Abstract
Purpose
Predicting the pattern of recurrence can aid in the development of targeted surveillance and treatment strategies. We identified patient populations that remain at risk for an event at a median follow-up of 24 years from the diagnosis of operable breast cancer.
Patients and Methods
International Breast Cancer Study Group clinical trials I to V randomly assigned 4,105 patients between 1978 and 1985. Annualized hazards were estimated for breast cancer–free interval (primary end point), disease-free survival, and overall survival.
Results
For the entire group, the annualized hazard of recurrence was highest during the first 5 years (10.4%), with a peak between years 1 and 2 (15.2%). During the first 5 years, patients with estrogen receptor (ER) – positive disease had a lower annualized hazard compared with those with ER-negative disease (9.9% v 11.5%; P = .01). However, beyond 5 years, patients with ER-positive disease had higher hazards (5 to 10 years: 5.4% v 3.3%; 10 to 15 years: 2.9% v 1.3%; 15 to 20 years: 2.8% v 1.2%; and 20 to 25 years: 1.3% v 1.4%; P < .001). Among patients with ER-positive disease, annualized hazards of recurrence remained elevated and fairly stable beyond 10 years, even for those with no axillary involvement (2.0%, 2.1%, and 1.1% for years 10 to 15, 15 to 20, and 20 to 25, respectively) and for those with one to three positive nodes (3.0%, 3.5%, and 1.5%, respectively).
Conclusion
Patients with ER-positive breast cancer maintain a significant recurrence rate during extended follow up. Strategies for follow up and treatments to prevent recurrences may be most efficiently applied and studied in patients with ER-positive disease followed for a long period of time.
INTRODUCTION
Almost 30% of patients with breast cancer who are free of disease after initial local and regional treatments present with disease recurrence during follow-up.1 The timing of breast cancer recurrence varies considerably, influenced by classic prognostic factors1 as well as adjuvant treatment strategies.2-6 In particular, estrogen receptor (ER) status provides a clinically useful distinction1,2; recurrences in patients with ER-negative disease occur earlier during follow-up, whereas in those with ER-positive disease, recurrences continue to occur later in follow-up (eg, years 5 to 10).7
The late relapses evident in ER-positive disease suggest that mechanisms related to disease relapse are different in ER-positive disease, where cancer cells may stay dormant for a protracted period of time despite adjuvant therapies.8 However, only recently have clinical studies begun to focus on late relapses,9,10 and little is known of the pattern of recurrence after 10 years of follow-up. Results from clinical trials are not generally reported after a median follow-up of 10 years, because of logistics and costs related to extended follow-up.
The limited evidence available on late outcomes has often focused on survival as opposed to recurrence,11 but survival is less informative about the history of the disease after prolonged follow-up, because it is influenced by the age and comorbidities of the patient at diagnosis. A better description of relapse patterns, resulting in a better understanding of time-specific risk, could lead to targeted therapeutic approaches and enhanced surveillance methods, ultimately leading to improved patient outcomes. The International Breast Cancer Study Group (IBCSG) observed enrolled patients for up to 25 years in its first generation of trials,12-14 offering a unique opportunity to better define the patterns of late breast cancer recurrence.
PATIENTS AND METHODS
Patients
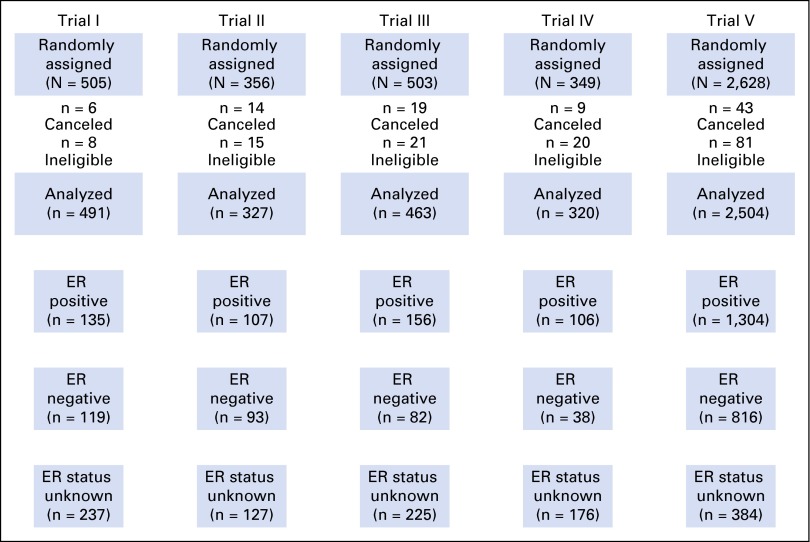
Data were analyzed from 4,105 eligible patients with breast cancer who entered the IBCSG (formerly Ludwig Group) randomized clinical trials I to V from 1978 to 1985 (Fig 1). Locoregional and adjuvant systemic therapies were assigned according to the design and conduct of the trials, as described elsewhere12-14 (Data Supplement). Trials I to IV addressed chemoendocrine questions in premenopausal (I and II) and postmenopausal women (III and IV) with node-positive early breast cancer using classical CMF (cyclophosphamide, methotrexate, and fluorouracil) with tamoxifen (12 months), oophorectomy, and/or prednisone (12 months).12 Prednisone was added to several regimens of chemotherapy and endocrine therapy as a result of information from a Canadian trial indicating that adrenal suppression induced by prednisone, when added to oophorectomy, led to improved treatment outcome.15 Trial V investigated the timing and duration of classic CMF in the node-positive population13 or a single cycle of perioperative CMF in the node-negative population.14 Tamoxifen and prednisone duration was 6 months. ER levels were measured centrally.16
Fig 1.
