Abstract
Recently several assays have been developed which allow the growth of colonies from cell suspensions prepared from human tumour biopsy specimens. It has been suggested that such assays will provide a reliable means of measuring the chemosensitivity of human tumours for predicting the response to treatment in patients. We have briefly reviewed the previous, largely unsuccessful, attempts at chemosensitivity testing and the potential place of the new assays. The measurement of the survival of clonogenic tumour cells after cytotoxic treatment probably reflects to some extent the survival of cells which in vivo are capable of proliferating to repopulate and regrow the tumour. This endpoint therefore has advantages over alternatives that do not directly measure reproductive cell death, and the assays also have the advantage of suppressing the growth of many non-malignant cells found in tumours. However, technical problems such as the preparation of cell suspensions and the artificial nature of the drug exposure phase of the assays have not been completely overcome and the plating efficiencies remain low in most systems. Work with model systems such as human tumour xenografts tends to support the usefulness of the assays but also highlights some difficulties. Clinical studies of chemosensitivity testing are in progress and initial results are encouraging but inconclusive.
Full text
PDF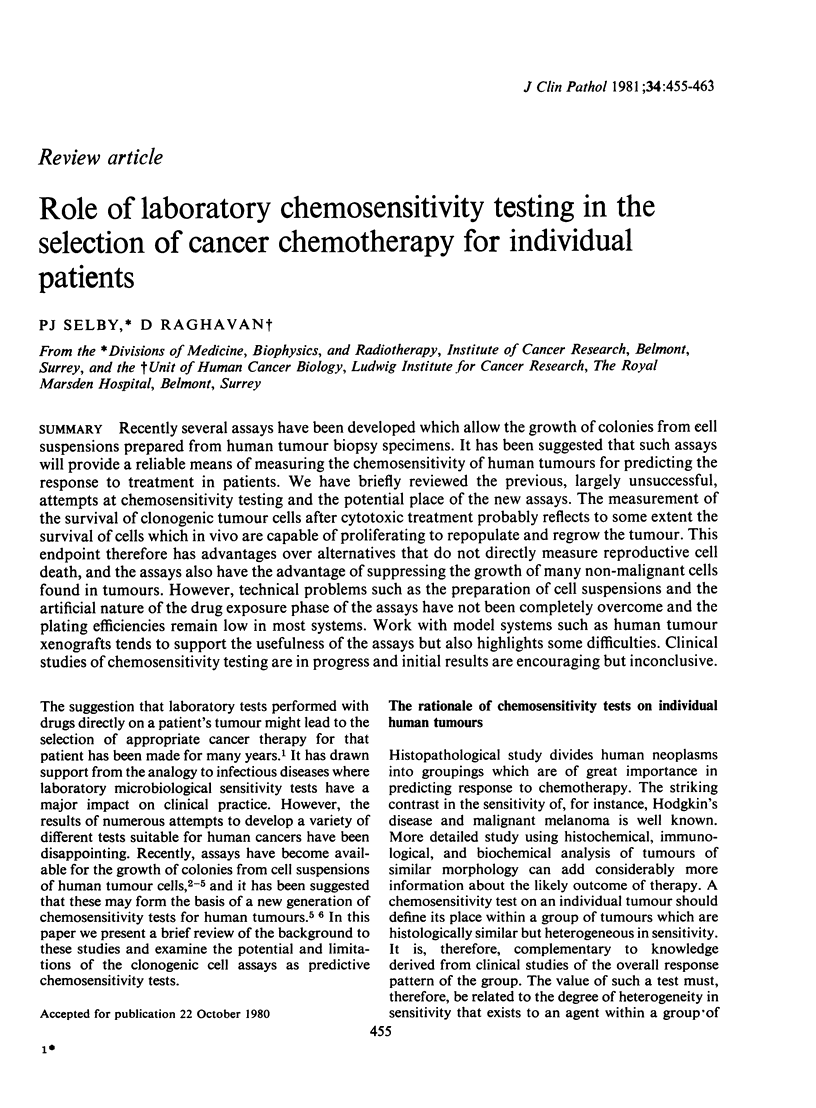






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts D. S., Samon S. E., Chen H. S., Surwit E. A., Soehnlen B., Young L., Moon T. E. In-vitro clonogenic assay for predicting response of ovarian cancer to chemotherapy. Lancet. 1980 Aug 16;2(8190):340–342. doi: 10.1016/s0140-6736(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Barendsen G. W. Analysis of tumour responses by excision and in vitro assay of cellular clonogenic capacity. Br J Cancer Suppl. 1980 Apr;4:209–216. [PMC free article] [PubMed] [Google Scholar]
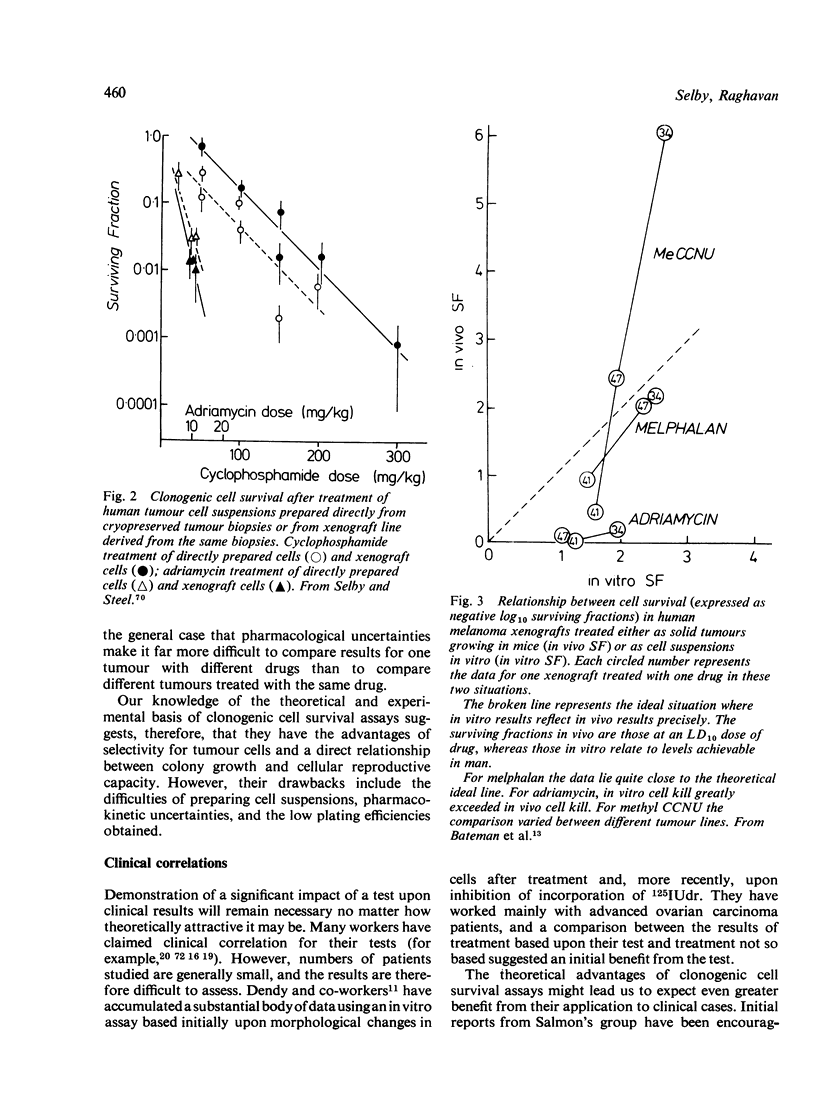
- Bateman A. E., Peckham M. J., Steel G. G. Assays of drug sensitivity for cells from human tumours: in vitro and in vivo tests on a xenografted tumour. Br J Cancer. 1979 Jul;40(1):81–88. doi: 10.1038/bjc.1979.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
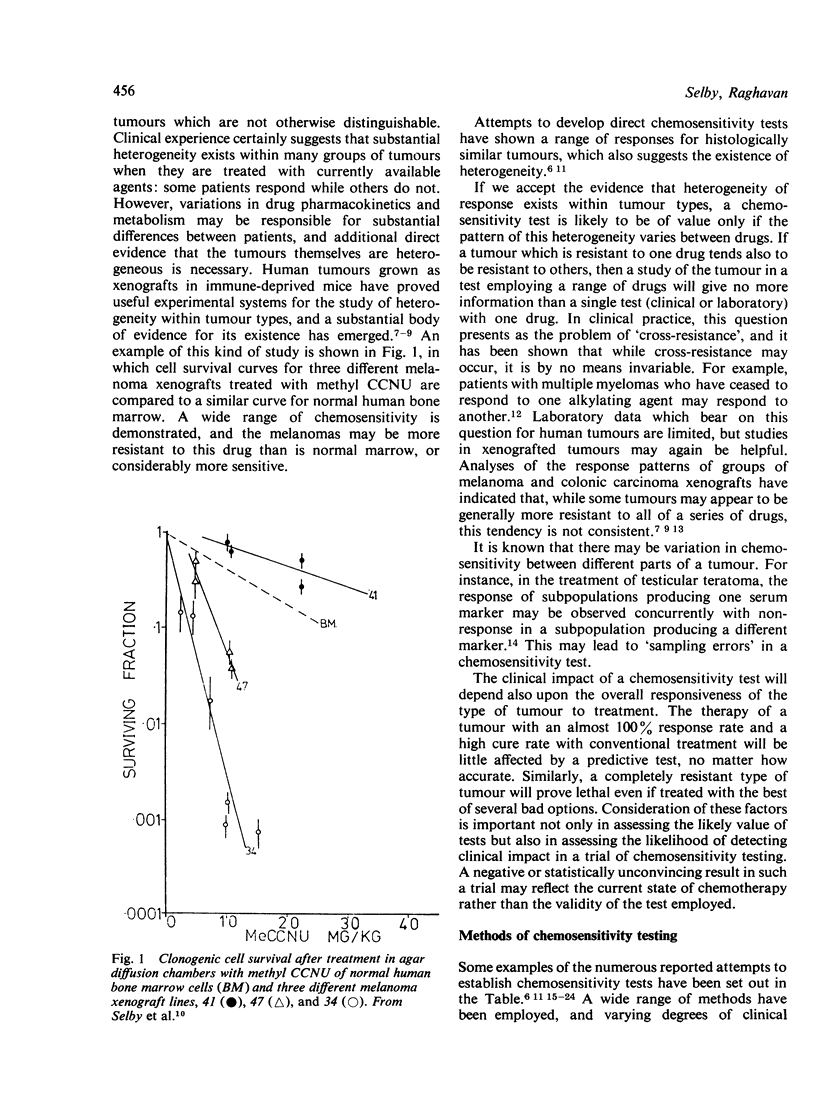
