Abstract
Viruses are frequent causes of lower respiratory infection (LRI). Programmed cell death-1 (PD-1) signaling contributes to pulmonary CD8+ T cell (TCD8) functional impairment during acute viral LRI, but the role of TCD8 impairment in viral clearance and immunopathology is unclear. We now find that human metapneumovirus (HMPV) infection induces virus-specific lung TCD8 that fail to produce effector cytokines or degranulate late after infection, with minimally increased function even in the absence of PD-1 signaling. Impaired lung TCD8 upregulated multiple inhibitory receptors, including PD-1, LAG-3, TIM-3, and 2B4. Moreover, co-expression of these receptors continued to increase even after viral clearance, with most virus-specific lung TCD8 expressing ≥3 inhibitory receptors on day 14 post-infection. Viral infection also increased expression of inhibitory ligands by both airway epithelial cells and antigen presenting cells, further establishing an inhibitory environment. In vitro antibody blockade revealed that multiple inhibitory receptors contribute to TCD8 impairment induced by either HMPV or influenza virus infection. In vivo blockade of TIM-3 signaling failed to enhance TCD8 function or reduce viral titers. However, blockade of LAG-3 in PD-1-deficient mice restored TCD8 effector functions but increased lung pathology, indicating that LAG-3 mediates lung TCD8 impairment in vivo and contributes to protection from immunopathology during viral clearance. These results demonstrate that an orchestrated network of pathways modifies lung TCD8 functionality during viral LRI, with PD-1 and LAG-3 serving prominent roles. Lung TCD8 impairment may prevent immunopathology but also contribute to recurrent lung infections.
Keywords: human metapneumovirus, paramyxovirus, influenza, T cell response, respiratory infections
INTRODUCTION
Respiratory viruses, including human metapneumovirus (HMPV), are leading causes of lower respiratory infection (LRI) and are associated with significant morbidity and mortality (1–6). Hospitalization with HMPV is more likely in patients with underlying conditions such as asthma, chronic obstructive pulmonary disease, HIV, or premature birth (7–12). HMPV causes severe and fatal disease in persons with depressed T cell function, highlighting the role of CD8+ T cells (TCD8) in viral clearance (7, 9, 11, 12). On the other hand, T cell-mediated inflammation contributes to lung damage and clinical disease during infection with RSV, influenza, and other respiratory viruses (1, 3, 13–18). Thus, a balance between immunoprotection and immunopathology is important for host defense against respiratory viruses.
Previous reports have identified functional impairment in acute and memory TCD8 from the lung, but not from draining lymph nodes or spleen, during murine infection with RSV, influenza, or pneumonia virus of mice, but the mechanism causing impairment was unknown (19–22). We recently demonstrated that signaling through the inhibitory receptor programmed cell death-1 (PD-1) dampens the effector functions of virus-specific lung TCD8, but not splenic TCD8, and impairs viral clearance during acute and recurrent infections (23, 24). The PD-1 pathway is thought to play a crucial role during chronic infections (25–27) and cancer (28, 29), with therapeutic blockade of PD-1 (“checkpoint inhibition” therapy) proving effective in several types of cancer (28–31). In addition, PD-1 was recently shown to be dispensable for the initiation of T cell exhaustion in a chronic infection model (32), highlighting the importance of better understanding additional pathways that modify T cell functions. Other inhibitory receptors that have been shown to mediate TCD8 exhaustion during chronic infection or cancer include lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin mucin-3 (TIM-3), and 2B4 (26, 33, 34). Growing evidence supports a role for PD-1 during acute infections (35), but it is not known whether these additional inhibitory receptors contribute to lung TCD8 impairment during acute viral LRI as well.
Expression of the major PD-1 ligand PD-L1 by respiratory epithelial cells alone is capable of impairing TCD8 in vitro (36, 37) and local blockade of PD-L1 in the respiratory tract restores TCD8 functions in vivo (38). However, given the immunologic complexity of the lung environment, we reasoned that additional mechanisms likely exist to control lung TCD8 responses. In the present study, we define the kinetics of pulmonary TCD8 impairment during viral LRI. We show that lung TCD8 become impaired even in the absence of PD-1 and that additional inhibitory receptors contribute to this impairment. Additionally, lung epithelial cells and antigen presenting cells upregulate the ligands for these receptors, inducing an inhibitory environment in the lung. We found that LAG-3 is capable of compensating for absent PD-1 signaling and that this inhibitory receptor may function to dampen lung TCD8 functions at later time points during the immune response to infection.
METHODS
Mice
C57BL/6 (B6) mice were purchased from the Jackson Laboratory. B6-Kb0Db0;B7.2 transgenic (B7tg) mice were obtained with permission from Drs. Alexander Sette (La Jolla Institute for Allergy and Immunology, La Jolla, CA) and Francois Lemonnier (Institut Pasteur, Paris, France). PD-1−/− mice were obtained with permission from Dr. Tasuku Honjo (Kyoto University, Kyoto, Japan). All animals were bred and maintained in specific pathogen-free conditions in accordance with the Vanderbilt Institutional Animal Care and Use Committee. 6–12 week old age- and gender-matched animals were used in all experiments.
Viruses and Infections
HMPV (pathogenic clinical strain TN/94-49, genotype A2) was grown and titered in LLC-MK2 cells as described (39). Influenza virus strains A/34/PR/8 (PR8; H1N1; ATCC) and HK/x31 (x31; H3N2; kindly provided by Drs. Jon McCullers and Paul Thomas, St. Jude Children’s Hospital, Memphis, TN) were grown in MDCK cells and titered on LLC-MK2 cells. For all experiments, mice were anesthetized with ketamine-xylazine and infected intranasally (i.n.) with 1×106 PFU of HMPV. Animals were euthanized on day 7 post-infection, and lung tissues collected and pulverized in glass homogenizers before centrifugation at 1200 rpm at 4°C for 10 min. Nasal turbinates (NT) were collected and ground with mortar and pestle prior to centrifugation. Supernatants were collected, aliquoted into cryovials, and snap-frozen in dry ice-ethanol for storage at −80°C until further use. Viral titers were quantified by plaque titration as previously described (39). For influenza virus challenge experiments, mice were primed i.p. with 2×105 PFU of PR8 and challenged i.n. with 5×102 PFU of x31 at least 15 weeks later.
Flow Cytometry Staining
Tetramers were generated for the following viral epitopes as described (23): HMPV (HLA-B*0702/M195–203 [APYAGLIMI], H2-Db/F528–536 [SGVTNNGFI], H2-Kb/N11–19 [LSYKHAIL], and influenza virus (H2-Db/NP366–374 [ASNENMETM]). Lymphocytes were isolated from spleens and lungs of infected animals and stained as described (23). Cells were stained with PE- or APC-labeled tetramers (0.1–1 µg/ml), anti-CD8α (clone 53-6.7, BD Biosciences), and anti-CD19 (clone 1D3, iCyt). In some experiments, cells were also stained for the inhibitory receptors PD-1 (clone RMP1-30), TIM-3 (clone RMT3-23), LAG-3 (clone C9B7W) and 2B4 (clone m2B4 (B6)458.1) or with appropriate isotype controls (all from Biolegend). Surface/tetramer staining was performed for 1 hour at RT in PBS containing 1% FBS and 50nM dasatinib. To stain for the ligands of each inhibitory receptor, lung cell suspensions were stained with LIVE/DEAD dye and Fc blocked in the presence of 20% mouse serum followed by surface staining for EpCAM (clone G8.8, Biolegend), CD11c (clone HL3, BD Biosciences), PD-L1 (clone MIH5, BD Biosciences), PD-L2 (clone TY25, Abcam), MHC-II (clone M5/114.15.2, eBiosciences) and CD48 (clone HM48-1, Biolegend). Some cells were also fixed and permeabilized to stain for intracellular Galectin-9 (clone 108A2, Biolegend). Intracellular cytokine staining was performed in parallel with tetramer staining as described (23). Flow cytometric data were collected using an LSRII or Fortessa (BD Biosciences) and analyzed with FlowJo software (Tree Star). Boolean gating in FlowJo was used to assess inhibitory receptor co-expression and patterns were visualized using the SPICE program (NIAID).
