Abstract
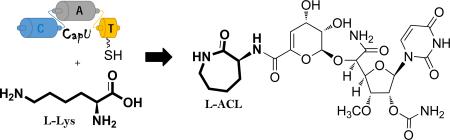
Capuramycins are one of several known natural products that contain an l-Lys-derived l-α-amino-ε-caprolactam (l-ACL). The α-amino group of l-ACL in capuramycins is linked to an unsaturated hexuronic acid component through an amide bond that was previously shown to originate via an ATP-independent enzymatic route. Using a combined in vivo and in vitro approach, a predicted tridomain nonribosomal peptide synthetase CapU is functionally characterized here as the ATP-dependent amide bond-forming catalyst responsible for the biosynthesis of the remaining amide bond found in l-ACL. The results are consistent with the adenylation domain of CapU as the essential catalytic component for l-Lys activation and thioesterification of the adjacent thiolation domain. However, in contrast to expectations, lactamization does not require any addi-tional domains or proteins and is likely a nonenzymatic event. The results set the stage to examine whether a similar NRPS-mediated mechanism is employed in the biosynthesis of other l-ACL-containing natural products and, just as intriguing, how spontaneous lactamization is avoided in the numerous NRPS-derived peptides that contain an unmodified l-Lys.
Keywords: Biosynthesis, nonribosomal peptide, antibiotic, natural product, nucleoside
Graphical Abstract
The biosynthetic mechanism for the aminocaprolactam that is a component of the capuramycin antimycobacterial antibiotics was delineated using an in vivo and in vitro approach. The process is initiated by adenylation and thioesterification of l-Lys and highlighted by an apparently nonenzymatic intramolecular aminolysis.
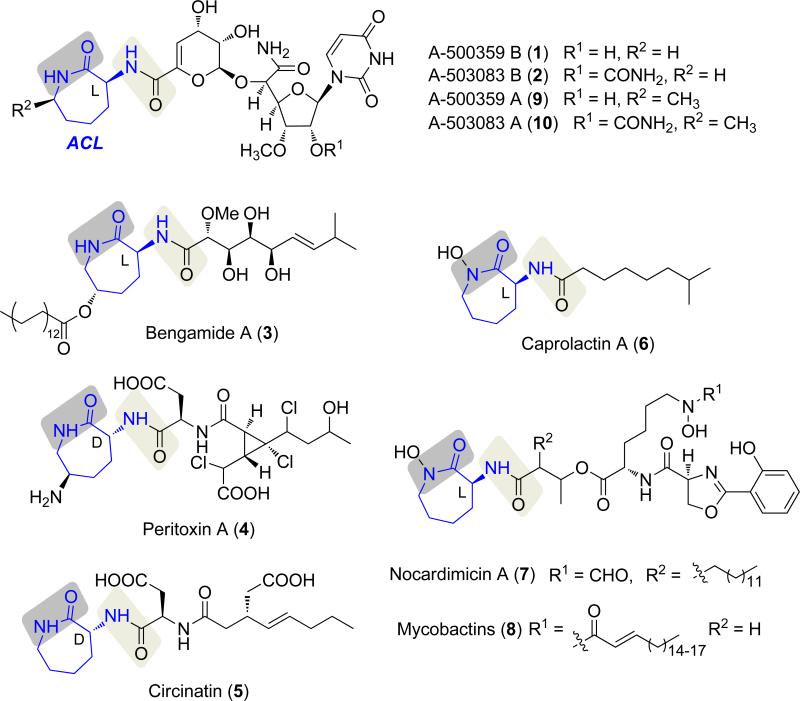
The capuramycin-type nucleoside antibiotics are natural products endowed with excellent antimycobacterial activity. Most members of the family, represented by A-500359 B (1) from Streptomyces griseus SANK 60196 and the 2′-O-carbamoylated derivative A-503083 B (2) from Streptomyces sp. SANK 62799 (Scheme 1),[1-5] contain an l-α-amino-ε-caprolactam (l-ACL) that is covalently linked to an uncommon unsaturated hexuronic acid component by an amide bond. Several other natural products are also known that contain an ACL component, including bengamide A (3), peritoxin A (4), circinatin (5), caprolactin A (6), and the siderophores nocardimicins (7) and mycobactins (8) (Scheme 1).[6-10] Similarly to 1 and 2, the 4-8 ACL, which is the l-isomer with the exception of 4 and 5, is covalently linked to the rest of the molecule by an amide bond. Often the ACL is further modified such as the stereoselective C-methylation that is observed in the capuramycins A-500359 A (9) and A-503083 A (10) or the unusual animation of 4.
Scheme 1.
Representative natural products that contain an α-amino- ε-caprolactam (ACL).
