Abstract
Mitochondrial dysfunction entailing decreased energy‐transducing capacity and perturbed redox homeostasis is an early and sometimes initiating event in ageing and age‐related disorders involving tissues with high metabolic rate such as brain, liver and heart. In the central nervous system (CNS), recent findings from our and other groups suggest that the mitochondrion‐centred hypometabolism is a key feature of ageing brains and Alzheimer's disease. This hypometabolic state is manifested by lowered neuronal glucose uptake, metabolic shift in the astrocytes, and alternations in mitochondrial tricarboxylic acid cycle function. Similarly, in liver and adipose tissue, mitochondrial capacity around glucose and fatty acid metabolism and thermogenesis is found to decline with age and is implicated in age‐related metabolic disorders such as obesity and type 2 diabetes mellitus. These mitochondrion‐related disorders in peripheral tissues can impact on brain functions through metabolic, hormonal and inflammatory signals. At the cellular level, studies in CNS and non‐CNS tissues support the notion that instead of being viewed as autonomous organelles, mitochondria are part of a dynamic network with close interactions with other cellular components through energy‐ or redox‐sensitive cytosolic kinase signalling and transcriptional pathways. Hence, it would be critical to further understand the molecular mechanisms involved in the communication between mitochondria and the rest of the cell. Therapeutic strategies that effectively preserves or improve mitochondrial function by targeting key component of these signalling cascades could represent a novel direction for numerous mitochondrion‐implicated, age‐related disorders.
Abbreviations
- Aβ
β‐amyloid
- Akt
protein kinase B
- AMPK
5’‐adenosine monophosphate‐activated protein kinase
- AP‐1
activator protein‐1
- BAT
brown adipose tissue
- ER
endoplasmic reticulum
- ERRα
oestrogen‐related receptor α
- GABA
γ‐aminobutyric acid
- GSK‐3β
glycogen synthase kinase‐3β
- IGF1
insulin‐like growth factor 1
- IIS
insulin/IGF1 signalling
- IRS
insulin receptor substrate
- JNK
c‐Jun N‐terminal kinase
- mTORc1
mammalian target of rapamycin complex 1
- NAA
N‐acetylaspartate
- NFκB
nuclear factor κ‐light‐chain‐enhancer of activated B cells
- NMR
nuclear magnetic resonance
- NRF
nuclear respiratory factor
- Nrf2
nuclear factor (erythroid‐derived 2)‐like 2
- OXPHOS
oxidative phosphorylation
- PDH
pyruvate dehydrogenase
- PET
positron emission tomography
- PGC1α
peroxisome‐proliferator‐activated receptor γ coactivator‐1α
- PI3K
phosphatidylinositide 3‐kinase
- PTEN
phosphatase and tensin homologue
- SCOT
succinyl‐CoA transferase
- Sirt1
sirtuin 1
- TCA
tricarboxylic acid
- TRE
tetradecanoyl phorbol acetate response element
- UCP
uncoupling protein
- WAT
white adipose tissue
Energy metabolism in brain ageing and Alzheimer's disease
Brain ageing
Brain utilizes 25% of the total body glucose to meet its energy demands; hence, maintenance of glucose homeostasis is critical for brain function, for glucose is the primary fuel meeting the energy demands of neurons and glial cells. Ketone bodies constitute a secondary fuel, especially during long fasting periods and starvation. Pronounced energy deficits are a feature of the ageing brain that are accompanied by neuronal loss, impaired cognition and memory, and an increased risk for neurodegenerative disorders. The gradual decline in energy metabolism during brain ageing and some neurodegenerative disorders results in a hypometabolic state, which is a function of deficits in (a) substrate supply, (b) mitochondrial catalysis and energy transduction, and (c) cytosolic metabolic and signalling pathways. Mitochondria play a central role for they integrate several signalling pathways and generate molecules that coordinate cytosolic signalling and transcriptional pathways (Fig. 1).
Figure 1. Coordination of insulin/IGF1 signalling and JNK signalling with substrate availability and mitochondrial catalytic machinery in brain .
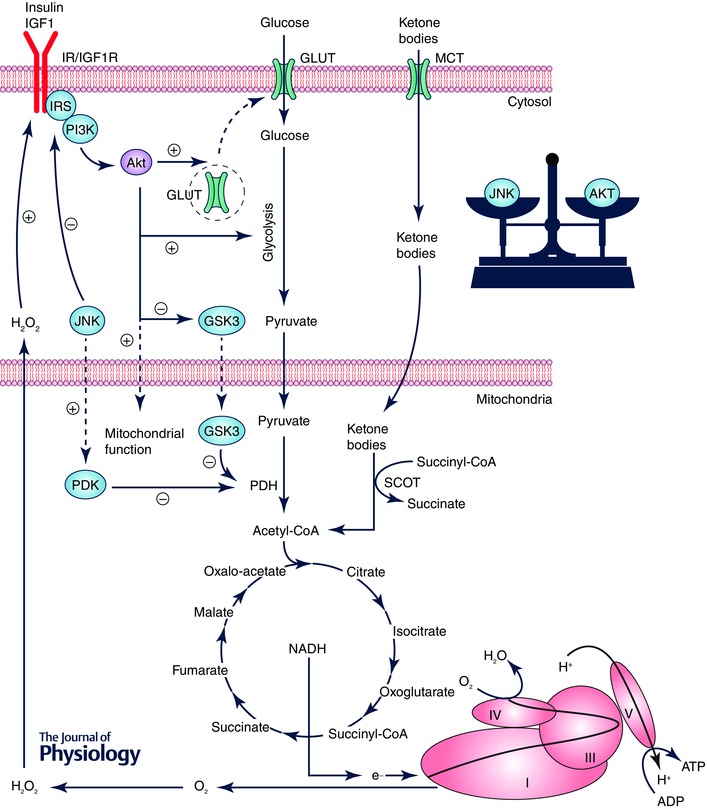
Pyruvate, generated from glucose by glycolysis in cytosol, undergoes oxidative decarboxylation by pyruvate dehydrogenase (PDH) to yield acetyl‐CoA. Ketone body metabolism is regulated by succinyl‐CoA transferase (SCOT) to yield acetyl‐CoA. Acetyl‐CoA generated by these pathways enters the TCA cycle to produce primarily NADH, which provides electrons to the electron transport chain to build up the proton motive force for ATP synthesis by complex V. Binding of insulin and IGF1 to their receptors activates the insulin receptor substrate (IRS) and the downstream phosphatidylinositide 3‐kinase (PI3K)/protein kinase B (Akt) pathway, which (1) facilitate the translocation of glucose transporters (GLUT3/4 in brain) to plasma membrane, (2) promote glycolytic reactions, and (3) enhance mitochondrial function through the translocation of Akt to mitochondria and the inhibition of glycogen synthase kinase‐3β (GSK3β, an inhibitor of PDH). O2.− generated by the electron transport chain at complex I and III is reduced to H2O2, which is released to the cytosol where it modulates the redox‐sensitive insulin/IGF1 signalling (IIS) and c‐Jun N‐terminal kinase (JNK) signalling. JNK can negatively regulate IIS by phosphorylating IRS at Ser307/312.
Dynamic micro‐positron emission tomography (PET) scanning using 18F‐labelled fluorodeoxyglucose (FDG) as a tracer showed a significant decline in glucose uptake during brain ageing in several rodent models. The decrease in glucose uptake (Fig. 2) is paralleled by a decrease in expression and translocation to the membrane of the insulin‐sensitive glucose transporters, GLUT4 and GLUT3, as well as the vascular endothelium glucose transporter GLUT1 (55 kDa); the expression of the glial glucose transporter GLUT1 (45 kDa) did not change or was increased as a function of age in Fischer 344 rats (Jiang et al. 2013). The increase in astrocytic GLUT1 (45 kDa) may account for the age‐dependent astrocytic metabolic shift (Jiang & Cadenas, 2014). Anaerobic glycolysis in astrocytes yields lactate from pyruvate reduction, and lactate released from astrocytes is utilized by neurons as an energy source (Bolaños et al. 2010; Bélanger et al. 2011). The age‐dependent astrocytic metabolic shift consists of an increase in their mitochondrial oxidative metabolism (Jiang & Cadenas, 2014) thereby depriving neurons of energy substrates (lactate) and exacerbating the inherent hypometabolic state in brain. Astrocytes are generally considered neurotrophic inasmuch as they provide neurons with energy substrates and recycle neurotransmitters (Jiang & Cadenas, 2014). Of note, the decline in glucose transport and metabolism was preceded by a shift to a ketogenic system in the female mouse brain during ageing as well as in a triple transgenic mouse model of Alzheimer's disease (Ding et al. 2013 a,b).
Figure 2. Brain glucose uptake decreases as a function of age .
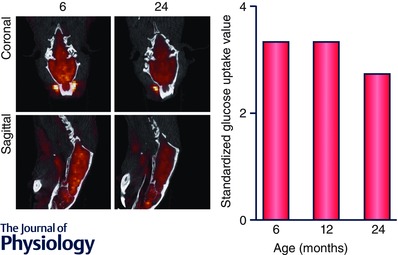
Dynamic microPET scanning using 18F‐labelled fluorodeoxyglucose (FDG)‐PET as a tracer in Fischer 344 rats demonstrated a significant decline in glucose uptake and metabolism in 24‐month‐old rats as compared to 6‐month‐old rats. Calculation of selective uptake values (SUV), which measures the kinetics of glucose uptake, demonstrates that the SUV for the 24‐month rat is lower than the 6‐month rat (at end of scan, 2.72 as compared to 3.34).
A decline in the mitochondrial catalytic machinery, in terms of deficits in expression and activity of respiratory chain complexes I and IV and an increase in mtDNA mutations, contributes further to the hypometabolic state (Drew & Leeuwenburgh, 2004; Navarro & Boveris, 2007; Boveris & Navarro, 2008). The age‐dependent phosphorylation of the E1α subunit of the pyruvate dehydrogenase complex results in its inactivation and, consequently, a decrease of acetyl‐CoA delivery to the tricarboxylic acid (TCA) cycle, increase reduction of pyruvate to lactate, and decrease in ATP production (Zhou et al. 2008, 2009). In addition, post‐translational modifications can impair mitochondrial function: the age‐dependent increase in neuronal nitric oxide synthase expression leads to nitration of mitochondrial proteins, such as succinyl‐CoA transferase (SCOT) and F1‐ATPase, thus resulting in a moderately impaired mitochondrial function (Lam et al. 2009). Mitochondria are highly dynamic organelles and undergo fusion and fission continuously, which regulates not only mitochondrial morphology, but also their biogenesis, trafficking and localization, quality control and degradation (Twig & Shirihai, 2011; Chan, 2012). Mitochondrial fusion and fission is found to be diminished or imbalanced in tissue ageing and neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease and Huntington's disease (Seo et al. 2010). Mitochondrial dynamics has some undefined role in organelle turnover, supposedly affecting the degradation pathways; in this regard, lysosomal autophagy declines in several tissues with age (Seo et al. 2010).