IFNγ ELISPOT
ELISPOT assays were performed as described (40) with slight modifications. 5×104 lung cells or 2×105 spleen cells were added to triplicate wells. Peptides were then added (10µM final concentration) followed by inhibitory receptor/ligand blocking antibodies (10µg/mL final concentration). The following blocking antibodies were used (all from Bio X-cell except as indicated): isotype control (clone LTF-2), anti-PD-L1 (clone 10F.9G2), anti-PD-L2 (clone TY-25), anti-PD-1 (clone J43), anti-TIM-3 (clone RMT3-23), anti-LAG-3 (clone C9B7W), anti-2B4 (clone m2B4 (B6)458.1, Biolegend), and anti-CD48 (clone HM48-1). Plates were incubated at 37C for 42–48 hours, developed, and then counted using the ImmunoSpot Micro Analyzer (Cellular Technology Limited). The average number of spots in wells stimulated with an irrelevant peptide was subtracted from each experimental value, which was then expressed as spot forming cells (SFC) per 106 lymphocytes.
In vivo Signaling Blockade
To block LAG-3 signaling, WT or PD-1−/− mice were injected i.p. with 200µg of rat anti-mouse LAG-3 antibody (clone C9B7W, Bio X-cell) on day 3 p.i. On days 5, 7 and 9 p.i., 100µg of anti-LAG-3 or isotype control antibody was given i.n. and 100µg were given i.p. To block TIM-3 and PD-1 signaling concurrently, mice were injected i.p. with 200µg of rat anti-mouse TIM-3 antibody (clone RMT3-23, Bio X-cell) and anti-PD-L1 (clone 10F.9G2) for 2 days prior to infection and then every other day during HMPV infection. To block galectin-9 (Gal-9) binding to TIM-3, WT mice were infected intranasally with HMPV-A2. At day 3 of infection and every day thereafter until euthanasia, mice were injected 2x/day (morning and night) with 277 mM sterile filtered alpha-lactose (Sigma) in PBS, or sterile PBS for controls. To block TIM-3 signaling, PD-1−/− mice were injected i.p. with 200µg of rat anti-mouse TIM-3 antibody (clone RMT3-23, Bio X-cell) or isotype control antibody on days 3, 5, 7, and 9 post-infection.
Lung histopathology
Mice were infected with HMPV as described. Ten days post-infection, the left lung lobes were inflated with 10% formalin and fixed for 24 hours, embedded in paraffin, sectioned at 5-µm, and stained with hematoxylin and eosin. Slides were evaluated by an experienced veterinary pathologist (KLB) blinded to the groups and scored using an established method (1-rare perivascular cuffs of lymphocytes and plasma cells 2–5 cells thick; 2-lymphoplasmacytic perivascular and peribronchiolar cuffing; 3-perivascular and peribronchiolar cuffing and focal neutrophilic infiltration and necrosis in the alveoli and bronchiole-alveolar junction; and 4-marked perivascular cuffing with interstitial inflammation and widespread necrosis) (41).
Statistical Analysis
Comparisons between tetramer staining and ICS within the same animals were performed using a paired t test or between two independent groups using an unpaired t test. Multiple group comparisons were performed using one-way ANOVA with a Bonferroni post-test for comparison of individual groups. One-way ANOVA and Dunnett contrast method was used for multiple comparisons of mean number of spot forming cells (SFC) stimulated with M195 peptide in the presence of the indicated inhibitory receptor blocking antibodies to the reference group. A p value <0.05 was considered statistically significant by convention. All p values are for two-sided tests and unadjusted for multiple comparisons unless otherwise noted. Error bars on each graph represented the standard error of the mean unless otherwise noted. All analyses were conducted using Prism 6 (GraphPad).
Study Approval
All animals were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were handled according to protocols approved by the Vanderbilt University and University of Pittsburgh IACUC.
RESULTS
T Cell Impairment Occurs in the Absence of PD-1 Signaling
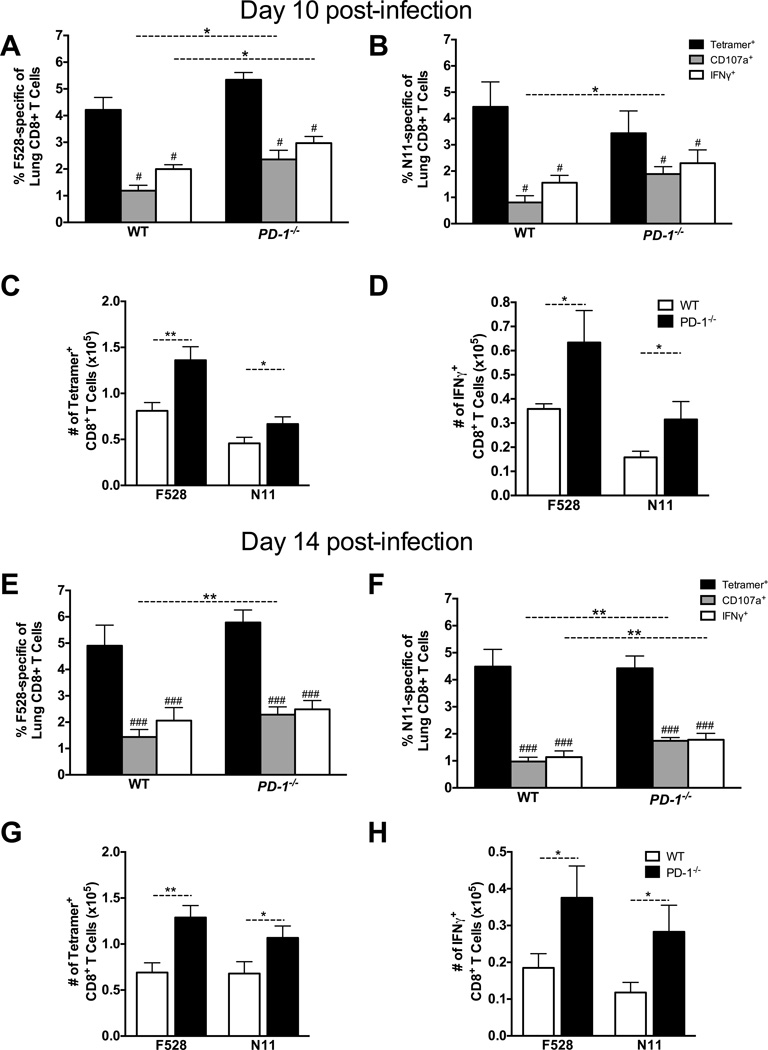
Antibody blockade or genetic ablation of PD-1 preserves TCD8 functionality on day 7 during acute viral LRI: in the absence of PD-1 signaling, lung TCD8 were 85–100% functional, leading to enhanced viral clearance (23). However, TCD8 impairment persists for many weeks beyond viral clearance (23, 24). We sought to determine whether PD-1 is the primary mediator of prolonged TCD8 impairment beyond day 7, as in the chronic LCMV infection model (27). We infected WT and PD-1−/− mice with HMPV and quantified TCD8 functionality on days 10 and 14 post-infection for the immunodominant epitopes H2-Db/F528–536 (F528) and H2-Kb/N11–19 (N11) using two separate assays performed in parallel: MHC-I tetramer staining to enumerate total epitope-specific TCD8 directly ex vivo and peptide restimulation followed by intracellular cytokine staining (ICS) for IFNγ (42) plus surface staining for CD107a (43) to quantify effector functions. Surprisingly, lung TCD8 exhibited an impaired phenotype on day 10 following primary HMPV infection despite absent PD-1 signaling (Fig. 1A–D); however, both the absolute number of epitope-specific TCD8 and functional TCD8 were still increased in PD-1−/− mice. This impairment persisted through day 14 post-infection (Fig. 1E–F), again with an increase in the percent and absolute number of epitope-specific and functional TCD8 in PD-1−/− mice. There was a significant but modest increase in functionality in PD-1−/− mice compared to WT, but the increased function in the absence of PD-1 was much less than that previously observed at day 7.