The mechanism for the formation, attachment, and modification of ACL in 1-8 remains, for the most part, unknown. Not surprisingly, isotopic enrichment studies using the 1-producing strain were consistent with l-Lys as the direct biosynthetic precursor to l-ACL.[4] The subsequent identification of the biosynthetic gene cluster for 1 and 2 revealed an orthologous set of genes (orf26 and orf27 for 1, capU and capV for 2, respectively) encoding nonribosomal peptide synthetases (NRPS) that were proposed to be involved in l-ACL maturation.[11-12] Orf26/CapU was bioinformatically predicted to consist of three domains found in NRPS, a condensation (C), adenylation (A), and thiolation (T) domain (sequential from N- to C-terminus), while Orf27/CapV was predicted to be a stand-alone C domain. The archetypical NRPS system orchestrates the assembly of peptides in a modular fashion, with the addition of each amino acid requiring a C, A, and T domain, the last of which is often termed a peptidyl carrier protein domain.[13-14] Prior to NRPS catalysis, a seryl residue on the apo-T domain is first posttranslationaly modified with a phosphopantetheine prosthetic group derived from coenzyme A (CoA), a reaction catalyzed by a phosphopantetheinyltransferase (PPTase). In turn the A domain selects an amino acid substrate, activates the carboxylic acid as the acyl adenylate at the expense of ATP, and attaches the amino acid to the sulfhydryl group of the holo-T domain to generate a thioesterified intermediate. Subsequently, the C domain catalyzes the oligomerization between adjacent thioester-linked substrates to form an amide bond. The resulting dipeptide, which remains attached to the acyl accepting T domain, then serves as a donor for aminolysis that is catalyzed by a downstream C domain, if present. Following complete elongation, which is dictated by the number of C-A-T modules in the NRPS, the resulting peptide is typically released by a terminal thioesterase domain by either hydrolysis or intramolecular cyclization, although other mechanisms of chain release are known.[15]
Using the traditional amino acid-dependent ATP-32PPi exchange assay to assess A domain substrate specificity,[16] preliminary characterization of the NRPS CapU was consistent with the A domain having a modest preference for l-Lys.[12] Surprisingly, however, was the discovery that the attachment of the l-ACL to the hexuronic acid did not involve the NRPS but was instead mediated by a carboxymethyltransferase, CapS and an ATP-independent transacylase, CapW.[12] Therefore the role of the NRPS in 2 biosynthes is appeared to be solely for lactamization of l-Lys, yet it was unclear why two C domains were present and which one was important for lactam formation. Using a combination of bioinformatic analysis and in vivo and in vitro approaches, we now demonstrate that neither C domain of CapU nor CapV is necessary for lactamization.
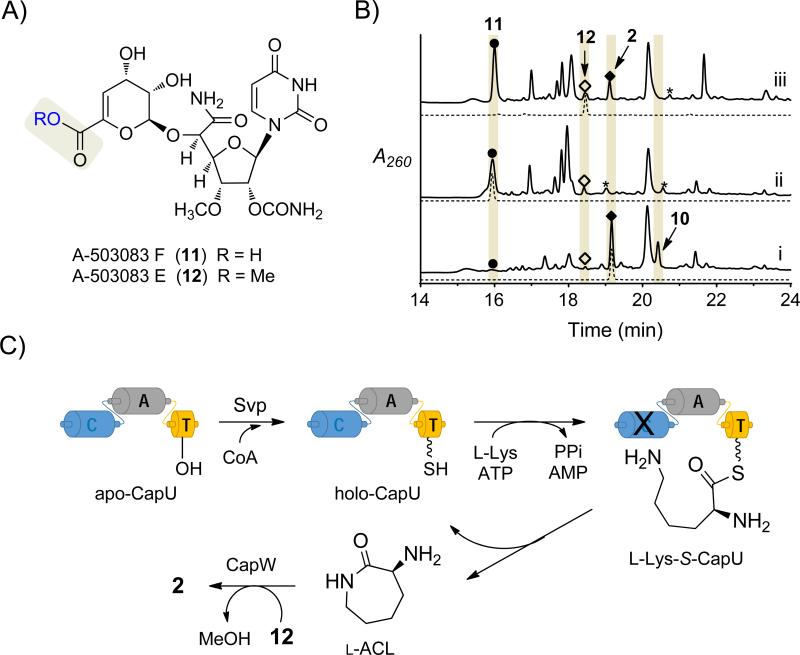
To interrogate the role of the NRPS system in capuramycin biosynthes is, the development of a genetic system within the 1-producing strain was initially explored.[11] However, the results suggested that this strain harbored multiple copies for several—if not all—of the required biosynthetic genes (Supporting Information Results and Figure S1), a phenomenon that has been observed for a few other natural product biosynthetic gene clusters.[17-19] Thus, we switched to the 2-producing strain, which makes the two aforementioned A CL-containing 2 and 10 as major congeners along with minor amounts of the deaminocaprolactam precursors A-503083 F (11), characterized by a carboxylic acid, and A-503083 E (12), the corresponding methyl ester of 11 (Figure 1A and Figure 1B, trace i).[5] The capU gene was targeted for inactivation, and the expected double-crossover genotype was confirmed by PCR and Southern Blot analysis (Supporting Information, Figure S2). As expected, HPLC analysis of the ΔcapU mutant strain revealed that the production of 2 and 10 was abolished with the concomitant increase in the peaks corresponding to 11 and 12 (Figure 1B, trace ii). Upon feeding of l-ACL to the ΔcapU mutant strain, the production of 2 was restored (Figure 1B, trace iii), demonstrating an essential role for CapU in l-ACL biosynthesis. Moreover, these results are consistent with the previous characterization of CapW as an l-ACL:12 transacylase.[12] To further support their role, capU and capV were cloned into pUW201 containing the ermE* constitutive promoter and expressed in the heterologous host Streptomyces lividans TK64. In contrast to the control consisting of the empty vector, expression of capU and capV in combination with feeding l-Lys resulted in the formation of l-ACL, which was detected by FMOC derivatization and LC-MS analysis (Supporting Information, Figure S3 and Table S1). The combined in vivo results suggest that, as initially posited, CapU and CapV orchestrate ACL biosynthesis.
Figure 1.
Role of the NRPS system in l-ACL biosynthesis. A) Structures of the deaminocaprolactam congeners. B) HPLC chromatograms of the methanol extract from the i) wild-type strain; ii) ΔcapU mutant strain; and iii) ΔcapU mutant strain cultured with exogenously supplied l-ACL. A260, absorbance at 260 nm; asterisk (*) denotes an unidentif ied metabolite with a unique UV/Vis spectrum relative to capuramycins. C) In vitro reconstitution of l-ACL biosynthesis starting from l-Lys and apo-CapU using Svp with or without additional enzymes.