Alzheimer's disease
Alzheimer's disease is a progressive neurodegenerative disease involving biochemical (Gottfries et al. 1983), metabolic (Mosconi, 2005) and physiological (Farkas & Luiten, 2001) changes that result in impairments of memory, thinking and behaviour. Alzheimer's disease is associated with β‐amyloid (Aβ) plaques and neurofibrillary tangles (hyperphosphorylated tau), the detection of which in post‐mortem tissue validates a definite diagnosis (Dubois et al. 2007). The aetiology of Alzheimer's disease has been hypothesized by several theories such as the β‐amyloid hypothesis (Tanzi & Bertram, 2005), cholinergic hypothesis (Francis et al. 1999), tau hypothesis (Maccioni et al. 2010), oxidative stress hypothesis (Markesbery, 1997), and mitochondrial cascade hypothesis (Swerdlow & Khan, 2009).
There is growing evidence for an early mitochondrial dysfunction preceding the classical Alzheimer's disease pathological hallmarks, i.e. Aβ plaques and neurofibrillary tangles (Brinton, 2008; Mosconi et al. 2011; Swerdlow, 2011). Post‐mortem tissues of individuals with Alzheimer's disease have been identified to have disruptions of mitochondrial functions in terms of altered morphology, compromised electron transfer chain complexes, and tricarboxylic acid cycle deficiencies (Perry et al. 1980; Blass et al. 2000). The mitochondrial cascade hypothesis proposes a late‐onset, sporadic Alzheimer's disease that reconciles the histopathological and pathophysiological features. This hypothesis proposes that the genetic makeup of an individual's electron transport train sets the basal rates for production of reactive oxygen species and thus sets the tone for oxidative damage. The cells respond to this oxidative stress by generating pathological features like β‐amyloid; this sets a cycle that results in aneuploidy, tau phosphorylation, and neurofibrillary tangle formation. The intrinsic need of brain for high energy dictates its dependence on functional mitochondria and also renders it sensitive to changes in mitochondrial function (Kann & Kovács, 2007). Because mitochondria also play an important role in cell signalling, changes in mitochondria are relayed to the entire cell and beyond. Several early pieces of evidence demonstrated the role of oxidative damage in Alzheimer's disease (Christen, 2000).
While oxidative stress and pathological manifestations clearly exist in Alzheimer's disease, there is growing research pointing towards the disturbances in energy metabolism being closely associated with this disease. Perturbations of glucose metabolism and mitochondrial bioenergetics apparently precede the development of Alzheimer's disease pathology (Gibson et al. 1998; Hauptmann et al. 2009; Yao et al. 2009; Galindo et al. 2010). Multiple clinical studies have demonstrated that decreased brain glucose uptake is a common condition in Alzheimer's disease and mild cognitive impairment (Mosconi, 2005; Mosconi et al. 2008, 2009). However, these disturbances also extend to the glycolytic pathway and intermediates of the tricarboxylic acid cycle and the several neurotransmitters derived from it, such as glutamate, glutamine, γ‐aminobutyric acid (GABA), and N‐acetylaspartate (NAA) (Moats et al. 1994; Lin et al. 2003; Sancheti et al. 2014 a).
Ninety per cent of glucose entering the brain is oxidized to CO2, primarily by mitochondrial metabolism (Mangialasche et al. 2010). The majority of this energy is utilized in maintaining neurotransmission and neuronal potential, and preventing excitotoxicity (Magistretti & Allaman, 2013). Thus, any disturbances in glucose uptake or metabolism would affect neurotransmission and neuronal function, and ultimately impinge on cognition, learning and memory. Long‐term potentiation is widely believed to be the cellular biochemical mechanism underlying synaptic plasticity (Bliss & Collingridge, 1993). Decreased brain glucose uptake has been demonstrated to be associated with substantially decreased long‐term potentiation in the 3xTg‐AD mouse model of Alzheimer's disease (Sancheti et al. 2013) and in a rat model of female perimenopausal ageing (Yin et al. 2015). The decline in glucose metabolism in 12‐month‐old 3xTg‐AD mice was also reflected by an approximately 50% decrease of glucose supported TCA cycle‐related metabolites including glutamate, glutamine, GABA and NAA (Sancheti et al. 2014 a). This led to a decrease in the flux of glucose being converted into TCA cycle metabolites, a process critical to generating neurotransmitters and maintaining synaptic plasticity. In fact, metabolic alterations in these mitochondrial TCA cycle metabolites have been demonstrated in different rodent models of Alzheimer's disease (Dedeoglu et al. 2004; Marjanska et al. 2005; Salek et al. 2010; Esteras et al. 2012; Tiwari & Patel, 2012; Haris et al. 2013; Nilsen et al. 2013; van Duijn et al. 2013; Doert et al. 2015) and clinical cases (Lin et al. 2003). These studies suggest that both lowered brain glucose uptake and alternations in mitochondrial TCA cycle function contribute to the hypometabolic state observed in ageing and Alzheimer's disease.
Although a hypometabolic state of mitochondrial TCA cycle metabolites is well studied in older rodent models of Alzheimer's disease, a relatively unexplored area is the presence of a hypermetabolic state that perhaps precedes this hypometabolic state of the mitochondrial TCA cycle metabolites. A hypermetabolic state was observed in the 7‐month‐old 3xTg‐AD rodent model of Alzheimer's disease at variance with the hypometabolic state that was reported in the 13‐month‐old 3xTg‐AD mice (Sancheti et al. 2014 b). This hypermetabolic state was hypothesized to be linked with the presence of Aβ plaques at 7 months of age in the 3xTg‐AD mouse model. Other Aβ‐based rodent models of Alzheimer's disease have also shown signs of hypermetabolism (Busche et al. 2008; Puzzo et al. 2008; Luo et al. 2012; Poisnel et al. 2012; Nilsen et al. 2013; Rojas et al. 2013); these studies provide several pieces of evidence that link hypermetabolism with Aβ plaques. This raises a question about the possible presence of a hypermetabolic state (encompassing mitochondrial hypermetabolism) in very early stages of Alzheimer's disease that have not been studied thoroughly. Overall, the mitochondrial perturbations in metabolism of glycolytic substrates (into the TCA cycle related metabolites) highlight the importance of metabolism in the coordination of pathology and cognitive decline associated with Alzheimer's disease.
Energy metabolism in adipose tissue ageing
Adipose tissue stores energy in the form of triglycerides and supplies energy in the process of fatty acid β‐oxidation under conditions of fasting or lowered liver glycogen levels (Girard & Lafontan, 2008). Mammals have two types of adipose tissue, the white and the brown adipose tissue (WAT and BAT, respectively), which can be distinguished by their morphology, metabolic activities, and cellular density of mitochondria (Saely et al. 2012). The WAT, with lower density of mitochondria, represents about 10% of body weight in lean humans as visceral and subcutaneous fat. WAT participates in the regulation of energy storage, insulin sensitivity and glucose metabolism in liver and muscle. BAT, with a higher numbers of mitochondria, dissipates energy as heat through adaptive thermogenesis (Virtanen et al. 2009).
Mitochondria in WAT ageing
Although their abundance is lower, mitochondria play essential roles in WAT function. Firstly, mitochondria in WAT provide substrates for fatty acids synthesis and fatty acid esterification, in the forms of acetyl‐CoA and glycerol‐3‐phosphate, respectively (Nye et al. 2008). Secondly, WAT mitochondria generate ATP to support lipogenic processes in differentiating pre‐adipocytes and adipocyte maturation (Lu et al. 2010). Moreover, mitochondrial H2O2 and enhanced biogenesis are causal factors that promote adipocyte differentiation in a mammalian target of rapamycin complex 1 (mTORc‐1)‐dependent manner (Tormos et al. 2011). Thirdly, mitochondria are involved in the synthesis of WAT‐generated adipokines (Trayhurn & Wood, 2004). For instance, mitochondrial dysfunction decreases adiponectin synthesis via the activation of a series of pathways that involve endoplasmic reticulum (ER) stress, c‐Jun N‐terminal kinase (JNK) and activating transcription factor 3 (ATF3) (Koh et al. 2007).
Mitochondrial dysfunction is associated with adipose tissue ageing. In parallel with the decline in lipolysis (Dax et al. 1981; Klein et al. 1986), both mtDNA content and mitochondrial oxidative phosphorylation (OXPHOS) proteins in WAT decrease with ageing and age‐related disorders, such as obesity and type 2 diabetes (Patti & Corvera, 2010; Donato et al. 2014). Calorie restriction is the only known reproducible experimental paradigm that extends maximal lifespan and delays the onset of many age‐related diseases (Masoro, 2005). Long‐term calorie restriction shifts WAT toward the activation of energy metabolism by upregulating genes required for glycolysis, lipogenesis, amino acid metabolism and mitochondrial energy metabolism including those involved in the TCA cycle, β‐oxidation, electron transport and OXPHOS (Higami et al. 2004). It is proposed that upon calorie restriction, WAT functions as an energy transducer that converts glucose to the high energy‐dense lipids (Okita et al. 2012). Long‐term calorie restriction also downregulates the expression of more than 50 pro‐inflammatory genes in mouse epididymal WAT (Higami et al. 2004; Higami et al. 2006). Fat‐specific insulin receptor knock‐out (FIRKO) mice exhibit reduced body weight and increased lifespan, despite normal or increased food intake and their WAT has higher expression of genes involved in glycolysis, the TCA cycle, β‐oxidation and OXPHOS, which are correlated with increased mitochondrial biogenesis (Katic et al. 2007). Data on caloric restriction and FIRKO mouse models suggest a close relationship between WAT ageing and mitochondrial function.