Fig. 1. T Cell Impairment Occurs Even in the Absence of PD-1 Signaling.
WT and PD-1−/− mice were infected with HMPV and the lung TCD8 responses were quantified at days 10 (A-D) and 14 (E-H) p.i. Black bars in A, B, E, and F indicate direct ex vivo H2-Db tetramer staining with F528 (A, E) or N11 (B, F). Lung cells were also restimulated with F528 or N11 peptide and the percentage that degranulate (i.e. mobilize CD107a to the cell surface) or produce IFNγ are shown in gray and white bars (A, B, E, and F), respectively. Absolute numbers of TET+ and IFNγ+ cells in WT (white bars) and PD-1−/− mice (black bars) are shown for day 10 (C, D) and 14 (G, H). Data are combined from 2 independent experiments with 5 mice per group per time point. # P<0.05, ### P<0.0005, paired t-test. * P<0.05, ** P<0.01, unpaired t-test.
Unlike lung TCD8, splenic TCD8 were not impaired on day 7 post-infection (23); here, we analyzed splenic HMPV-specific TCD8 on days 10 and 14 post-infection (Fig. S1). As in the lung, there was an increase in the percent of epitope-specific and functional TCD8 in PD-1−/− mice as well as an increase in the absolute number (not shown). However, splenic TCD8 in WT or PD-1−/− mice were not significantly impaired for degranulation or IFNγ secretion on days 10 and 14 (Fig. S1). Similar to these findings of lung TCD8 impairment in the absence of PD-1, we previously discovered a limited role for PD-1 during secondary HMPV and influenza virus infection in mice. During heterologous influenza challenge infection (PR8 H1N1 prime with x31 H3N2 challenge), lung TCD8 specific for the immunodominant NP366 epitope in PD-1−/− mice were slightly more functional than in WT mice, but nonetheless continued to exhibit significant impairment on day 10 post-infection (24). Moreover, blockade of PD-1 during secondary HMPV infection only partially restored TCD8 function (44). Taken together, these data suggest additional impairment mechanisms exist beyond that mediated by PD-1.
Kinetics of TCD8 Inhibitory Receptor and Ligand Expression
Gene expression analysis of lung and spleen TCD8 revealed that numerous immunomodulatory receptors were upregulated by lung TCD8 during acute viral LRI (45). To determine whether some of these inhibitory receptors increased on the cell surface during acute viral LRI, we performed flow cytometric analysis of lung and spleen HMPV-specific TCD8 at days 7, 10, and 14 p.i. To gain enough lung and spleen epitope-specific cells, we utilized B6-Kb0Db0;B7.2 transgenic (B7tg) mice, which can only recognize TCD8 epitopes restricted by human HLA-B*0702. In these mice, the immunodominant TCD8 epitope is M195–204 (M195).
The percentage of TCD8 expressing surface PD-1 was greater for lung TCD8 at day 7 and remained elevated over spleen at all time points (Fig. 2A). The same was true for TIM-3, LAG-3, and 2B4, with 2B4 increasing over time. We also determined the amount of surface inhibitory receptor expressed per cell by measuring mean fluorescence intensity (MFI). The amount of each receptor per cell was significantly increased on lung TCD8 over spleen, particularly for LAG-3, where expression was >4-fold greater in the lung (Fig. 2B). 2B4 expression was similar at day 7, but increased steadily in the lung over time.
Fig. 2. Kinetics of TCD8 Inhibitory Receptor and Ligand Expression in Lung and Spleen.
(A) Expression of individual inhibitory receptors on immunodominant M195-specific TCD8 from the lung and spleen at indicated times p.i. in B7tg mice. Lines connect lung and spleen samples from the same mouse. Data are combined from two independent experiments. (B) Mean fluorescence intensity (MFI) of individual inhibitory receptors on M195-specific TCD8 compared to isotype control stained cells (ISO) from the lung and spleen at indicated times p.i. Data are combined from two independent experiments with 4–5 mice per group per time point. (C) Boolean gating analysis for the co-expression of inhibitory receptors on lung M195-specific TCD8 at indicated times p.i. Percent of cells is indicated in each pie slice. Relative expression of possible inhibitory receptor expression combinations in lung (D) and spleen (E) at indicated times p.i. The expression of possible inhibitory receptor combinations is shown. # P<0.05, paired t-test. * P<0.05, unpaired t-test.
We next quantified the co-expression of inhibitory receptors and found that the majority of spleen TCD8 express ≤1 inhibitory receptor at each time point, with ~80% expressing ≤1 of the analyzed receptors by day 14 (Fig. 2C). In contrast, only ~30% of lung TCD8 express ≤1 inhibitory receptor at day 7, with many expressing 2, a large population expressing 3, and a smaller but substantial population expressing all 4. Further, the proportion of lung TCD8 expressing 3 or 4 inhibitory receptors increased from day 7 to 14. Analysis of the actual combinations of inhibitory receptors expressed by lung TCD8 revealed a shifting landscape over time (Fig. 2D, Fig. S2). PD-1 was consistently high and co-expressed with TIM-3 and LAG-3 at day 7, but shifted to co-expression with TIM-3 and 2B4 by day 10. Also, by day 14, PD-1neg populations emerged that were characterized by expression of TIM-3 and 2B4, only 2B4, or no inhibitory receptors. PD-1 was the most commonly expressed inhibitory receptor in the spleen, but the expression was much lower than in the lung and lack of expression of these markers dominated (Fig. 2E, Fig. S2). Thus, impaired lung TCD8 co-express multiple inhibitory receptors, which increase over time even after viral clearance.
We previously demonstrated that PD-1 upregulation without a concomitant increase in its ligand did not result in lung TCD8 impairment (23). We therefore infected mice with HMPV and stained primary lung cells for EpCAM (epithelial cell marker) and CD11c (APC marker) to quantify expression of each inhibitory receptor’s ligand on the cell types most likely encountered by Ag-specific lung TCD8. EpCAM expression was restricted to CD45− cells while all CD11c+ cells were CD45+ (not shown). PD-L1 was upregulated on both cell types at day 7 p.i. compared to mock infected animals (though with much higher overall expression on CD11c+ cells), decreased on day 10, and returned to near baseline by day 14 (Fig. 3). PD-L2 was undetectable on both cell types (not shown). Intracellular galectin-9, the soluble ligand for TIM-3 (46), was upregulated by infection and remained elevated. MHC-II, the ligand for LAG-3, was highly expressed even at baseline, increased following infection, and remained elevated on both populations. Finally, CD48, the ligand for 2B4, was only expressed by CD11c+ cells and was minimally altered by infection. These results indicate that the ligands for each inhibitory receptor are constitutively expressed in mouse lungs and most are increased by HMPV infection.
Fig. 3. Lung Cells Express Multiple Inhibitory Ligands.
Expression of the ligands for each inhibitory receptor on lung epithelial cells (Ep-CAM+) or APCs (CD11c+) at the indicated times p.i. Ligand:Receptor pairs are PD-L1:PD-1, MHC-II:LAG-3, CD48:2B4, Galectin-9:TIM-3. Representative data from 2 independent experiments with 4–5 mice per time point are shown. Isotype indicates staining with isotype control antibody. Mock indicates mock-infection with cell lysate.