As previously noted, prior results demonstrated recombinant CapU has a modest preference for l-Lys compared to other proteinogenic amino acids, 11, or d-Lys.[12] This was consistent with a mechanism wherein the A domain directly loads l-Lys in cis to the holo-T domain, and one of the two available C domains catalyzes lactamization to release l-ACL (Figure 1C). Analysis of model C domains by others has revealed a conserved His is essential for not only intermolecular aminolysis but also an unnatural intramolecular macrolactamization catalyzed by an excised C domain of the tyrocidin NRPS.[20-22] Amino acid sequence alignments of CapU and CapV with C domains whose structures have been solved revealed this His is found in CapV (H119) but is substituted with Gln in CapU (Q184; Supporting Information, Figure S4). Therefore, CapV was initially predicted to be the amide bond-forming catalyst, and was therefore targeted for in vitro analysis along with CapU. Similarly to CapU, soluble CapV was obtained from E. coli using standard expression conditions (Supporting Information, Figure S5).
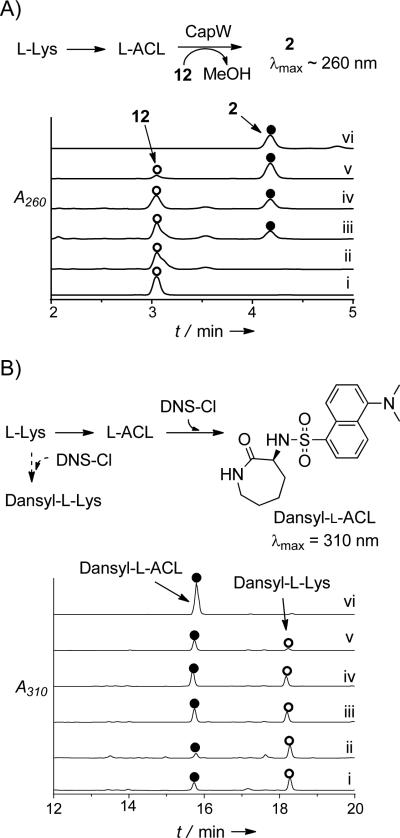
To simplify the detection of the expected l-ACL product, activity was initially assessed by enzymatically coupling the reactions of CapU and/or CapV with CapW, which transfers l-ACL to 12 and was previously determined to have no activity with l-Lys as an acyl acceptor (Figure 2A).[12] Unlike CapU and CapV, however, soluble, recombinant CapW was only attainable from Streptomyces lividans TK24 (Supporting Information, Figure S5). Svp, a well characterized and promiscuous PPTase,[23] was also included for the in situ generation of holo-CapU from CoA and apo-CapU (Figure 1C). In comparison to controls (e.g., Figure 2A, traces i and ii), HPLC analysis of the reactions containing all four proteins starting with substrates l-Lys and 12 revealed a new peak that coeluted with authentic 2 that was generated by CapW or purified from the producing strain (Figure 2A, traces iii-v). LC-MS analysis of the product yielded an [M+H]+ ion at m/z 612.6, which is consistent with the molecular formula of 2 (calc. 612.2) (all conditions tested are summarized in the Supporting Information, Table S2). When CoA and Svp were omitted, 2 was still detected, albeit at a significantly reduced level. This suggested that some CapU is produced in the holo-form upon heterologous expression in E. coli, a phenomenon that has been reported for T domains of other NRPS systems.[24-26] The heterologous production of some holo-CapU may also explain why l-Lys was only moderately preferred in the amino acid-dependent ATP-32PPi exchange assay, as turnover to l-ACL (vide infra) would counteract the incorporation of the radiolabel into ATP. As expected, no product was detected when ATP or CapU was excluded, consistent with the hypothesis that l-Lys is first activated as an acyl-adenylate and transferred to the T domain of holo-CapU. In contrast to our expectations, however, CapV was not essential nor enhanced 2 formation when starting with l-Lys (Figure 2A, trace iv), suggesting that CapU was the sole responsible catalyst for l-Lys activation and lactamization.
Figure 2.
Detection of l-ACL f ormation. A) Expected amide bond-forming reactions starting from l-Lys and enzymatic coupling with CapW. Representative HPLC traces for the four enzyme reaction including i) authentic 12; ii) reactions omitting CapU, CapV, and Svp, thus confirming CapW is unable to incorporate l-Lys directly; iii) reactions containing all of the necessary enzymes (CapU, CapV, CapW, and Svp) and substrates/cofactors (l-Lys, ATP, 12, CoA, and MgCl2); iv) reaction omitting CapV; v) positive control confirming CapW-catalyzed transacylation starting from l-ACL and 12; vi) authentic 2. A260, absorbance at 260 nm. B) Expected amide bond-forming reactions starting from l-Lys and post-reaction derivatization with dansyl chloride. Representative HPLC traces of l-ACL formation by dansyl chloride modification including i) reactions omitting CapV, thus confirming CapV is not necessary for lactamization; ii) reactions omitting CapV and using truncated CapU_AT (A+T domains); iii) reactions using l-Lys-SNAC (13) as the substrate in place of l-Lys; d) reactions starting from 13 without inclusion of any enzymes, demonstrating nonenzymatic lactamization and hydrolysis in buffered aqueous conditions; v) reactions using 14 as the substrate, which favors lactamization over hydrolysis; vi) reaction omitting all of enzymes only with the l-Lys, and vi) control of l-ACL modified with dansyl chloride. A310, absorbance at 310 nm. Dansyl-l-Lys is l-Lys modified with two dansyl groups based on MS analysis.
