Abstract
Introduction:
Polycystic ovarian syndrome (PCOS) constitutes most cases of endocrine disorder among females.
Objectives:
This study was done to assess the proportion of university students with PCOS and to study its risk factors.
Materials and Methods:
Data were collected from students of a private medical, dental, and nursing college using a self-administered questionnaire. Height and weight of all participants were recorded by standard procedures.
Results:
The mean age of students was 20.4 1.5 years. Of the 480 participants, 39 (8.1%) were already diagnosed with PCOS. Out of the remaining 441 participants, 40 (9.1%) were at high risk, and 401 (90.9%) were at low risk for PCOS. Greater proportion of PCOS cases was seen in the age group 23-25 years (P = 0.026), among those with family history of PCOS (P = 0.002), among those who were permanent residents of urban areas (P = 0.048), and among those who were overweight or obese (P = 0.004). About 90% of PCOS cases and those at high risk for PCOS, each had difficulty in controlling excess weight or were experiencing difficulty in maintaining ideal weight. About 36 (92.3%) of PCOS cases and all those at high risk had emotional problems such as feeling moody or experiencing fatigability over the previous 2 weeks.
Conclusion:
PCOS is a common disorder among young women in this settings and this warrants periodic screening activities. A multidisciplinary approach is required to bring about lifestyle modification and help those with emotional problems due to this endocrine disorder.
Key words: Polycystic ovarian syndrome, risk factors, university students
INTRODUCTION
Polycystic ovarian syndrome (PCOS) constitutes most cases of endocrine disorder among females.[1] It affects approximately 4-6% of adolescent girls and young women.[2]
This syndrome constitutes a variable combination of menstrual irregularity, hirsutism, acne, and obesity.[3] Women with this condition are known to have an average of about five visits to different consultants before actual diagnosis.[4] They would have consulted a gynecologist for menstrual irregularity and infertility, physician for obesity, dietician for nutritional counseling, dermatologist for hair and skin ailments, and psychiatrist for depression as a result of body image disturbances. Each time they get treated for isolated morbidities, further delaying the diagnosis of this multidimensional syndrome.[5] PCOS accounts for significant healthcare costs,[6] distress and has been found to impact quality of life of patients.[7] Even though a common problem among women, it is a complex trait with unclear etiology.[8,9,10] A number of cases in the community also remain undiagnosed.[5,11,12,13] Thus, risk assessment in the form of a survey would be the ideal strategy to identify this condition early so as to encourage young women to seek timely treatment and prevent its long-term complications. Studies during early adulthood would also provide new insights about natural history of the disease and might also reveal any different insights of its health risks.[14] With this background, this study was done to assess the proportion of university students with PCOS and to study its risk factors in a coastal city in South India.
MATERIALS AND METHODS
This cross-sectional study was done in a private medical, dental, and nursing college in Mangalore City situated in Karnataka State of South India in February 2014. Approval was taken from the Institutional Ethics Committee.
Based on the findings of a previous study,[15] where proportion of college students with PCOS was 9.13%, the sample size of 956 was calculated at 95% confidence level and relative precision of 20%. The sample size was then adjusted for finite population to 436 by assuming the total female students in all these colleges to be 800. Adding a nonresponse rate of 10%, the final sample size was calculated as 480 students. The sample was further stratified into 300 medical, 100 dental, and 80 nursing students' proportion to their population size at these colleges. Using convenience sampling method, all participants who gave written informed consent were enrolled for this study. A total of 510 participants took part in this study. Students who were known case of thyroid disorders, Cushing's syndrome, and who were nonconsenting were excluded from this study.
Use of a simple questionnaire-based survey was found to be sufficient and appropriate tool for initial screening for PCOS.[16] As there is no validated tool available for making clinical diagnosis of PCOS,[17] a modified version of the Cronin et al. questionnaire[18] was used in this study.