In Vitro Blockade Reveals Multiple Inhibitory Receptors Mediate Impairment
PD-1 signaling impairs lung TCD8 at early time points, but the absence of PD-1 failed to prevent impairment later (Fig. 1), suggesting that the lung environment has multiple mechanisms to control T cell functionality. To test this, we first determined whether inhibitory receptor expression identifies all epitope-specific TCD8 or just the impaired subset. We performed ICS for IFNγ and TNF after peptide restimulation of lung TCD8 on day 10 p.i. and stained for inhibitory receptor expression. We found that the subset of M195-specific TCD8 that were functional, i.e., capable of producing IFNγ+ (Fig. 4A) or IFNγ+TNF+ (not shown), expressed a similar number and pattern of inhibitory receptors as the entire population of M195-specific TCD8 identified by tetramer staining (Fig. 2C). These results suggest that the functional subset of lung TCD8 may be susceptible to future impairment due to continued inhibitory receptor expression.
Fig. 4. Multiple Inhibitory Receptors Contribute to TCD8 impairment.
(A). B7tg mice were infected with HMPV and lung TCD8 were isolated at day 10 p.i. ICS and inhibitory receptor staining was then performed following brief M195 peptide restimulation. The percentage of M195-specific TCD8 that produce IFNγ was quantified and populations were grouped based on the total number of inhibitory receptors expressed. M195-specific TCD8 inhibitory receptor expression did not change during the stimulation (not shown). Data are combined from two independent experiments with 5 mice per experiment. (B) Mice were infected with HMPV and then lungs and spleen harvested at day 7 p.i. Splenic or lung suspension cells were added to anti-IFNγ-coated ELISPOT plates and restimulated with M195 peptide in the presence of blocking antibody or isotype control antibody for 48 hours. Mock indicates wells stimulated with an irrelevant peptide. Data are expressed as the number of spot forming cells (SFC) per 106 lymphocytes. (C) Spleen suspension cells were harvested at day 10 post-HMPV infection and restimulated in the presence of antibody as in (B). Lung suspension cells at days 7 (D) and 10 (E) post-HMPV infection were restimulated in the presence of antibody as in (B) and the number of spot forming cells (SFC) per 106 lymphocytes calculated. The mean spot size is shown for M195-restimulated lung TCD8 harvested at days 7 (F) and 10 p.i. (G) in the presence of the indicated inhibitory receptor blocking antibodies. (H) WT B6 mice were primed with PR8 influenza (H1N1) i.p, and then challenged with X31 influenza (H3N2) i.n. Lung suspension cells harvested at day 7 post-infection were restimulated with NP366 peptide in an IFNγ ELISPOT as in (B). Each symbol represents the mean from one of five or six total independent experiments with 2–3 mice per experiment and the horizontal bar indicates the mean. Dotted line indicates the mean number of spots from M195-stimulated (or NP366 in E), isotype control antibody treated cells as a reference. One-way ANOVA and Dunnett contrast method were used for multiple comparisons of mean number of SFC to reference group, * P<0.05.
To screen inhibitory receptors for functional relevance to TCD8 impairment, we utilized an in vitro method to block individual or combinations of receptors (24). Splenocytes or lung suspension cells from HMPV-infected mice were added to an IFNγ-detecting ELISPOT assay, restimulated with M195-peptide and incubated with monoclonal antibodies against various inhibitory receptors or their ligands. M195 restimulation of lung cells plus PD-L1 blockade resulted in significantly more IFNγ-secreting cells (i.e. more spots; Fig. 4B), confirming our previous in vivo results (23). Blockade of PD-1 or PD-L1 increased IFNγ by ~2-fold on day 7 but only ~30% on day 10. Importantly, PD-L1 blockade of restimulated splenocytes (which are unimpaired in vivo) had no effect (Fig. 4C). Blockade of TIM-3 and LAG-3 resulted in more spots in restimulated lung cells at both day 7 (Fig. 4D) and day 10 p.i. (Fig. 4E). Blocking 2B4 had no effect at day 7 but a significant effect at day 10 p.i. Combining PD-L1 blockade with TIM-3, LAG-3 or PD-L2 blockade resulted in even greater numbers of IFNγ-producing cells. Combination blockade also resulted in larger spots (i.e. more IFNγ production per cell, Fig. 4B), which was more pronounced at day 7 p.i. (Fig. 4F) than day 10 p.i. (Fig. 4G). To determine if additional inhibitory receptors also play a role in the PD-1-independent impairment we observed previously during secondary influenza virus infection, we harvested lung cells from mice undergoing influenza virus challenge infection and tested blocking antibodies against the four inhibitory receptors. We found that blocking TIM-3 had the greatest effect, followed next by LAG-3, both of which were greater than PD-1 blockade (Fig. 4H). A role for TIM-3 in impairing influenza-specific TCD8 is consistent with a previous report (47). These results indicate that multiple inhibitory receptors cooperate to inhibit lung TCD8 cytokine production and to maintain this impairment.
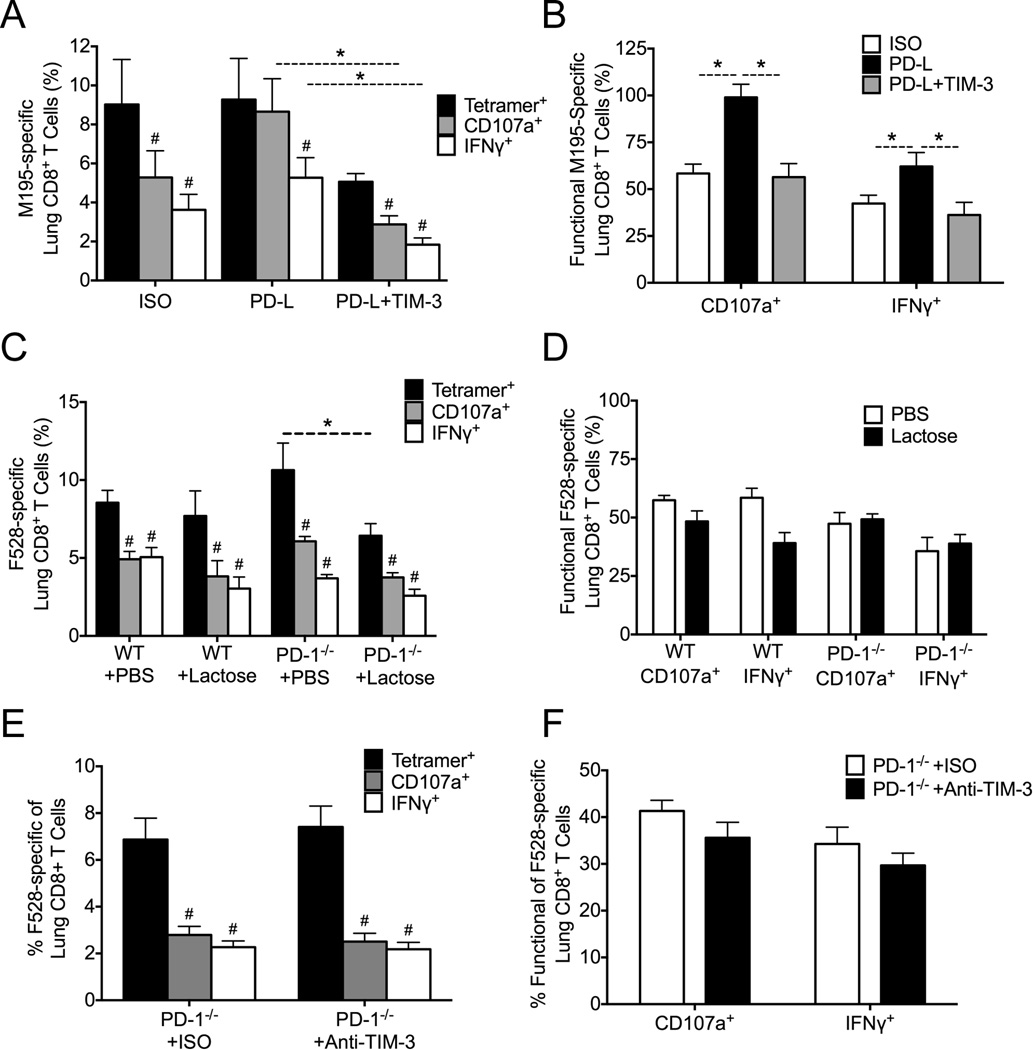
TIM-3 Does Not Contribute to TCD8 Impairment During HMPV Infection
