
| Average | 4,863 | 3.29 | 1.16 | 0.703901 | 0.512921 | 19.2318 | −0.20 | 0.05 | −0.11 | 0.03 |
| 06MT32 | −0.20 | 0.07 | −0.09 | 0.05 | ||||||
| Duplicate | −0.18 | 0.09 | −0.09 | 0.06 | ||||||
| Duplicate | −0.20 | 0.06 | −0.10 | 0.04 | ||||||
| Average | 5,130 | 3.3 | 0.7 | 0.703867 | 0.512941 | 19.1985 | −0.20 | 0.04 | −0.10 | 0.03 |
| Intermediate arc | ||||||||||
| 06MT60* | 8,760 | 2.75 | 1.29 | 0.706689 | 0.512318 | 19.8770 | −0.18 | 0.07 | −0.09 | 0.06 |
| 06MT71 | −0.16 | 0.07 | −0.06 | 0.05 | ||||||
| Duplicate | −0.17 | 0.09 | −0.09 | 0.06 | ||||||
| Duplicate | −0.12 | 0.06 | −0.06 | 0.04 | ||||||
| Duplicate | −0.12 | 0.08 | −0.04 | 0.05 | ||||||
| Replicate* | −0.14 | 0.11 | −0.08 | 0.10 | ||||||
| Duplicate | −0.13 | 0.06 | −0.06 | 0.04 | ||||||
| Average | 10,300 | 3.8 | 0.93 | 0.704936 | 0.512806 | 19.7037 | −0.14 | 0.03 | −0.06 | 0.02 |
| 06MT69* | 10,640 | 5.25 | 1.95 | 0.704831 | 0.512837 | 19.6896 | −0.14 | 0.07 | −0.06 | 0.06 |
| Old arc | ||||||||||
| 06MT54* | 20,800 | 4.76 | 1.23 | 0.704092 | 0.512973 | 19.1876 | −0.15 | 0.07 | −0.11 | 0.06 |
| 06MT53* | −0.13 | 0.07 | −0.05 | 0.06 | ||||||
| Duplicate | −0.13 | 0.06 | −0.06 | 0.04 | ||||||
| Average | 23,400 | 2.85 | 1.36 | 0.704014 | 0.512967 | 19.1900 | −0.13 | 0.05 | −0.06 | 0.03 |
| 06MT68* | −0.21 | 0.07 | −0.10 | 0.07 | ||||||
| Duplicate* | −0.17 | 0.07 | −0.07 | 0.06 | ||||||
| Average | 24,800 | 6.47 | 1.47 | 0.703701 | 0.513030 | 18.9866 | −0.19 | 0.05 | −0.08 | 0.04 |
The age, MgO, LOI, and Sr, Nd, Pb isotopic data are from Labanieh et al. (4, 5); 2 SD is 2 times the SD of the population of n (n > 20) repeated measurements of the standards during an analytical session. Duplicate refers to repeated measurement of Mg isotopic ratios on the same purified Mg cuts at different days. Replicate refers to repeated column chemistry and measurement of different aliquots of a stock solution. The average value and associated 2 SD are error-weighted values calculated by Isoplot.
Data that were measured on a Nu Plasma HR-MC-ICP-MS; all other data were measured on a Nu Plasma II MC-ICP-MS.
Partial melting of a peridotite source and fractional crystallization of olivine, pyroxene, and plagioclase can be ruled out too, as these processes do not fractionate Mg isotopes (18, 28–30). Nonetheless, arc lavas could potentially be isotopically heavier than MORB if they were produced by partial melting of a subducted oceanic crust, with garnet as a residual phase, e.g., adakite (1). This is because garnet has much lower δ26Mg relative to coexisting pyroxene, as was observed in cratonic and orogenic eclogites (31–33). However, this cannot be the cause of the high δ26Mg values of Martinique lavas, because their chemical compositions are inconsistent with derivation from slab melting, i.e., adakite (4–7).