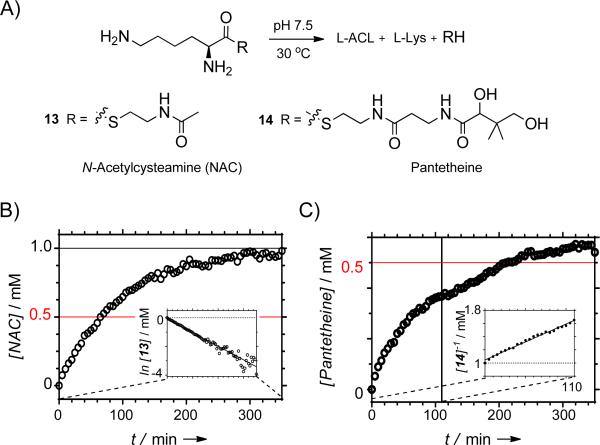
We next aimed to directly monitor l-ACL formation by removing 12 from the reaction and derivatizing any product and remaining l-Lys with dansyl chloride prior to analysis (Figure 2B). In comparison to the appropriate controls, HPLC analysis clearly revealed that l-ACL was formed under the condition that CapU and ATP were included in the reaction (Figure 2B, trace i). Moreover, a truncated didomain version of CapU was prepared that consisted of the A and T domains (CapU_AT). Similarly to the native protein, CapU_AT catalyzed the formation of l-ACL from l-Lys (Figure 2B, trace ii), clearly demonstrating a functional A and T domain for this recombinant protein. With hopes to further simplify the interpretation of the results, l-Lys-S-N-acetylcysteamine 13 and the l-Lys-S-pantetheine thioester 14 (Figure 3A) were synthesized and used as potential surrogate substrates for l-Lys loaded onto the pantetheine group of the T domain (l-Lys-S-CapU in Figure 1C). However, when 13 and 14 were transferred to aqueous Tris or phosphate buffer (pH 7.5), l-ACL was almost immediately detected in the absence of enzyme. Following completion of the reaction, a ratio of 62:38 and 90:10 of l-ACL:l-Lys was detected starting from 13 and 14, respectively. By monitoring the formation of the free sulfhydryl upon modification with 5,5’-dithiobis(2-nitrobenzoate) as previously described during the characterization of Nε-OH-Lys:acetyl-CoA Nε-transacetylase,[27] the time dependence of substrate consumption for the parallel reactions displayed apparent first order kinetics for 13 (k = 1.0 × 10−2 min−1; Figure 3B). The time dependency for 14 appeared more complex under the reaction conditions, displaying a potential mixed kinetics with second order at higher concentrations of 14 (k = 5.5 × 10−3 mM−1min−1; Figure 3B) and first order at low er concentrations (k = 1.7 × 10−3 min−1; Supporting Information, Figure S6). These kinetic parameters are comparable to the reported enzymatic N-acetylation of Nε-OH-Lys using acetyl-CoA (kcat = 2.9 × 10−3 min−1 and kcat/Km = 52 × 10−3 mM−1 min−1).[27] Although additional kinetic investigations will be required to infer any mechanistic insight from this data, it is nevertheless clear that l-ACL is nonenzymatically formed from l-Lys-thioesters at a significant rate.
Figure 3.
Nonenzymatic lactamization/hydrolysis. A) Structures of the surrogate substrates 13 and 14. B) Time dependence of the nonenzymatic reaction of 13; the inset is suggestive of first order kinetics. C) Time dependence of the nonenzymatic reaction of 14; the inset depicts the initial stages of the reaction (t < 110 min) that is suggestive of second order kinetics.
Despite the nonenzymatic lactamization/hydrolysis, we finally tested if any enzyme or combination thereof accelerates intramolecular aminolysis of 14. Whether using CapU and/or CapV, the rate of product formation was the same as control reactions without any enzyme (Supporting Information, Figure S6C). Additionally, CapW had no effect on the rate except for a slight increase under the condition that 12 was including (Supporting Information, Figure S6D). Given the lack of additional candidates within the biosynthetic gene cluster, the results suggest lactamization is a nonenzy matic process during 2 biosynthesis. This phenomenon is not unlike the recent report of nonenzymatic acylation of Lys residues in bacterial peptides by acyl-CoA thioesters[28] and acetyl phosphate.[29]
The revelation that neither C domain of CapU or CapV is essential for lactamization of l-Lys raises the question of the role of these domains in 1 and 2 biosynthesis, if any. In this particular case, we propose that they indeed have no role and are evolutionary remnants of horizontal gene transfer. In support of this, close bioinformatic inspection of the 2 biosynthetic locus reveals capU, V, and W form a subcluster that are flanked by genes encoding putative transposases followed by long intergenic regions (Supporting Information, Figure S7). Perhaps more indicative, however, is that the calculated codon adaptation index for capV (0.401) and capU (0.495) is substantially lower than the predicted 17 open reading frames involved in 2 biosynthesis (0.521-0.779) when referenced against the codon usage of Streptomyces griseus subs. griseus (Table S3).30 A similar trend is observed when using the housekeeping glucokinase gene from S. griseus subs. griseus as a reference (Table S3). The observation of a low codon adaptation index is strongly indicative of genes of heterologous origin and that capV, and to a lesser extent capU, have undergone minimal selection for optimizing the codon usage in a Streptomyces host as might be expected for a nonfunctional gene. The genetic and heterologous biotransformation systems that were developed here can now be employed to further interrogate the unexpected lack of requirement of CapV and the C domain of CapU for 2 biosynthesis in vitro.