- Bateman A. E., Selby P. J., Steel G. G., Towse G. D. In vitro chemosensitivity tests on xenografted human melanomas. Br J Cancer. 1980 Feb;41(2):189–198. doi: 10.1038/bjc.1980.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsagel D. E., Cowan D. H., Hasselback R. Plasma cell myeloma: response of melphalan-resistant patients to high-dose intermittent cyclophosphamide. Can Med Assoc J. 1972 Nov 4;107(9):851–855. [PMC free article] [PubMed] [Google Scholar]
- Berry R. J., Laing A. H., Wells J. Fresh explant culture of human tumours in vitro and the assessment of sensitivity to cytotoxic chemotherapy. Br J Cancer. 1975 Feb;31(2):218–227. doi: 10.1038/bjc.1975.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuyan B. K., Loughman B. E., Fraser T. J., Day K. J. Comparison of different methods of determining cell viability after exposure to cytotoxic compounds. Exp Cell Res. 1976 Feb;97(2):275–280. doi: 10.1016/0014-4827(76)90617-0. [DOI] [PubMed] [Google Scholar]
- Billiau A., Cassiman J. J., Willems D., Verhelst M., Heremans H. In vitro cultivation of human tumor tissues. Oncology. 1975;31(5-6):257–272. doi: 10.1159/000225032. [DOI] [PubMed] [Google Scholar]
- Bloomfield C. D., Smith K. A., Peterson B. A., Hildebrandt L., Zaleskas J., Gajl-Peczalska K. J., Frizzera G., Munck A. In-vitro glucocorticoid studies for predicting response to glucocorticoid therapy in adults with malignant lymphoma. Lancet. 1980 May 3;1(8175):952–956. doi: 10.1016/s0140-6736(80)91405-1. [DOI] [PubMed] [Google Scholar]
- Boone C. W., Harell G. S., Bond H. E. The resolution of mixtures of viable mammalian cells into homogeneous fractions by zonal centrifugation. J Cell Biol. 1968 Feb;36(2):369–378. doi: 10.1083/jcb.36.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce W. R., Meeker B. E., Valeriote F. A. Comparison of the sensitivity of normal hematopoietic and transplanted lymphoma colony-forming cells to chemotherapeutic agents administered in vivo. J Natl Cancer Inst. 1966 Aug;37(2):233–245. [PubMed] [Google Scholar]
- Buick R. N., Fry S. E., Salmon S. E. Application of in vitro soft agar techniques for growth of tumor cells to the study of colon cancer. Cancer. 1980 Mar 15;45(5 Suppl):1238–1242. doi: 10.1002/1097-0142(19800315)45:5+<1238::aid-cncr2820451333>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Buick R. N., Stanisic T. H., Fry S. E., Salmon S. E., Trent J. M., Krasovich P. Development of an agar-methyl cellulose clonogenic assay for cells in transitional cell carcinoma of the human bladder. Cancer Res. 1979 Dec;39(12):5051–5056. [PubMed] [Google Scholar]
