Significance
Phosphatase and tensin homolog (PTEN) is a phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] and phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] 3-phosphatase that plays important roles in cell polarization, division, and development as well as in tumor suppression. Voltage-sensing phosphatases (VSPs) are PTEN family members but have catalytic activity in response to membrane depolarization with a wider range of substrates: They cleave the 5-phosphate of PI(3,4,5)P3 and phosphatidylinositol 4,5-bisphosphate as well as the 3-phosphate of PI(3,4)P2 and perhaps PI(3,4,5)P3. Using in-cell observations accompanied by quantitative analysis, we demonstrated that VSPs clearly cleave 3-phosphate from PI(3,4,5)P3 as PTEN does. Our results suggest that VSPs might act as surrogates for PTEN in several biological events related to membrane depolarization in addition to their inherent role.
Keywords: phosphoinositide; Dr-VSP; Ci-VSP; PI(3,4,5)P3; PI(4,5)P2
Abstract
Voltage-sensing phosphatases (VSPs) are homologs of phosphatase and tensin homolog (PTEN), a phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] and phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] 3-phosphatase. However, VSPs have a wider range of substrates, cleaving 3-phosphate from PI(3,4)P2 and probably PI(3,4,5)P3 as well as 5-phosphate from phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and PI(3,4,5)P3 in response to membrane depolarization. Recent proposals say these reactions have differing voltage dependence. Using Förster resonance energy transfer probes specific for different PIs in living cells with zebrafish VSP, we quantitate both voltage-dependent 5- and 3-phosphatase subreactions against endogenous substrates. These activities become apparent with different voltage thresholds, voltage sensitivities, and catalytic rates. As an analytical tool, we refine a kinetic model that includes the endogenous pools of phosphoinositides, endogenous phosphatase and kinase reactions connecting them, and four exogenous voltage-dependent 5- and 3-phosphatase subreactions of VSP. We show that apparent voltage threshold differences for seeing effects of the 5- and 3-phosphatase activities in cells are not due to different intrinsic voltage dependence of these reactions. Rather, the reactions have a common voltage dependence, and apparent differences arise only because each VSP subreaction has a different absolute catalytic rate that begins to surpass the respective endogenous enzyme activities at different voltages. For zebrafish VSP, our modeling revealed that 3-phosphatase activity against PI(3,4,5)P3 is 55-fold slower than 5-phosphatase activity against PI(4,5)P2; thus, PI(4,5)P2 generated more slowly from dephosphorylating PI(3,4,5)P3 might never accumulate. When 5-phosphatase activity was counteracted by coexpression of a phosphatidylinositol 4-phosphate 5-kinase, there was accumulation of PI(4,5)P2 in parallel to PI(3,4,5)P3 dephosphorylation, emphasizing that VSPs can cleave the 3-phosphate of PI(3,4,5)P3.
This paper concerns the substrate specificity and voltage dependence of a unique voltage-sensitive phosphoinositide (PI) phosphatase in intact live cells. Bioelectricity, caused by ion channels and differences in ion concentrations between the inside and outside of a cell, regulates essential biological activities like generation, propagation, and processing of neuronal signals; muscle contraction; and secretion of hormones. Voltage-gated ion channels were the first protein family identified that possessed bioelectric voltage-sensing domains (VSDs) and participated in these signaling activities. Recently, a quite unanticipated voltage-sensing enzyme with a VSD was cloned from the sea squirt Ciona intestinalis (1). Biochemical and electrophysiological examination revealed a voltage-dependent phosphatase activity toward polyphospho-PIs that was given the name C. intestinalis voltage-sensing phosphatase (Ci-VSP). Since then, homologs have been discovered in other vertebrates, for example, Danio rerio (zebrafish; Dr-VSP), African frog (Xi-VSP and Xt-VSP), chicken (Gg-VSP), and salamander (Hn-VSP and Cp-VSP) (2–5). In addition, two mammalian homologs have been discovered in human Hs-transmembrane phosphatase with tensin homology (TPTE) and mouse (Mm-VSP) tissues (6, 7). Although knowledge about the physiological function of these two proteins is still lacking, a recent study showed that mouse VSP seems to be localized to intracellular membranes of neuronal cells, suggesting a different role for mammalian VSPs than for plasma membrane-localized Ci-VSP or Dr-VSP (7). As in voltage-gated ion channels, the VSD of VSPs consists of four transmembrane segments, S1–S4, with the charged voltage-sensing S4 segment being moved by the intense electric fields across the plasma membrane upon depolarization (8). However, VSPs are monomeric and have a cytosolic catalytic domain instead of the pore-forming domain (S5–S6) of ion channels. This enzyme domain is homologous to tumor suppressor phosphatase and tensin homolog (PTEN), a phosphoinositide 3-phosphatase that dephosphorylates both phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] and phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] (1). Three distinctive regions of PTEN, an N-terminal phospholipid-binding motif that anchors the protein at the plasma membrane, a phosphatase domain that has the enzymatic site, and a C-terminal lipid-interacting C2 domain (9, 10), are well conserved in sequence and structure in the VSPs (11–14).
Propagation of the depolarization-induced conformational changes of the VSD to the cytosolic catalytic domain activates the unique voltage-activated phosphoinositide phosphatase activity (12, 14, 15). Unlike its analog PTEN, which has only 3-phosphatase activity (9, 16), Ci-VSP was seen initially to cleave the 5-phosphate from phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and PI(3,4,5)P3 in response to membrane depolarization, generating phosphatidylinositol 4-phosphate [PI(4)P] and PI(3,4)P2, respectively (1, 17–19). Subsequently, VSPs were reported to cleave 3-phosphate from PI(3,4)P2, generating PI(4)P upon larger depolarization (20), and, very recently, Ci-VSP was indicated to cleave 3-phosphate from PI(3,4,5)P3, generating PI(4,5)P2 (21, 22). Because different substrate reactions are best seen at different voltages, several authors have suggested that changing electric fields can drive VSPs successively through several catalytically active states that favor one set of substrates or reactions over another (20–22). The active site of VSPs is well conserved among species (12) and shows only a single amino acid difference from the active site of PTEN (1). When this residue in Ci-VSP was mutated to the corresponding amino acid of the PTEN active site, the mutated Ci-VSP still showed both 5- and 3-phosphatase activities toward polyphosphoinositides (14). Therefore, the observed substrate specificity and the voltage-dependent dual phosphatase activity of VSPs might be determined by the environment surrounding the active site rather than only by the active site itself (14). When the entire enzymatic domain of Ci-VSP was replaced by PTEN, the resulting chimera, called VSPTEN, had the enzymatic properties of a voltage-dependent pure 3-phosphatase (23).
VSPs are found in remarkably diverse tissues, including the testis and brain of mice (7, 24, 25); testis, brain, and stomach of humans (6); testis and neuronal complex of the ascidian (1); and testis, ovary, kidney, and liver of the African frog (26). Surprisingly, the physiological functions of this widespread enzyme remain a puzzle. Recent work suggests that VSPs might have roles in egg fertilization (26) and in neuronal signaling in the brain (7). To understand its physiological enzymology more completely, we screened Dr-VSP–induced phosphoinositide changes in living cells by engineered Förster resonance energy transfer (FRET) probes that specifically report the cellular dynamics of PI(4)P, PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3. Our results show that when exogenous expression of PI(4)P 5-kinase type Iγ (PIPKIγ) was used selectively to counteract the 5-phosphatase activity of Dr-VSP, PI(4,5)P2 accumulated, revealing an intrinsic 3-phosphatase activity of VSP toward PI(3,4,5)P3. The voltage dependence and substrate specificity of 3- and 5-phosphatase subreactions of Dr-VSP were then extracted quantitatively by a comprehensive kinetic systems analysis. Together, our data demonstrate that Dr-VSP possesses both 3- and 5-phosphatase activities toward PI(3,4,5)P3, with the same voltage dependence but with quite different absolute catalytic rates. These results should help clarify the roles of VSPs in fertilization, neural computations, and other signaling events that involve voltage changes.
Results
Voltage-Dependent 3- and 5-Phosphatase Activities.
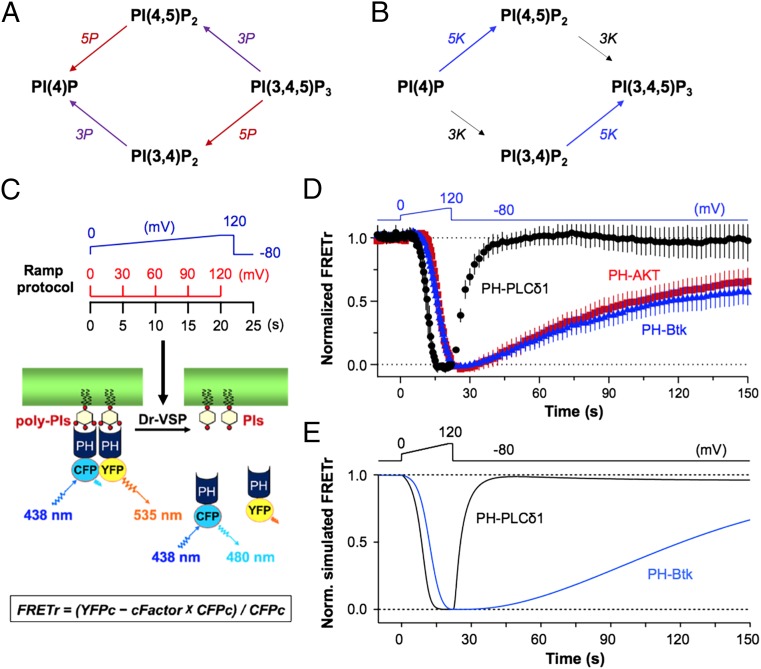
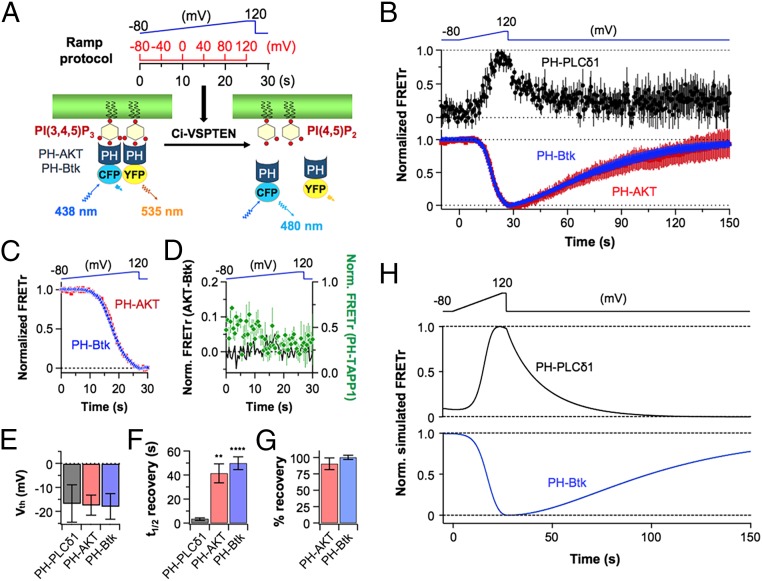
We tested the phosphatase activity of Dr-VSP on the phosphoinositides PI(3,4,5)P3, PI(3,4)P2, PI(4,5)P2, and PI(4)P using the specific probes in Table 1. Each lipid was detected by FRET between diffusible enhanced cyan fluorescent protein (ECFP) and enhanced yellow fluorescent protein (EYFP) versions of the pleckstrin homology (PH) domain probes. Separate tsA201 cell batches were cotransfected with Dr-VSP and individual FRET pairs of each PH domain. Unlike Xenopus oocytes in standard media used in previous studies (1, 23, 27), tsA201 cells have enough PI(3,4,5)P3 in the plasma membrane to localize the PI(3,4,5)P3-specific PH-AKT and PH-Bruton’s tyrosine kinase (Btk) probes to the membrane without additional treatment (28). On the other hand, we did not detect any basal PI(3,4)P2. A slow depolarizing ramp voltage stimulus (Fig. 1C) progressively activated Dr-VSP, and the changing membrane lipid concentrations were measured by FRET (Fig. 1C). FRET is expressed as the FRET ratio (FRETr), defined approximately as long-wavelength light divided by short-wavelength light (Materials and Methods). Decreasing FRET signifies a loss of the monitored lipid at the plasma membrane. Fig. 1A diagrams the possible 5-phosphatase (red) and 3-phosphatase (purple) reactions of Dr-VSP on three of these lipids. During a strong depolarizing activation of Dr-VSP, the three lipids would be converted in one or two steps to PI(4)P. After Dr-VSP is turned off again, the endogenous 5-kinase (blue) and 3-kinase (black) enzymes of the cell (Fig. 1B) would restore the original status quo. Fig. 1D is an overview of the results for three probes. All probes start at the plasma membrane (high FRETr), go gradually to the cytoplasm during the depolarizing ramp, and return to the membrane after the ramp, reflecting lipid dephosphorylation by VSP followed by lipid rephosphorylation by endogenous kinases. It is clear from the time courses that the different phosphoinositides become depleted at different times during the ramp and are restored at different rates afterward, properties that we will have to explain.