The final version of the instrument after item reduction, for most important parameters to assess risk of PCOS, had 10 items from four domains, namely menstrual problems, weight, skin problems, and hair distribution.[5,19] Each item was rated on a 7-point Likert scale, in which level of functioning when poorest was scored as 7 and when optimal without any problem was scored as 1. Question on acne was scored nil when absent, one for mild, two for moderate, and three for severe. Questions on skin changes and variation of pimples with menstrual cycle were scored 1 if present and 0 if absent. The time frame was set as past 2 weeks for most items in the questionnaire. However, for few items such as menstrual and weight-related problems, the time frame was referred to previous 6 months.
The schedule also contained questions relating to the sociodemographic information of the participant, awareness, and source of information about PCOS, age at menarche, family history of PCOS, and other related comorbidities. Two items to assess emotional problems such as feeling moody or easy fatigability were also added to the questionnaire designed in a 7-point Likert scale. Questionnaire was content validated by experts and later pretested among ten students before its use in this study.
Range of scores (allotted to the 10 items) from 7 to 32 was categorized as low risk and from 33 to 54 as high-risk status for PCOS among participants. No invasive procedure for the diagnosis of PCOS was used in this study as the diagnostic criteria for PCOS in adults may be controversial in youngsters. This primarily because the diagnostic pathological features in adults may be normal physiological pubertal events in younger age groups.[20,21] Height was recorded using a stadiometer, and weight was recorded using a standard weighing scale. Body mass index (BMI) was categorized as per Asian classification.
Data were entered and analyzed using SPSS Inc., version 17.0, Illinois, USA. Test of association between various determinants with risk/presence of PCOS among students was done using Chi-square test. P ≤ 0.05 was taken as the cutoff for significant association.
RESULTS
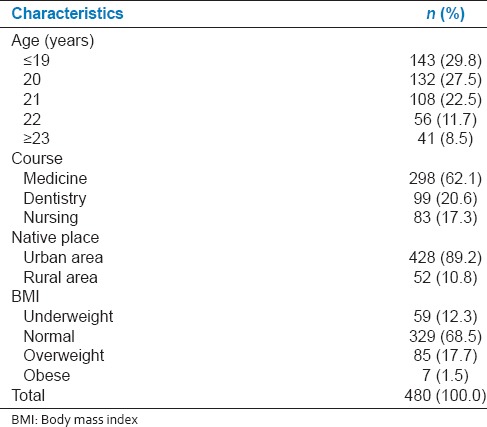
Of the 510 participants who took part in this study, 25 were excluded as there were known cases of thyroid disorders and five were excluded as their forms were incomplete. The mean age of the rest 480 participants was 20.4 ± 1.5 years ranging from 18 to 25 years. Majority of these participants were medical students (298 [62.1%]) and were from urban areas (428 [89.2%]) [Table 1].
Table 1.
Sociodemographic and body mass index distribution of study participants
All participants had heard about this disease. The most common source of information about PCOS was from doctors (192 [40%]), friends (93 [19.4%]), internet (37 [7.7%]), books (33 [6.9%]), relatives (23 [4.8%]), newspapers (18 [3.8%]), and television (7 [1.5%]).
Of the 480 participants, 39 (8.1%) were already diagnosed with PCOS. Among them, 24 (61.5%) were not on any medications, 9 (23.1%) were on hormonal treatment with either progesterone or estrogen, 4 (10.3%) were on metformin, and 2 (5.1%) underwent surgery.
Among the rest 441 participants, 40 (9.1%) were at high risk and 401 (90.9%) were at low risk for PCOS.