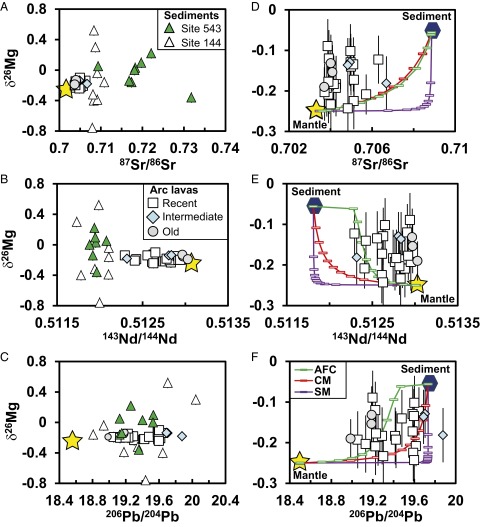
The forearc sediments that enter the Lesser Antilles Trench have, on average, a heavy Mg isotopic composition (−0.10 ± 0.61, 2 SD) (Fig. 2); they could thus be a potential source for the heavy Mg isotopic compositions of the Martinique lavas. Equivalent sediments in arc crust through which the Martinique lavas erupted could provide such a source, as well, if they were assimilated into the lavas. Furthermore, due to the lack of Mg isotope fractionation during prograde metamorphism (31, 33, 34), the metamorphic counterparts of the subducting sediments should preserve their original Mg isotopic signature. Previous isotopic studies of Martinique lavas show that the sedimentary input increases with age from old to intermediate lavas whereas it is much more variable in the recent lavas (4). However, Mg isotopic compositions of the Martinique lavas do not correlate with either age or any radiogenic isotopic system (Fig. 3), suggesting that the presence of heavy Mg is not caused by sediment addition to the subarc mantle source or directly to the lavas themselves. Furthermore, neither binary mixing between subarc mantle peridotite and sediments nor assimilation and fractional crystallization of arc magma can explain the data (Fig. 3). In all modeled mixing arrays, the amount of sediments required to account for the δ26Mg measured in the lavas is unrealistically high (>50%) due to the generally much lower Mg concentration in sediment (2–3%) compared with basalt (8%) and peridotite (48%) (25). Presence of such a large amount of sediment in a source producing basalts and andesites is impossible from a major element point of view. The opposite is true for elements such as Nd, Sr, Pb, or Li, which are drastically more enriched in sediment than in peridotite. In other words, a small addition of sedimentary materials into a peridotite or basalt can change their Nd, Sr, Pb, or Li isotopic compositions significantly, whereas a very large amount of sediment is required to change their Mg isotopic composition. The fact that δ26Mg varies little in Martinique arc lavas, whereas their Nd, Sr, and Pb isotopes change significantly, implies that (i) the peridotite in the mantle wedge has an unusual Mg isotopic composition and (ii) the impact of sedimentary material, if any, is invisible from the Mg isotope perspective because of the large concentration contrast.
Fig. 3.
Variations of Mg isotopic composition with Sr, Nd, and Pb isotopic compositions of Martinique arc lavas and subducting sediments from the Lesser Antilles arc (Tables S1 and S2). A−C include data of both Martinique arc lavas and subducting sediments, and D−F focus on the range observed in the lavas. The yellow star represents the hypothesized composition of the normal mantle. The hexagon represents an estimate of the average composition of subducting sediments (Tables S1–S3). D−F show mixing curves corresponding to three different types of mixing: Source mixing (SM) is depleted mantle−sediment mixing in the subarc mantle; crust mixing (CM) is primitive magma−sediment mixing; and AFC is assimilation and fractional crystallization. The ticks on modeling curves represent 10% increments during fractional crystallization or mixing. These mixing curves clearly do not fit the data measured on the Martinique arc lavas. A and D are 87Sr/86Sr, B and E are 143Nd/144Nd, and C and F are 206Pb/204Pb. See Table S3 for modeling details.
Table S3.