CONSORT diagram showing the analytic population according to estrogen receptor (ER) status for the five clinical trials.
Staging included hematologic evaluation, renal and liver function tests, chest x-ray, and bone scan. All patients were carefully observed for evidence of relapse or survival. Clinical, hematologic, and biochemical assessments (blood urea nitrogen and serum creatinine, bilirubin, alkaline phosphatase, AST or ALT, γ-glutamyl transferase, and serum calcium) of each patient were required every 3 months for 2 years, every 6 months until the end of the fifth year, and yearly thereafter until death. Chest x-rays and bone scans were required every 6 months for 2 years and once yearly for up to 5 years. These tests were recommended only if clinically indicated beyond the fifth year. Yearly follow-up forms were collected until 2008 or 2009, when the IBCSG decided to stop follow-up.
Categories of Sites of Invasive Relapse
All first reoccurring breast cancer events were classified according to site as follows: local recurrences confined to the ipsilateral chest wall, including mastectomy scar; regional relapses, including ipsilateral axillary, supraclavicular, and internal mammary lymph node metastases; and distant metastases, including soft tissue or nodal metastases in distant sites, bone metastases, and visceral metastases in all other organs or diffuse intra-abdominal metastases. Other first events included invasive contralateral breast cancer, second non–breast cancer malignancies, and deaths without malignancies. Any site was considered to be a component of a first event if diagnosed within a 2-month timeframe, with first site of breast cancer recurrence specified hierarchically according to worst prognosis: viscera, bone, distant nodes or soft tissue, regional, contralateral breast, and local.
Statistical Methods
The primary end point for this analysis was breast cancer–free interval (BCFI), with disease-free survival (DFS) and overall survival (OS) considered secondary end points. BCFI was defined as time from the date of random assignment to any invasive breast cancer recurrence (including ipsilateral or contralateral breast recurrence) and was censored at date of last follow-up or at date of death without recurrence. DFS was defined as time from the date of random assignment to any invasive relapse (including ipsilateral breast recurrence), appearance of a second primary cancer (including contralateral breast cancer), or death, whichever occurred first. OS was defined as time from the date of random assignment to death resulting from any cause. Event-time distributions were estimated using the Kaplan-Meier method. To evaluate whether the hazard ratio changed over time, interaction term of survival times by ER status was tested in a Cox model, where the interaction term was treated as a time-varying variable.
Annualized hazard rates for events of interest were calculated using the maximum likelihood estimate from a piece-wise exponential model. These estimates (reported as percentages) are number of events occurring within a time interval divided by the total years of follow-up during the interval accrued by patients at risk during the interval. To compare hazard rates between time intervals and among patient populations, a constant hazard was estimated within each cohort, and the tests were based on normal approximation of the difference between estimates.
The cumulative incidences of site-specific first breast cancer events were calculated based on a competing-risk model,17,18 treating recurrences in other sites and death without recurrence as competing events. Comparisons of subgroups within time intervals were based on Gray’s test,17,18 restricted to follow-up just from that specific time interval. All P values are two sided.
RESULTS
A total of 4,105 eligible patients from IBCSG trials I to V were included in the analysis. Table 1 lists the distribution of patient characteristics according to ER status. ER levels of 10 fmol/mg or greater of cytosol protein based on biochemical assay were classified as positive (n = 1,808; 40%). ER status was unknown for 1,149 patients (28%).
Table 1.
Patient Characteristics According to ER Status
| Characteristic | ER Positive (n = 1,808) | ER Negative (n = 1,148) | ER Unknown (n = 1,149) | Total (N = 4,105) | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| No. of positive axillary nodes | |||||||||
| 0 | 640 | 35.4 | 409 | 35.6 | 226 | 19.7 | 1,275 | 31.1 | |
| 1-3 | 671 | 37.1 | 399 | 34.8 | 573 | 49.9 | 1,643 | 40.0 | |
| ≥ 4 | 497 | 27.5 | 340 | 29.6 | 350 | 30.5 | 1,187 | 28.9 | |
| Tumor size, cm | |||||||||
| ≤ 2 | 809 | 44.7 | 433 | 37.7 | 531 | 46.2 | 1,773 | 43.2 | |
| > 2 | 907 | 50.2 | 650 | 56.6 | 582 | 50.7 | 2,139 | 52.1 | |
| Unknown | 92 | 5.1 | 65 | 5.7 | 36 | 3.1 | 193 | 4.7 | |
| Menopausal status | |||||||||
| Premenopausal | 909 | 50.3 | 714 | 62.2 | 602 | 52.4 | 2,225 | 54.2 | |
| Postmenopausal | 899 | 49.7 | 434 | 37.8 | 547 | 47.6 | 1,880 | 45.8 | |
| Tumor grade | |||||||||
| 1 | 335 | 18.5 | 93 | 8.1 | 222 | 19.3 | 650 | 15.8 | |
| 2 | 904 | 50.0 | 380 | 33.1 | 510 | 44.4 | 1,794 | 43.7 | |
| 3 | 482 | 26.7 | 591 | 51.5 | 339 | 29.5 | 1,412 | 34.4 | |
| Unknown | 87 | 4.8 | 84 | 7.3 | 78 | 6.8 | 249 | 6.1 | |
| Adjuvant systemic treatment | |||||||||
| Observation | 309 | 17.1 | 188 | 16.4 | 239 | 20.8 | 736 | 17.9 | |
| Hormonal therapy ± chemotherapy | 362 | 20.0 | 172 | 15.0 | 281 | 24.5 | 815 | 19.9 | |
| Chemotherapy without hormonal therapy | 1,137 | 62.9 | 788 | 68.6 | 629 | 54.7 | 2,554 | 62.2 | |
| Age, years | |||||||||
| Median | 53 | 49 | 52 | 52 | |||||
| Range | 22-80 | 24-79 | 21-80 | 21-80 | |||||
Abbreviation: ER, estrogen receptor.