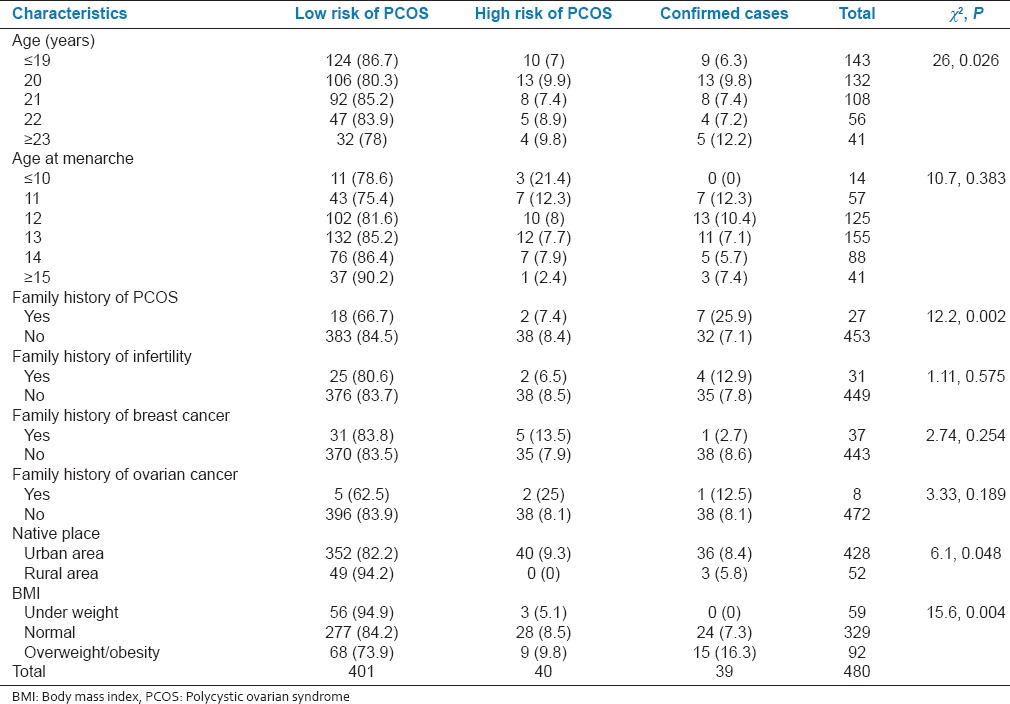
PCOS cases were significantly more in the age group 23-25 years (P = 0.026), among those with family history of PCOS (P = 0.002), those who were permanent residents of urban areas (P = 0.048), and participants who were overweight or obese (P = 0.004) [Table 2].
Table 2.
Association between sociodemographic variables and body mass index with polycystic ovarian syndrome risk status among participants
Family history of PCOS was reported by 27 participants. History was reported positive among mothers in six, sisters in ten, cousins in seven, aunts in three, and relative in one case by the participants.
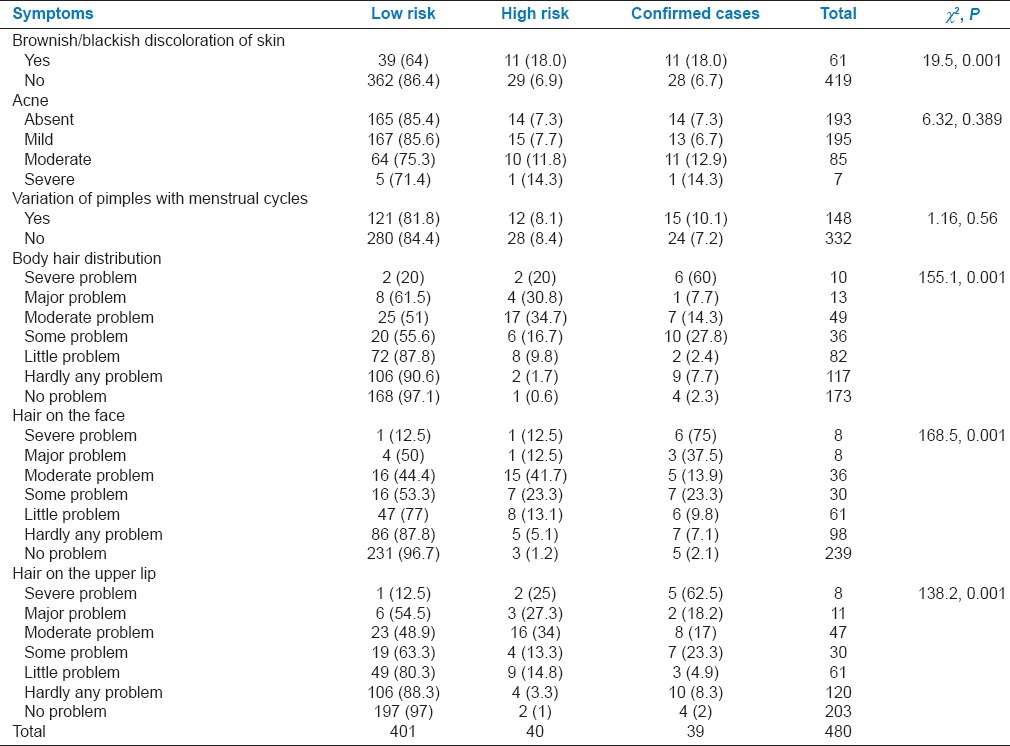
Hyperpigmented patches over the skin were reported by 11 (28.2%) PCOS cases, and it was significantly associated with the presence of PCOS (P < 0.001). Hirsutism was reported by 35(89.7%) PCOS cases, and its presence was significantly associated with PCOS (P < 0.001) [Table 3].