- Buick R. N., Till J. E., McCulloch E. A. Colony assay for proliferative blast cells circulating in myeloblastic leukaemia. Lancet. 1977 Apr 16;1(8016):862–863. doi: 10.1016/s0140-6736(77)92818-5. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D., Selby P. J., Smith I. E., Mills J., Peckham M. J. Growth of human tumour cell colonies from biopsies using two soft-agar techniques. Br J Cancer. 1978 Jul;38(1):77–81. doi: 10.1038/bjc.1978.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D., Smith I. E., Peckham M. J., Steel G. G. In vitro and in vivo radiosensitivity of human tumour cells obtained from a pancreatic carcinoma xenograft. Nature. 1976 Oct 28;263(5580):771–772. doi: 10.1038/263771a0. [DOI] [PubMed] [Google Scholar]
- DIPAOLO J. A. In vitro test systems for cancer chemotherapy. I. Inhibition of dehydrogenases and growth in the Ehrlich ascites tumor. Cancer Res. 1963 Feb;23:184–190. [PubMed] [Google Scholar]
- Dendy P. P. The use of in vitro methods to predict tumour response to chemotherapy. Br J Cancer Suppl. 1980 Apr;4:195–198. [PMC free article] [PubMed] [Google Scholar]
- Freshney R. I., Paul J., Kane I. M. Assay of anti-cancer drugs in tissue culture: conditions affecting their ability to incorporate 3H-leucine after drug treatment. Br J Cancer. 1975 Jan;31(1):89–99. doi: 10.1038/bjc.1975.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney R. I. Tumour cells disaggregated in collagenase. Lancet. 1972 Sep 2;2(7775):488–489. doi: 10.1016/s0140-6736(72)91885-5. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y. Quantitation of haemopoietic cells from normal and leukaemic RFM mice using an in vivo colony assay. Br J Cancer. 1974 Nov;30(5):421–428. doi: 10.1038/bjc.1974.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guner M., Freshney R. I., Morgan D., Freshney M. G., Thomas D. G., Graham D. I. Effects of dexamethasone and betamethasone on in vitro cultures from human astrocytoma. Br J Cancer. 1977 Apr;35(4):439–447. doi: 10.1038/bjc.1977.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C. Prediction of responses to therapy and mechanisms of resistance. Semin Oncol. 1977 Jun;4(2):193–202. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E., Kim M. B., Trent J. M., Soehnlen B. J., Alberts D. S., Schmidt H. J. Direct cloning of human ovarian carcinoma cells in agar. Cancer Res. 1978 Oct;38(10):3438–3444. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Holmes H. L., Little J. M. Tissue-culture microtest for predicting response of human cancer to chemotherapy. Lancet. 1974 Oct 26;2(7887):985–987. doi: 10.1016/s0140-6736(74)92075-3. [DOI] [PubMed] [Google Scholar]
