Abstract
Cytochromes P450 are members of a superfamily of hemoproteins that are involved in the metabolism of various physiologic and xenobiotic organic compounds. This superfamily of proteins can be divided into two classes based on the electron donor proximal to the P450: an iron-sulfur protein for class I P450s or a flavoprotein for class II. The only known tertiary structure of any of the cytochromes P450 is that of P450cam, a class I soluble enzyme isolated from Pseudomonas putida (product of the CYP101 gene). To understand the details of the structure-function relationships within and between the two classes, structural studies on additional cytochromes P450 are crucial. We report here characterization of the crystal forms of two soluble, bacterial enzymes: cytochrome P450terp [class I enzyme from a Pseudomonas species (product of CYP108 gene)] and the hemoprotein domain of cytochrome P450BM-3 [class II enzyme from Bacillus megaterium (product of the CYP102 gene)]. The crystals of cytochrome P450terp are hexagonal and belong to the space group P6(1)22 (or its enantiomorph, P6(5)22) with unit cell dimensions a = b = 68.9 A and c = 458.7 A. The crystals of the hemoprotein domain of cytochrome P450BM-3 are monoclinic and belong to the space group P2(1) with unit cell dimensions a = 59.4 A, b = 154.0 A, c = 62.2 A, and beta = 94.7 degrees. Diffraction data for the crystals of these two proteins were obtained to a resolution better than 2.2 A. Assuming the presence of two molecules in the asymmetric unit for the hemoprotein domain of P450BM-3 and one molecule for P450terp, the calculated values of Vm are 2.6 and 3.3 A3/Da, respectively.
Full text
PDF

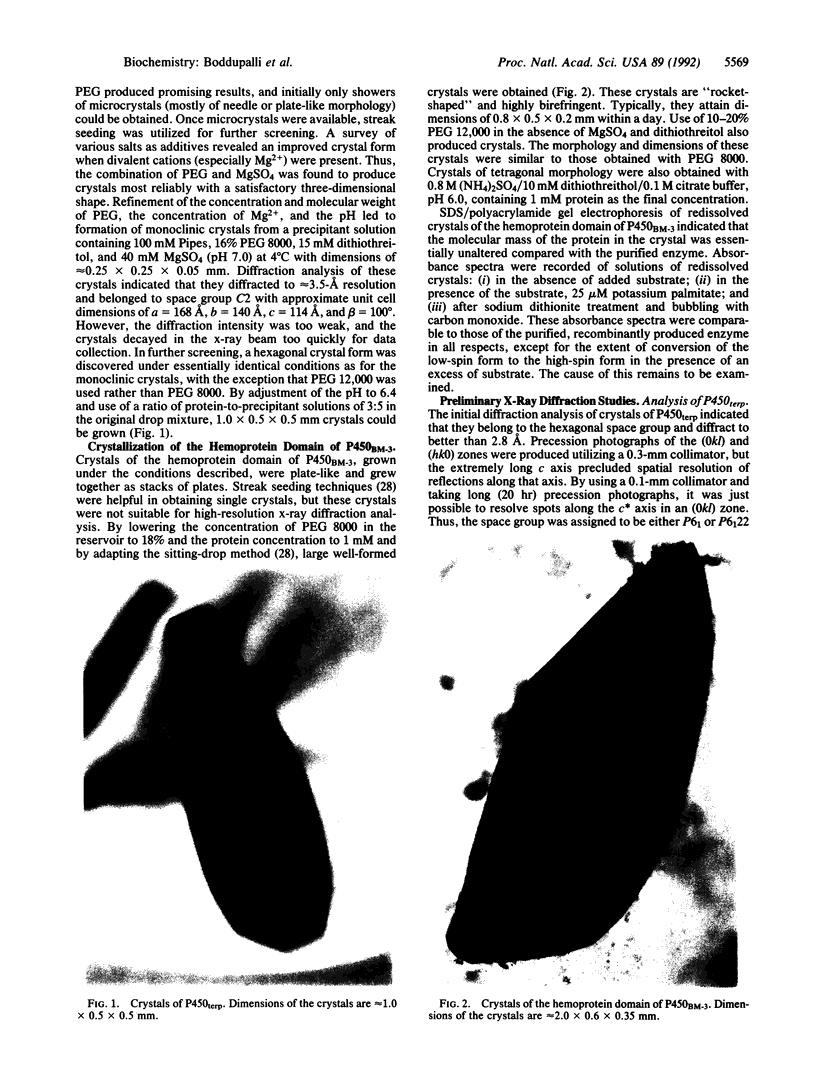


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boddupalli S. S., Estabrook R. W., Peterson J. A. Fatty acid monooxygenation by cytochrome P-450BM-3. J Biol Chem. 1990 Mar 15;265(8):4233–4239. [PubMed] [Google Scholar]
- Boddupalli S. S., Pramanik B. C., Slaughter C. A., Estabrook R. W., Peterson J. A. Fatty acid monooxygenation by P450BM-3: product identification and proposed mechanisms for the sequential hydroxylation reactions. Arch Biochem Biophys. 1992 Jan;292(1):20–28. doi: 10.1016/0003-9861(92)90045-x. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. P450BM-3 and other inducible bacterial P450 cytochromes: biochemistry and regulation. Annu Rev Pharmacol Toxicol. 1991;31:177–203. doi: 10.1146/annurev.pa.31.040191.001141. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Guengerich F. P. Reactions and significance of cytochrome P-450 enzymes. J Biol Chem. 1991 Jun 5;266(16):10019–10022. [PubMed] [Google Scholar]
- Gunsalus I. C., Bhattacharyya P. K., Suhara K. Bioregulation of binding and dynamics: the cytochrome P-450CAM model. Curr Top Cell Regul. 1985;26:295–309. doi: 10.1016/b978-0-12-152826-3.50029-2. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Sligar S. G. Oxygen reduction by the P450 monoxygenase systems. Adv Enzymol Relat Areas Mol Biol. 1978;47:1–44. doi: 10.1002/9780470122921.ch1. [DOI] [PubMed] [Google Scholar]
- Hamlin R. Multiwire area X-ray diffractometers. Methods Enzymol. 1985;114:416–452. doi: 10.1016/0076-6879(85)14029-2. [DOI] [PubMed] [Google Scholar]
- Howard A. J., Nielsen C., Xuong N. H. Software for a diffractometer with multiwire area detector. Methods Enzymol. 1985;114:452–472. doi: 10.1016/0076-6879(85)14030-9. [DOI] [PubMed] [Google Scholar]