Several NRPS-derived peptides contain a Lys wherein the ε-amine is either unmodified, hydroxylated and acylated, or undergoes lactamization to form a macrocyclic peptide or ACL. A domains of the NRPS involved in the biosynthesis of 8, which contains two Lys-derived amino acids (Figure 1), are the only enzymes for these ACL-containing compounds that have been biochemically interrogated in vitro.31 When using the amino acid-dependent ATP-32PPi exchange assay, the results with the full-length mycobactin NRPS proteins MbtE (C-A-T-C-T domains) and MbtF (C-A-T-C domains), which are responsible for incorporating the two l-Lys units per final product, have revealed that in both cases the ε-amine is modified prior to A domain activation. MbtE is most active with Nε-acyl-Nε-OH-l-Lys, suggesting it is responsible for incorporating the internal l-Lys unit, and the modifications prior to thioesterification potentially protect against lactamization. On the other hand, MbtF is most active with Nε-OH-l-Lys, suggesting that this NRPS component is responsible for incorporating the terminal l-Lys unit that is converted to l-ACL. Similarly to the capuramycin NRPS system, there is an extra C domain at the terminus of MbtF that was proposed to catalyze lactamization. Whether lactamization with concomitant peptide release is indeed a catalyzed process or nonenzymatic as revealed here remains unknown. Juxtaposed with l-Lys lactamization is the process for introducing an unmodified l-Lys into NRPS-derived peptides. Currently, this mechanism has not been elucidated, and it will be of general interest to decipher how Lys is incorporated and whether this process involves a high degree of kinetic control during peptide assembly or possibly a natural protection/deprotection strategy is used analogous to the biosynthesis of the 4-amino-2-hydroxybutyrate component found in the aminoglycoside butirosin.[32]
Experimental Section
Gene inactiv ation of capU
The capU gene was inactivated using REDIRECT technology.[33] Briefly, cosmid pN-1[12] containing capU was introduced into E. coli BW25141/pKD78 to generate E. coli BW-N-1. A linear PCR fragment, obtained with template pIJ773 and the following primer pair: (forward) 5′- CCTCGCGGCGCACCTCCGCGGCAACGTCTCTGAGGTGCCATTCCGGGGATCCGTCGACC-3′ / (reverse) 5′- GCGGTACGCCGAACTTCTCCACCACCCCTCCGACGATTTTGTAGGCTGGAGCTGCTTC-3′ (underlined sequence corresponds to target gene), was introduced into E. coli BW-N-1 by electroporation. The double-crossover was selected by apramycin resistance and confirmed by PCR. The modified cosmid was transformed into E. coli ET12567(pUZ8002) and introduced into Streptomyces sp. SANK 62799 by conjugation. The double-crossover event was selected by apramycin resistance on ISP4 agar. The genotype was subsequently confirmed by PCR and Southern blot analysis. For Southern blot analysis, genomic DNA of either wild-type or the ΔcapU mutant strain was isolated, digested with XhoI, and applied to agarose gel electrophoresis for detection with a DIG labeled probe generated from PCR using primers complementary to regions flanking the capU gene.
Biotransformation with CapU and CapV
The capU and capV genes were amplified by PCR as a single fragment using Expand Long Template PCR System from KOD PLUS Neo with supplied Buffer, 200 μM dNTPs, 5 % DMSO, 10 ng DNA template pN-4,[12] 5 U DNA polymerase, and 400 nM each of the following primer pairs: (forward) 5′-GGGAATTCCATATGGTGGACGCCCCGCGTCACTGGA-3′ (NdeI site underlined)/ (reverse) 5′-CCCAAGCTTTCACCAACGGAATGTCCCGGCGATT-3′ (HindIII site underlined). The PCR program included an initial hold at 94 °C for 4 min, followed by 30 cycles of (96 °C for 30 s, 63 °C for 30 s, 68 °C for 5 min), and then hold at 68 °C for 7 min. The gel-purified PCR product was ligated to the identical sites of pUWL201pw to yield pUWL201pw-capUV.
After sequencing to confirm PCR fidelity, pUWL201pw-capUV was transformed into S. lividans TK64 using PEG-mediated protoplast transformation.[34] After 20 h at 28 °C, plates were overlaid with 1 mL of soft nutrient agar supplemented with 200 μg of thiostrepton. After 3 additional days at 28 °C, single colonies were transferred to fresh R2YE plates supplemented with 50 μg/mL thiostrepton. After 4 days at 28 °C, positive transformants were confirmed by colony PCR using InstaGene Matrix from Bio-Rad (Hercules, CA) and LA-Taq polymerase with GC buffer II from Takara Bio Inc. (Shiga, Japan). Positive transformants were used to inoculate 50 mL R2YE containing 25 μg/mL thiostrepton and grown for 2 days at 28 °C at 250 rpm, at which time 2 mL was transferred to fresh 100 mL R2YE containing 25 μg/mL thiostrepton. Following growth for 3 days at 28 °C at 250 rpm, l-Lys was added (25 mg/mL final concentration), and the culture was grown for an additional 72 h. The culture was separated by centrifugation, and the pH of the supernatant was adjusted to 7 with 1 M NaOH. The mixture was applied to a column containing XAD16 resin and sequentially washed with 5 volumes each of water, 20 % acetone, 50 % acetone, 80 % acetone. Fractions eluting with 20 % acetone were collected and dried under vacuum. The dried solid containing l-ACL from a 100 mL culture was dissolved in 500 μL of sodium bicarbonate solution (20 mM), and an equal volume of FMOC-OSU acetonitrile solution (2 mM) was added. After the mixture was stirred for 5 min, samples were analyzed using an Agilent 1200 Series Quaternary LC system equipped with an Eclipse XDB-C18 column (150 mm × 4.6 mm, 5 μm, 80Å). A series of linear gradients was developed from 0.1 % TFA in water (A) to 0.1 % in 90 % acetonitrile (B) in the following manner (beginning time and ending time with linear increase to % B): 0-20 min, 50-90 % B; 20-25 min, 90 % B. The flow rate was kept constant at 1.0 mL/min, and elution was monitored at 254 nm.