Elemental and isotopic compositions of the end members used for mixing and AFC models
| Element/isotopes | Depleted mantle | Primitive magma | Sediment | D value (AFC) |
| MgO, wt% | 37.8 | 7.95 | 2.36 | 3 |
| Sr, ppm | 15.5 | 178 | 220 | 3.2 |
| Nd, ppm | 0.713 | 5.5 | 22.6 | 0.22 |
| Pb, ppm | 0.02 | 1.34 | 7.9 | 0.61 |
| δ26Mg | −0.25 | −0.25 | −0.06 | |
| 87Sr/86Sr | 0.70346 | 0.70360 | 0.70887 | |
| 143Nd/144Sr | 0.51301 | 0.51301 | 0.51181 | |
| 206Pb/204Pb | 18.5 | 18.5 | 19.735 |
The MgO content of the depleted mantle is from McDonough and Sun (20). The MgO content of the sediment is from Plank (25), and MgO of the primitive magma is from Kelemen (3). The δ26Mg of the depleted mantle and primitive magma are from Teng et al. (18). The δ26Mg of the sediment is the MgO-weighted average of sediments measured in this study. The assimilation/crystallization ratio of 0.25, Sr, Nd, and Pb content and isotopic compositions of the primitive magma and crustal contaminant, as well as D values for Sr, Nd, and Pb, are from Labanieh et al. (4).
Our conclusions above are consistent with the few available Mg isotopic data for arc peridotites. Thus far, the only arc peridotites analyzed for Mg isotopes come from Avacha Volcano in Kamchatka, and they represent fragments of the subarc mantle that has been metasomatized by fluids released from the subducting Pacific plate (35). Their δ26Mg values vary from −0.25 to −0.06 with an average of −0.18 ± 0.10 (2 SD) (36), overlapping the Martinique arc lava range but significantly different from values reported for normal mantle peridotites (18) (Fig. 2). Although the mechanism responsible for the heavy Mg isotopic composition of these arc peridotites is uncertain, it is possible that fluids coming from the subducted slab could modify the peridotite present in the mantle wedge.
The few available δ26Mg data on altered MORB and abyssal peridotite are shown in Fig. 2. Abyssal peridotites are of particular interest because they have high Mg concentrations and their Mg isotopic compositions are on average heavier than fresh MORB (−0.25 to 0.10, with an average of −0.12 ± 0.14, 2 SD).†,‡ The most likely explanation for their high δ26Mg is that they were altered to various degrees by hydrothermal circulation during and after emplacement onto the seafloor.†,‡ As subducted altered basalts and abyssal peridotites contain large amounts of fluids (their LOI is in the order of 10 wt.%), they can be the source of vast volumes of Mg-rich fluids (37, 38) released to the mantle wedge during dehydration of the subducted slab. These fluids infiltrate the mantle wedge through fluid−peridotite interactions and modify its Mg isotopic composition toward a heavy value, which comprises the source of the arc lavas.
Our interpretation that fluid−peridotite interactions in the subarc mantle have shifted mantle wedge and Martinique lavas to heavy Mg isotopic composition is also consistent with other independent observations. Dehydration of the subducting slab has been called on to explain the high concentration of fluid-mobile elements in Martinique lava samples (5, 39) and, more generally, in a large number of island arcs (40). In addition, the Sr isotopic composition of Martinique lavas remains quite low, at about 0.7035, indicating that the leached material is basaltic or peridotitic rather than sedimentary (see ref. 5 for more details). It could, however, be argued that the large amount of fluids needed to modify the δ26Mg of the mantle wedge should also impact other geochemical parameters. For example, ratios of mobile to immobile trace elements should differ from normal mantle melts. This is indeed the case for Martinique lavas that have generally high Ba/Th and Pb/Ce ratios (85.3 ± 48.2 and 0.23 ± 0.06, 1 SD, respectively) relative to the values of MORB (71.93 ± 8.32 and 0.0402 ± 0.0016, 95% confidence, respectively) (41). However, no clear correlation exists between δ26Mg and Ba/Th or Pb/Ce ratios in our dataset, mainly reflecting the combined effects of low concentrations of incompatible trace elements in the dehydrated subducted slab and the residual mineralogy that could retain some trace elements during melting and fractionation processes.