Mitochondria in BAT ageing
BAT was considered to wane fast after birth in humans; however, recent studies using positron emission tomography demonstrated that BAT remains present during adulthood (Zingaretti et al. 2009). In mammals, BAT plays an important role in thermogenesis with mitochondria at the centre stage burning fatty acids to generate heat to maintain body temperature in cold environments, a process driven by uncoupling protein 1 (UCP1) by stimulating H+ leak across the mitochondrial inner membrane without ATP production (Rousset et al. 2004). UCP1 is regulated by mitochondrion‐associated histone deacetylase SIRT3 through peroxisome‐proliferator‐activated receptor γ coactivator‐1α (PGC1α) and transcription factor CREB (Shi et al. 2005). During cold exposure, mitochondrial dynamin‐related protein 1 is activated, promoting fission and sensitizing the mitochondria to free fatty acids (Wikstrom et al. 2014). In addition to heat generation, BAT thermogenesis is capable of protecting against diet‐induced obesity (Hamann et al. 1998; Kontani et al. 2005)
The mass and thermogenesis capability of BAT declines with age in humans (Saito et al. 2009; Pfannenberg et al. 2010). Ageing reduces mitochondrial biogenesis, which, in turn, impairs the formation of thermogenic brown adipocytes (Graja & Schulz, 2014). As seen in WAT, caloric restriction is also effective in preventing BAT activity loss during ageing by preserving mitochondrial function. Long‐term caloric restriction delays the age‐related decline in mitochondrial mass, complex IV activity, uncoupling levels and mitochondrial transcription factor A (Tfam) in BAT of rats (Valle et al. 2008). Caloric restriction also increased fatty acid biosynthesis but not mitochondrial respiratory capacity (Okita et al. 2012). Exercise is another potent inducer of BAT mass and adrenergic brown recruitment of adipocytes in aged animals, without changing the mRNA or protein levels of mitochondrial UCPs (Scarpace et al. 1994; Oh‐ishi et al. 1996; De Matteis et al. 2013).
Liver energy metabolism and metabolic diseases
These age‐related changes in liver modulate digestion, metabolism, immunity, storage of nutrients and clearance of drugs (Le Couteur & McLean, 1998). Ageing exhibits a significant negative correlation with liver volume, ascribed to the age‐dependent decrease in hepatic blood flow (Wynne et al. 1989). The gross appearance of liver from elderly subjects is similar to younger individuals with malnutrition and cachexia. It has a brown colour due to the accumulation of lipofuscin within hepatocytes. There is also the presence of macrohepatocytes and polyploidy along with an increase in nuclei and nucleoli (Schmucker, 1998; Anantharaju et al. 2002). Liver triglycerides and cholesterol levels increase with age and are correlated with declining metabolism of low‐density lipoprotein and decrease in their receptors (Aaronson & Woo, 1981).
During ageing, liver mitochondria show increased size (Sastre et al. 1996), decreased matrical density and decreased number (Schmucker, 1998). Additionally, there is a decrease in membrane potential (Sastre et al. 1996) and respiratory chain enzymes (Muller‐Hocker et al. 1997). Around 87% of those above 50 years of age were found to have defects in the respiratory chain caused by a loss of enzyme proteins involving both nuclear and mitochondrial coded subunits. The majority of these subjects (94%) had a defect in the complex IV subunit, whereas 4% had defects in the complex III subunit (Muller‐Hocker et al. 1997). Additionally, the content of cytochrome oxidase also declined with age along with age‐related decline in the mtRNA synthesis in heart, lungs, brain, liver and skeletal muscle (Anantharaju et al. 2002). These decreases in respiratory chain enzymes are correlated with a decrease in mitochondrial respiratory capacity. A significant negative correlation between age and respiratory control ratio was observed in Chinese populations of various ages (Yen et al. 1989).
Ageing liver mitochondria are also accompanied by increased oxidative modifications that negatively impact their function (Richter, 1995; Sastre et al. 2000; Navarro & Boveris, 2004; Castro et al. 2012). Multiple mitochondrial proteins also undergo oxidative damage in an age‐related manner (Kolosova et al. 2003). Interestingly, Lon protease, a key enzyme in the degradation of oxidized proteins within the mitochondrial matrix and typically highly induced under stress, declines with age. Thus, Lon protease has been suggested to be a significant factor in age and age‐related diseases (Ngo et al. 2013). Mice expressing proofreading‐deficient version of the mitochondrial DNA polymerase γ accumulated mtDNA mutations that resulted in accelerated ageing and correlated with the induction and increase of apoptotic markers in an age‐related manner (Kujoth et al. 2005). There is also an age‐related decline of mtRNA synthesis in brain, liver, heart, lungs and skeletal muscle (Anantharaju et al. 2002). Additionally, rat liver Kupffer cells show decreased function (Brouwer et al. 1985) and efficiency to phagocytose and degrade radiolabelled mitochondria (Martin et al. 1994). This perhaps leads to more severely damaged mitochondria that accumulate with age. Thus, the age‐related macro changes in liver are accompanied by several subcellular micro changes in the liver mitochondria.
The liver–brain axis
The liver senses blood glucose levels adequately to control utilization of glucose by regulating glycogenesis and glycogenolysis; when the liver glycogen reserves are running low, hepatocytes maintain an adequate supply of glucose to the brain by activating gluconeogenesis from non‐carbohydrate carbon sources. Importantly, mitochondria take centre‐stage in this liver‐centric energy homeostasis and a multi‐level regulation ensures a constant supply of energy to the brain, thus forming the core of a metabolic ‘liver–brain axis’.
The neurotoxic role of liver‐generated ceramides is an example of an impaired ‘liver–brain axis’ with implications for Alzheimer's disease (de la Monte et al. 2009 a, 2010; de la Monte, 2012). On the one hand, ceramides contribute to cell membrane structure and have roles in growth, proliferation, motility, apoptosis, differentiation, senescence (Zheng et al. 2006; de la Monte, 2012) and maintenance of the skin barrier (Wartewig & Neubert, 2007). On the other hand, ceramides function as a lipid signals that can cause insulin resistance (Teruel et al. 2001; Chavez et al. 2005; Summers, 2006; Delarue & Magnan, 2007), cytotoxicity and inflammation (de Mello et al. 2009; Gill & Sattar, 2009). These effects of ceramides have been hypothesized to cause a ‘triangulated mal‐signalling in Alzheimer's disease’ (de la Monte, 2012): toxic ceramides generated from extra‐CNS tissues (e.g. liver) are released into the blood, bypass the blood–brain barrier, and cause brain insulin resistance, inflammation and cell death, all of which impair synaptic plasticity. In support of this hypothesis, Long–Evans rat pups administered ceramide analogues by intraperitoneal injection developed hyperglycaemia, hyperlipidaemia, mild steatohepatitis, reduced brain lipid content, increased ceramide levels in liver, brain and serum, and significant abnormalities in spatial learning and memory (de la Monte et al. 2010). Impairment of the phosphatidylinositide 3‐kinase (PI3K)/protein kinase B (Akt) signalling cascade by ceramides led to cognitive and motor dysfunctions (de la Monte et al. 2012). A similar pattern is also observed with alcohol‐associated neurodegeneration: chronic alcohol consumption produced steatohepatitis, promoting insulin resistance and pro‐inflammatory cytokines that lead to an increased release of toxic lipids such as ceramides (de la Monte et al. 2009 a). Other compounds directly affecting the liver, i.e. nitrosamines (commonly found in fried fast foods), induced insulin resistance, type 2 diabetes (in liver and brain), non‐alcoholic steatohepatitis, deficits in spatial learning and neurodegeneration (de la Monte et al. 2009 b).
Several studies have examined the role of insulin resistance in Alzheimer's disease; however, the mechanistic/pathological aspects of the disease can be modulated by agents that combat insulin resistance (McClean et al. 2011; Businaro et al. 2012; Sancheti et al. 2014 a). Thus, the signalling pathways involved in the liver–brain axis form an intricate communication network that ensures adequate supply of energy substrates to maintain a healthy brain; pathological or chemical intrusions to the liver seem to play an important role in initiation and progression of neurodegeneration.
What is the role of mitochondria in the liver–brain axis? It is well known that the mitochondrial function is impaired during insulin resistance (Petersen et al. 2004; Lowell & Shulman, 2005; Parish & Petersen, 2005; Højlund et al. 2008). Insulin resistance is mainly characterized by the inability of insulin to stimulate glucose uptake by peripheral tissues and/or control the synthesis of glucose by liver, the actions of which manifest as hyperglycaemia, hyperinsulinaemia and dyslipidaemia. This dysregulation leads to obesity, type 2 diabetes, cardiovascular disease, and neurodegeneration (Saltiel & Kahn, 2001; White, 2003; Cheng et al. 2010). From the point‐of‐view of the liver–brain axis, mitochondrial functions connected with maintenance of energy metabolism and redox control seem to be of the outmost importance.
Owing to these pivotal roles of mitochondria, it is not surprising that perturbation of mitochondrial function is involved in metabolic disorders like type 2 diabetes, insulin resistance, cardiovascular complications and obesity. Subjects with obesity or type 2 diabetes have mitochondria with an impaired bioenergetic capacity (Kelley et al. 2002). The linkage between mitochondrial dysfunction and type 2 diabetes has been reviewed earlier (Lowell & Shulman, 2005). Nuclear magnetic resonance (NMR) studies showed that elderly subjects had a 40% decrease in mitochondrial oxidative phosphorylation capacity (Petersen et al. 2003) and insulin‐resistant subjects had a 60% decrease in insulin‐stimulated rate of glucose uptake and a 30% reduction in mitochondrial oxidative phosphorylation (Petersen et al. 2004). Another major link between these metabolic conditions is their association with dysfunctional liver mitochondria and/or increased fat accumulation in the liver tissue (Petersen et al. 2003). In fact, liver mitochondrial dysfunction has been shown to precede hepatic steatosis and insulin resistance (Rector et al. 2010). Transgenic mice with liver‐specific overexpression of lipoprotein lipase were insulin resistant with twofold increased liver triglyceride content. They were also associated with an impaired ability of insulin to suppress endogenous glucose production due to inactivated insulin receptor substrate‐2 and PI3K activity (Kim et al. 2001 b). Interestingly, chronic leptin treatment reversed insulin resistance and hepatic steatosis in patients with severe lipodystrophy. Thus, modulation of energy homeostasis presents an interesting target against metabolic conditions like insulin resistance, obesity and type 2 diabetes (Petersen et al. 2002). Overall, mitochondrial activity is a prime modulator of the liver–brain axis in maintaining adequate substrate supply for the brain and its dysfunction is associated with the pathologies that impinge on the liver–brain axis.
Metformin, widely used for the treatment of type 2 diabetes, reduces hepatic gluconeogenesis and enhances peripheral insulin sensitivity; its mechanism of action entails inhibition of complex I of the mitochondrial respiratory chain (Owen et al. 2000) and this may account partly for activation of 5'‐adenosine monophosphate‐activated protein kinase (AMPK) (Zhou et al. 2001). Inhibition of complex I results in decreased ATP levels and increased AMP, which binds to the γ subunit of AMPK, thereby activating it. However, inhibition of the mitochondrial glycerol‐P dehydrogenase activity (a component of the glycerol‐P shuttle) by metformin is another mechanism to augment the cytosol reducing environment leading to the overproduction of lactate and inhibition of gluconeogenesis; this together with other metformin‐sensitive processes, such as suppression of glucagon signalling, activation of autophagy and lessening of inflammasome‐dependent cytokine production, may suggest new target pathways for the treatment of type 2 diabetes (Hur & Lee, 2015).