Cloning and expression of capU, capV, and capU_AT
The genes for CapU, CapV and CapU_AT (truncated CapU consisting of the A and T domains) were amplified by PCR using Expand Long Template PCR System from Roche (Indianapolis, IN, USA) with supplied Buffer 2, 200 μM dNTPs, 5 % DMSO, 10 ng pN-4,[12] 5 U DNA polymerase, and 400 nM each of the following primer pair: capU (forward) 5′-GGTATTGAGGGTCGCATGGACGCCCCGCGTCACTG-3′/(reverse) 5′-AGAGGAGAGTTAGAGCCTCAGGGCGAGGAGTCGACATAG-3′; capV (forward) 5′-GGTATTGAGGGTCGCATGCCCGGACCGCAGAATG-3′/(reverse) 5′-AGAGGAGAGTTAGAGCCTCACCAACGGAATGTCCCG-3′; and capU_AT (forward) 5′-AAAAAACATATGGGTGATGTCATCGGCCCC-3′/(reverse) 5′-AAAAAAGGATCCTCAGGGCGAGGAGTCGACA-3′. The PCR program included an initial hold at 94 °C for 2 min, followed by 30 cycles of 94 °C for 10 s, 56 °C for 15 s, and 68 °C for 60 s. The gel-purified PCR product of capU and capV was inserted into pET-30 Xa/LIC using ligation-independent cloning as described by Novagen (Madison, WI, USA) to yield pET30-capU and pET30-capV, which were subsequently sequenced to confirm PCR fidelity; The gel-purified PCR product of capU_AT was digested with NdeI and BamHI and inserted into similarly digested Pdb.His.MBP using T4 DNA ligase (New England Biolabs) to yield Pdb.His.MBP-capU_AT, which was sequenced to confirm PCR fidelity. The capV gene, engineered to be expressed as an N-terminal His6-protein, was subcloned by PCR using pET30-capV as the template and the following primer pair: (forward) 5′-GATAGGCATATGCCCGGACCGCAGAATG-3′ (NdeI site underlined)/(reverse) 5′-CGAGTTAAGCTTTCCCCAACGGAATGTCCCGG-3′ (HindIII site underlined). After restriction digestion the PCR-product was ligated into a pET30 DNA-fragment originating from pET30-capV that was digested with the same enzymes. The resulting plasmid, pET30-CcapV, was used for production of the C-terminal His6-tagged CapV.
One-pot reaction with CapU, CapV and CapW
Reactions (100 μL) consisted of 50 mM Tris-Cl (pH 7.5), 5 mM l-Lys, 0.05 mM CoA, 5 mM ATP, 20 mM MgCl2, 2 mM 12, 3.5 μM Svp, 3.5 μM CapU, 3.5 μM CapV, and 3.5 μM CapW at 30 °C for 8 h. Following removal of protein by ultrafiltration, the reaction components were analyzed using reverse-phase chromatography using the Dionex Ultimate 3000 system equipped with an Acclaim 120 C-18 column (4.6 mm × 100 mm, 3 μm). A series of linear gradients from 0.1 % TFA in 2.5 % acetonitrile (C) to 0.1 % TFA in 90 % acetonitrile (D) was developed in the following manner (beginning time and ending time with linear increase to % D): 0-5 min, 0 % D; 5-10 min, 0-50 % D; 10-15 min, 50-100 % D; 15-18 min, 100 % D. The flow rate was kept constant at 1 mL/min, and elution was monitored at 260 nm. Peaks were identified by retention time and MS by comparison to control reactions or authentic standards.
Formation and analysis of l-ACL
Reactions (100 μL) consisted of 50 mM Tris-Cl or phosphate (pH 7.5), 5 mM l-Lys, 5 mM ATP, 20 mM MgCl2, 3.5 μM Svp, 3.5 μM CapU/Truncated CapU (A+T domains), and 3.5μM CapV or CapW at 30 °C for 2 h. Following removal of the protein by ultrafiltration, the reaction components (50 μL) were treated with 1 M sodium bicarbonate (50 μL) and dansyl chloride (25 μL of 10 mg/mL in DMF). After incubating 1 h at 42 °C, reaction components were analyzed using the Agilent system described above. A series of linear gradients from 0.1 % formic acid in water (E) to 0.1 % formic acid in acetonitrile (F) was developed in the following manner (beginning time and ending time with linear increase or decrease of % F): 0-18 min, 5-90 % F and 18-20 min, 90-5 % F. The flow rate was kept constant at 0.4 mL/min, and elution was monitored at 310 nm. Peaks were identified by comparison to the control reaction and authentic standards.
Synthesis of l-Lys-SNAC (13)
Diisopropylethylamine (1.4 mL, 8.0 mmol) and N-acetylcysteamine (0.22 mL, 2.2 mmol) were sequentially added to a solution of N-Boc-l-Lys (0.692 g, 2.0 mmol) and PyBOP (2.0 g, 4.0 mmol) in DCM (10 mL). The mixture was stirred at room temperature, and the reaction was monitored by TLC until completion (~ 2 h). The solvent was removed under vacuum, and the residue was dissolved in ethyl acetate (50 mL). The organic layer was washed with brine (2 × 10 mL) and dried to give a colorless oil. N-Boc-l-Lys-SNAC was purified by silica column chromatography (50–100 % ethyl acetate/hexane mixture) as a white solid. N-Boc-l-Lys-SNAC was dissolved in DCM (2 mL) at room temperature, and TFA (2 mL) was added dropwise. The resulting mixture was stirred for 1 h at room temperature, and the solvent removed under vacuum. 13 was purified by silica column chromatography as a colorless solid in 62 % yield over two steps. Chemical characterization: 1H NMR (400 MHz, Methanol-d4) δ ppm 4.18 (t, J=6.3 Hz, 1 H), 3.32-3.43 (m, 2 H), 3.03-3.19 (m, 2 H), 2.93 (t, J=7.6 Hz, 2 H), 1.91-2.06 (m, 2 H), 1.90 (s, 3 H), 1.65-1.75 (m, 2 H), 1.43-1.58 (m, 2 H); 13C NMR (100 MHz, D2O with methanol as standard) δ 197.92, 173.65, 60.33, 49.99, 40.29, 39.71, 32.27, 29.82, 28.07, 22.76. HRMS (ESI): C10H21N3O2S+H+, Calc: 248.1427, Found: 248.1424.