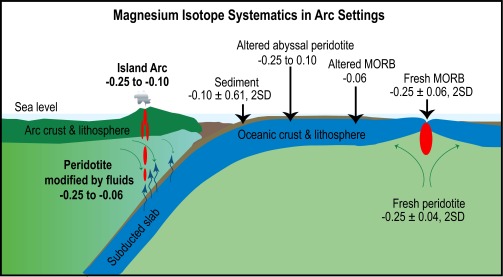
Our study shows, for the first time to our knowledge, that the Mg isotopic composition of some arc lavas differs from that of MORB. Although both crustal assimilation during magma ascent and sediment addition in the mantle wedge likely occur (4–17), neither of them can be the major process responsible for the Mg isotopic variation in Martinique lavas due to the large concentration contrast between sediment and arc lava or peridotite. Instead, the difference between Martinique lavas and MORB likely results from massive flux of dehydration fluids that leave the subducted oceanic plate to invade the mantle wedge and change its overall isotopic composition (Fig. 4). By combining Mg isotopes with radiogenic isotopes such as Sr, Nd, or Pb, a better picture of the processes occurring during arc genesis can be obtained: In contrast to trace elements that track down the presence of enriched sedimentary materials, a major element such as Mg may help pinpoint the role of subducted products in the overall composition of arc magmas.
Fig. 4.
Sketch of a subduction zone displaying the Mg isotopic compositions of the main components involved in arc magmatism. Seafloor alteration produces isotopically heterogeneous marine sediments, altered MORB, and altered abyssal peridotites. Subduction transports these components into the mantle. Fluids released from these components inherit the isotopically heterogeneous Mg, infiltrate the mantle wedge, and modify its Mg isotopic composition by fluid−rock interactions. Partial melting of this isotopically heterogeneous mantle wedge produced the heterogeneous island arc lavas. Data sources are the same as in Fig. 2.
Analytical Methods
All experiments were performed at the Isotope Laboratory of the University of Washington, Seattle. The detailed procedures for routine sample dissolution, column chemistry, and instrumental analysis of Mg isotopes have been reported in our previous publications (18, 29, 30, 44–48). Below, only a brief description is provided.
All samples were dissolved by using concentrated acids in the following sequence: (i) HF-HNO3, (ii) HNO3−HCl, (iii) HNO3, or (iv) HCl in Teflon vials on a hot plate. A few drops of concentrated HClO4 were also added in step i for samples containing organic materials. Separation of Mg was achieved by cation exchange chromatography, loaded with precleaned Bio-Rad AG 50W-X8 (200−400 mesh) resin. Magnesium was eluted in 1 N HNO3 media (18, 29, 30, 48). The purified sample solution was analyzed by using a Nu Plasma high-resolution–multicollector–inductively coupled plasma mass spectrometry (HR-MC-ICPMS) and/or Nu Plasma II MC-ICPMS with a “wet” plasma, consisting of a Cinnabar spray chamber and a MicroMist microuptake glass concentric nebulizer (45). Magnesium isotopic ratios were analyzed in the low-resolution mode, with 26Mg, 25Mg, and 24Mg measured simultaneously in separate Faraday cups.
Magnesium isotopic compositions were analyzed by the sample−standard bracketing method. The average value of the two bracketing standards is used to correct the sample analysis for instrumental mass fractionation. The isotopic ratios are then reported in δ-notation,
where X refers to mass 25 or 26 and standard refers to deep sea magnesium. The standard−sample sequences were repeated n times. The reported isotopic composition for a given sample is the average of n (≥2) repeat analyses. The 2 SD based on multiple analyses of standards is used here, as there are more standard analyses than samples during a batch run. The SD is thus better known and is more conservative (44).