- Jones S. E., Hamburger A. W., Kim M. B., Salmon S. E. Development of a bioassay for putative human lymphoma stem cells. Blood. 1979 Feb;53(2):294–303. [PubMed] [Google Scholar]
- Kao W. W., Prockop D. J. Proline analogue removes fibroblasts from cultured mixed cell populations. Nature. 1977 Mar 3;266(5597):63–64. doi: 10.1038/266063a0. [DOI] [PubMed] [Google Scholar]
- Kessel D., Hall T. C., Roberts D., Wodinsky I. Uptake as a determinant of methotrexate response in mouse leukemias. Science. 1965 Nov 5;150(3697):752–754. doi: 10.1126/science.150.3697.752. [DOI] [PubMed] [Google Scholar]
- Knock F. E., Galt R. M., Oester Y. T., Sylvester R. In vitro estimate of sensitivity of individual human tumors to antitumor agents. Oncology. 1974;30(1):1–22. doi: 10.1159/000224937. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W., Lippman M. E. Glucocorticoid sensitivity and receptors in cells of human myelogenous leukemia lines. Cancer Res. 1980 Mar;40(3):563–566. [PubMed] [Google Scholar]
- Lin H., Stewart C. C. Colony formation by mouse peritoneal exudate cells in vitro. Nat New Biol. 1973 Jun 6;243(127):176–177. doi: 10.1038/newbio243176a0. [DOI] [PubMed] [Google Scholar]
- MADDEN R. E., BURK D. Production of viable single cell suspensions from solid tumors. J Natl Cancer Inst. 1961 Oct;27:841–861. [PubMed] [Google Scholar]
- Marzotko F., Krafft W., Preibsch W., Schröder M. Die in-vitro-Testung und klinische Erfahrungen mit 5-Fluor-Uracil bei gynäkologischen Karzinomen. Arch Geschwulstforsch. 1976;46(2):140–145. [PubMed] [Google Scholar]
- McAllister R. M., Reed G. Colonial growth in agar of cells derived from neoplastic and non-neoplastic tissues of children. Pediatr Res. 1968 Sep;2(5):356–360. doi: 10.1203/00006450-196809000-00004. [DOI] [PubMed] [Google Scholar]
- McGuire W. L. Steroid receptors in human breast cancer. Cancer Res. 1978 Nov;38(11 Pt 2):4289–4291. [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Salmon S. E. Inhibition of human melanoma colony formation by retinoids. Cancer Res. 1979 Oct;39(10):4055–4057. [PubMed] [Google Scholar]
- Nowak K., Peckham M. J., Steel G. G. Variation in response of xenografts of colo-rectal carcinoma to chemotherapy. Br J Cancer. 1978 Apr;37(4):576–584. doi: 10.1038/bjc.1978.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Bergsagel D. E., McCulloch E. A. Chemotherapy of mouse myeloma: quantitative cell cultures predictive of response in vivo. Blood. 1973 Jan;41(1):7–15. [PubMed] [Google Scholar]