Synthesis of l-Lys-PANT (14)
a) 2,2-dimethoxypropane (4.88 mL, 40 mmol) was added to a solution of d-pantethine (1.526 g, 2 mmol) and PTSA (0.038 g, 0.2 mmol) in acetone (10 mL). The mixture was stirred at room temperature for 8 h, at which time the solvent was removed under vacuum. Isopropyl-d-pantethine was purified by silica column chromatography (5-10 % methanol/ethyl acetate mixture) as a white powder. b) Isopropyl-d-pantethine (0.635 g, 1.0 mmol) was dissolved into a 1 M sodium bicarbonate solution (5 mL) containing dithiothreitol (0.185 g, 1.2 mmol) and stirred at room temperature for 2 h. The mixture was freeze-dried and the crude powder containing isopropyl-d-pantetheine was used in the next step without further purification. c) Boc-l-Lys(Boc)-OH (0.415g, 1.2 mmol), N,N-diisopropylethylamine (0.7 mL, 4.0 mmol), and PyBOP (1.04g, 2.0 mmol) were sequentially added to a solution of isopropyl-d-pantetheine (0.318 g, 1.0 mmol) in DCM (10 mL). The mixture was stirred at room temperature, and the reaction was monitored by TLC until completion (~3 h). The solvent was removed under vacuum, and the residue dissolved in ethyl acetate (30 mL). The organic layer was washed with 5 % KHSO4 (2 × 10 mL), 5 % NaHCO3 (2 × 10 mL), and brine (10 mL) and dried to give a colorless oil. N-Boc-l-Lys-PANT was purified by silica column chromatography (50-100 % ethyl acetate/hexane mixture) as a hygroscopic white solid. d) N-Boc-l-Lys-PANT (0.323g, 0.5 mmol) was dissolved in 6 M hydrogen chloride (2 mL) and stirred at room temperature for 30 min. The reaction was quenched with 1 M sodium bicarbonate until pH = 5 was reached. 14 was purified by semi-preparative HPLC (water/methanol) as a white powder in 24 % yield over four steps. Chemical characterization: 1H NMR (400 MHz, Methanol-d4) δ ppm 4.33 (t, J=6.5 Hz, 1 H), 3.98-4.06 (m, 2 H), 3.42-3.49 (m, 2 H), 3.19-3.26 (m, 5 H), 2.99-3.03 (m, 3 H), 2.65-2.71 (m, 3 H), 2.04 - 2.15 (m, 2 H), 1.92-2.04 (m, 2 H), 1.78 (dt, J=15.1, 7.3 Hz, 3 H), 1.50-1.67 (m, 4 H), 1.20 (s, 3 H), 1.03 (s, 3 H); 13C NMR (100 MHz, D2O with methanol as standard) δ 197.66, 179.47, 172.55, 77.35, 76.70, 60.32, 41.95, 40.38, 39.73, 37.25, 33.11, 32.21, 29.86, 28.16, 22.98, 22.93, 19.36. HRMS (ESI): C17H34N4O5S+H+, Calc: 407.2323, Found: 407.2329
Nonenzymatic hydrolysis or lactamization of 13 and 14
Reactions (100 μL) consisted of 50 mM Tris-Cl or phosphate (pH 7.5), 1 mM 13 or 14, and 2 mM 5,5-dithiobis-(2-nitrobenzoic acid) at 30 °C. Identical reactions were performed with the inclusion of 2 mM 12 and 10 μM CapW. The production of 2-nitro-5-thiobenzoate was monitored at 412 nm using ε = 14,150 M−1cm−1. To validate the assay and determine the relative amounts of amine-containing products, identical reactions were performed with phosphate buffer and without 5,5′ dithiobis-(2-nitrobenzoic acid), and the components derivatized with dansyl chloride as described in the previous section. The reported data are an average of three replicates. To obtain rate constants, data were fitted to equations describing simple parallel/competitive reactions where k is equal to the sum of the individual rate constants for the formation of l-ACL and l-Lys.
Codon adaptation index
The codon adaptation index was calculated for the genes involved in 2 biosynthesis using http://genomes.urv.es/CAIcal/ when using the codon usage from Streptomyces griseus subs. griseus as a reference or http://www.umbc.edu/codon/cai/cais.php when using the codon usage of Streptomyces griseus subs. griseus glucokinase gene as a reference.
Supplementary Material
Acknowledgements
This work was supported by a National Institute of Health grant AI087849 and UL1TR000117 (S.V.L.) and a National Natural Science Foundation of China grant 81261120417, 81321004, and 81273414 (Z.Y.).