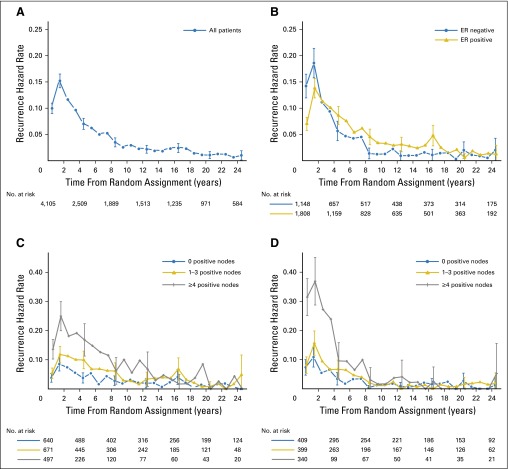
At a median follow-up of 24.2 years, 2,590 patients (63.1%) had died, and 2,451 (59.7%) had experienced a breast cancer recurrence, either a first recurrence at a local site (n = 420; 10.2% of all patients), contralateral breast cancer (n = 219; 5.3% of all patients), a recurrence at a regional site (n = 351; 8.6% of all patients), or a recurrence at a distant site (n = 1,461; 40.7% of all patients). One hundred ninety-nine patients (4.9%) had a nonbreast second primary malignancy before breast cancer recurrence; 485 patients (11.9%) died before breast cancer recurrence; and 76% of the 1,169 patients last known to be alive without recurrence were observed for at least 15 years (Data Supplement). The hazards of BCFI, DFS, and OS events by 5-year intervals according to ER status are listed in Table 2, and annual hazard of recurrence plots are shown in Fig 2. For the entire group, the annualized hazard of breast cancer recurrence was greatest for the first 5 years (10.4%), with a peak interval between years 1 and 2 after surgery (15.2%; Fig 2A). The hazard decreased consistently during years 5 to 10 (4.5%) and then remained stable. During years 10 to 15, 15 to 20, and 20 to 25, the hazard of recurrence was 2.2%, 1.5%, and 0.7%, respectively (Table 2).
Table 2.
Annualized Hazards of Recurrence Estimated Within 5-Year Intervals From Random Assignment
| Outcome | Hazard (%; SE) | ||||
|---|---|---|---|---|---|
| Years 0-5 | Years 5-10 | Years 10-15 | Years 15-20 | Years 20-25 | |
| BCFI | |||||
| All patients | 10.4 (0.2) | 4.5 (0.2) | 2.2 (0.2) | 1.5 (0.3) | 0.7 (0.2) |
| ER positive | 9.9 (0.4) | 5.4 (0.3) | 2.9 (0.3) | 2.8 (0.4) | 1.3 (0.3) |
| ER negative | 11.5 (0.5) | 3.3 (0.3) | 1.3 (0.2) | 1.2 (0.3) | 1.4 (0.4) |
| DFS | |||||
| All patients | 11.2 (0.3) | 5.4 (0.2) | 3.7 (0.2) | 4.2 (0.3) | 4.0 (0.3) |
| ER positive | 10.6 (0.4) | 6.6 (0.4) | 4.5 (0.4) | 5.5 (0.5) | 4.2 (0.6) |
| ER negative | 12.1 (0.5) | 3.8 (0.4) | 2.3 (0.3) | 3.1 (0.4) | 4.2 (0.6) |
| OS | |||||
| All patients | 5.7 (0.2) | 5.3 (0.2) | 3.6 (0.2) | 4.0 (0.2) | 4.1 (0.3) |
| ER positive | 4.3 (0.2) | 6.1 (0.3) | 4.0 (0.3) | 4.7 (0.4) | 4.6 (0.5) |
| ER negative | 7.9 (0.4) | 4.2 (0.4) | 2.2 (0.3) | 2.7 (0.4) | 3.3 (0.5) |
NOTE. Data are shown as percentage (SE).
Abbreviations: BCFI, breast cancer–free interval; DFS, disease-free survival; OS, overall survival.
Fig 2.
Annual hazard of breast cancer recurrence (A) for 4,105 patients included in International Breast Cancer Study Group trials I to V overall, (B) according to known estrogen receptor (ER) status (n = 2,956), (C) according to number of positive nodes for patients with ER-positive disease (n = 1,808), and (D) according to number of positive nodes for patients with ER-negative disease (n = 1,148). Vertical lines indicate 95% CI of the hazard.