Mitochondrial signalling molecules and redox‐sensitive kinase signalling
Mitochondrial H2O2 as a signalling molecule
The redox‐regulating capacity of the mitochondria generates second messengers such as H2O2 that regulate multiple cell signalling pathways and a range of cell functions (Fig. 3) (Ghafourifar & Cadenas 2005; Yin et al. 2012 a, 2014). H2O2 acts as an efficient redox molecule since it can easily pass the mitochondrial membranes. Additionally, H2O2 produced by one mitochondrion can diffuse to other mitochondria, thus relaying signals among mitochondria (Murphy, 2009). Mitochondrial O2.– (more likely a long‐lived species) was proposed as the critical response of cells to hyperglycaemia (Brownlee, 2005) leading to the activation of the four major pathways of hyperglycaemic damage: inhibition of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) by the O2.–‐mediated activation of poly ADP ribose polymerase (PARP) seems to be the triggering event. It was emphasized that the cells damaged by hyperglycaemia are those that cannot decrease the transport of glucose inside the cell when exposed to a hyperglycaemic environment (i.e. the cell types involved in diabetic complications). However, the mitochondrial formation of ROS seems to be of importance in the pathogenesis of diabetes mellitus and its complications through modification of various cellular events in many tissues, including vessels, kidney, pancreatic β cells and liver (Piconi et al. 2006; Nishikawa & Araki, 2007; Palmeira et al. 2007). There is some correlative evidence that certain cell types that depend on dehydroascorbate uptake through GLUT have their ability to counteract oxidative stress impaired (Root‐Bernstein et al. 2002). This decrease in cellular transport of dehydroascorbate is implicated in worsening the hyperglycaemia‐induced oxidative stress. Of course, this would be applicable to those cell types that rely on dehydroascorbate uptake through GLUT.
Figure 3. Mitochondrion‐derived energy and redox signals regulate multiple cytosolic and nuclear pathways .
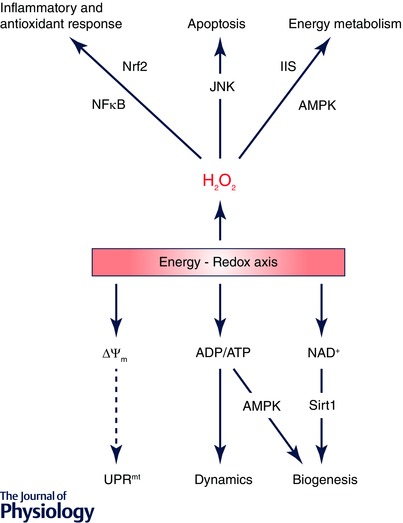
Redox signals, primarily H2O2, modulate cellular energetic pathways through IIS and AMPK signalling; high levels of H2O2 activate apoptotic pathways through JNK. H2O2 also induces multiple inflammatory and antioxidants pathways in the nucleus via transcription factors such as nuclear factor κ‐light‐chain‐enhancer of activated B cells (NFκB) and nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2). On the other hand, energy signals transduced from ADP/ATP and NAD+ to AMPK and sirtuin 1 (Sirt1) control the biogenesis and dynamic remodelling of mitochondria. The mitochondrial unfolded protein response (UPRmt) represents another mechanism through which mitochondria communicate with the nucleus monitoring the organelles’ protein import efficiency, a process dependent on mitochondrial inner membrane potential (ΔΨm).
Insulin/IGF1 signalling
Insulin/IGF1 signalling (IIS) is responsive to H2O2 owing to the presence of several redox‐sensitive cysteine residues on the insulin receptor and IGF‐1 receptor (Fig. 1). Oxidation of these cysteine residues to cystine by H2O2 promotes their tyrosine autophosphorylation and activates downstream signalling cascades that promote metabolic pathways (Loh et al. 2009). Additionally, H2O2 inhibits tyrosine phosphatase (e.g. PTP1B) and lipid phosphatase (PTEN), which are both negative regulators of IIS through the dephosphorylation of insulin/IGF1 receptors and phosphatidylinositol‐3,4,5‐trisphosphate (PIP3), respectively (Elchebly et al. 1999). In cerebellar granule neurons, the mitochondrial respiratory chain‐generated H2O2 was responsible for insulin receptor activation (Storozhevykh et al. 2007); similarly, in hepatocytes, H2O2 activates insulin signalling, demonstrated by increased phosphorylation of insulin receptor (on tyrosine), Akt and glycogen synthase kinase‐3β (GSK‐3β). It is noteworthy that while lower doses of H2O2 (5–10 μm) led to activation of insulin signalling, higher doses of H2O2 (25–50 μm) led to its inactivation (Iwakami et al. 2011). This is likely to be due to the activation of JNK by higher H2O2 concentration, considering JNK as a negative regulator of the IIS (Karpac & Jasper, 2009; Yin et al. 2013).
JNK
Another signalling pathway critically regulated by H2O2 is the stress‐activated JNK signalling. JNKs are multifunctional kinases involved in a variety of pathological conditions due to their role of inducing apoptosis (Cui et al. 2007). H2O2 was shown to activate JNK and decrease cell viability in primary neurons (Zhou et al. 2008) and in hepatocytes (Iwakami et al. 2011). Importantly, we have shown that JNK can be specifically activated by mitochondrially originated H2O2 in nicotinamide nucleotide transhydrogenase (NNT)‐silenced PC12 cells (Yin et al. 2012 b).
AMPK
Interestingly, H2O2 also seems to have a role in the activation of the energy sensor and regulator AMPK, which is typically activated by increased AMP/ATP ratio. The AMPK pathway was activated by increased H2O2 concentrations in HEK cells and in mice (Zmijewski et al. 2010). Under hypoxic conditions, an increase in mitochondrial H2O2 also leads to AMPK activation that is not dependent on the AMP/ATP ratio (Emerling et al. 2009). In early diabetic nephropathy, a decrease in mitochondrial ROS resulted in decreased AMPK activity with downstream consequences such as decrease in PGC1α and mitochondrial biogenesis (Dugan et al. 2013); an alternative explanation (Nishikawa et al. 2015) that considers the substantial increase of ROS in diabetes suggests that a decrease in AMPK activity can account for the increase in mitochondrial ROS.
Regulation of mitochondrial function by cytosolic signalling
Mitochondria modulate cytosolic components through redox‐sensitive signalling; on the other hand, mitochondria are also recipients of cytosolic signalling that in turn regulates mitochondrial metabolic and redox functions.
IIS
The cytosolic modulation of mitochondrial bioenergetic functions is primarily carried out by components of the IIS. It is well known that the mitochondrial function is impaired during insulin resistance, an indicator of compromised insulin signalling (Lowell & Shulman, 2005). In addition to its role in regulating glucose metabolism, in the central nervous system, IIS has also been shown to influence neuronal survival and synaptic plasticity (van der Heide et al. 2006). Recent studies in our laboratory have shown that α‐lipoic acid, an insulin mimetic nutriceutical, is able to rescue the brain metabolic deficits and mitochondrial dysfunction that occur in brain ageing (Jiang et al. 2013) and in a mouse model of Alzheimer's disease (Sancheti et al. 2013).
An important downstream component of the IIS that facilitates energy metabolism is Akt; Akt has been shown to directly translocate to mitochondria and enhance mitochondrial function in hepatocytes (Li et al. 2013). In neuroblastoma cells, insulin stimulates the translocation of phosphorylated Akt to the mitochondria within minutes. Two mitochondrial proteins, GSK‐3β and the β‐subunit of ATP synthase, are phosphorylated as a result of Akt translocation (Bijur & Jope, 2003). Activated GSK‐3β also phosphorylates pyruvate dehydrogenase (PDH) and inhibits its activity (Hoshi et al. 1996). Another prominent feature of IIS is its promotion of survival by directly inactivating components of the mitochondrial dependent intrinsic apoptosis. This entails phosphorylation and inactivation of the pro‐apoptotic members of the Bcl‐2 family (Linseman et al. 2002). The prevention of neuronal death could thus have implications for brain ageing and neurodegeneration which are characterized by significant neuronal loss (Kanazawa, 2001).
JNK
JNK is a negative regulator of both mitochondrial metabolic function and the IIS pathway. Anisomycin‐ or H2O2‐activated JNK translocates to mitochondria in primary cortical neurons; JNK associated with the outer mitochondrial membrane initiates a cascade that leads to the inhibitory phosphorylation of the E1α subunit of the PDH complex, which results in a decrease in cellular ATP levels and a metabolic shift toward anaerobic glycolysis (Zhou et al. 2008). The inactivation of IIS by JNK is due to the inhibitory phosphorylation of the IRS at Ser307 (Ser312 in human IRS) by JNK, which prevents the insulin/IGF1 mediated tyrosine phosphorylation of IRS (Karpac & Jasper, 2009). Intriguingly, the IIS also inhibits JNK activation through multiple mechanisms including the phosphorylation of MLK3 (Barthwal et al. 2003) and the suppression of ASK1 (Kim et al. 2001 a). This is consistent with our findings that aged rats fed with α‐lipoic acid to enhance IIS also exhibit decreased JNK activation compared to age‐matched controls (Jiang et al. 2013).
Coordination of the energy‐redox axis with nuclear transcription
Energy charge and mitochondrial biogenesis
Mitochondrial energy charge is linked to the nuclear transcription pathways and modulates mitochondrial biogenesis (Fig. 3) (Yin & Cadenas, 2015). Mitochondrial biogenesis entails the replication of mtDNA, as well as the synthesis, transport and integration of proteins and lipids to the existing mitochondrial population (Attardi & Schatz, 1988). Most of the 1500 mitochondrial proteins are encoded by the nuclear genome (Calvo et al. 2006). Transcription factors such as nuclear respiratory factor‐1 and ‐2 (NRF‐1 and NRF‐2) and oestrogen‐related receptors (ERR) (Scarpulla, 2002) regulate the transcription of these genes, directly or indirectly. These transcriptional pathways are coordinated by members of the peroxisome‐proliferator‐activated receptor γ coactivator‐1 (PGC‐1) family, primarily PGC‐1α (Handschin & Spiegelman, 2006). As the master regulator of mitochondrial biogenesis, PGC1‐α activity is regulated by its transcription, post‐translational modification, and degradation (Puigserver & Spiegelman, 2003). PGC1α activity is highly regulated by energy charge‐related signals from mitochondria. These regulations involve energy messengers such as NAD+ and AMP/ADP, and energy sensors including sirtuin 1 (Sirt1) and AMPK (Fig. 3) (Fernandez‐Marcos & Auwerx, 2011).