Table 1.
FRET probes for phosphoinositides
Fig. 1.
Measuring voltage-dependent changes of phosphoinositides using FRET probes in living cells. (A) Polyphosphoinositide dephosphorylation by 3-phosphatase (3P, purple) and 5-phosphatase (5P, red) activities of Dr-VSP. (B) Phosphoinositide resynthesis by endogenous phosphoinositide 5-kinase (5K, blue) and 3-kinase (3K, black) enzymes after Dr-VSP activation. (C) Protocol for FRET measurements. A ramp depolarization (blue line) rising from 0 to 120 mV over 20 s (rising rate of change, dV/dt = 6 mV/s) followed by a 2-s hold at the apex is given to tsA201 cells expressing Dr-VSP and a CFP/YFP pair of phosphoinositide FRET indicators chosen from Table 2. The holding potential was −80 mV. (D) Normalized time courses of FRETr responses of PH-PLCδ1 (black circles, n = 5), PH-AKT (red squares, n = 5), or PH-Btk (blue triangles, n = 6) during ramp Dr-VSP activation. Four points were averaged during repolarization (after 22 s) for better visibility. (E) Normalized simulated FRETr responses from the model of PH-PLCδ1 and PH-Btk domains for the experimental conditions in D.
We turn to quantitative analysis of such experiments. The goal is to determine the substrate preferences and voltage dependence of the Dr-VSP phosphatase activities and the interactions with cellular lipid metabolism. In many of our figures, we have included simulated traces like those for PH-PLCδ1 and PH-Btk of Fig. 1E. They come from a kinetic description of phosphoinositide metabolism that includes the phosphatase and kinase reactions of Fig. 1 A and B for endogenous cellular enzymes and for exogenous Dr-VSP, as well as other enzymes that will be overexpressed and other endogenous enzymes that feed or deplete these lipid pools. The simulated traces serve as a qualitative and quantitative test of how well we understand the in situ phosphatase subreactions of Dr-VSP. The derived rate constants are summarized in Table 2. Each experiment helped to inform the values of rate constants. For example, the recovery kinetics during long recordings like Fig. 1D were particularly helpful in setting the rate constants for the endogenous lipid kinases.
Table 2.
Rate constants and parameters for modeling at 22 °C
| Parameter | Value | Rationale for parameter |
| Free PI(4,5)P2 (density) | 5,000 μm−2 | Xu et al., 2003 (40) |
| Bound PI(4,5)P2 (density) | 10,000 μm−2 | 2 × 5,000 μm−2 |
| PI(4)P (density) | 4,000 μm−2 | Xu et al., 2003 (40) |
| PI (density) | 140,000 μm−2 | Xu et al., 2003 (40) |
| PI(3,4,5)P3 (density) | 1,338 μm−2 | FRETr response PH-Btk |
| PI(3,4)P2 (density) | 22 μm−2 | Steady state of basal PLC and DAGase |
| Surface (area plasma membrane) | 1,500 μm2 | As previously reported by Falkenburger et al., 2013 (72) |
| Size_cytosol (volume) | 2,500 μm3 | From surface/volume ratio |
| Size_ER (volume) | 462 μm3 | 18% cytosol, Falkenburger et al., 2013 (72) |
| k_4K | 0.000026 s−1 | PH-PLCδ1 recovery after VSP |
| k_4P | 0.003 s−1 | Steady state of basal PI(4,5)P2 |
| k_5K | 0.05 s−1 | PH-PLCδ1 recovery after VSP |
| k_5P | 0.036 s−1 | Steady state of basal PI(4,5)P2 |
| k_VSP [5P on PI(4,5)P2] | 11.3 s−1 | Based on PH-PLCδ1 FRETr |
| k_VSP [5P on PI(3,4,5)P3] | 2.0 s−1 | Based on PH-Btk and PH-TAPP1 FRETr |
| k_VSP [3P on PI(3,4)P2] | 0.25 s−1 | Based on PH-TAPP1 and P4M FRETr |
| k_VSP [3P on PI(3,4,5)P3] | 0.2 s−1 | Based on PH-Btk and PH-PLCδ1 FRETr |
| PH-PLCδ1-PI(4,5)P2 | 700 μm−2 | Based on FRETr |
| PH-PLCδ1 (concentration) | 1 μM | Falkenburger et al., 2013 (72) |
| PH-Btk-PI(3,4,5)P3 | 4,402 μm−2 | Based on FRETr |
| PH-Btk (concentration) | 1 μm−2 | Based on fluorescence intensity |
| PH-TAPP1-PI(3,4)P2 | 213 μm−2 | Based on FRETr |
| PH-TAPP1 (concentration) | 1 μM | Based on fluorescence intensity |
| P4M-PI(4)P | 400 μm−2 | Based on FRETr |
| P4M (concentration) | 1 μM | Based on fluorescence intensity |
| PIPKIγ_PI(4)P_max | 15 s−1 | Based on FRETr response |
| PIPKIγ_PI(3,4)P2_max | 70 s−1 | Based on FRETr response |
| Kd_PLCδ1 | 2 μM | As previously reported by Falkenburger et al., 2013 (72) |
| Kd_Btk | 0.3 μM | Based on FRETr and Hamman et al., 2002 (76) |
| Kd_TAPP-1 | 0.1 μM | Based on FRETr and Dowler et al., 2000 (74) |
| Kd_P4M | 1.5 μM | Based on P4M-FRETr |
| k_CiVSPTEN [3P on PI(3,4,5)P3] | 0.2 s−1 | Based on PH-Btk and PH-PLCδ1 FRETr |
| k_CiVSPTEN [3P on PI(3,4)P2] | 0.25 s−1 | As k_VSP [3P on PI(3,4)P2] |
| V_0.5_CiVSPTEN | 55 mV | Falkenburger et al., 2010 (29) |
| z_CiVSPTEN | 1.0 | Falkenburger et al., 2010 (29) |
| V_0.5_Dr-VSP | 100 mV | Falkenburger et al., 2010 (29) |
| z_Dr-VSP | 1.5 | Falkenburger et al., 2010 (29) |
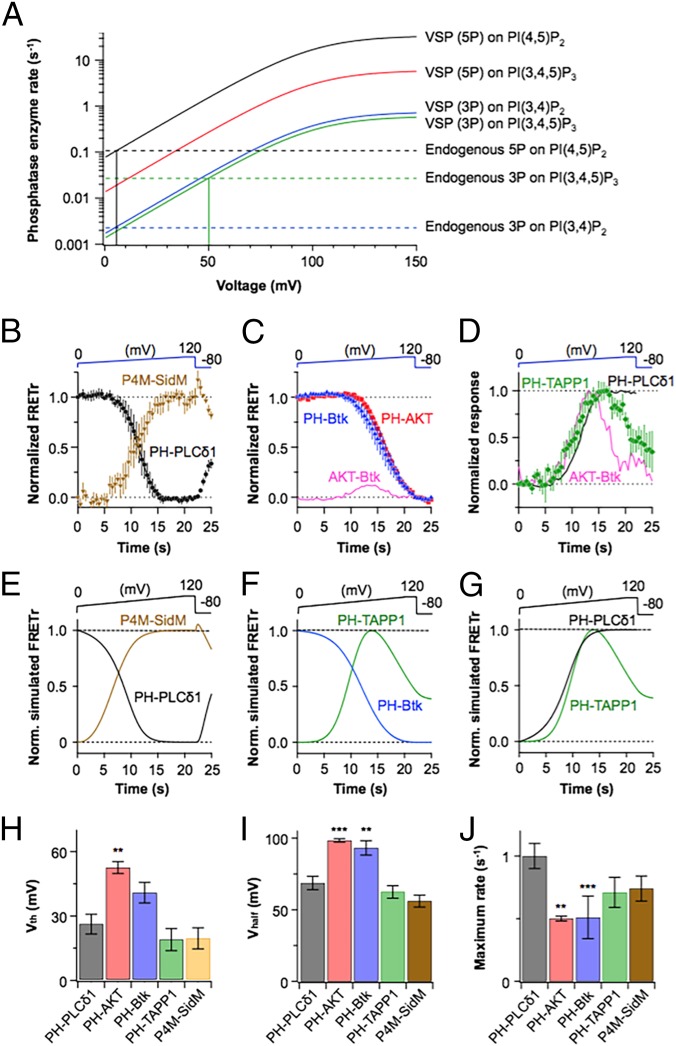
During the voltage ramp, each probe began to leave the plasma membrane as the membrane potential rose past some critical value. Qualitatively, we can consider this voltage a threshold (Vth) for seeing one of the enzymatic subreactions of Dr-VSP. At this potential, the phosphatase activity of Dr-VSP has risen enough to perturb the dynamic steady state set up by the endogenous metabolism for that lipid species noticeably. For example, one might anticipate that when the increasing VSP activity becomes equal to the endogenous activity so that the total phosphatase activity is twice as much as normal, the FRET probes should be reporting a noticeable perturbation of lipid pools. Our empirical definition for measuring Vth is given in Materials and Methods. It is helpful to understand this threshold first by considering the model. Fig. 2A plots phosphatase rate constants from the model against voltage. Three horizontal dashed lines represent the endogenous voltage-independent phosphatase activities of the cell, and the four rising solid curves represent the modeled four voltage-dependent phosphatase subreactions of the expressed Dr-VSP (note semilogarithmic axis). The VSP curves follow identical Boltzmann functions with a single voltage dependence taken from our earlier electrophysiological measurements of VSP sensing current (29). They differ only by vertical scaling factors that reflect our derived conclusions for Dr-VSP (developed later): (i) that the 5-phosphatase subreactions are much faster than the 3-phosphatase subreactions and (ii) that PI(4,5)P2 is a better substrate for the 5-phosphatase reaction than is PI(3,4,5)P3. Two vertical lines mark the voltages where two of the voltage-dependent phosphatase subreactions become faster than their corresponding endogenous phosphatase reaction. They are very widely separated along the voltage axis. At the crossing point, the total phosphatase activity (endogenous plus VSP) has been approximately doubled. The separation of such crossings on the diagram illustrates one explanation for different apparent Vth values in our experiments. It is clear that each crossing point depends both on the rate constant of the VSP subreaction as well as the rate constant of the endogenous phosphatase. Now we can consider the experiments.
Fig. 2.
Dynamic changes of FRET probes during ramp activation of Dr-VSP. (A) Postulated voltage dependence of phosphatase enzyme rate constants of Dr-VSP (colored curves) and endogenous phosphatases (dashed lines) in the model. (B) Comparison of FRETr responses for PH-PLCδ1 (black) and P4M-SidM (brown, n = 5) during ramp depolarization. (C) Magnified view of PH-AKT (red squares) and PH-Btk (blue triangles) FRETr changes during the ramp depolarization from Fig. 1D. The pink line shows the FRETr difference between PH-AKT and PH-Btk, obtained by subtracting the PH-Btk trace from PH-AKT. (D) Superposition of PH-TAPP1 (green diamonds, n = 5), PH-PLCδ1 (black trace), and the subtracted difference between PH-AKT and BH-Btk signals from C (pink trace). (E–G) Normalized simulated FRETr changes of P4M-SidM, PH-PLCδ1, PH-TAPP1, and PH-Btk domains for experimental conditions shown in B–D. (H) Membrane potential at which FRETr of each probe starts to change (voltage threshold). Time at the beginning of descent was converted to voltage threshold by Vth = (tthreshold × 6). The analyzed membrane potentials of Vhalf (I) and rmax (J) were obtained from fitted traces of FRETr data (B–D) with a sigmoid function. **P < 0.01; ***P < 0.001, compared with values of PH-PLCδ1. All data are mean ± SEM.