Table 3.
Association between dermatological ailments with level of risk of polycystic ovarian syndrome among participants
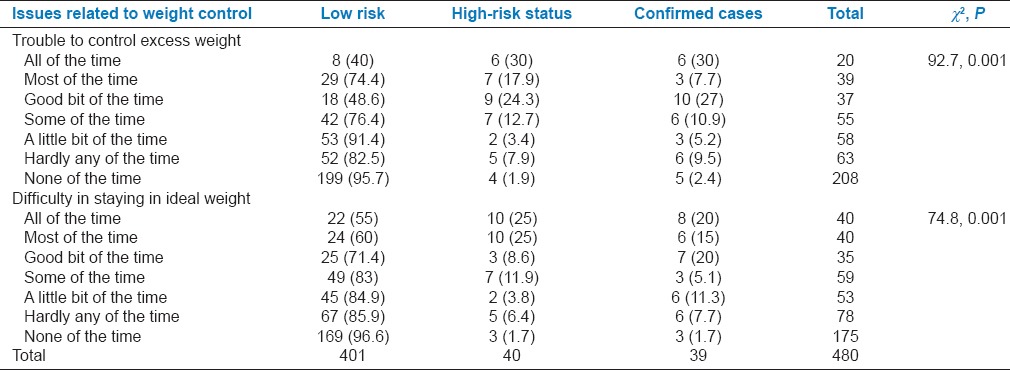
As many as 36 (90%) participants at high risk and 34 (87.2%) PCOS cases had difficulty to control excess weight, whereas 37 (92.5%) at high risk and 36 (92.3%) PCOS cases had difficulty to maintain ideal weight over the previous 6 months. These items were also significantly associated with the presence of PCOS (P < 0.001) [Table 4].
Table 4.
Association between domain related to weight with risk status of polycystic ovarian syndrome among participants
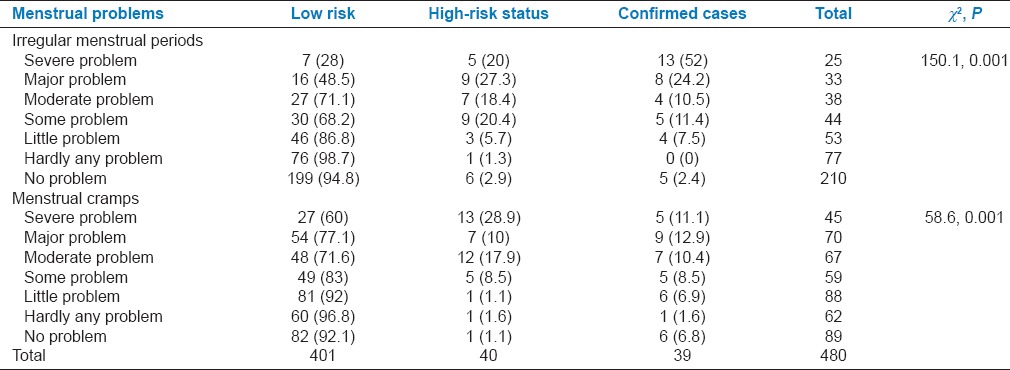
As many as 34 (87.2%) PCOS cases had irregular menstrual periods and 33 (84.6%) had menstrual cramps during the previous 6 months. Presence of these symptoms was significantly associated with the presence of PCOS (P < 0.001) [Table 5].