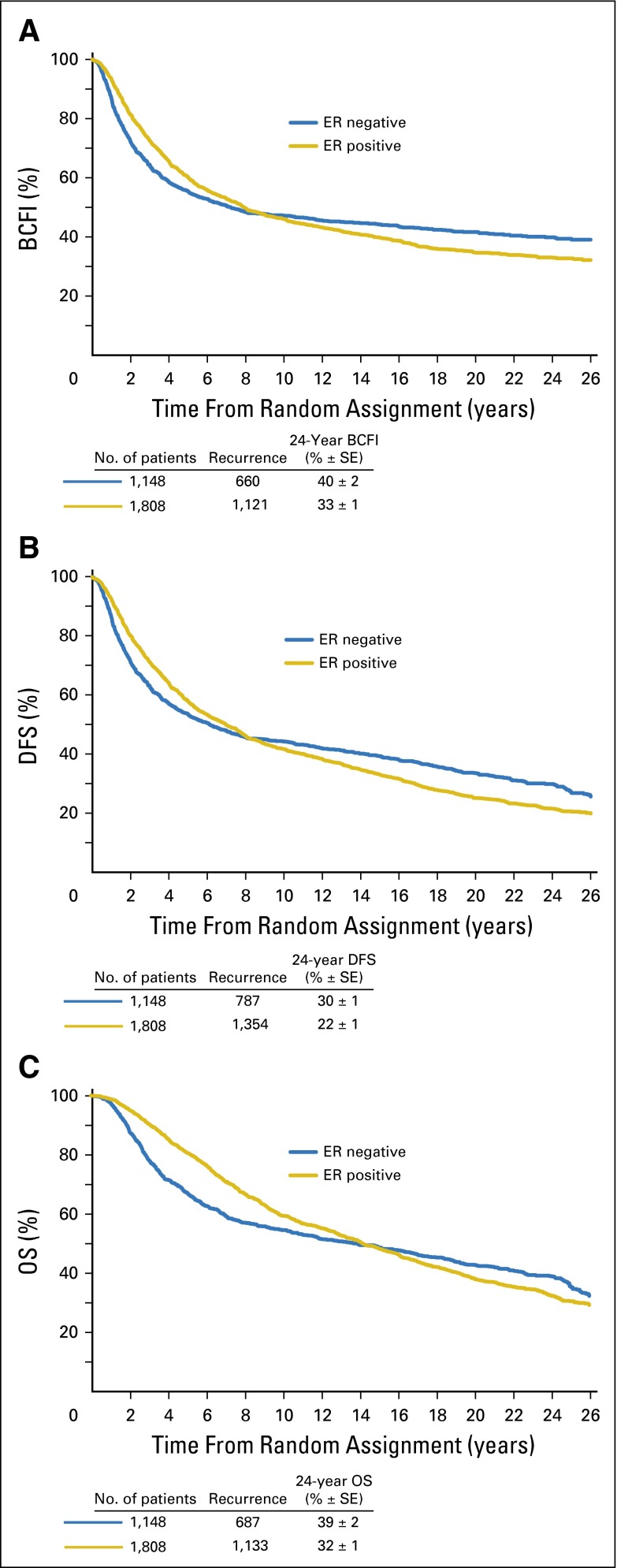
We observed a statistically significantly lower hazard of breast cancer recurrence for patients presenting with ER-positive disease versus those with ER-negative disease during the first 5 years (9.9% v 11.5%; P = .01). However, the hazards of ER-positive and ER-negative disease crossed between years 2 and 3 (Fig 2B). Beyond 5 years, the hazard of recurrence was higher for patients with ER-positive disease compared with that of those with ER-negative disease (5 to 10 years: 5.4% v 3.3%; 10 to 15 years: 2.9% v 1.3%; 15 to 20 years: 2.8% v 1.2%; and 20 to 25 years: 1.3% v 1.4%; P < .001; Table 2). As shown in the Kaplan-Meier curves (Fig 3) as well, the curves of ER-positive and ER-negative cohorts cross, and the hazard ratio of ER-positive versus ER-negative disease changes over time for BCFI, DFS, and OS (P < .001 for interactions of ER status and survival time).
Fig 3.
Kaplan-Meier curves according to known estrogen receptor (ER) status for the end points of (A) breast cancer–free interval (BCFI), (B) disease-free survival (DFS), and (C) overall survival (OS). Two-sided P values based on tests for interaction of ER status and survival times are each P < .001, indicating significant nonproportionality of hazards over time.
Table 3 lists the cumulative incidence rates of site-specific first breast cancer events over time according to ER status. During the first 5 years, distant recurrences occurred more frequently in patients with ER-negative versus ER-positive disease (27.1% v 23.4%; P = .005). Beyond 5 years, the cumulative incidence of distant recurrence increased more rapidly in the ER-positive subgroup than in the ER-negative subgroup (cumulative incidence rates at years 10, 15, 20, and 25 were 31.9%, 35%, 37.4%, and 38.3% for ER-positive disease v 31.8%, 33.4%, 34.1%, and 35.3% for ER-negative disease, respectively; P < .001). Cumulative incidence for local and contralateral recurrences was also higher in later years for the ER-positive cohort (Table 3), and the same crossing pattern seen for other end points was also observed for locoregional first recurrences (Data Supplement).
Table 3.