During a ramp, the FRETr trace for the PI(4,5)P2-binding domain PH-PLCδ1 (Figs. 1D and 2B) began to show a fast decrease at 26 ± 5 mV (Vth) reflecting the canonical 5-phosphatase activity of VSPs depleting PI(4,5)P2. Note that the x axis of these traces is labeled in time, but it corresponds to the voltages on the ramp inset drawn above. The trace was fitted to a sigmoid function giving a half-maximal membrane potential [half-maximal effective voltage (Vhalf)] at 69 ± 5 mV and a maximum rate (rmax) of 1.0 ± 0.1 s−1 (Materials and Methods). The FRETr trace for the PI(4)P-reporter P4M-SidM was complementary (Fig. 2B, brown). It increased during the depolarization, reflecting the generation of PI(4)P from PI(4,5)P2. As expected, the kinetics closely tracked the kinetics for PH-PLCδ1 (Fig. 2 H–J) but were opposite in direction (Fig. 2B). A small unexpected spike appeared consistently at the top of the P4M-SidM trace after the ramp depolarization. A control experiment with VSP showed that the spike did not occur unless Dr-VSP was expressed. As an ad hoc explanation, the spike might be due to a final release of some trapped PI(4)P during deactivation of Dr-VSP, including possible delivery of excess PI(4)P from the Golgi. The kinetic simulations (Fig. 2E) reproduce the PH-PLCδ1 and P4M-SidM traces, including a final spike when making this latter empirical assumption.
Now we consider changes of PI(3,4,5)P3 and PI(3,4)P2, starting with the PH-AKT probe, which binds to these two species with similar affinity (30, 31). The PH-AKT trace began a slow decrease only at 52 ± 3 mV (Figs. 1D and 2 C and H). Compared with PH-PLCδ1, the sigmoid fits gave a much more positive Vhalf (99 ± 1 mV) and a slower rmax (0.5 ± 0.02 s−1) (Fig. 2 I and J). The FRETr trace for the PI(3,4,5)P3-specific PH-Btk probe (32) started to decrease from a low voltage (41 ± 5 mV) close to the Vth of PH-PLCδ1, but its slow kinetics and overall changes tracked the slow kinetics and overall changes of PH-AKT (Vhalf = 93 ± 5 mV and rmax = 0.5 ± 0.02 s−1) (Figs. 1D and 2 C and H–J). Thus, the detectable effects of this mostly 5-phosphatase subreaction on PI(3,4,5)P3 (red line in Fig. 2A) show quite different parameters from the parameters for the 5-phosphatase subreaction on PI(4,5)P2 (black line in Fig. 2A).
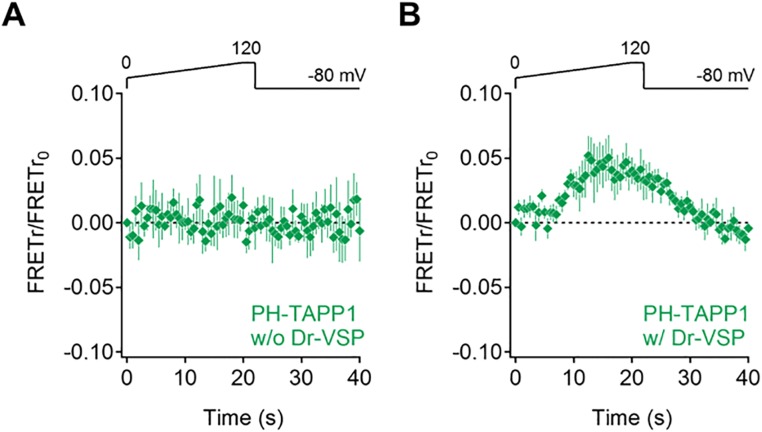
To confirm that the loss of PI(3,4,5)P3 is primarily via a 5-phosphatase step, we looked for the expected product, PI(3,4)P2, by constructing a pair of PH tandem PH domain-containing protein 1 (TAPP1) FRET probes. The result was a bell-shaped curve (Fig. 2D, green) reporting at rest no PI(3,4)P2, but at voltages between 30 and 90 mV, the expected production of PI(3,4)P2 [complementary to loss of PI(3,4,5)P3], and then at high voltages, a secondary loss of PI(3,4)P2. We will be showing that this secondary loss is a further dephosphorylation by a Dr-VSP 3-phosphatase subreaction of PI(3,4)P2 to PI(4)P. The model curves (Fig. 2 F and G) predict the same result. The PH-AKT and PH-Btk traces were not identical (Fig. 2C, red and blue). The same graph shows a difference curve, AKT minus Btk (pink line). Because PH-AKT binds both PI(3,4)P2 and PI(3,4,5)P3 and PH-Btk is more specific for PI(3,4,5)P3, the difference curve might represent the PI(3,4)P2 pool. Indeed, as predicted, the AKT-minus-Btk difference curve (Fig. 2D, pink) is bell-shaped, and it tracks the PH-TAPP1 FRET curve well (Fig. 2D, green) up to a membrane potential near 90 mV. Control experiments using the PH-TAPP1 FRET probes without expression of Dr-VSP showed that unlike Xenopus oocytes (14, 20), our cells had no endogenous voltage-dependent phosphatase activity in this kind of experiment (Fig. S1).
Fig. S1.
Evaluating endogenous voltage-dependent phosphatase activity on PI(3,4)P2 in tsA201. PH-TAPP1 FRET traces during ramp depolarization in control (A) and Dr-VSP coexpressing (B) cells (n = 5). Data are mean ± SEM.
These observations begin to specify the 3- and 5-phosphatase subreactions of Dr-VSP as they become visible with different apparent voltage thresholds and different rates during a ramp. Our conclusions from the experiments are drawn through the kinetic model described in Materials and Methods and Table 2. The key findings are that (i) each of the Dr-VSP enzyme subreactions has the same voltage-dependent activation (Fig. 2A) given by the sensing charge movement measurements in a study by Falkenburger et al. (29) for Dr-VSP, and (ii) the four subreactions have very different fully activated rates: 11.3, 2.0, 0.25, and 0.2 s−1 for 5-phosphatase activity on PI(4,5)P2 and PI(3,4,5)P3 and for 3-phosphatase activity on PI(3,4)P2 and PI(3,4,5)P3, respectively. These numbers express the deduced substrate and catalytic selectivities of Dr-VSP. Even with identical catalytic voltage dependence, the predicted apparent voltage thresholds are quite different, as is observed. We continue with tests of this description.
Perturbations with Wortmannin.
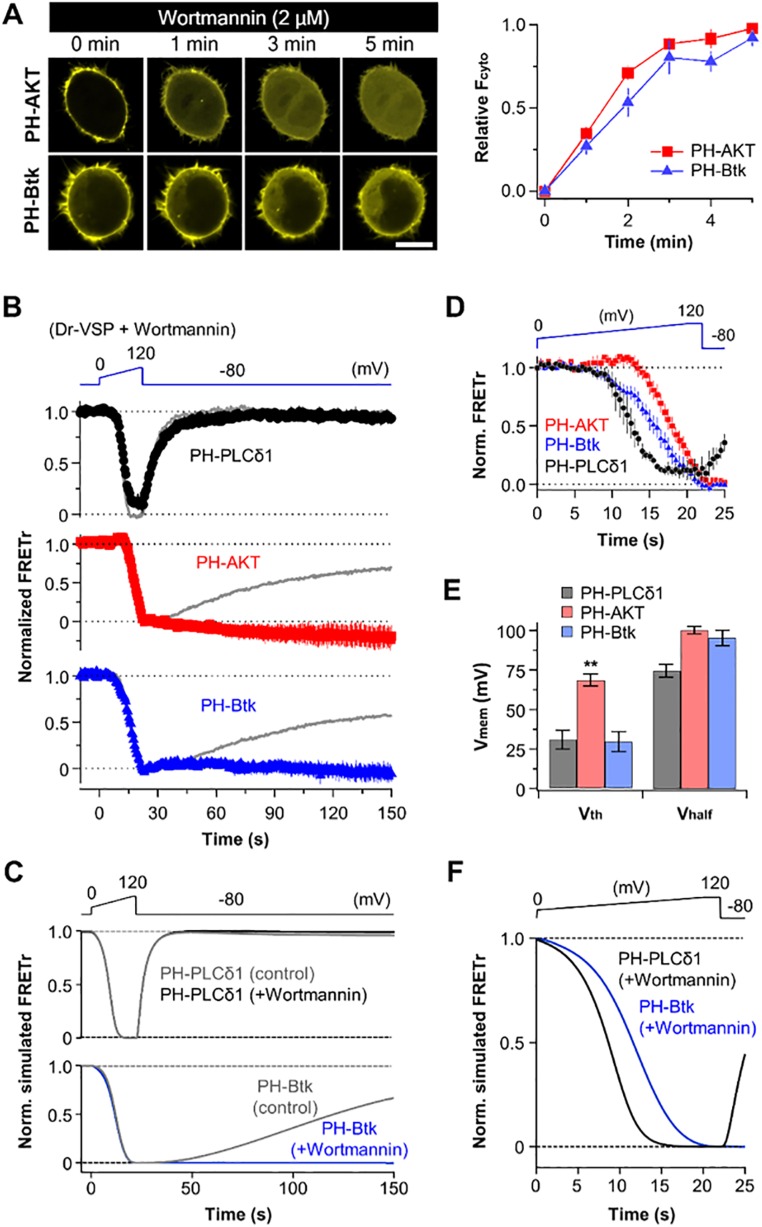
To characterize the 3-phosphatase activities of Dr-VSP more directly, we first need to examine the opposing endogenous cellular 3-kinase enzyme activity. We used the PI kinase inhibitor wortmannin. When cells were incubated with 2 μM wortmannin to inhibit PI3 kinases preferentially, both the AKT probes and the Btk probes gradually translocated away from the plasma membrane (Fig. S2A), reflecting a slow spontaneous loss of PI(3,4,5)P3. In experiments with Dr-VSP and voltage ramps, wortmannin did not affect the recovery of PI(4,5)P2 as reported by PH-PLCδ1, but it abrogated recovery of PI(3,4,5)P3 as reported by PH-AKT and PH-Btk (Fig. S2B). Even without wortmannin, the recovery was quite slow. Such observations guide our choice of 3-kinase rate for the model (Fig. S2C). They mean that an endogenous background phosphoinositide 3-kinase (PI3K) synthesizes PI(3,4,5)P3 continuously in normal cells, filling pools slowly with a time course of more than a minute, which would also tell us the turnover time of PI(3,4,5)P3 molecules in resting cells. As we saw in Fig. 2, with low-voltage depolarizations, this constitutive 3-kinase activity is able to counteract and mask the slow 3-phosphatase activity of Dr-VSP against PI(3,4,5)P3. Wortmannin had some effects on the dynamic dephosphorylation parameters during the ramp (Fig. S2 D and E). The principal change was that Vth and Vhalf for PH-Btk reporting PI(3,4,5)P3 were shifted by 12 and 26 mV to the left, respectively, implying that the 3-phosphatase activity of Dr-VSP became more effective. This result is expected when the masking endogenous 3-kinase activity is reduced by wortmannin. As could be anticipated, there was no change in the Vth and Vhalf for PH-PLCδ1 reporting PI(4,5)P2 [i.e., 3-kinases do not affect PI(4,5)P2 production]. The kinetic modeling describes these results well (Fig. S2 C and F).
Fig. S2.