NAD+–Sirt1
PGC1α is inactivated by acetylation and activated by Sirt1‐mediated deacetylation. The deacetylation is required for sequestering PGC1α to the nucleus and for activating the above‐mentioned transcription factors (NRF‐1, NRF2, ERRα) (Gerhart‐Hines et al. 2007). Sirt1 removes the acetyl group on lysine residues using NAD+ as a substrate and generates O‐acetyl‐ADP‐ribose, and nicotinamide (Houtkooper et al. 2010). As the major domain of NAD+/NADH metabolism, the mitochondrial energy component is thus capable of regulating the NAD+‐dependent sirtuin pathways and the activity of PGC1α.
AMP‐AMPK
PGC1α expression and activity are also regulated by AMPK. AMPK, an energy sensor in cells, is activated when the cellular AMP/ATP or ADP/ATP ratio is high (Oakhill et al. 2011; Xiao et al. 2011). The cytosolic ADP/ATP ratio is determined by the consumption of ATP and the synthesis of ATP as a function of mitochondrial bioenergetic status. It is known that activation of AMPK leads to an increase in PGC1α transcription. More importantly, AMPK enhances mitochondrial biogenesis by activating PGC1α through the phosphorylation of threonine177 and serine538, which impacts the ability of PGC1α to dock on certain transcription factors and affects the binding or function of other cofactors in the PGC1α coactivator complex (Jäger et al. 2007). Whereas AMPK directly enhances PGC‐1α expression and activation, another indirect way that AMPK modulates PGC‐1α is to increases NAD+ levels by upregulating fatty acid oxidation, thereby enhancing Sirt1 activity and PGC1α deacetylation (Cantó et al. 2009).
Redox‐sensitive transcription factors
In addition to the energy charge‐sensitive transcriptional pathways that induce mitochondrial biogenesis, a variety of transcriptional pathways are redox sensitive and can be activated upon intracellular redox changes (Fig. 4).
Figure 4. Mitochondrial regulation of redox‐sensitive transcription factors .
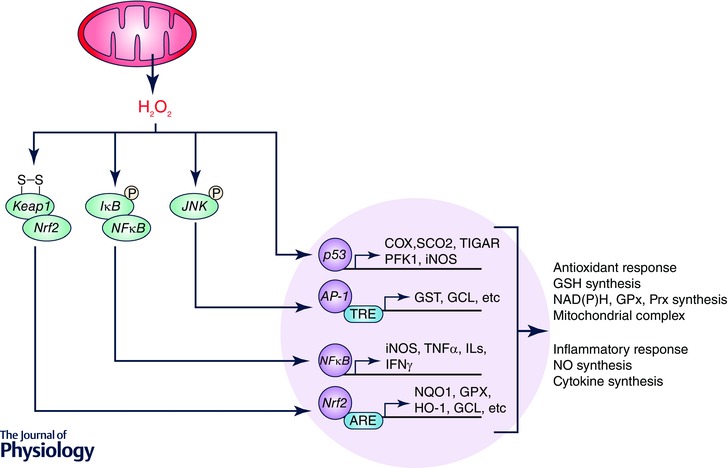
Redox‐sensitive transcription factors such as NFκB, AP‐1 and Nrf2 can be activated by H2O2 generated from mitochondria. H2O2 also inhibits p53 DNA binding activity. These transcription factors, in turn, master the synthesis of glutathione (GSH), NAD(P)H, glutathione peroxidase (GPx), peroxiredoxin (Prx), and mitochondrial complex subunits, and thus regulate the cellular redox status. These factors also manage inflammatory response by controlling the transcription of iNOS and a wide‐range of cytokines.
Nrf2
Transcriptional regulation of antioxidant or detoxifying genes is predominantly mediated by a redox‐sensitive transcription factor nuclear factor‐erythroid derived 2 (NF‐E2) related factor‐2 (Nrf2) (Kensler et al. 2007). Oxidants released from mitochondria induce activation of Nrf2, and this process can be inhibited by the mitochondrion‐specific redox enzyme Trx2) (Imhoff & Hansen, 2009). Under basal conditions, Nrf2 interacts with Kelch‐like ECH‐associated protein 1 (Keap1) in the cytosol where it undergoes ubiquitin‐mediated degradation. Upon oxidative modification of its cysteine residues, Keap1 dissociates from Nrf2, allowing the translocation of Nrf2 into the nucleus. By binding to the antioxidant response elements (AREs) of a range of phase II antioxidant defense genes, Nrf2 induces their expression such as peroxiredoxins, thioredoxins, glutathione S‐transferase (GST), NAD(P)H: quinone oxidoreductase (NQO1), haem oxygenase‐1 (HO‐1), glutathione peroxidase (GPX), and glutamate‐cysteine ligase (GCL) (Fig. 4). These genes play major roles in the removal of cytotoxic oxidants or electrophiles (Kensler et al. 2007). The Nrf–ARE pathway has also been found to be involved in the attenuation of inflammation‐associated conditions, such as rheumatoid arthritis, asthma, emphysema, gastritis, colitis, autoimmune diseases and atherosclerosis (Kim et al. 2010). There is also a crosstalk between nuclear factor κ‐light‐chain‐enhancer of activated B cells (NFκB)‐mediated inflammatory‐ and Nrf2‐driven antioxidant response pathways. For instance, Nrf2 deficiency leads to disrupted cellular redox balance and increased susceptibility to NFκB activation when the cells are challenged with inflammatory stimuli (Kensler et al. 2007). In endothelial cells, it was also reported that overexpression of Nrf2 abolished TNF‐α mediated p38 MAPK activation and the downstream VCAM‐1 expression (Chen et al. 2006).
NFκB
The transcription factor NFκB family comprises five well‐characterized proteins, namely p50 (NFκB1), p52 (NFκB2), p65 (RelA), c‐Rel and RelB, which form a variety of homo‐ and heterodimeric combinations under different circumstances (Baeuerle & Baltimore, 1996). NFκB plays a central role in immune and inflammatory responses, through the transcriptional regulation of a large number of cytokines and other immune response genes (Fig. 4) (Janssen‐Heininger et al. 2000). NFκB is redox‐sensitive. Oxidants including O2 , H2O2 and the hydroxyl radical (OH·) can positively or negatively modulate NFκB activation. Mitochondria‐derived H2O2 plays a critical role in the activation of NFκB (Csiszar et al. 2008). Under basal conditions, NFκB is localized in the cytoplasm in an inactive form binding with the inhibitor of NFκB (IκB); in response to stimuli, NFκB disassociates from the complex and translocates into the nucleus where it induces the transcription of its target genes (Kabe et al. 2005). In the cytosol, oxidative stress can stimulate phosphorylation (serine or tyrosine) of IκB and MAPKs, which in‐turn induce NFκB activation.
AP‐1
Activator protein‐1 (AP‐1) is another redox‐sensitive transcription factor. AP‐1 can be formed by the dimeric combinations of basic leucine zipper proteins that belong the Jun or Fos families (Gius et al. 1999). AP‐1 protein binds to the tetradecanoyl phorbol acetate response elements (TREs), which are within the regulatory sequence of target genes and control their basal and inducible expression (Fig. 4) (Rahman et al. 1999). AP‐1 is activated in response to oxidative and pro‐inflammatory stimuli, via the MAPK signalling pathways. Mitochondrial oxidant‐mediated JNK activation mediates the activation of the c‐Jun component of AP‐1, which then combines with the c‐Fos subunit. The resulting AP‐1 heterodimer induces the production of various inflammatory mediators (Sandireddy et al. 2014). Interestingly, while some studies show that AP‐1 is activated by oxidants, other work also showed that antioxidants such as pyrrolidine dithiocarbamate and N‐acetyl‐cysteine stimulate the activation of AP‐1 (Meyer et al. 1993; Janssen et al. 1995).
p53
p53 is the key factor that maintains genomic stability by regulating the cell cycle and DNA repair process. p53 promotes aerobic metabolism by targeting mitochondria. It has been found that p53 directly regulates mitochondrial oxygen consumption through transcriptional regulation of an assembly factor for the cytochrome c oxidase complex (Complex IV), synthesis of cytochrome c oxidase 2 (SCO2) (Zhuang et al. 2012). p53 activity is redox sensitive, due to the 10 cysteine residues (human) existing exclusively in its DNA‐binding domain. Oxidation of these cysteine residues to disulfide bonds suppresses tetramerization and its DNA binding activity of p53. Mitochondrial function thus regulates p53 activity by modulating cellular redox status (Sun et al. 2003). p53 also regulates the expression of inducible nitric oxide synthase (iNOS), which produces ∙NO and promotes inflammation in tissues including hepatocytes (Ambs et al. 1998).
Additional information
Competing interests
None declared.
Funding
Supported by NIH grant RO1AG016718.
Biography
Enrique Cadenas is Professor of Pharmacology and Pharmaceutical Sciences at the University of Southern California School of Pharmacy. Fei Yin is a senior research associate at the same institution. Harsh Sancheti obtained his PhD from the same institution. Zhigang Liu is a visiting scholar from Northwest A&F University, China. The authors conduct research on mechanisms inherent in brain ageing and Alzheimer's disease focusing on mitochondria as a cellular hub of dynamic coordinated networks of signalling and transcriptional pathways.