Cumulative Incidence of Site-Specific First Breast Cancer Events by Year From Random Assignment
| Event | Cumulative Incidence (%; SE) | ||||
|---|---|---|---|---|---|
| Year 5 | Year 10 | Year 15 | Year 20 | Year 25 | |
| Local only | |||||
| ER positive | 8.8 (0.7) | 11.2 (0.7) | 11.9 (0.8) | 12.5 (0.8) | 12.6 (0.8) |
| ER negative | 6.9 (0.8) | 8.0 (0.8) | 8.1 (0.8) | 8.3 (0.8) | 8.3 (0.8) |
| Contralateral breast ± local | |||||
| ER positive | 2.2 (0.3) | 3.9 (0.5) | 5.1 (0.5) | 6.2 (0.6) | 6.5 (0.6) |
| ER negative | 1.3 (0.3) | 2.7 (0.5) | 3.8 (0.6) | 5.3 (0.7) | 6.2 (0.8) |
| Regional ± above | |||||
| ER positive | 5.0 (0.5) | 6.2 (0.6) | 7.0 (0.6) | 7.3 (0.6) | 7.5 (0.6) |
| ER negative | 9.0 (0.8) | 10.0 (0.9) | 10.0 (0.9) | 10.0 (0.9) | 10.1 (0.9) |
| Distant ± above | |||||
| ER positive | 23.4 (1.0) | 31.9 (1.1) | 35.0 (1.1) | 37.4 (1.2) | 38.3 (1.2) |
| ER negative | 27.1 (1.3) | 31.8 (1.4) | 33.4 (1.4) | 34.1 (1.4) | 35.3 (1.5) |
NOTE. Data are shown as percentage (SE).
Abbreviation: ER, estrogen receptor.
Table 4 lists the hazards of recurrence by 5-year interval according to ER status for subgroups defined by number of positive axillary nodes, tumor size, menopausal status, tumor grade, and treatment. For every subgroup, consistent with the overall populations, patients with ER-positive disease had a lower hazard of recurrence compared with those with ER-negative disease during the first 5 years. Beyond 5 years, patients with ER-positive disease had a higher hazard of recurrence than those with ER-negative disease. Within the ER-positive subgroup, those with no axillary involvement (hazards during years 10 to 15, 15 to 20, and 20 to 25 were 2.0%, 2.1%, and 1.1%, respectively) or one to three positive nodes (hazards during years 10 to 15, 15 to 20, and 20 to 25 were 3.0%, 3.5%, and 1.5%, respectively) maintained a stable hazard of recurrence, whereas for patients with ER-positive tumors with more than four involved nodes, the hazard of recurrence continued to decrease gradually (hazards during years 10 to 15, 15 to 20, and 20 to 25 were 5.9%, 3.8%, and 1.3%, respectively; Fig 2C).
Table 4.
Hazard of Recurrence by 5-Year Interval According to ER Status for Subgroups Defined by Number of Positive Axillary Nodes, Tumor Size, Menopausal Status, Tumor Grade, and Treatment
| Variable | Hazard (%; SE) | ||||
|---|---|---|---|---|---|
| Years 0-5 | Years 5-10 | Years 10-15 | Years 15-20 | Years 20-25 | |
| No. of positive axillary nodes | |||||
| 0 | |||||
| ER positive | 5.8 (0.5) | 3.3 (0.4) | 2.0 (0.4) | 2.1 (0.4) | 1.1 (0.4) |
| ER negative | 6.7 (0.6) | 2.1 (0.4) | 1.1 (0.3) | 1.6 (0.4) | 1.1 (0.5) |
| 1-3 | |||||
| ER positive | 9.5 (0.6) | 5.8 (0.6) | 3.0 (0.5) | 3.5 (0.7) | 1.5 (0.6) |
| ER negative | 9.2 (0.7) | 3.7 (0.6) | 1.1 (0.4) | 0.9 (0.4) | 1.9 (0.7) |
| ≥ 4 | |||||
| ER positive | 17.2 (0.9) | 10.9 (1.2) | 5.9 (1.2) | 3.8 (1.2) | 1.3 (0.9) |
| ER negative | 22.8 (1.2) | 6.2 (1.3) | 2.8 (1.0) | 0.5 (0.5) | 0.8 (0.8) |
| Tumor size, cm | |||||
| ≤ 2 | |||||
| ER positive | 7.0 (0.4) | 4.8 (0.4) | 2.9 (0.4) | 2.7 (0.5) | 1.5 (0.5) |
| ER negative | 8.3 (0.7) | 3.3 (0.5) | 1.3 (0.4) | 1.5 (0.4) | 0.6 (0.4) |
| > 2 | |||||
| ER positive | 12.9 (0.6) | 6.1 (0.6) | 2.9 (0.5) | 2.7 (0.5) | 1.1 (0.5) |
| ER negative | 14.0 (0.7) | 3.2 (0.5) | 1.4 (0.4) | 0.9 (0.3) | 2.1 (0.6) |
| Menopausal status | |||||
| Premenopausal | |||||
| ER positive | 9.6 (0.5) | 5.1 (0.5) | 3.2 (0.4) | 2.8 (0.5) | 1.4 (0.4) |
| ER negative | 11.0 (0.6) | 3.6 (0.4) | 1.6 (0.3) | 1.3 (0.3) | 1.8 (0.5) |
| Postmenopausal | |||||
| ER positive | 10.3 (0.5) | 5.8 (0.5) | 2.5 (0.4) | 2.8 (0.5) | 1.0 (0.5) |
| ER negative | 12.4 (0.8) | 2.7 (0.5) | 0.8 (0.3) | 1.0 (0.4) | 0.6 (0.4) |
| Tumor grade | |||||
| 1 | |||||
| ER positive | 5.8 (0.6) | 4.9 (0.7) | 3.6 (0.7) | 4.0 (0.9) | 0.7 (0.5) |
| ER negative | 4.9 (1.1) | 2.5 (0.9) | 1.6 (0.8) | 2.4 (1.1) | 4.7 (1.9) |
| 2 | |||||
| ER positive | 9.6 (0.5) | 6.3 (0.5) | 2.8 (0.4) | 2.7 (0.5) | 1.8 (0.5) |
| ER negative | 11.7 (0.9) | 5.2 (0.8) | 2.5 (0.6) | 2.3 (0.7) | 1.0 (0.6) |
| 3 | |||||
| ER positive | 14.1 (0.8) | 4.1 (0.6) | 2.5 (0.6) | 2.4 (0.7) | 0.4 (0.4) |
| ER negative | 13.0 (0.7) | 2.4 (0.4) | 0.7 (0.2) | 0.3 (0.2) | 1.0 (0.4) |
| Treatment | |||||
| Observation | |||||
| ER positive | 9.5 (0.9) | 4.1 (0.7) | 2.5 (0.7) | 2.7 (0.8) | 1.2 (0.7) |
| ER negative | 11.8 (1.2) | 2.8 (0.8) | 0.3 (0.3) | 2.2 (0.8) | 1.0 (0.7) |
| Hormonal therapy ± chemotherapy | |||||
| ER positive | 9.6 (0.4) | 5.2 (0.4) | 2.8 (0.4) | 3.0 (0.4) | 1.3 (0.4) |
| ER negative | 10.6 (0.6) | 3.3 (0.4) | 1.7 (0.3) | 1.1 (0.3) | 1.5 (0.4) |
| Chemotherapy without hormonal therapy | |||||
| ER positive | 11.3 (0.9) | 7.4 (1.0) | 3.4 (0.8) | 1.8 (0.7) | 1.4 (1) |
| ER negative | 16.1 (1.5) | 3.4 (1.1) | 0.4 (0.4) | 0.0 (0) | 1.0 (1) |
NOTE. Data are shown as percentage (SE).