Effect of PI3K inhibition on phosphoinositide dynamics after Dr-VSP activation. (A) Time-lapse confocal images of cells expressing YFP-tagged PH-AKT (Top) or PH-Btk (Bottom) after application of wortmannin (Left) and their relative response of cytosolic fluorescence intensity (Right). (Scale bar, 10 μm.) Data are mean ± SEM (n = 6). (B) Dr-VSP–induced FRETr responses of PH-PLCδ1 (Top, black circles), PH-AKT (Middle, red squares), or PH-Btk (Bottom, blue triangles) were plotted in the presence of wortmannin (2 μM). Wortmannin was perfused 40 to 60 s before the ramp depolarization. Cells held at −80 mV were depolarized from 0 to 120 mV for 20 s and then sustained for an additional 2 s. Gray lines indicate averaged control traces shown in Fig. 1D. Data are mean ± SEM (n = 4–5). (C) Normalized simulated FRETr responses of the experiment shown in B. (D) Horizontal magnification of FRETr signals (B) between 0 and 25 s. Note that the FRETr traces decrease with different kinetics depending on the PI probes. (E) Vth and Vhalf of PH-PLCδ1 (black), PH-AKT (red), and PH-Btk (blue) FRETr traces. Data are mean ± SEM. (F) Normalized simulated FRETr responses of PH-PLCδ1 and PH-Btk domains in the presence of Wortmannin as shown in C.
Voltage-Dependent PI(3,4,5)P3 3-Phosphatase Activity of Ci-VSPTEN.
The 3-phosphatase activities of Dr-VSP are difficult to analyze in living cells because of the accompanying stronger 5-phosphatase activities. We decided to begin with the simpler chimeric enzyme construct Ci-VSPTEN (23) that combines the pure 3-phosphatase enzyme PTEN with the voltage sensor of Ci-VSP. With Ci-VSPTEN, we could start to disentangle voltage-dependent effects of 3-phosphatase activities alone. This PTEN enzyme dephosphorylates PI(3,4,5)P3 to PI(4,5)P2. Because the chimera is based on Ci-VSP, which has a more negative midpoint voltage for activation, V0.5, than Dr-VSP, the activation does not require as strong depolarizations (2, 29). Note, for example, the 45-mV shift in the modeling parameters between V_0.5_Dr-VSP and V_0.5_Ci-VSPTEN in Table 2. The modified ramp depolarization started from −80 mV to 120 mV for 25 s (rising rate of change, dV/dt = 8 mV/s) with a 2-s final hold (Fig. 3A). As anticipated, PI(3,4,5)P3 was dephosphorylated relatively slowly and at relatively positive voltages for the Ci-based voltage sensor (Vhalf = 53 ± 9 mV, rmax = 0.44 ± 0.06 s−1 for PH-AKT and Vhalf = 62 ± 4 mV, rmax = 0.41 ± 0.02 s−1 for PH-Btk). In a complementary manner, the expected product PI(4,5)P2 increased as seen by FRETr of PH-PLCδ1 (23). The FRETr changes of all PH pairs initiated around −20 mV (Fig. 3 B and E), and there was no longer any difference between PH-AKT and PH-Btk kinetics (Fig. 3C). No PI(3,4)P2 generation was detected by PH-TAPP1 (Fig. 3D). After the repolarization, the recovery of PH-PLCδ1 FRETr was fast [time to half-maximal (t1/2) = 3.6 ± 1.1 s], whereas the recovery of PH-Btk and PH-AKT FRETr was quite slow (t1/2 = 50 ± 5 s and 41 ± 8 s, respectively) (Fig. 3F). Unlike for Dr-VSP–induced responses, the FRETr of PH-Btk and PH-AKT recovered fully after Ci-VSPTEN activation (Fig. 3G). Remarkably, all of these effects of Ci-VSPTEN could be reproduced in the Dr-VSP model by making only two changes: (i) that the only voltage-dependent enzyme activity to consider is the PI(3,4,5)P3 3-phosphatase, and (ii) that the maximum 3-phosphatase activity is the same as it was for Dr-VSP but with a 45-mV shifted voltage dependence given by our previous measurements of sensing currents of wild-type Ci-VSP (29). Thus, here, the VSPTEN chimera copies just one subreaction of intact VSP remarkably precisely.
Fig. 3.
Dynamic voltage-dependent actions of Ci-VSPTEN 3-phosphatase. (A) Ramp protocol for Ci-VSPTEN. Cells cotransfected with Ci-VSPTEN and PH-PLCδ1, PH-AKT, PH-Btk, or PH-TAPP1, labeled with CFP or YFP, are held at −80 mV and gradually depolarized to 120 mV over 25 s (dV/dt = 8 mV/s), and then held for another 2 s. (B) Normalized FRETr responses to depolarization of PH-PLCδ1 (black circles, n = 5), PH-AKT (red squares, n = 4), and PH-Btk (blue triangles, n = 7). Traces of PH-PLCδ1 FRETr were normalized using 0 s as minimum and a point between 25 and 32 s as maximum. PH-AKT and PH-Btk traces were normalized between 0 s (maximum) and 27 s (minimum). Data are mean ± SEM. (C) Magnified view of PH-AKT and PH-Btk FRETr signals between 0 s and 30 s. (D) Difference between PH-AKT and PH-Btk FRETr traces (black line) of C compared with normalized FRETr response of PH-TAPP1 (green diamonds, n = 3). The difference trace is scaled to match the signal-to-noise ratio for PH-TAPP1. (E) Threshold voltages for change of probe signals. One-way ANOVA showed no significant difference in Vth among probes. (F) t1/2 recovery of each PH-probe. **P < 0.01; ****P < 0.0001, compared with the value for PH-PLCδ1. (G) Relative amplitude of recovery compared with whole FRETr decrease. The signal-to-noise ratio was too small to fit the recovery of PH-PLCδ1 FRETr. Data are mean ± SEM. (H) Simulated FRETr responses of PH-PLCδ1 (black trace) and PH-Btk (blue trace) upon Ci-VSPTEN activation with the voltage protocol shown.
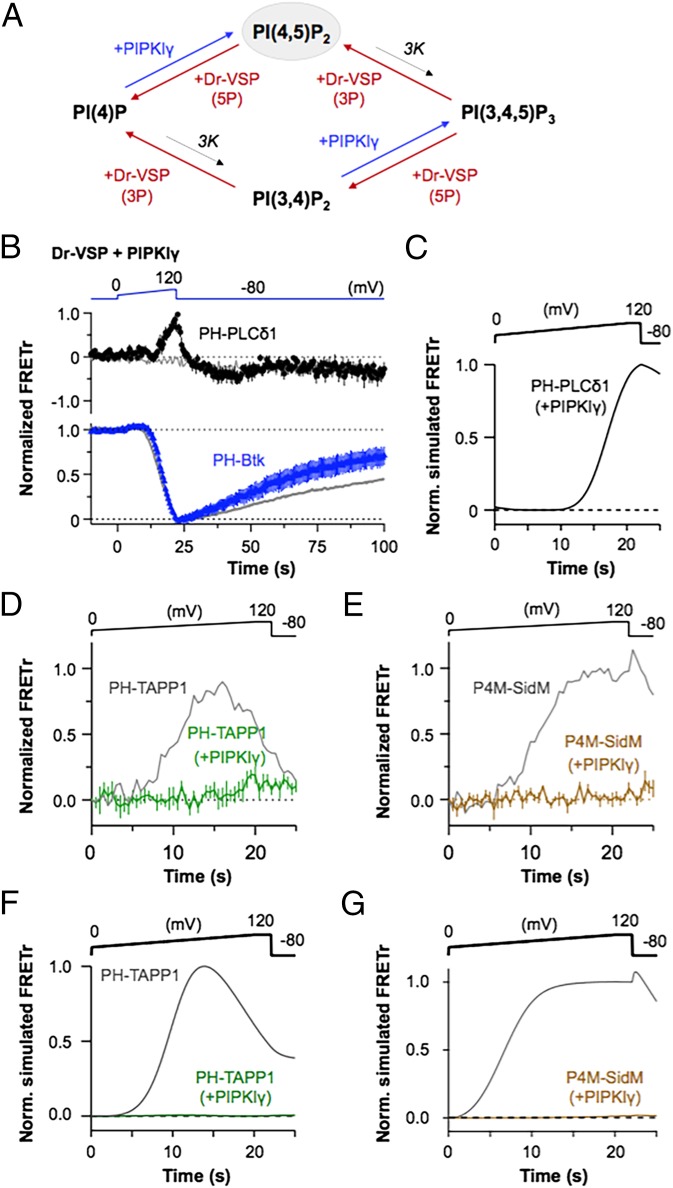
PIPKIγ Nullifies 5-Phosphatase Effects of Dr-VSP.
We next returned to Dr-VSP and tried to mask its 5-phosphatase reactions by countering them with a coexpressed 5-kinase. Such masking would allow us to study the voltage-dependent 3-phosphatase subreactions in isolation. PIPKIγ is a phosphoinositide 5-kinase that can phosphorylate PI(4)P to PI(4,5)P2 and PI(3,4)P2 to PI(3,4,5)P3 (Fig. 4A). Its activity would elevate the standing levels of PI(4,5)P2 (33) and potentially PI(3,4,5)P3 in the plasma membrane, and would counteract Dr-VSP 5-phosphatase activity (34, 35). As soon as a lipid species lost its 5-phosphate, the 5-kinase would put it back on again. Cells were cotransfected with Dr-VSP and PIPKIγ in a 1:1 plasmid ratio together with FRET probes. When a ramp depolarization was given, PH-Btk FRETr, reporting PI(3,4,5)P3, fell (Vth = 53 ± 6 mV), whereas PH-PLCδ1 FRETr, reporting PI(4,5)P2, began to rise above control (Vth = 52 ± 3 mV) in a fully complementary manner (Fig. 4B; n = 5–9). These responses resembled the responses just described with Ci-VSPTEN as if now the 5-phosphatase activities of Dr-VSP indeed had been totally masked. The results are well described by simulations using the kinetic model with a boosted 5-kinase activity (Fig. 4C). The observed changes of the two FRET probes occurred at the same high, right-shifted voltage compared with the normal cells as if the PI(4,5)P2 increase was mediated by Dr-VSP 3-phosphatase acting on PI(3,4,5)P3. Apparently, under normal conditions with no exogenous 5-kinase, any PI(4,5)P2 generated from PI(3,4,5)P3 by the VSP 3-phosphatase activity at high voltage is immediately dephosphorylated to PI(4)P by the high 5-phosphatase activity of Dr-VSP, but, here, PI(4,5)P2 is continually restored. Further, consistent with highly elevated 5-kinase activity, there was no detectable buildup of PI(3,4)P2 (PH-TAPP1, Fig. 4D) or of PI(4)P (P4M-SidM, Fig. 4E), again much as we saw with Ci-VSPTEN. Those results are well described in the kinetic model simply by boosting the basal 5-kinase activity (Fig. 4 F and G).
Fig. 4.
Expression of PIPKIγ unmasks innate 3-phosphatase activity of Dr-VSP toward PI(3,4,5)P3. (A) Relationship between Dr-VSP 3- and 5-phosphatase activities (red), endogenous PI3K (3K, black), and PIPKIγ (blue). (B) Cells held at −80 mV were depolarized from 0 to 120 mV for 20 s (dV/dt = 6 mV/s) followed by a hold at 120 mV for 2 s. The FRETr of PI(4,5)P2 and PI(3,4,5)P3 probes during the ramp depolarization and repolarization in cells expressing Dr-VSP and PIPKIγ in a plasmid ratio of 1:1 is shown. PH-PLCδ1 FRETr increased (black, n = 6), whereas PH-Btk FRETr decreased (blue, n = 5) during ramp depolarization. Gray lines indicate PH-PLCδ1 FRETr response without Dr-VSP (Upper) and PH-Btk FRETr response in control cells without PIPKIγ (Lower, from Fig. 1D). Points were averaged in groups of four after 22 s for better visibility. (C) Normalized simulated FRETr of PH-PLCδ1 domains in the presence of PIPKIγ. Simulated Dr-VSP was activated by the voltage protocol shown. The FRETr response of PH-TAPP1 (D, n = 6) and P4M-SidM (E, n = 5) probes in cells expressing Dr-VSP and PIPKIγ is shown. Gray lines indicate FRETr responses in control cells without PIPKIγ (from Fig. 2 D and B, respectively). Unresponsive traces were scaled down to match the signal-to-noise ratio of normal traces. Data are mean ± SEM. The normalized simulated FRETr of PH-TAPP1 (F) and P4M-SidM (G) in the presence of PIPKIγ is shown. Gray lines indicate FRETr responses in control cells without PIPKIγ (as shown in Fig. 2 G and E, respectively). Simulated Dr-VSP was activated by the voltage protocol shown.