Table 5.
Association between domain related to menstrual problems with risk status of polycystic ovarian syndrome among participants
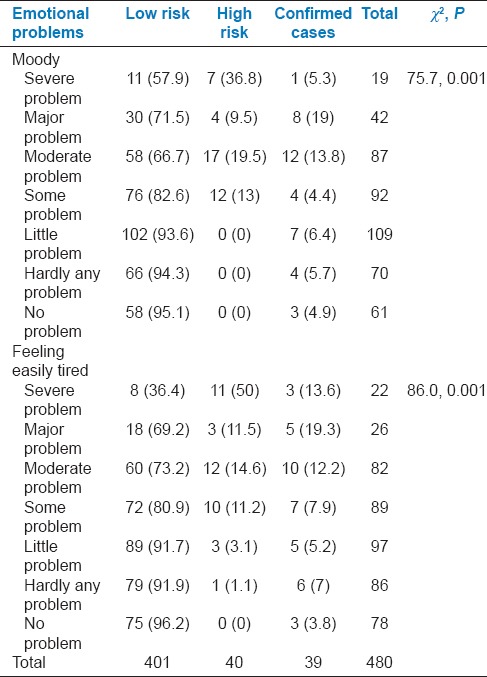
Among PCOS cases, 36 (92.3%) each had emotional problems such as feeling moody or easy fatigability over the previous 2 weeks and presence of these symptoms were significantly associated with the presence of PCOS (P < 0.001). All cases at high risk of PCOS also had emotional problems [Table 6].
Table 6.
Association with domain related to emotional problems with risk status of polycystic ovarian syndrome among participants
DISCUSSION
In this study, 8.1% of university students were confirmed cases of PCOS. In other Indian studies, it was 15% among university students in a study done in Kottayam, Kerala,[22] and 3.7% among university students in Lucknow, India.-[19] Among preuniversity college students, it was reported by 9.13% in a study done in Andhra Pradesh,[15] and 9.8% in a study done in Trivandrum, Kerala.[20] However, the prevalence of PCOS among university students in Korea was only 4.9%.[23] The reason for a greater proportion of PCOS in Indian population could be the greater prevalence of insulin resistance which is central to the pathogenesis of PCOS.[19]
It is important that every such diagnosed cases have to be made fully aware of their condition, and they require support from various groups for improving their quality of life. The support should be in relation to overcoming depression, weight management, and medication therapy.
In this study, the probable number of cases with PCOS among the 441 unconfirmed participants was 9.1% which was lower than that detected in studies done among university students in Lucknow, India,[19] (13.1%) and in Udupi, India[12] (13.6%).