Abbreviation: ER, estrogen receptor.
DISCUSSION
In this study, we evaluated patterns of recurrence for patients included in the first generation of IBCSG trials of adjuvant treatment. Our focus was on events occurring during extended follow-up to consider treatments, such as prolonged endocrine therapies for ER-positive disease, that might target this specific type of relapse. The limited amount of clinical trial data assessing the magnitude of the hazard ratios for late recurrence may in fact result in unnecessary extended adjuvant endocrine therapy for some or, conversely, a lack of effective extended treatment for others.
Although several studies of hazard ratios after 10 years are available in the literature,19-22 limited data have been reported after a careful extended follow-up within the context of clinical trials. Other limitations include no description of biologic features20 or adjuvant systemic treatment received19 and limited sample size.21
Since 1978, the IBCSG has had the policy of regular lifelong follow-up and collection of sites and dates of first and all subsequent events. The member institutions maintained collaboration with the IBCSG during the years and carried the costs for the follow-up of the patients, regarding it as a moral obligation. To our knowledge, the current study, based on prospectively defined and quality-controlled databases for the first generation of IBCSG randomized clinical trials, provides the largest population of patients included in clinical trials with extended follow-up (median, 24 years) available.
We have demonstrated that the hazards of recurrence and death decreased consistently until year 10 but then remained stable after year 10 through year 25. We have also shown that patients with ER-positive tumors continued to have a higher risk of relapse, including distant metastases, during years 5 to 25, reinforcing the need for long-term clinical follow-up to understand the pattern of recurrence of breast cancer and confirm that micrometastases are completely eradicated.
Regarding locoregional events, it is important to note that all patients entered the trials after mastectomy and axillary clearance, with at least eight axillary nodes removed. At that time, this standardized surgical approach excluded locoregional radiotherapy. A recently published overview, without results according to ER status, demonstrated that for women with node-positive disease, postmastectomy radiotherapy reduced locoregional recurrence as first relapse site within a 10-year follow-up period.6 Our study shows a locoregional first recurrence risk at 24 years of follow-up that is slightly lower than that seen in the overview control group, with a pattern of late events almost exclusively seen in the ER-positive cohort (Data Supplement). The incidence of locoregional recurrence as first recurrence site might have been reduced had radiotherapy been used for patients at high risk for recurrence.
In the seminal study by Saphner et al,1 a significant relapse rate was maintained after year 5 for patients with ER-positive disease, although because of the small number of patients evaluable after year 10, no definite conclusion could be drawn on outcome after prolonged follow-up. Subsequently, other studies showed a different pattern of relapse for ER-positive and ER-negative disease after a median follow-up generally not exceeding 10 years.9 We have reported that the hazard rates for ER-negative and ER-positive disease started to diverge quite early and then crossed between years 2 and 3, after which women with ER-negative tumors had a lower rate of death resulting from breast cancer than those with ER-positive tumors. Consequently, the Kaplan-Meier estimates of BCFI rates crossed between the eighth and ninth years, after which the percentage of women with ER-negative tumors who were breast cancer free was higher than that of those with ER-positive tumors (Fig 3A).
Notably, the recurrence rate was different according to nodal status. Others have demonstrated that the contrast between early and late recurrences is larger in the subsets with the greatest tumor burden, where patients have a more abrupt decrease in recurrence rate,1 as shown in our study. Conversely, patients with the lowest tumor burdens, namely those with no axillary involvement or one to three positive nodes, exhibited the minimum contrast between early and late hazards of recurrence, maintaining a constant risk of relapse after 10 years of follow-up (Fig 2C). It is of interest that, in our series, late events were similar for patients with ER-positive disease who initially presented with high or low burden of metastatic lymph nodes.