Step Response of Dr-VSP in PIPKIγ-Coexpressing Cells.
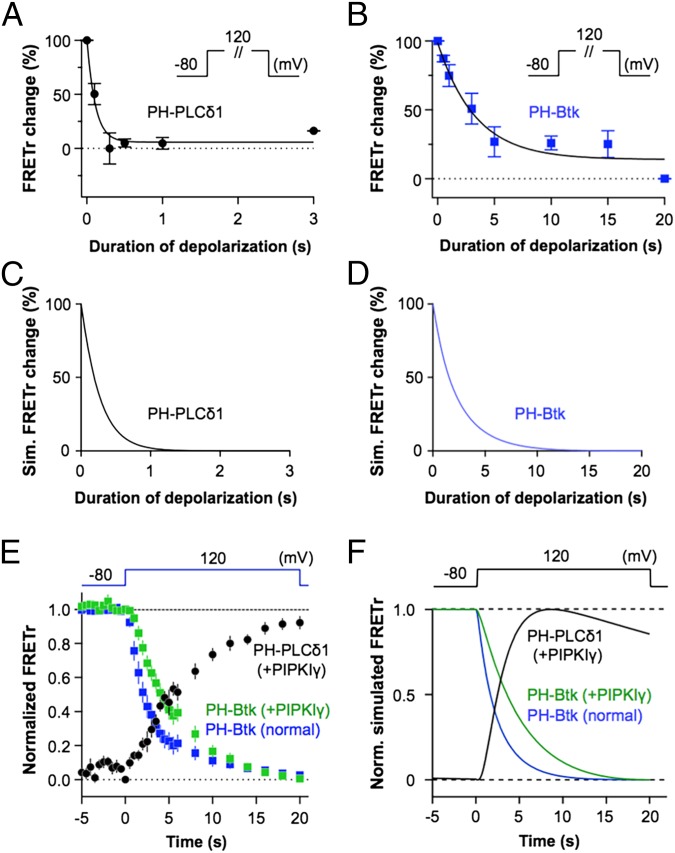
So far, we have used depolarizing ramps. However, most VSP-related studies used step depolarizations, and during the process of fertilization, a possible arena for physiological action of VSPs, a steady egg depolarization develops within 3 s and lasts many minutes (36). Therefore, we wanted to extend our analysis to step-voltage stimuli. We gave 120-mV step depolarizations of various durations to cells coexpressing PH-PLCδ1 or PH-Btk with Dr-VSP. According to our heuristic diagram (Fig. 2A), at this high voltage, both the 3-phosphatase and 5-phosphatase activities should be well above the endogenous cellular activities. The experimental results showed that a 0.5-s step depolarization was enough for nearly complete PI(4,5)P2 depletion (Fig. 5A), whereas the same step decreased PI(3,4,5)P3 levels by only 15%, and a 20-s depolarization was needed for full depletion (Fig. 5B). The kinetic predictions are in full concordance (Fig. 5 C and D). Step voltages were also applied to cells cotransfected with a 5-kinase. Fig. 5E compares FRETr changes of PH-Btk [reporting PI(3,4,5)P3] with (green) and without (blue) overexpression of PIPKIγ (1:1 ratio of Dr-VSP and PIPKIγ plasmids)]. The kinase, which counteracts the 5-phosphatase activity of Dr-VSP as we have seen, slows the depletion of PI(3,4,5)P3 because now only the 3-phosphatase activity remains productive. At the same time, there is accumulation of the product PI(4,5)P2 (black), confirming the conversion of PI(3,4,5)P3 to PI(4,5)P2 at this high voltage. Our kinetic description duplicates this result (Fig. 5F). Thus, we have confirmed our quantitative analysis of an enzyme with four subreactions tested by voltage ramps and by voltage steps yielding a single self-consistent description of all experiments.
Fig. 5.
Step response of Dr-VSP in the absence and presence of PIPKIγ. Dr-VSP step responses on the dephosphorylation of PI(4,5)P2 (A, n = 5 at each point) and PI(3,4,5)P3 (B, n = 2–5 at each point) against the duration of depolarization were measured using FRETr probes PH-PLCδ1 and PH-Btk, respectively, in control cells. Cells were held at −80 mV and then depolarized to 120 mV for the time duration indicated on the x axis. (C and D) Simulated FRETr changes in the percentage of PH-PLCδ1 and PH-Btk domains upon step responses shown in A and B. (E) Dr-VSP step response upon PI(3,4,5)P3 in normal (blue, n = 5) or PIPKIγ-coexpressing (green, n = 5) cells and upon PI(4,5)P2 in PIPKIγ cotransfected cells (black, n = 5). (F) Normalized simulated FRETr of PH-PLCδ1 (black) and PH-Btk (green) domains in the presence of PIPKIγ or PH-Btk domains in the absence of PIPKIγ (blue) upon Dr-VSP step responses as shown in E.
Discussion
We have measured voltage-dependent enzyme activity of Dr-VSP against PI(3,4,5)P3, PI(4,5)P2, and PI(3,4)P2 in situ by expressed FRET indicators. Analysis with kinetic modeling allowed clear resolution and a full quantitative specification of each of four subreactions. Together, they generate PI(4)P as a final product. Among them, the 5-phosphatase activities of Ci-VSP against PI(4,5)P2 and PI(3,4,5)P3 were previously well recognized (17–20), but the 3-phosphatase activities were less fully characterized. Previous work with Ci-VSP revealed PI(3,4)P2 3-phosphatase activity at high voltages in addition to the PI(4,5)P2 and PI(3,4,5)P3 5-phosphatase activity at lower voltages (20). Older, in vitro studies with catalytic domains of mammalian VSP homologs also revealed a PI(3,4,5)P3 3-phosphatase activity (6, 24), and very recent live-cell experiments with Ci-VSP showed short-lived transient signals attributed to that activity as well (21, 22). Under appropriate conditions, we could now show that Dr-VSP has the same 3-phosphatase activity against PI(3,4,5)P3 as the chimeric Ci-VSPTEN enzyme, even with the same catalytic rate constant. Thus, when the 5-phosphatase activity of Dr-VSP was counteracted by overexpression of PIPKIγ, we could see generation of PI(4,5)P2 from PI(3,4,5)P3 during depolarization (Fig. 4B). In this way, we support catalytic pathways from PI(3,4,5)P3 to PI(4)P through both PI(3,4)P2 and PI(4,5)P3 for Dr-VSP (Fig. 1A). Sequence similarity suggests that this feature is likely conserved in other VSP homologs.
Our quantitative approach now allows us to summarize the enzymology of Dr-VSP as follows. It is unnecessary to suppose that the several 5- and 3-phosphatase subreactions have different intrinsic voltage dependence even though the 3-phosphatase against PI(3,4)P2 is slower and needs a larger depolarization to become evident in experiments with intact cells. It suffices to regard the enzyme as existing in only two principal catalytic states at the plasma membrane, one active and the other not active. Like a voltage-gated ion channel, the enzyme activity is switched between these states in a stochastic manner by transitions of the charged voltage sensor, giving graded activity. The probability of being in the active state as a function of voltage follows the same Boltzmann distribution as the sensing charge movement that can be recorded electrophysiologically. The one active state can catalyze at least four subreactions. As described in Fig. 2A and Table 2, the four catalytic rate constants of the active form of the Dr-VSP enzyme are: 11.3, 2.0, 0.25, and 0.2 s−1 for the 5-phosphatase activity against PI(4,5)P2 and PI(3,4,5)P3 and for the 3-phosphatase activity against PI(3,4)P2 and PI(3,4,5)P3, respectively. These catalytic rate constants are in the context of a living cell at room temperature and at the expression level we achieved. Possible additional VSP activity against PI(5)P and PI(3)P has not been assessed.
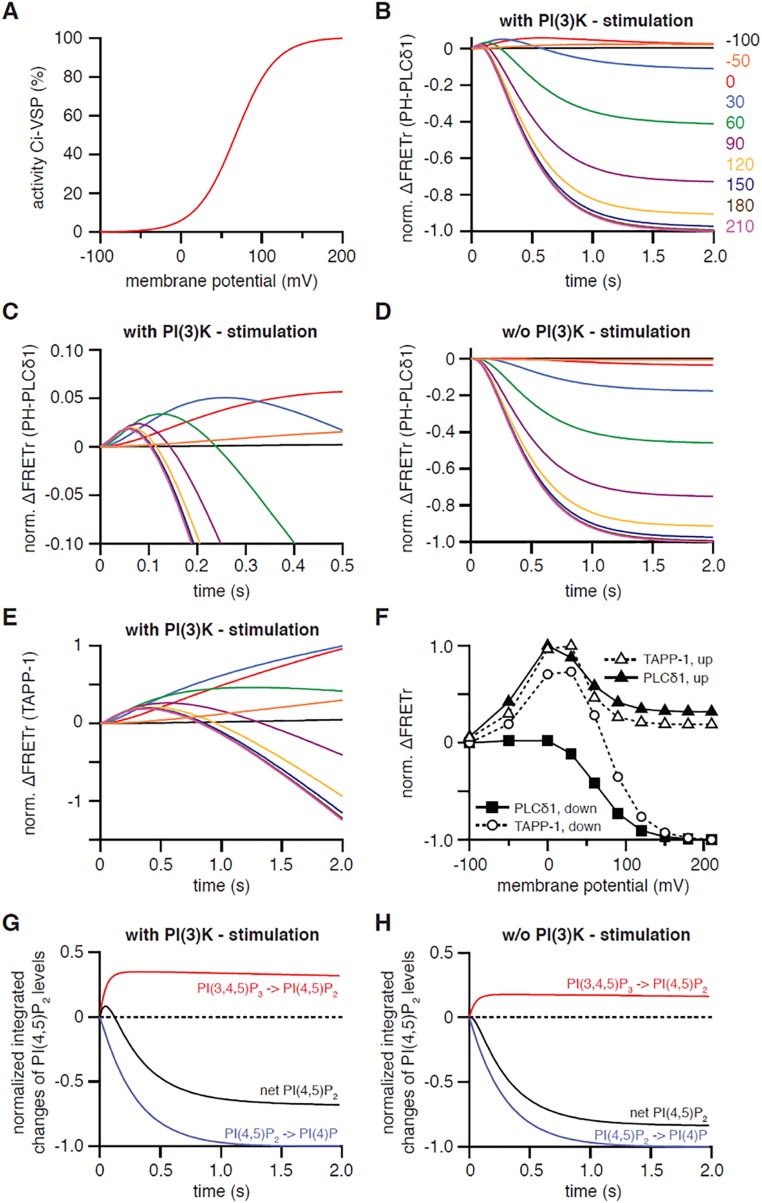
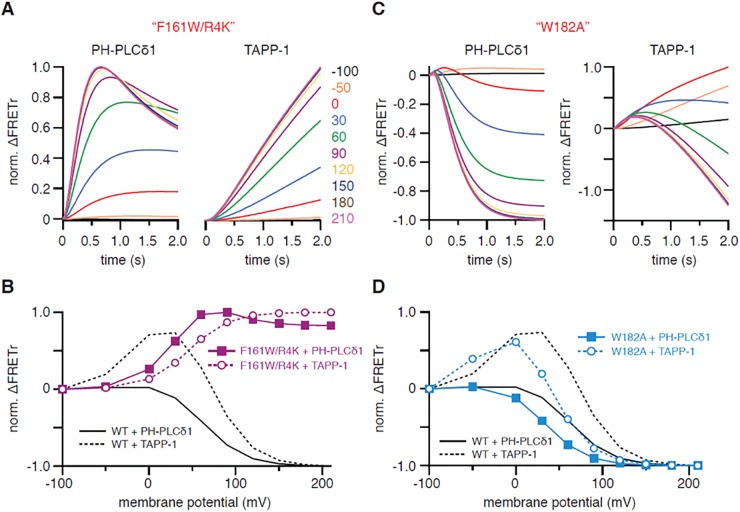
Our description of VSP as having only one active state contrasts with suggestions of multiple active states in the literature (20, 21), presented most explicitly in a new study published while our manuscript was under review. There, Grimm and Isacoff (22) analyzed the activity of Ci-VSP in oocytes of Xenopus laevis with PH domain probes and proposed an initial substrate preference for PI-trisphosphate [PI(3,4,5)P3] at less depolarized membrane potentials followed by a preference for PI-bisphosphates [PI(4,5)P2 and PI(3,4)P2] at more depolarized potentials, with sequential transitions from “off” to “active state 1” and continuing to “active state 2.” A key experiment used the PH-PLCδ1 probe to monitor PI(4,5)P2 during the step depolarizations. The striking finding was an initial transient burst increase of the probe signal reflecting net PI(4,5)P2 synthesis by rapid dephosphorylation of PI(3,4,5)P3, followed by a slower profound decrease reflecting the dephosphorylation of PI(4,5)P2 to PI(4)P. This effect was visible in the records of Liu et al. (14) and clearly noted with some discussion by Castle et al. (21), but by developing PH-domain probes that were prelocalized to the plasma membrane, Grimm and Isacoff (22) could improve the time resolution, which was previously much slowed by diffusion in large oocytes. We found that all of the results in figure 3 of ref. 22 could be reproduced qualitatively with our model (Fig. S3 and its legend) and did not require the hypothesis of two active states. The model required three changes (Table S1): (i) The sizes of some lipid pools had to be adjusted for X. laevis oocytes based on published analyses (37); notably, there was a modestly higher density of PI(3,4,5)P3 in the oocytes (Table S1) because they had been treated with insulin to boost the endogenous lipid 3-kinase; (ii) the rate constants for some individual subreactions catalyzed by Ci-VSP were adjusted compared with Dr-VSP; in particular, the 3-phosphatase activity against PI(3,4,5)P3 was made more than 100-fold faster than for Dr-VSP; and (iii) the voltage dependence of the Boltzmann term was shifted and lowered in slope to match Ci-VSP (29) as we had done for Ci-VSPTEN.