We next sought to determine what role these receptors played in vivo. We first determined that LAG-3 and TIM-3 were not upregulated to a higher level at later time points during infection of PD-1−/− mice compared to WT mice (data not shown), suggesting that increased expression of either of these receptors alone was not able to account for late impairment in the absence of PD-1. Both TIM-3 and LAG-3 can collaborate with PD-1 to cause T cell exhaustion during chronic infections; however, neither has been described to fully compensate for blockade of PD-1. We used several complementary approaches to test the hypothesis that TIM-3 mediates TCD8 impairment in vivo. First, we treated B7tg mice during HMPV infection with isotype control Ab, anti-PD-L Ab alone, or anti-PD-L combined with anti-TIM-3 Ab, and analyzed pulmonary TCD8 responses on day 10 post-infection. As expected, isotype-treated mice were impaired and PD-L blockade increased TCD8 function, though the effect was more pronounced for degranulation than for IFNγ secretion (Fig. 5A, B). However, the addition of TIM-3 blockade reduced TCD8 function significantly compared with PD-L blockade alone. As an alternative approach, we treated WT and PD-1−/− mice with α-lactose, which blocks Gal-9 binding to TIM-3 (47, 48), during HMPV infection and determined lung TCD8 responses on day 10 post-infection. All groups exhibited significant TCD8 impairment for degranulation and IFNγ secretion (Fig. 5C), with a slightly increased percentage of F528-specific TCD8 in PD-1−/− mice treated with PBS compared with α-lactose treatment. However, the percentage of functional TCD8 between groups was not significantly different (Fig. 5D). Finally, we treated PD-1−/− mice with isotype control Ab or anti-TIM-3 Ab during HMPV infection. Lung TCD8 analyzed on day 10 post-infection were significantly impaired in both groups, with no increase in the anti-TIM-3 treated mice (Fig. 5E, F). Collectively, these data suggest that TIM-3 does not contribute to late TCD8 impairment during HMPV infection.
Fig. 5. TIM-3 Does Not Contribute to TCD8 Impairment During HMPV Infection.
(A) B7tg mice were infected with HMPV and treated in vivo with isotype control Ab, anti-PD-L Ab, or anti-PD-L plus anti-TIM-3 Ab for 2 days prior to infection and then every other day during HMPV infection. At day 10 p.i., lungs were harvested and lung M195-specific TCD8 responses quantified via tetramer staining and ICS for CD107a and IFNγ. (B) The percentage of functional M195-TCD8 was calculated as in Fig. 1. (C) WT mice were infected with HMPV and injected with α-lactose twice daily from days 3–10. At day 10 p.i., lungs were harvested and lung F528-specific TCD8 responses quantified via tetramer staining and ICS. (D) The percentage of functional F528-specific TCD8 was calculated. (E) PD-1−/− mice were injected i.p. with anti-TIM-3 Ab or isotype control Ab on days 3, 5, 7, and 9 post-infection. At day 10 p.i., lungs were harvested and lung F528-specific TCD8 responses quantified via tetramer staining and ICS. (F) The percent functional F528-specific TCD8 was calculated. # P<0.05, paired t-test. * P<0.05, unpaired t-test.
LAG-3 Compensates for PD-1 in vivo to Impair Lung TCD8
LAG-3 enhanced the in vitro response of virus-specific lung TCD8 (Fig. 4), and further we were struck by the dramatic upregulation of MHC-II (a LAG-3 ligand) by both epithelial and APCs (Fig. 3). We thus tested LAG-3 Ab blockade in vivo during acute HMPV infection and analyzed pulmonary TCD8 responses on day 10. WT B6 and PD-1−/− mice treated with an isotype control Ab were similarly impaired (Fig. 6A). Whereas in Fig. 1 there was a significant yet modest increase in function in PD-1−/− mice, we did not observe any increase in function in these experiments, likely due to a more effective viral inoculation and greater overall T cell response. However, a higher percentage of HMPV-specific lung TCD8 degranulated and secreted both IFNγ and TNF in PD-1−/− mice treated with anti-LAG-3 Ab versus isotype control Ab treated animals (Fig. 6A). LAG-3 blockade also improved the ability of lung TCD8 to degranulate, as measured by CD107a mobilization. Notably, the percentage (Fig. 6A) and absolute number (Fig. 6B) of tetramer+ cells was not significantly different between groups, indicating that LAG-3 blockade did not increase the percentage of epitope-specific cells, but rather restored functionality to these cells. We further demonstrated this point by calculating the percentage of functional F528-specific TCD8 by dividing the % with each effector function by the % staining tetramer+. LAG-3 blockade increased the percent of functional TCD8 over WT or PD-1−/− mice treated with isotype Ab (Fig. 6C) as well as the absolute number of functional TCD8 (Fig. 6D). To confirm a role for LAG-3 in TCD8 impairment, we treated WT mice with isotype Ab or anti-LAG-3 Ab blockade in vivo during acute HMPV infection and analyzed lung TCD8 on day 10. While both groups of mice were impaired, the percentage of F528-specific lung TCD8 capable of producing the cytokines IFNγ and TNF was significantly increased in WT mice treated with anti-LAG-3 alone (Fig. 6E). Lung TCD8 from anti-LAG-3 treated mice were also significantly more functional (Fig. 6F). Thus, LAG-3 contributes to TCD8 impairment and can compensate for the loss of PD-1 signaling just days after PD-1 is the primary driver of impairment, returning lung TCD8 to an impaired state. Additionally, LAG-3 alone is also capable of mediating impairment of cytokine production by virus specific TCD8.
Fig. 6. LAG-3 Compensates for PD-1 in vivo to Impair Lung TCD8.
(A) PD-1−/− mice were infected with HMPV and treated in vivo with isotype control or anti-LAG-3 Ab on days 3, 5, 7, and 9. At day 10 p.i., lungs from these infected animals and WT isotype-treated control animals were harvested and the lung F528-specific TCD8 responses were quantified via tetramer staining and ICS for CD107a, IFNγ and TNF. (B) Absolute numbers of tetramer+ (TET+) cells are shown. (C) The percentage and (D) number of functional F528-TCD8 was calculated as in Fig. 1. Results are combined from two independent experiments with 5–7 mice per group. (E) WT mice were infected with HMPV and treated in vivo with isotype control or anti-LAG-3 Ab on days 3, 5, 7, and 9. At day 10 p.i., lungs from were harvested and the lung F528-specific TCD8 responses quantified via tetramer staining and ICS for CD107a, IFNγ and TNF. (F) The percent functional F528-specific TCD8 was calculated. * P<0.05, ** P<0.01, *** P<0.001, unpaired t-test.