The accuracy was checked by analysis of well-characterized standards, including San Carlos (SC) olivine, Hawaiian seawater, basalt standard JB-1, and shale standards SCo-1 and SGR-1, which were processed together with arc lava and sediment samples in the same chemical and analytical sessions. All of them agree with recommended values from previous studies (Table S1).
Acknowledgments
We thank Roberta Rudnick, Ming Tang, and Stu McCallum for helpful discussion, and Matt Jackson, an anonymous reviewer, and the editor for constructive and detailed comments, which greatly improved the quality of our manuscript. This work is financially supported by the National Science Foundation (EAR-1340160) and the Agence Nationale de la Recherche (ANR-10-BLAN-0603M&Ms).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Hu Y, Teng F-Z, Plank T, Huang K-J, AGU Fall Meeting, December 12−16, 2015, San Francisco, CA.
†Wimpenny J, Harvey J, Yin Q, AGU Fall Meeting, December 3−7, 2012, San Francisco, CA.
‡Liu P-P, Teng F-Z, Zhou M-F, Dick HJB, Goldschmidt Conference, June 26 through July 1, 2016, Yokohama, Japan.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518456113/-/DCSupplemental.
References
- 1.Castillo PR. An overview of adakite petrogenesis. Chin Sci Bull. 2006;51(3):257–268. [Google Scholar]
- 2.Ryan JG, Chauvel C. In: The Mantle. Carlson RW, editor. Vol 3. Elsevier; Oxford: 2014. pp. 1727–1741. [Google Scholar]
- 3.Kelemen PB, Hanghoj K, Greene AR. In: The Crust. Rudnick RL, editor. Vol 4. Elsevier; Oxford: 2014. pp. 749–805. [Google Scholar]
- 4.Labanieh S, Chauvel C, Germa A, Quidelleur X, Lewin E. Isotopic hyperbolas constrain sources and processes under the Lesser Antilles arc. Earth Planet Sci Lett. 2010;298(1-2):35–46. [Google Scholar]
- 5.Labanieh S, Chauvel C, Germa A, Quidelleur X. Martinique: A clear case for sediment melting and slab dehydration as a function of distance to the trench. J Petrol. 2012;53(12):2441–2464. [Google Scholar]
- 6.Carpentier M, Chauvel C, Mattielli N. Pb−Nd isotopic constraints on sedimentary input into the Lesser Antilles arc system. Earth Planet Sci Lett. 2008;272(1-2):199–211. [Google Scholar]
- 7.Carpentier M, Chauvel C, Maury RC, Mattielli N. The “zircon effect” as recorded by the chemical and Hf isotopic compositions of Lesser Antilles forearc sediments. Earth Planet Sci Lett. 2009;287(1-2):86–99. [Google Scholar]
- 8.Tang M, Rudnick RL, Chauvel C. Sedimentary input to the source of Lesser Antilles lavas: A Li perspective. Geochim Cosmochim Acta. 2014;144:43–58. [Google Scholar]
- 9.White WM, Patchett PJ. Hf-Nd-Sr isotopes and incompatible element abundances in island arcs: Implications for magma origins and crust-mantle evolution. Earth Planet Sci Lett. 1984;67(2):167–185. [Google Scholar]
- 10.White WM, Dupre B, Vidal P. Isotope and trace element geochemistry of sediments from the Barbados Ridge Demerara Plain region, Atlantic Ocean. Geochim Cosmochim Acta. 1985;49(9):1875–1886. [Google Scholar]
- 11.White WM, Dupre B. Sediment subduction and magma genesis in the Lesser Antilles: Isotopic and trace element constraints. J Geophys Res Solid Earth Planets. 1986;91(B6):5927–5941. [Google Scholar]
- 12.Davidson JP. Lesser Antilles isotopic evidence of the role of subducted sediment in island arc magma genesis. Nature. 1983;306(5940):253–256. [Google Scholar]