Fig. S3.
Simulation of FRETr responses to activation of Ci-VSP in oocytes of X. laevis. These simulations are meant to imitate figures 2C and 3 A and B of ref. 22. (A) Voltage dependence of simulated wild-type Ci-VSP activity. (B) Simulated FRETr response of PH-PLCδ1 domains to activation of Ci-VSP by a 2-s-long voltage step to various potentials as indicated. (C) Same simulation as for B, but enlarged and showing only the first 0.5 s of the voltage steps. (D) Same calculations as for B, but without insulin stimulation of PI(3)K. (E) Modeled FRETr response of TAPP-1 domains to activation of Ci-VSP with PI(3)K stimulated. Voltage steps are as in B. (F) Normalized ΔFRETr at the end of a 2-s depolarization for simulations shown in B and E for PH-PLCδ1 and TAPP-1 domains (“down”) as well as peak ΔFRETr for both reporters (“up”). (G and H) Dissection of the underlying subreactions as normalized integrals of the indicated fluxes during activation of Ci-VSP by a 2-s-long depolarization to 60 mV from a holding potential of −100 mV. Traces show production of PI(4,5)P2 by dephosphorylation of PI(3,4,5)P3 (red), reduction of PI(4,5)P2 levels due to dephosphorylation of PI(4,5)P2 to PI(4)P (blue), and the net change in PI(4,5)P2 (black) upon stimulation of PI(3)K (G) or without stimulation of PI(3)K (H). Note that increased PI(3,4,5)P3 levels in the simulation with PI(3)K stimulation in G allow for observation of the transient net PI(4,5)P2 production at the beginning of the depolarizing voltage step, whereas such an increase in PI(4,5)P2 is lacking in H due to the absence of PI(3)K stimulation.
Table S1.
Rate constants and parameters for Ci-VSP-models
| Parameter | Value | Rationale for parameter |
| PI(3,4,5)P3 (density) [+PI(3)K-stimulation] | 2,944 μm−2 | (37) |
| PI(3,4)P2 (density) [+PI(3)K-stimulation] | 1,800 μm−2 | (37) |
| PI(3,4,5)P3 (density) [−PI(3)K-stimulation] | 1,338 | (37) |
| PI(3,4)P2 (density) [− PI(3)K-stimulation] | 1,200 | (37) |
| k_4K | 0.000087 s−1 | Based on PH-PLCδ1 FRETr |
| k_4P | 0.003 s−1 | Based on PH-PLCδ1 FRETr |
| k_5K | 0.625 s−1 | Based on PH-PLCδ1 FRETr |
| k_5P | 0.45 s−1 | Steady state of basal PI(4,5)P2 |
| k_Ci-VSP [5P on PI(4,5)P2] | 8.0 s−1 | Based on PH-PLCδ1 FRETr |
| k_Ci-VSP [5P on PI(3,4,5)P3] | 2.0 s−1 | Based on PH-TAPP1 FRETr |
| k_Ci-VSP [3P on PI(3,4)P2] | 0.2 s−1 | Based on PH-TAPP1 FRETr |
| k_Ci-VSP [3P on PI(3,4,5)P3] | 44.0 s−1 | Based on PH-PLCδ1 FRETr |
| k_Ci-VSP-F161W/R4K [5P on PI(4,5)P2] | 0.4 s−1 | (22) |
| k_Ci-VSP-F161W/R4K [5P on PI(3,4,5)P3] | 0.1 s−1 | (22) |
| k_Ci-VSP-F161W/R4K [3P on PI(3,4)P2] | 0.01 s−1 | (22) |
| k_Ci-VSP-F161W/R4K [3P on PI(3,4,5)P3] | 2.2 s−1 | (22) |
| V_0.5_Ci-VSP [wt and F161W/R4K] | 67.7 mV | (22) |
| z_Ci-VSP [wt and F161W/R4K] | 1.0 | (29) |
| V_0.5_Ci-VSP (W182A) | 37.7 mV | (22) |
| z_Ci-VSP (W182A) | 1.0 | (29) |
Parameters are altered compared with the model for Dr-VSP in tsA201-cells. wt, wild type.
With these changes, there is an initial rapid net production of PI(4,5)P2 from PI(3,4,5)P3 as the faster 3-kinase acts on the enlarged PI(3,4,5)P3 pool. The production of PI(4,5)P2 is transient, not because the enzyme changes to a new active state with different substrate preference, but because the PI(3,4,5)P3 pool is rapidly consumed and, all of the time, the 5-phosphatase activity is depleting PI(4,5)P2.
Grimm and Isacoff (22) also mutated the voltage sensor in the VSD of Ci-VSP, attempting to favor one or the other of their postulated voltage-sensitive active states (figure 5 of ref. 22). Fig. S4 indicates that those results also are consistent with a one-active-state model. To mimic the results, it sufficed to shift the voltage dependence of all reactions equally or to slow all rate constants 20-fold without altering the ratios of the rates of the catalyzed subreactions (legend of Fig. S4). Our simulations show that a fixed substrate preference and a single Boltzmann voltage dependence suffice to describe a broad range of findings with Dr-VSP and Ci-VSP.
Fig. S4.
Simulation of activation of mutant variants of Ci-VSP. These simulations are meant to imitate figure 5 of ref. 22. (A) Simulated FRETr responses of PH-PLCδ1 (Left) and TAPP-1 (Right) domains to a 2-s-long activation of Ci-VSP-F161W/R4K by various voltage steps as indicated. The activity of Ci-VSP-F161W/R4K was modeled by reducing all Vmax values of wild-type (WT) Ci-VSP by a factor of 20 (Table S1). (B) Normalized ΔFRETr at the end of a 2-s depolarization in simulations shown in A for PH-PLCδ1 and TAPP-1 domains (down) as well as peak ΔFRETr for both reporters (up). (C) Same as in A, but for Ci-VSP-W182A. For simulation of Ci-VSP-W182A, the voltage dependence of WT Ci-VSP was shifted by −30 mV. (D) Same type of display as in B, but for data plotted in C.
Taking a more critical view, although the qualitative experimental transitions of the FRET probes are nicely captured by our simplified model, the curves we present do not reproduce the experimental data precisely. Does that mean we should go to a two-active state model? First, we should remember that our simple model does reproduce all of the qualitative phenomena that had inspired the proposed more complex models and that the alternative more complex concepts have not been given mathematical form by their proposers to allow an actual evaluation. Second, our model has not been optimized by some global-fitting criterion; rather, it was adjusted manually. Finally, our modeling omits several significant cell-biological complexities that would compromise a desire for more perfect fits. Our previous work, as well as the work of others, shows that the four phosphoinositide species we have discussed actually have several dynamic pools on different membranes and organelles that exchange with each other by trafficking or lipid exchange in tens of seconds (38, 39). Further, we and others have shown that when phosphoinositide pools are depleted by activating phospholipase C, the cellular lipid kinases are accelerated considerably (29, 40). Is it possible that analogous rate changes of endogenous metabolic enzymes occur in response to the dynamic PI changes that follow activation of VSP? Our simple model did not include interchanging lipid compartments or changes of endogenous enzyme properties. Our kinetic description presumably lumps together dynamic pools to some degree. Some of our FRET probes might be reporting, in part, from intracellular membranes; however, the voltage activation of VSP would be confined to the plasma membrane and all of the depolarization-stimulated 3- and 5-phosphatase subreactions we have described would be taking place there.
Our attempt to explain the results of Grimm and Isacoff (22) led to the unexpected conclusion that Ci-VSP has a disproportionately faster 3-phosphatase activity against PI(3,4,5)P3 in oocytes than either Dr-VSP or Ci-VSPTEN itself in tsA201 cells. Perhaps this higher enzyme rate is important in the physiology of Ciona. However, in selecting model parameters, the choice of the 3-phosphatase rate constant depends reciprocally on the postulated PI(3,4,5)P3 pool size. If this pool were actually fivefold larger in the insulin-treated oocytes than assumed here, the chosen 3-phosphatase rate constant for Ci-VSP would be fivefold lower. Thus, this parameter is not well determined by the available data, although it is likely to be faster than for Dr-VSP in any version. In the concept suggested by Grimm and Isacoff (22), the enzyme makes a transition probably quickly from a PI(3,4,5)P3-preferring state to a PI-bisphosphate–preferring state. Because the PI(3,4,5)P3 is already depleted at that time, we are not able to test whether the 3-phosphatase activity against PI(3,4,5)P3 does change in time from the records available. It can be noted that fluorescence measurements with the VSD of VSP and functional measurements of other voltage-gated ion channels have suggested that voltage sensors do make more than one step on the way to the fully activated state (41, 42).
Cells maintain a specific mix of phosphoinositide pools at the plasma membrane at rest. The lipids, in turn, regulate activities of many membrane proteins determining the physiological state of the cell. This status quo can be perturbed by raising the expression or activity of enzymes like PIPKI or VSPs. VSPs would impart a membrane-potential dependence to the phosphoinositide mix. Small depolarizations might favor depletion of PI(4,5)P2 and accumulation of PI(3,4)P2, and higher depolarization can deplete PI(3,4)P2 and favor accumulation of PI(4)P. Ci-VSP might favor depletion of PI(3,4,5)P3. However, if a 5-kinase activity is also elevated, as we saw, the changes with VSP are again different, and it is even possible to have an accumulation of PI(4,5)P2 due to 3-phosphatase activity against PI(3,4,5)P3. Previous studies report a range of PI(3,4)P2 dynamics during Ci-VSP activation. Variously, PI(3,4)P2 may be increased (19) decreased (20), or even decreased after an initial increase (14) by similar depolarizations. Based on our results, such a diversity of outcomes is possible depending on the other activities in the cells.
VSPs may be involved during the process of fertilization (6, 24, 26, 43). Considering that PIPKI isoforms may be highly activated during egg fertilization (44–47), we could envision why previous studies found PI(4,5)P2 increases following the rapid membrane depolarization (42) during fertilization (48–51). Taken together, VSPs possibly regulate the spatial and temporal distribution of phosphoinositides in a cell membrane in response to voltage (52) or pH changes in the cell (7, 53). If the distribution is localized and dynamic, VSPs may determine binding of target proteins at specific regions and time, resulting in, for example, cell migration or polarization (54–56).