- Osieka R., Houchens D. P., Goldin A., Johnson R. K. Chemotherapy of human colon cancer xenografts in athymic nude mice. Cancer. 1977 Nov;40(5 Suppl):2640–2650. doi: 10.1002/1097-0142(197711)40:5+<2640::aid-cncr2820400938>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Park C. H., Bergsagel D. E., McCulloch E. A. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971 Feb;46(2):411–422. [PubMed] [Google Scholar]
- Puck T. T., Marcus P. I. A RAPID METHOD FOR VIABLE CELL TITRATION AND CLONE PRODUCTION WITH HELA CELLS IN TISSUE CULTURE: THE USE OF X-IRRADIATED CELLS TO SUPPLY CONDITIONING FACTORS. Proc Natl Acad Sci U S A. 1955 Jul 15;41(7):432–437. doi: 10.1073/pnas.41.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan D., Gibbs J., Nogueira Costa R., Kohn J., Orr A. H., Barrett A., Peckham M. J. The interpretation of marker protein assays: a critical appraisal in clinical studies and a xenograft model. Br J Cancer Suppl. 1980 Apr;4:191–194. [PMC free article] [PubMed] [Google Scholar]
- Rasey J. S., Nelson N. J. Response of an in vivo-in vitro tumour to X-rays and cytotoxic drugs: effect of tumour disaggregation method on cell survival. Br J Cancer Suppl. 1980 Apr;4:217–221. [PMC free article] [PubMed] [Google Scholar]
- Roper P. R., Drewinko B. Cell survival following treatment with antitumor drugs. Cancer Res. 1979 Apr;39(4):1428–1430. [PubMed] [Google Scholar]
- Roper P. R., Drewinko B. Comparison of in vitro methods to determine drug-induced cell lethality. Cancer Res. 1976 Jul;36(7 Pt 1):2182–2188. [PubMed] [Google Scholar]
- Rosenblum M. L., Dougherty D. A., Deen D. F., Hoshino T., Wilson C. B. Analysis of clonogenic human brain tumour cells: preliminary results of tumour sensitivity testing with BCNU. Br J Cancer Suppl. 1980 Apr;4:181–185. [PMC free article] [PubMed] [Google Scholar]
- Rupniak H. T., Hill B. T. The poor cloning ability in agar of human tumour cells from biopsies of primary tumours. Cell Biol Int Rep. 1980 May;4(5):479–486. doi: 10.1016/0309-1651(80)90035-1. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W., Soehnlen B., Durie B. G., Alberts D. S., Moon T. E. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978 Jun 15;298(24):1321–1327. doi: 10.1056/NEJM197806152982401. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Thompson E. B. Glucocorticoid receptors and glutamine synthetase in leukemic Sézary cells. Cancer Res. 1979 Feb;39(2 Pt 1):376–382. [PubMed] [Google Scholar]