- 13.Davidson JP. Isotopic and trace element constraints on the petrogenesis of subudction-related lavas from Martinique, Lesser Antilles. J Geophys Res Solid Earth Planets. 1986;91(B6):5943–5962. [Google Scholar]
- 14.Davidson JP. Crustal contamination versus subduction zone enrichment: Examples from the Lesser Antilles and implications for mantle source compositions of island arc volcanic rocks. Geochim Cosmochim Acta. 1987;51(8):2185–2198. [Google Scholar]
- 15.Davidson JP, Harmon RS. Oxygen isotope constraints on the petrogenesis of volcanic arc magmas from Martinique, Lesser Antilles. Earth Planet Sci Lett. 1989;95(3-4):255–270. [Google Scholar]
- 16.Woodhead JD, Hergt JM, Davidson JP, Eggins SM. Hafnium isotope evidence for ‘conservative’ element mobility during subduction zone processes. Earth Planet Sci Lett. 2001;192(3):331–346. [Google Scholar]
- 17.Bezard R, et al. Assimilation of sediments embedded in the oceanic arc crust: Myth or reality? Earth Planet Sci Lett. 2014;395:51–60. [Google Scholar]
- 18.Teng F-Z, et al. Magnesium isotopic composition of the Earth and chondrites. Geochim Cosmochim Acta. 2010;74(14):4150–4166. [Google Scholar]
- 19.Tipper ET, et al. The magnesium isotope budget of the modern ocean: Constraints from riverine magnesium isotope ratios. Earth Planet Sci Lett. 2006;250(1-2):241–253. [Google Scholar]
- 20.McDonough WF, Sun SS. The composition of the Earth. Chem Geol. 1995;120(3-4):223–253. [Google Scholar]
- 21.White WM, Klein EM. In: The Crust. Rudnick RL, editor. Vol 4. Elsevier; Oxford: 2014. pp. 457–496. [Google Scholar]
- 22.Ling M-X, et al. Homogeneous magnesium isotopic composition of seawater: An excellent geostandard for Mg isotope analysis. Rapid Commun Mass Spectrom. 2011;25(19):2828–2836. doi: 10.1002/rcm.5172. [DOI] [PubMed] [Google Scholar]
- 23.Bourdon B, Tipper ET, Fitoussi C, Stracke A. Chondritic Mg isotope composition of the Earth. Geochim Cosmochim Acta. 2010;74(17):5069–5083. [Google Scholar]
- 24.Huang K-J. 2013. The behavior of magnesium isotopes during low-temperature water-rock interactions. PhD dissertation (China University of Geosciences, Wuhan, China)
- 25.Plank T. In: The Crust. Rudnick RL, editor. Vol 4. Elsevier; Oxford: 2014. pp. 607–629. [Google Scholar]
- 26.Huang K-J, Teng F-Z, Elsenouy A, Li W-Y, Bao Z-Y. Magnesium isotopic variations in loess: Origins and implications. Earth Planet Sci Lett. 2013;374:60–70. [Google Scholar]
- 27.Wimpenny J, Yin Q-Z, Tollstrup D, Xie L-W, Sun J. Using Mg isotope ratios to trace Cenozoic weathering changes: A case study from the Chinese Loess Plateau. Chem Geol. 2014;376:31–43. [Google Scholar]
- 28.Handler MR, Baker JA, Schiller M, Bennett VC, Yaxley GM. Magnesium stable isotope composition of Earth’s upper mantle. Earth Planet Sci Lett. 2009;282(1-4):306–313. [Google Scholar]
- 29.Yang W, Teng F-Z, Zhang H-F. Chondritic magnesium isotopic composition of the terrestrial mantle: A case study of peridotite xenoliths from the North China craton. Earth Planet Sci Lett. 2009;288(3-4):475–482. [Google Scholar]
- 30.Teng F-Z, Wadhwa M, Helz RT. Investigation of magnesium isotope fractionation during basalt differentiation: Implications for a chondritic composition of the terrestrial mantle. Earth Planet Sci Lett. 2007;261(1-2):84–92. [Google Scholar]