LAG-3 Mediated Impairment Does Not Affect Viral Clearance
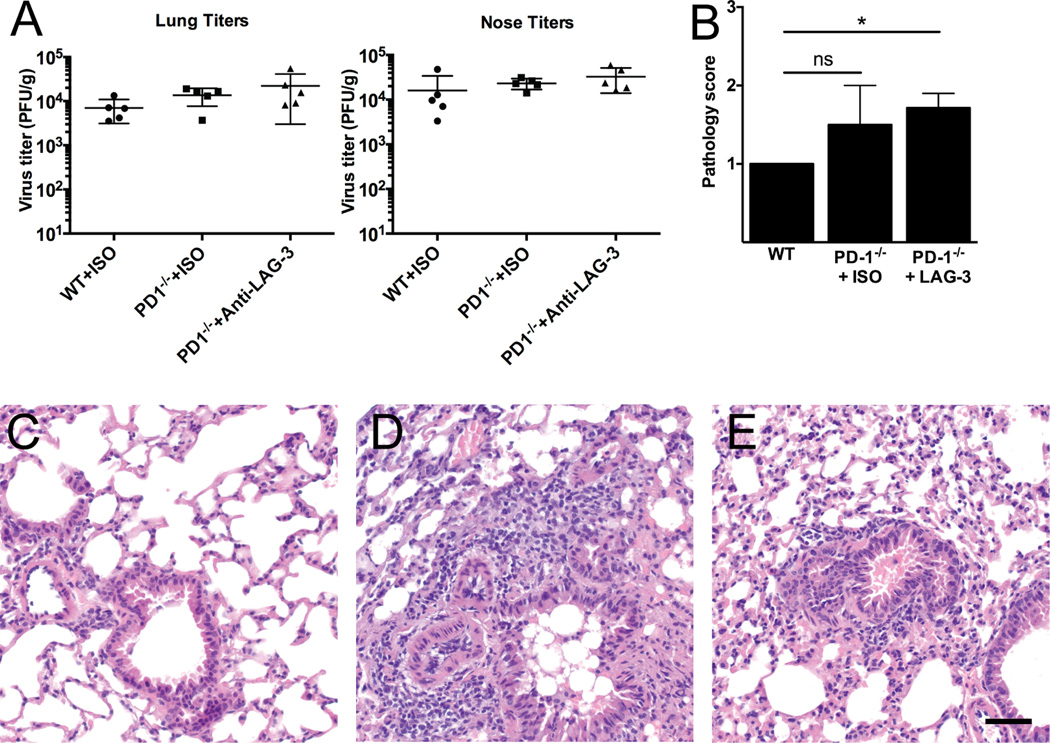
To determine the functional consequences of the increased TCD8 function we observed with LAG-3 blockade, we infected WT or PD-1−/− mice with HMPV, treated with isotype or anti-LAG-3 mAb as in Fig. 6, and measured lung viral titers on day 7 and histopathology on day 10 post-infection (infectious virus is cleared from the lungs by day 10 in this model). There were no significant differences in viral titer in the upper or lower respiratory tract between any of the groups (Fig. 7A and not shown). However, there was similarly increased lung inflammation in both PD-1−/− groups, though the difference only reached statistical significance compared with WT for the PD-1−/− mice treated with anti-LAG-3 (Fig. 7B–E).
Fig. 7. LAG-3 Blockade in vivo Does Not Reduce Viral Titer.
WT or PD-1−/− mice in groups of 5 were infected with HMPV and treated in vivo with isotype control or anti-LAG-3 Ab on days 3, 5, 7, and 9. At day 7 p.i., mice were euthanized and noses and lungs collected for virus titration. On day 10, separate groups of mice were euthanized and lung histopathology was formally scored using an established method by a group-blinded observer. (A) Lung (left) and nose (right) viral titers are shown. Horizontal bar indicates mean ± SEM. (B) Lung pathology scores. * P<0.05, unpaired t-test; only PD-1−/− + anti-LAG-3 Ab is significantly different from WT. Representative histology images from WT (C), PD-1−/− + ISO (D), and PD-1−/− + anti-LAG-3 Ab (E). All images at 400× magnification; scale bar indicates 50 µm.
DISCUSSION
Respiratory virus infections are common causes of morbidity and mortality. Moreover, many respiratory viruses are capable of causing recurrent infections throughout life, despite the presence of serum antibody (3, 8, 9, 13, 16–18). Conversely, numerous mechanisms exist to ensure tolerance to the vast majority of foreign antigens (49). A complex interaction therefore exists between the lung, pathogens, and other inhaled substances that must balance inflammatory anti-pathogen responses with tolerance to inert substances. Further knowledge of lung immunology is critical to better understanding the pathogenesis of LRI and the development of novel interventions.
We observed rapid impairment of lung TCD8 under the control of PD-1, which until recently was thought to operate only under conditions of prolonged antigen stimulation. However, by day 7 post-HMPV infection, lung TCD8 lost most of their ability to make IL-2 and TNF and less than half retained the capacity to make IFNγ or degranulate (23). This is in contrast to splenic or draining lymph node TCD8, which maintain functionality during respiratory viral infection (19–24, 38). Here, we confirmed that splenic TCD8 remain functional at later time points; we focused on splenic TCD8 because transcriptome analyses showed significant differential gene regulation between lung and spleen TCD8 (45). Moreover, there were insufficient numbers of cells in the draining lymph nodes during HMPV infection to perform ex vivo tetramer and ICS assays (data not shown). Here we show further decline in functionality of lung TCD8 over time (days 10 and 14), despite viral clearance, and dependence on inhibitory receptors other than PD-1. Previous work by our group and other shows that pulmonary TCD8 functional impairment persists for at least 6–12 weeks post-infection (20, 22–24) and that PD-1 signaling limited the protective efficacy of memory TCD8 against HMPV and influenza in mice (24).
We have uncovered a rapidly acting and redundant network utilized by the lung to regulate acute TCD8 responses in response to viral infection. Despite the utilization of inhibitory receptors to dampen T cell responses during both acute viral LRI and chronic infections, important differences exist. First, TCD8 during acute viral LRI rapidly become impaired by day 7 post-infection, while exhaustion takes several weeks to develop fully (50). Additionally, we found that LAG-3 is capable of compensating for PD-1 to impair lung TCD8 during viral LRI. LAG-3 shares homology with CD4 and also binds MHC-II (51), impairing T cell activation and proliferation (52). Combined blockade of PD-1 and LAG-3 restores TCD8 functions during chronic viral infection (53) and boosts T follicular helper cell function during malaria (54). Importantly, LAG-3 is also expressed on CD4+ conventional and regulatory T cells plus plasmacytoid dendritic cells and LAG-3 signaling on these cells regulates immune responses (53–59). The mechanism of how LAG-3 impairs T cells is unclear, but we found that both lung epithelial cells and APCs abundantly expressed its ligand, MHC-II. During acute LRI, we found that LAG-3 was capable of compensating for a lack of PD-1 signaling as early as day 10 post-infection, and blockade of LAG-3 enhanced TCD8 effector functions. LAG-3 blockade alone in WT animals also restored cytokine production to TCD8. These findings suggest that lung TCD8 functional responses to infection are tightly regulated by a layered system of inhibitory receptors.
We found that TIM-3 does not contribute to TCD8 impairment during HMPV infection. Previous studies have suggested a role for TIM-3 in mediating TCD8 exhaustion during chronic viral infection (48, 53) and in cancer, TIM-3 can compensate for PD-1 blockade (60). However, some reports identified an immunostimulatory function of TIM-3 in other settings (61, 62). Thus, the effects of TIM-3 signaling in different immune cells may be context-dependent (63).