PTEN is a well-characterized phosphoinositide 3-phosphatase acting on PI(3,4,5)P3 and PI(3,4)P2 (9, 16). Hence, as a negative regulator of the PI3K/AKT/mTOR pathway, PTEN enhances cell survival and suppresses tumorigenesis (57, 58). Two other mammalian enzymes, TPTE (24) and PTEN homologous inositol lipid phosphatase (TPIP) (6), are PTEN homologs. The cytosolic enzyme domains of TPTE and TPIP have 35% and 37% sequence identity with PTEN and almost the same catalytic active site, suggesting that all may have similar substrate specificity. Unlike PTEN, TPTE and TPIP have N-terminal transmembrane domains that resemble the voltage sensor of VSP and of voltage-gated ion channels (59). Recently, a chimera of the voltage sensor of TPTE with the pore domain of the bacterial voltage-gated potassium channel was shown to reconstitute functional voltage-gated channel activity (7).
Despite the structural similarity between VSP and PTEN, the substrate selectivity and subreactions of VSP are broader than the substrate selectivity and subreactions of PTEN. The demonstration of a 3-phosphatase activity for VSP against PI(3,4,5)P3 fills in an important connection between VSP and PTEN. We could speculate that VSP might participate in PTEN-related cellular signaling, including balancing tumor suppression vs. tumorigenesis (57, 58, 60), stem cell differentiation (61–63), and axon regeneration (64–66). Such processes might even be triggered and regulated by electrochemical signals, giving VSPs important previously unidentified roles in pathobiology and normal development.
Materials and Methods
Cell Culture and Transfection.
HEK293-derived tsA201 cells were grown in DMEM supplemented with 10% (vol/vol) FBS and 0.2% penicillin/streptomycin. They were subcultured every 6 or 7 d (70–80% confluence), transfected 1–4 d after subculture using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions, and then plated on a poly-l-lysine–coated coverslip 12–24 h after transfection using trypsin. Experiments were carried out 24–36 h after transfection. The following cDNAs were generously given to us: Dr-VSP without eGFP (from Björn Falkenburger, University of Aachen, Aachen, Germany), human PTEN conjugated with the VSD of Ci-VSP (Ci-VSPTEN16) (from Christian Halaszovich, University of Marburg, Marburg, Germany), ECFP- and EYFP-tagged PH domains derived from phospholipase C-δ1 (PH-PLCδ1) (from Kees Jalink, The Netherlands Cancer Institute, Amsterdam), AKT/protein kinase B (PH-AKT) (from Tamas Balla, NIH, Bethesda), Bruton’s tyrosine kinase (PH-Btk) and tandem PH domain-containing protein 1 (PH-TAPP1) [originating from Tamas Balla and then subcloned into pECFP-C1 and pEYFP-C1 vector (Clontech) by the authors], the P4M domain of PI(4)P binding domain of SidM (P4M-SidM) (from Tamas Balla), and PIPKIγ (from Thomas F. J. Martin, University of Wisconsin–Madison, Madison, WI).
Electrophysiology.
The whole-cell configuration of the patch-clamp method was used to depolarize the membrane using an EPC-10 patch-clamp amplifier (HEKA Elektronik) at room temperature. Pipette resistance was 1.3–3.0 MΩ, and series resistance was between 3.4 and 6.0 MΩ with 60% compensation. The bath solution contained 160 mM NaCl, 2.5 mM KCl, 2 mM CaCl2-2H2O, 1 mM MgCl2, 10 mM Hepes, and 8 mM glucose. The pipette solution contained 175 mM KCl, 5 mM MgCl2, 5 mM Hepes, 0.1 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-K4, 3 mM Na2ATP, and 0.1 mM Na3GTP, titrated to pH 7.4 with NaOH. The holding potential in all experiments was −80 mV, and depolarizing electrical stimulation patterns were either voltage ramps or voltage steps. We mainly used ramp protocols (Fig. 1C) to study the voltage dependence of the 3- and 5-phosphatase activities of Dr-VSP. In most cases, voltage ramps started at 0 mV and rose to 120 mV over 20 s (dV/dt = 6 mV/s), with a final 2-s hold at 120 mV (Fig. 1C).
FRET.
Our FRET setup was described briefly in our previous publication (67). In detail, FRET between ECFP and EYFP fused to PH domains was measured by a photomultiplier tube (PMT)-based photometry system (Till Photonics GmbH). Indigo exciting light (438 ± 12 nm) from a PolychromeV monochrometer (Till Photonics GmbH) was applied for 45 ms every 500 ms. Simultaneously, the emission was split into short (480 ± 40 nm) and long (535 ± 15 nm) wavelengths by a dichroic mirror (505DCLP) and band-pass filters (D480-40 for short wavelengths and ET535-30 for long wavelengths; Chroma Technology) and detected by two PMTs connected with an FDU-2 fluorescence detection unit (Till Photonics, GmbH). The analog outputs were digitized by the data acquisition board (PCI-6221; National Instruments) at a sampling rate of 10 kHz and averaged. Then, FRETr was calculated by the following equation: FRETr = (YFPC − cFactor × CFPC)/CFPC (67, 68). The numerator is the voltage output of the long-wavelength PMT minus calculated bleed-through from the donor, indicating excitation of acceptor by FRET, and the denominator is the voltage output of the short-wavelength PMT, indicating direct donor excitation by the light source. The bleed-through from CFP to the long-band PMT was 0.55, and from YFP to short-band PMT, it was 0.02, which was ignored. Timing control, data acquisition, and real-time calculation of FRETr, including background compensation, were performed using an in-house program that also controlled the monochromator and patch-clamp amplifier through the data acquisition interface. Photobleaching of donor and acceptor were compensated during postprocessing.
Changes of the membrane density of each lipid were monitored by FRET between ECFP- and EYFP-tagged versions of the same lipid probe. Because probe density at the plasma membrane is dependent on the abundance of the lipid in the cytoplasmic leaflet of the plasma membrane, the FRETr falls as the density falls (68–70).
Normalization and Fitting of Traces.
The FRETr traces were normalized to their minima and maxima as follows. The point at t = 0 s was set to the initial minimum (0.0) or maximum (1.0) value depending on the direction of subsequent changes, and the trace maximum or minimum during depolarization was set to the opposite extremum. For empirical characterization of the FRETr responses to applied voltage ramps in the presence of Dr-VSP, FRETr traces were fitted to a sigmoid curve against voltage:
Here, V is voltage during the ramp, FRETrV0 is the FRETr value at t = 0 s, ΔFRETrpeak is the peak amplitude of the change during depolarization, Vhalf is the voltage for half-maximal change, and rmax is maximum steepness of the change (V−1). In the text and figures, rmax is converted to a rate of change (s−1) by multiplying by dV/dt. We also used an empirical parameter that we call the voltage threshold (Vth) to denote the voltage at which the effects of the VSP phosphatase first became apparent. It was determined as follows: A sequential sliding t test was performed between two groups of points each containing 10 samples around the target point. Vth is defined as the first point that shows statistically significant deviation in the transition region.
Confocal Microscopy.
A confocal microscope (LSM700; Carl Zeiss) was used for time-lapse images. The interval between images was 1 min for wortmannin-mediated PI3K blocking experiments in Fig. 4. Successive pairs of 512 × 512-pixel images were averaged, and the fluorescence intensity was obtained with ImageJ (NIH) and plotted using IGOR Pro-6.0 (WaveMetrics).
Mathematical Modeling.
The Virtual Cell software environment (University of Connecticut Health Center, available at www.nrcam.uchc.edu) was used to develop a kinetic description of modulation of phosphoinositide levels in tsA201 cells by the voltage-sensitive lipid phosphatases in situ. The model allowed extraction of all reaction rates from the recordings from this complex system. For comparison with experimental results, such a description must include the endogenous phosphoinositide metabolism of the cell; the effects of exogenous enzymes that we transfect into the cells; the properties of the lipid-binding probes that are used to measure the dynamic changes of phosphoinositides; and, most interesting for this paper, the deduced voltage dependence and catalytic rates of the VSPs. As a starting point, we used a compartmental model (71, 72) that had been developed to describe phosphoinositide metabolism upon modulation by muscarinic receptors and Dr-VSP in the tsA201 cell line. Final conditions used to simulate the results obtained from FRET recordings are shown in Table 2, including the dissociation constants for each of the fluorescent probes, taken from the literature. Rate constants were adjusted manually to fit experimental data. The modeled traces approximate changes in FRETr as a cooperative square law of the predicted membrane-bound fraction of FRET domains, as recently described (70). The voltage dependence of the enzymatic activities of VSP was described by a Boltzmann equation with no residual baseline activity at very negative voltages. Thus, for each of the four enzymatic subreactions of VSP, the rate constants varied as a function of membrane voltage (V) as follows
| [1] |
for which the four subreaction-specific fully activated rate constants k_VSP and the subreaction-independent half-activation voltage V0.5 and equivalent valence z are listed in Table 2. For Dr-VSP, the midpoint potential, V0.5, was +100 mV, and for Ci-VSP, it was +55 mV. Τhe Virtual Cell model, “hillelab: KeumKruseKimHilleSuh_2016,” is publicly available at www.vcell.org/.
Analysis and Statistics.
All data were analyzed using Excel (Microsoft), IGOR Pro-6.0, ImageJ, or GraphPad Prism 6.0 (GraphPad Software). Statistics in text or figures represent mean ± SEM. Statistical comparisons were made by one-way ANOVA with or without Tukey’s post hoc test depending on the number of experimental groups.
Materials.
Wortmannin (Sigma–Aldrich) was dissolved in DMSO, stored at −20 °C with protection from light, and then diluted to 2 μM in Ringer’s solution just before the experiments. Wortmannin was used for less than 1 mo after unpacking.
Acknowledgments
We thank Eamonn J. Dickson, Seung-Ryoung Jung, Mario G. Rosasco, Daewon Moon, and Oscar Vivas for comments on the manuscript, and Lea M. Miller for technical help. This study was supported by the National Institute of Neurological Disorders and Stroke of the NIH (Grant R37NS008174), the Wayne E. Crill Endowed Professorship, the Korean Brain Research Institute basic research program funded by the Ministry of Science, Information and Communications Technology and Future Planning (Grant 2231-415), and the Daegu Gyeongbuk Institute of Science and Technology (DGIST) R&D Program of the Ministry of Science, Information and Communications Technology & Future Planning (Grant 16-BD-06). The Virtual Cell is supported by NIH Grant P41GM103313 from the National Institute of General Medical Sciences.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 7012.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606472113/-/DCSupplemental.