- Selby P. J., Courtenay V. D., McElwain T. J., Peckham M. J., Steel G. G. Colony growth and clonogenic cell survival in human melanoma xenografts treated with chemotherapy. Br J Cancer. 1980 Sep;42(3):438–447. doi: 10.1038/bjc.1980.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby P. J., Thomas J. M. Clonogenic cell survival curves for human melanoma xenografts using agar diffusion chamber and lung colony assays. Br J Cancer Suppl. 1980 Apr;4:150–153. [PMC free article] [PubMed] [Google Scholar]
- Selby P. J., Thomas J. M., Peckham M. J. A comparison of the chemosensitivity of a primary tumour and its metastases using a human tumour xenograft. Eur J Cancer. 1979 Dec;15(12):1425–1429. doi: 10.1016/0014-2964(79)90020-3. [DOI] [PubMed] [Google Scholar]
- Shorthouse A. J., Peckham M. J., Smyth J. F., Steel G. G. The therapeutic response of bronchial carcinoma xenografts: a direct patient-xenograft comparison. Br J Cancer Suppl. 1980 Apr;4:142–145. [PMC free article] [PubMed] [Google Scholar]
- Smith I. E., Courtenay V. D., Gordon M. Y. A colony-forming assay for human tumour xenografts using agar in diffusion chambers. Br J Cancer. 1976 Nov;34(5):476–483. doi: 10.1038/bjc.1976.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. C., Currie G. A., Peacock J. H. Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. Br J Cancer. 1978 Nov;38(5):573–582. doi: 10.1038/bjc.1978.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Whitescarver J., Briggs L., Anson J. H. Separation of tumor cells from fibroblasts with use of discontinuous density gradients. J Natl Cancer Inst. 1970 Apr;44(4):855–864. [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Tanneberger S., Bacigalupo G. Die Benutzung von Zellkulturen zur Ermittlung der Sensibilität menschlicher Tumoren gegenüber Zytostatika. Dtsch Gesundheitsw. 1967 Jan 5;22(1):11–15. [PubMed] [Google Scholar]
- Valeriote F., van Putten L. Proliferation-dependent cytotoxicity of anticancer agents: a review. Cancer Res. 1975 Oct;35(10):2619–2630. [PubMed] [Google Scholar]
- WRIGHT J. C., COBB J. P., GUMPORT S. L., GOLOMB F. M., SAFADI D. Investigation of the relation between clinical and tissue-culture response to chemotherapeutic agents on human cancer. N Engl J Med. 1957 Dec 19;257(25):1207–1211. doi: 10.1056/NEJM195712192572502. [DOI] [PubMed] [Google Scholar]
- Wright J. C., Walker D. A predictive test for the selection of cancer chemotherapeutic agents for the treatment of human cancer. J Surg Oncol. 1975;7(5):381–393. doi: 10.1002/jso.2930070509. [DOI] [PubMed] [Google Scholar]