PD-1 appears to be a master regulator that exerts its major inhibitory effect on TCD8 functionality early, though PD-1 signaling does still contribute to impairment at later time points. LAG-3 plays a prominent role in impairing cells even in the absence of PD-1. In addition, blocking LAG-3 or TIM-3 in vitro restored function to lung TCD8, although not to the degree of PD-1 blockade. This suggests redundant inhibitory pathways for impairing effector functions, especially since TCD8 remain impaired despite a complete lack of PD-1 signaling in vivo. Interestingly, blockade of LAG-3 in mice lacking PD-1 failed to reduce viral titers (23). It is possible that LAG-3 blockade might have had an effect on the duration of viral shedding rather than the peak, as we previously observed for PD-1 blockade (23). Moreover, mice are semi-permissive hosts for HMPV replication (64), and thus may imperfectly reflect the antiviral functions of TCD8 in humans.
We previously reported a nonsignificant increase in lung pathology in the absence of PD-1 alone on day 7 (23). Here, we found that the increased TCD8 function resulting from genetic ablation of PD-1 was associated with enhanced lung inflammation on day 10 post-infection, with or without LAG-3 blockade. These results have important implications for the deployment of therapeutic interventions targeting the PD-1 pathway. If other inhibitory receptors can quickly compensate for a blocked PD-1 pathway, then a combination approach might be more effective. In addition, respiratory adverse events have been reported in recipients of PD-1 inhibition therapy (30, 31, 65) and these events are more common in patients treated with combination checkpoint inhibition (66). There are several ongoing clinical trials using combination checkpoint inhibition, including anti-PD-1 and anti-LAG-3 (www.clinicaltrials.gov), and more combination therapy trials are likely based on preclinical studies (67). Recent studies show that in the murine LCMV model, other inhibitory receptors can compensate for the genetic absence of PD-1 (32). Further elucidation of the mechanisms utilized by TIM-3 and LAG-3 to impair T cells may identify a common downstream molecule that can be effectively targeted and suggest combinations that may be associated with immune-mediated adverse events.
Moreover, these results suggest the lung epithelium may possess more immunoregulatory mechanisms to respond to infection than previously appreciated. AECs are the initially infected cells during viral LRI and produce cytokines including IL-25 (59), IL-33 (68), and TSLP (69) that help shape the adaptive immune response. IL-33 is an alarmin that has recently been shown to promote antiviral TCD8 responses (70) and thus its release by infected AECs may factor prominently into the development of lung immunity. Besides secreted mediators, AECs also express numerous cell surface molecules capable of modulating immune responses; for example, AECs can act as APCs via MHC expression while also displaying co-stimulatory molecules like B7-1, B7-2 and ICAM-1 (71). Data exist suggesting that MHC-I expression is localized to the basolateral compartment of AECs (72) where it could potentially interact with interstitial and/or vascular TCD8. Further, PD-L1 is induced upon viral infection and can modify T cell functionality (36–38). We have previously shown PD-L1 to be expressed by AEC during LRI in humans (23) and here we show it is upregulated and remains elevated following viral infection. Interestingly, AECs can also express MHC class II molecules (73), which could both interact with CD4+ T cells and also modulate CD8+ T cells via interaction with LAG-3.
In summary, we show that the lung possesses rapid and overlapping mechanisms for controlling TCD8 effector functions in response to viral infection. The approach of identifying inhibitory receptors expressed by impaired TCD8 followed by in vitro and in vivo screening could lead to the identification of additional novel therapeutic targets. Our results suggest that an orchestrated network of inhibitory receptors keeps lung TCD8 in check, and that therapeutically it may be necessary to target multiple pathways to achieve durable clinical outcomes. However, modulation of T cell impairment may lead to enhanced immunopathology during common viral respiratory infections.
Supplementary Material
Acknowledgments
We thank D. Flaherty, B. Matlock, and K. Weller at the Vanderbilt Flow Cytometry Shared Resource for their assistance with flow cytometry. We also thank Drs. A. Sette, F. Lemonnier, and T. Honjo for providing mice used in these experiments. We are grateful to Mark Boothby and Stokes Peebles for helpful discussions of the work.
Financial support: Supported by NIH grants AI085062 (JVW) and GM007347 for the Vanderbilt Medical Scientist Training Program (JJE). The VUMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
Footnotes
AUTHOR CONTRIBUTIONS
J.J.E. designed and performed the experiments, analyzed results, and wrote the manuscript. S.J.T. and M.C.R. performed experiments, analyzed results, and critically reviewed the manuscript. K.L.B. analyzed data and critically reviewed the manuscript. J.V.W. designed the experiments, analyzed the results, and wrote the manuscript.
REFERENCES
- 1.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esper F, Martinello RA, Boucher D, Weibel C, Ferguson D, Landry ML, Kahn JS. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189:1388–1396. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE., Jr Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, Lee JH, Song EK, Kim SH, Park JY, Sung JY. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams JV, Wang CK, Yang CF, Tollefson SJ, House FS, Heck JM, Chu M, Brown JB, Lintao LD, Quinto JD, Chu D, Spaete RR, Edwards KM, Wright PF, Crowe JE., Jr The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, Staat MA, Iwane M, Prill MM, Williams JV. Burden of human metapneumovirus infection in young children. The New England journal of medicine. 2013;368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 8.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of Hospitalizations for Respiratory Syncytial Virus, Human Metapneumovirus, and Influenza Virus in Older Adults. J Infect Dis. 2012 doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–2496. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhi SA, Ludewick H, Kuwanda L, van Niekerk N, Cutland C, Klugman KP. Seasonality, incidence, and repeat human metapneumovirus lower respiratory tract infections in an area with a high prevalence of human immunodeficiency virus type-1 infection. Pediatr Infect Dis J. 2007;26:693–699. doi: 10.1097/INF.0b013e3180621192. [DOI] [PubMed] [Google Scholar]
- 11.Englund JA, Boeckh M, Kuypers J, Nichols WG, Hackman RC, Morrow RA, Fredricks DN, Corey L. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 12.Shahda S, Carlos WG, Kiel PJ, Khan BA, Hage CA. The human metapneumovirus: a case series and review of the literature. Transpl Infect Dis. 2011;13:324–328. doi: 10.1111/j.1399-3062.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlin JA, Hickey AC, Ulbrandt N, Chan YP, Endy TP, Boukhvalova MS, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Ennis FA, Jarman R, Gibbons RV, Broder CC. Human metapneumovirus reinfection among children in Thailand determined by ELISA using purified soluble fusion protein. J Infect Dis. 2008;198:836–842. doi: 10.1086/591186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebihara T, Endo R, Ishiguro N, Nakayama T, Sawada H, Kikuta H. Early reinfection with human metapneumovirus in an infant. J Clin Microbiol. 2004;42:5944–5946. doi: 10.1128/JCM.42.12.5944-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung J, Esper F, Weibel C, Kahn JS. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. Journal of clinical microbiology. 2005;43:1213–1219. doi: 10.1128/JCM.43.3.1213-1219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 17.Kravetz HM, Knight V, Chanock RM, Morris JA, Johnson KM, Rifkind D, Utz JP. Respiratory syncytial virus. III. Production of illness and clinical observations in adult volunteers. Jama. 1961;176:657–663. [PubMed] [Google Scholar]
- 18.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 19.Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- 20.Claassen EA, van der Kant PA, Rychnavska ZS, van Bleek GM, Easton AJ, van der Most RG. Activation and inactivation of antiviral CD8 T cell responses during murine pneumovirus infection. Journal of immunology. 2005;175:6597–6604. doi: 10.4049/jimmunol.175.10.6597. [DOI] [PubMed] [Google Scholar]