References
- Aaronson RP & Woo E (1981). Organization in the cell nucleus: divalent cations modulate the distribution of condensed and diffuse chromatin. J Cell Biol 90, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambs S, Ogunfusika MO, Merriam WG, Bennett WP, Billiar TR & Harris CC (1998). Up‐regulation of inducible nitric oxide synthase expression in cancer‐prone p53 knockout mice. Proc Natl Acad Sci USA 95, 8823–8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaju A, Feller A & Chedid A (2002). Aging liver. Gerontology 48, 343–353. [DOI] [PubMed] [Google Scholar]
- Attardi G & Schatz G (1988). Biogenesis of mitochondria. Annu Rev Cell Biol 4, 289–331. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA & Baltimore D (1996). NF‐κB: ten years after. Cell 87, 13–20. [DOI] [PubMed] [Google Scholar]
- Barthwal MK, Sathyanarayana P, Kundu CN, Rana B, Pradeep A, Sharma C, Woodgett JR & Rana A (2003). Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival. J Biol Chem 278, 3897–3902. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Allaman I & Magistretti PJ (2011). Brain energy metabolism: focus on astrocyte‐neuron metabolic cooperation. Cell Metab 14, 724–738. [DOI] [PubMed] [Google Scholar]
- Bijur GN & Jope RS (2003). Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3‐kinase activation. J Neurochem 87, 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass J, Sheu R & Gibson G (2000). Inherent abnormalities in energy metabolism in alzheimer disease: interaction with cerebrovascular compromise. Ann N Y Acad Sci 903, 204–221. [DOI] [PubMed] [Google Scholar]
- Bliss T & Collingridge G (1993). A synaptic model of memory: long‐term potentiation in the hippocampus. Nature 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Bolaños JP, Almeida A & Moncada S (2010). Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35, 145–149. [DOI] [PubMed] [Google Scholar]
- Boveris A & Navarro A (2008). Brain mitochondrial dysfunction in aging. IUBMB Life 60, 308–314. [DOI] [PubMed] [Google Scholar]
- Brinton R (2008). Estrogen regulation of glucose metabolism and mitochondrial function: Therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev 60, 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A, Barelds R & Knook D (1985). Age‐related changes in the endocytic capacity of rat liver kupffer and endothelial cells. Hepatology 5, 362–366. [DOI] [PubMed] [Google Scholar]
- Brownlee M (2005). The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625. [DOI] [PubMed] [Google Scholar]
- Busche M, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold K, Haass C, Staufenbiel M, Konnerth A & Garaschuk O (2008). Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science 321, 1686–1689. [DOI] [PubMed] [Google Scholar]
- Businaro R, Ippoliti F, Ricci S, Canitano N & Fuso A (2012). Alzheimer's disease promotion by obesity: Induced mechanisms—Molecular links and perspectives. Curr Gerontol Geriatr Res 2012, 986823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo S, Jain M, Xie X, Sheth SA, Chang B, Goldberger OA, Spinazzola A, Zeviani M, Carr SA & Mootha VK (2006). Systematic identification of human mitochondrial disease genes through integrative genomics. Nat Genet 38, 576–582. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart‐Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P & Auwerx J (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro MdelR, Suarez E, Kraiselburd E, Isidro A, Paz J, Ferder L & Ayala‐Torres S (2012). Aging increases mitochondrial DNA damage and oxidative stress in liver of rhesus monkeys. Exp Gerontol 47, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC (2012). Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46, 265–287. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Holland WL, Bär J, Sandhoff K & Summers SA (2005). Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 280, 20148–20153. [DOI] [PubMed] [Google Scholar]
- Chen X‐L., Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH & Kunsch C (2006). Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol 290, H1862–H1870. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Tseng Y & White MF (2010). Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab 21, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen Y (2000). Oxidative stress and Alzheimer disease. Am J Clin Nutr 71, 621s–629s. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG & Ungvari Z (2008). Inflammation and endothelial dysfunction during aging: role of NF‐κB. J Appl Physiol 105, 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Zhang M, Zhang YQ & Xu ZH (2007). JNK pathway: diseases and therapeutic potential. Acta Pharmacol Sin 28, 601–608. [DOI] [PubMed] [Google Scholar]
- Dax EM, Partilla JS & Gregerman RI (1981). Mechanism of the age‐related decrease of epinephrine‐stimulated lipolysis in isolated rat adipocytes: beta‐adrenergic receptor binding, adenylate cyclase activity, and cyclic AMP accumulation. J Lipid Res 22, 934–943. [PubMed] [Google Scholar]
- de la Monte SM (2012). Triangulated mal‐signaling in Alzheimer's disease: Roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis 30, S231–S249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Derdak Z & Wands JR (2012). Alcohol, insulin resistance and the liver–brain axis. J Gastroenterol Hepatol 27, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Longato L, Tong M, DeNucci S & Wands JR (2009. a). The liver–brain axis of alcohol‐mediated neurodegeneration: role of toxic lipids. Int J Environ Res Public Health 6, 2055–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Lawton M & Longato L (2009. b). Nitrosamine exposure exacerbates high fat diet‐mediated type 2 diabetes mellitus, non‐alcoholic steatohepatitis, and neurodegeneration with cognitive impairment. Mol Neurodegener 4, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Nguyen V, Setshedi M, Longato L & Wands JR (2010). Ceramide‐mediated insulin resistance and impairment of cognitive‐motor functions. J Alzheimers Dis 21, 967–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis R, Lucertini F, Guescini M, Polidori E, Zeppa S, Stocchi V, Cinti S & Cuppini R (2013). Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr Metab Cardiovasc Dis 23, 582–590. [DOI] [PubMed] [Google Scholar]
- de Mello VDF, Lankinen M, Schwab U, Kolehmainen M, Lehto S, Seppänen‐Laakso T, Orešič M, Pulkkinen L, Uusitupa M & Erkkilä AT (2009). Link between plasma ceramides, inflammation and insulin resistance: association with serum IL‐6 concentration in patients with coronary heart disease. Diabetologia 52, 2612–2615. [DOI] [PubMed] [Google Scholar]
- Dedeoglu A, Choi J, Cormier K, Kowall N & Jenkins B (2004). Magnetic resonance spectroscopic analysis of Alzheimer's disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain Res 1012, 60–65. [DOI] [PubMed] [Google Scholar]
- Delarue J & Magnan C (2007). Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care 10, 142–148. [DOI] [PubMed] [Google Scholar]
- Ding F, Yao J, Rettberg JR, Chen S & Brinton RD (2013. a). Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer's mouse brain: implication for bioenergetic intervention. PLoS One 8, e79977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Yao J, Zhao L, Mao Z, Chen S & Brinton RD (2013. b). Ovariectomy induces a shift in fuel availability and metabolism in the hippocampus of the female transgenic model of familial Alzheimer's. PLoS One 8, e59825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doert A, Pilatus U, Zanella F, Müller W & Eckert G (2015). 1H‐ and 13C‐NMR spectroscopy of Thy‐1‐APPSL mice brain extracts indicates metabolic changes in Alzheimer's disease. J Neural Transm 122, 541–550. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Henson GD, Hart CR, Layec G, Trinity JD, Bramwell RC, Enz RA, Morgan RG, Reihl KD, Hazra S, et al (2014). The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: evidence of significant multisystem dysfunction. J Physiol 592, 4083–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew B & Leeuwenburgh C (2004). Ageing and subcellular distribution of mitochondria: role of mitochondrial DNA deletions and energy production. Acta Physiol Scand 182, 333–341. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman H, Jacova C, DeKosky S, Barberger‐Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, et al (2007). Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS–ADRDA criteria. Lancet Neurol 6, 734–746. [DOI] [PubMed] [Google Scholar]
- Dugan LL, You YH, Ali SS, Diamond‐Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, et al (2013). AMPK dysregulation promotes diabetes‐related reduction of superoxide and mitochondrial function. J Clin Invest 123, 4888–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms‐Hagen J, Chan CC, et al (1999). Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase‐1B gene. Science 283, 1544–1548. [DOI] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR & Chandel NS (2009). Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med 46, 1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteras N, Alquézar C, Bartolomé F, Antequera D, Barrios L, Carro E, Cerdán S & Martín‐Requero Á (2012). Systematic evaluation of magnetic resonance imaging and spectroscopy techniques for imaging a transgenic model of Alzheimer's disease (AβPP/PS1). J Alzheimers Dis 30, 337–353. [DOI] [PubMed] [Google Scholar]
- Farkas E & Luiten P (2001). Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol 64, 575–611. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Marcos PJ & Auwerx J (2011). Regulation of PGC‐1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93, 884S–890S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis P, Palmer A, Snape M & Wilcock G (1999). The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry 66, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo M, Ikuta I, Zhu X, Casadesus G & Jordán J (2010). Mitochondrial biology in Alzheimer's disease pathogenesis. J Neurochem 114, 933–945. [DOI] [PubMed] [Google Scholar]
- Gerhart‐Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z & Puigserver P (2007). Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC‐1α. EMBO J 26, 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafourifar P & Cadenas E (2005). Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26, 190–195. [DOI] [PubMed] [Google Scholar]
- Gibson G, Sheu K & Blass J (1998). Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm 105, 855–870. [DOI] [PubMed] [Google Scholar]
- Gill JMR & Sattar N (2009). Ceramides: A new player in the inflammation‐insulin resistance paradigm? Diabetologia 52, 2475–2477. [DOI] [PubMed] [Google Scholar]
- Girard J & Lafontan M (2008). Impact of visceral adipose tissue on liver metabolism and insulin resistance. Part II: Visceral adipose tissue production and liver metabolism. Diabetes Metab 34, 439–445. [DOI] [PubMed] [Google Scholar]
- Gius D, Botero A, Shah S & Curry HA (1999). Intracellular oxidation/reduction status in the regulation of transcription factors NF‐κB and AP‐1. Toxicol Lett 106, 93–106. [DOI] [PubMed] [Google Scholar]
- Gottfries C, Adolfsson R, Aquilonius S, Carlsson A, Eckernas S, Nordberg A, Oreland L, Svennerholm L, Wiberg Å & Winblad B (1983). Biochemical changes in dementia disorders of Alzheimer type (AD/SDAT). Neurobiol Aging 4, 261–271. [DOI] [PubMed] [Google Scholar]
- Graja A & Schulz TJ (2014). Mechanisms of aging‐related impairment of brown adipocyte development and function. Gerontology 61, 211–217. [DOI] [PubMed] [Google Scholar]
- Hamann A, Flier JS & Lowell BB (1998). Obesity after genetic ablation of brown adipose tissue. Z Ernahrungswiss 37 Suppl 1, 1–7. [PubMed] [Google Scholar]
- Handschin C & Spiegelman BM (2006). Peroxisome proliferator‐activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27, 728–735. [DOI] [PubMed] [Google Scholar]
- Haris M, Nath K, Cai K, Singh A, Crescenzi R, Kogan F, Verma G, Reddy S, Hariharan H, Melhem E, et al (2013). Imaging of glutamate neurotransmitter alterations in Alzheimer's disease. NMR Biomed 26, 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann S, Scherping I, Dröse S, Brandt U, Schulz K, Jendrach M, Leuner K, Eckert A & Müller W (2009). Mitochondrial dysfunction: An early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging 30, 1574–1586. [DOI] [PubMed] [Google Scholar]
- Higami Y, Barger JL, Page GP, Allison DB, Smith SR, Prolla TA & Weindruch R (2006). Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr 136, 343–352. [DOI] [PubMed] [Google Scholar]
- Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA & Weindruch R (2004). Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long‐term caloric restriction. FASEB J 18, 415–417. [DOI] [PubMed] [Google Scholar]
- Højlund K, Mogensen M, Sahlin K & Beck‐Nielsen H (2008). Mitochondrial dysfunction in type 2 diabetes and obesity. Endocrinol Metab Clin North Am 37, 713–731. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Takashima A, Noguchi K, Murayama M, Sato M, Kondo S, Saitoh Y, Ishiguro K, Hoshino T & Imahori K (1996). Regulation of mitochondrial pyruvate dehydrogenase activity by tau protein kinase I/glycogen synthase. kinase 3β in brain. Proc Natl Acad Sci USA 93, 2719–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Cantó C, Wanders RJ & Auwerx J (2010). The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev 31, 194–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur K‐Y & Lee M‐S (2015). New mechanisms of metformin action: focusing on mitochondria and the gut. J Diabetes Invest 6, 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff B & Hansen J (2009). Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J 424, 491–500. [DOI] [PubMed] [Google Scholar]
- Iwakami S, Misu H, Takeda T, Sugimori M, Matsugo S, Kaneko S & Takamura T (2011). Concentration‐dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS One 6, e27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger S, Handschin C, St‐Pierre J & Spiegelman BM (2007). AMP‐activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC‐1α. Proc Natl Acad Sci USA 104, 12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen‐Heininger YMW, Poynter ME & Baeuerle PA (2000). Recent advances torwards understanding redox mechanisms in the activation of nuclear factor κb. Free Radic Biol Med 28, 1317–1327. [DOI] [PubMed] [Google Scholar]
- Janssen YMW, Heintz NH & Mossman BT (1995). Induction of c‐fos and c‐jun proto‐oncogene expression by asbestos is ameliorated by N‐acetyl‐L‐cysteine in mesothelial cells. Cancer Res 55, 2085–2089. [PubMed] [Google Scholar]
- Jiang T & Cadenas E (2014). Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13, 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yin F, Yao J, Brinton RD & Cadenas E (2013). Lipoic acid restores age‐associated impairment of brain energy metabolism through the modulation of Akt/JNK signaling and PGC1a transcriptional pathway. Aging Cell 12, 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabe Y, Ando K, Hirao S, Yoshida M & Handa H (2005). Redox regulation of NF‐κB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal 7, 395–403. [DOI] [PubMed] [Google Scholar]
- Kanazawa I (2001). How do neurons die in neurodegenerative diseases? Trends Mol Med 7, 339–344. [DOI] [PubMed] [Google Scholar]
- Kann O & Kovács R (2007). Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292, C641–C657. [DOI] [PubMed] [Google Scholar]
- Karpac J & Jasper H (2009). Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol Metab 20, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos‐Flier E & Kahn CR (2007). Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat‐specific insulin receptor knock‐out mice. Aging Cell 6, 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV & Ritov VB (2002). Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N & Biswal S (2007). Cell survival responses to environmental stresses via the Keap1‐Nrf2‐ARE pathway. Annu Rev Pharmacol Toxicol 47, 89–116. [DOI] [PubMed] [Google Scholar]
- Kim AH, Khursigara G, Sun X, Franke TF & Chao MV (2001. a). Akt phosphorylates and negatively regulates apoptosis signal‐regulating kinase 1. Mol Cell Biol 21, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cha Y‐N & Surh Y‐J (2010). A protective role of nuclear factor‐erythroid 2‐related factor‐2 (Nrf2) in inflammatory disorders. Mutat Res 690, 12–23. [DOI] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez‐Carrasco W, Goldberg IJ, et al (2001. b). Tissue‐specific overexpression of lipoprotein lipase causes tissue‐specific insulin resistance. Proc Natl Acad Sci USA 98, 7522–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Young VR, Blackburn GL, Bistrian BR & Wolfe RR (1986). Palmitate and glycerol kinetics during brief starvation in normal weight young adult and elderly subjects. J Clin Invest 78, 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, et al (2007). Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 56, 2973–2981. [DOI] [PubMed] [Google Scholar]
- Kolosova N, Grishanova A, Krysanova Z, Zueva T, Sidorova I & Sinitsyna O (2003). [Age‐related changes in protein and lipid oxidation in the liver of prematurely aging rats OXYS]. Biomed Khim 50, 73–78. [PubMed] [Google Scholar]
- Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki‐Miura T, Wang Z, Sato Y, Mori N & Yamashita H (2005). UCP1 deficiency increases susceptibility to diet‐induced obesity with age. Aging Cell 4, 147–155. [DOI] [PubMed] [Google Scholar]
- Kujoth G, Hiona A, Pugh T, Someya S, Panzer K, Wohlgemuth S, Hofer T, Seo A, Sullivan R & Jobling W (2005). Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484. [DOI] [PubMed] [Google Scholar]
- Lam PY, Yin F, Hamilton RT, Boveris A & Cadenas E (2009). Elevated neuronal nitric oxide synthase expression during ageing and mitochondrial energy production. Free Radic Res 43, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur D & McLean A (1998). The aging liver. Clin Pharmacokinet 34, 359–373. [DOI] [PubMed] [Google Scholar]
- Li C, Li Y, He L, Agarwal AR, Zeng N, Cadenas E & Stiles BL (2013). PI3K/AKT signaling regulates bioenergetics in immortalized hepatocytes. Free Radic Biol Med 60, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Shic F, Enriquez C & Ross B (2003). Reduced glutamate neurotransmission in patients with Alzheimer's disease–an in vivo 13C magnetic resonance spectroscopy study. MAGMA 16, 29–42. [DOI] [PubMed] [Google Scholar]
- Linseman DA, Phelps RA, Bouchard RJ, Le SS, Laessig TA, McClure ML & Heidenreich KA (2002). Insulin‐like growth factor‐I blocks Bcl‐2 interacting mediator of cell death (Bim) induction and intrinsic death signaling in cerebellar granule neurons. J Neurosci 22, 9287–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, et al (2009). Reactive oxygen species enhance insulin sensitivity. Cell Metab 10, 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB & Shulman GI (2005). Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387. [DOI] [PubMed] [Google Scholar]
- Lu RH, Ji H, Chang ZG, Su SS & Yang GS (2010). Mitochondrial development and the influence of its dysfunction during rat adipocyte differentiation. Mol Biol Rep 37, 2173–2182. [DOI] [PubMed] [Google Scholar]
- Luo F, Rustay N, Ebert U, Hradil V, Cole T, Llano D, Mudd S, Zhang Y, Fox G & Day M (2012). Characterization of 7‐ and 19‐month‐old Tg2576 mice using multimodal in vivo imaging: limitations as a translatable model of Alzheimer's disease. Neurobiol Aging 33, 933–944. [DOI] [PubMed] [Google Scholar]
- Maccioni R, Farías G, Morales I & Navarrete L (2010). The revitalized tau hypothesis on Alzheimer's disease. Arch Med Res 41, 226–231. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ & Allaman I (2013). Brain energy metabolism In Neuroscience in the 21st Century, ed. Pfaff DW, pp 1591–1620. Springer. [Google Scholar]
- Mangialasche F, Solomon A, Winblad B, Mecocci P & Kivipelto M (2010). Alzheimer's disease: clinical trials and drug development. Lancet Neurol 9, 702–716. [DOI] [PubMed] [Google Scholar]
- Marjanska M, Curran G, Wengenack T, Henry P, Bliss R, Poduslo J, Jack C, Uğurbil K & Garwood M (2005). Monitoring disease progression in transgenic mouse models of Alzheimer's disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci USA 102, 11906–11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery W (1997). Oxidative stress hypothesis in alzheimer's disease. Free Radic Biol Med 23, 134–147. [DOI] [PubMed] [Google Scholar]
- Martin G, Sewell R, Yeomans N, Morgan D & Smallwood R (1994). Hepatic Kupffer cell function: the efficiency of uptake and intracellular degradation of 14C‐labelled mitochondria is reduced in aged rats. Mech Ageing Dev 73, 157–168. [DOI] [PubMed] [Google Scholar]
- Masoro EJ (2005). Overview of caloric restriction and ageing. Mech Ageing Dev 126, 913–922. [DOI] [PubMed] [Google Scholar]
- McClean PL, Parthsarathy V, Faivre E & Hölscher C (2011). The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci 31, 6587–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Schreck R & Baeuerle PA (1993). H2O2 and antioxidants have opposite effects on activation of NF‐κB and AP‐1 in intact cells: AP‐1 as secondary antioxidant‐responsive factor. EMBO J 12, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moats R, Ernst T, Shonk T & Ross B (1994). Abnormal cerebral metabolite concentrations in patients with probable Alzheimer disease. Magn Reson Med 32, 110–115. [DOI] [PubMed] [Google Scholar]
- Mosconi L (2005). Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. Eur J Nucl Med Mol Imaging 32, 486–510. [DOI] [PubMed] [Google Scholar]
- Mosconi L, de Leon M, Murray J, Lu J, Javier E, McHugh P & Swerdlow R (2011). Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer's disease. J Alzheimers Dis 27, 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Tsui W, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, et al (2009). FDG‐PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imaging 36, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Tsui W, Herholz K, Pupi A, Drzezga A, Lucignani G, Reiman E, Holthoff V, Kalbe E, Sorbi S, et al (2008). Multicenter standardized 18F‐FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J Nucl Med 49, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller‐Hocker J, Aust D, Rohrbach H, Napiwotzky J, Reith A, Link T, Seibel P, Holzel D & Kadenbach B (1997). Defects of the respiratory chain in the normal human liver and in cirrhosis during aging. Hepatology 26, 709–719. [DOI] [PubMed] [Google Scholar]
- Murphy M (2009). How mitochondria produce reactive oxygen species. Biochem J 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A & Boveris A (2004). Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol 287, R1244–R1249. [DOI] [PubMed] [Google Scholar]
- Navarro A & Boveris A (2007). The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292, C670–C686. [DOI] [PubMed] [Google Scholar]
- Ngo J, Pomatto L & Davies K (2013). Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol 1, 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen L, Rae C, Ittner L, Gotz J & Sonnewald U (2013). Glutamate metabolism is impaired in transgenic mice with tau hyperphosphorylation. J Cereb Blood Flow Metab 33, 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T & Araki E (2007). Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 9, 343–353. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Brownlee M & Araki E (2015). Mitochondrial reactive oxygen species in the pathogenesis of early diabetic nephropathy. J Diabetes Investig 6, 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye C, Kim J, Kalhan SC & Hanson RW (2008). Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol Metab 19, 356–361. [DOI] [PubMed] [Google Scholar]
- Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S & Kemp BE (2011). AMPK is a direct adenylate charge‐regulated protein kinase. Science 332, 1433–1435. [DOI] [PubMed] [Google Scholar]
- Oh‐ishi S, Kizaki T, Toshinai K, Haga S, Fukuda K, Nagata N & Ohno H (1996). Swimming training improves brown‐adipose‐tissue activity in young and old mice. Mech Ageing Dev 89, 67–78. [DOI] [PubMed] [Google Scholar]
- Okita N, Hayashida Y, Kojima Y, Fukushima M, Yuguchi K, Mikami K, Yamauchi A, Watanabe K, Noguchi M, Nakamura M, et al (2012). Differential responses of white adipose tissue and brown adipose tissue to caloric restriction in rats. Mech Ageing Dev 133, 255–266. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E & Halestrap AP (2000). Evidence that metformin exerts its anti‐diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348, 607–614. [PMC free article] [PubMed] [Google Scholar]
- Palmeira CM, Rolo AP, Berthiaume J, Bjork JA & Wallace KB (2007). Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol Appl Pharmacol 225, 214–220. [DOI] [PubMed] [Google Scholar]