- Imai M., Shimada H., Watanabe Y., Matsushima-Hibiya Y., Makino R., Koga H., Horiuchi T., Ishimura Y. Uncoupling of the cytochrome P-450cam monooxygenase reaction by a single mutation, threonine-252 to alanine or valine: possible role of the hydroxy amino acid in oxygen activation. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7823–7827. doi: 10.1073/pnas.86.20.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughton C. A., Neidle S., Zvelebil M. J., Sternberg M. J. A molecular model for the enzyme cytochrome P450(17 alpha), a major target for the chemotherapy of prostatic cancer. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1160–1167. doi: 10.1016/0006-291x(90)90806-x. [DOI] [PubMed] [Google Scholar]
- Li H. Y., Darwish K., Poulos T. L. Characterization of recombinant Bacillus megaterium cytochrome P-450 BM-3 and its two functional domains. J Biol Chem. 1991 Jun 25;266(18):11909–11914. [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Okey A. B. Enzyme induction in the cytochrome P-450 system. Pharmacol Ther. 1990;45(2):241–298. doi: 10.1016/0163-7258(90)90030-6. [DOI] [PubMed] [Google Scholar]
- Oster T., Boddupalli S. S., Peterson J. A. Expression, purification, and properties of the flavoprotein domain of cytochrome P-450BM-3. Evidence for the importance of the amino-terminal region for FMN binding. J Biol Chem. 1991 Nov 25;266(33):22718–22725. [PubMed] [Google Scholar]
- Peterson J. A., Lu J. Y. Bacterial cytochromes P450: isolation and identification. Methods Enzymol. 1991;206:612–620. doi: 10.1016/0076-6879(91)06131-l. [DOI] [PubMed] [Google Scholar]
- Porter T. D. An unusual yet strongly conserved flavoprotein reductase in bacteria and mammals. Trends Biochem Sci. 1991 Apr;16(4):154–158. doi: 10.1016/0968-0004(91)90059-5. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Coon M. J. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991 Jul 25;266(21):13469–13472. [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Howard A. J. Crystal structure of substrate-free Pseudomonas putida cytochrome P-450. Biochemistry. 1986 Sep 9;25(18):5314–5322. doi: 10.1021/bi00366a049. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Howard A. J. High-resolution crystal structure of cytochrome P450cam. J Mol Biol. 1987 Jun 5;195(3):687–700. doi: 10.1016/0022-2836(87)90190-2. [DOI] [PubMed] [Google Scholar]
- Poulos T. L. Modeling of mammalian P450s on basis of P450cam X-ray structure. Methods Enzymol. 1991;206:11–30. doi: 10.1016/0076-6879(91)06073-c. [DOI] [PubMed] [Google Scholar]
- Raag R., Poulos T. L. Crystal structure of the carbon monoxide-substrate-cytochrome P-450CAM ternary complex. Biochemistry. 1989 Sep 19;28(19):7586–7592. doi: 10.1021/bi00445a013. [DOI] [PubMed] [Google Scholar]
- Raag R., Poulos T. L. The structural basis for substrate-induced changes in redox potential and spin equilibrium in cytochrome P-450CAM. Biochemistry. 1989 Jan 24;28(2):917–922. doi: 10.1021/bi00428a077. [DOI] [PubMed] [Google Scholar]
- Ruettinger R. T., Wen L. P., Fulco A. J. Coding nucleotide, 5' regulatory, and deduced amino acid sequences of P-450BM-3, a single peptide cytochrome P-450:NADPH-P-450 reductase from Bacillus megaterium. J Biol Chem. 1989 Jul 5;264(19):10987–10995. [PubMed] [Google Scholar]
- Yu C., Gunsalus I. C. Crystalline cytochrome P-450cam. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1431–1436. doi: 10.1016/0006-291x(70)90027-6. [DOI] [PubMed] [Google Scholar]
- Zvelebil M. J., Wolf C. R., Sternberg M. J. A predicted three-dimensional structure of human cytochrome P450: implications for substrate specificity. Protein Eng. 1991 Feb;4(3):271–282. doi: 10.1093/protein/4.3.271. [DOI] [PubMed] [Google Scholar]