- 31.Wang S-J, Teng F-Z, Li S-G, Hong J-A. Magnesium isotopic systematics of mafic rocks during continental subduction. Geochim Cosmochim Acta. 2014;143:34–48. [Google Scholar]
- 32.Wang S-J, Teng F-Z, Williams HM, Li S. Magnesium isotopic variations in cratonic eclogites: Origins and implications. Earth Planet Sci Lett. 2012;359-360:219–226. [Google Scholar]
- 33.Li W-Y, Teng F-Z, Xiao Y, Huang J. High-temperature inter-mineral magnesium isotope fractionation in eclogite from the Dabie orogen, China. Earth Planet Sci Lett. 2011;304(1-2):224–230. [Google Scholar]
- 34.Li W-Y, Teng F-Z, Wing BA, Xiao Y. Limited magnesium isotope fractionation during metamorphic dehydration in metapelites from the Onawa contact aureole, Maine. Geochem Geophys Geosyst. 2014;15(2):408–415. [Google Scholar]
- 35.Ionov DA. Petrology of mantle wedge lithosphere: New data on supra-subduction zone peridotite xenoliths from the andesitic Avacha volcano, Kamchatka. J Petrol. 2010;51(1-2):327–361. [Google Scholar]
- 36.Pogge von Strandmann PAE, et al. Variations of Li and Mg isotope ratios in bulk chondrites and mantle xenoliths. Geochim Cosmochim Acta. 2011;75(18):5247–5268. [Google Scholar]
- 37.Niu Y. Bulk-rock major and trace element compositions of abyssal peridotites: Implications for mantle melting, melt extraction and post-melting processes beneath Mid-Ocean Ridges. J Petrol. 2004;45(12):2423–2458. [Google Scholar]
- 38.Staudigel H. In: The Crust. Rudnick RL, editor. Vol 4. Elsevier; Oxford: 2014. pp. 583–606. [Google Scholar]
- 39.Turner S, et al. U-series isotopes and destructive plate margin magma genesis in the Lesser Antilles. Earth Planet Sci Lett. 1996;142(1-2):191–208. [Google Scholar]
- 40.Elliott T, Plank T, Zindler A, White W, Bourdon B. Element transport from slab to volcanic front at the Mariana arc. J Geophys Res Solid Earth. 1997;102(B7):14991–15019. [Google Scholar]
- 41.Gale A, Dalton CA, Langmuir CH, Su Y, Schilling J-G. The mean composition of ocean ridge basalts. Geochem Geophys Geosyst. 2013;14(3):489–518. [Google Scholar]
- 42.Liu S-A, Teng F-Z, Yang W, Wu FY. High-temperature inter-mineral magnesium isotope fractionation in mantle xenoliths from the North China craton. Earth Planet Sci Lett. 2011;308(1-2):131–140. [Google Scholar]
- 43.Wang S-J, Teng F-Z, Rudnick RL, Li S-G. Magnesium isotope evidence for a recycled origin of cratonic eclogites. Geology. 2015;43(12):1071–1074. [Google Scholar]
- 44.Teng F-Z, et al. Magnesium isotopic compositions of international geological reference materials. Geostand Geoanal Res. 2015;39(3):329–339. [Google Scholar]
- 45.Teng F-Z, Yang W. Comparison of factors affecting the accuracy of high-precision magnesium isotope analysis by multi-collector inductively coupled plasma mass spectrometry. Rapid Commun Mass Spectrom. 2014;28(1):19–24. doi: 10.1002/rcm.6752. [DOI] [PubMed] [Google Scholar]
- 46.Ling M-X, et al. Formation of the world’s largest REE deposit through protracted fluxing of carbonatite by subduction-derived fluids. Sci Rep. 2013;3:1776. [Google Scholar]
- 47.Sedaghatpour F, Teng F-Z, Liu Y, Sears DW, Taylor LA. Magnesium isotopic composition of the Moon. Geochim Cosmochim Acta. 2013;120:1–16. [Google Scholar]
- 48.Li W-Y, et al. Heterogeneous magnesium isotopic composition of the upper continental crust. Geochim Cosmochim Acta. 2010;74(23):6867–6884. [Google Scholar]