Several factors might in fact influence the lag time between surgery and appearance of metastases. It is intuitive that recurrences in locally advanced cancers are related to metastases already evident or appearing soon. Conversely, the late appearance of metastases in ER-positive low-stage breast cancer occurring many years after diagnosis might not be related exclusively to the low rate of proliferation.23-25 Several mechanisms have been proposed, including angiogenic switch, immunosurveillance, and interaction with extracellular matrix and stromal cells,26,27 but late relapses and related mechanisms still represent a major clinical challenge and a research priority.28
Our findings are also useful for formulating strategies for breast cancer follow-up and management in asymptomatic patients after surgery, supporting the need for long-term clinical follow-up in selected subpopulations. A noted outcome of proper follow-up is improvement in quality of life. In particular, in previously reported studies, the value of follow-up was related to diagnosis of recurrence, patient anxiety, and maintenance of health-related quality of life.29,30 This outcome could be related not only to the investigation of symptoms and communication of test results, but also to physician awareness of the results of ongoing investigations as well as history of the disease.31 Long-term follow-up is an orphan item that is not given sufficient priority or attention in the clinical trials community. Data from long-term observation of trial populations might provide valuable information on morbidity and delayed costs of treatments.
To our knowledge, our retrospective study represents the largest series reporting outcomes of patients included in randomized clinical trials at a median follow-up of 24 years. However, our study of long-term follow-up has the same limitation that affects all such analyses; the adjuvant systemic therapies studied in trials that accrued patients from 1978 to 1985 are not the same as those routinely offered to patients today. A significant improvement in breast cancer relapse–free survival was in fact shown for patients treated in the modern era, compared with patients treated several decades ago, possibly related to the better efficacy of modern adjuvant treatment approaches.32 For example, in the reported trials, aromatase inhibitors were not used; endocrine treatment duration was shorter than recommended by current guidelines, which advise up to 10 years, especially for node-positive disease; and few premenopausal patients were treated with oophorectomy and none with ovarian suppression. Moreover, commonly used adjuvant chemotherapy regimens act primarily on the early hazard peak of recurrences and deaths (reducing the risk of events during the first years after initial diagnosis).33 A similar effect with treatment affecting primarily the earlier hazard peak has been shown for adjuvant endocrine treatments.34 Thus, although modern therapies might reduce the hazard of early recurrences like those shown in our report, their influence on later recurrence risk is less certain, and in fact, the newer treatments might delay rather than eliminate recurrence risk. Unfortunately, with current clinical trial designs terminating follow-up at 10 years, we may never know the answer.
Another limitation of our study is that factors used for treatment choice today, such as human epidermal growth factor receptor 2/neu expression, were not available in our database. Consequently, the tumor subgroups identified in our analysis include heterogeneous groups of tumors, and the identification of additional tumor subtypes amenable to targeted treatments represents a research priority. In particular, the ER-positive subgroup is highly heterogeneous and might be separated into different subpopulations (eg, luminal A or B) that could behave differently during extended follow-up.35
In conclusion, the risk of breast cancer recurrence continues through 24 years after primary treatment, supporting the importance of continuing care for patients with breast cancer. We identified a population (ER positive) that maintains a significant risk of relapse even after more than 10 years of follow-up. New targeted treatments and different modes of breast cancer surveillance for preventing late recurrences within this population should be studied. Developing cost-effective mechanisms to maintain the follow-up of patients enrolled in current randomized clinical trials is essential.
Supplementary Material
Acknowledgment
We thank the patients, physicians, nurses, and data managers who participate in the International Breast Cancer Study Group (IBCSG) trials. In addition, we acknowledge IBCSG participating institutions and investigators for trials I to V, in particular Jurij Lindtner, MD, and Carl-Magnus Rudenstam, MD; Marvin Zelen, PhD, for his efforts to launch these trials; and the Ludwig Breast Cancer Study Group.
Footnotes
Written on behalf of the International Breast Cancer Study Group.
Supported initially by the Ludwig Institute for Cancer Research and the Cancer League of Ticino; supported currently in central coordination, data management, and statistics by the Swedish Cancer League, Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group, Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, Swiss Cancer League, and US National Cancer Institute (Grant No. CA-75362); by the Cancer Association of South Africa for Cape Town participants; and by the Foundation for Clinical Cancer Research of Eastern Switzerland for St Gallen participants.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
See accompanying editorial on page 895
AUTHOR CONTRIBUTIONS
Conception and design: Marco Colleoni, Lorenzo Gianni, Richard D. Gelber, Aron Goldhirsch
Collection and assembly of data: Karen N. Price, Per Karlsson, John F. Forbes, Beat Thürlimann, Monica Castiglione, Richard D. Gelber, Alan S. Coates, Aron Goldhirsch
Data analysis and interpretation: Marco Colleoni, Zhuoxin Sun, Richard D. Gelber, Aron Goldhirsch
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Marco Colleoni
Honoraria: Novartis
Consulting or Advisory Role: Boehringer Ingelheim, Taiho Pharmaceutical, AbbVie, AstraZeneca, Pierre Fabre, Pfizer
Zhuoxin Sun
No relationship to disclose
Karen N. Price
No relationship to disclose
Per Karlsson
No relationship to disclose
John F. Forbes
No relationship to disclose
Beat Thürlimann
Stock or Other Ownership: Roche, Novartis, Roche (I), Novartis (I)