References
- 1.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435(7046):1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 2.Hossain MI, et al. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J Biol Chem. 2008;283(26):18248–18259. doi: 10.1074/jbc.M706184200. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi S, et al. Potential role of voltage-sensing phosphatases in regulation of cell structure through the production of PI(3,4)P2. J Cell Physiol. 2014;229(4):422–433. doi: 10.1002/jcp.24463. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsui H, et al. Improved detection of electrical activity with a voltage probe based on a voltage-sensing phosphatase. J Physiol. 2013;591(18):4427–4437. doi: 10.1113/jphysiol.2013.257048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutua J, et al. Functional diversity of voltage-sensing phosphatases in two urodele amphibians. Physiol Rep. 2014;2(7):e12061. doi: 10.14814/phy2.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker SM, Downes CP, Leslie NR. TPIP: A novel phosphoinositide 3-phosphatase. Biochem J. 2001;360(Pt 2):277–283. doi: 10.1042/0264-6021:3600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosasco MG, Gordon SE, Bajjalieh SM. Characterization of the functional domains of a mammalian voltage-sensitive phosphatase. Biophys J. 2015;109(12):2480–2491. doi: 10.1016/j.bpj.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villalba-Galea CA, Frezza L, Sandtner W, Bezanilla F. Sensing charges of the Ciona intestinalis voltage-sensing phosphatase. J Gen Physiol. 2013;142(5):543–555. doi: 10.1085/jgp.201310993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell RB, Liu F, Ross AH. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278(36):33617–33620. doi: 10.1074/jbc.C300296200. [DOI] [PubMed] [Google Scholar]
- 10.Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem. 2004;279(16):16606–16613. doi: 10.1074/jbc.M312098200. [DOI] [PubMed] [Google Scholar]
- 11.Okamura Y, Dixon JE. Voltage-sensing phosphatase: Its molecular relationship with PTEN. Physiology (Bethesda) 2011;26(1):6–13. doi: 10.1152/physiol.00035.2010. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda M, et al. Crystal structure of the cytoplasmic phosphatase and tensin homolog (PTEN)-like region of Ciona intestinalis voltage-sensing phosphatase provides insight into substrate specificity and redox regulation of the phosphoinositide phosphatase activity. J Biol Chem. 2011;286(26):23368–23377. doi: 10.1074/jbc.M110.214361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobiger K, Utesch T, Mroginski MA, Friedrich T. Coupling of Ci-VSP modules requires a combination of structure and electrostatics within the linker. Biophys J. 2012;102(6):1313–1322. doi: 10.1016/j.bpj.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, et al. A glutamate switch controls voltage-sensitive phosphatase function. Nat Struct Mol Biol. 2012;19(6):633–641. doi: 10.1038/nsmb.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villalba-Galea CA. New insights in the activity of voltage sensitive phosphatases. Cell Signal. 2012;24(8):1541–1547. doi: 10.1016/j.cellsig.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 16.McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda) 2009;24(1):8–16. doi: 10.1152/physiol.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Physiol. 2007;583(Pt 3):875–889. doi: 10.1113/jphysiol.2007.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki H, et al. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2008;105(23):7970–7975. doi: 10.1073/pnas.0803936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halaszovich CR, Schreiber DN, Oliver D. Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5′-phosphatase. J Biol Chem. 2009;284(4):2106–2113. doi: 10.1074/jbc.M803543200. [DOI] [PubMed] [Google Scholar]
- 20.Kurokawa T, et al. 3′ Phosphatase activity toward phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] by voltage-sensing phosphatase (VSP) Proc Natl Acad Sci USA. 2012;109(25):10089–10094. doi: 10.1073/pnas.1203799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castle PM, Zolman KD, Kohout SC. Voltage-sensing phosphatase modulation by a C2 domain. Front Pharmacol. 2015;6:63. doi: 10.3389/fphar.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimm SS, Isacoff EY. Allosteric substrate switching in a voltage-sensing lipid phosphatase. Nat Chem Biol. 2016;12(4):261–267. doi: 10.1038/nchembio.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacroix J, et al. Controlling the activity of a phosphatase and tensin homolog (PTEN) by membrane potential. J Biol Chem. 2011;286(20):17945–17953. doi: 10.1074/jbc.M110.201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, et al. PTEN 2, a Golgi-associated testis-specific homologue of the PTEN tumor suppressor lipid phosphatase. J Biol Chem. 2001;276(24):21745–21753. doi: 10.1074/jbc.M101480200. [DOI] [PubMed] [Google Scholar]
- 25.Reymond A, et al. Human chromosome 21 gene expression atlas in the mouse. Nature. 2002;420(6915):582–586. doi: 10.1038/nature01178. [DOI] [PubMed] [Google Scholar]
- 26.Ratzan WJ, Evsikov AV, Okamura Y, Jaffe LA. Voltage sensitive phosphoinositide phosphatases of Xenopus: Their tissue distribution and voltage dependence. J Cell Physiol. 2011;226(11):2740–2746. doi: 10.1002/jcp.22854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villalba-Galea CA, Sandtner W, Starace DM, Bezanilla F. S4-based voltage sensors have three major conformations. Proc Natl Acad Sci USA. 2008;105(46):17600–17607. doi: 10.1073/pnas.0807387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314(5804):1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falkenburger BH, Jensen JB, Hille B. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J Gen Physiol. 2010;135(2):99–114. doi: 10.1085/jgp.200910345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James SR, et al. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315(Pt 3):709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frech M, et al. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272(13):8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 32.Rameh LE, et al. A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J Biol Chem. 1997;272(35):22059–22066. doi: 10.1074/jbc.272.35.22059. [DOI] [PubMed] [Google Scholar]
- 33.Wenk MR, et al. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32(1):79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 34.Halstead JR, et al. A novel pathway of cellular phosphatidylinositol(3,4,5)-trisphosphate synthesis is regulated by oxidative stress. Curr Biol. 2001;11(6):386–395. doi: 10.1016/s0960-9822(01)00121-x. [DOI] [PubMed] [Google Scholar]
- 35.Rudge SA, Wakelam MJO. SnapShot: Lipid kinases and phosphatases. Cell. 2013;155(7):1654–1654.e1. doi: 10.1016/j.cell.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe LA. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976;261(5555):68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- 37.Liu XJ, Sorisky A, Zhu L, Pawson T. Molecular cloning of an amphibian insulin receptor substrate 1-like cDNA and involvement of phosphatidylinositol 3-kinase in insulin-induced Xenopus oocyte maturation. Mol Cell Biol. 1995;15(7):3563–3570. doi: 10.1128/mcb.15.7.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickson EJ, Jensen JB, Hille B. Golgi and plasma membrane pools of PI(4)P contribute to plasma membrane PI(4,5)P2 and maintenance of KCNQ2/3 ion channel current. Proc Natl Acad Sci USA. 2014;111(22):E2281–E2290. doi: 10.1073/pnas.1407133111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson EJ, et al. Dynamic formation of ER-PM junctions presents a lipid phosphatase to regulate phosphoinositides. J Cell Biol. 2016;213(1):33–48. doi: 10.1083/jcb.201508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C, Watras J, Loew LM. Kinetic analysis of receptor-activated phosphoinositide turnover. J Cell Biol. 2003;161(4):779–791. doi: 10.1083/jcb.200301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bezanilla F, Villalba-Galea CA. The gating charge should not be estimated by fitting a two-state model to a Q-V curve. J Gen Physiol. 2013;142(6):575–578. doi: 10.1085/jgp.201311056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui J. Voltage-Dependent Gating: Novel Insights from KCNQ1 Channels. Biophys J. 2016;110(1):14–25. doi: 10.1016/j.bpj.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamura Y. Biodiversity of voltage sensor domain proteins. Pflugers Arch. 2007;454(3):361–371. doi: 10.1007/s00424-007-0222-6. [DOI] [PubMed] [Google Scholar]
- 44.Jarquin-Pardo M, Fitzpatrick A, Galiano FJ, First EA, Davis JN. Phosphatidic acid regulates the affinity of the murine phosphatidylinositol 4-phosphate 5-kinase-Ibeta for phosphatidylinositol-4-phosphate. J Cell Biochem. 2007;100(1):112–128. doi: 10.1002/jcb.21027. [DOI] [PubMed] [Google Scholar]
- 45.Sharma D, Kinsey WH. Fertilization triggers localized activation of Src-family protein kinases in the zebrafish egg. Dev Biol. 2006;295(2):604–614. doi: 10.1016/j.ydbio.2006.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bates RC, et al. Activation of Src and release of intracellular calcium by phosphatidic acid during Xenopus laevis fertilization. Dev Biol. 2014;386(1):165–180. doi: 10.1016/j.ydbio.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stith BJ. Phospholipase C and D regulation of Src, calcium release and membrane fusion during Xenopus laevis development. Dev Biol. 2015;401(2):188–205. doi: 10.1016/j.ydbio.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner PR, Sheetz MP, Jaffe LA. Fertilization increases the polyphosphoinositide content of sea urchin eggs. Nature. 1984;310(5976):414–415. doi: 10.1038/310414a0. [DOI] [PubMed] [Google Scholar]
- 49.Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P(2) at fertilization of mouse eggs. J Cell Sci. 2002;115(Pt 10):2139–2149. doi: 10.1242/jcs.115.10.2139. [DOI] [PubMed] [Google Scholar]
- 50.Kamel LC, Bailey J, Schoenbaum L, Kinsey W. Phosphatidylinositol metabolism during fertilization in the sea urchin egg. Lipids. 1985;20(6):350–356. doi: 10.1007/BF02534201. [DOI] [PubMed] [Google Scholar]
- 51.Ciapa B, Borg B, Whitaker M. Polyphosphoinositide metabolism during the fertilization wave in sea urchin eggs. Development. 1992;115(1):187–195. doi: 10.1242/dev.115.1.187. [DOI] [PubMed] [Google Scholar]
- 52.Chang F, Minc N. Electrochemical control of cell and tissue polarity. Annu Rev Cell Dev Biol. 2014;30(1):317–336. doi: 10.1146/annurev-cellbio-100913-013357. [DOI] [PubMed] [Google Scholar]
- 53.Mavrantoni A, et al. A method to control phosphoinositides and to analyze PTEN function in living cells using voltage sensitive phosphatases. Front Pharmacol. 2015;6:68. doi: 10.3389/fphar.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Comer FI, Parent CA. Phosphoinositides specify polarity during epithelial organ development. Cell. 2007;128(2):239–240. doi: 10.1016/j.cell.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27(41):5464–5476. doi: 10.1038/onc.2008.243. [DOI] [PubMed] [Google Scholar]
- 56.Fabian L, et al. Phosphatidylinositol 4,5-bisphosphate directs spermatid cell polarity and exocyst localization in Drosophila. Mol Biol Cell. 2010;21(9):1546–1555. doi: 10.1091/mbc.E09-07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96(8):4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4(4):257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 59.Kumánovics A, Levin G, Blount P. Family ties of gated pores: Evolution of the sensor module. FASEB J. 2002;16(12):1623–1629. doi: 10.1096/fj.02-0238hyp. [DOI] [PubMed] [Google Scholar]
- 60.Kuemmel A, et al. Humoral immune responses of lung cancer patients against the Transmembrane Phosphatase with TEnsin homology (TPTE) Lung Cancer. 2015;90(2):334–341. doi: 10.1016/j.lungcan.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Lachyankar MB, et al. A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20(4):1404–1413. doi: 10.1523/JNEUROSCI.20-04-01404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441(7092):518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 63.Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3(11):e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferreira LMR, Floriddia EM, Quadrato G, Di Giovanni S. Neural regeneration: Lessons from regenerating and non-regenerating systems. Mol Neurobiol. 2012;46(2):227–241. doi: 10.1007/s12035-012-8290-9. [DOI] [PubMed] [Google Scholar]
- 65.Liu K, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13(9):1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keum D, Baek C, Kim DI, Kweon HJ, Suh BC. Voltage-dependent regulation of CaV2.2 channels by Gq-coupled receptor is facilitated by membrane-localized β subunit. J Gen Physiol. 2014;144(4):297–309. doi: 10.1085/jgp.201411245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jensen JB, Lyssand JS, Hague C, Hille B. Fluorescence changes reveal kinetic steps of muscarinic receptor-mediated modulation of phosphoinositides and Kv7.2/7.3 K+ channels. J Gen Physiol. 2009;133(4):347–359. doi: 10.1085/jgp.200810075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Wal J, Habets R, Várnai P, Balla T, Jalink K. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J Biol Chem. 2001;276(18):15337–15344. doi: 10.1074/jbc.M007194200. [DOI] [PubMed] [Google Scholar]
- 70.Itsuki K, et al. PLC-mediated PI(4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels. J Gen Physiol. 2014;143(2):183–201. doi: 10.1085/jgp.201311033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dickson EJ, Falkenburger BH, Hille B. Quantitative properties and receptor reserve of the IP3 and calcium branch of Gq-coupled receptor signaling. J Gen Physiol. 2013;141(5):521–535. doi: 10.1085/jgp.201210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falkenburger BH, Dickson EJ, Hille B. Quantitative properties and receptor reserve of the DAG and PKC branch of Gq-coupled receptor signaling. J Gen Physiol. 2013;141(5):537–555. doi: 10.1085/jgp.201210887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Várnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143(2):501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dowler S, et al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J. 2000;351(Pt 1):19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hammond GRV, Machner MP, Balla T. A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J Cell Biol. 2014;205(1):113–126. doi: 10.1083/jcb.201312072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamman BD, Pollok BA, Bennett T, Allen J, Heim R. Binding of a Pleckstrin homology domain protein to phosphoinositide in membranes: A miniaturized FRET-based assay for drug screening. J Biomol Screen. 2002;7(1):45–55. doi: 10.1177/108705710200700107. [DOI] [PubMed] [Google Scholar]