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.Muramatsu Y, Muramatsu A, Ohnuki T, Ishii M, Kizuka M, Enokita R, Tsutsumi S, Arai M, Ogawa Y, Suzuki T, Takatsu T, Inukai M. J. Antibiot. 2003;56:243–252. doi: 10.7164/antibiotics.56.243. [DOI] [PubMed] [Google Scholar]
- 2.Muramatsu Y, Ishii MM, Inukai M. J. Antibiot. 2003;56:253–258. doi: 10.7164/antibiotics.56.253. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu Y, Miy akoshi S, Ogawa Y, Ohnuki T, Ishii MM, Arai M, Takatsu T, Inukai M. J. Antibiot. 2003;56:259–267. doi: 10.7164/antibiotics.56.259. [DOI] [PubMed] [Google Scholar]
- 4.Ohnuki T, Muramatsu Y, Miy akoshi S, Takatsu T, Inukai M. J. Antibiot. 2003;56:268–279. doi: 10.7164/antibiotics.56.268. [DOI] [PubMed] [Google Scholar]
- 5.Muramatsu Y, Ohnuki T, Ishii MM, Kizuka M, Enokita R, Shunichi M, Takatsu T, Inukai M. J. Antibiot. 2004;57:639–646. doi: 10.7164/antibiotics.57.639. [DOI] [PubMed] [Google Scholar]
- 6.Adamczeski M, Quiñoà E, Crews PJ. Am. Chem. Soc. 1989;111:647–654. [Google Scholar]
- 7.Ikeda Y, Nonaka H, Furumai T, Onaka H, Igarashi Y. J. Nat. Prod. 2005;68:1061–1065. doi: 10.1021/np050091j. [DOI] [PubMed] [Google Scholar]
- 8.Ratledge C, Snow GA. Biochem. J. 1974;139:407–413. doi: 10.1042/bj1390407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macko V, Stimmel MB, Wolpert TJ, Dunkle LD, Acklin W, Bänateli R, Juan B, Arigoni D. Proc. Natl. Acad. Sci. 1992;89:9574–9578. doi: 10.1073/pnas.89.20.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dav idson BD, Schumacher RW. Tetrahedron. 1993;49:6569–6574. [Google Scholar]
- 11.Funabashi M, Nonaka K, Yada C, Hosobuchi M, Masuda N, Shibata T, Van Lanen SG. J. Antibiot. 2009;62:325–332. doi: 10.1038/ja.2009.38. [DOI] [PubMed] [Google Scholar]
- 12.Funabashi M, Yang Z, Nonaka K, Hosobuchi M, Fujita Y, Shibata T, Chi X, Van Lanen SG. Nat. Chem. Biol. 2010;6:581–586. doi: 10.1038/nchembio.393. [DOI] [PubMed] [Google Scholar]
- 13.Felnagle EA, Jackson EE, Chan YA, Podev els AM, Berti AD, McMahon MD, Thomas MG. Mol. Pharm. 2008;5:191–211. doi: 10.1021/mp700137g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koglin A, Walsh CT. Nat. Prod. Rep. 2009;26:987–1000. doi: 10.1039/b904543k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du, L. L, Lou Nat. Prod. Rep. 2010;27:255–278. doi: 10.1039/b912037h. [DOI] [PubMed] [Google Scholar]
- 16.Itoh H, Yamada M, Tomino S, Kurahashi K. J. Biochem. 1969;64:259–261. doi: 10.1093/oxfordjournals.jbchem.a128888. [DOI] [PubMed] [Google Scholar]
- 17.Peschke U, Schmidt H, Ahang HZ, Piepersberg W. Mol. Microbiol. 1995;16:1137–1156. doi: 10.1111/j.1365-2958.1995.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Nonaka K, Nie L, Zhang J, Christenson SD, Bae J, Van Lanen SG, Zazopoulos E, Farnet CM, Yang CF, Shen B. Chem. Biol. 2005;12:293–302. doi: 10.1016/j.chembiol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Yanai K, Murakami T, Bibb M. Proc. Natl. Acad. Sci. USA. 2006;103:9661–9666. doi: 10.1073/pnas.0603251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergendahl V, Linne U, Marahiel MA. Eur. J. Biochem. 2002;269:620–629. doi: 10.1046/j.0014-2956.2001.02691.x. [DOI] [PubMed] [Google Scholar]
- 21.Samel SA, Schoenaf inger G, Knappe TA, Marahiel MA, Essen L-O. Structure. 2007;15:781–792. doi: 10.1016/j.str.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Tanov ic A, Samel SA, Essen L-O, Marahiel, M. A. MA. Science. 2008;321:659–663. doi: 10.1126/science.1159850. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez C, Du L, Edwards DJ, Toney MD, Shen B. Chem. Biol. 2001;8:725–738. doi: 10.1016/s1074-5521(01)00047-3. [DOI] [PubMed] [Google Scholar]
- 24.Stachelhaus T, Hüser A, Marahiel MA. Chem. Biol. 1996;3:913–921. doi: 10.1016/s1074-5521(96)90180-5. [DOI] [PubMed] [Google Scholar]
- 25.Gocht M, Marahiel MA. J. Bacteriol. 1994;176:2654–2662. doi: 10.1128/jb.176.9.2654-2662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stachelhaus T, Marahiel MA. J. Biol. Chem. 1995;318:927–936. doi: 10.1074/jbc.270.11.6163. [DOI] [PubMed] [Google Scholar]
- 27.Coy C, Paw BH, Bindereif A, Nielands JB. Biochemistry. 1986;25:2485–2489. doi: 10.1021/bi00357a030. [DOI] [PubMed] [Google Scholar]
- 28.Simic Z, Weiwad M, Schierhorn A, Steegborn C, Schutkowski M. ChemBioChem. 2015;16:2337–2347. doi: 10.1002/cbic.201500364. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn ML, Zemaitaitis B, Hu LI, Sahu A, Sorensen D, Minasov G, Lima BP, Scholle M, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ. PLOS ONE. 2014;9:e94816. doi: 10.1371/journal.pone.0094816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp PM, Li W-H. Nucl. Acid Res. 1987;15:1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon MD, Rush JS, Thomas MG. J. Bacteriol. 2012;194:2809–2818. doi: 10.1128/JB.00088-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llewelly n NM, Li Y, Spencer JB. Chem. Biol. 2007;14:379–386. doi: 10.1016/j.chembiol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Gust B, Chandra G, Jakimowicz D, Yuqing T, Bruton CJ, Chater KF. Adv. Appl. Microbiol. 2004;54:107–128. doi: 10.1016/S0065-2164(04)54004-2. [DOI] [PubMed] [Google Scholar]
- 34.Kieser T, Bibb M, Buttner M, Chater KF, Hopwood DA. Practical Streptomyces Genetics. The John Innes Foundation; Norwich, UK: 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.