- Parish R & Petersen KF (2005). Mitochondrial dysfunction and type 2 diabetes. Curr Diab Rep 5, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME & Corvera S (2010). The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev 31, 364–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E, Perry R, Tomlinson B, Blessed G & Gibson P (1980). Coenzyme a‐acetylating enzymes in Alzheimer's disease: Possible cholinergic ‘compartment’ of pyruvate dehydrogenase. Neurosci Lett 18, 105–110. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW & Shulman GI (2003). Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 300, 1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R & Shulman GI (2004). Impaired mitochondrial activity in the insulin‐resistant offspring of patients with type 2 diabetes. N Engl J Med 350, 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, et al (2002). Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109, 1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Haring HU, Claussen CD & Stefan N (2010). Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 59, 1789–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconi L, Quagliaro L, Assaloni R, Da Ros R, Maier A, Zuodar G & Ceriello A (2006). Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev 22, 198–203. [DOI] [PubMed] [Google Scholar]
- Poisnel G, Hérard A, El Tannir El Tayara N, Bourrin E, Volk A, Kober F, Delatour B, Delzescaux T, Debeir T, Rooney T, et al (2012). Increased regional cerebral glucose uptake in an APP/PS1 model of Alzheimer's disease. Neurobiol Aging 33, 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P & Spiegelman BM (2003). Peroxisome proliferator‐activated receptor‐γ coactivator 1α (PGC‐1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24, 78–90. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A & Arancio O (2008). Picomolar amyloid‐β positively modulates synaptic plasticity and memory in hippocampus. J Neurosci 28, 14537–14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Antonicelli F & MacNee W (1999). Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor‐α and dexamethasone in human alveolar epithelial cells. J Biol Chem 274, 5088–5096. [DOI] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, et al (2010). Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non‐alcoholic fatty liver disease in an obese rodent model. J Hepatol 52, 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C (1995). Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol 27, 647–653. [DOI] [PubMed] [Google Scholar]
- Rojas S, Herance J, Gispert J, Abad S, Torrent É, Jiménez X, Pareto D, Perpiña U, Sarroca S, Rodríguez E, et al (2013). In vivo evaluation of amyloid deposition and brain glucose metabolism of 5XFAD mice using positron emission tomography. Neurobiol Aging 34, 1790–1798. [DOI] [PubMed] [Google Scholar]
- Root‐Bernstein R, Busik JV & Henry DN (2002). Are diabetic neuropathy, retinopathy and nephropathy caused by hyperglycemic exclusion of dehydroascorbate uptake by glucose transporters? J Theor Biol 216, 345–359. [DOI] [PubMed] [Google Scholar]
- Rousset S, Alves‐Guerra MC, Mozo J, Miroux B, Cassard‐Doulcier AM, Bouillaud F & Ricquier D (2004). The biology of mitochondrial uncoupling proteins. Diabetes 53 Suppl 1, S130–135. [DOI] [PubMed] [Google Scholar]
- Saely CH, Geiger K & Drexel H (2012). Brown versus white adipose tissue: a mini‐review. Gerontology 58, 15–23. [DOI] [PubMed] [Google Scholar]
- Saito M, Okamatsu‐Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio‐Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al (2009). High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek R, Xia J, Innes A, Sweatman B, Adalbert R, Randle S, McGowan E, Emson P & Griffin J (2010). A metabolomic study of the CRND8 transgenic mouse model of Alzheimer's disease. Neurochem Int 56, 937–947. [DOI] [PubMed] [Google Scholar]
- Saltiel AR & Kahn CR (2001). Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806. [DOI] [PubMed] [Google Scholar]
- Sancheti H, Akopian G, Yin F, Brinton RD, Walsh JP & Cadenas E (2013). Age‐dependent modulation of synaptic plasticity and insulin mimetic effect of lipoic acid on a mouse model of Alzheimer's disease. PLoS One 8, e69830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancheti H, Kanamori K, Patil I, Brinton RD, Ross BD & Cadenas E (2014. a). Reversal of metabolic deficits by lipoic acid in a triple transgenic mouse model of Alzheimer's disease: A 13C NMR study. J Cereb Blood Flow Metab 34, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancheti H, Patil I, Kanamori K, Diaz Brinton R, Zhang W, Lin A & Cadenas E (2014. b). Hypermetabolic state in the 7‐month‐old triple transgenic mouse model of Alzheimer's disease and the effect of lipoic acid: a 13C‐NMR study. J Cereb Blood Flow Metab 34, 1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandireddy R, Yerra VG, Areti A, Komirishetty P & Kumar A (2014). Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol 2014, 674987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre J, Pallardó F, Plá R, Pellín A, Juan G, O'Connor J, Estrela J, Miquel J & Viña J (1996). Aging of the liver: Age‐associated mitochondrial damage in intact hepatocytes. Hepatology 24, 1199–1205. [DOI] [PubMed] [Google Scholar]
- Sastre J, Pallardó F & Viña J (2000). Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life 49, 427–435. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Yenice S & Tumer N (1994). Influence of exercise training and age on uncoupling protein mRNA expression in brown adipose tissue. Pharmacol Biochem Behav 49, 1057–1059. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC (2002). Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576, 1–14. [DOI] [PubMed] [Google Scholar]
- Schmucker D (1998). Aging and the liver: an update. J Gerontol A Biol Sci Med Sci 53A, B315–B321. [DOI] [PubMed] [Google Scholar]
- Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP & Leeuwenburgh C (2010). New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci 123, 2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Wang F, Stieren E & Tong Q (2005). SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280, 13560–13567. [DOI] [PubMed] [Google Scholar]
- Storozhevykh TP, Senilova YE, Persiyantseva NA, Pinelis VG & Pomytkin IA (2007). Mitochondrial respiratory chain is involved in insulin‐stimulated hydrogen peroxide production and plays an integral role in insulin receptor autophosphorylation in neurons. BMC Neurosci 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA (2006). Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45, 42–72. [DOI] [PubMed] [Google Scholar]
- Sun XZ, Vinci C, Makmura L, Han S, Tran D, Nguyen J, Hamann M, Grazziani S, Sheppard S & Gutova M (2003). Formation of disulfide bond in p53 correlates with inhibition of DNA binding and tetramerization. Antioxid Redox Signal 5, 655–665. [DOI] [PubMed] [Google Scholar]
- Swerdlow R (2011). Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta 1812, 1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R & Khan S (2009). The Alzheimer's disease mitochondrial cascade hypothesis: An update. Exp Neurol 218, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R & Bertram L (2005). Twenty years of the Alzheimer's disease amyloid hypothesis: A genetic perspective. Cell 120, 545–555. [DOI] [PubMed] [Google Scholar]
- Teruel T, Hernandez R & Lorenzo M (2001). Ceramide mediates insulin resistance by tumor necrosis factor‐α in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 50, 2563–2571. [DOI] [PubMed] [Google Scholar]
- Tiwari V & Patel A (2012). Impaired glutamatergic and GABAergic function at early age in AβPPswe‐PS1dE9 mice: Implications for Alzheimer's disease. J Alzheimers Dis 28, 765–769. [DOI] [PubMed] [Google Scholar]
- Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B & Chandel NS (2011). Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P & Wood IS (2004). Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92, 347–355. [DOI] [PubMed] [Google Scholar]
- Twig G & Shirihai OS (2011). The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A, Guevara R, Garcia‐Palmer FJ, Roca P & Oliver J (2008). Caloric restriction retards the age‐related decline in mitochondrial function of brown adipose tissue. Rejuvenation Res 11, 597–604. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Ramakers GM & Smidt MP (2006). Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol 79, 205–221. [DOI] [PubMed] [Google Scholar]
- van Duijn S, Nabuurs R, van Duinen S, Natté R, van Buchem M & Alia A (2013). Longitudinal monitoring of sex‐related in vivo metabolic changes in the brain of Alzheimer's disease transgenic mouse using magnetic resonance spectroscopy. J Alzheimers Dis 34, 1051–1059. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al (2009). Functional brown adipose tissue in healthy adults. N Engl J Med 360, 1518–1525. [DOI] [PubMed] [Google Scholar]
- Wartewig S & Neubert RHH (2007). Properties of ceramides and their impact on the stratum corneum structure: A review. Skin Pharmacol Physiol 20, 220–229. [DOI] [PubMed] [Google Scholar]
- White MF (2003). Insulin signaling in health and disease. Science 302, 1710–1711. [DOI] [PubMed] [Google Scholar]
- Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, Twig G, Petrovic N, Zingaretti C, Graham A, et al (2014). Hormone‐induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33, 418–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne H, Cope L, Mutch E, Rawlins M, Woodhouse K & James O (1989). The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 9, 297–301. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al (2011). Structure of mammalian AMPK and its regulation by ADP. Nature 472, 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin R, Zhao L, Nilsen J, Hamilton R & Brinton R (2009). Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 106, 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T, Chen Y, King K, Yeh S & Wei Y (1989). Liver mitochondrial respiratory functions decline with age. Biochem Biophys Res Commun 165, 994–1003. [DOI] [PubMed] [Google Scholar]
- Yin F, Boveris A & Cadenas E (2014). Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal 20, 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F & Cadenas E (2015). Mitochondria: the cellular hub of the dynamic coordinated network. Antioxid Redox Signal 22, 961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Jiang T & Cadenas E (2013). Metabolic triad in brain aging: mitochondria, insulin/IGF‐1 signalling, and JNK signalling. Biochem Soc Trans 41, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Sancheti H & Cadenas E (2012. a). Mitochondrial thiols in the regulation of cell death pathways. Antioxid Redox Signal 17, 1714–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Sancheti H & Cadenas E (2012. b). Silencing of nicotinamide nucleotide transhydrogenase impairs cellular redox homeostasis and energy metabolism in PC12 cells. Biochim Biophys Acta 1817, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yao J, Sancheti H, Feng T, Melcangi RC, Morgan TE, Finch CE, Pike CJ, Mack WJ, Cadenas E, et al (2015). The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging 36, 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, et al (2006). Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758, 1864–1884. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk‐Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al (2001). Role of AMP‐activated protein kinase in mechanism of metformin action. J Clin Invest 108, 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Lam PY, Han D & Cadenas E (2008). c‐Jun N‐terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem 104, 325–335. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Lam PY, Han D & Cadenas E (2009). Activation of C‐Jun‐N‐terminal kinase and decline of mitochondrial pyruvate dehydrogenase activity during brain aging. FEBS Lett 583, 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Ma W, Lago CU & Hwang PM (2012). Metabolic regulation of oxygen and redox homeostasis by p53: Lessons from evolutionary biology? Free Radic Biol Med 53, 1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J & Cinti S (2009). The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23, 3113–3120. [DOI] [PubMed] [Google Scholar]
- Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER & Abraham E (2010). Exposure to hydrogen peroxide induces oxidation and activation of AMP‐activated protein kinase. J Biol Chem 285, 33154–33164. [DOI] [PMC free article] [PubMed] [Google Scholar]