In a study done in Korea among university students, 20% cases with PCOS were obese in comparison to 38.5% cases found to be either overweight or obese in this study.[23] In a study done in Trivandrum, India, among PUC students, 8.6% PCOS cases were overweight and 2.6% were obese.[20]
BMI was significantly higher in students confirmed with PCOS in this study as also observed in the study done in Korea.[23] However, the study done in Trivandrum, India, reported no association.[20] Previous studies have reported that young women with PCOS described excessive weight as the factor that had the greatest impact on their quality of life.[5,24] This concern was expressed by PCOS patients to be even more concerning than other issues such as menstrual problems.[5]
The BMI was also significantly associated with high risk of PCOS among participants in this study. However, no association was seen in another study done in Udupi, India.[12] Previous studies have found that a minimal loss of weight of 5% was sufficient to help in restoring menstrual cycle regularity and ovulation by lowering androgen levels as well as by reducing the insulin resistance. The overall mental well-being was also found to improve.[25,26] These observations support the importance of weight control in improving the clinical condition in PCOS. In this study also significantly greater proportion of PCOS cases and participants at high risk for PCOS reported trouble in controlling excess weight and difficulty in staying at ideal weight. PCOS though an incurable condition is controllable with healthy lifestyle choices. Hence, a lifestyle modification program that promotes healthy diet, exercise, and that which supports behavioral change is the best first line treatment for PCOS.[27,28]
In this study, 34 (87.2%) PCOS cases had irregular menstrual periods as compared to the study done in Lucknow, India, where all the confirmed cases had this problem.[19]
In this study, among PCOS cases, acne was present in 64.1% cases and variation in pimples with menstrual cycle in 38.5% cases. In a study done in Trivandrum, India, among PUC students, 50% of PCOS cases had acne but was not associated with PCOS which was similar to our findings.[20]
In this study, 89.7% participants already diagnosed with PCOS had hirsutism which was much higher than the observations of other studies where it was found in 11.4%[20] and 20%[23] college students. This is another concern in the management of PCOS as hirsute women have been found to have greater emotional problems such as having low self-esteem, feeling moody, worried, and depressed.[5]
Emotional problems were present in 92.3% cases with PCOS and all cases at high risk of PCOS which supports the need for counseling services in the management of this condition.
In this study, family history of PCOS was significantly associated with development of PCOS. Increased frequency of PCOS in first-degree relatives of affected women has been supported by genetic studies. These studies have proposed several candidate genes involved in the development of this heterogeneous syndrome.[29]
CONCLUSION
From the observations in this study, it is clear that PCOS is a common disorder among young women. Therefore, periodic screening is required particularly for those with positive family history of PCOS. This will enable early diagnosis of this condition so as to facilitate prompt referral and management to prevent long-term complications. Lifestyle modification for weight reduction and dietary modification along with counseling services for addressing emotional problems should support other management options. Hence, a multidisciplinary approach involving professionals is required to manage young women with this endocrine disorder.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We authors thank the principals and students of the respective colleges for their cooperation in the conduct of this study. No source of support was acquired for this study.
REFERENCES
- 1.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 2.Emans SJ, Laufer MR, Goldstein DP. Pediatric and Adolescent Gynecology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. Androgen abnormalities in the adolescent girl. [Google Scholar]
- 3.Buggs C, Rosenfield RL. Polycystic ovary syndrome in adolescence. Endocrinol Metab Clin North Am. 2005;34:677–705, x. doi: 10.1016/j.ecl.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecchine R. Scrambled: A journey through polycystic ovarian syndrome. Boston: Fanlight Productions; 2001. [Last accessed on 2014 Apr 03]. Available from: http://www.scrambledthewebsite.com . [Google Scholar]