- 21.DiNapoli JM, Murphy BR, Collins PL, Bukreyev A. Impairment of the CD8+ T cell response in lungs following infection with human respiratory syncytial virus is specific to the anatomical site rather than the virus, antigen, or route of infection. Virology journal. 2008;5:105. doi: 10.1186/1743-422X-5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray PM, Arimilli S, Palmer EM, Parks GD, Alexander-Miller MA. Altered function in CD8+ T cells following paramyxovirus infection of the respiratory tract. Journal of virology. 2005;79:3339–3349. doi: 10.1128/JVI.79.6.3339-3349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson JJ, Gilchuk P, Hastings AK, Tollefson SJ, Johnson M, Downing MB, Boyd KL, Johnson JE, Kim AS, Joyce S, Williams JV. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest. 2012;122:2967–2982. doi: 10.1172/JCI62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erickson JJ, Rogers MC, Hastings AK, Tollefson SJ, Williams JV. Programmed Death-1 Impairs Secondary Effector Lung CD8+ T Cells during Respiratory Virus Reinfection. Journal of immunology. 2014 doi: 10.4049/jimmunol.1302208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. Journal of virology. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 28.Henick BS, Herbst RS, Goldberg SB. The PD-1 pathway as a therapeutic target to overcome immune escape mechanisms in cancer. Expert Opin Ther Targets. 2014:1–14. doi: 10.1517/14728222.2014.955794. [DOI] [PubMed] [Google Scholar]
- 29.Harshman LC, Choueiri TK, Drake C, Stephen Hodi F., Jr Subverting the B7-H1/PD-1 pathway in advanced melanoma and kidney cancer. Cancer J. 2014;20:272–280. doi: 10.1097/PPO.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. The New England journal of medicine. 2012 doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. 2015;212:1125–1137. doi: 10.1084/jem.20142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Current opinion in immunology. 2010;22:397–401. doi: 10.1016/j.coi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telcian AG, Laza-Stanca V, Edwards MR, Harker JA, Wang H, Bartlett NW, Mallia P, Zdrenghea MT, Kebadze T, Coyle AJ, Openshaw PJ, Stanciu LA, Johnston SL. RSV-induced bronchial epithelial cell PD-L1 expression inhibits CD8+ T cell nonspecific antiviral activity. J Infect Dis. 2011;203:85–94. doi: 10.1093/infdis/jiq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanciu LA, Bellettato CM, Laza-Stanca V, Coyle AJ, Papi A, Johnston SL. Expression of programmed death-1 ligand (PD-L) 1, PD-L2, B7-H3, and inducible costimulator ligand on human respiratory tract epithelial cells and regulation by respiratory syncytial virus and type 1 and 2 cytokines. J Infect Dis. 2006;193:404–412. doi: 10.1086/499275. [DOI] [PubMed] [Google Scholar]
- 38.McNally B, Ye F, Willette M, Flano E. Local blockade of epithelial PDL-1 in the airways enhances T cell function and viral clearance during influenza virus infection. Journal of virology. 2013;87:12916–12924. doi: 10.1128/JVI.02423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams JV, Tollefson SJ, Johnson JE, Crowe JE., Jr The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. Journal of virology. 2005;79:10944–10951. doi: 10.1128/JVI.79.17.10944-10951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rock MT, Crowe JE., Jr Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology. 2003;108:474–480. doi: 10.1046/j.1365-2567.2003.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen SC, Schuster JE, Gilchuk P, Boyd KL, Joyce S, Williams JV. Lung CD8+ T cell impairment occurs during human metapneumovirus infection despite virus-like particle (VLP) induction of functional CD8+ T cells. Journal of virology. 2015 doi: 10.1128/JVI.00670-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton H, Russell N, Moore E, Frank I, Baydo R, Havenar-Daughton C, Lee D, Deers M, Hudgens M, Weinhold K, McElrath MJ. Correlation between interferon-gamma secretion and cytotoxicity, in virus-specific memory T cells. J Infect Dis. 2004;190:1692–1696. doi: 10.1086/424490. [DOI] [PubMed] [Google Scholar]
- 43.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 44.Erickson JJ, Rogers MC, Hastings AK, Tollefson SJ, Williams JV. Programmed Death-1 Impairs Secondary Effector Lung CD8+ T Cells during Respiratory Virus Reinfection. J Immunol. 2014;193:5108–5117. doi: 10.4049/jimmunol.1302208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erickson JJ, Lu P, Wen S, Hastings AK, Gilchuk P, Joyce S, Shyr Y, Williams JV. Acute Viral Respiratory Infection Rapidly Induces a CD8+ T Cell Exhaustion-like Phenotype. Journal of immunology. 2015;195:4319–4330. doi: 10.4049/jimmunol.1403004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345–349. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma S, Sundararajan A, Suryawanshi A, Kumar N, Veiga-Parga T, Kuchroo VK, Thomas PG, Sangster MY, Rouse BT. T cell immunoglobulin and mucin protein-3 (Tim-3)/Galectin-9 interaction regulates influenza A virus-specific humoral and CD8 T-cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19001–19006. doi: 10.1073/pnas.1107087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sehrawat S, Reddy PB, Rajasagi N, Suryawanshi A, Hirashima M, Rouse BT. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010;6:e1000882. doi: 10.1371/journal.ppat.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tournoy KG, Provoost S, Van Hove C, Joos G. The role of immune tolerance in asthma pathogenesis. Curr Allergy Asthma Rep. 2006;6:437–443. doi: 10.1007/s11882-996-0018-3. [DOI] [PubMed] [Google Scholar]
- 50.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. Journal of virology. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, Piatier-Tonneau D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327–337. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. Journal of immunology. 1998;161:4058–4065. [PubMed] [Google Scholar]
- 53.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durham NM, Nirschl CJ, Jackson CM, Elias J, Kochel CM, Anders RA, Drake CG. Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PloS one. 2014;9:e109080. doi: 10.1371/journal.pone.0109080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camisaschi C, De Filippo A, Beretta V, Vergani B, Villa A, Vergani E, Santinami M, Cabras AD, Arienti F, Triebel F, Rodolfo M, Rivoltini L, Castelli C. Alternative activation of human plasmacytoid DCs in vitro and in melanoma lesions: involvement of LAG-3. The Journal of investigative dermatology. 2014;134:1893–1902. doi: 10.1038/jid.2014.29. [DOI] [PubMed] [Google Scholar]
- 57.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, Roncarolo MG. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 58.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. Journal of immunology. 2009;182:1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorman JV, Starbeck-Miller G, Pham NL, Traver GL, Rothman PB, Harty JT, Colgan JD. Tim-3 directly enhances CD8 T cell responses to acute Listeria monocytogenes infection. Journal of immunology. 2014;192:3133–3142. doi: 10.4049/jimmunol.1302290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phong BL, Avery L, Sumpter TL, Gorman JV, Watkins SC, Colgan JD, Kane LP. Tim-3 enhances FcepsilonRI-proximal signaling to modulate mast cell activation. J Exp Med. 2015;212:2289–2304. doi: 10.1084/jem.20150388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferris RL, Lu B, Kane LP. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. Journal of immunology. 2014;193:1525–1530. doi: 10.4049/jimmunol.1400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams J, Tollefson S, Johnson J, Crowe J. The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. Journal of virology. 2005;79:10944–10951. doi: 10.1128/JVI.79.17.10944-10951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J, He B, Kansra V, McPhee F, Wind-Rotolo M, Grasela D, Selby M, Korman AJ, Lowy I. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PloS one. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA-4 and PD-1 blockade and new combinations. Semin Oncol. 2015;42:363–377. doi: 10.1053/j.seminoncol.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 68.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. Journal of immunology. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D, Lohning M, Pinschewer DD. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 71.Zdrenghea MT, Johnston SL. Role of PD-L1/PD-1 in the immune response to respiratory viral infections. Microbes Infect. 2012;14:495–499. doi: 10.1016/j.micinf.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 73.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14:719–730. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.