Honoraria: Roche
Expert Testimony: Celgene, Roche
Travel, Accommodations, Expenses: Pierre Fabre
Lorenzo Gianni
Travel, Accommodations, Expenses: Roche, Novartis
Monica Castiglione
No relationship to disclose
Richard D. Gelber
Research Funding: AstraZeneca (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Roche, (Inst), Celgene (Inst), Merck (Inst), Pfizer (Inst)
Alan S. Coates
No relationship to disclose
Aron Goldhirsch
No relationship to disclose
REFERENCES
- 1.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Gelber RD, Price KN, et al. Effect of systemic adjuvant treatment on first sites of breast cancer relapse. Lancet. 1994;343:377–381. doi: 10.1016/s0140-6736(94)91221-1. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Davies C, Godwin J, Gray R, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peto R, Davies C, Godwin J, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGale P, Taylor C, Correa C, et al. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagani O, Price KN, Gelber RD, et al. International Breast Cancer Study Group (IBCSG) Patterns of recurrence of early breast cancer according to estrogen receptor status: A therapeutic target for a quarter of a century. Breast Cancer Res Treat. 2009;117:319–324. doi: 10.1007/s10549-008-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang XH, Giuliano M, Trivedi MV, et al. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res. 2013;19:6389–6397. doi: 10.1158/1078-0432.CCR-13-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sestak I, Dowsett M, Zabaglo L, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–1511. doi: 10.1093/jnci/djt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzger-Filho O, Sun Z, Viale G, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: Results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31:3083–3090. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods LM, Rachet B, Lambert PC, et al. “Cure” from breast cancer among two populations of women followed for 23 years after diagnosis. Ann Oncol. 2009;20:1331–1336. doi: 10.1093/annonc/mdn791. [DOI] [PubMed] [Google Scholar]
- 12.Castiglione-Gertsch M, Johnsen C, Goldhirsch A, et al. The International (Ludwig) Breast Cancer Study Group Trials I-IV: 15 years follow-up. Ann Oncol. 1994;5:717–724. doi: 10.1093/oxfordjournals.annonc.a058976. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig Breast Cancer Study Group Combination adjuvant chemotherapy for node-positive breast cancer: Inadequacy of a single perioperative cycle. N Engl J Med. 1988;319:677–683. doi: 10.1056/NEJM198809153191104. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig Breast Cancer Study Group Prolonged disease-free survival after one course of perioperative adjuvant chemotherapy for node-negative breast cancer. N Engl J Med. 1989;320:491–496. doi: 10.1056/NEJM198902233200804. [DOI] [PubMed] [Google Scholar]
- 15.Meakin JW, Hayward JL, Panzarella T, et al. Ovarian irradiation and prednisone following surgery and radiotherapy for carcinoma of the breast. Breast Cancer Res Treat. 1996;37:11–19. doi: 10.1007/BF01806627. [DOI] [PubMed] [Google Scholar]
- 16.Jordan VC, Zava DT, Eppenburger U, et al. Reliability of steroid hormone receptor assays: An international study. Eur J Cancer Clin Oncol. 1983;19:357–363. doi: 10.1016/0277-5379(83)90133-5. [DOI] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 18.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Tubiana M, Koscielny S. Natural history of human breast cancer: Recent data and clinical implications. Breast Cancer Res Treat. 1991;18:125–140. doi: 10.1007/BF01990028. [DOI] [PubMed] [Google Scholar]
- 20.Brenner H, Hakulinen T. Are patients diagnosed with breast cancer before age 50 years ever cured? J Clin Oncol. 2004;22:432–438. doi: 10.1200/JCO.2004.04.067. [DOI] [PubMed] [Google Scholar]
- 21.Bonadonna G, Moliterni A, Zambetti M, et al. 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: Cohort study. BMJ. 2005;330:217–220. doi: 10.1136/bmj.38314.622095.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green S. Do estimates of long-term survival tell us whether patients diagnosed with breast cancer before age 50 years are ever cured? J Clin Oncol. 2004;22:392–394. doi: 10.1200/JCO.2004.11.972. [DOI] [PubMed] [Google Scholar]
- 23.Quesnel B. Tumor dormancy and immunoescape. APMIS. 2008;116:685–694. doi: 10.1111/j.1600-0463.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 24.Retsky MW, Demicheli R, Hrushesky WJ, et al. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS. 2008;116:730–741. doi: 10.1111/j.1600-0463.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 25.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park) 2012;26:688–694, 696. [PubMed] [Google Scholar]
- 26.Kim RS, Avivar-Valderas A, Estrada Y, et al. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS One. 2012;7:e35569. doi: 10.1371/journal.pone.0035569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumour dormancy. APMIS. 2008;116:754–770. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 28.Gnant M, Filipits M, Greil R, et al. Austrian Breast and Colorectal Cancer Study Group Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: Using the PAM50 risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]
- 29.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 30.National Institute for Clinical Excellence Guidance on cancer services: Improving outcomes in breast cancer—manual update. https://www.nice.org.uk/guidance/csg1/resources/improving-outcomes-in-breast-cancer-update-773371117.
- 31.Dixon JM, Montgomery DA. Extended follow-up of breast cancer patients in clinic wastes time for both patients and doctors: The case for. Breast Cancer Res. 2008;10(suppl 4):S7. doi: 10.1186/bcr2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cossetti RJ, Tyldesley SK, Speers CH, et al. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol. 2015;33:65–73. doi: 10.1200/JCO.2014.57.2461. [DOI] [PubMed] [Google Scholar]
- 33.Jatoi I, Anderson WF, Jeong JH, et al. Breast cancer adjuvant therapy: Time to consider its time-dependent effects. J Clin Oncol. 2011;29:2301–2304. doi: 10.1200/JCO.2010.32.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong JH, Jung SH, Wieand S. A parametric model for long-term follow-up data from phase III breast cancer clinical trials. Stat Med. 2003;22:339–352. doi: 10.1002/sim.1349. [DOI] [PubMed] [Google Scholar]
- 35.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.