- 5.McCook JG, Reame NE, Thatcher SS. Health-related quality of life issues in women with polycystic ovary syndrome. J Obstet Gynecol Neonatal Nurs. 2005;34:12–20. doi: 10.1177/0884217504272945. [DOI] [PubMed] [Google Scholar]
- 6.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell K, Antoni MH. Insulin resistance, obesity, inflammation, and depression in polycystic ovary syndrome: Biobehavioral mechanisms and interventions. Fertil Steril. 2010;94:1565–74. doi: 10.1016/j.fertnstert.2010.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matzke A. The Underdiagnosis of Polycystic Ovarian Syndrome in Normal Weight Adolescent Females [Dissertation] Minneapolis: St. Catherine University; 2011. [Google Scholar]
- 9.Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–96. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 10.Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond) 2008;32:1035–41. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- 11.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 12.Shobha K, Devi ES, Prabhu A. An exploratory survey to identify the adolescents with high risk for Polycystic Ovarian Syndrome (PCOS) and to find the effectiveness of an awareness programme among students of selected pre university colleges of Udupi District. IOSR J Nurs Health Sci. 2014;3:66–9. [Google Scholar]
- 13.Ma YM, Li R, Qiao J, Zhang XW, Wang SY, Zhang QF, et al. Characteristics of abnormal menstrual cycle and polycystic ovary syndrome in community and hospital populations. Chin Med J (Engl) 2010;123:2185–9. [PubMed] [Google Scholar]
- 14.Michelmore KF. Polycystic ovary syndrome in adolescence and early adulthood. Hum Fertil (Camb) 2000;3:96–100. doi: 10.1080/1464727002000198771. [DOI] [PubMed] [Google Scholar]
- 15.Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011;24:223–7. doi: 10.1016/j.jpag.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen SD, Brar S, Faris P, Corenblum B. Polycystic ovary syndrome: Validated questionnaire for use in diagnosis. Can Fam Physician. 2007;53:1041–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, et al. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS) J Clin Endocrinol Metab. 1998;83:1976–87. doi: 10.1210/jcem.83.6.4990. [DOI] [PubMed] [Google Scholar]
- 19.Gill H, Tiwari P, Dabadghao P. Prevalence of polycystic ovary syndrome in young women from North India: A Community-based study. Indian J Endocrinol Metab. 2012;16(Suppl 2):S389–92. doi: 10.4103/2230-8210.104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair MK, Nirmala C, Padma K, Sarma PS, Renjit M. Prevalence of Polycystic Ovary Syndrome (PCOS) Among Plus Two Girls with Menstrual Dysfunction. Trivandrum: INCLEN/IndiaCLEN/USAID Infectious Disease Initiative; 2004. [Last cited on 2014 Apr 05]. Available from: http://www.inclentrust.org/inclen/uploadedbyfck/file/complete%20Project/29_FinalPCOS1227(1).pdf2 . [Google Scholar]
- 21.Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibanez L, et al. The diagnosis of Polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83:376–89. doi: 10.1159/000375530. [DOI] [PubMed] [Google Scholar]
- 22.Vijayan CP, Sonia A. A prevalence of Polycysic ovary syndrome among students of a teaching collegiate hospital. Health Sci. 2013;2:1–12. [Google Scholar]
- 23.Byun EK, Kim HJ, Oh JY, Hong YS, Sung YA. The prevalence of Polycystic ovary syndrome in college students from Seoul. J Korean Soc Endocrinol. 2005;20:120–6. [Google Scholar]
- 24.Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: Body mass index as mediator of quality of life. Ambul Pediatr. 2005;5:107–11. doi: 10.1367/A04-130R.1. [DOI] [PubMed] [Google Scholar]
- 25.Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113:1148–59. doi: 10.1111/j.1471-0528.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 26.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13:251–7. doi: 10.1016/s1043-2760(02)00612-4. [DOI] [PubMed] [Google Scholar]
- 27.Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BG, Wong JL, et al. Assessment and management of polycystic ovary syndrome: Summary of an evidence-based guideline. Med J Aust. 2011;195:S65–112. doi: 10.5694/mja11.10915. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Blasco F, Botella-Carretero JI, San Millán JL, Escobar-Morreale HF. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med. 2006;166:2081–6. doi: 10.1001/archinte.166.19.2081. [DOI] [PubMed] [Google Scholar]
- 29.American Association of Clinical Endocrinologists Polycystic Ovary Syndrome Writing Committee. American association of clinical endocrinologists position statement on metabolic and cardiovascular consequences of Polycystic ovary syndrome. Endocr Pract. 2005;11:126–34. doi: 10.4158/EP.11.2.125. [DOI] [PubMed] [Google Scholar]


